Abstract
To establish a causal role for locally produced IGF-I in the mechanical strain response in the bone, we have generated mice with conditional disruption of the insulin-like growth factor (IGF) I gene in type 1α2 collagen-expressing cells using the Cre-loxP approach. At 10 wk of age, loads adjusted to account for bone size difference were applied via four-point bending or axial loading (AL) in mice. Two wk of bending and AL produced significant increases in bone mineral density and bone size at the middiaphysis of wild-type (WT), but not knockout (KO), mice. In addition, AL produced an 8–25% increase in trabecular parameters (bone volume-tissue volume ratio, trabecular thickness, and trabecular bone mineral density) at the secondary spongiosa of WT, but not KO, mice. Histomorphometric analysis at the trabecular site revealed that AL increased osteoid width by 60% and decreased tartrate-resistance acidic phosphatase-labeled surface by 50% in the WT, but not KO, mice. Consistent with the in vivo data, blockade of IGF-I action with inhibitory IGF-binding protein (IGFBP4) in vitro completely abolished the fluid flow stress-induced MC3T3-E1 cell proliferation. One-way ANOVA revealed that expression levels of EFNB1, EFNB2, EFNA2, EphB2, and NR4a3 were different in the loaded bones of WT vs. KO mice and may, in part, be responsible for the increase in bone response to loading in the WT mice. In conclusion, IGF-I expressed in type 1 collagen-producing bone cells is critical for converting mechanical signal to anabolic signal in bone, and other growth factors cannot compensate for the loss of local IGF-I.
Keywords: mechanical loading, insulin-like growth factor I, micro-computed tomography, gene expression, osteoid
osteoporosis, a significant health problem characterized by low bone mass, affects millions of elderly individuals. To some extent, this disease is both preventable and treatable. Physical exercise has been used as one of the strategies to maintain bone mass and prevent osteoporosis in humans. Although a number of studies using in vitro and in vivo models have shown that mechanical loading (ML) promotes bone formation and that unloading results in reduction in bone mass (1, 4, 6, 9, 14, 15, 17, 18, 21, 33), the molecular components that mediate skeletal anabolic response to ML in vivo have not been fully elucidated. We believe that identification of signaling pathways for ML-induced bone formation could lead to pharmacological treatment strategies to enhance or maximize the osteogenic effects of exercise.
In terms of potential mediators of bone anabolic response to ML, there is considerable evidence that insulin-like growth factor (IGF) I is a strong candidate. 1) Gene expression work involving microarray, in situ hybridization, and real-time RT-PCR approaches has shown that loading increases IGF-I production in bone cells in vitro and in vivo (26, 32, 35). 2) Gross et al. (13) showed that transgenic mice with elevated IGF-I expression in osteoblasts exhibited an increased bone formation response to ML compared with control mice. 3) IGF-I administration induced a bone formation response in growth hormone-deficient normally loaded rats, but not in unloaded rats (27, 28). Although these data provide indirect evidence for an IGF-I role in mediating the bone anabolic response to ML, the cause-and-effect relationship between loading-induced increases in IGF-I expression and skeletal anabolic changes in vivo is lacking. Since ML increases IGF-I production in bone cells and because IGF-I is an important regulator of bone formation, we hypothesize that anabolic effects of ML on bone formation are mediated by osteoblast-derived IGF-I. Using mice with conditional disruption of the IGF-I gene in type 1 collagen-producing osteoblasts and their corresponding control mice, we tested this hypothesis in the present study.
MATERIALS AND METHODS
Animals and genotyping.
Breeding pairs of transgenic mice in which Cre recombinase is driven by the entire regulatory region of procollagen type 1α2 gene (Col1α2-Cre) were bred with transgenic mice in which exon 4 of the IGF-I gene is flanked by the loxP gene (IGF-Ilox/lox) to generate Cre+ loxP+/− and Cre− loxP+/− mice in the F1 generation. (Both the Cre and loxP mice have been backcrossed several generations with C57BL/6J mice to minimize the effect of mixed genetic background in the skeletal response to ML.) In the F2 generation, we crossed Cre+ loxP+/− [heterozygous conditional knockout (KO)] with IGF-Ilox/lox mice to generate Cre+ IGF-Ilox/lox (homozygous conditional KO) and Cre− IGF-Ilox/lox (littermate controls) mice for our studies (12). The experimental procedures were approved by the Institutional Animal Care and Use Committee at the Jerry L. Pettis Memorial Veterans Affairs Medical Center. we refer to the homozygous IGF-I conditional KO mice as IGF-I KO and Cre− littermate controls as wild-type (WT) mice.
