Abstract
Improved diagnosis and treatment of traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) are needed for our military and veterans, their families, and society at large. Advances in brain imaging offer important biomarkers of structural, functional, and metabolic information concerning the brain. This article reviews the application of various imaging techniques to the clinical problems of TBI and PTSD. For TBI, we focus on findings and advances in neuroimaging that hold promise for better detection, characterization, and monitoring of objective brain changes in symptomatic patients with combat-related, closed-head brain injuries not readily apparent by standard computed tomography or conventional magnetic resonance imaging techniques.
Keywords: diagnosis, diffusion tensor imaging, fMRI, neuroimaging, OIF/OEF, posttraumatic stress disorder, PTSD, TBI, traumatic brain injury, veterans
TRAUMATIC BRAIN INJURY AND POSTTRAUMATIC STRESS DISORDER: “INVISIBLE WOUNDS”
Improved diagnosis and treatment of traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) are needed for our military and veterans, their families, and society at large. According to a RAND Corporation study based on screening questionnaire data, nearly one out of five Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) servicemembers (300,000) are estimated to experience symptoms of PTSD or depression and more than 320,000 OIF/OEF servicemembers have sustained a TBI [1]. Similarly, 23 percent (907/3,973) of a returning brigade combat team were clinician-identified to have a history of TBI [2].
The majority of cases of TBI in civilian and combat-related settings are categorized as “mild,” a category based primarily on the characteristics of the acute sequelae following the injury. The criteria for the classification of mild can vary, but the Department of Defense/Department of Veterans Affairs March 2009 Clinical Practice Guideline has adopted the following criteria: (1) brief loss of consciousness (30 minutes or less), (2) brief alteration of consciousness (up to 24 hours), (3) posttraumatic amnesia for 0 to 1 days, or (4) Glasgow Coma Score (best score within the first 24 hours) of 13 to 15 (15 = normal), and (5) a normal-appearing brain on computed tomography (CT) scan [344].
In contrast to civilian TBIs due to falls, sports, etc., nearly 70 percent of combated-related TBIs are a result of blast “plus” injuries, i.e., the effects of blast plus another modality [3]. In mild TBI, the underlying pathology is not well understood and the lesion(s) may be subtle, scattered, varied, and, as indicated above, not detected on conventional brain CT studies. Further diagnostic challenges are posed by virtue of the varied and nonspecific postconcussion symptoms (e.g., concentration problems, irritability, headaches) that are also found in PTSD, depression, sleep disorders, or in otherwise healthy persons. However, improving the sensitivity of neuroimaging to subtle brain perturbations and combining these objective measures with careful clinical characterization of patients may facilitate better understanding of the neural bases and treatment of the signs and symptoms of mild TBI.
For combat-related PTSD, the clinical manifestations include not only intrusive recurrent memories and hypervigilance but also nonspecific symptoms, including insomnia, concentration difficulties, irritability, impaired decision-making abilities, and memory problems. Moreover, overlap of symptoms and the comorbidities of PTSD, TBI, depression, and their sequelae (e.g., sleep deprivation, drug or alcohol abuse) make assessment, diagnosis, and management of these patients very difficult. As in the case of TBI, objective and specific biological or anatomical markers would be invaluable in the diagnosis of PTSD. Neuroimaging assays could also aid in the monitoring and evaluation of treatment approaches. In addition, these data may also provide information on brain vulnerability to subsequent injury and help establish guidelines for safe return to duty.
Brain imaging offers an important class of biomarkers because of its ability to obtain structural, functional, and metabolic information concerning the brain with various X-ray CT, magnetic resonance (MR) imaging (MRI), and positron emission tomography (PET) scanning techniques. CT remains an extremely valuable and the most commonly utilized imaging modality. It is very sensitive to fractures of the skull and facial bones and can rapidly assess the possible need for urgent neurosurgical interventions, such as evacuation of hematomas [4]. MRI has exquisite soft-tissue contrast and also can measure function and metabolism. Various PET scanning techniques can measure brain function and amyloid deposition.
This article reviews the application of various imaging techniques to the clinical problems of TBI and PTSD. For TBI, we focus on findings and advances in neuroimaging that hold promise for better detection, characterization, and monitoring of objective brain changes in symptomatic patients with combat-related, closed-head brain injuries not readily apparent by standard CT or conventional MRI techniques.
OVERVIEW: NEUROIMAGING IN TRAUMATIC BRAIN INJURY
Advanced neuroimaging techniques are finding increased use in the study of TBI. Whereas CT and standard MRI structural images can readily demonstrate large focal contusions or bleeds, diffuse axonal injury may be detected indirectly by brain volume loss (volumetric analysis) or diffusion tensor imaging (DTI). DTI studies have shown reductions in fractional anisotropy (FA) at sites of traumatic axonal shearing injury, corresponding to a loss of microstructural fiber integrity, resulting in the reduced directionality of microscopic water motion [5–6]. More recently, an increasing number of DTI studies in TBI have been emerging [5–33], a few of which also indicate correlations between DTI findings and neurocognition [10,32,34]. Several studies have confirmed the potential of single-voxel proton MR spectroscopy (1H-MRS or MRS) for the detection of neuronal injury following TBI [35–44]. One common finding includes altered metabolite concentrations in regions that appear normal on structural MR images, suggesting widespread and diffuse tissue damage. In particular, studies using single-voxel techniques have shown a significant correlation between unfavorable clinical outcome and reduced N-acetylaspartate (NAA), a marker of neuronal integrity [40,45–47], and increased choline (Cho) [45,47–48]. Proton MR spectroscopic imaging (MRSI) is a technique similar to MRS, except instead of acquiring data from a single region or voxel, spectroscopic information is collected from multiple voxels during the same imaging acquisition. MRSI, like MRS, has been found useful in the detection of metabolic abnormalities that predict outcome [36]. In addition, a few investigators have studied relationships between metabolic and neurocognitive effects with the use of MRS [49–50] and MRSI [42]. Susceptibility-weighted imaging (SWI) has been applied on a clinical 1.5 T MRI scanner in several studies of pediatric TBI [41,51–53]. These studies demonstrated that SWI allows detection of hemorrhagic lesions in children with TBI with significantly higher sensitivity than conventional gradient-echo MRI [52]. The number and volume of hemorrhagic lesions correlated with the Glasgow Coma Scale score [54] as well as with other clinical measures of TBI severity and with outcome at 6 to 12 months postinjury [53]. Significant differences were detected between children with normal outcome or mild disability and children with moderate or severe disability when regional injury was compared with clinical variables [53]. In addition, negative correlations between lesion number and volume with measures of neuropsychological functioning at 1 to 4 years postinjury were demonstrated [41]. Studies using functional MRI (fMRI) in patients with TBI show abnormal patterns of brain activation in patients compared with healthy control subjects [55–73]. While dynamic contrast-enhanced perfusion-weighted MRI (PW-MRI) has shown that regions of both normal-appearing and contused brain may have an abnormal regional cerebral blood volume (rCBV) and that alterations in rCBV may play a role in determining the clinical outcome of patients [74], to our knowledge, no studies using arterial spin labeling PW-MRI in TBI have been published to date. PET studies in TBI demonstrate that early reductions in cerebral perfusion can result in cerebral ischemia that is associated with poor outcome [75–82]. Finally, a potential new avenue of research in TBI involves imaging amyloid plaque depositions in TBI, particularly using Pittsburgh Compound B (PIB). Currently, no published studies have employed imaging with PIB in combat-related TBI.
OVERVIEW: NEUROIMAGING IN POSTTRAUMATIC STRESS DISORDER
Brain imaging studies in PTSD have implicated a circuit of brain regions, including the hippocampus, prefrontal cortex (including anterior cingulate), and amygdala, in the symptoms of PTSD.
Numerous studies used structural MRI to show smaller volume of the hippocampus and/or used MRS to show reduced NAA in the hippocampus, a brain area that mediates verbal declarative memory [83–100]. However, some studies of adults did not show smaller hippocampal volume to be specific to PTSD [101–103] and studies in children have not found smaller hippocampal volume to be associated with PTSD [104–106]. Results are mixed regarding whether new onset or recent PTSD is associated with smaller hippocampal volumes [107–109]. Two meta-analyses pooled data from all the published studies and found smaller hippocampal volume for both the left and right sides equally in adult men and women with chronic PTSD and no change in children [110–111]. Interestingly, paroxetine, a selective serotonin reuptake inhibitor, appears to effectively improve short-term memory deficits and possibly reverse hippocampal atrophy [112]. These data suggest that PTSD is associated with deficits in verbal declarative memory and with smaller hippocampal volume.
Multiple studies have shown smaller volume of the anterior cingulate in PTSD [113–118]. A recent twin study of combat-related PTSD suggests that atrophic changes in the pregenual anterior cingulate cortex (ACC) and both insula may represent (or at least be contributed to by) an acquired stress-induced loss rather than a preexisting condition [115]. In contrast, the authors concluded that the reduced hippocampal volume found in these subjects represented a pretrauma vulnerability factor and was not related to stress-induced losses [90].
Regarding functional neuroimaging data of PTSD, exposure to traumatic reminders in the form of traumatic slides and/or sounds or traumatic scripts is associated with increased PTSD symptoms and decreased blood flow and/ or failure of activation in the medial prefrontal cortex/anterior cingulate, including Brodmann (area 25) or the subcallosal gyrus (areas 32 and 24), as measured with PET or fMRI [93,119–126]. Other findings in studies of traumatic-reminder exposure include decreased function in the hippocampus [119], visual association cortex [119,125], parietal cortex, and inferior frontal gyrus [119,124–125,127] and increased function in the amygdala [127–128], posterior cingulate [119,121–122,125], and parahippocampal gyrus [119,121,123]. Several studies have shown that PTSD patients have deficits in hippocampal activation while performing a verbal declarative memory task [88,93] or a virtual water-maze task [129]. Other studies found increased posterior cingulate and parahippocampal gyrus activation and decreased medial prefrontal and dorsolateral prefrontal activation during an emotional Stroop paradigm [130] and increased amygdala function with exposure to masked fearful faces [131] or during classical fear conditioning, with decreased medial prefrontal function with extinction in PTSD [132]. Retrieval of words with emotional valence [133] or emotional Stroop tasks [134] were associated with decreased medial prefrontal function. The findings point to a network of related regions mediating symptoms of PTSD, including the medial prefrontal cortex, anterior cingulate, hippocampus, and amygdala [135].
Neuroreceptor studies are consistent with prefrontal dysfunction in PTSD. Bremner et al. used single photon emission CT (SPECT) and the benzodiazepine receptor ligand [123I] iomazenil and found decreased prefrontal cortical binding in Vietnam combat veterans with PTSD [136]. Another study by Fujita et al. in First Gulf War veterans with PTSD showed no difference in binding with SPECT [123I] iomazenil from controls [137], although this study did show a significant negative correlation between binding in the right superior temporal gyrus and severity of childhood trauma in PTSD patients. In this study, the subjects also had less severe PTSD than those included in the study by Bremner and colleagues.
In summary, these studies are consistent with dysfunction of the prefrontal cortex, hippocampus, and amygdala in PTSD.
IMAGING MODALITIES
The following sections will discuss applications of advanced neuroimaging modalities to TBI and PTSD. The primary focus will be on advanced MRI techniques (Table).
Table.
Magnetic resonance imaging (MRI) neuroimaging techniques.
| Technique | What It Measures | Applications |
|---|---|---|
| BOLD fMRI | Indirect measure of blood flow, BOLD signal changes originate in venules. BOLD fMRI takes advantage of susceptibility differences between oxygenated and deoxygenated blood. | Evaluate regional brain activity related to particular cognitive tasks or sensory/motor stimulation. Evaluate brain networks related to cognitive states. Evaluate brain “resting state” or “default” networks. |
| PW-MRI | Direct measure of blood flow, allows quantification of blood perfusion. | Assess brain perfusion or resting cerebral blood flow. Evaluate brain function in manner similar to fMRI. |
| DTI | Indirectly measures diffusion of water molecules. Mean diffusion, diffusion direction, and anisotropy white matter tracts. | Use diffusion anisotropy measures as marker of disease. Improved visualization of edema. Evaluate structural “connectivity” between brain regions. |
| MRS | Proton (1H) MRI spectra typically contain signals from the metabolites N-acetylaspartate, creatine, choline, glutamate/glutamine, and myo-inositol. | Evaluate changes in brain metabolites related to myelination, neuronal density, edema, etc. |
| SWI | MRI sequences that are especially sensitive to changes in magnetic susceptibility, in particular blood. | Improved detection of hemorrhages. Improved imaging of blood vessels. |
BOLD = blood oxygen level dependent, DTI = diffusion tensor imaging, fMRI = functional MRI, MRS = magnetic resonance spectroscopy, PW-MRI = perfusion weighted MRI, SWI = susceptibility-weighted imaging.
Diffusion Magnetic Resonance Imaging
The ability to visualize anatomical connections between different parts of the brain, noninvasively and on an individual basis, has opened a new era in the field of functional neuroimaging. This major breakthrough for neuroscience and related clinical fields has developed over the past 10 years through the advance of “diffusion magnetic resonance imaging” or D-MRI. D-MRI produces MRI quantitative maps of microscopic, natural displacements of water molecules that occur in brain tissues as part of the physical diffusion process. Water molecules are thus used as a probe that can reveal microscopic details about tissue architecture, either normal or diseased.
Concept of Molecular Diffusion
Molecular diffusion refers to the random translational motion of molecules (also called Brownian motion) that results from the thermal energy carried by these molecules. Molecules travel randomly in space over a distance that is statistically well described by a “diffusion coefficient.” This coefficient depends only on the size (mass) of the molecules, the temperature, and the nature (viscosity) of the medium.
D-MRI is, thus, deeply rooted in the concept that during their diffusion-driven displacements, molecules probe tissue structure at a microscopic scale well beyond the usual millimeter image resolution. During typical diffusion times of about 50 to 100 ms, water molecules move in brain tissues on average over distances around 1 to 15 m, bouncing, crossing, or interacting with many tissue components, such as cell membranes, fibers, or macromolecules. Because of the tortuous movement of water molecules around those obstacles, the actual diffusion distance is reduced compared with free water. Hence, the noninvasive observation of the water diffusion-driven displacement distributions in vivo provides unique clues to the fine structural features and geometric organization of neural tissues and to changes in those features with physiological or pathological states.
Imaging Diffusion with Magnetic Resonance Imaging: Principles
While early water diffusion measurements were made in biological tissues with the use of Nuclear Magnetic Resonance in the 1960s and 1970s, it was not until the mid-1980s that the basic principles of D-MRI were laid out [138–140]; see, for instance, Le Bihan [141] for a review. MRI signals can be made sensitive to diffusion through the use of a pair of sharp magnetic field gradient pulses, the duration and separation of which can be adjusted. The result is a signal (echo) attenuation that is precisely and quantitatively linked to the amplitude of the molecular displacement distribution: fast diffusion results in a large distribution and a large signal attenuation, while slow diffusion results in a small distribution and a small signal attenuation. Of course, the effect also depends on the intensity of the magnetic field gradient pulses.
In practice, any MRI imaging technique can be sensitized to diffusion by the insertion of the adequate magnetic field gradient pulses [142]. By acquiring data with various gradient pulse amplitudes, one gets images with different degrees of diffusion sensitivity (Figure 1). Contrast in these images depends not only on diffusion but also on other MRI parameters, such as the water relaxation times. Hence, these images are often numerically combined to determine, with use of a global diffusion model, an estimate of the diffusion coefficient in each image location. The resulting images are maps of the diffusion process and can be visualized with a quantitative scale.
Figure 1.
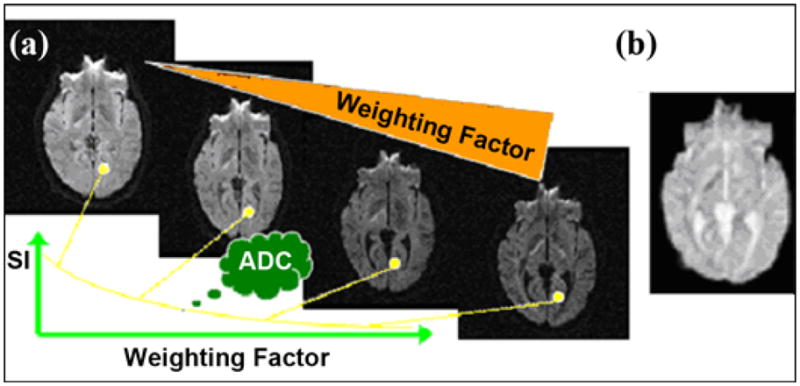
Diffusion-weighting. In practice, different degrees of diffusion-weighted images can be obtained by varying the weighting factor, which is carried out by varying time and strength of gradient pulses (represented by orange triangle). (a) The larger the weighting factor, the more the signal intensity (SI) becomes attenuated in image. This attenuation, though, is modulated by the diffusion coefficient: signal in structures with fast diffusion (e.g., water-filled ventricular cavities) decays very fast with the weighting factor, while signal in tissues with low diffusion (e.g., gray and white matter) decreases more slowly. By fitting signal decay as a function of weighting factor, one obtains the Apparent Diffusion Coefficient (ADC) for each elementary volume (voxel) of image. (b) Calculated diffusion images (ADC maps), depending solely on diffusion coefficient, can then be generated and displayed using gray (or color) scale: high diffusion, as in ventricular cavities, appears bright, while low diffusion appears dark.
Because the overall signal observed in a “diffusion” MRI image voxel, at a millimetric resolution, results from the statistical integration of all the microscopic displacement distributions of the water molecules present in this voxel, Le Bihan et al. suggested portraying the complex diffusion processes that occur in a biological tissue on a voxel scale by using a global statistical parameter, the Apparent Diffusion Coefficient (ADC) [143]. The ADC concept has been largely used since then in the literature. The ADC now depends not only on the actual diffusion coefficients of the water molecular populations in the voxel but also on experimental technical parameters, such as the voxel size and the diffusion time.
Although the first diffusion images of the brain were obtained in the mid-1980s in normal subjects and in patients [143], D-MRI did not really take off until the mid-1990s. Initially, the specifications of the clinical MRI scanners made obtaining reliable diffusion images difficult because acquisition times were long (10 to 20 minutes) and the large gradient pulses required for diffusion also made the images very sensitive to macroscopic motion artifacts, such as those induced by head motion, breathing, or even cardiac-related brain pulsation [144]. Therefore, although D-MRI was shown to be potentially useful in the clinic, demonstrative clinical studies started only later, when better MRI scanners equipped with echo-planar imaging (EPI) became available. Exploiting gradient hardware EPI makes it possible to collect a whole-brain image in a single “shot” lasting a few tens of milliseconds and images of the whole brain in less than a second, virtually freezing macroscopic motion.
