Abstract
Considerable evidence implicates the renin-angiotensin system (RAS) in the regulation of energy balance. To evaluate the role of the RAS in the central nervous system regulation of energy balance, we used osmotic minipumps to chronically administer angiotensin II (Ang II; icv; 0.7 ng/min for 24 days) to adult male Long-Evans rats, resulting in reduced food intake, body weight gain, and adiposity. The decrease in body weight and adiposity occurred relative to both ad libitum- and pair-fed controls, implying that reduced food intake in and of itself does not underlie all of these effects. Consistent with this, rats administered Ang II had increased whole body heat production and oxygen consumption. Additionally, chronic icv Ang II increased uncoupling protein-1 and β3-adrenergic receptor expression in brown adipose tissue and β3-adrenergic receptor expression in white adipose tissue, which is suggestive of enhanced sympathetic activation and thermogenesis. Chronic icv Ang II also increased hypothalamic agouti-related peptide and decreased hypothalamic proopiomelanocortin expression, consistent with a state of energy deficit. Moreover, chronic icv Ang II increased the anorectic corticotrophin- and thyroid-releasing hormones within the hypothalamus. These results suggest that Ang II acts in the brain to promote negative energy balance and that contributing mechanisms include an alteration in the hypothalamic circuits regulating energy balance, a decrease in food intake, an increase in energy expenditure, and an increase in sympathetic activation of brown and white adipose tissue.
Keywords: food intake, obesity, renin-angiotensin system
millions of people suffer from obesity and the concomitant susceptibility to cardiovascular disease and type 2 diabetes, and emerging evidence has implicated the renin-angiotensin system (RAS) as contributing to these disorders; one consequence is that the RAS has emerged as a novel target for the treatment of these comorbidities (5, 6, 30, 68, 70, 83). The RAS is best known as an endocrine system important in the regulation of cardiovascular function and hydromineral balance; however, recent evidence suggests that RAS also plays a substantial role in the regulation of energy and glucose homeostasis (5, 6, 30, 68, 70, 83). Most physiological actions of the RAS are exerted by angiotensin II (Ang II), a peptide that is synthesized from angiotensinogen (AGT) via a series of proteolytic cleavage events. Drugs that reduce Ang II synthesis [angiotensin-converting enzyme (ACE) inhibitors] or action [angiotensin type 1 receptor blockers (ARBs)] have been used to treat hypertension for decades, and more recently, clinical and preclinical studies have explored the utility of these pharmacological agents to promote insulin sensitivity (1, 57). Interestingly, we and others have identified a potential novel utility of these pharmacological agents as therapeutics for obesity using animal models (4, 16, 21, 76, 84, 85).
Although interference with systemic RAS activity consistently decreases body weight and fat in rodent studies, it is also evident that Ang II can exert diverse effects on metabolism, depending on the tissues it accesses. In addition to the classically described endocrine RAS, all of the components necessary for Ang II synthesis and action also exist within several specific tissues where local Ang II is thought to exert autocrine and paracrine actions (17, 62). There is substantial evidence that, within adipose tissue, the local RAS facilitates energy storage. Ang II is hypertrophic for adipocytes (36, 53), and elevated adipose tissue-specific RAS activity is associated with obesity (53). The brain also expresses all components of the RAS, and Ang II has dynamic effects on the nervous system (27, 42, 49). The brain is consequently influenced by both circulating Ang II, which can activate receptors in circumventricular organs, and locally produced Ang II that acts as a neurotransmitter or neuromodulator (49). However, Ang II's role in the neural regulation of energy balance is not well worked out, and it is complicated by the fact that angiotensin receptors are expressed on sympathetic nerve terminals in the periphery as well as in brain regions that have critical roles in energy balance (49). Accumulating evidence suggests that increased RAS activity locally within the brain promotes negative energy balance, possibly in a negative feedback manner. In this regard, chronic central administration of Ang II or transgenic upregulation of brain RAS activity promotes negative energy balance (29, 64, 65). Conversely, downregulation of AGT (the precursor gene for Ang II) via the expression of AGT antisense oligonucleotides in brain glial cells increases food intake (38). Moreover, we have provided evidence that the peripherally acting ACE inhibitor captopril, which does not itself readily access the brain, increases circulating angiotensin I that does enter the brain, where it is converted to Ang II by local ACE, and that the locally generated Ang II then results in hypophagia and weight loss (16).
In the present studies, we directly assessed the role of Ang II in the neural regulation of energy balance by using a chronic intracerebroventricular (icv) Ang II infusion paradigm in adult male Long-Evans rats. We hypothesized that increasing brain angiotensin receptor signaling would enhance hypothalamic circuits that regulate energy balance, thereby decreasing food intake and augmenting energy expenditure and sympathetic outflow to brown (BAT) and white adipose tissue (WAT). We predicted that this in turn would result in decreased body weight and body fat. Results from these studies support the hypothesis that Ang II, which becomes elevated in obesity and promotes energy storage within adipose tissue, also acts in a negative feedback manner on the central nervous system to reduce adiposity.
MATERIALS AND METHODS
Animals.
Adult male Long-Evans rats (Harlan, Indianapolis, IN), weighing 250–300 g upon arrival, were individually housed and maintained on a 12:12-h light-dark cycle (lights on at 0600). Unless otherwise noted, rats had ad libitum access to water and standard rodent chow (∼5% calories by fat, 3.4 kcal/g; Harlan Teklad). All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Stereotaxic surgery and icv miniosmotic pump implantation.
Miniosmotic pumps (Alzet Model 2004; Durect, Cupertino, CA) and brain infusion kits (Durect) were used to chronically administer Ang II. The osmotic pumps were prefilled with Ang II or saline vehicle and attached to brain infusion cannulas using 7.5 cm of catheter tubing. This brain infusion ensemble was then allowed to prime in 37°C saline for ≥48 h, as recommended in the manufacturer's instructions. Subsequently, while under ketamine (∼60.6 mg/kg) and xylazine (∼7.9 mg/kg) anesthesia, rats were placed in a stereotaxic device and implanted with the brain infusion cannula in the lateral cerebral ventricle. The attached osmotic minipumps were implanted in the rostral midscapular region. With lambda and bregma at the same vertical coordinate, the coordinates from bregma were as follows: anterior 0.9 mm, lateral 1.4 mm, and ventral 3.5 mm. The cannulas were then attached to the skull using anchor screws and dental acrylic, and the skin was closed using suture. Subsequent to cannula and minipump implantation, water intake was monitored daily (by weighing the water bottles at the same time each day for the duration of the studies) for behavioral verification of cannula placement.
Subcutaneous miniosmotic pump implantation.
In a control experiment, Ang II was administered subcutaneously (sc). Primed minipumps loaded with the same amount of Ang II or saline were implanted sc in anesthetized rats. A 1.5-cm incision was made in the rostral midscapular region, and the minipumps were inserted through the incision such that the minipump rested between the scapulae. The wound was closed using wound clips, and assessment of food and water intake began immediately. Food and water intakes were assessed by weighing the water bottles and food hoppers at the same time each day for the duration of the study.
Body composition and fat pad weights.
Body composition was determined using NMR technology (Echo NMR, Waco, TX) on unanesthetized rats, as described previously (80). Distribution of adipose tissue in the epidydimal (eWAT), mesenteric/omental (mWAT), retroperitoneal (rpWAT), and inguinal (iWAT) depots was determined by carefully dissecting and weighing the fat pads at the time of euthanization.
Indirect calorimetry.