Genotyping of Cre/loxP mice.
At 3 wk of age, DNA was extracted from tail tissue using a Puregene DNA purification kit (Gentra Systems, Minneapolis, MN), and PCR was performed to identify mice with Cre recombinase and/or loxP sites, as described elsewhere (12).
Strain calculation.
Prior to the four-point bending method of loading, mechanical strain produced by varying loads at the cortical site (middiaphysis) in tibias of 10-wk-old female IGF-I KO and WT mice was calculated using a mathematical approach (30). Similarly, prior to the axial method of loading, the amount of mechanical strain produced by loads axially at the trabecular site was measured using a strain-gauge technique, as described elsewhere (15), in isolated tibias of male and female IGF-I KO and WT mice. Briefly, a portable strain indicator (model P-3500) and a strain gauge of a specific range (EP-XX-015DJ-120) were used to measure the amount of mechanical strain produced by loads. The ends of the strain-gauge circuits were soldered to a copper wire and glued 5 mm from the tibia-fibula junction on the medial surface of the tibia in a longitudinal alignment. The copper wires were connected to the indicator, and the amounts of strain produced by different loads (n = 3–4 mice) on the loading zone were recorded. To avoid variation or overestimation of the measured strain, care was taken to ensure that the copper wires were properly soldered to the strain gauges or that the strain gauges did not come in direct contact with the loading parts of the Instron mechanical tester during measurement of the mechanical strain.
Four-point bending and in vivo peripheral quantitative CT measurements.
To evaluate cortical bone response to ML in IGF-I KO and WT mice, we used a four-point bending method of loading. Briefly, the four-point bending device (Instron, Canton, MA) consists of two upper vertically movable points covered with rubber pads, which are 4 mm apart, and two 12-mm lower nonmovable points covered with rubber pads. After the mice were anesthetized, the ankle of the tibia was positioned on the second lower immobile points of the device, such that the region of tibia loaded did not vary in different mice. During bending, the two upper pads touch the lateral surface of the tibia through overlaying muscle and soft tissue, while the lower pads touch the medial surface of the proximal and distal parts of the tibia. One of the limitations of this model is that force applied over soft tissue may have some local effect on blood and fluid flow. We took care to minimize this by changing the rubber pads frequently in the Instron mechanical tester.
At 10 wk of age, a 9-N load was applied to female WT mice and a 6-N load to female IGF-I KO mice. The loading was performed at 2-Hz frequency, for 36 cycles, once per day, 6 days/wk with 1 day of rest for 2 wk on both groups of mice (15). The right tibia was used for loading (externally loaded) and the left tibia as a contralateral internal control nonexternally loaded). On day 15, mice were euthanized, and tibias were collected and stored in 10% formalin for further analysis. Skeletal changes in loaded and externally nonloaded tibiae were measured by peripheral quantitative CT (Stratec XCT 960M, Norland Medical System, Ft. Atkinson, WI), as described previously (15).
Tibia axial loading.
To study the anabolic effects of loading on trabecular and cortical bone, we used a tibia axial loading model, which was originally developed for rat ulna and subsequently adapted for mouse ulna and tibia, as described previously (2, 10, 23, 29, 31, 34). The apparatus and protocol for dynamically loading the mouse have been adapted from previously published studies (10, 29). Briefly, the axial method of loading was performed on mice using servo-hydraulic mechanical testing (Instron). This model consists of one upper vertical movable cup covered with soft pads and another lower nonmovable cup (cups were made in-house using denture material) covered with soft pads (to prevent skin damage caused by loading axially) attached to the load cell. Mice were anesthetized using isoflurane (4% isoflurane and 2 l/min medical oxygen), and the tibia was positioned between the cups in a 90°C angle: the proximal end of the tibia to the upper movable point and the distal part to the lower immovable point. A 2-N load was applied to hold the mouse tibia in position to avoid twisting during axial loading. ML was applied over the growth plate, cartilage followed by skin using a trapezoidal waveform [trapezoidal-shaped pulse period = 0.1 s (loading 0.025 s, hold 0.05 s, and unloading 0.025 s)] for 40 cycles with 10 s of rest between each cycle for 2 wk (3 alternate days/wk) in the axial direction on the tibia. When the downward load is applied to the tibia, the axial load is translated to bending moment, producing compressive strain on the medial surface and tensile strain on the lateral surface of the tibia in mice. This bending strain is higher in the midshaft than in the proximal area of the tibia.