Diffusion Tensor Magnetic Resonance Imaging
Diffusion is truly a three-dimensional process; therefore, water molecular mobility in tissues is not necessarily the same in all directions. This diffusion anisotropy may result from obstacles that limit molecular movement in some directions. It was not until the advent of D-MRI that anisotropy was detected for the first time in vivo, at the end of the 1980s, in spinal cord and brain white matter [145–146]. Diffusion anisotropy in white matter grossly originates from its specific organization in bundles of more or less myelinated axonal fibers running in parallel: diffusion in the direction of the fibers (whatever the species or the fiber type) is about three to six times faster than in the perpendicular direction. However, the relative contributions of the intra-axonal and extracellular spaces, as well as the presence of the myelin sheath, to the ADC and the exact mechanism for the anisotropy are still not completely understood and remain the object of active research (see, for instance, Beaulieu [147] for a review). It quickly became apparent, however, that this anisotropy effect could be exploited to map out the orientation in space of the white matter tracts in the brain, assuming that the direction of the fastest diffusion would indicate the overall orientation of the fibers [148]. The work on diffusion anisotropy really took off with the introduction into the field of D-MRI of the more rigorous formalism of the diffusion tensor by Basser et al. [149–150]. With DTI, diffusion is no longer described by a single diffusion coefficient but by an array of nine coefficients that fully characterize how diffusion in space varies according to direction (see, for instance, Le Bihan and Van Zijl [151] for a review on DTI). Hence, diffusion anisotropy effects can be fully extracted and exploited, providing even more exquisite details on tissue microstructure.
DTI data are often summarized in three ways to provide information on tissue microstructure and architecture for each voxel [141,152]: (1) the mean diffusivity or ADC characterizes the overall mean-squared displacement of molecules and the overall presence of obstacles to diffusion, (2) the degree of anisotropy describes how much molecular displacements vary in space and is related to the presence and coherence of oriented structures, and (3) the main direction of diffusivities is linked to the orientation in space of the structures. For instance, in stroke, the average diffusion and the diffusion anisotropy in white matter had different time courses, potentially enhancing the use of D-MRI for the accurate diagnosis and prognosis of stroke [153]. The diffusion along the main direction of diffusion is often termed axial diffusion, whereas radial diffusion is the diffusion along directions perpendicular to the main direction. Early studies with mice have indicated that changes in radial diffusion may be more specific to myelination than are changes in axial diffusion or other measures of anisotropy [154].
Diffusion Anisotropy in White Matter: Toward Brain Connectivity
Studies of neuronal connectivity are important in order to interpret fMRI data and establish the networks underlying cognitive processes. Basic DTI provides a means to determine the overall orientation of white matter bundles in each voxel, assuming that only one direction is present or predominant in each voxel and that diffusivity is the highest along this direction. Three-dimensional vector field maps representing fiber orientation in each voxel can then be obtained back from the image data through the diagonalization (a mathematical operation that provides orthogonal directions coinciding with the main diffusion directions) of the diffusion tensor determined in each voxel. A second step after this “inverse problem” is solved consists in “connecting” subsequent voxels on the basis of their respective fiber orientation to infer some continuity in the fibers (Figure 2). Several algorithms have been proposed (see Mori [155] and van Zijl and Jones [156] for reviews). Line propagation algorithms reconstruct tracts from voxel to voxel from a seed point [157–158]. Another approach is based on regional energy minimization (minimal bending) to select the most likely trajectory among several possible [159]. Finally, a promising approach is probabilistic tracking using Bayesian [160] or bootstrapping [161] methodologies. In any case, one has to keep in mind that at this stage only white matter bundles made of somewhat large numbers of axons are visible (and not intracortical connections). The application of tractography to PTSD and TBI studies is an area for future research. While tractography yields very nice pictures, how this technology will be best applied to research is still unclear. A possibility would be the use of probabilistic tractography to determine whether a reduction or break occurs in the anatomical connectivity between two regions, or nodes, of a functional network. These nodes are normally chosen either a priori or empirically from fMRI results. This is an area in which the combination of DTI and fMRI could be particularly useful in both PTSD and TBI [162–163].
Figure 2.
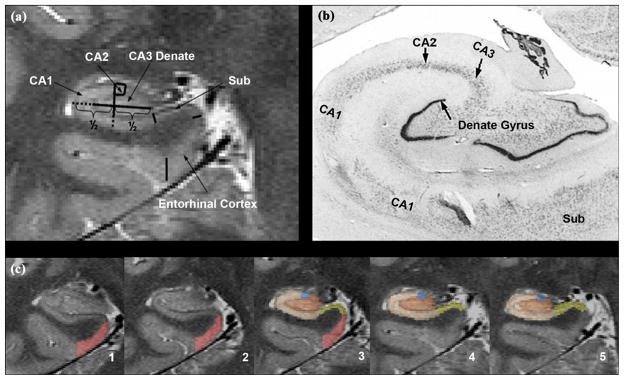
Imaging the hippocampal subfields. (a) High-resolution magnetic resolution imaging. (b) Histological section. (c) Manual marking. CA = cornu ammonis, Sub = subiculum.
Clinical Applications
In white matter, any change in tissue orientation patterns inside the MRI voxel would probably result in a change in the degree of anisotropy. A growing literature body supports this assumption: many clinical studies of patients with white matter diseases have shown the exquisite sensitivity of DTI to detect abnormalities at an early stage or to characterize them in terms of white matter fiber integrity (e.g., multiple sclerosis [164]). Further DTI analysis using other indexes, such as the trace of the diffusion tensor, which reflects overall water content, and anisotropy indexes, which point toward myelin fiber integrity, can be useful. Clinical examples include multiple sclerosis [165–168], leukoencephalopathies [169–170], Wallerian degeneration, HIV-1 (Human immunodeficiency virus 1) infection [171], Alzheimer disease (AD) [172–173], or CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) [174] (see Horsfield and Jones [175] for a review).
However, D-MRI could also unravel more subtle functional disorders that do not necessarily translate into macroanatomical anomalies. For instance, anisotropy measurements may highlight subtle anomalies in the microorganization of white matter tracts otherwise not visible with plain anatomical MRI. The potential is enormous for patients with functional symptoms linked to disconnectivity, for instance, in patients with psychiatric disorders (see Lim and Helpern [176] for a review), TBI, or potentially PTSD.
Hence, water diffusion patterns within and between white matter tissue are highly sensitive to microstructural abnormalities/pathologies. However, it is important to emphasize that DTI abnormalities are not specific and may reflect a host of conditions, including demyelination, axonal pathology/loss, gliosis, inflammation, or edema.
Diffusion Tensor Imaging in Mild Traumatic Brain Injury
Several studies have investigated DTI abnormalities in patients with mild TBI [5,20–22,31,33,177–178]. Arfanakis et al. studied five patients within 24 hours of injury, and two of these patients were also studied 1 month later [5]. Five white matter regions of interest (ROIs) were analyzed bilaterally in patients, and comparisons between hemispheres as well as with a control group were performed. Some patients’ ROIs had reduced FA values at the anterior corpus callosum and anterior internal capsule (with normal conventional MRI) compared with controls. Further, two subjects had “normalized” FA values in some ROIs 1 month later. However, no clinical correlative data were reported.
Bazarian et al. studied six patients with mild TBI and six orthopedic controls within 72 hours of injury by using both a whole-brain and an ROI approach [177]. In the whole-brain analysis, the first percentile (histogram) showed significantly lower trace values (or ADC) in mild TBI patients. Further, these trace values correlated with symptoms consistent with postconcussion syndrome (PCS) in patients with mild TBI, although the symptoms were not significantly greater than those in the control group. Except for impulse control, psychometric tests (verbal and visual memory, visual motor speed, reaction time) did not significantly differ between the mild TBI group and the controls. However, ROIs showed mild TBI subjects to have significantly lower mean trace in the left anterior internal capsule and higher maximum ROI-specific median FA values in the posterior corpus callosum. These FA values correlated with the 72-hour PCS score and two neurobehavioral tests (visual motor speed and impulse control). The authors speculated that the data represented axonal swelling.
Wilde et al. studied 10 adolescents with mild TBI within 1 week of MRI scanning [178]. They calculated average FA, ADC, and radial diffusivity within the corpus callosum. When compared with that of 10 healthy, age-matched control subjects, the FA for the mild TBI subjects was significantly increased while the ADC and radial diffusivity were significantly decreased. In addition, the FA values correlated with postconcussion symptoms and emotional distress. The authors argued, similar to Bazarian et al., that the increased FA and decreased ADC were likely due to edema that occurs during the acute stage of TBI.
Miles et al. also studied adult patients with mild TBI in the acute stage [33]. These authors compared DTI data from nondisabled control subjects with that of 17 mild TBI patients who were on average 4 days postinjury. They calculated FA and ADC summary values by averaging FA and ADC for voxels over multiple ROIs: centrum semiovale, the genu and splenium of the corpus callosum, and the posterior limb of the internal capsule. This group found significantly higher ADC and lower FA values for the mild TBI group.
More studies are necessary to reconcile the findings of Miles et al. and Arfanakis et al. to those of Wilde et al. and Bazarian et al. Time since injury, age (adolescent vs adult patient), ROI selection, and other factors may influence these discrepancies in the FA/ADC changes in acute mild TBI.
While the previous studies mentioned here studied patients with mild TBI in the acute stage, Rutgers et al. divided 24 mild TBI subjects into two groups: 12 subjects less than 3 months postinjury and 12 subjects more than 3 months postinjury [22]. The authors calculated the average FA and ADC within three regions of the corpus callosum: genu, body, and splenium. When compared with 10 control subjects, mild TBI subjects who were less than 3 months from injury showed significantly increased ADC and decreased FA within the genu ROI. However, the mild TBI subjects imaged more than 3 months after their injury did not show any significant differences in FA or ADC compared with the control group.
In studies focused more on chronic subjects, Kraus et al. studied 55 patients with chronic TBI (more than 6 months postinjury), 20 of whom had mild TBI (average 92 months postinjury) [31]. The authors acquired DTI data and evaluated 3 estimates of anisotropy (FA, axial and radial diffusivity) from 13 ROIs and also estimated total white matter load (total number of regions with decreased FA). A battery of more than 20 neuropsychological tests was also administered. For the mild TBI group, performance was impaired compared with controls in only the Conner’s continuous performance test and did not differ from the controls in the domains of attention, memory, or overall executive function. Decreased FA was found in the corticospinal tract, sagittal stratum, and superior longitudinal fasciculus for the mild TBI group (with no clinical correlate).
Lipton et al. studied DTI data from 17 mild TBI patients with cognitive impairment who were at least 8 months postinjury [20]. The authors used voxelwise morphometry analyses and whole-brain histograms to compare FA and ADC between the mild TBI subjects and 10 healthy control subjects. The histograms showed an overall downward shift in FA for the mild TBI patients, and the voxel-wise analyses revealed significantly reduced FA for the mild TBI patients in the corpus callosum and internal capsule (bilaterally). The areas showing decreased FA also showed significantly increased ADC.
Chappell and colleagues reported widespread FA reductions and ADC increases in professional boxers, mainly in white matter, despite negative conventional MRI scans [179]. Abnormalities were seen in the internal capsule, medial temporal lobes, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and midbrain and are interpreted to represent injury from chronic blows to the head. No neuropsychometric testing or report of “major trauma to the head” (undefined) was described.
A recent combined imaging-neuropsychometric study of patients with chronic mild TBI performed jointly between Weill Cornell Medical College and the University of California, San Francisco (UCSF) examined the spatial extent of microstructural white matter injury and its relationship with global cognitive processing speed [9]. All subjects had a Modified Glasgow Coma Scale score of 13 to 15 at the time of original assessment postinjury, a history of loss of consciousness shorter than 30 minutes, and posttraumatic amnesia. All had at least one postconcussion symptom persisting at least 1 month (range 1–65 months) at the time of imaging and cognitive assessment for the study. Subjects were excluded if they had a history of prior TBI, drug/alcohol abuse, or other preexisting neurological or psychiatric conditions. A white matter tract in a TBI patient was considered “damaged” if DTI demonstrated an FA value more than 2.5 standard deviations below the mean FA of that tract in a group of normal volunteers. The measure of cognitive processing speed was reaction time (RT) in the Attention Network Task (ANT) [180], which involves pressing a button to indicate the direction of an arrow flashed on a computer monitor. A robust and statistically significant correlation was found between increasing number of white matter tracts with microstructural injury and poorer RT (r = 0.49, p = 0.012). In contradistinction, the number of traumatic microhemorrhages detected by T2*-weighted gradient echo imaging at 3 T did not correlate with RT (r = –0.08; p = 0.701). These results demonstrate that, in chronic mild TBI, increasing spatial extent of regional white matter injury on DTI is associated with slower cognitive processing speed, whereas the number of focal hemorrhagic shearing lesions on conventional 3 T MRI is not.
The two most frequently damaged white matter tracts in this cohort of mild TBI patient were the anterior corona radiata (ACR) and the uncinate fasciculus (UF). This finding was not surprising, since the two most common cognitive symptoms in PCS, in addition to slowed overall processing speed, are impairments in attention and memory [181]. The ACR contains fibers that connect the anterior cingulate with the prefrontal cortex and therefore plays a critical role in attentional processes. The UF connects temporal lobe structures with prefrontal cortex and is vital to working memory.
The relationship of these two tracts to attention and memory was examined for 43 chronic mild TBI patients in a follow-up 3 T DTI study performed jointly at Cornell and UCSF [34]. Attentional performance was gauged with the conflict measure of the ANT [180], which measures the difference in RT between congruent and incongruent trials requiring the subject to determine the direction of an arrow flashed on a computer monitor in the presence of flanking arrows. Verbal memory was assessed with the long-delay free recall (LDFR) subtest of the California Verbal Learning Test, 2nd Edition, which requires the subject to recall a list of 16 words after a delay of 20 minutes after presentation. FA of the UF in both hemispheres correlated significantly with memory performance on the LDFR in mild TBI subjects. The bilateral average ACR FA correlated significantly with attentional control, as measured by the conflict score on the ANT. A closer examination of the contributions of each hemisphere showed that the left ACR FA was the primary contributor to this relationship. These results show that in mild TBI, lower FA of the UF is related to poorer verbal memory performance and lower FA of the ACR is related to poorer attentional control. These findings form a double dissociation, because FA of the UF did not correlate with attentional control, nor did FA of the ACR correlate with verbal memory. These results show that DTI is sensitive to microstructural white matter injury in chronic mild TBI that correlates with functional disability. The spatial extent of axonal injury is associated with impairments in global cognitive processing, whereas damage to specific white matter tracts can account for deficits in specific cognitive domains, such as memory and attention.
Larger-scale longitudinal investigations are needed to determine whether DTI in the acute phase of TBI can predict long-term functional outcomes, which would represent a first step toward validation of this methodology as a biomarker for TBI for use in applications such as assessment of neuroplasticity during recovery and monitoring of the efficacy of therapeutic interventions and rehabilitation.
Early studies support the notion that DTI combined with behavioral assessments may indeed provide useful prognostic information. Sidaros et al. reported in a longitudinal study of 30 patients with severe TBI studied acutely and after 1 year (n = 23) that FA in the cerebral peduncle correlated with Glasgow Outcome Scale scores at 1 year (r = 0.60, p < 0.001). Moreover, favorable dichotomized outcomes at 1 year were accurately predicted when FA was used in combination with clinical evaluation at the time of the first scan [27] (but see Bendlin et al. [19]).
Hence, it is important to underscore that the underlying processes mediating DTI disturbances may vary in the acute and chronic states, and prognostic information might be gleaned from these data.
Diffusion Tensor Imaging in Posttraumatic Stress Disorder
In recent years, DTI has shown promise in PTSD applications. Two studies used morphometry to compare FA values between PTSD and healthy control subjects on a voxelwise basis [182–183]. Abe et al. compared 25 subjects who were victims of the Tokyo subway sarin attack, 9 of whom were diagnosed with PTSD [182]. These authors found increased FA in the left anterior cingulum with the morphometry analysis and followed this analysis with a post hoc ROI analysis of the FA values in the anterior cingulum. The ROI analysis confirmed the significant increase in FA, providing support that the increase in FA was not an artifact created by misalignment or excessive smoothing in the morphometry procedures. Kim et al. compared 20 survivors of a subway fire who developed PTSD with 20 healthy control subjects [183]. These authors reported a decrease in FA in the left anterior cingulate of the PTSD subjects. Further, a correlation analysis within the PTSD subjects revealed that both lifetime and current-experience scores of the Clinician-Administered PTSD Scale (CAPS) were negatively correlated with FA values in the anterior cingulate white matter.
Jackowski et al. used DTI to investigate possible changes in myelination or white matter coherence in the corpus callosum in maltreated children with PTSD as compared with healthy children [184]. These authors used an ROI analysis and divided the corpus callosum into seven regions: rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium. The ROI analysis revealed significant reductions in FA within the anterior and posterior midbody regions of the maltreated PTSD group. However, since the control group was comprised of healthy children as opposed to maltreated children without a PTSD diagnosis, the differences could be attributed to either PTSD or maltreatment. These early DTI studies show promise for the further characterization of PTSD-related brain abnormalities.
BRAIN VOLUMETRICS
By differentiating tissues on their MRI signal intensities, compartment segmentation algorithms in combination with either voxel-based measures or ROI analyses can indirectly measure local volume loss.
Detection and quantification of loss of both white and gray matter have been demonstrated in TBI through MRI volumetric analyses [19,185–191]. Both whole-brain volume and regional volume decreases have been demonstrated and appear to correlate with clinical injury severity (e.g., Levine et al. [185]). However, longitudinal changes may not necessarily correlate with behavioral/cognitive measures [19]. Advances in volumetric methodology in combination with other modalities will, nonetheless, likely provide new insights into structural-functional relations in both TBI and PTSD (see below).
Many studies have investigated volumetric differences between PTSD and control subjects in whole-brain [192–193], amygdala [194], corpus callosum [192,195], insula [115], anterior cingulate [113–118], and hippocampal [87,92,118,133,194] analyses.
Volumetric Analyses of Hippocampus
The fact that the hippocampus is involved in learning and memory—processes requiring a high degree of neuronal plasticity—and is capable of life-long neurogenesis, renders it particularly vulnerable to all types of insults. As a consequence, hippocampal atrophy is found in different diseases such as AD, epilepsy, schizophrenia, and hypothyroidism, as well as in PTSD and chronic TBI, even in cases in which the primary impact site was remote from the hippocampus. The hippocampus, however, is not a homogeneous structure but consists of several histologically and functionally distinct but tightly interconnected subfields: the subiculum with the subdivisions presubiculum, parasubiculum, and subiculum proper; the four cornu ammonis sectors (CA1–4); and the dentate gyrus (DG) [196]. Animal studies have shown that different disease processes affect these subfields differently; e.g., AD is associated with a prominent neuronal cell loss in CA1, whereas temporal lobe epilepsy is typically characterized by cell loss in the DG. Evidence also exists that TBI and PTSD affect certain subfields more than others. Acute stress is associated with activation of the sympathetic-adrenomedullary system and the hypothalamo-pituitary-adrenal axis, resulting in increased levels of catecholamines and adrenal steroids that are restored to baseline levels by a negative feedback mechanism once the stressor has been removed. Evidence exists that this feedback is pathologically enhanced in PTSD, which results in chronically lowered basal cortisol levels but increased sensitivity of glucocorticoid receptors in target tissues [197]; one of those is the hippocampus. Adrenal steroids play a crucial role in the hippocampus, where they modulate short-term functions (excitability, long-term potentiation, and depression) as well as long-term, delayed effects (neuronal plasticity, neurogenesis) [198–199].