Energy expenditure during fasting, refeeding, and ad libitum feeding conditions was assessed by indirect calorimetry using a Physioscan system (AccuScan Instruments, Columbus, OH). On the first day of energy expenditure assessment, rats were fasted for 16 h, during which time energy expenditure was monitored continuously. Subsequently, ad libitum-fed (AL) control and Ang II-treated rats were given ad libitum access to chow for 3 days. Pair-fed (PF) control rats continued to receive twice daily rations of chow averaging the same daily food intake as Ang II-treated rats. Due to the initial hyperphagic response to the fast, day 1 of ad libitum feeding was considered refeeding, whereas subsequent days of ad libitum chow access were used to determine ad libitum feeding energy expenditure. Oxygen consumption (V̇o2) and CO2 production (V̇co2) were normalized to lean body mass. Energy expenditure was determined using the equation 3.815 × V̇o2 + 1.232 × V̇co2 and reported in kcal·kg lean mass−1·h−1 for the dark and light phases (69).
Tissue collection.
At the end of each study, rats were fasted for 6 h to ensure minimal variability in energy status, and they were then euthanized by conscious decapitation for tissue collection at 1400 (4 h prior to the onset of dark). Whole brains were removed and flash-frozen in dry ice-cooled isopentane. Samples of rpWAT and BAT were obtained and flash-frozen in dry ice-cooled isopentane for gene expression analysis. Plasma was separated from 5 ml of trunk blood collected in 125 μl of EDTA (100 mM).
RNA isolation and cDNA synthesis.
RNAeasy columns (Qiagen, Valencia, CA) were used to isolate RNA from the hypothalamus, BAT, and rpWAT. DNAase treatment (Qiagen) was performed to minimize genomic DNA contamination of RNA extracts. For hypothalamic gene expression analysis, the hypothalamus was quickly dissected from the frozen brains and submerged in 700 μl of RLT buffer from the Qiagen RNAeasy kit on the day of RNA extraction. RNA extraction and DNAase treatment procedures were then performed according to the manufacturer's instructions.
A slightly modified protocol was used for RNA extraction from BAT and WAT. A small sample (<100 mg) of frozen adipose tissue was submerged in 1 ml of TriReagent (Applied Biosystems/Ambion, Austin, TX) and homogenized. Bromo-3-chloro-propane (200 μl) was then added, and the samples were centrifuged (10,000 rpm) for 10 min. Ethanol (70%, 500 μl) was added to the supernatant, the mixture was applied to the RNeasy columns, and RNA extraction and DNAase treatment were performed according to the manufacturer's instructions. Subsequently, iScript (Bio-Rad, Hercules, CA) was used to synthesize cDNA from 1 μg of total RNA.
Semiquantitative real-time PCR.
Gene expression was assessed in the hypothalamus, BAT, and WAT using semiquantitative real-time PCR. For semiquantitative real-time PCR analysis of agouti-related peptide (AgRP), AGT, fatty acid synthase (FAS), β3-adrenergic receptor (β3-AR), lipoprotein lipase (LPL), neuropeptide Y (NPY), proopiomelanocortin (POMC), and uncoupling protein-1 (UCP1), diluted (1:3) cDNA samples were run in triplicate using an iCycler (Bio-Rad) and the iQ SYBR Green Supermix (Bio-Rad). Primers (IDT, Coralville, IA) used for gene expression analysis are listed in Table 1. For the analysis of the remaining genes [angiotensin type-1a receptor (AT1a), arginine vasopressin (AVP), corticotrophin-releasing hormone (CRH), angiotensin (1–7) receptor (Mas1), oxytocin (OXT) and thyroid-releasing hormone (TRH)], diluted (1:5) cDNA samples were run in duplicate using a 7900HT Fast Real-time PCR system, Taqman Gene Expression Master Mix and validated Taqman probes (Applied Biosystems, Foster City, CA). Expression patterns of genes of interest were normalized to constitutively expressed ribosomal protein L32 (assessed using both the Bio-Rad and Applied Biosystems techniques), and relative expression was quantified using the 2ΔΔCT method.
Table 1.
Primers (IDT, Coralville, IA)
| Gene | Forward Primer | Reverse Primer | Temperature, °C |
|---|---|---|---|
| β3-AR | 5′-CAG-AAC-TCA-CCG-CTC-AAC-AG | 5′-CCT-TCA-TGT-GGG-AAA-TGG-AC | 57.1 |
| AgRP | 5′-TTC-CCA-GAG-TTC-TCA-GGT-CTA | 5′-ATC-TAG-CAC-CTC-TGC-CAA-A | 55.0 |
| AGT | 5′-CTG-GGC-AAG-ATG-GGT-GAC | 5′-CTG-GCC-TGC-TTG-GAG-TTC | 55.8 |
| FAS | 5′-ATA-TGA-AGG-TGG-TGG-AGG-TG | 5′-TGC-AGC-TTG-GTC-TGA-ACA-TC | 55.8 |
| L32 | 5′-CAG-ACG-CAC-CAT-CGA-AGT-TA | 5′-AGC-CAC-AAA-GGA-CGT-GTT-TC | 61.2 |
| LPL | 5′-ACA-CTG-GAA-ACG-CTG-TTG-TG | 5′-TTC-CGG-ATA-AAA-CGT-TCT-CG | 55.8 |
| NPY | 5′-CTC-TGC-GAC-ACT-ACA-TCA-A | 5′-GGG-GCA-TTT-TCT-GTG-CTT-T | 61.2 |
| POMC | 5′-TCC-ATA-GAC-GTG-TGG-AGC-TG | 5′-ACT-TCC-GGG-GAT-TTT-CAG-TC | 57.1 |
| UCP1 | 5′-GTG-TAC-CCA-GCT-GTG-CAA-TGA | 5′-CGC-AAA-CCC-TTT-GAA-AAA-GG | 61.2 |
β3-AR, β3-adrenergic receptor; AgRP, agouti-related peptide; AGT, angiotensinogen; FAS, fatty acid synthase; LPL, lipoprotein lipase; NPY, neuropeptide Y; POMC, proopiomelanocortin; UCP1, uncoupling protein 1.
Analysis of plasma hormones.
Plasma insulin and leptin (subsequent to a 6-h fast) were assessed using ELISA kits from Crystal Chem, and plasma glucose was determined using the glucose oxidation method, as described previously (16). Plasma renin activity (PRA) levels were determined using a 125I RIA kit from Diasorin, and corticosterone levels were assessed using a 125I RIA kit from MP Biomedicals (16, 42).
Experimental design.
Ang II was chronically infused into the lateral cerebral ventricle (icv) to allow it to readily access angiotensin receptors in circumventricular organs (primarily the subfornical organ) as well as those located within midline hypothalamic nuclei [primarily the paraventricular nucleus (PVN)]. The dose of Ang II was predetermined by conducting a dose effect study, in which Ang II was administered chronically at three different doses [0.1 (0.096 pmol/min), 1 (0.96 pmol/min), and 10 ng/min (9.6 pmol/min)] for 2 wk, and food intake and body weight were assessed daily at the same time each day by the same laboratory personnel. The doses used for this preliminary study were based on those used in the literature (64, 65). These doses also took into account the approximate half-life of icv-administered Ang II (∼26.8 min), as well as the difference in hypothalamic Ang II levels between spontaneously hypertensive and normotensive control rats (∼0.375 nmol/g) (19, 56), and aimed to cause an increase in central Ang II that was within the physiological range. Based on these preliminary studies, a dose of 0.7 ng/min (0.67 pmol/min) was used for the experiments. Importantly, this dose had no significant effect on daily food intake, water intake, body weight, or body composition when administered sc, as determined in the control experiment.
