Abstract
Islet damage from glucose toxicity is implicated in the pathogenesis of type 2 diabetes, but the sequence of events leading to islet cell dysfunction and hyperglycemia remains unclear. To examine the early stages of islet pathology resulting from increased basal glucose loads, normal awake rats were infused with glucose continuously for 10 days. Plasma glucose and markers of islet and liver function were monitored throughout the infusion. After initial hyperglycemia, rats adapted to the infusion and maintained euglycemia for approximately 4 days. Continued infusion led to worsening hyperglycemia in just 5% of rats after 6 days, but 69% after 8 days and 89% after 10 days, despite unchanged basal and stimulated plasma insulin and C-peptide concentrations. In contrast, plasma glucagon concentrations increased fivefold. Endogenous glucose production (EGP) was appropriately suppressed after 4 days (2.8 ± 0.7 vs. 6.1 ± 0.4 mg·kg−1·min−1 on day 0, P < 0.001) but tripled between days 4 and 8 (9.9 ± 1.7 mg·kg−1·min−1, P < 0.01). Surprisingly, the increase in EGP was accompanied by increased mitochondrial phosphoenolpyruvate carboxykinase expression with appropriate suppression of the cytosolic isoform. Infusion of anti-glucagon antibodies normalized plasma glucose to levels identical to those on day 4 and ∼300 mg/dl lower than controls. This improved glycemia was associated with a 60% reduction in EGP. These data support the novel concept that glucose toxicity may first manifest as α-cell dysfunction prior to any measurable deficit in insulin secretion. Such hyperglucagonemia could lead to excessive glucose production overwhelming the capacity of the β-cell to maintain glucose homeostasis.
Keywords: glucagon, hepatic glucose production, type 2 diabetes
the mechanisms that underlie the early stages of the progression to type 2 diabetes (T2D) are not fully understood. Inappropriately elevated endogenous glucose production (EGP) derived primarily from increased gluconeogenesis associated with hyperglucagonemia is an early feature in the progression to diabetes (16, 27, 28). Unlike the periodic and interrupted nature of prandial contributions to plasma glucose, EGP uniquely can contribute continuously to the glucose pool. Consequently, the sustained increase in insulin secretion throughout the day required to dispose of the increased EGP will increase the “basal workload” of islet cells. Although the mechanism is unclear, hyperglucagonemia has also been well documented in patients (2, 31, 33) and animal models (17, 19, 35, 38) of diabetes with both hyper- and hypoinsulinemia, and it contributes to hyperglycemia by augmenting EGP. Because of the multiple physiological abnormalities in diabetic patients and most animal models, it is difficult to determine the relative contribution of hyperglucagonemia to loss of glycemic control. In this study, we examined the effect of a sustained glucose load on islet α- and β-cell function in normal, healthy rats. In our glucose-infused rats, hyperglycemia, hyperglucagonemia, and excessive hepatic glucose production developed after 8 days. Our study stands in contrast to animal studies in which inappropriately elevated glucagon levels occurred concurrently with hypoinsulinemia resulting from streptozotocin treatment (7, 8, 30) and implies that hyperglucagonemia and inappropriate hepatic glucose output may play a causative role in hyperglycemia even in the setting of hyperinsulinemia.
A chronic, intravenous, glucose-infused (CIGI) rat model was used to assess whether a sustained increase in the rate of glucose appearance per se could impair islet function. An advantage of the CIGI rat is that, unlike models that raise glucose appearance by either impairing β-cell function or increasing insulin resistance, the effect of increased glucose load is direct. Dual intravenous catheters, one for infusion and one for plasma sampling, were placed in healthy, awake, adult Sprague-Dawley rats with free access to food and water. Rats were infused over a period of 10 days with either glucose or an equal volume of half-normal saline. The CIGI rats progressively adapted to the infusion and achieved a new steady state of glycemia on the 4th day that was sustained until the 8th day of infusion when they consistently developed hyperglycemia, hyperglucagonemia in the setting of hyperinsulinemia, and excessive hepatic glucose production. Herein we report our findings of the CIGI rat during the transition from adaptation to overt hyperglycemia.
MATERIALS AND METHODS
Animals.