Animal models of chronic stress have shown that adrenal steroids can adversely influence basal synaptic excitability and neuronal plasticity in CA1, cause reversible dendritic atrophy in CA3, and impair neurogenesis in DG. Interestingly, TBI can lead to a similar pattern of neuronal dysfunction/damage in the hippocampus as PTSD. Animal studies have shown that TBI is associated with hippocampal damage in CA3, DG, and, to a lesser degree, CA1 [200] and that although the mechanisms leading to hippocampal damage in TBI are complex, it is partially mediated by posttraumatically increased adrenal steroids and altered adrenocorticosteroid receptor properties in those subfields [201–202]. A review of the broader distribution of injuries in a well-studied animal model of TBI may be found in Thompson et al. [203].
In accordance with the findings in animal studies, MR studies in chronic TBI have reported smaller total hippocampal volumes that were inversely associated with memory problems [204–206], although one study found that the memory problems were related more to diffuse brain injury than to hippocampal injury [207]. Similar findings have been reported in PTSD, although not consistently so [208]. Several reasons exist for this inconsistency in PTSD, e.g., presence of comorbidity affecting hippocampal volume, different severity of PTSD, and time since traumatic event. However, measurements of total hippocampal volume may also not be sensitive enough to detect subtle atrophic changes restricted to a relatively circumscribed region of the hippocampus (CA3 and DG) and leaving the majority of the structure otherwise intact. The same might be true for very mild cases of TBI. Therefore, volumetric measurements of hippocampal subfields might provide a better measure of the hippocampal pathology in PTSD and mild TBI than volumetric measurements of the whole hippocampus.
Measurement of Hippocampal Subfields with High Resolution Imaging at High Field
Measurements of hippocampal subfields require that details of the internal structure of the hippocampal formation can be depicted in vivo. Recent advancements with high field MRI (3–4 T), achieving increased gray/white matter contrast because of the increased signal sensitivity at high fields, additional magnetization transfer effects, and T1 weighting, have resulted in excellent in vivo anatomical images at submillimeter resolution that can be acquired within a few minutes [209]. The manual marking scheme depends on anatomical landmarks, particularly on a hypointense line representing the leptomeningeal tissue in the vestigial hippocampal sulcus, which can be reliably visualized on these high-resolution images (cf. Figure 2). For a detailed discussion on a marking procedure measuring hippocampal subfields that provides good to excellent inter- and intrarater reliability, refer to Mueller et. al [210].
FUNCTIONAL MAGNETIC RESONANCE IMAGING
Advances in MRI methods make extension from imaging of structure toward inferences about function possible. fMRI studies typically measure signal changes due to changes in blood flow or oxygenation while a person is performing a task, making use of the link between blood oxygenation and neural activity to determine task-related neural activity [211–212]. The signal changes in fMRI related to blood oxygenation is referred to as blood oxygen dependent level (BOLD) contrast. Experimental paradigms for fMRI generally involve comparison of a baseline condition with two or more experimental conditions consisting of specific cognitive tasks or sensory stimulation. These conditions can occur during extended periods of time (e.g., 10–60 s) in “blocked” design experiments or during very brief periods of time (e.g., 1–2 s) in “event-related” design experiments. Blocked-design experimental paradigms allow for the BOLD signal to add up over time because local neuronal firing constantly elevates during the experimental block. The increase in BOLD signal yields a larger effect size and higher detection rates than in event-related designs, allowing for adequate detection rates for shorter scan durations. This is important clinically because limiting scan duration is often necessary for clinical populations. Other methods that emphasize perfusion as opposed to BOLD contrast are also being tested, e.g., Kim et al. [213].
fMRI techniques have been used extensively for investigating mechanisms of brain function in health and may be useful for examining changes in brain function after injury as well as changes that occur over recovery or as a result of treatment interventions. However, the application of fMRI to various clinical populations, such as TBI and PTSD, is more complicated than fMRI experiments using healthy populations, and many variables need to be considered, such as hemodynamic changes due to injury, medications, and increased movement artifacts with patients, when fMRI studies are being designed and analyzed (see Hillary et al., D’Esposito et al., and Bartsch et al. [214–216]).
Thus far, most applications of fMRI to TBI and PTSD patients have emphasized techniques for “mapping” brain activations. These techniques have the advantage of allowing exploratory analyses, such as comparing differences between a patient group and a control group on a voxelwise basis, asking the open-ended question of which regions differ in activity. This inquiry may provide hypothesis-generating information about sources of dysfunction with TBI. Standard statistical parametric mapping approaches are suited to this type of question. However, potential confounders, such as changes in vasculature postinjury in TBI, may affect across-group comparisons.
Longitudinal Studies
fMRI is noninvasive and does not require injection of a radioisotope into the bloodstream; therefore, it is suitable for repeated studies and potentially useful for investigating the nature of longitudinal changes during recovery or with treatment interventions, such as pharmacotherapy [217]. Within-subjects comparisons also make across-group confounders less of an issue.
Functional Magnetic Resonance Imaging Studies of Mild Traumatic Brain Injury
A handful of studies have utilized functional MRI methods to examine functional activation patterns in patients with TBI [57–58,61–62,218–219]. Because cognitive dysfunction is of paramount concern with TBI, these studies have primarily used tasks that challenge working memory with conventional blocked-design fMRI tasks. Results have been mixed. Chrisodoulou et al. found that patients with severe TBI showed more widespread activation as a group on the Paced Auditory Serial Addition Task [57]. McAllister and colleagues found that patients had somewhat greater extent of activation with easier n-back tasks but, unlike healthy controls, seemed not to activate larger areas of cortex with increasing task load; this finding was corroborated by Perlstein et al. [58,61–62]. The specific interpretation of these studies is controversial but overall may suggest that patients with even mild TBI require larger areas of cortex to perform a given task, and those with moderate-severe TBI may have difficulty recruiting additional cortical resources when needed for more difficult tasks.
An active area of fMRI research with mild TBI comes from studies of athletes and sports-related concussions (see review by Ptito et al. [219]). Lovell et al. studied 28 athletes with concussions who were evaluated within approximately 1 week of injury and again after clinical recovery [67]. They used an n-back (0-, 1-, and 2-back) fMRI task along with a computer-based battery of neurocognitive tests and subjective symptom scales. The authors found that athletes who demonstrated hyperactivation on fMRI scans at the time of their first fMRI scan demonstrated a more prolonged clinical recovery than athletes who did not demonstrate hyperactivation.
Chen et al. used a working memory task (externally ordered task) to study a group of 16 athletes with concussions (15 symptomatic, 1 asymptomatic) and 8 matched healthy control subjects [72]. The authors reported that the activation pattern of the asymptomatic athlete was similar to that of the healthy controls while the activation patterns for the symptomatic athletes were abnormal in one manner or another (differences were not consistent). The authors also longitudinally followed one subject who had multiple concussions and found that as the subject’s symptoms improved, the subject’s ability to do the task improved and fMRI activation in the dorsolateral pre-frontal region increased (i.e., activation pattern became normalized or similar to that of healthy control subjects).
Chen et al. later studied a group of 28 male athletes, 18 with concussion and 10 without [73]. The concussion group was further divided into two subgroups, those with mild concussions (PCS score 6–21) and those with moderate concussions (PCS score >21). The moderate PCS group showed significantly slower response times than the control group on matching and 1-back behavioral tasks, while the mild group did not show any significant behavioral differences. However, both groups showed reduced fMRI task-related activation within the dorsolateral prefrontal regions. Also, the activation patterns for both concussion groups were more dispersed compared with the control group. The authors also reported a negative relationship between the PCS scores for the concussion group and BOLD signal changes within prefrontal regions. Further, the same authors have shown that the differential activation within the dorsolateral prefrontal regions can be affected by depression [220], which underscores the complexity of fMRI research in TBI and PTSD populations, because depression symptoms are often associated with both groups.
Functional Magnetic Resonance Imaging Studies of Posttraumatic Stress Disorder
The majority of fMRI studies of PTSD use traumatic stimuli or emotional pictures. The most common methods for traumatic stimuli involve traumatic scripts [221–222] or traumatic pictures [220–226]. Tasks involving emotional stimuli normally use either fearful or emotional faces [131,221–230]. However, more recently, researchers have been using more cognitive tasks without an emotional component, such as an auditor oddball task [231], visual working memory task [232], Stroop task [233], and Go No-Go task [234–235]. Most of these studies showed differential activation in at least one of the areas most often implicated in PTSD: amygdala, anterior cingulate, and medial temporal lobe.
Functional Magnetic Resonance Imaging Studies of Mild Traumatic Brain Injury Recovery and Rehabilitation
Discovery of the dynamic brain mechanisms of recovery and rehabilitation through neuroimaging is anticipated to advance both the rationale and methods of brain injury treatment in particular and learning and memory in general. Many studies, some of which performed repeated imaging during spontaneous recovery, have described fMRI patterns of activation in patients with mild TBI [67,72,218]. MRI studies of moderate-severe TBI have also been performed [236–239]. However, we are aware of only a few groups that have conducted fMRI neuroimaging investigations during rehabilitation in TBI [71,240–250].
Strangman et al. studied 54 patients with chronic TBI (>1 year postinjury, mean 11 years), 14 of whom had mild TBI, and found that after 12 sessions of rehabilitation focusing on internal strategies of improving memory, severe versus mild baseline injury (p = 0.049) or extreme abnormal activation (under- or overactivation) in the left ventral lateral prefrontal cortex at baseline (p = 0.007) predicted poorer responses to ~6 weeks of rehabilitative training [241]. No posttraining fMRI acquisitions were obtained to correlate with performance changes.
Kim et al. studied 17 subjects with moderate TBI (Glasgow Coma Scale 9–12) of variable duration (3–57 months, mean 16 months), 10 of whom underwent cognitive training for 4 weeks as well as pre- and post-fMRI imaging [71]. Improved performances in attentional tasks were accompanied by attentional network changes, i.e., decreased frontal lobe activity and increased activity in the anterior cingulate and precuneus areas. Given the heterogeneity in duration since injury (some less than 6 months postinjury), some changes in activation patterns may have reflected spontaneous recovery.
Before and after 4 to 8 months of individualized cognitive therapy, Laatsch et al. studied a case series of five patients with mild TBI and suggested that changes in activation for visual saccade and reading comprehension tasks might be related to, or due to, training [239]. The study suffered from many design and methodological concerns, including that the subjects were dissimilar in age, histories, duration and frequencies of brain injury events, and MRI findings (e.g., frontal lobe atrophy in a 20-year-old male). Further, this study lacked standardized training, duration, and control subjects. Laatsch and colleagues also used this testing paradigm in a case study of severe TBI [239] and in a study of three other patients with severe TBI [240] and reported “diffuse and variable activation patterns” compared with qualitative imaging stability between sessions for controls.
Functional Magnetic Resonance Imaging Studies of Posttraumatic Stress Disorder Rehabilitation
A few functional imaging studies have investigated the effects of cognitive-behavior therapy (CBT) on PTSD. Felmingham et al. studied eight patients with PTSD before and after eight once-weekly sessions of CBT with an fMRI paradigm consisting of neutral and fearful faces [242]. The authors reported an increase in fMRI activation in the rostral anterior cingulate cortex (bilaterally) after therapy. A significant positive correlation was also found between the changes in CAPS scores and the right anterior cingulate activation as well as a negative correlation between the CAPS scores and the amygdala activation. However, although a significant correlation existed between the CAPS scores and BOLD response within the amygdala, there were no significant activations compared with the fMRI baseline control condition within the amygdala during the individual sessions (before or after treatment).
The same authors performed a similar study in which they compared fMRI activation before and after eight once-weekly sessions of CBT in 14 subjects with PTSD (8 with comorbid depression) [243]. In this study, the authors altered the fMRI paradigm to increase the amygdala activation. In their previous study, each stimulus (blocks of fearful or neutral faces) was presented for 500 ms with an interstimulus interval of 768 ms [242]. In the latter study, the stimuli were presented for 16.7 ms with an interstimulus interval of 163.3 ms [243]. This rapid presentation of stimuli had been shown previously to activate the amygdala in PTSD patients [131,228]. The short duration of the stimulus was just long enough for unconscious processing of the stimulus but precluded conscious awareness. With this paradigm, not only was activation detected within the amygdala region, but the amygdala activation was also much greater than that for a group of healthy, nontrauma-exposed control subjects. In addition, PTSD subjects who did not significantly improve with therapy had significantly greater activation in the bilateral amygdala and right ventral anterior cingulate regions before therapy than did those subjects who did significantly improve with therapy (defined as at least a 50% reduction in pretreatment scores). Also, a significant positive correlation was found between posttreatment CAPS scores and both amygdala (bilateral) and ventral anterior cingulate activation. This study shows the potential for using fMRI to help predict the efficacy of certain therapies.
Functional Connectivity
Although most fMRI studies have focused on detection of regional brain activations, many cognitive functions affected by TBI are understood to require interactions across brain regions via the very white matter tracts that are vulnerable to injury. Multivariate statistical methods [244–246] or independent component analyses [247–249] may be used to assess injury-related changes in the functional connections across brain regions. Better characterization and understanding of the effects of TBI (and/or PTSD) on the coherence of these functional networks in both task and resting states may also provide insights into recovery and rehabilitation (see reviews by He et al. and Fox and Raichle [250–251]).
MAGNETIC RESONANCE SPECTROSCOPIC IMAGING
MRSI methods represent a fusion of MRI and MRS. While changes in contrast on clinical MRI images may indicate a structural abnormality, signal alterations in the MRS spectrum can give additional information about its nature. In certain cases, such alterations can even appear in regions that look normal on MRI images. Furthermore, as a combination of MRI and MRS, MRSI holds great potential for providing such additional information for a larger volume than is possible with MRS alone [252–253]. Most MRSI applications employ a two-dimensional approach, providing spectra from within one or multiple sections through the structure of interest. Three-dimensional MRSI methods allow coverage of larger volumes or an entire organ [254–256]. Typically, spectra are obtained from a regular array of subvolumes, which allows generation of images from individual peaks. Besides TBI and PTSD applications, other clinical applications of MRSI include the study of tumors [257–259], neurodegenerative disorders [260–262], neuropsychiatric diseases [263–265], epilepsy [266–268], and substance use disorders [269–271].
Magnetic Resonance Spectroscopy in Traumatic Brain Injury
Several metabolites are of interest as markers of injury. NAA is believed to reflect neuronal and axonal integrity [272]. Cho peaks reflect Cho-containing phospholipid constituents of cell membranes, including myelin. A heightened Cho peak may represent increased levels of free Cho, which can be expected in membrane disruption or turnover, as in inflammation, demyelination, and remyelination [273]. Pig models of TBI indicate that NAA/creatine (Cr) is diminished by at least 20 percent in regions of histologically confirmed axonal pathology in the face of negative findings from conventional MRI [274–275]. These findings suggest that MRS may be highly sensitive to microscopic pathology following diffuse brain injury.
Although MRS findings in moderate to severe TBI suggest a correlation with neuropsychological and functional outcomes (e.g., Brooks et al. [50]), such correlations have not been reported for mild TBI. Garnett et al. found that, compared with controls, Cho/Cr ratios of patients with mild TBI (n = 6 or 8, depending on classification) were elevated in frontal lobe white matter free of T1 or T2 lesions but NAA levels were not significantly different from normal [276]. All patients were scanned within 18 days of injury and no neuropsychometric testing was reported. Three of these patients had a repeat study about 4 months later [40], but no individual behavioral data or Cho/Cr data points were specified. Son et al. reported that NAA levels were reduced and lactate/Cr ratios were elevated at pericontusional white matter about 1 month after injury (no report of clinical correlate) and that these values improved after 2 months [277]. Cecil et al. reported decreased NAA in a single-voxel analysis in 35 patients with TBI (26 with mild TBI) in the splenium of the corpus callosum and lobar white matter, but clinical correlation was not established [48]. The subjects were scanned 9 days to 4.5 years (mean 1 year) postinjury, and the authors did not separate the mild TBI patients from the more severe TBI patients and do a separate analysis. Studies using MRS to account for cognitive deficits in mild TBI are sparse. In a group of 14 patients within 1 month of sustaining a mild TBI (many of whom had positive CT findings), NAA levels were lower in the parietal white matter bilaterally and Cho/Cr ratios were higher within two separate regions of the occipital gray matter [39]. No significant correlation was found in this small sample for Glasgow Outcome Scale at discharge or at 6 months after injury. In summary, MRS abnormalities have been described in mild TBI, but structural, functional, and clinical relevance need to be established.
Magnetic Resonance Spectroscopy in Posttraumatic Stress Disorder
Researchers have focused primarily on the medial temporal lobe/hippocampus and the anterior cingulate cortex (ACC) for MRS studies in PTSD. Many of these studies used single-voxel techniques and were thus limited to examining one large region at a time. For example, Ham et al. acquired spectroscopic data of a 15 × 15 × 15 mm3 volume in the ACC and bilaterally in the medial temporal lobe (each volume acquired separately) [278]. The authors found significant NAA decreases within all three volumes when comparing the metabolite levels of 26 survivors of a subway train fire who were diagnosed with PTSD with those of 25 healthy control subjects. Moreover, the NAA levels for the PTSD subjects were correlated with symptom severity. While the authors looked at NAA, Cho, and Cr levels, they only found significant differences in NAA levels. Other studies have found similar results in the ACC [97,279–280] and medial temporal lobe [91,97,280]. However, not all studies have found these differences. Seedat et al. compared NAA/Cr, Cho/Cr, and myo-inositol (mI)/Cr ratios from the ACC in female domestic violence victims (seven with PTSD, nine control subjects) [279]. In this small study, the authors did not find significant differences in NAA/Cr, but they did find a significant increase in Ch/Cr and mI/Cr ratios for the women with PTSD. Also, Freeman et al. examined 20 prisoners of war (10 with PTSD) and 6 controls and did not find any significant group differences in NAA/Cr or Cho/Cr ratios [281].
The most comprehensive spectroscopy study to date used spectroscopic and volumetric data to compare the association of PTSD and alcohol abuse with metabolite ratios and hippocampal volumes [280]. The authors used MRI, MRS (hippocampus), and MRSI (frontal/parietal region) to acquire data from 55 patients with PTSD (28 tested positive for alcohol abuse) and 49 control subjects (23 tested positive for alcohol abuse). The authors found that PTSD was associated with reduced NAA/Cr within both the hippocampus and ACC. Despite finding NAA reductions, the authors did not find any significant volume reductions. Also, the decrease in NAA/Cr associated with the PTSD patients could not be attributed to alcohol abuse.