Rats received Ang II (0.7 ng/min, 0.67 pmol/min) or saline icv via Alzet osmotic minipumps for 24 days (n = 9–10/group). One control group had ad libitum access to chow (AL), whereas the other was PF (2 rations of food/day) to match the total daily intake of the icv Ang II-treated rats. All of the PF rats consumed their entire rations of food each day. Body weight, food intake, and water intake were monitored daily. Body composition was assessed by NMR prior to the start of Ang II administration and on day 21 of the study. At the end of the study, rats were fasted for 6 h and euthanized, and tissue was collected. In a control experiment, rats were given the same dose of Ang II sc for 7 days, and body weight, food intake, and water intake were assessed.
A separate cohort of rats received Ang II (0.7 ng/min) or saline icv via Alzet osmotic minipumps for 10 days (n = 7–9/group). Ten days of Ang II administration was selected based on the analysis of the body weight curve from the previous cohort and allowed for tissue collection during the dynamic portion of body weight loss (relative to controls). Starting on the 4th day of Ang II infusion, energy expenditure was monitored via indirect calorimetry for 4 consecutive days. At the end of the study, rats were euthanized, and BAT and rpWAT were collected.
Data analysis.
For the chronic icv Ang II infusion studies, all data were analyzed with the appropriate ANOVA using GraphPad (Prism, San Diego, CA). Food intake, water intake, body weight, and energy expenditure were analyzed using a two-way repeated-measures ANOVA. Body composition, plasma measurements, and gene expression analyses were performed using a one-way ANOVA. Post hoc analyses of main effects and interactions were assessed using the Bonferroni test or Tukey's test. For the control sc Ang II infusion study, body weight, food intake, and water intake were analyzed using a t-test. P < 0.05 was considered statistically significant.
RESULTS
Chronic icv Ang II dose-dependently decreases food intake and body mass.
Chronic icv Ang II caused a significant decrease in cumulative food intake (F3,11 = 6.57, P < 0.01) over 14 days of infusion at the intermediate (1 ng/min, 214.0 ± 7.4 g) and high (10 ng/min, 201.2 ± 10.6 g) doses; however, rats receiving the low dose (0.1 ng/min, 261.0 ± 20.8 g) had cumulative food intakes similar to vehicle-treated controls (274.8 ± 9.78 g). Body weight gain over the 14 days (F3,11 = 8.30, P < 0.01) was decreased in the rats receiving the intermediate (−5.89 ± 13.12 g) and high (−14.43 ± 8.31 g) icv Ang II doses but not in the rats receiving the low dose (23.8 ± 5.76 g, vehicle-treated = 31.95 ± 5.15 g). Based on this dose response, we utilized a 0.7 ng/min dose for future experiments.
Chronic icv Ang II decreases food intake and increases water intake.
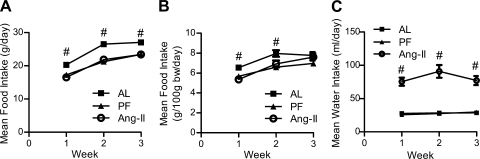
Chronic icv Ang II (0.7 ng/min) significantly reduced mean daily food intake [effect of condition (F2,50 = 22.19, P < 0.0001) and time (F2,50 = 127.4, P < 0.0001); Fig. 1A]. Chronic icv Ang II also reduced mean daily food intake when normalized to body weight [effect of condition (F2,50 = 5.49, P < 0.05), time (F2,50 = 81.16, P < 0.0001), and interaction (F4,50 = 4.033, P < 0.01); Fig. 1B]; however, this reduction in normalized food intake occurred only during the initial 2 wk. Consistent with previous studies (65), chronic icv Ang II elicited a robust increase in mean daily water intake [effect of condition (F2,50 = 22.19; P < 0.0001); Fig. 1C] that lasted for the duration of the study. When administered sc, this same dose did not alter mean daily food [22.85 ± 0.52 (sc Ang II) vs. 23.38 ± 0.33 (AL), P = 0.41] or water intake [22.67 ± 0.68 (sc Ang II) vs. 22.65 ± 0.98 (AL), P = 0.98].
Fig. 1.
Food and water intake. Mean daily food intake (A), mean daily food intake normalized to body mass (B), and water intake (C) of rats chronically administered angiotensin II (Ang II) intracerebroventricularly (icv; 0.7 ng/min) or saline vehicle [ad libitum-fed (AL) or pair-fed (PF) controls] for 24 days. #Significantly different from other groups (P < 0.05); n = 9–10/group. bars represent 1 SE.
Chronic icv Ang II decreases body weight and percent fat mass.
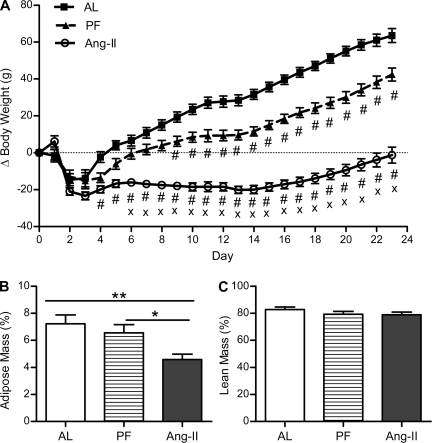
Chronic icv Ang II treatment caused a significant reduction in body mass relative to both AL and PF controls [effect of time (F23,575 = 196.44, P < 0.0001), treatment (F2,575 = 84.51, P < 0.0001), and interaction between these (F46,575 = 42.90, P < 0.0001); Fig. 2A]. Specifically, after an initial period of postsurgical weight loss (3 days), the control groups began to gain weight at an average of 4.1 g/day for AL controls and 2.4 g/day for PF controls, whereas the icv Ang II-treated rats maintained a consistent body weight (∼20 g less than their presurgical levels). This persisted until day 14, after which the Ang II-treated rats began to gain weight at an average of 2.1 g/day for the remainder of the study, significantly less than the weight gain of the control groups (3.5 g/day for AL controls and 3.4 g/day for PF controls). As a result, body mass was decreased in the Ang II-treated rats relative to AL controls (P < 0.05) starting on day 4 and relative to PF controls (P < 0.05) starting on day 6. PF controls weighed significantly less than AL controls (P < 0.05) starting on day 9. When administered sc, this same dose of Ang II did not affect body weight gain during the 7-day study [15.22 ± 1.87 (sc Ang II) vs. 14.67 ± 1.82 (AL), P = 0.84].
Fig. 2.
Body mass and body composition. Mean change in body mass (A), %adipose mass (B), and %lean mass (C) of rats chronically administered Ang II (icv; 0.7 ng/min) or saline vehicle (AL or PF controls) for 24 days. %Adipose and %lean mass were assessed on day 21. Body mass was significantly decreased in the Ang II-treated rats relative to AL controls starting on day 4 (P < 0.01) and relative to PF controls starting on day 6 (P < 0.05). PF controls weighed significantly less than AL controls starting on day 9, and all differences in body mass persisted for the duration of the study. *P < 0.05; **P < 0.01; #significantly different from AL (P < 0.05); ×significantly different from PF (P < 0.05); n = 9–10/group. Bars represent 1 SE.
The chronic icv Ang II-induced decrease in body mass was due to a significant reduction in percent fat mass (F2,25 = 5.75, P < 0.01; Fig. 2B) relative to both AL and PF controls, as assessed on day 21 [4.57 ± 0.40 (Ang II) vs. 7.22 ± 0.66 (AL; P < 0.01) and 6.55 ± 0.61% (PF; P < 0.05)]. Pair feeding did not cause a significant reduction in percent adipose mass relative to AL. Percent lean mass was similar among all groups (F2,25 = 1.137, P = 0.337; Fig. 2C).
Adipose tissue distribution.