A 6- and 12-N load was applied to 10-wk-old female IGF-I KO and corresponding WT mice, respectively. Similarly, a 6.5- and 12-N load was applied to male IGF-I KO and corresponding WT mice. The right tibia was used for loading and the left tibia as a contralateral control. Mice were anesthetized during loading using 4% isoflurane and 2 l/min medical oxygen. On day 15, mice were euthanized, and the bones were collected and stored at −80°C or in 10% formalin for further analyses.
CT.
To measure microarchitectural changes of trabecular bone, as well as cortical bone, in response to axial loading, we used micro-CT (μ-CT), a high-resolution tomography image system (Invivo CT40, Scanco). Routine calibration was performed once per week using a three-point calibration phantom corresponding to the density range from air to cortical bone. Bones were immersed in 1× PBS to prevent them from drying, and scanning was performed using 75-kV X-ray at a resolution of 10.5 μm. To minimize the position error (slice positioning) and to be consistent in our sampling site from mouse to mouse, we undertook several precautionary steps, which include the use of 1) scout view of the whole tibia to determine landmarks and precise selections of measurement sites, 2) the growth plate of the tibia as the reference point, 3) a 0.525-mm sampling site that represented a distance of 0.315 mm from the growth plate for measurement of trabecular bone parameters, and 4) a 1.05-mm sampling site that represented a distance 5.5 mm from the growth plate for measurement of cortical bone parameters. After radiographic data were acquired, images were reconstructed by using two-dimensional image software provided by Scanco. The area of the trabecular analysis was outlined within the trabecular compartment. Every 10 sections were outlined, and the intermediate sections were interpolated with the contouring algorithm to create a volume of interest, followed by a three-dimensional analysis using Scanco in vivo software. Parameters such as bone volume (BV, mm3), BV fraction [BV-tissue volume (TV) ratio, %], apparent density (mg hydroxyapatite/cm3), trabecular number (mm−1), trabecular thickness (Tb.Th, μm), and trabecular space (μm) were evaluated in the externally loaded right and nonloaded left tibia of IGF-I KO and WT mice. Since IGF-I KO mice are smaller and exhibit reduced bone length compared with WT mice, we adjusted for this difference in length using standard calculations, such that the sampling site is the same for both sets of mice.
RNA extraction.
An RNA extraction kit (Qiagen, Valencia, CA) was used to extract total RNA from the loaded and externally nonloaded bones (tibias) with the following modifications, as described elsewhere (15). Quality and quantity of RNA were analyzed using a bioanalyzer (model 2100, Agilent, Palo Alto, CA) and a NanoDrop device (Thermo scientific, Wilmington, DE).
Gene expression: RT and real-time RT-PCR.
Quantitative real-time RT-PCR was used to determine expression levels of genes, as previously described (15). Briefly, purified total RNA (200 μg/μl) was used to synthesize the first-strand cDNA by RT according to the manufacturer's instructions (Bio-Rad, Hercules, CA). The gene-specific primers were designed by using Vector NTI software and ordered from Integrated DNA Technologies. The data were analyzed using SDS software, version 2.0, and the results were exported to Microsoft Excel for further analysis. Data normalization was accomplished using the endogenous control (β-actin or peptidylprolyl isomerase A) to correct for variation in the RNA quality among samples. The normalized cycle threshold (Ct) values were subjected to a 2−ΔΔCt formula to calculate the fold change between the externally loaded and non externally loaded tibias groups. The formula and its derivations were obtained from the instrument user guide.
Histomorphometric analysis.
IGF-I KO and WT mice were subjected to 2 wk of axial loading, as described above. On day 15, mice were euthanized, and tibias were collected and fixed overnight with 10% cold neutral-buffered formalin. After 24 h, bones were rinsed with 1× PBS to remove formalin and embedded in methyl methacrylate. Cross sections (0.5 mm thick) were cut from the middiaphysis of the bones with a wire saw (Delaware Diamond Knives), and this cross section was then ground lightly. For metaphyseal sections, thin longitudinal sections (5 μm) were cut, stained with Goldner's trichrome stain, mounted in Fluoromount G (Fisher Scientific, Pittsburgh, PA), or left unstained and examined under an Olympus BH-2 fluorescence/bright-field microscope. These bones were subjected to histomorphometric analysis, as described elsewhere (16).
Fluid shear stress.