ARTERIAL SPIN LABELING
Perfusion refers to the delivery of blood to a tissue or organ. Brain perfusion is also termed cerebral blood flow (CBF) and is expressed in units of milliliters/gram/minute, reflecting the volume of flow per gram of brain tissue per unit time. Primary alterations in CBF occur in a number of central nervous system (CNS) disorders, most notably cerebrovascular disease, but because CBF changes are also coupled to neural activity, regional CBF measurements can be used to indirectly monitor neural activity both at rest and during task or pharmacological manipulations.
For MRI, the two most common methods for imaging perfusion are the dynamic susceptibility contrast approach, which detects the first passage of an intravascular contrast agent such as Gd-DTPA, and arterial spin labeling (ASL), which utilizes magnetically labeled arterial blood water as a diffusible flow tracer. Absolute quantification of tissue perfusion requires a tracer that can diffuse from the vasculature into tissue.
ASL techniques are completely noninvasive (and thus avoid the use of exogenous contrast agents) and can provide quantitative CBF images in standard physiological units of milliliters/gram/minute. ASL perfusion MRI should be particularly useful for multisite or longitudinal studies of brain function in which absolute quantification is critical. Absolute quantification also allows the resting state to be characterized, in contrast to BOLD fMRI, which primarily detects differences between two or more experimentally manipulated conditions. Thus, resting ASL perfusion MRI is useful for characterizing behavioral [213,282] or pharmacological [283] “states” and genetic “traits” [284] and complements BOLD fMRI studies of stimulus-evoked activity. For studies of task activation, ASL also provides sensitivity at extremely low task frequencies where BOLD fMRI becomes insensitive using standard parametric statistics because of low frequency noise [285]. Continual technical advances have dramatically improved the sensitivity of ASL perfusion MRI [282,286–291], and its use is likely to increase in the coming years.
In ASL techniques, arterial blood water is magnetically “labeled” using radio-frequency pulses. It is highly analogous to PET CBF measurements, which use water labeled with radioactive 15O, except that the magnetically labeled arterial water “decays” with T1 relaxation rather than a radioactive decay. Depending on field strength, the T1 relaxation rate for water in blood or tissue is 1 to 2 seconds, which is much more rapid than the ~2 minute decay rate for 15O. As a result, only small amounts of arterial spin-labeled water accumulate in the brain, though the temporal resolution is much faster than with H215O-PET. A postlabeling delay allows labeled arterial spins to exchange with brain microvasculature and tissue [292]. The effects of ASL on brain image intensity are measured by comparison with a control image in which arterial blood is not labeled. Quantification of CBF requires a model that accounts for a variety of parameters, including T1 rates for blood and tissue, arterial transit times, and labeling efficiency [292–294]. Good correlations between ASL and 15O-PET have been demonstrated both for resting CBF [295] and CBF during task activation [296].
ASL perfusion MRI has been used to study rat models of TBI [297–302], and more recently, it has begun to be applied to studies of TBI in humans. A wide variety of potential applications of ASL to TBI exist, including characterization of regional brain function in severe TBI for which task-evoked responses may be difficult to obtain, correlations of changes in regional CBF with attentional and other cognitive deficits to try to identify potential targets for pharmacological or transcranial magnetic stimulation therapy, and use as a biomarker of regional brain function for pharmaceutical trials (Figure 3).
Figure 3.
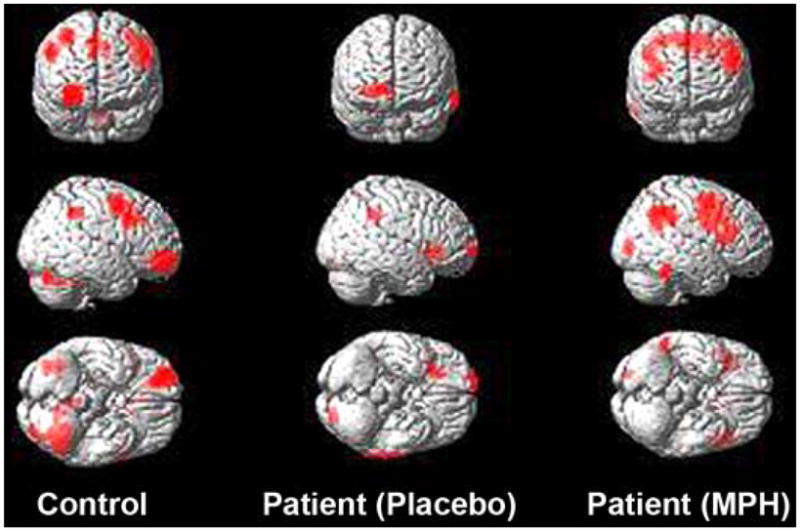
Arterial spin labeling perfusion. Magnetic resonance imaging perfusion-based group activation maps obtained during letter 2-back working-memory task from control subjects (left) and patients with traumatic brain injury studied following either placebo (middle) or methylphenidate (MPH) (right). Frontal activation in patients is reduced on placebo when compared with activation in controls, but normal-appearing activation is restored after MPH administration. Source: Unpublished data courtesy of Junghoon Kim and John Whyte, Moss Rehabilitation Institute.
High-speed helical CT scanners have facilitated the advent of CT perfusion studies to provide data such as CBF, blood volume, and mean transit times after intravenous administration of iodinated contrast material, which is an approach that is analogous to perfusion MRI based on dynamic susceptibility tracking. Although the need for exogenous contrast and exposure to ionizing radiation limits the number of measurements that can be made, CT perfusion imaging is emerging as a powerful and cost-effective tool that avoids logistical problems posed by monitoring equipment or surgical hardware required for MR studies [303].
SUSCEPTIBILITY-WEIGHTED IMAGING
The last of the MRI techniques that we will discuss in this review is SWI and its ability to recognize damage to the brain caused by bleeding, shearing, and loss of oxygen saturation [304–306]. SWI is an imaging technique that is exquisitely sensitive to microhemorrhaging and the presence of hemosiderin and deoxyhemoglobin. Data are usually collected with a resolution of 1.0 mm3 at 1.5 T and 0.5 mm3 at 3 T. The entire brain can be covered in less than 5 minutes with the use of parallel imaging with an excellent signal-to-noise ratio (SNR). Special processing incorporates the phase information into the magnitude information to enhance the contrast. Studies have shown that SWI is more sensitive to hemorrhagic lesions than are traditional MRI scans [41,51]. Figure 4 shows an example of a conventional T2 scan, followed by three different images emanating from the SWI scan. In this case, multiple small lesions can be seen in the frontal lobe and another smaller microhemorrhage in the right side of the brain. SWI has been shown to have three to six times more sensitivity to microhemorrhages than conventional gradient echo imaging or any other imaging in MRI. To date, it has been used predominantly to study patients with severe head trauma, including coma patients [306]. It has also been used to image children [36,52,307].
Figure 4.
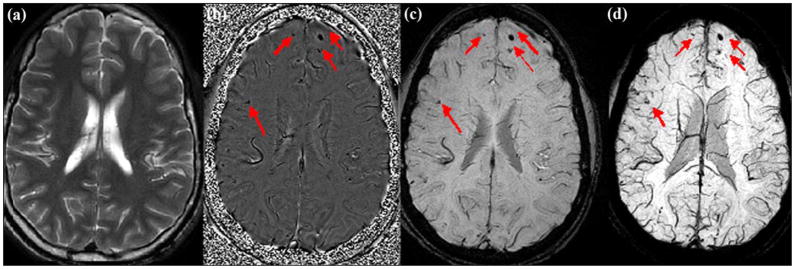
Susceptibility-weighted image (SWI) example. Comparison of (a) T2-weighted, (b) SWI filtered phase, (c) processed magnitude, and (d) maximum intensity projection images on patient with traumatic brain injury, acquired on 3 T TRIO Siemens system. SWI has the following acquisition parameters: echo time/repetition time (TR/TE): 29/20 ms, flip angle: 15°, bandwidth: 120 Hz/pixel, 8-channel phased array coil with a parallel imaging factor of two, field of view (FOV): 256 × 256 mm2, slice thickness: 2 mm, acquisition matrix: 512 × 416 × 64, spatial resolution: 0.5 × 0.5 × 2 mm3. T2-weighted image acquired with T2 fast spin echo with TR/TE: 5000/113 ms, FOV: 256 × 256 mm2, slice thickness: 2 mm, acquisition matrix: 320 × 320. Red arrows label multiple possible microhemorrhages invisible on both T1- and T2-weighted images (some not labeled). In this case, SWI data clearly demonstrate multiple possible microhemorrhages in brain.
MULTISITE STUDIES USING MAGNETIC RESONANCE IMAGING
Medical imaging for patient care invokes considerations at the levels of the individual patient and the individual imaging site. However, medical imaging in clinical research studies is often performed at multiple sites. This is true for large observational studies and is particularly true for therapeutic trials, which are nearly always conducted at multiple sites. The reasons for the this are fairly obvious. If one wishes to capture the relevant variation present across an entire population for a natural history study, then subjects must be recruited from multiple sites. The same considerations hold for therapeutic trials in which therapeutic efficacy across a representative range of the population must be demonstrated. In addition, recruiting the number of subjects needed to power a study is often impossible at a single site.
Like other imaging modalities, MRI captures morphometric or functional data that provide useful information about pathological processes of relevance. That is, imaging measures serve as in vivo surrogates of relevant pathologies. Because of its flexible nature, MRI can provide information about a variety of anatomical and physiological brain processes. These processes include brain morphology, changes in relaxation properties, perfusion, diffusion, and metabolite concentration.
Variability in imaging data collected across different subjects or across individual subjects over time can be considered in three categories. First is the data variability due to the effect of the pathology one seeks to measure. For example, variability in brain volume may be due to presence and severity of AD or due to TBI. Second is data variability due to biology that is irrelevant to the pathology of interest. For example, brain volume may vary with hydration or nutritional status. Third is data variability due to technical or engineering-related factors. In any study, whether single site or multisite, the objective is to maximize the impact of biologically relevant data variability and minimize the impact of other sources of variability. The reason for this objective is self-evident; the more variability in a data set that is directly due to the pathology of interest, the more useful the imaging will be in probing the biologically relevant relationships. Conversely, irrelevant biological variability and engineering-related variability will only obscure the relationships between imaging and the pathology of interest. In designing multisite trials, unwanted data variability due to the irrelevant biology can be minimized to some extent by rigorous inclusion and exclusion criteria. But the source of undesirable data variability that is under greatest control is that due to technical or engineering-related factors.
A variety of specific items should be considered in the design of multisite trials. However, an overarching principle is that unwanted data variability due to technical factors can be minimized by standardization. And the principle of standardization applies in two dimensions: (1) across sites/scanners and (2) across time. MRI has been employed in numerous different multisite observational and therapeutic CNS studies, including studies on AD [308–311], mild cognitive impairment [312], cerebral vascular disease [308–314], and schizophrenia [315]. The remainder of this section will focus on specific features of the design of multisite CNS studies that were gleaned from the experience of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [316]. In multisite studies, enrollment sites are selected on the basis of their ability to recruit and retain subjects meeting specific clinical criteria. Sites are typically not selected on the basis of access to advanced MRI equipment or expertise. Consequently, multisite studies must be performed across a broad array of hardware/software platforms and across sites with variable levels of expertise. State-of-the-art applications may not be available on all systems.
Some basic principles in the design and conduct of multisite CNS MRI studies follow. The main confound to address when different scanners at different sites are used is ensuring that equivalent sequences are being run with equivalent parameters on each scanner to ensure the same contrasts, artifacts, noise, etc. Further, each scanner used in the study should be qualified at baseline before subject enrollment and requalified after any hardware or software upgrades. “Qualified” means using a specific quality assurance (QA) protocol [317] with a specific phantom to ensure that the scanner is performing adequately. Each site should use the same type of phantom and QA protocol. Ideally, a single scanner should be used at each site for the entire study.
Tesla strength of MRI scanners at different sites may vary. If so, the sequences and specific parameters will need to be adjusted to maintain equivalent T1 and T2 weighting for all scanners across sites. Also, in the case of functional imaging, differences in SNR fluctuations between scanners need to be accounted for in postprocessing before activation across scanners/sites is compared [318].
Before start-up of the study, it might be highly useful to conduct a small pilot study of the proposed acquisition protocol on a representative group of systems. Such a pilot study will avoid the unfortunate situation in which incompatibilities between the study protocol and certain platforms are discovered after enrollment has begun. Electronically distributing system-specific protocols to each scanner used in the study is also helpful. Electronic distribution avoids the situation in which protocols are built manually from paper protocols on individual scanners, which dramatically increases the likelihood of protocol errors at individual sites. Imaging sequences in the protocol should be easy to prescribe and use by MRI technologists with a wide range of experience.
Ideally, the study protocol should avoid nonproduct imaging sequences to minimize administrative and regulatory overhead. A central quality control center is recommended. All protocol scans should be checked for protocol compliance, image quality, and medically significant abnormalities. Identification of medically significant abnormalities by the MRI center should be used for subject inclusion and exclusion purposes. However, clinical interpretations of study scans should be the responsibility of the local enrollment site—i.e., medicolegal responsibility should reside locally, not with the research study.
Some means of monitoring scanner performance for site qualification purposes and throughout the study is useful. In the ADNI, a phantom scan is acquired along with each patient study. Measurements from these phantom scans allow identification of scanning errors that elude detection because the relevant information is not recorded electronically with the imaging data. Measurements from phantom scans can be applied retrospectively to correct drifts or discontinuities in the coupled human images, provided certain assumptions are met. Finally, despite detailed attention to standardization of acquisition and quality control, correcting residual abnormalities in the image data after the fact remains important. The types of corrections that may be useful are corrections for gradient nonlinearity, intensity nonuniformity, and drift or discontinuities in scanner calibration.
The preceding comments are largely recommendations derived from the experience of the ADNI. However, these considerations are relevant for any multisite study. To the extent that technical variation is reduced in a data set, the data set becomes a more useful and powerful tool for achieving the ultimate objective, which is the use of imaging as an in vivo surrogate of specific pathologies of interest. The large patient-population capabilities of multisite studies could be invaluable to combat-related TBI and PTSD research. For more information regarding multisite studies, see the ADNI (www.loni.ucla.edu/ADNI) or the Biomedical Informatics Research Network (www.nbirn.net) Web sites.
POSITRON EMISSION TOMOGRAPHY
PET imaging with the tracer [18F] fluorodeoxyglucose (FDG) has been used for 3 decades to provide information about glucose metabolism in the human brain. Beginning with early studies that carefully validated tracer kinetic models [319] and extending through applications that involved studies of both normal cognition [320] and many disease states, the technique has become a robust approach to human clinical neuroscience. While studies of brain activation and cognition have largely been supplanted by fMRI, which has wider availability, higher spatial and temporal resolution, and no ionizing radiation, FDG-PET has continued to be applied to the study of human disease. This application is largely based on findings that show reductions in metabolism that are often regionally specific in numerous diseases. In addition, a host of basic studies strongly suggest that FDG-PET is a measure that parallels synaptic function [321], providing a good basis for interpretation of image findings.
One example is AD, in which reductions in posterior parietal, temporal, and posterior cingulate/precuneus cortex predominate [322–323]. These metabolic reductions are related to the neuropathological accumulation of amyloid plaques and neurofibrillary tangles, as has been revealed by several studies correlating the pattern of glucose hypometabolism with postmortem pathological evaluation [324–326]. While the recent introduction of techniques to image the accumulation of beta-amyloid offer the promise of a far more specific molecular approach to diagnosis of dementia [327–329], FDG-PET remains a popular technique for brain imaging because of its wide availability and large accumulated experience. Applications to a variety of diseases, including Huntington’s [330], epilepsy [331], and brain tumors [332], have been published on extensively over decades.
A limited number of studies have been performed with FDG-PET to investigate TBI. Metabolic rates in the striatum and thalamus are lower in patients soon after episodes of TBI, and in subjects in coma, the thalamus, brainstem, and cerebellum are particularly affected and related to level of consciousness [333]. Dynamic PET studies suggest that these metabolic reductions are related to alterations in hexokinase activity and not to changes in glucose transport [334]. In chronic TBI patients with diffuse axonal injury, metabolic decreases are pronounced in the frontal cortex, temporal cortex, thalamus, and cerebellum and the severity of frontal lobe hypometabolism is related to cognitive function [335]. In particular, abnormalities of glucose metabolism in the cingulate gyrus may be related to neuropsychological function after TBI [336]. FDG-PET studies of patients with mild TBI have not revealed a consistent pattern of disturbances (see review in Belanger et al. [236]). However, with the advent of novel radioligands, new imaging techniques have emerged that may provide new insights into TBI and PTSD. For example, preliminary data suggest that amyloid-β (Aβ) plaques may be useful biomarkers of TBI.
IMAGING AMYLOID IN TRAUMATIC BRAIN INJURY
Head trauma has been identified as a potent risk factor for subsequent development of AD [337], and the connection between TBI and AD neuropathology has been reviewed recently [338]. In particular, cortical Aβ deposits have been observed in about one-third of biopsied temporal cortexes in patients with severe TBI as soon as 2 hours after injury, while tau-positive neurofibrillary tangles have been observed less frequently [339]. Recent advances in the development of imaging agents capable of quantifying regional Aβ plaque densities in living human brain by using PET or SPECT have made Aβ imaging studies in TBI subjects feasible.
The Aβ plaque deposition in TBI subjects and its relationship to long-term cognitive sequelae remain to be fully elucidated. Noninvasive longitudinal imaging studies capable of assessing Aβ plaque changes in TBI could play an important role in this regard.
The most utilized human Aβ imaging agents to date are the PET radioligands [18F] FDDNP (fluoroethyl (methyl)amino]-2-naphthyl} ethylidene) malononitrile) and [11C] PIB [326,338–342]. This area of research is active, and several other radioligands have been reported in human studies, including 123I-labeled and other 18F-labeled agents. Example of PET images from a study using [11C] PIB to assess Aβ plaque pathology in AD is shown in Figure 5 [342].
Figure 5.
Positron emission tomography (PET) example. PET images obtained with the amyloid-imaging agent Pittsburgh Compound B ([11C] PIB) in normal control (far left), three different patients with mild cognitive impairment (MCI) (three center images), and patient with mild Alzheimer disease (AD) (far right). Some MCI patients have control-like levels of amyloid, some have AD-like levels, and some have intermediate levels. DVR = distribution volume ratio.
Future prospective investigations of long-term cognitive declines or AD development in subjects with TBI may be done in parallel with studies of amyloid (Aβ) deposition to see whether the latter can account for the former. Similar longitudinal studies are underway to determine whether amyloid-burden (Aβ) increases over time correlate with cognitive consequences in elderly patients at risk of AD. An example of a 2-year follow-up study using [11C] PIB to assess Aβ plaque pathology in an elderly control subject is shown in Figure 6 (University of Pittsburgh, unpublished data).