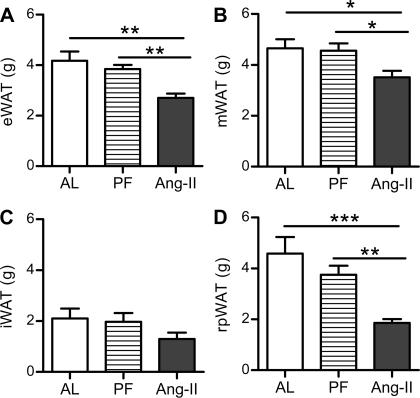
As depicted in Fig. 3, 24 days of chronic icv Ang II administration resulted in a significant reduction in rpWAT (F2,25 = 9.116, P < 0.01), eWAT (F2,25 = 8.606, P < 0.01), and mWAT (F2,25 = 4.092, P = 0.05) mass relative to both the AL and PF controls. Conversely, there was no significant difference in the weight of these adipose tissue depots between the PF and AL controls. Moreover, the weight of the sc iWAT was similar among the groups (F2,25 = 1.665, P = 0.22).
Fig. 3.
Adipose tissue distribution. The mass of the epidydimal (eWAT; A), mesenteric (mWAT; B), inguinal (iWAT; C) and retroperitoneal white adipose tissue (rpWAT; D) depots after 24 days of chronic Ang II (icv; 0.7 ng/min) or saline vehicle (AL or PF controls) infusion. *P < 0.05; **P < 0.01; ***P < 0.001; n = 9–10/group. Bars represent 1 SE.
Energy expenditure.
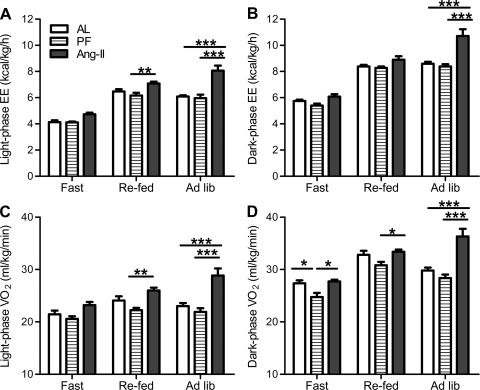
Energy expenditure (kcal·kg−1·h−1) and V̇o2 (ml·kg−1·min−1) were assessed from days 4 through 8. During the light phase, there was an effect of treatment (F2,38 = 26.47, P < 0.0001; Fig. 4A) and feeding condition (i.e., fasted vs. refed vs. ad libitum, F2,38 = 152.74, P < 0.0001; Fig. 4A) on energy expenditure as well as an interaction between these (F4,38 = 5.88, P < 0.001; Fig. 4A). Specifically, during the light phase, icv Ang II elevated energy expenditure relative to both control groups (AL and PF) during ad libitum feeding and relative to the PF controls during refeeding. Similarly, there was an effect of treatment (F2,38 = 13.76, P < 0.001; Fig. 4B) and feeding condition (F2,38 = 333.02, P < 0.0001; Fig. 4B) on dark-phase energy expenditure as well as an interaction between these (F4,38 = 10.30, P < 0.0001; Fig. 4B). Specifically, dark-phase whole animal energy expenditure was elevated in Ang II rats, particularly during ad libitum feeding conditions (relative to PF and AL controls).
Fig. 4.
Energy expenditure and oxygen consumption (V̇o2). Mean energy expenditure (A and B) and V̇o2 (C and D) of rats chronically administered Ang II (icv; 0.7 ng/min) and of AL and PF controls during the dark and light phases when subjected to fasting, refeeding, or ad libitum feeding conditions. Mean V̇o2 and heat production were assessed between days 4 and 8 of Ang II infusion. *P < 0.05; **P < 0.01; ***P < 0.001; n = 7–9/group. Bars represent 1 SE.
Chronic icv Ang II infusion also significantly increased light-phase [effect of treatment (F2,38 = 12.6, P < 0.001), feeding condition (F2,38 = 29.6, P < 0.0001), and the interaction (F4,38 = 7.36, P < 0.001); Fig. 4C] and dark-phase V̇o2 [effect of treatment (F2,38 = 12.13; P < 0.001), feeding condition (F2,38 = 80.97, P < 0.0001), and the interaction (F4,38 = 9.81, P < 0.0001); Fig. 4D]. Specifically, when fed ad libitum, Ang II elevated dark- and light-phase V̇o2 relative to AL and PF controls. Ang II also elevated dark-phase V̇o2 relative to PF controls during refeeding and light-phase V̇o2 during fasting.
BAT and rpWAT gene expression.
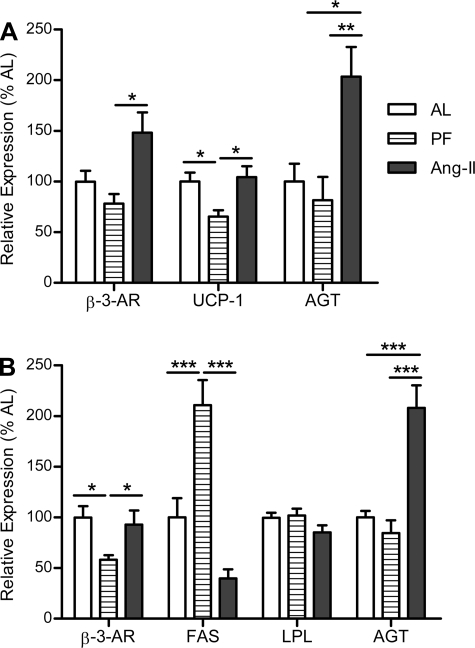
Consistent with the hypothesis that a compensatory decrease in energy expenditure occurs as a consequence of food restriction, PF controls expressed significantly less UCP1 mRNA in BAT than AL controls (F2,23 = 5.421, P < 0.05; Fig. 5A). Conversely, rats given icv Ang II had no such decrease in UCP1 relative to AL controls despite the same decrease in energy consumption. Rather, icv Ang II resulted in a significant increase in UCP1 and β3-AR (F2,22 = 6.388, P < 0.01) expression in BAT relative to what occurred in PF controls, indicative of increased thermogenesis and enhanced sympathetic activity (Fig. 5A). Additionally, there was a twofold increase in AGT expression in BAT of rats given icv Ang II relative to PF and AL controls (F2,22 = 7.728, P < 0.01; Fig. 5A).
Fig. 5.
Adipose tissue gene expression. Assessment of brown (BAT) and white adipose tissue (WAT) gene expression in rats chronically administered Ang II (icv; 0.7 ng/min) for 10 days. A: relative expression of β3-adrenergic receptor (β3-AR), uncoupling protein 1 (UCP1), and angiotensinogen (AGT) in BAT. B: relative expression of β3-AR, fatty acid synthase (FAS), AGT, and lipoprotein lipase (LPL) in rpWAT. *P < 0.05; **P < 0.01; ***P < 0.001; n = 7–10/group. Bars represent 1 SE.
PF controls had decreased β3-AR in rpWAT relative to AL controls (F2,23 = 4.296, P < 0.05; Fig. 5B). No such decrease was observed in Ang II-treated rats, implying that icv Ang II-treated rats also have increased sympathetic activation in rpWAT. Similar to what occurred in BAT, AGT expression was also elevated in the rpWAT of icv Ang II-treated rats (F2,23 = 22.7, P < 0.0001; Fig. 5B). Moreover, FAS was elevated in the PF controls (210.7 ± 24.7%) relative to the AL controls (100 ± 19.0%), whereas it was reduced in the Ang II-treated rats (39.8 ± 9.1%, F2,23 = 16.94, P < 0.0001; Fig. 5B). Conversely, there was no significant difference in LPL expression among the groups (F2,23 = 1.93, P = 0.17; Fig. 5B).
Hypothalamic gene expression.