In vitro mechanical stress was applied on MC3T3 osteoblast cells derived from mouse calvaria, as described elsewhere (14). Four groups (n = 5 replicates for each group) of MC3T3 cells were plated on glass slides (75 × 38 mm) at 5 × 104 cells/slide in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum. At ∼80% confluency, the cells were serum-deprived for 24 h and subjected to a steady fluid flow stress (FFS) of 20 dyn/cm2 for 30 min in Cytodyne flow chambers, as previously described (14).
Cell proliferation assay.
Cell proliferation was assessed by [3H]thymidine incorporation into cell DNA, as described previously (14).
Statistical analysis.
Data are presented as means ± SE. Regression analysis, ANOVA (Newman-Keuls post hoc test), and standard t-test were used to evaluate the effects of loading as well as to compare loading response between IGF-I KO and WT mice. Since changes in bone parameters in response to loading were not significantly different between male and female mice, data from male and female mice were pooled for the statistical analyses. Values are expressed as percent change in the externally loaded vs. nonexternally loaded bones [(loaded − nonloaded)/nonloaded ∗ 100]. We used STATISTICA software for our analyses, and the results were considered significantly different at P < 0.05.
RESULTS
Strain calculation, four-point bending, and changes in cortical bone parameters between WT and IGF-I KO mice.
Since bones of IGF-I KO mice show 30% (P < 0.05) reduction in periosteal circumference compared with WT mice, we predicted that mice with bones of smaller circumference would tend to receive higher mechanical strain than mice with bones of larger circumference at any given load. To ensure that the difference in skeletal anabolic response to loading is due to lack of local IGF-I, and not differences in mechanical strain, we used a mathematical approach to calculate the amount of mechanical strain produced by varying loads in both sets of mice. The calculated data show that a 6-N load (4,871 ± 113 με, n = 3) in IGF-I KO mice produced mechanical strain equivalent to a 9-N load (4,730 ± 14 με, n = 3) in the WT mice. On the basis of these data, a four-point bending device was used to apply adjusted loads to tibias of IGF-I KO and WT mice, such that mouse strains received similar amounts of mechanical strain.
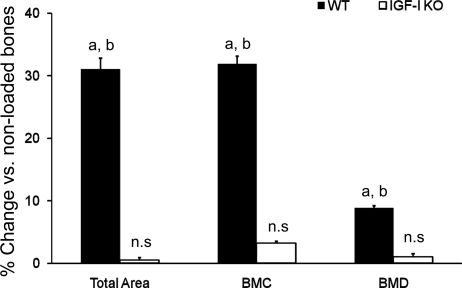
In response to 2 wk of four-point bending, the externally loaded bones of WT mice showed significant increases in skeletal parameters, such as total area (cross-sectional area), bone mineral content, and bone mineral density (BMD), measured by peripheral quantitative CT, compared with nonloaded bones (Fig. 1). In contrast, the externally loaded bones of IGF-I KO mice did not show a significant increase in any of these skeletal parameters compared with nonloaded bones.
Fig. 1.
Changes in skeletal parameters measured by peripheral quantitative CT in response to 2 wk of 4-point bending in insulin-like growth factor (IGF)-I knockout (KO) and wild-type (WT) mice. y-Axis, percent increase; x-axis, bone parameters. BMC, bone mineral content; BMD, bone mineral density; ns, not significant. Values are means ± SE (n = 8 per group). aP < 0.05 vs. corresponding non-externally loaded tibiae. bP < 0.05 vs. IGF-I KO.
Strain measurements, axial loading, and changes in trabecular and cortical parameters between WT and IGF-I KO mice.
Measurements of the amount of mechanical strain produced by axial loading protocol using a strain-gauge approach (15) revealed that a 6-N load produced 780 ± 86 με in female IGF-I KO mice, which is comparable to the amount of mechanical strain produced by a 12-N load (825 ± 56 με, n = 3) in the female WT mice. Similarly, male IGF-I KO mice produced 745 ± 22 με for a 6.5-N load, equivalent to the mechanical strain produced by a 12-N load (773 ± 48.6 με, n = 2–3) in the male WT mice. While the amount of measured strain in our study is similar to that reported by Sugiyama et al. (29), it is different from that reported by Brodt and Silva (8). One potential explanation for this could be the differences in the site (metaphysis vs. diaphysis) used for measurement of strain in the different studies. On the basis of these strain data, adjusted axial loads were applied on tibias of IGF-I KO and WT mice, such that both mouse strains received similar amounts of mechanical strain.