Figure 6.
Positron emission tomography (PET) amyloid-β (Aβ) plaque example. Increased retention of tracer in right frontal cortical area is seen over 2 years during which 74-year-old subject remained cognitively normal. Similar longitudinal PET imaging studies in traumatic brain injury subjects may help assess role and dynamics of Aβ deposition. Source: Unpublished data courtesy of University of Pittsburgh.
CONCLUSIONS
Detection and objective characterization of subtle but clinically significant abnormalities in mild TBI and PTSD are important objectives of modern neuroimaging. Several MRI methods have excellent potential to help visualize metabolic (via MRSI), microstructural (via DTI or, more specifically, FA), and functional network changes related to resting and cognitive states (fMRI, MR perfusion). In addition, new MRI methods (SWI) allow for better detection of microhemorrhaging and could be particularly useful in the screening of servicemembers and veterans for lesions related to head trauma. The development of high-resolution imaging of hippocampal subfields may contribute to improved understanding of memory mechanisms in cerebral trauma or PTSD. PET methods may also provide insights into the metabolic and/or degenerative changes that may accompany these disorders.
Multimodal techniques may emerge as helpful orthogonal approaches to enhance the yield of detection and characterization of abnormalities. These methods may provide complementary information about the neural, glial, vascular, and network conditions that subserve cognitive and behavioral states. The development of multivariable models combining structural, neurochemical, physiological, and psychophysical or neurobehavioral findings can be anticipated to be an active area of future research. Future imaging studies of prospective, controlled clinical trials with parallel neuropsychological testing and rehabilitation also hold promise for elucidating mechanisms of learning or relearning. Potential information about predilection, distinguishing neural signatures, objective assays for monitoring recovery and response to treatment, and prognosis for patients may all come to fruition as a result of the continued advances in neuroimaging. Thus, neuroimaging may provide information that contributes substantially to the development of improved, rational approaches to the management of patients with TBI and PTSD.
Acknowledgments
Funding/Support: This material was unfunded at the time of manuscript preparation.
Abbreviations
- Aβ
amyloid-β
- ACC
anterior cingulate cortex
- ACR
anterior corona radiata
- AD
Alzheimer disease
- ADC
Apparent Diffusion Coefficient
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- ANT
Attention Network Task
- ASL
arterial spin labeling
- BOLD
blood oxygen dependent level
- CA
cornu ammonis
- CAPS
Clinician-Administered PTSD Scale
- CBF
cerebral blood flow
- CBT
cognitive-behavior therapy
- Cho
choline
- CNS
central nervous system
- Cr
creatine
- CT
computed tomography
- DG
dentate gyrus
- D-MRI
diffusion magnetic resonance imaging
- DTI
diffusion tensor imaging
- EPI
echo-planar imaging
- FA
fractional anisotropy
- FDG
fluorodeoxyglucose
- fMRI
functional magnetic resonance imaging
- LDFR
long-delay free recall
- mI
myo-inositol
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MRSI
magnetic resonance spectroscopic imaging
- NAA
N-acetylaspartate
- OIF/OEF
Operation Iraqi Freedom/ Operation Enduring Freedom
- PCS
postconcussion syndrome
- PET
positron emission tomography
- PIB
Pittsburgh Compound B
- PTSD
posttraumatic stress disorder
- PW-MRI
perfusion-weighted magnetic resonance imaging
- QA
quality assurance
- rCBV
regional cerebral blood volume
- ROI
region of interest
- RT
reaction time
- SNR
signal-to-noise ratio
- SPECT
single photon emission computed tomography
- SWI
susceptibility-weighted imaging
- TBI
traumatic brain injury
- UCSF
University of California, San Francisco
- UF
uncinate fasciculus
Footnotes
Financial Disclosures: The authors have declared that no competing interests exist.
Author Contributions:
Composed and critically revised manuscript for important intellectual content: R. W. Van Boven, G. S. Harrington, D. B. Hackney, A. Ebel, G. Gauger, J. D. Bremner, M. D’Esposito, J. A. Detre, E. M. Haacke, C. R. Jack Jr, W. J. Jagust, D. Le Bihan, C. A. Mathis, S. Mueller, P. Mukherjee, N. Schuff, A. Chen, M. W. Weiner.
Additional Contributions: Dr. Van Boven is now with the Mild Traumatic Brain Injury Clinic, Irwin Army Community Hospital, Fort Riley, Kansas.
References
- 1.Tanielian TL, Jaycox L RAND Corporation. Invisible wounds of war: Psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica (CA): RAND; 2008. [Google Scholar]
- 2.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: Preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 3.Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21(5):398–402. doi: 10.1097/00001199-200609000-00004. 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Zee CS, Go JL. CT of head trauma. Neuroimaging Clin N Am. 1998;8(3):525–39. [PubMed] [Google Scholar]
- 5.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 6.Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wu O, Sorensen AG. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(3):370–76. [PMC free article] [PubMed] [Google Scholar]
- 7.Naganawa S, Sato C, Ishihra S, Kumada H, Ishigaki T, Miura S, Watanabe M, Maruyama K, Takizawa O. Serial evaluation of diffusion tensor brain fiber tracking in a patient with severe diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(9):1553–56. [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, Iwama T. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006;77(7):850–55. doi: 10.1136/jnnp.2005.077875. 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29(5):967–73. doi: 10.3174/ajnr.A0970. 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, Pickard JD. Diffusion tensor imaging in chronic head injury survivors: Correlations with learning and memory indices. Neuroimage. 2006;29(1):117–24. doi: 10.1016/j.neuroimage.2005.07.012. 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J Neurosurg. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 12.Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, Millis SR, Makki M, Latif Z, Coplin W, Meythaler J, Haacke EM. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24(3):446–59. doi: 10.1089/neu.2006.0153. 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- 13.Ducreux D, Huynh I, Fillard P, Renoux J, Petit-Lacour MC, Marsot-Dupuch K, Lasjaunias P. Brain MR diffusion tensor imaging and fibre tracking to differentiate between two diffuse axonal injuries. Neuroradiology. 2005;47(8):604–8. doi: 10.1007/s00234-005-1389-1. 10.1007/s00234-005-1389-1. [DOI] [PubMed] [Google Scholar]
- 14.Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins dis-concordant for traumatic brain injury. Am J Neuroradiol. 2006;27(4):879–81. [PMC free article] [PubMed] [Google Scholar]
- 15.Le TH, Mukherjee P, Henry RG, Berman JI, Ware M, Manley GT. Diffusion tensor imaging with three-dimensional fiber tractography of traumatic axonal shearing injury: An imaging correlate for the posterior callosal “disconnection” syndrome: Case report. Neurosurgery. 2005;56(1):189. [PubMed] [Google Scholar]
- 16.Xu J, Rasmussen IA, Lagopoulos J, Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 2007;24(5):753–65. doi: 10.1089/neu.2006.0208. 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- 17.Tisserand DJ, Stanisz G, Lobaugh N, Gibson E, Li T, Black SE, Levine B. Diffusion tensor imaging for the evaluation of white matter pathology in traumatic brain injury. Brain Cogn. 2006;60(2):216–17. [PubMed] [Google Scholar]
- 18.Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey CM, Narayana PA. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26(4):481–95. doi: 10.1089/neu.2008.0461. 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 19.Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42(2):503–14. doi: 10.1016/j.neuroimage.2008.04.254. 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, Bello JA, Branch CA. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25(11):1335–42. doi: 10.1089/neu.2008.0547. 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 21.Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29(3):514–19. doi: 10.3174/ajnr.A0856. 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutgers DR, Fillard P, Paradot G, Tadié M, Lasjaunias P, Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008;29(9):1730–35. doi: 10.3174/ajnr.A1213. 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akpinar E, Koroglu M, Ptak T. Diffusion tensor MR imaging in pediatric head trauma. J Comput Assist Tomogr. 2007;31(5):657–61. doi: 10.1097/RCT.0b013e318033df1a. 10.1097/RCT.0b013e318033df1a. [DOI] [PubMed] [Google Scholar]
- 24.Avants B, Duda JT, Kim J, Zhang H, Pluta J, Gee JC, Whyte J. Multivariate analysis of structural and diffusion imaging in traumatic brain injury. Acad Radiol. 2008;15(11):1360–75. doi: 10.1016/j.acra.2008.07.007. 10.1016/j.acra.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galloway NR, Tong KA, Ashwal S, Oyoyo U, Obenaus A. Diffusion-weighted imaging improves outcome prediction in pediatric traumatic brain injury. J Neurotrauma. 2008;25(10):1153–62. doi: 10.1089/neu.2007.0494. 10.1089/neu.2007.0494. [DOI] [PubMed] [Google Scholar]
- 26.Ptak T, Sheridan RL, Rhea JT, Gervasini AA, Yun JH, Curran MA, Borszuk P, Petrovick L, Novelline RA. Cerebral fractional anisotropy score in trauma patients: A new indicator of white matter injury after trauma. AJR Am J Roentgenol. 2003;181(5):1401–7. doi: 10.2214/ajr.181.5.1811401. [DOI] [PubMed] [Google Scholar]
- 27.Sidaros A, Endberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain. 2008;131(Pt 2):559–72. doi: 10.1093/brain/awm294. 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 28.Han BS, Kim SH, Kim OL, Cho SH, Kim YH, Jang SH. Recovery of corticospinal tract with diffuse axonal injury: A diffusion tensor image study. NeuroRehabilitation. 2007;22(2):151–55. [PubMed] [Google Scholar]
- 29.Hou DJ, Tong KA, Ashwal S, Oyoyo U, Joo E, Shutter L, Obenaus A. Diffusion-weighted magnetic resonance imaging improves outcome prediction in adult traumatic brain injury. J Neurotrauma. 2007;24(10):1558–69. doi: 10.1089/neu.2007.0339. 10.1089/neu.2007.0339. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy MR, Wozniak JR, Muetzel RL, Mueller BA, Chiou HH, Pantekoek K, Lim KO. White matter and neurocognitive changes in adults with chronic traumatic brain injury. J Int Neuropsychol Soc. 2009;15(1):130–36. doi: 10.1017/S1355617708090024. 10.1017/S1355617708090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–19. doi: 10.1093/brain/awm216. 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 32.Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, Chia J, Vasquez AC, Hunter JV. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22(2):115–22. doi: 10.1080/02699050801888816. 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- 34.Niogi S, Mukherjee P, Kolster R, Sarkar R, Ghajar J, McCandliss B. Diffusion tensor imaging measures of white matter integrity dissociate damage to memory versus attention networks in mild traumatic brain injury. Proceedings of the ISMRM 15th Scientific Meeting and Exhibition; 2007 May 19–25; Berlin, Germany. Berkeley (CA): ISMRM; 2007. [Google Scholar]
- 35.Holshouser BA, Tong KA, Ashwal S. Proton MR spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR Am J Neuroradiol. 2005;26(5):1276–85. [PMC free article] [PubMed] [Google Scholar]
- 36.Holshouser BA, Tong KA, Ashwal S, Oyoyo U, Ghamsary M, Saunders D, Shutter L. Prospective longitudinal proton magnetic resonance spectroscopic imaging in adult traumatic brain injury. J Magn Reson Imaging. 2006;24(1):33–40. doi: 10.1002/jmri.20607. 10.1002/jmri.20607. [DOI] [PubMed] [Google Scholar]
- 37.Hunter JV, Thornton RJ, Wang ZJ, Levin HS, Roberson G, Brooks WM, Swank PR. Late proton MR spectroscopy in children after traumatic brain injury: Correlation with cognitive outcomes. Am J Neuroradiol. 2005;26(3):482–88. [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon SJ, Lee JH, Kim ST, Chun MH. Evaluation of traumatic brain injured patients in correlation with functional status by localized 1H-MR spectroscopy. Clin Rehabil. 2005;19(2):209–15. doi: 10.1191/0269215505cr813oa. 10.1191/0269215505cr813oa. [DOI] [PubMed] [Google Scholar]
- 39.Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, Maudsley AA. Volumetric proton spectroscopic imaging of mild traumatic brain injury. Am J Neuroradiol. 2004;25(5):730–37. [PMC free article] [PubMed] [Google Scholar]
- 40.Garnett MR, Blamire AM, Corkill RG, Cadoux-Hudson TA, Rajagopalan B, Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123(Pt 10):2046–54. doi: 10.1093/brain/123.10.2046. 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- 41.Babikian T, Freier MC, Tong KA, Nickerson JP, Wall CJ, Holshouser BA, Burley T, Riggs ML, Ashwal S. Susceptibility weighted imaging: Neuropsychologic outcome and pediatric head injury. Pediatr Neurol. 2005;33(3):184–94. doi: 10.1016/j.pediatrneurol.2005.03.015. 10.1016/j.pediatrneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: Predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006;24(4):801–11. doi: 10.1002/jmri.20696. 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- 43.Cohen BA, Inglese M, Rusinek H, Babb JS, Grossman RI, Gonen O. Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. AJNR Am J Neuroradiol. 2007;28(5):907–13. [PMC free article] [PubMed] [Google Scholar]
- 44.Sinson G, Bagley LJ, Cecil KM, Torchia M, McGowan JC, Lenkinski RE, McIntosh TK, Grossman RI. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: Correlation with clinical outcome after traumatic brain injury. AJNR Am J Neuroradiol. 2001;22(1):143–51. [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil. 2001;16(2):149–64. doi: 10.1097/00001199-200104000-00005. 10.1097/00001199-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Shutter L, Tong KA, Holshouser BA. Proton MRS in acute traumatic brain injury: Role for glutamate/ glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–1705. doi: 10.1089/neu.2004.21.1693. 10.1089/neu.2004.21.1693. [DOI] [PubMed] [Google Scholar]
- 47.Shutter L, Tong KA, Lee A, Holshouser BA. Prognostic role of proton magnetic resonance spectroscopy in acute traumatic brain injury. J Head Trauma Rehabil. 2006;21(4):334–49. doi: 10.1097/00001199-200607000-00005. 10.1097/00001199-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Cecil KM, Hills EC, Sandel ME, Smith DH, McIntosh TK, Mannon LJ, Sinson GP, Bagley LJ, Grossman RI, Lenkinski RE. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 1998;88(5):795–801. doi: 10.3171/jns.1998.88.5.0795. 10.3171/jns.1998.88.5.0795. [DOI] [PubMed] [Google Scholar]
- 49.Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol. 1998;19(10):1879–85. [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks WM, Stidley CA, Petropoulos H, Jung RE, Weers DC, Friedman SD, Barlow MA, Sibbitt WL, Jr, Yeo RA. Metabolic and cognitive response to human traumatic brain injury: A quantitative proton magnetic resonance study. J Neurotrauma. 2000;17(8):629–40. doi: 10.1089/089771500415382. 10.1089/089771500415382. [DOI] [PubMed] [Google Scholar]
- 51.Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: A review of clinical applications in children. AJNR Am J Neuroradiol. 2008;29(1):9–17. doi: 10.3174/ajnr.A0786. 10.3174/ajnr.A0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, Kido DK. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: Improved detection and initial results. Radiology. 2003;27(2):332–39. doi: 10.1148/radiol.2272020176. 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 53.Tong KA, Ashwal S, Holshouser BA, Nickerson JP, Wall CJ, Shutter LA, Osterdock RJ, Haacke EM, Kido D. Diffuse axonal injury in children: Clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56(1):36–50. doi: 10.1002/ana.20123. 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- 54.Tong KA, Holshouser BA, Ashwal S. Evaluation of pediatric diffuse axonal injury using susceptibility weighted imaging (SWI) and MR spectroscopy. Proceedings of the ISMRM 14th Scientific Meeting and Exhibition; 2006 May 6–12; Toronto, Canada. Berkeley (CA): ISMRM; 2006. [Google Scholar]
- 55.Prigatano GP, Johnson SC, Gale SD. Neuroimaging correlates of the Halstead Finger Tapping Test several years post-traumatic brain injury. Brain Inj. 2004;18(7):661–69. doi: 10.1080/02699050310001646170. 10.1080/02699050310001646170. [DOI] [PubMed] [Google Scholar]
- 56.Wiese H, Tönnes C, De Greiff A, Nebel K, Diener HC, Stude P. Self-initiated movements in chronic prefrontal traumatic brain injury: An event-related functional MRI study. Neuroimage. 2006;30(4):1292–1301. doi: 10.1016/j.neuroimage.2005.11.012. 10.1016/j.neuroimage.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, Kalnin AJ, Liu WC, Steffener J, Diamond BJ, Ni AC. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;71(2):161–68. doi: 10.1136/jnnp.71.2.161. 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, Briggs RW. Parametric manipulation of working memory load in traumatic brain injury: Behavioral and neural correlates. J Int Neuropsychol Soc. 2004;10(5):724–41. doi: 10.1017/S1355617704105110. 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz TW, Rowley HA, Kawahara TN, Johnson SC. Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia. 2006;44(5):762–73. doi: 10.1016/j.neuropsychologia.2005.07.012. 10.1016/j.neuropsychologia.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: A functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47(7):501–6. doi: 10.1007/s00234-005-1372-x. 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- 61.McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53(6):1300–8. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 62.McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–12. doi: 10.1006/nimg.2001.0899. 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- 63.Newsome MR, Scheibel RS, Hunter JV, Wang ZJ, Chu Z, Li X, Levin HS. Brain activation during working memory after traumatic brain injury in children. Neurocase. 2007;13(1):16–24. doi: 10.1080/13554790601186629. 10.1080/13554790601186629. [DOI] [PubMed] [Google Scholar]
- 64.Newsome MR, Scheibel RS, Steinberg JL, Troyanskaya M, Sharma RG, Rauch RA, Li X, Levin HS. Working memory brain activation following severe traumatic brain injury. Cortex. 2007;43(1):95–111. doi: 10.1016/s0010-9452(08)70448-9. 10.1016/S0010-9452(08)70448-9. [DOI] [PubMed] [Google Scholar]
- 65.Scheibel RS, Pearson DA, Faria LP, Kotrla KJ, Aylward E, Bachevalier J, Levin HS. An fMRI study of executive functioning after severe diffuse TBI. Brain Inj. 2003;17(11):919–30. doi: 10.1080/0269905031000110472. 10.1080/0269905031000110472. [DOI] [PubMed] [Google Scholar]
- 66.Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Troyanskaya M, Sharma RG, Levin HS. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil Neural Repair. 2007;21(1):36–45. doi: 10.1177/1545968306294730. 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- 67.Lovell MR, Pardini JE, Welling J, Collins MW, Bakal J, Lazar N, Roush R, Eddy WF, Becker JT. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2):352–60. doi: 10.1227/01.NEU.0000279985.94168.7F. 10.1227/01.NEU.0000279985.94168.7F. [DOI] [PubMed] [Google Scholar]
- 68.Maruishi M, Miyatani M, Nakao T, Muranaka H. Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: A functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2007;78(2):168–73. doi: 10.1136/jnnp.2006.097345. 10.1136/jnnp.2006.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cazalis F, Feydy A, Valabrègue R, Pélégrini-Issac M, Pierot L, Azouvi P. fMRI study of problem-solving after severe traumatic brain injury. Brain Inj. 2006;20(10):1019–28. doi: 10.1080/02699050600664384. 10.1080/02699050600664384. [DOI] [PubMed] [Google Scholar]