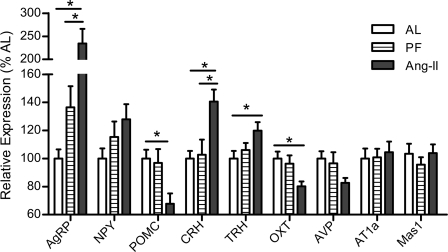
As depicted in Fig. 6, after 24 days of central Ang II infusion, rats had elevated hypothalamic AgRP (F2,23 = 12.21, P < 0.001) and reduced POMC (F2,22 = 4.66, P < 0.05) expression relative to both PF and AL controls, consistent with their state of energy deficit. Additionally, hypothalamic CRH and TRH were elevated in the Ang II-treated rats (F2,24 = 7.10, P < 0.01, and F2,24 = 4.62, P < 0.05, respectively), suggestive of these hormones contributing to Ang II-induced anorexia. Conversely, OXT was reduced significantly (F2,24 = 4.68, P < 0.05). Hypothalamic expression of AVP (F2,24 = 3.39, P = 0.051), NPY (F2,23 = 2.01, P = 0.16), AT1a (F2,24 = 0.70, P = 0.50), and Mas1 (F2,24 = 0.79, P = 0.47) was not significantly altered among the groups.
Fig. 6.
Hypothalamic gene expression. Hypothalamic proopiomelanocortin (POMC), agouti-related peptide (AgRP), neuropeptide Y (NPY), corticotropin-releasing hormone (CRH), oxytocin (OXT), arginine vasopressin (AVP), thyroid-releasing hormone (TRH), angiotensin type-1a receptor (AT1a), and angiotensin (1–7) receptor (Mas1) mRNA levels of rats chronically administered Ang II (icv; 0.7 ng/min) or saline vehicle (AL or PF controls) for 24 days. Data were normalized to the constitutively expressed ribosomal protein L32 and expressed as %AL control. *P < 0.05; n = 8–9/group. Bars represent 1 SE.
Plasma hormone levels.
At the end of the study, rats receiving icv Ang II had decreased plasma renin activity (F2,26 = 8.65, P < 0.01) relative to AL and PF controls (Table 2). Pair feeding induced a significant elevation in corticosterone levels relative to AL controls, whereas icv Ang II reduced corticosterone levels relative to PF controls (F2,23 = 6.61, P < 0.01; Table 2). Similarly, glucose (F2,23 = 5.32, P < 0.05), insulin (F2,25 = 5.99, P < 0.01) and leptin (F2,25 = 12.01, P < 0.001) levels were also reduced (Table 2).
Table 2.
Plasma measurements
| AL Controls | PF Controls | Ang-II Treated | |
|---|---|---|---|
| Glucose, mg/dl | 142.5 ± 2.14 | 140.1 ± 2.52 | 131.4 ± 3.04* |
| Insulin, ng/ml | 2.37 ± 0.23 | 2.65 ± 0.27 | 1.58 ± 0.14*# |
| Leptin, ng/ml | 3.14 ± 0.43 | 3.73 ± 0.33 | 1.44 ± 0.18*# |
| Renin activity, ng•ml−1•h−1 | 34.86 ± 6.04 | 25.40 ± 2.72 | 10.41 ± 2.20*# |
| Corticosterone, ng/ml | 111.2 ± 30.4 | 151.9 ± 22.5 ng/ml* | 56.1 ± 11.1 ng/ml# |
Values are means ± SE.
Ang II, angiotensin II; AL, ad libitum fed; PF, pair fed. Rats were administered Ang II intracerebroventricularly (icv; 0.7 ng/min) or saline vehicle (AL or PF controls) for 24 days, and plasma was collected after a 6-h fast.
Significantly different from AL controls;
significantly different from PF controls.
DISCUSSION
The RAS is increasingly acknowledged as a key player in the regulation of energy balance. Obese humans and animals have elevated activity of the adipose tissue RAS (6, 14, 66), which acts locally to promote energy storage (36). Moreover, peripheral inhibition of the RAS using ACE inhibitors or ARBs reduces body weight and adiposity (16, 76, 85). Contrary to its actions in adipose tissue, elevated RAS activity within the brain promotes negative energy balance (16, 65). This divergence suggests the possibility of a negative feedback system that is activated when the spillover of Ang II from adipose tissue accesses the brain, thereby counteracting peripheral Ang II action. Consistent with this hypothesis, the principal findings of the present study indicate that chronic elevation of central Ang II signaling reduces food intake, body weight, and body fat. The decreased body weight and adiposity occurs relative to both PF and AL controls, demonstrating that reduced food intake in and of itself does not underlie these effects. Consistent with this, icv Ang II increased whole animal energy expenditure and oxygen consumption as well as indices of elevated sympathetic activation of BAT and rpWAT. Moreover, in support of a hypothalamic mechanism, Ang II elevated the expression of the anorectic hormones CRH and TRH and modified the expression patterns of several genes within the hypothalamus. Collectively, these data support a role for the RAS in the neural regulation of energy balance.
The observation in the present studies that chronic icv Ang II reduces body weight and food intake and increases water intake is consistent with previous research from our laboratory (16) and others (64, 65). Porter and colleagues (64, 65) reported that chronic icv administration of a high dose of Ang II decreased body weight and food intake in young and adult rats independent of increases in water intake, and Kasper et al. (38) observed that decreasing central Ang II via the glial expression of AGT antisense oligonucleotides increased food intake in rats. Additionally, the anorexia induced by an orally administered ACE inhibitor that does not access the brain is due in part to elevated central RAS activity (16). However, to our knowledge, this is the first report that an increase in central Ang II reduces body weight primarily because of a reduction in percent body adiposity rather than lean mass and that chronic icv Ang II does not preferentially reduce adipose mass within certain depots. The present results also suggest that some underlying mechanisms of this reduction in adiposity include decreased food intake, elevated energy expenditure, augmented sympathetic activation of adipose tissue, and increased expression of CRH and TRH expression within the hypothalamus.
An important novel observation in these studies is that elevated energy expenditure and V̇o2 contribute to the effect of icv Ang II to reduce body and adipose mass. That is, the difference in body mass and adiposity observed between the PF controls and the Ang II-treated animals can be attributed, at least in part, to elevated energy expenditure. Chronic mild food restriction is accompanied typically by compensatory decreases in energy expenditure and overall sympathetic tone that maintain energy stores (26). Consistent with this, the PF controls exhibited reduced dark-phase V̇o2 relative to AL controls, particularly during fasting. Conversely, Ang II-treated animals did not compensate for reduced food intake by decreasing V̇o2 or energy expenditure. Rather, icv Ang II elevated V̇o2 and whole animal energy expenditure, particularly during the refeeding and ad libitum feeding conditions regardless of whether it was the dark phase (when rats are typically more active) or light phase. Previous studies have determined that acute sc administration of a substantially higher amount of Ang II also results in elevations in oxygen consumption (9), although the increase was of a lesser magnitude than we observed with a smaller dose of icv Ang II. Moreover, transgenic upregulation of brain RAS activity results in elevations in V̇o2 during both the light and dark phases (29). Nonetheless, to our knowledge, this is the first report of V̇o2 and whole animal energy expenditure during multiple feeding conditions in rats chronically administered icv Ang II. An elevation in activity may also serve as a contributing mechanism for the negative energy balance. In this regard, a recent report considering the effect of aging on the hypertensive effects of Ang II noted that a similar dose of Ang II administered icv leads to elevated locomotor activity in both young and old rats (24).
In addition to its effects on energy expenditure, chronic mild food restriction also elevated corticosterone levels in the PF control group, implying that hypophagia per se activates the hypothalamic-pituitary-adrenal (HPA) axis. Nonetheless, although icv Ang II-treated rats had elevated CRH, this did not translate into an elevation in plasma corticosterone. This disconnect in levels of the HPA axis has been observed previously and may be due to an alteration in adrenal sensitivity to ACTH or the modulation of corticosterone secretion through a neural mechanism (82, 87).