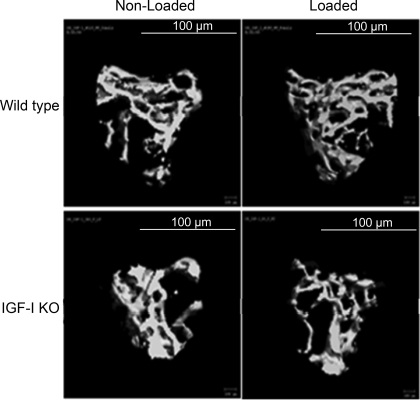
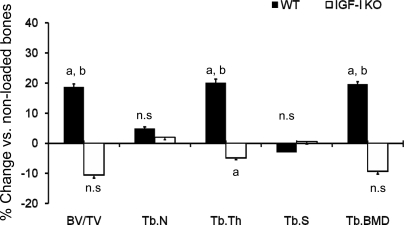
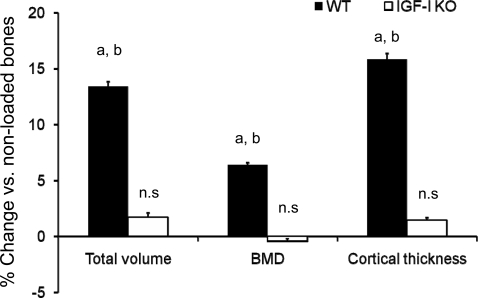
μ-CT images of trabecular bone from loaded and nonloaded tibia reveal that alternate days of axial loading for 2 wk caused an increase in the amount of trabecular bone in the loaded tibia compared with nonloaded tibia of the WT, but not IGF-I KO, mice (Fig. 2). μ-CT measurements revealed significant increases in BV/TV (23%), trabecular BMD (21%), and Tb.Th (19%) in response to axial loading in WT mice (Fig. 3). In contrast, neither trabecular BV/TV nor trabecular BMD was significantly different in the loaded bones of IGF-I KO mice. However, Tb.Th was decreased by 5% (P < 0.03) in the IGF-I KO mice (Fig. 3). In addition to trabecular parameters of secondary spongiosa, we also measured cortical parameters and found that the tissue volume, density, and cortical thickness were increased by 7–13% in WT mice (Fig. 4), while in the IGF-I KO mice, no change was observed in these parameters (Fig. 4).
Fig. 2.
Representative μ-CT images of nonexternally loaded and externally loaded metaphyseal region of tibia containing secondary spongiosa after 2 wk of axial loading in IGF-I KO and WT mice.
Fig. 3.
Changes in trabecular parameters of secondary spongiosa measured by μ-CT after 2 wk of axial loading in IGF-I KO and WT mice. y-Axis, percent increase; x-axis, bone parameters. BV/TV, bone volume/tissue volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular space; Tb.BMD, trabecular bone mineral density. Values are means ± SE (n = 10 per group). aP < 0.05 vs. corresponding non-externally loaded tibiae. bP < 0.05 vs. IGF-I KO.
Fig. 4.
Changes in cortical bone parameters measured by μ-CT at the diaphysis after 2 wk of axial loading on 10-wk-old IGF-I KO and WT mice. y-Axis, percent increase; x-axis, bone parameters. Values are means ± SE (n = 15 per group). aP < 0.05 vs. corresponding non-externally loaded tibiae. bP < 0.05 vs. IGF-I KO.
Histomorphometric analysis of skeletal parameters in response to axial loading.
To determine the impact of IGF-I disruption in type 1 collagen-producing bone cells on bone formation response to ML, we performed histomorphometric measurements at the secondary spongiosa of trabecular bone in the loaded and nonloaded bones of IGF-I KO and WT mice. We found that osteoid perimeter, a measure of length of forming surface, and Tb.Th were increased by 40–60% in the loaded bones of WT, but not IGF-I KO, mice (Fig. 5). Tartrate-resistant acid phosphatase (TRAP)-labeled surface was reduced significantly in the loaded bones of WT, but not IGF-I KO, mice (Fig. 5).
Fig. 5.
Histomorphometric analyses of externally loaded and nonexternally loaded bones from IGF-I KO and WT mice. Values are means ± SE (n = 9 per group). O.pm, osteiod perimeter; TRAP, tartrate-resistant acid phosphatase.
Gene expression analysis in response to axial loading.