- 70.Rasmussen IA, Xu J, Antonsen IK, Brunner J, Skandsen T, Axelson DE, Berntsen EM, Lydersen S, Håberg A. Simple dual tasking recruits prefrontal cortices in chronic severe traumatic brain injury patients, but not in controls. J Neurotrauma. 2008;25(9):1057–70. doi: 10.1089/neu.2008.0520. 10.1089/neu.2008.0520. [DOI] [PubMed] [Google Scholar]
- 71.Kim YH, Yoo WK, Ko MH, Park CH, Kim ST, Na DL. Plasticity of the attentional network after brain injury and cognitive rehabilitation. Neurorehabil Neural Repair. 2008;23(5):468–77. doi: 10.1177/1545968308328728. 10.1177/1545968308328728. [DOI] [PubMed] [Google Scholar]
- 72.Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: An fMRI study. Neuroimage. 2004;22(1):68–82. doi: 10.1016/j.neuroimage.2003.12.032. 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Chen JK, Johnston KM, Collie A, McCrory P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry. 2007;78(11):1231–38. doi: 10.1136/jnnp.2006.110395. 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garnett MR, Blamire AM, Corkill RG, Rajagopalan B, Young JD, Cadoux-Hudson TA, Styles P. Abnormal cerebral blood volume in regions of contused and normal appearing brain following traumatic brain injury using perfusion magnetic resonance imaging. J Neurotrauma. 2001;18(6):585–93. doi: 10.1089/089771501750291828. 10.1089/089771501750291828. [DOI] [PubMed] [Google Scholar]
- 75.Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, Downey SP, Williams GB, Aigbirhio F, Hutchinson PJ, Rice K, Carpenter TA, Clark JC, Pickard JD, Monon DK. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24(2):202–11. doi: 10.1097/01.WCB.0000103022.98348.24. 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- 76.Coles JP, Fryer TD, Smielewski P, Rice K, Clark JC, Pickard JD, Menon DK. Defining ischemic burden after traumatic brain injury using 15O PET imaging of cerebral physiology. J Cereb Blood Flow Metab. 2004;24(2):191–201. doi: 10.1097/01.WCB.0000100045.07481.DE. 10.1097/01.WCB.0000100045.07481.DE. [DOI] [PubMed] [Google Scholar]
- 77.Coles JP, Minhas PS, Fryer TD, Smielewski P, Aigbirhio F, Donovan T, Downey SP, Williams G, Chatfield D, Matthews JC, Gupta AK, Carpenter TA, Clark JC, Pickard JD, Menon DK. Effect of hyperventilation on cerebral blood flow in traumatic head injury: Clinical relevance and monitoring correlates. Crit Care Med. 2002;30(9):1950–59. doi: 10.1097/00003246-200209000-00002. 10.1097/00003246-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Cunningham AS, Salvador R, Coles JP, Chatfield DA, Bradley PG, Johnston AJ, Steiner LA, Fryer TD, Aigbirhio FI, Smielewski P, Williams GB, Carpenter TA, Gillard JH, Pickard JD, Menon DK. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Brain. 2005;128(Pt 8):1931–42. doi: 10.1093/brain/awh536. 10.1093/brain/awh536. [DOI] [PubMed] [Google Scholar]
- 79.Menon DK, Coles JP, Gupta AK, Fryer TH, Smielewski P, Chatfield DA, Aigbirhio F, Skepper JN, Minhas PS, Hutchinson PJ, Carpenter TA, Clark JC, Pickard JD. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32(6):1384–90. doi: 10.1097/01.ccm.0000127777.16609.08. 10.1097/01.CCM.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- 80.O’Connell MT, Seal A, Nortje J, Al-Rawi PG, Coles JP, Fryer TH, Menon DK, Pickard JD, Hutchinson PJ. Glucose metabolism in traumatic brain injury: A combined microdialysis and [18F]-2-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) study. Acta Neurochir Suppl. 2005;95:165–68. doi: 10.1007/3-211-32318-x_35. 10.1007/3-211-32318-X_35. [DOI] [PubMed] [Google Scholar]
- 81.Owen AM, Coleman MR, Monon DK, Johnsrude IS, Rodd JM, Davis MH, Taylor K, Pickard JD. Residual auditory function in persistent vegetative state: A combined PET and fMRI study. Neuropsychol Rehabil. 2005;15(3–4):290–306. doi: 10.1080/09602010443000579. 10.1080/09602010443000579. [DOI] [PubMed] [Google Scholar]
- 82.Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25(6):763–74. doi: 10.1038/sj.jcbfm.9600073. 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973–81. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. 10.1016/S0006-3223(96)00162-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related post-traumatic stress disorder. Biol Psychiatry. 1996;40(11):1091–99. doi: 10.1016/S0006-3223(96)00229-6. 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27(4):951–59. doi: 10.1017/s0033291797005242. 10.1017/S0033291797005242. [DOI] [PubMed] [Google Scholar]
- 87.Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, Den Heeten GJ, Gersons BP. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56(5):356–63. doi: 10.1016/j.biopsych.2004.05.021. 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 88.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53(10):879–89. doi: 10.1016/s0006-3223(02)01891-7. 10.1016/S0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 89.Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40(1):66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 90.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–47. doi: 10.1038/nn958. 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):952–59. doi: 10.1016/s0006-3223(01)01245-8. 10.1016/S0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52(2):119–25. doi: 10.1016/s0006-3223(02)01359-8. 10.1016/S0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 93.Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14(3):292–300. doi: 10.1002/hipo.10183. 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 94.Emdad R, Bonekamp D, Sondergaard HP, Bjorklund T, Agartz I, Ingvar M, Theorell T. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychother Psychosom. 2006;75(2):122–32. doi: 10.1159/000090897. 10.1159/000090897. [DOI] [PubMed] [Google Scholar]
- 95.Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Cariler IV, Majoie CB, Den Heeten GJ, Gersons BP. Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: A MRI investigation. Psychol Med. 2005;35(10):1421–31. doi: 10.1017/S0033291705005246. 10.1017/S0033291705005246. [DOI] [PubMed] [Google Scholar]
- 96.Lindauer RJ, Olff M, Van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59(2):171–77. doi: 10.1016/j.biopsych.2005.06.033. 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 97.Mahmutyaziciolu K, Konuk N, Ozdemir H, Atasoy N, Atik L, Gündodu S. Evaluation of the hippocampus and the anterior cingulate gyrus by proton MR spectroscopy in patients with post-traumatic stress disorder. Diagn Interv Radiol. 2005;11(3):125–29. [PubMed] [Google Scholar]
- 98.Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biol Psychiatry. 2005;57(2):173–82. doi: 10.1016/j.biopsych.2004.10.004. 10.1016/j.biopsych.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Li L, Chen S, Liu J, Zhang J, He Z, Lin X. Magnetic resonance imaging and magnetic resonance spectroscopy study of deficits in hippocampal structure in fire victims with recent-onset posttraumatic stress disorder. Can J Psychiatry. 2006;51(7):431–37. doi: 10.1177/070674370605100704. [DOI] [PubMed] [Google Scholar]
- 100.Hedges DW, Allen S, Tate DF, Thatcher GW, Miller MJ, Rice SA, Cleavinger HB, Sood S, Bigler ED. Reduced hippocampal volume in alcohol and substance naïve Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16(4):219–24. doi: 10.1097/00146965-200312000-00003. 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 101.Golier JA, Yehuda R, De Santi S, Segal S, Dolan S, De Leon MJ. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without post-traumatic stress disorder. Psychiatry Res. 2005;139(1):53–64. doi: 10.1016/j.pscychresns.2005.02.007. 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 102.Yehuda R, Golier JA, Tischler L, Harvey PD, Newmark R, Yang RK, Buchsbaum MS. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: Relation to risk and resilience factors. J Psychiatr Res. 2007;41(5):435–45. doi: 10.1016/j.jpsychires.2005.12.002. 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004;161(12):2194–2200. doi: 10.1176/appi.ajp.161.12.2194. 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- 104.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45(10):1271–84. doi: 10.1016/s0006-3223(99)00045-1. 10.1016/S0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 105.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):943–51. doi: 10.1016/s0006-3223(01)01218-5. 10.1016/S0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 106.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50(4):305–9. doi: 10.1016/s0006-3223(01)01105-2. 10.1016/S0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 107.Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD, Woodruff PW. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry. 2004;56(11):832–36. doi: 10.1016/j.biopsych.2004.09.015. 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 108.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158(8):1248–51. doi: 10.1176/appi.ajp.158.8.1248. 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52(11):1089–1101. doi: 10.1016/s0006-3223(02)01413-0. 10.1016/S0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 110.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 111.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 2005;15(6):798–807. doi: 10.1002/hipo.20102. 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 112.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. 10.1016/S0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14(7):913–16. doi: 10.1097/01.wnr.0000071767.24455.10. 10.1097/00001756-200305230-00002. [DOI] [PubMed] [Google Scholar]
- 114.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90(2–3):171–74. doi: 10.1016/j.jad.2005.11.006. 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63(6):550–56. doi: 10.1016/j.biopsych.2007.06.022. 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100(15):9039–43. doi: 10.1073/pnas.1530467100. 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Corbo V, Clément MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: Contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry. 2005;58(2):119–24. doi: 10.1016/j.biopsych.2005.02.032. 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 118.Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59(7):582–87. doi: 10.1016/j.biopsych.2005.07.033. 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 119.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156(11):1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol Psychiatry. 2003;53(3):204–10. doi: 10.1016/s0006-3223(02)01466-x. 10.1016/S0006-3223(02)01466-X. [DOI] [PubMed] [Google Scholar]
- 121.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45(7):806–16. doi: 10.1016/s0006-3223(98)00297-2. 10.1016/S0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am J Psychiatry. 2001;158(11):1920–22. doi: 10.1176/appi.ajp.158.11.1920. 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 123.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45(7):817–26. doi: 10.1016/s0006-3223(98)00246-7. 10.1016/S0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 124.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156(4):575–84. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 125.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54(3):233–41. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 126.Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63(1):65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 127.Rauch SL, Van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53(5):380–87. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 128.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–76. doi: 10.1001/archpsyc.61.2.168. 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 129.Astur RS, St Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9(2):234–40. doi: 10.1089/cpb.2006.9.234. 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 130.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–42. doi: 10.1016/s0006-3223(01)01215-x. 10.1016/S0006-3223(01)01215-X. [DOI] [PubMed] [Google Scholar]
- 131.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. 10.1016/S0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 132.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. 10.1017/S0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160(5):924–32. doi: 10.1176/appi.ajp.160.5.924. 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 134.Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55(6):612–20. doi: 10.1016/j.biopsych.2003.10.001. 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 135.Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep. 2002;4(4):254–63. doi: 10.1007/s11920-996-0044-9. 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- 136.Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am J Psychiatry. 2000;157(7):1120–26. doi: 10.1176/appi.ajp.157.7.1120. 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 137.Fujita M, Southwick SM, Denucci CC, Zoghbi SS, Dillon MS, Baldwin RM, Bozkurt A, Kugaya A, Verhoeff NP, Seibyl JP, Innis RB. Central type benzodiazepine receptors in Gulf War veterans with posttraumatic stress disorder. Biol Psychiatry. 2004;56(2):95–100. doi: 10.1016/j.biopsych.2004.03.010. 10.1016/j.biopsych.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 138.Le Bihan D, Breton E. [Imagerie de diffusion in vivo par résonance magnétique nucléaire] C R Acad Sc Paris. 1985;301(15):1109–12. French. [Google Scholar]
- 139.Merboldt KD, Hanicke W, Frahm J. Self-diffusion NMR imaging using stimulated echoes. J Magn Reson. 1985;64(3):479–86. [Google Scholar]
- 140.Taylor DG, Bushell MC. The spatial mapping of translational diffusion coefficients by the NMR imaging technique. Phys Med Biol. 1985;30(4):345–49. doi: 10.1088/0031-9155/30/4/009. 10.1088/0031-9155/30/4/009. [DOI] [PubMed] [Google Scholar]
- 141.Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995;8(7–8):375–86. doi: 10.1002/nbm.1940080711. 10.1002/nbm.1940080711. [DOI] [PubMed] [Google Scholar]
- 142.Le Bihan D. Applications to functional MRI. New York (NY): Raven Press; 1995. Diffusion and perfusion magnetic resonance imaging. [Google Scholar]
- 143.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 144.Anderson AW, Gore JC. Analysis and correction of motion artifacts in diffusion weighted imaging. Magn Reson Med. 1994;32(3):379–87. doi: 10.1002/mrm.1910320313. 10.1002/mrm.1910320313. [DOI] [PubMed] [Google Scholar]
- 145.Moseley ME, Cohen Y, Mintorovich J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR. Early detection of regional cerebral ischemia in cats: Comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14(2):330–46. doi: 10.1002/mrm.1910140218. 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- 146.Chenevert TL, Brunberg JA, Pipe JG. Anisotropic diffusion in human white matter: Demonstration with MR techniques in vivo. Radiology. 1990;177(2):401–5. doi: 10.1148/radiology.177.2.2217776. [DOI] [PubMed] [Google Scholar]
- 147.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002;15(7–8):435–55. doi: 10.1002/nbm.782. 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 148.Douek P, Turner R, Pekar J, Patronas N, Le Bihan D. MR color mapping of myelin fiber orientation. J Comput Assist Tomogr. 1991;15(6):923–29. doi: 10.1097/00004728-199111000-00003. 10.1097/00004728-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 149.Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–67. doi: 10.1016/S0006-3495(94)80775-1. 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Basser PJ, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–54. doi: 10.1006/jmrb.1994.1037. 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 151.Le Bihan D, Van Zijl P. From the diffusion coefficient to the diffusion tensor. NMR Biomed. 2002;15(7–8):431–34. doi: 10.1002/nbm.798. 10.1002/nbm.798. [DOI] [PubMed] [Google Scholar]
- 152.Basser PJ. Imaging brain structure and function. In: Lester DS, Felder CC, Lewis EN, editors. Imaging brain structure and function: Emerging Technologies in the Neurosciences. New York (NY): New York Academy of Sciences; 1997. [PubMed] [Google Scholar]
- 153.Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury—A review. NMR Biomed. 2002;15(7–8):561–69. doi: 10.1002/nbm.786. 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- 154.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–36. doi: 10.1006/nimg.2002.1267. 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 155.Mori S, Van Zijl PC. Fiber tracking: Principles and strategies—A technical review. NMR Biomed. 2002;15(7–8):468–80. doi: 10.1002/nbm.781. 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 156.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936–52. doi: 10.1016/j.cortex.2008.05.002. 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 157.Mori S, Crain BJ, Chacko VP, Van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–69. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. 10.1002/1531-8249(199902)45:2<265::AID-ANA21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 158.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96(18):10422–27. doi: 10.1073/pnas.96.18.10422. 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poupon C, Clark CA, Frouin V, Régis J, Bloch I, Le Bihan D, Mangin J. Regularization of diffusion-based direction maps for the tracking of brain white matter fascicles. Neuroimage. 2000;12(2):184–95. doi: 10.1006/nimg.2000.0607. 10.1006/nimg.2000.0607. [DOI] [PubMed] [Google Scholar]
- 160.Jbabdi S, Woolrich MW, Andersson JL, Behrens TE. A Bayesian framework for global tractography. Neuroimage. 2007;37(1):116–29. doi: 10.1016/j.neuroimage.2007.04.039. 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 161.Jones DK, Pierpaoli C. Confidence mapping in diffusion tensor magnetic resonance imaging tractography using a bootstrap approach. Magn Reson Med. 2005;53(5):1143–49. doi: 10.1002/mrm.20466. 10.1002/mrm.20466. [DOI] [PubMed] [Google Scholar]
- 162.Cherubini A, Luccichenti G, Péran P, Hagberg GE, Barba C, Formisano R, Sabatini U. Multimodal fMRI tractography in normal subjects and in clinically recovered traumatic brain injury patients. Neuroimage. 2007;34(4):1331–41. doi: 10.1016/j.neuroimage.2006.11.024. 10.1016/j.neuroimage.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 163.Jang SH, Ahn SH, Ha JS, Lee SJ, Lee J, Ahn YH. Peri-infarct reorganization in a patient with corona radiata infarct: A combined study of functional MRI and diffusion tensor image tractography. Restor Neurol Neurosci. 2006;24(2):65–68. [PubMed] [Google Scholar]
- 164.Ono J, Harada K, Mano T, Sakurai K, Okada S. Differentiation of dys- and demyelination using diffusional anisotropy. Pediatr Neurol. 1997;16(1):63–66. doi: 10.1016/s0887-8994(96)00249-4. 10.1016/S0887-8994(96)00249-4. [DOI] [PubMed] [Google Scholar]
- 165.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52(8):1626–32. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 166.Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol. 1999;20(8):1491–99. [PMC free article] [PubMed] [Google Scholar]
- 167.Iwasawa T, Matoba H, Ogi A, Kurihara H, Saito K, Yoshida T, Matsubara S, Nozaki A. Diffusion-weighted imaging of the human optic nerve: A new approach to evaluate optic neuritis in multiple sclerosis. Magn Reson Med. 1997;38(3):484–91. doi: 10.1002/mrm.1910380317. 10.1002/mrm.1910380317. [DOI] [PubMed] [Google Scholar]
- 168.Horsfield MA, Larsson HB, Jones DK, Gass A. Diffusion magnetic resonance imaging in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(Suppl 1):S80–S84. [PubMed] [Google Scholar]
- 169.Ay H, Buonanno FS, Schaefer PW, Le DA, Wang B, Gonzalez RG, Koroshetz WJ. Posterior leukoencephalopathy without severe hypertension: Utility of diffusion-weighted MRI. Neurology. 1998;51(5):1369–76. doi: 10.1212/wnl.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 170.Eichler FS, Itoh R, Barker PB, Mori S, Garrett ES, Van Zijl PC, Moser HW, Raymond GV, Melhem ER. Proton MR spectroscopic and diffusion tensor brain MR imaging in X-linked adrenoleukodystrophy: Initial experience. Radiology. 2002;225(1):245–52. doi: 10.1148/radiol.2251011040. 10.1148/radiol.2251011040. [DOI] [PubMed] [Google Scholar]