Systemic Ang II is thought to influence the central nervous system by activating neurons within circumventricular organs such as the subfornical organ, which has been implicated in the regulation of energy balance (42, 77, 78). However, within the brain, Ang II is synthesized locally and acts as a neurotransmitter (49). Consistent with this, in addition to being expressed in circumventricular organs, angiotensin receptors are located in several other brain areas that are important in the control of energy balance and autonomic function. Based on the current route of administration, we cannot definitively identify which population(s) of angiotensin receptor-containing neurons is responsible for the phenotype, since icv Ang II can readily access several populations of Ang II receptor-containing neurons. Moreover, based on these studies, we also cannot determine receptor specificity, since Ang II activates both AT1 and AT2. Nonetheless, AT1 is densely expressed within many key brain nuclei critical for the regulation of energy balance (45, 73).
For example, the hypothalamic arcuate nucleus (ARC), which is considered a major integrative site for the central regulation of energy balance (88), expresses AT1 (45, 73). ARC neurons respond directly to circulating adiposity signals such as insulin and leptin, and there are two major subcategories of these neurons. One group of ARC neurons expresses the anorexigenic precursor peptide POMC, and the other group coexpresses the orexigenic peptides NPY and AgRP. Modulation of the relative activity/expression profiles of these two groups of neurons is thought to underlie the anorectic effects of insulin and leptin (72) and has also been implicated in the sympathetic activation of adipose tissue (7).
Although central Ang II elicits some responses that are similar to those of insulin and leptin (e.g., reduced food intake and body weight) (72), and levels of insulin, leptin, and Ang II are often correlated (e.g., all are typically elevated in obesity), our data suggest that the underlying neural circuitry and molecular mechanism(s) mediating Ang II's anorectic and sympathoexcitatory actions within adipose tissue likely differ from those of insulin and leptin. In contrast to what happens following central insulin or leptin administration (72), icv Ang II results in elevated AgRP and reduced POMC expression within the hypothalamus, consistent with a compensatory homeostatic response to energy deficit. Moreover, the expression profiles of AgRP and POMC are reflective of the reduced plasma levels of insulin and leptin in the Ang II rats (i.e., the Ang II-treated rats have less insulin and leptin, and the hypothalamic targets of insulin responded accordingly). As such, the underlying mechanism(s) of this altered expression profile could be the reduced insulin/leptin, or it could be due to a direct effect of Ang II on these neurons (or neurons that project to these). However, Ang II receptors are only sparsely expressed within the ARC. Although the expressions of NPY and AgRP within the ARC are often correlated, in the present study we did not observe any differences in NPY. This can likely be attributed to the fact that whereas AgRP is localized primarily in the ARC, NPY is expressed in many other hypothalamic nuclei, and its expression may be differentially regulated within these areas. As a consequence, NPY may indeed be elevated specifically within the ARC, but we were not able to detect this when assessing gene expression in the entire hypothalamus.
Because many other brain areas that are critically involved in energy balance regulation also express AT1 and utilize Ang II as a neurotransmitter (49), including the paraventricular (PVN) and lateral hypothalamic (LH) nuclei (45), it is possible that one or more of these brain regions mediates Ang II's anorectic effects. Because these particular AT1-expressing areas are important in regulating autonomic function, it is reasonable to hypothesize that activation of AT1 within the PVN and/or LH contributes to Ang II-induced negative energy balance. Within the PVN, AT1 is expressed on CRH-containing neurons (2, 79), and CRH induces anorexia, elevates energy expenditure, and enhances sympathetic outflow (67). Interestingly, expression of CRH was increased in animals chronically administered Ang II icv, and this alteration in CRH expression is consistent with CRH signaling contributing to the observed negative energy balance. On the other hand, CRH antagonism produced the opposite effect (32, 41). Additionally, TRH, another anorexigenic factor (40, 71), was also elevated in the Ang II-treated rats. It should be noted that chronic Ang II did not similarly affect expression of all hypothalamic neurotransmitters. Rats treated with Ang II had decreased expression of OXT and no difference in AVP, both of which induce negative energy balance (44, 58). However, OXT and AVP are also critical for maintaining hydromineral balance, and the reduced expression of OXT is consistent with a compensatory response (i.e., decreased sodium excretion) to the elevated water intake in the Ang II-treated rats. Taken together, the results suggest that the specific upregulation of hypothalamic CRH and TRH contribute to the negative energy balance resulting from augmented central RAS activity.
An important finding of this report is that chronic icv Ang II elevates UCP1 and β3-AR, indices of sympathetic activation of BAT and WAT. Although it is well established that Ang II is a potent activator of the sympathetic nervous system to some tissues (23, 60), the impact of central RAS activity on sympathetic tone to BAT and WAT has not been unequivocally discerned. Increased sympathetic outflow to BAT is typically associated with thermogenesis, and activation of AT1 on sympathetic nerve terminals facilitates sympathetic activation of BAT (10–12). Although there is some evidence that Ang II acts centrally to decrease thermogenesis (50, 75), we and others (29, 64) have found that well-accepted indices of BAT thermogenesis are elevated with increased brain RAS activity. Of particular relevance to these studies, neurons within the PVN reduce sympathetic outflow to BAT (51). Moreover, Ang II acts within the PVN to attenuate synaptic GABA release, thereby enhancing sympathoexcitation (13, 46, 47). It is possible that Ang II influences this circuit to regulate BAT thermogenesis.
Importantly, this is the first report that there may be heterogeneity among the ability of icv Ang II to reduce to mass of specific WAT depots and that central Ang II elevates β3-AR in rpWAT, suggesting enhanced sympathetic activation of WAT. Increased sympathetic outflow to WAT typically produces lipolysis and thereby reduces energy stores within this tissue. As a consequence, a coordinated increase in sympathetic activation of BAT and WAT likely contributes to the increased energy expenditure and decreased adiposity observed in rats given central Ang II. Consistent with this possibility, administration of β-agonists increases lipolysis and thermogenesis and produces weight loss (43), whereas β-adrenergic blockade results in the converse (86). As discussed, AT1 is located in hypothalamic brain regions heavily implicated in autonomic function (45), and it is likely that activation of AT1 within one or more of these brain regions contributes to the elevations in sympathetic outflow and energy expenditure.
Increased sympathetic nerve activity augments the rate of renin release from the kidney (28), but chronic central Ang II, which elevated indices of sympathetic tone in adipose tissue, actually decreased PRA in the present experiments. This decrease in PRA has been reported in similar paradigms (25, 52, 55) and is likely a compensatory negative feedback response to the high Ang II levels within the brain. This implies that central Ang II likely does not drive renal sympathetic activity in our paradigm (22, 37, 54) and raises the possibility that Ang II may discriminately facilitate sympathetic nerve transmission to some tissues but not others in our model. This notion of differential effects of Ang II on sympathetic activation of various tissues has been studied extensively with regard to the role of Ang II in hypertension (60). For example, King et al. (39), Osborn et al. (61), Toney et al. (81), and Yoshimoto et al. (89) have determined that on a high-salt diet Ang II reduces sympathetic activation of kidneys, does not affect the activation of skeletal muscle, and enhances sympathetic outflow to the splanchnic circulation. Importantly, and relevant to the present findings, Ang II's actions on PVN neurons play a key role in regulating sympathetic nervous system activity in relation to cardiovascular function, particularly hypertension (8, 48). Some mechanisms for these actions include elevations of inflammation and oxidant stress locally within the brain (8, 33, 63, 74), which have also been implicated in the central regulation of energy balance (18, 34). In fact, the effects of icv Ang II on energy balance are strikingly similar to those of the cytokine TNFα (3, 18). This, coupled with the fact that TNFα's secretion is also regulated by Ang II (15), implies that TNFα may be involved in some of Ang II's effects on energy balance.