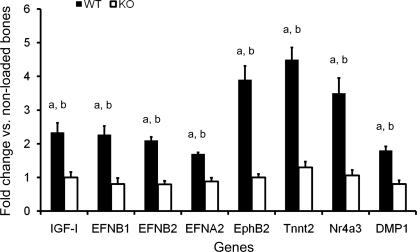
To identify the genes that contribute to bone anabolic response to loading, using real-time RT-PCR, we measured expression levels of several selected candidate genes (bone sialoprotein, IGF-I, Tnnt2, ephrin-B2, ephrin-A2, ephrin-A4, β-catenin, EphB2, EphB4, Nr4a3, SOST, osterix, and stromal cell-derived factor-1) that have been implicated in the ML signaling pathway in the externally and nonexternally loaded tibias of IGF-I KO and WT mice. Among these genes, expression levels of IGF-I, ephrin-B1, -B2, and -A2, EphB2, Nr4a3, and Tnnt2 were significantly increased in the loaded tibias of WT mice (1.5- to 3.3-fold, P < 0.05), but not IGF-I KO mice, compared with corresponding nonloaded tibias (Fig. 6). Expression levels of ephrin-A4, β-catenin, SOST, and SDF-1 were not affected by loading in both groups of mice.
Fig. 6.
Quantitative analysis of mRNA levels of genes measured by real-time RT-PCR after 2 wk of axial loading in 10-wk-old IGF-I KO and WT mice. Values are means ± SE (n = 5 or 6 per group). aP < 0.05 vs. nonexternally loaded bones; bP < 0.05 vs. IGF-I KO (ANOVA, Newman-Keuls post hoc test).
IGF-binding protein (IGFBP4) inhibits fluid flow stress response in MC3T3 cells.
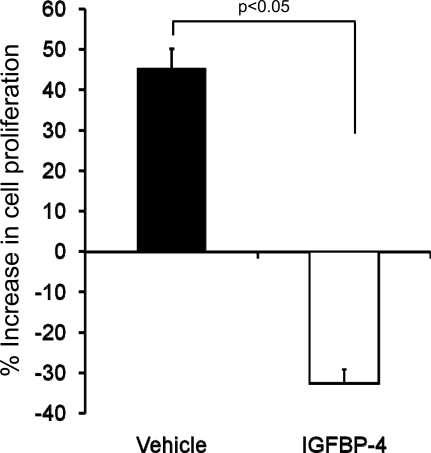
To determine if locally produced IGF-I is involved in mediating the proliferative response to FFS, we added inhibitory IGFBP4 to neutralize IGF-I action. The rationale for selecting IGFBP4 to block IGF-I action was based on the well-established action of this binding protein to block binding of IGFs to their receptors (22). We found that preincubation with IGFBP4 significantly reduced FFS-induced cell proliferation (Fig. 7).
Fig. 7.
Effect of IGF-binding protein (IGFBP4) treatment on fluid flow shear (FFS)-induced MC3T3 cell proliferation. In the vehicle group, MC3T3 cells were subjected to FFS without IGFBP4; corresponding static control cells were kept in identical conditions in a Cytodyne chamber but without exposure to FFS. In the IGFBP group, MC3T3 cells were incubated with IGFBP4 (300 ng) for 1 h prior to FFS, and its corresponding static control group was also incubated with IGFBP4 and kept in identical conditions in a Cytodyne chamber but without exposure to FFS. Percent change in cell proliferation for cells treated with and without IGFBP4 were calculated using the following formula: [(FFS − non-FFS)/(non-FFS) ∗100]. Values are means ± SE (n = 5).
DISCUSSION
In previous studies, we and others showed that mechanical stimulation increased IGF-I expression in monolayer cultures of osteoblast line cells in vitro (14, 18). Similarly, ML has been shown to stimulate IGF-I expression in bone cells in vivo (7, 18, 25). Although there is a considerable body of data in the literature to implicate a role for IGF-I in mediating the mechanical strain response in bone, direct evidence to demonstrate a cause-and-effect relationship between an increase in IGF-I expression and skeletal changes is lacking. To test a causal role for IGF-I in bone anabolic response to ML, we generated conditional IGF-I KO mice using a Cre-loxP approach and subjected these mice to ML. Initially, we used a four-point bending method of loading, which produces a robust increase in cortical bone, to evaluate the involvement of local IGF-I in cortical bone response (1, 15). Our findings demonstrate, for the first time, that local IGF-I expression in type 1 collagen-producing osteoblasts is critical for bone anabolic response to ML. The lack of cortical bone response in the female IGF-I KO mice cannot be explained on the basis of inadequate mechanical strain, because we applied loads that produced similar amounts of mechanical strain in the KO and WT mice. The findings that mechanical strain exerts an important effect on periosteal expansion during postnatal development (15) and that periosteal bone formation is compromised in mice with disruption of IGF-I in type 1 collagen-producing cells (12) are consistent with the lack of cortical bone response to ML observed in this study using IGF-I conditional KO mice. Consistent with an important role for locally produced IGF-I in bone cell response to ML, we found that the increase in proliferation of MC3T3-E1 cells caused by FFS was significantly reduced by neutralization of IGF-I action by inhibitory IGFBP4.