- 171.Filippi CC, Ulug AM, Ryan E, Ferrando SJ, Van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol. 2001;22(2):277–83. [PMC free article] [PubMed] [Google Scholar]
- 172.Hanyu H, Shindo H, Kakizaki D, Abe K, Iwamoto T, Takasaki M. Increased water diffusion in cerebral white matter in Alzheimer’s disease. Gerontology. 1997;43(6):343–51. doi: 10.1159/000213874. 10.1159/000213874. [DOI] [PubMed] [Google Scholar]
- 173.Hanyu H, Sakurai H, Iwamoto T, Takasaki M, Shindo H, Abe K. Diffusion-weighted MR imaging of the hippocampus and temporal white matter in Alzheimer’s disease. J Neurol Sci. 1998;156(2):195–200. doi: 10.1016/s0022-510x(98)00043-4. 10.1016/S0022-510X(98)00043-4. [DOI] [PubMed] [Google Scholar]
- 174.Chabriat H, Pappata S, Poupon C, Clark CA, Vahedi K, Poupon F, Mangin JF, Pachot-Clouard M, Jobert A, Le Bihan D, Bousser MG. Clinical severity in CADASIL related to ultrastructural damage in white matter: In vivo study with diffusion tensor MRI. Stroke. 1999;30(12):2637–43. doi: 10.1161/01.str.30.12.2637. [DOI] [PubMed] [Google Scholar]
- 175.Horsfield MA, Jones DK. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases—A review. NMR Biomed. 2002;15(7–8):570–77. doi: 10.1002/nbm.787. 10.1002/nbm.787. [DOI] [PubMed] [Google Scholar]
- 176.Lim KO, Helpern JA. Neuropsychiatric applications of DTI—A review. NMR Biomed. 2002;15(7–8):587–93. doi: 10.1002/nbm.789. 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- 177.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J Neurotrauma. 2007;24(9):1447–59. doi: 10.1089/neu.2007.0241. 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 178.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–55. doi: 10.1212/01.wnl.0000305961.68029.54. 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 179.Chappell MH, Ulu AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, Watts R. Distribution of microstructural damage in the brains of professional boxers: A diffusion MRI study. J Magn Reson Imaging. 2006;24(3):537–42. doi: 10.1002/jmri.20656. 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- 180.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–47. doi: 10.1162/089892902317361886. 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 181.Lundin A, De Boussard C, Edman G, Borg J. Symptoms and disability until 3 months after mild TBI. Brain Inj. 2006;20(8):799–806. doi: 10.1080/02699050600744327. 10.1080/02699050600744327. [DOI] [PubMed] [Google Scholar]
- 182.Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, Ohtani T, Masutani Y, Kato N, Ohtomo K. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. 2006;146(3):231–42. doi: 10.1016/j.pscychresns.2006.01.004. 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 183.Kim SJ, Jeong DU, Sim ME, Bae SC, Chung A, Kim MJ, Chang KH, Ryu J, Renshaw PF, Lyoo IK. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54(2):120–25. doi: 10.1159/000098262. 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- 184.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. 2008;162(3):256–61. doi: 10.1016/j.pscychresns.2007.08.006. 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Levine B, Kovacevic N, Nica EI, Cheung G, Gao F, Schwartz ML, Black SE. The Toronto traumatic brain injury study: Injury severity and quantified MRI. Neurology. 2008;70(10):771–78. doi: 10.1212/01.wnl.0000304108.32283.aa. 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- 186.Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 1997;18(1):11–23. [PMC free article] [PubMed] [Google Scholar]
- 187.Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 2002;23(2):255–66. [PMC free article] [PubMed] [Google Scholar]
- 188.Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, Fearing MA, Cleavinger HB, Li X, Swank PR, Pedroza C, Roberson GS, Bachevalier J, Levin HS. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma. 2005;22(3):333–44. doi: 10.1089/neu.2005.22.333. 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- 189.Fearing MA, Bigler ED, Wilde EA, Johnson JL, Hunter JV, Xiaogi Li, Hanten G, Levin HS. Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. J Child Neurol. 2008;23(7):729–37. doi: 10.1177/0883073808314159. 10.1177/0883073808314159. [DOI] [PubMed] [Google Scholar]
- 190.Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, Detre JA, Gee JC. Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage. 2008;39(3):1014–26. doi: 10.1016/j.neuroimage.2007.10.005. 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sidaros A, Skimminge A, Liptrot MG, Sidaros K, Engberg AW, Herning M, Paulson OB, Jernigan TL, Rostrup E. Long-term global and regional brain volume changes following severe traumatic brain injury: A longitudinal study with clinical correlates. Neuroimage. 2009;44(1):1–8. doi: 10.1016/j.neuroimage.2008.08.030. 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 192.Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, Petropoulos H, Brooks WM. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131(3):227–35. doi: 10.1016/j.pscychresns.2004.05.002. 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 193.Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, Wald LL, Renshaw PF, Frederick BB, Lane B, Sheikh JI, Stegman WK, Kutter CJ, Stewart LP, Prestel RS, Arsenault NJ. Brain, skull, and cerebrospinal fluid volumes in adult posttraumatic stress disorder. J Trauma Stress. 2007;20(5):763–74. doi: 10.1002/jts.20241. 10.1002/jts.20241. [DOI] [PubMed] [Google Scholar]
- 194.Weniger G, Lange C, Sachsse U, Irle E. Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand. 2008;118(4):281–90. doi: 10.1111/j.1600-0447.2008.01246.x. 10.1111/j.1600-0447.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 195.Kitayama N, Brummer M, Hertz L, Quinn S, Kim Y, Bremner JD. Morphologic alterations in the corpus callosum in abuse-related posttraumatic stress disorder: A preliminary study. J Nerv Ment Dis. 2007;195(12):1027–29. doi: 10.1097/NMD.0b013e31815c044f. 10.1097/NMD.0b013e31815c044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Duvernoy HM. Functional anatomy, vascularization and serial sections with MRI. 3. Berlin, Heidelberg (Germany): Springer-Verlag Berlin Heidelberg; 2005. The human hippocampus. [Google Scholar]
- 197.Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- 198.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: Implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 199.Alfarez DN, Wiegert O, Krugers HJ. Stress, corticosteroid hormones and hippocampal synaptic function. CNS Neurol Disord Drug Targets. 2006;5(5):521–29. doi: 10.2174/187152706778559345. 10.2174/187152706778559345. [DOI] [PubMed] [Google Scholar]
- 200.Anderson KJ, Miller KM, Fugaccia I, Scheff SW. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp Neurol. 2005;193(1):125–30. doi: 10.1016/j.expneurol.2004.11.025. 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 201.McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947(1):41–49. doi: 10.1016/s0006-8993(02)02904-9. 10.1016/S0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- 202.McCullers DL, Sullivan PG, Scheff SW, Herman JP. Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience. 2002;109(2):219–30. doi: 10.1016/s0306-4522(01)00477-8. 10.1016/S0306-4522(01)00477-8. [DOI] [PubMed] [Google Scholar]
- 203.Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: A 15-year review and evaluation. J Neurotrauma. 2005;22(1):42–75. doi: 10.1089/neu.2005.22.42. 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 204.Ariza M, Serra-Grabulosa JM, Junqué C, Ramírez B, Mataró M, Poca A, Bargalló N, Sahuquillo J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44(10):1956–61. doi: 10.1016/j.neuropsychologia.2005.11.007. 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 205.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Inj. 2005;19(2):93–100. doi: 10.1080/02699050410001720031. 10.1080/02699050410001720031. [DOI] [PubMed] [Google Scholar]
- 206.Tomaiuolo F, Carlesimo GA, Di Paola M, Petrides M, Fera F, Bonanni R, Formisano R, Pasqualetti P, Caltagirone C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: A T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75(9):1314–22. doi: 10.1136/jnnp.2003.017046. 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Serra-Grabulosa JM, Junqué C, Verger K, Salgado-Pineda P, Mañeru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J Neurol Neurosurg Psychiatry. 2005;76(1):129–31. doi: 10.1136/jnnp.2004.027631. 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shin LM, Rauch SL, Pitman RK. Amygdala, medial pre-frontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 209.De Vita E, Thomas DL, Roberts S, Parkes HG, Turner R, Kinchesch P, Shmueli K, Yousry TA, Ordidge RJ. High resolution MRI of the brain at 4.7 Tesla using fast spin echo imaging. Br J Radiol. 2003;76(909):631–37. doi: 10.1259/bjr/69317841. 10.1259/bjr/69317841. [DOI] [PubMed] [Google Scholar]
- 210.Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weinder MW. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28(5):719–26. doi: 10.1016/j.neurobiolaging.2006.03.007. 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–79. doi: 10.1073/pnas.89.12.5675. 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–55. doi: 10.1073/pnas.89.13.5951. 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: Quantification of tonic CBF changes during sustained attention and working memory tasks. Neuroimage. 2006;31(1):376–85. doi: 10.1016/j.neuroimage.2005.11.035. 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hillary FG, Steffener J, Biswal BB, Lange G, DeLuca J, Ashburner J. Functional magnetic resonance imaging technology and traumatic brain injury rehabilitation: Guidelines for methodological and conceptual pitfalls. J Head Trauma Rehabil. 2002;17(5):411–30. doi: 10.1097/00001199-200210000-00004. 10.1097/00001199-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 215.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci. 2003;4(11):863–72. doi: 10.1038/nrn1246. 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 216.Bartsch AJ, Homola G, Biller A, Solymosi L, Bendszus M. Diagnostic functional MRI: Illustrated clinical applications and decision-making. J Magn Reson Imaging. 2006;23(6):921–32. doi: 10.1002/jmri.20579. 10.1002/jmri.20579. [DOI] [PubMed] [Google Scholar]
- 217.McDowell S, Whyte J, D’Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121(Pt 6):1155–64. doi: 10.1093/brain/121.6.1155. 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- 218.Jantzen KJ, Anderson B, Steinberg FL, Kelso JA. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am J Neuroradiol. 2004;25(5):738–45. [PMC free article] [PubMed] [Google Scholar]
- 219.Ptito A, Chen JK, Johnston KM. Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation. 2007;22(3):217–27. [PubMed] [Google Scholar]
- 220.Chen JK, Johnston KM, Petrides M, Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch Gen Psychiatry. 2008;65(1):81–89. doi: 10.1001/archgenpsychiatry.2007.8. 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- 221.Frewen P, Lane RD, Neufeld RW, Densmore M, Stevens T, Lanius R. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosom Med. 2008;70(1):27–31. doi: 10.1097/PSY.0b013e31815f66d4. 10.1097/PSY.0b013e31815f66d4. [DOI] [PubMed] [Google Scholar]
- 222.Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: A functional MRI investigation. Psychiatry Res. 2007;155(1):45–56. doi: 10.1016/j.pscychresns.2006.11.006. 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 223.Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64(8):681–90. doi: 10.1016/j.biopsych.2008.05.027. 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: A functional MRI study. Neuroimage. 2005;26(3):813–21. doi: 10.1016/j.neuroimage.2005.02.032. 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 225.Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: A functional MRI study. Neurosci Lett. 2004;370(1):13–18. doi: 10.1016/j.neulet.2004.07.033. 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 226.Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. Sensing the invisible: Differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19(3):587–600. doi: 10.1016/s1053-8119(03)00141-1. 10.1016/S1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 227.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 228.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961–63. doi: 10.1176/appi.ajp.162.10.1961. 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 229.Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp. 2008;29(5):517–23. doi: 10.1002/hbm.20415. 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, Bryant RA, Williams LM. Influence of comorbid depression on fear in posttraumatic stress disorder: An fMRI study. Psychiatry Res. 2007;155(3):265–69. doi: 10.1016/j.pscychresns.2007.01.010. 10.1016/j.pscychresns.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 231.Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, Gordon E, Williams LM. Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58(2):111–18. doi: 10.1016/j.biopsych.2005.03.021. 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 232.Morey RA, Petty CM, Cooper DA, Labar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res. 2008;162(1):59–72. doi: 10.1016/j.pscychresns.2007.07.007. 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, Martis B, Macklin ML, Lasko NB, Orr SP, Pitman RK, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20(5):701–12. doi: 10.1002/jts.20231. 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- 234.Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: An fMRI study in youth. Depress Anxiety. 2008;25(6):514–26. doi: 10.1002/da.20346. 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- 235.Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33(5):413–22. [PMC free article] [PubMed] [Google Scholar]
- 236.Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19(1):5–20. doi: 10.1176/jnp.2007.19.1.5. 10.1176/appi.neuropsych.19.1.5. [DOI] [PubMed] [Google Scholar]
- 237.Strangman G, O’Neil-Pirozzi TM, Burke D, Cristina D, Goldstein R, Rauch SL, Savage CR, Glenn MB. Functional neuroimaging and cognitive rehabilitation for people with traumatic brain injury. Am J Phys Med Rehabil. 2005;84(1):62–75. doi: 10.1097/01.phm.0000150787.26860.12. 10.1097/01.PHM.0000150787.26860.12. [DOI] [PubMed] [Google Scholar]
- 238.Metting Z, Rödiger LA, De Keyser J, Van der Naalt J. Structural and functional neuroimaging in mild-to-moderate head injury. Lancet Neurol. 2007;6(8):699–710. doi: 10.1016/S1474-4422(07)70191-6. 10.1016/S1474-4422(07)70191-6. [DOI] [PubMed] [Google Scholar]
- 239.Laatsch L, Little DM, Thulborn KR. Changes in fMRI following cognitive rehabilitation in severe traumatic brain injury: A case study. Rehabil Psychol. 2004;49(3):262–67. 10.1037/0090-5550.49.3.262. [Google Scholar]
- 240.Laatsch L, Krisky C. Changes in fMRI activation following rehabilitation of reading and visual processing deficits in subjects with traumatic brain injury. Brain Inj. 2006;20(13–14):1367–75. doi: 10.1080/02699050600983743. 10.1080/02699050600983743. [DOI] [PubMed] [Google Scholar]
- 241.Strangman GE, O’Neil-Pirozzi TM, Goldstein R, Kelkar K, Katz DI, Burke D, Rauch SL, Savage CR, Glenn MB. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Arch Phys Med Rehabil. 2008;89(5):974–81. doi: 10.1016/j.apmr.2008.02.011. 10.1016/j.apmr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 242.Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127–29. doi: 10.1111/j.1467-9280.2007.01860.x. 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 243.Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med. 2008;38(4):555–61. doi: 10.1017/S0033291707002231. 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- 244.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 245.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–63. doi: 10.1016/j.neuroimage.2004.06.035. 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 246.Sun FT, Miller LM, D’Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004;21(2):647–58. doi: 10.1016/j.neuroimage.2003.09.056. 10.1016/j.neuroimage.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 247.Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, Beckmann CF. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum Brain Mapp. 2009;30(1):256–66. doi: 10.1002/hbm.20505. 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–13. doi: 10.1098/rstb.2005.1634. 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rajapakse JC, Tan CL, Zheng X, Mukhopadhyay S, Yang K. Exploratory analysis of brain connectivity with ICA. IEEE Eng Med Biol Mag. 2006;25(2):102–11. doi: 10.1109/memb.2006.1607674. 10.1109/MEMB.2006.1607674. [DOI] [PubMed] [Google Scholar]
- 250.He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol. 2007;20(6):655–60. doi: 10.1097/WCO.0b013e3282f1c720. 10.1097/WCO.0b013e3282f1c720. [DOI] [PubMed] [Google Scholar]
- 251.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 252.Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proc Natl Acad Sci U S A. 1982;79(11):3523–26. doi: 10.1073/pnas.79.11.3523. 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Maudsley AA, Hilal SK, Perman WH, Simon HE. Spatially resolved high-resolution spectroscopy by “four-dimensional” NMR. J Magn Reson. 1983;51:147–51. [Google Scholar]
- 254.Posse S, DeCarli C, Le Bihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology. 1994;192(3):733–38. doi: 10.1148/radiology.192.3.8058941. [DOI] [PubMed] [Google Scholar]
- 255.Adalsteinsson E, Irarrazabal P, Spielman DM, Macovski A. Three-dimensional spectroscopic imaging with time-varying gradients. Magn Reson Med. 1995;33(4):461–66. doi: 10.1002/mrm.1910330402. 10.1002/mrm.1910330402. [DOI] [PubMed] [Google Scholar]
- 256.Gonen O, Hu J, Stoyanova R, Leigh JS, Goelman G, Brown TR. Hybrid three dimensional (1D-Hadamard, 2D-chemical shift imaging) phosphorus localized spectroscopy of phantom and human brain. Magn Reson Med. 1995;33(3):300–308. doi: 10.1002/mrm.1910330304. 10.1002/mrm.1910330304. [DOI] [PubMed] [Google Scholar]
- 257.Segebarth CM, Balériaux DF, Luyten PR, Den Hollander JA. Detection of metabolic heterogeneity of human intracranial tumors in vivo by 1H NMR spectroscopic imaging. Magn Reson Med. 1990;13(1):62–76. doi: 10.1002/mrm.1910130108. 10.1002/mrm.1910130108. [DOI] [PubMed] [Google Scholar]
- 258.Fulham MJ, Bizzi A, Dietz MJ, Shih HH, Raman R, Sobering GS, Frank JA, Dwyer AJ, Alger JR, Di Chiro G. Mapping of brain tumor metabolites with proton MR spectroscopic imaging: Clinical relevance. Radiology. 1992;185(3):675–86. doi: 10.1148/radiology.185.3.1438744. [DOI] [PubMed] [Google Scholar]
- 259.Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7-cm3) spatial resolution. Radiology. 1996;198(3):795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 260.Meyerhoff DJ, MacKay S, Constans JM, Norman D, Van Dyke C, Fein G, Weiner MW. Axonal injury and membrane alterations in Alzheimer’s disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1994;36(1):40–47. doi: 10.1002/ana.410360110. 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 261.Tedeschi G, Bertolino A, Lundbom N, Bonavita S, Patronas NJ, Duyn JH, Metman LV, Chase TN, Di Chiro G. Cortical and subcortical chemical pathology in Alzheimer’s disease as assessed by multislice proton magnetic resonance spectroscopic imaging. Neurology. 1996;47(3):696–704. doi: 10.1212/wnl.47.3.696. [DOI] [PubMed] [Google Scholar]