Interestingly, both BAT and WAT AGT expression were elevated in our paradigm. A similar increase in BAT AGT was reported recently by Grobe et al. (29) in mice that had a transgenic upregulation of brain RAS activity. However, since plasma renin (the rate-limiting enzyme for Ang II synthesis) activity was decreased in our study as well as in those transgenic mice, this is likely a compensatory increase in adipose AGT synthesis. Additionally, renin itself negatively regulates AGT expression such that elevations in renin, as which occur with captopril treatment or direct administration of renin, reduce AGT, whereas reduced renin elevates AGT (16, 31).
Another novel finding from the present studies was that, within WAT, FAS expression was elevated in the PF controls. Conversely, chronic icv Ang II treatment caused a robust reduction in the expression of this lipogenic enzyme relative to the PF controls. Consistent with the result that pair feeding leads to elevated FAS expression, there is evidence that increased body fat is associated with reductions in lipogenic enzymes in WAT (20, 35, 59). These results suggest that increased brain RAS activity also reduces the lipogenic potential of WAT.
The RAS is critically involved in many aspects of the metabolic syndrome, rendering it a potential target for treating obesity and its comorbidities (17). Whereas the influence of the RAS in hypertension and insulin sensitivity has been studied extensively, detailed investigation of the RAS with regard to obesity per se is somewhat of a new endeavor. Although interference with RAS activity consistently decreases body weight and body fat in rodent studies, it has recently become evident that Ang II can exert diverse effects on metabolism, depending on the tissues it accesses. As a consequence, it is imperative to determine the unique roles of the RAS in energy balance regulation within specific tissues to ascertain the therapeutic utility of targeting the RAS in obese patients. In this regard, the present study provides substantial insight on the control of energy balance by the brain RAS.
To summarize, chronic icv Ang II infusion reduces body mass and adipose mass. This reduction in energy storage is consequent to both a decrease in food intake and an increase in energy expenditure. Moreover, indices of sympathetic activation are elevated in both BAT and WAT, implying that Ang II acts centrally to enhance BAT thermogenesis and WAT lipolysis, presumably contributing to the state of negative energy balance. The negative energy balance observed in Ang II-treated animals was accompanied by increased hypothalamic expression of CRH and TRH. Given the anorexigenic effects of CRH and TRH, it is likely that increased RAS activity promotes negative energy balance by altering the actions of these neurotransmitters in the hypothalamus. Collectively, the present data elucidate the impact of the RAS on the neural regulation of energy balance, thereby highlighting a divergence between the influence of the central RAS from the systemic and adipose tissue RAS pools on energy balance. These data are consistent with the hypothesis that there is a negative feedback system that is activated when the spillover of Ang II from adipose tissue becomes sufficiently high to allow it to access the brain, thereby providing a brake on peripheral Ang II action.
GRANTS
This study was supported by the following National Institutes of Health grants/fellowships: NS-681222 (A. D. de Kloet), HL-096830 (E. G. Krause), DK-087816 (M. T. Foster), DK-54890 (R. J. Seeley), DK-056863 (R. J. Seeley and S. C. Woods), DK-078201 (S. C. Woods), and DK-017844 (S. C. Woods).
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1. Abuissa H, Jones PG, Marso SP, O'Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol 46: 821–826, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 61: 437–444, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Arruda AP, Milanski M, Romanatto T, Solon C, Coope A, Alberici LC, Festuccia WT, Hirabara SM, Ropelle E, Curi R, Carvalheira JB, Vercesi AE, Velloso LA. Hypothalamic actions of tumor necrosis factor alpha provide the thermogenic core for the wastage syndrome in cachexia. Endocrinology 151: 683–694, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Berne C. Metabolic effects of ACE inhibitors. J Intern Med Suppl 735: 119–125, 1991 [PubMed] [Google Scholar]
- 5. Bokil HS, Porter JP. Brain angiotensin type 1 receptor expression and function in the Zucker obese rat. Neurosci Lett 281: 139–142, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 287: R943–R949, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–5347, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension 57: 289–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cassis L, Helton M, English V, Burke G. Angiotensin II regulates oxygen consumption. Am J Physiol Regul Integr Comp Physiol 282: R445–R453, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Cassis LA. Angiotensin II in brown adipose tissue from young and adult Zucker obese and lean rats. Am J Physiol Endocrinol Metab 266: E453–E458, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Cassis LA. Role of angiotensin II in brown adipose thermogenesis during cold acclimation. Am J Physiol Endocrinol Metab 265: E860–E865, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Cassis LA, Dwoskin LP. Presynaptic modulation of neurotransmitter release by endogenous angiotensin II in brown adipose tissue. J Neural Transm Suppl 34: 129–137, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Cooper R, Forrester T, Ogunbiyi O, Muffinda J. Angiotensinogen levels and obesity in four black populations. ICSHIB Investigators. J Hypertens 16: 571–575, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Danielyan L, Lourhmati A, Verleysdonk S, Kabisch D, Proksch B, Thiess U, Umbreen S, Schmidt B, Gleiter CH. Angiotensin receptor type 1 blockade in astroglia decreases hypoxia-induced cell damage and TNF alpha release. Neurochem Res 32: 1489–1498, 2007 [DOI] [PubMed] [Google Scholar]
- 16. de Kloet AD, Krause EG, Kim DH, Sakai RR, Seeley RJ, Woods SC. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology 150: 4114–4123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav 100: 525–534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Kloet AD, Pacheco-López G, Langhans W, Brown LM. The effect of TNFα on food intake and central insulin sensitivity in rats. Physiol Behav 103: 17–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dewey AL, Wright JW, Hanesworth JM, Harding JW. Effects of aminopeptidase inhibition on the half-lives of [125I]angiotensins in the cerebroventricles of the rat. Brain Res 448: 369–372, 1988 [DOI] [PubMed] [Google Scholar]
- 20. Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 282: E46–E51, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Dominguez JR, de la Calle H, Hurtado A, Robles RG, Sancho-Rof J. Effect of converting enzyme inhibitors in hypertensive patients with non-insulin-dependent diabetes mellitus. Postgrad Med J 62, Suppl 1: 66–68, 1986 [PubMed] [Google Scholar]
- 22. Dorward PK, Rudd CD. Influence of brain renin-angiotensin system on renal sympathetic and cardiac baroreflexes in conscious rabbits. Am J Physiol Heart Circ Physiol 260: H770–H778, 1991 [DOI] [PubMed] [Google Scholar]
- 23. English V, Cassis L. Facilitation of sympathetic neurotransmission contributes to angiotensin regulation of body weight. J Neural Transm 106: 631–644, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Erdos B, Cudykier I, Woods M, Basgut B, Whidden M, Tawil R, Cardounel AJ, Tumer N. Hypertensive effects of central angiotensin II infusion and restraint stress are reduced with age. J Hypertens 28: 1298–1306, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Eriksson L, Fyhrquist F. Plasma renin activity following central infusion of angiotensin II and altered CSF sodium concentration in the conscious goat. Acta Physiol Scand 98: 209–216, 1976 [DOI] [PubMed] [Google Scholar]
- 26. Even PC, Nicolaidis S. Adaptive changes in energy expenditure during mild and severe feed restriction in the rat. Br J Nutr 70: 421–431, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89: 370–376, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Gordon RD, Kuchel O, Liddle GW, Island DP. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J Clin Invest 46: 599–605, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hainault I, Nebout G, Turban S, Ardouin B, Ferré P, Quignard-Boulangé A. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. Am J Physiol Endocrinol Metab 282: E59–E66, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Herrmann HC, Dzau VJ. The feedback regulation of angiotensinogen production by components of the renin-angiotensin system. Circ Res 52: 328–334, 1983 [DOI] [PubMed] [Google Scholar]