The four-point bending method of loading induces mostly a periosteal bone response, as the load is applied to the middiaphysis of bone, which lacks trabecular bone. We, therefore, evaluated if the bone cell-produced IGF-I is also involved in inducing trabecular bone formation in response to ML. μ-CT measurements revealed that 2 wk of axial loading caused a dramatic 27% increase in trabecular BV in WT, but not IGF-I KO, mice. In terms of the mechanism for the ML-induced trabecular BV, we found that axial loading significantly increased thickness of existing trabeculae but not generation of new trabeculae. A similar observation has also been reported in mice overexpressing osteoblast-produced IGF-I (38). Since IGF-I is involved in proliferation and differentiation, we predict that both mechanisms could contribute to the increase in Tb.Th in the loaded bones of WT mice.
Surprisingly, Tb.Th was decreased in the loaded bones of IGF-I KO mice compared with nonloaded controls. To address whether this decrease in Tb.Th is caused by increased bone resorption, we measured the TRAP-labeled surface on the bones (externally loaded and non-externally loaded tibia) of both sets of mice. However, the results from our study revealed no such increase in the TRAP-labeled surface in the loaded bones of IGF-I KO mice. As expected, the TRAP-labeled surface was significantly decreased in the loaded bones of WT mice, thus suggesting that axial loading results in a decrease in bone resorption, as well as an increase in bone formation. These findings together demonstrate that loss of trabecular bone in the loaded bones of IGF-I KO mice is not due to an increase in bone resorption. However, further study is required to explain the small loss of trabecular BV in the IGF-I KO mice in response to loading.
To address if expression levels of genes that are known to be induced by ML are affected by knockdown of IGF-I in type 1 collagen-producing cells, we measured mRNA levels of a number of potential candidates known to be involved in the mechanical signaling pathway. Our results revealed that several genes were differentially expressed between externally loaded and nonexternally loaded tibias of WT, but not IGF-I KO, mice (Fig. 6). The ML-induced changes in the expression of some of the genes in the WT mice are consistent with those previously reported by us and others (5, 11, 14, 37). Several genes in the ephrin/Eph signaling pathway were upregulated by ML in the WT, but not IGF-I conditional KO, mice, thus suggesting that IGF-I is upstream of ephrin signaling in the ML signaling pathway. On the basis of previous findings that ephrin signaling is important in the bone formation process (3, 24, 36), it is possible that the lack of skeletal anabolic response in IGF-I conditional KO mice is caused by the failure to activate ephrin/Eph signaling in the loaded bones of IGF-I KO mice. Future studies will address the relative contribution of ephrin/Eph signaling in bone adaptive response to ML.
Several studies have shown that osteocyte-derived IGF-I is critical for bone anabolic response to ML (19, 20, 26). Thus one could question whether the skeletal effects observed in our study in response to loading in the WT and KO mice are due to osteoblast- or osteocyte-derived IGF-I. In our study, we have knocked out IGF-I in type 1 collagen-producing osteoblasts, and since many of these osteoblasts become osteocytes, the osteocytes will also be deficient in IGF-I. However, further study is necessary to delineate the relative contribution of osteocyte- vs. osteoblast-derived IGF-I in mediating bone anabolic response to loading.
The limitations of this study are as follows. 1) Static, but not dynamic, histomorphometry studies could be performed, since calcein was not administered at appropriate times to measure rate of formation and mineralization. 2) We found that the amounts of mechanical strain produced by a 6-N load were different for the four-point bending and axial loading methods. Future studies are needed to address whether difference in bone site and/or orientation of loading contributes to this strain difference by the two loading protocols. 3) We used 10-wk-old mice for four-point bending and axial loading experiments. Bones in younger mice are growing at a much higher pace, and application of ML to these mice will reveal only smaller response to loading because of the accelerated bone growth. In 10-wk-old mice, bones continue to grow, but at a much slower pace. Previously, we found that the bone response to a given ML did not vary among the age groups (10, 16, and 36 wk). Therefore, we used 10-wk-old mice as an early age group to perform the ML study.
In summary, our data demonstrate that bone-derived local IGF-I is essential for the ML-induced increase in new bone formation, thus suggesting that other growth factors cannot compensate for the lack of local IGF-I to produce skeletal anabolic response to ML.