- 262.Cruz CJ, Aminoff MJ, Meyerhoff DJ, Graham SH, Weiner MW. Proton MR spectroscopic imaging of the striatum in Parkinson’s disease. Magn Reson Imaging. 1997;15(6):619–24. doi: 10.1016/s0730-725x(97)00079-9. 10.1016/S0730-725X(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 263.Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CT, Frank JA, Tedeschi G, Weinberger DR. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1996;153(12):1554–63. doi: 10.1176/ajp.153.12.1554. [DOI] [PubMed] [Google Scholar]
- 264.Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. The hippocampus in patients treated with electroconvulsive therapy: A proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57(10):937–43. doi: 10.1001/archpsyc.57.10.937. 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 265.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61(5):450–58. doi: 10.1001/archpsyc.61.5.450. 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 266.Hugg JW, Laxer KD, Matson GB, Maudsley AA, Husted CA, Weiner MW. Lateralization of human focal epilepsy by 31P magnetic resonance spectroscopic imaging. Neurology. 1992;42(10):2011–18. doi: 10.1212/wnl.42.10.2011. [DOI] [PubMed] [Google Scholar]
- 267.Hetherington HP, Kuzniecky RI, Pan JW, Vaughan JT, Twieg DB, Pohost GM. Application of high field spectroscopic imaging in the evaluation of temporal lobe epilepsy. Magn Reson Imaging. 1995;13(8):1175–80. doi: 10.1016/0730-725x(95)02029-s. 10.1016/0730-725X(95)02029-S. [DOI] [PubMed] [Google Scholar]
- 268.Cendes F, Stanley JA, Dubeau F, Andermann F, Arnold DL. Proton magnetic resonance spectroscopic imaging for discrimination of absence and complex partial seizures. Ann Neurol. 1997;41(1):74–81. doi: 10.1002/ana.410410113. 10.1002/ana.410410113. [DOI] [PubMed] [Google Scholar]
- 269.Obergriesser T, Ende G, Braus DF, Henn FA. Hippocampal 1H-MRSI in ecstasy users. Eur Arch Psychiatry Clin Neurosci. 2001;251(3):114–16. doi: 10.1007/s004060170044. 10.1007/s004060170044. [DOI] [PubMed] [Google Scholar]
- 270.O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: Quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25(11):1673–82. 10.1111/j.1530-0277.2001.tb02174.x. [PubMed] [Google Scholar]
- 271.Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: A preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28(12):1849–60. doi: 10.1097/01.alc.0000148112.92525.ac. 10.1097/01.ALC.0000148112.92525.AC. [DOI] [PubMed] [Google Scholar]
- 272.Wolinsky JS, Narayana PA. Magnetic resonance spectroscopy in multiple sclerosis: Window into the diseased brain. Curr Opin Neurol. 2002;15(3):247–51. doi: 10.1097/00019052-200206000-00004. 10.1097/00019052-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 273.Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):46S–57S. doi: 10.1177/1051228405284200. 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cecil KM, Lenkinski RE, Meaney DF, McIntosh TK, Smith DH. High-field proton magnetic resonance spectroscopy of a swine model for axonal injury. J Neurochem. 1998;70(5):2038–44. doi: 10.1046/j.1471-4159.1998.70052038.x. [DOI] [PubMed] [Google Scholar]
- 275.Smith DH, Cecil KM, Meaney DF, Chen XH, McIntosh TK, Gennarelli TA, Lenkinski RE. Magnetic resonance spectroscopy of diffuse brain trauma in the pig. J Neurotrauma. 1998;15(9):665–74. doi: 10.1089/neu.1998.15.665. 10.1089/neu.1998.15.665. [DOI] [PubMed] [Google Scholar]
- 276.Garnett MR, Blamire AM, Rajagopalan B, Styles P, Cadoux-Hudson TA. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: A magnetic resonance spectroscopy study. Brain. 2000;123(Pt 7):1403–9. doi: 10.1093/brain/123.7.1403. 10.1093/brain/123.7.1403. [DOI] [PubMed] [Google Scholar]
- 277.Son BC, Park CK, Choi BG, Kim EN, Choe BY, Lee KS, Kim MC, Kang JK. Metabolic changes in pericontusional oedematous areas in mild head injury evaluated by 1H MRS. Acta Neurochir Suppl. 2000;76:13–16. doi: 10.1007/978-3-7091-6346-7_3. [DOI] [PubMed] [Google Scholar]
- 278.Ham BJ, Chey J, Yoon SJ, Sung Y, Jeong DU, Ju Kim S, Sim ME, Choi N, Choi IG, Renshaw PF, Lyoo IK. Decreased N-acetyl-aspartate levels in anterior cingulate and hippocampus in subjects with post-traumatic stress disorder: A proton magnetic resonance spectroscopy study. Eur J Neurosci. 2007;25(1):324–29. doi: 10.1111/j.1460-9568.2006.05253.x. 10.1111/j.1460-9568.2006.05253.x. [DOI] [PubMed] [Google Scholar]
- 279.Seedat S, Videen JS, Kennedy CM, Stein MB. Single voxel proton magnetic resonance spectroscopy in women with and without intimate partner violence-related post-traumatic stress disorder. Psychiatry Res. 2005;139(3):249–58. doi: 10.1016/j.pscychresns.2005.06.001. 10.1016/j.pscychresns.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 280.Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, Kornak J, Marmar CR, Weiner MW. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162(2):147–57. doi: 10.1016/j.pscychresns.2007.04.011. 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Freeman T, Kimbrell T, Booe L, Myers M, Cardwell D, Lindquist DM, Hart J, Komoroski RA. Evidence of resilience: Neuroimaging in former prisoners of war. Psychiatry Res. 2006;146(1):59–64. doi: 10.1016/j.pscychresns.2005.07.007. 10.1016/j.pscychresns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 282.Wang Z, Wang J, Connick TJ, Wetmore GS, Detre JA. Continuous ASL (CASL) perfusion MRI with an array coil and parallel imaging at 3T. Magn Reson Med. 2005;54(3):732–37. doi: 10.1002/mrm.20574. 10.1002/mrm.20574. [DOI] [PubMed] [Google Scholar]
- 283.Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, Detre J. Remifentanil-induced cerebral blood flow effects in normal humans: Dose and ApoE genotype. Anesth Analg. 2007;105(1):167–75. doi: 10.1213/01.ane.0000266490.64814.ff. 10.1213/01.ane.0000266490.64814.ff. [DOI] [PubMed] [Google Scholar]
- 284.Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, Brodkin ES, Detre JA, Farah MJ. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62(6):600–606. doi: 10.1016/j.biopsych.2006.11.028. 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 285.Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- 286.Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn Reson Med. 2000;44(1):92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. 10.1002/1522-2594(200007)44:1<92::AID-MRM14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 287.Duyn JH, Van Gelderen P, Talagala L, Koretsky A, De Zwart JA. Technological advances in MRI measurement of brain perfusion. J Magn Reson Imaging. 2005;22(6):751–53. doi: 10.1002/jmri.20450. 10.1002/jmri.20450. [DOI] [PubMed] [Google Scholar]
- 288.Fernández-Seara MA, Wang Z, Wang J, Rao HY, Guenther M, Feinberg DA, Detre JA. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magn Reson Med. 2005;54(5):1241–47. doi: 10.1002/mrm.20674. 10.1002/mrm.20674. [DOI] [PubMed] [Google Scholar]
- 289.Garcia DM, Duhamel G, Alsop DC. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med. 2005;54(2):366–72. doi: 10.1002/mrm.20556. 10.1002/mrm.20556. [DOI] [PubMed] [Google Scholar]
- 290.Garcia DM, De Bazelaire C, Alsop D. Pseudo-continuous flow driven adiabatic inversion for arterial spin labeling. Proc Intl Soc Mag Reson Med. 2005;13:37. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: Feasibility study. Radiology. 2005;235(1):218–28. doi: 10.1148/radiol.2351031663. 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 292.Alsop DC, Detre JA. Reduced transit-time sensitivity in non-invasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16(6):1236–49. doi: 10.1097/00004647-199611000-00019. 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 293.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383–96. doi: 10.1002/mrm.1910400308. 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 294.Parkes LM, Tofts PS. Improved accuracy of human cerebral blood perfusion measurements using arterial spin labeling: Accounting for capillary water permeability. Magn Reson Med. 2002;48(1):27–41. doi: 10.1002/mrm.10180. 10.1002/mrm.10180. [DOI] [PubMed] [Google Scholar]
- 295.Ye FQ, Berman KF, Ellmore T, Esposito G, Van Horn JD, Yang Y, Duyn J, Smith AM, Frank JA, Weinberger DR, McLaughlin AC. H215O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44(3):450–56. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. 10.1002/1522-2594(200009)44:3<450::AID-MRM16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 296.Feng CM, Narayana S, Lancaster JL, Jerabek PA, Arnow TL, Zhu F, Tan LH, Fox PT, Gao JH. CBF changes during brain activation: fMRI vs. PET Neuroimage. 2004;22(1):443–46. doi: 10.1016/j.neuroimage.2004.01.017. 10.1016/j.neuroimage.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 297.Forbes ML, Hendrich KS, Kochanek PM, Williams DS, Schiding JK, Wisniewski SR, Kelsey SF, DeKosky ST, Graham SH, Marion DW, Ho C. Assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact by perfusion magnetic resonance imaging using arterial spin-labeling in rats. J Cereb Blood Flow Metab. 1997;17(8):865–74. doi: 10.1097/00004647-199708000-00005. 101097/00004647-199708000-00005. Erratum in: J Cereb Blood Flow Metab 1997 17, 11–1263. [DOI] [PubMed] [Google Scholar]
- 298.Forbes ML, Hendrich KS, Schiding JK, Williams DS, Ho C, DeKosky ST, Marion DW, Kochanek PM. Perfusion MRI assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact in rats. Adv Exp Med Biol. 1997;411:7–12. doi: 10.1007/978-1-4615-5865-1_2. [DOI] [PubMed] [Google Scholar]
- 299.Hendrich KS, Kochanek PM, Williams DS, Schiding JK, Marion DW, Ho C. Early perfusion after controlled cortical impact in rats: Quantification by arterial spin-labeled MRI and the influence of spin-lattice relaxation time heterogeneity. Magn Reson Med. 1999;42(4):673–81. doi: 10.1002/(sici)1522-2594(199910)42:4<673::aid-mrm8>3.0.co;2-b. 10.1002/(SICI)1522-2594(199910)42:4<673::AID-MRM8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 300.Robertson CL, Hendrich KS, Kochanek PM, Jackson EK, Melick JA, Graham SH, Marion DW, Williams DS, Ho C. Assessment of 2-chloroadenosine treatment after experimental traumatic brain injury in the rat using arterial spin-labeled MRI: A preliminary report. Acta Neurochir Suppl. 2000;76:187–89. doi: 10.1007/978-3-7091-6346-7_37. [DOI] [PubMed] [Google Scholar]
- 301.Kochanek PM, Hendrich KS, Dixon CE, Schiding JK, Williams DS, Ho C. Cerebral blood flow at one year after controlled cortical impact in rats: Assessment by magnetic resonance imaging. J Neurotrauma. 2002;19(9):1029–37. doi: 10.1089/089771502760341947. 10.1089/089771502760341947. [DOI] [PubMed] [Google Scholar]
- 302.Kochanek PM, Hendrich KS, Jackson EK, Wisniewski SR, Melick JA, Shore PM, Janesko KL, Zacharia L, Ho C. Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spin-labeled magnetic resonance imaging. J Cereb Blood Flow Metab. 2005;25(12):1596–1612. doi: 10.1038/sj.jcbfm.9600154. 10.1038/sj.jcbfm.9600154. [DOI] [PubMed] [Google Scholar]
- 303.Wintermark M, Sincic R, Sridhar D, Chien JD. Cerebral perfusion CT: Technique and clinical applications. J Neuroradiol. 2008;35(5):253–60. doi: 10.1016/j.neurad.2008.03.005. 10.1016/j.neurad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 304.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204(1):272–77. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 305.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52(3):612–18. doi: 10.1002/mrm.20198. 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 306.Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, Xu Y, Neelavalli J, Haddar D, Reichenbach JR. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22(4):439–50. doi: 10.1002/jmri.20404. 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- 307.Sigmund GA, Tong KA, Nickerson JP, Wall CJ, Oyoyo U, Ashwal S. Multimodality comparison of neuroimaging in pediatric traumatic brain injury. Pediatr Neurol. 2007;36(4):217–26. doi: 10.1016/j.pediatrneurol.2007.01.003. 10.1016/j.pediatrneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 308.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. AN1792(QS-21)-201 Study Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64(9):1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 309.Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry. 2005;162(4):676–82. doi: 10.1176/appi.ajp.162.4.676. 10.1176/appi.ajp.162.4.676. [DOI] [PubMed] [Google Scholar]
- 310.Jack CR, Jr, Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, Xu Y, Shiung M, O’Brien PC, Cha R, Knopman D, Petersen RC. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60(2):253–60. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Krishnan KR, Charles HC, Doraiswamy PM, Mintzer J, Weisler R, Yu X, Perdomo C, Ieni JR, Rogers S. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry. 2003;160(11):2003–11. doi: 10.1176/appi.ajp.160.11.2003. 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 312.Jack CR, Jr, Petersen RC, Grundman M, Jin S, Gamst A, Ward CP, Sencakova D, Doody RS, Thal LJ Members of the Alzheimer’s Disease Cooperative Study (ADCS) Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging. 2007;29(9):1285–95. doi: 10.1016/j.neurobiolaging.2007.03.004. 10.1016/j.neurobiolaging.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: Establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 314.Lopez OL, Kuller LH, Becker JT, Jagust WJ, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Kawas C, Carlson M. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology. 2005;64(9):1539–47. doi: 10.1212/01.WNL.0000159860.19413.C4. 10.1212/01.WNL.0000159860.19413.C4. [DOI] [PubMed] [Google Scholar]
- 315.Potkin SG, Ford JM. Widespread cortical dysfunction in schizophrenia: The FBIRN imaging consortium. Schizophr Bull. 2009;35(1):15–18. doi: 10.1093/schbul/sbn159. 10.1093/schbul/sbn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Friedman L, Glover GH. Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging. 2006;23(6):827–39. doi: 10.1002/jmri.20583. 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- 318.Friedman L, Glover GH FBIRN Consortium. Reducing interscanner variability of activation in a multicenter fMRI study: Controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006;33(2):471–81. doi: 10.1016/j.neuroimage.2006.07.012. 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 319.Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann Neurol. 1979;6(5):371–88. doi: 10.1002/ana.410060502. 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 320.Mazziotta JC, Phelps ME, Carson RE, Kuhl DE. Tomographic mapping of human cerebral metabolism: Auditory stimulation. Neurology. 1982;32(9):921–37. doi: 10.1212/wnl.32.9.921. [DOI] [PubMed] [Google Scholar]
- 321.Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: A study in baboons. Neuroimage. 2003;20(3):1894–98. doi: 10.1016/j.neuroimage.2003.07.002. 10.1016/j.neuroimage.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 322.Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, Ober BA, Huesman RH, Derenzo SE. Regional cerebral metabolic alterations in dementia of the Alzheimer type: Positron emission tomography with [18F]fluorodeoxy-glucose. J Comput Assist Tomogr. 1983;7(4):590–98. doi: 10.1097/00004728-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 323.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 324.Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, Coleman RE. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41(11):1920–28. [PubMed] [Google Scholar]
- 325.Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, Czernin J, Rapoport SI, Pietrini P, Alexander GE, Schapiro MB, Jagust WJ, Hoffman JM, Welsh-Bohmer KA, Alavi A, Clark CM, Salmon E, De Leon MJ, Mielke R, Cummings JL, Kowell AP, Gambhir SS, Hoh CK, Phelps ME. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–27. doi: 10.1001/jama.286.17.2120. 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 326.Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69(9):871–77. doi: 10.1212/01.wnl.0000269790.05105.16. 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- 327.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. doi: 10.1002/ana.20009. 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 328.Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, Klunk WE, Mathis CA, Rosen HJ, Miller BL, Jagust WJ. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68(15):1205–12. doi: 10.1212/01.wnl.0000259035.98480.ed. 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 329.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemange VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 330.Mazziotta JC, Phelps ME, Pahl JJ, Huang SC, Baxter LR, Riege WH, Hoffman JM, Kuhl DE, Lanto AB, Wapenski JA, et al. Reduced cerebral glucose metabolism in asymptomatic subjects at risk for Huntington’s disease. N Engl J Med. 1987;316(7):357–62. doi: 10.1056/NEJM198702123160701. [DOI] [PubMed] [Google Scholar]
- 331.Van Paesschen W, Dupont P, Sunaert S, Goffin K, Van Laere K. The use of SPECT and PET in routine clinical practice in epilepsy. Curr Opin Neurol. 2007;20(2):194–202. doi: 10.1097/WCO.0b013e328042baf6. 10.1097/WCO.0b013e328042baf6. [DOI] [PubMed] [Google Scholar]
- 332.Herholz K, Pietrzyk U, Voges J, Schröder R, Halber M, Treuer H, Sturm V, Heiss WD. Correlation of glucose consumption and tumor cell density in astrocytomas. A stereotactic PET study. J Neurosurg. 1993;79(6):853–58. doi: 10.3171/jns.1993.79.6.0853. 10.3171/jns.1993.79.6.0853. [DOI] [PubMed] [Google Scholar]
- 333.Hattori N, Huang SC, Wu HM, Yeh E, Glenn TC, Vespa PM, McArthur D, Phelps ME, Hovda DA, Bergsneider M. Correlation of regional metabolic rates of glucose with Glasgow Coma Scale after traumatic brain injury. J Nucl Med. 2003;44(11):1709–16. [PubMed] [Google Scholar]
- 334.Hattori N, Huang SC, Wu HM, Liao W, Glenn TC, Vespa PM, Phelps ME, Hovda DA, Bergsneider M. Acute changes in regional cerebral 18F-FDG kinetics in patients with traumatic brain injury. J Nucl Med. 2004;45(5):775–83. [PubMed] [Google Scholar]
- 335.Kato T, Nakayama N, Yasokawa Y, Okumura A, Shinoda J, Iwama T. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma. 2007;24(6):919–26. doi: 10.1089/neu.2006.0203. 10.1089/neu.2006.0203. [DOI] [PubMed] [Google Scholar]
- 336.Nakashima T, Nakayama N, Miwa K, Okumura A, Soeda A, Iwama T. Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. AJNR Am J Neuroradiol. 2007;28(2):236–42. [PMC free article] [PubMed] [Google Scholar]
- 337.Jellinger KA. Traumatic brain injury as a risk factor for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(3):511–12. [PMC free article] [PubMed] [Google Scholar]
- 338.Szczygielski J, Mautes A, Steudel WI, Falkai P, Bayer TA, Wirths O. Traumatic brain injury: Cause or risk of Alzheimer’s disease? A review of experimental studies. J Neural Transm. 2005;112(11):1547–64. doi: 10.1007/s00702-005-0326-0. 10.1007/s00702-005-0326-0. [DOI] [PubMed] [Google Scholar]
- 339.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clarks RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190(1):192–203. doi: 10.1016/j.expneurol.2004.06.011. 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 340.Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10(1):24–35. [PubMed] [Google Scholar]
- 341.Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelph ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355(25):2652–63. doi: 10.1056/NEJMoa054625. 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 342.Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Meltzer CC, Schimmel K, Tsopelas ND, DeKosky ST, Price JC. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: A comparative analysis. J Nucl Med. 2005;46(12):1959–72. [PubMed] [Google Scholar]
- 343.Nelissen N, Vandenbulcke M, Fannes K, Verbruggen A, Peeters R, Dupont P, Van Laere K, Bormans G, Vandenberghe R. Abeta amyloid deposition in the language system and how the brain responds. Brain. 2007;130(Pt 8):2055–69. doi: 10.1093/brain/awm133. 10.1093/brain/awm133. [DOI] [PubMed] [Google Scholar]
- 344.The Management of Concussion/mTBI Working Group. VA/DOD Clinical practice guidelines for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46(6):CP1–CP68. [PubMed] [Google Scholar]