- 32. Hotta M, Shibasaki T, Arai K, Demura H. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res 823: 221–225, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763–1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaillard T, Roger M, Galinier A, Guillou P, Benani A, Leloup C, Casteilla L, Penicaud L, Lorsignol A. Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes 58: 1544–1549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang L, Wang Q, Yu Y, Zhao F, Huang P, Zeng R, Qi RZ, Li W, Liu Y. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS One 4: e6884, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 138: 1512–1519, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Kannan H, Nakamura T, Jin XJ, Hayashida Y, Yamashita H. Effects of centrally administered angiotensin on sympathetic nerve activity and blood flow to the kidney in conscious rats. J Auton Nerv Syst 34: 201–210, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Kasper SO, Carter CS, Ferrario CM, Ganten D, Ferder LF, Sonntag WE, Gallagher PE, Diz DI. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin-angiotensin systems. Physiol Genomics 23: 311–317, 2005 [DOI] [PubMed] [Google Scholar]
- 39. King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Kow LM, Pfaff DW. The effects of the TRH metabolite cyclo(His-Pro) and its analogs on feeding. Pharmacol Biochem Behav 38: 359–364, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res Bull 17: 285–289, 1986 [DOI] [PubMed] [Google Scholar]
- 42. Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology 149: 6416–6424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar MV, Moore RL, Scarpace PJ. Beta3-adrenergic regulation of leptin, food intake, and adiposity is impaired with age. Pflugers Arch 438: 681–688, 1999 [PubMed] [Google Scholar]
- 44. Langhans W, Delprete E, Scharrer E. Mechanisms of vasopressin's anorectic effect. Physiol Behav 49: 169–176, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18: 383–439, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther 313: 1035–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Li H, Gao Y, Qi Y, Katovich MJ, Jiang N, Braseth LN, Scheuer DA, Shi P, Sumners C. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. FASEB J 22: 3175–3185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265: R302–R309, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Lin MT, Chandra A, Jou JJ. Angiotensin II inhibits both heat production and heat loss mechanisms in the rat. Can J Physiol Pharmacol 58: 909–914, 1980 [DOI] [PubMed] [Google Scholar]
- 51. Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Malayan SA, Keil LC, Ramsay DJ, Reid IA. Mechanism of suppression of plasma renin activity by centrally administered angiotensin II. Endocrinology 104: 672–675, 1979 [DOI] [PubMed] [Google Scholar]
- 53. Massiéra F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15: 2727–2729, 2001 [DOI] [PubMed] [Google Scholar]
- 54. May CN, McAllen RM. Baroreceptor-independent renal nerve inhibition by intracerebroventricular angiotensin II in conscious sheep. Am J Physiol Regul Integr Comp Physiol 273: R560–R567, 1997 [DOI] [PubMed] [Google Scholar]
- 55. McKinley MJ, McBurnie MI, Mathai ML. Neural mechanisms subserving central angiotensinergic influences on plasma renin in sheep. Hypertension 37: 1375–1381, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Meyer JM, Felten DL, Weyhenmeyer JA. Measurement of immunoreactive angiotensin II levels in microdissected brain nuclei from developing spontaneously hypertensive and Wistar Kyoto rats. Exp Neurol 107: 164–169, 1990 [DOI] [PubMed] [Google Scholar]
- 57. Niklason A, Hedner T, Niskanen L, Lanke J; Captopril Prevention Project Study Group Development of diabetes is retarded by ACE inhibition in hypertensive patients–a subanalysis of the Captopril Prevention Project (CAPPP). J Hypertens 22: 645–652, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785–791, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Ortega FJ, Mayas D, Moreno-Navarrete JM, Catalán V, Gómez-Ambrosi J, Esteve E, Rodriguez-Hermosa JI, Ruiz B, Ricart W, Peral B, Fruhbeck G, Tinahones FJ, Fernández-Real JM. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 18: 13–20, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol 95: 61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep 13: 221–228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin II on body weight gain in young rats. Brain Res 959: 20–28, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol 287: R422–R428, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab 286: E891–E895, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 440: 189–197, 2002 [DOI] [PubMed] [Google Scholar]
- 68. Ruano M, Silvestre V, Castro R, García-Lescún MC, Rodríguez A, Marco A, García-Blanch G. Morbid obesity, hypertensive disease and the renin-angiotensin-aldosterone axis. Obes Surg 15: 670–676, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59: 1817–1824, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet 369: 1208–1219, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Schuhler S, Warner A, Finney N, Bennett GW, Ebling FJ, Brameld JM. Thyrotrophin-releasing hormone decreases feeding and increases body temperature, activity and oxygen consumption in Siberian hamsters. J Neuroendocrinol 19: 239–249, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 73. Seltzer A, Tsutsumi K, Shigematsu K, Saavedra JM. Reproductive hormones modulate angiotensin II AT1 receptors in the dorsomedial arcuate nucleus of the female rat. Endocrinology 133: 939–941, 1993 [DOI] [PubMed] [Google Scholar]
- 74. Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shido O, Nagasaka T. Effects of intraventricular angiotensin II on heat balance at various ambient temperatures in rats. Jpn J Physiol 35: 163–167, 1985 [DOI] [PubMed] [Google Scholar]
- 76. Shimabukuro M, Tanaka H, Shimabukuro T. Effects of telmisartan on fat distribution in individuals with the metabolic syndrome. J Hypertens 25: 841–848, 2007 [DOI] [PubMed] [Google Scholar]
- 77. Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol 296: R512–R520, 2009 [DOI] [PubMed] [Google Scholar]
- 78. Smith PM, Rozanski G, Ferguson AV. Acute electrical stimulation of the subfornical organ induces feeding in satiated rats. Physiol Behav 99: 534–537, 2010 [DOI] [PubMed] [Google Scholar]
- 79. Sumitomo T, Suda T, Nakano Y, Tozawa F, Yamada M, Demura H. Angiotensin II increases the corticotropin-releasing factor messenger ribonucleic acid level in the rat hypothalamus. Endocrinology 128: 2248–2252, 1991 [DOI] [PubMed] [Google Scholar]
- 80. Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem 377: 990–1002, 2003 [DOI] [PubMed] [Google Scholar]
- 81. Toney GM, Pedrino GR, Fink GD, Osborn JW. Does enhanced respiratory-sympathetic coupling contribute to peripheral neural mechanisms of angiotensin II-salt hypertension? Exp Physiol 95: 587–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76: 79–92, 2002 [DOI] [PubMed] [Google Scholar]
- 83. Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA 93: 12490–12495, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weisinger HS, Begg DP, Egan GF, Jayasooriya AP, Lie F, Mathai ML, Sinclair AJ, Wark JD, Weisinger RS. Angiotensin converting enzyme inhibition from birth reduces body weight and body fat in Sprague-Dawley rats. Physiol Behav 93: 820–825, 2008 [DOI] [PubMed] [Google Scholar]
- 85. Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiol Behav 98: 192–197, 2009 [DOI] [PubMed] [Google Scholar]
- 86. Welle SL, Thompson DA, Campbell RG. β-Adrenergic blockade inhibits thermogenesis and lipolysis during glucoprivation in humans. Am J Physiol Regul Integr Comp Physiol 243: R379–R382, 1982 [DOI] [PubMed] [Google Scholar]
- 87. Wilkinson CW, Shinsako J, Dallman MF. Rapid decreases in adrenal and plasma corticosterone concentrations after drinking are not mediated by changes in plasma adrenocorticotropin concentration. Endocrinology 110: 1599–1606, 1982 [DOI] [PubMed] [Google Scholar]
- 88. Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383, 1998 [DOI] [PubMed] [Google Scholar]
- 89. Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]