GRANTS
This research was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01 AR-48139 (S. Mohan) and R03 AR-056827 (C. Kesavan) and US Army Assistance Award DAMD17-01-1-744.
DISCLAIMER
The information contained in this publication does not necessarily reflect the position or the policy of the US Government, and no official endorsement should be inferred.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C. K. and S. M. are responsible for conception and design of the research; C. K. performed the experiments; C. K., J. E. W., and S. M. analyzed the data; C. K. and S. M. interpreted the results of the experiments; C. K. prepared the figures; C. K. drafted the manuscript; C. K., K. -H. W. L., and S. M. edited and revised the manuscript; C. K., J. E. W., and S. M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the Department of Veterans Affairs for the use of their facilities. We thank Catrina Alarcon for animal work and Anil Kapoor for μ-CT scanning of the bone specimens.
REFERENCES
- 1. Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int 63: 442–449, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Alam I, Warden SJ, Robling AG, Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner Res 20: 438–446, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE, Martin TJ. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 23: 1170–1181, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull 16: 45–54, 2003 [PubMed] [Google Scholar]
- 5. Boudignon BM, Bikle DD, Kurimoto P, Elalieh H, Nishida S, Wang Y, Burghardt A, Majumdar S, Orwoll BE, Rosen C, Halloran BP. Insulin-like growth factor I stimulates recovery of bone lost after a period of skeletal unloading. J Appl Physiol 103: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem 279: 30588–30599, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bravenboer N, Engelbregt MJ, Visser NA, Popp-Snijders C, Lips P. The effect of exercise on systemic and bone concentrations of growth factors in rats. J Orthop Res 19: 945–949, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res 25: 2006–2015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 536: 193–197, 2003 [DOI] [PubMed] [Google Scholar]
- 10. De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37: 810–818, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 18: 807–817, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 148: 5706–5715, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res 17: 493–501, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapur S, Mohan S, Baylink DJ, Lau KH. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem 280: 20163–20170, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kesavan C, Mohan S, Oberholtzer S, Wergedal JE, Baylink DJ. Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol 99: 1951–1957, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Kim J, Xing W, Wergedal J, Chan JY, Mohan S. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol Genomics 40: 100–110, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Kunnel JG, Igarashi K, Gilbert JL, Stern PH. Bone anabolic responses to mechanical load in vitro involve COX-2 and constitutive NOS. Connect Tissue Res 45: 40–49, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Lau KH, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem 281: 9576–9588, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol Endocrinol Metab 268: E318–E327, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Lean JM, Mackay AG, Chow JW, Chambers TJ. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol Endocrinol Metab 270: E937–E945, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun 349: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology 140: 5719–5728, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res 17: 284–292, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Pirih FQ, Nervina JM, Pham L, Aghaloo T, Tetradis S. Parathyroid hormone induces the nuclear orphan receptor NOR-1 in osteoblasts. Biochem Biophys Res Commun 306: 144–150, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Raab-Cullen DM, Thiede MA, Petersen DN, Kimmel DB, Recker RR. Mechanical loading stimulates rapid changes in periosteal gene expression. Calcif Tissue Int 55: 473–478, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Reijnders CM, Bravenboer N, Tromp AM, Blankenstein MA, Lips P. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol 192: 131–140, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone 32: 669–680, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res 19: 436–446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone 43: 238–248, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Stephen CC. Bone Mechanics Handbook (2nd ed.). London, UK: Informa Healthcare, 2001 [Google Scholar]
- 31. Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periosteal pressure. Calcif Tissue Int 54: 241–247, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Triplett JW, O'Riley R, Tekulve K, Norvell SM, Pavalko FM. Mechanical loading by fluid shear stress enhances IGF-1 receptor signaling in osteoblasts in a PKCζ-dependent manner. Mol Cell Biomech 4: 13–25, 2007 [PubMed] [Google Scholar]
- 33. Umemura Y, Baylink DJ, Wergedal JE, Mohan S, Srivastava AK. A time course of bone response to jump exercise in C57BL/6J mice. J Bone Miner Metab 20: 209–215, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5–10 Hz. Bone 34: 261–270, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem 96: 1049–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol Cell Biol 30: 711–721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaman G, Saxon LK, Sunters A, Hilton H, Underhill P, Williams D, Price JS, Lanyon LE. Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor-α and disuse. Bone 46: 628–642, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141: 2674–2682, 2000 [DOI] [PubMed] [Google Scholar]