Normal male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing ∼300 g were housed in a 12:12-h dark-light cycle. Animals underwent surgery for the placement of indwelling femoral and jugular venous catheters using 50- and 90-gauge polyethylene tubing, respectively, and were allowed to recover for ≥3 days in individual cages. The rats were harnessed, and the two catheters, protected by a flexible spring, were attached to a dual channel swivel on a balanced lever arm (Instech Solomon, Plymouth Meeting, PA). This arrangement enabled the rodents to have full access to the cage during the infusion. Calibrated syringe pumps (Harvard Apparatus, Holliston, MA) were used to infuse all rats at the same constant rate of 0.49% NaCl or either 2.5 (CIGI-low) or 3.3 (CIGI-high) g·kg−1·h−1 dextrose (0.35 and 0.5 g/ml, respectively) through the 50-gauge catheter throughout the study. Half-normal saline was used instead of normal saline to reduce the possibility of activating the renin-angiotensin system with a large sodium load, leading to confounding physiological changes. Daily blood samples were collected from the 90-gauge catheter into prechilled tubes containing aprotinin. Plasma was separated by centrifugation and stored at −80°C. The patency of the blood sampling catheter was maintained with a constant (0.25 ml/h) infusion of heparinized saline (1.25 U/h) using Razel pumps (Mansfield, St. Albans, VT). Intake of regular chow (Harlan-Teklad 2018, 77% carbohydrate-5% fat-18% protein) was monitored daily. The daily total caloric intakes were calculated based on the nutritional composition of the chow consumed plus the additional caloric input from the infusion. Rats were fasted overnight (14–16 h) where designated. All protocols were approved by the Yale University Animal Care and Use Committee.
Biochemical analysis of plasma.
Plasma glucose concentrations were measured using the glucose oxidase method on a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA). Insulin was measured using normal range ELISA kits (Mercodia, Uppsala, Sweden). Glucagon and amylin were measured by LINCOplex assay (Millipore, Billerica, MA). C-peptide and corticosterone were measured via RIA (Millipore). Chemistry analysis to measure plasma triglyceride, nonesterified fatty acid, sodium, potassium, chloride, albumin, alanine aminotransferase, and ketone concentrations was performed using the COBAS MIRA Plus (Roche Diagnostics, Pleasanton, CA).
EGP.
After an overnight fast prior to days 0, 4, or 8, the harnessed rats had three plasma samples collected in the basal state without discontinuation of the glucose infusion (−30, −20, and −5 min). Day 0 rats received a primed, continuous infusion of 99% enriched [6,6 2H2]glucose (prime 3.0 mg·kg−1·min−1 × 5 min, continuous 0.3 mg·kg−1·min−1 × 90 min). CIGI-high rats also received a 3.0 mg·kg−1·min−1 prime, but the 3.3 g·kg−1·h−1 continuous infusion of natural abundance glucose was changed to 5% [6,6-2H2]glucose without the infusion rate being altered. Plasma was obtained after steady state was reached at 60, 75, and 90 min. Samples were deproteinized with 5 volumes of 100% methanol, dried, and derivatized with 1:1 acetic anhydride-pyridine to produce the pentacetate derivative of glucose. The atom percent enrichment of glucose M + 1 and M + 2 were measured by GC-MS using a Hewlett-Packard 5890 gas chromatograph interfaced to a Hewlett-Packard 5971A mass-selective detector operating in the electron-ionization mode (3). Glucose M + 1 and M + 2 enrichments were determined from the mass-to-charge ratios 201–200 and 202–200 respectively. EGP was calculated using Steele's equation (39).
Intravenous glucose tolerance test.
Rats were fasted overnight prior to intravenous glucose tolerance test (IVGTTs) on days 0, 4, or 8. A 1 g/kg dextrose bolus was administered through the femoral venous catheter, and samples were collected at 0, 2.5, 5, 10, 15, 20, 30, 45, 60, and 90 min for measurement of plasma glucose and insulin concentrations. The basal glucose infusion rate was not altered. Insulin area under the curve (AUC) from time point a (timea) to subsequent time point b (timeb) was calculated using the following formula: total insulin AUC = ∑ [(timea − timeb) × ½ (insulina + insulinb)]. The baseline insulin AUC and insulin AUC above baseline were calculated as follows: baseline insulin AUC = 90 min × insulin time 0, insulin AUC above baseline = total insulin AUC − baseline insulin AUC. First-phase insulin AUC was calculated with data from 0 to 15 min using the above formulas.
Glucagon antibody study.
Following an overnight fast, on day 8, an EGP assay was performed on fasted hyperglycemic rats (plasma glucose ≥200 mg/dl). Immediately afterward, an intravenous injection of an anti-glucagon or a control antibody (anti-DNP, 4 mg/kg) was administered (a kind gift from Erica Nishimura at Novo Nordisk). Six hours later, a second EGP assay was performed. Glucose infusion was continued, and plasma was obtained 24 h after antibody treatment for plasma glucose and insulin measurement. The animal was euthanized immediately.
Tissue analysis.
At the end of infusions, animals were euthanized using pentobarbital sodium and tissues harvested in situ and frozen in a liquid nitrogen bath. Samples were powdered in a prechilled mortar and pestle and stored at −80°C for further processing. Liver glycogen content was measured by digesting liver homogenates with amyloglucosidase and measuring the resulting glucose concentration with the Beckman glucose analyzer (18). Liver triglyceride was measured using a DCL Triglyceride Reagent (Diagnostic Chemicals, Oxford, CT). Liquid chromatography-mass spectrometry-mass spectrometry was performed to measure long-chain fatty acyl-CoA and diacylglycerol content, as described previously (44). To measure liver mRNA levels, cDNA was extracted using the Qiacube (Qiagen, Valencia, CA), and reverse transcription was performed with the Peltier Thermal Cycler (MJ Research, Waltham, MA). mRNA was quantified using the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Liver proteins were detected by Western blot, loading 30–50 μg of protein lysate on a 4–12% Tris-glycine gel (Invitrogen, Carlsbad, CA) and transferring to a polyvinylidene difluoride membrane (Millipore). The membranes were incubated with a sheep polyclonal cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) antibody (a kind gift from Daryl Granner at Vanderbilt University Medical Center) and a goat anti-mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) antibody (Abcam, Cambridge, MA). Rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (Abcam) was used as a loading control. The blots were developed and quantified using Adobe Photoshop CS4 (Adobe Systems, San Jose, CA) software.
Statistics and data analysis.
Because of the technical challenges of this model, the study was performed as an intention to treat analysis. If an infusion line was lost, all data were kept up until the time of line closure. Similarly, if a sampling line was lost part of the way through the study, the infusion was continued, but the rodent was used only for the end-point tissue analysis study. All data are reported as means ± SW. Unpaired two-tailed Student t-tests and one-way ANOVA were performed using the Prism software package version 5 (GraphPad, La Jolla, CA). Mixed-model analysis was performed using the IPSS software package. Differences were considered to be significant at P < 0.05.
RESULTS
CIGI rats have modestly increased total caloric balance with compensating decreases in food intake.
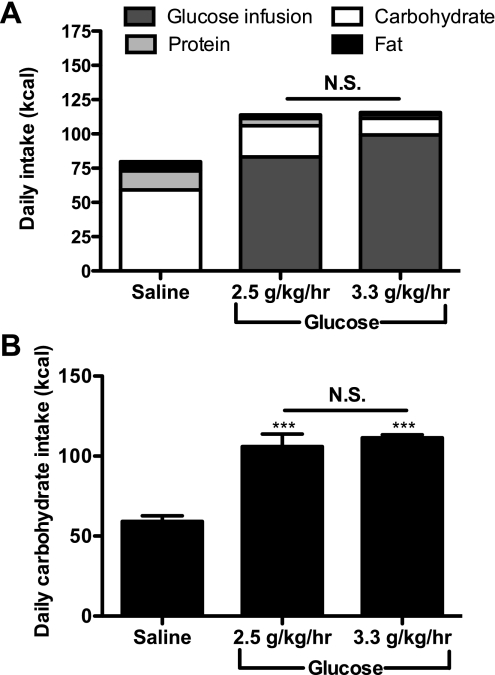
Rats infused with glucose at both 2.5 and 3.3 g·kg−1·h−1 (CIGI-low and CIGI-high, respectively) had on average a 45% increased caloric balance compared with saline-infused controls over the duration of the experiment (Fig. 1A). Both CIGI groups significantly decreased their chow intake at the outset of the infusion inversely to the rate of glucose infusion such that there was no difference in total caloric balance between them. The total carbohydrate balance was roughly double in the CIGI rats, with no difference (P = 0.35) between the CIGI-low and -high groups (Fig. 1B).
Fig. 1.
Caloric intake over the first 8 days of glucose or saline infusion. A: daily caloric intake, including glucose infusion and food consumption. Statistics are for total caloric intake [n = 17 saline infused, 7 chronic, intravenous, glucose-infused (CIGI)-low, and 40 CIGI-high rats]. B: daily carbohydrate intake. ***P < 0.001 vs. saline; 1-way ANOVA with Bonferroni's multiple comparison test was used to determine significance. In this and all of the following figures, data are presented as means ± SE. NS, not significant.
After adaptation, CIGI-high rats develop hyperglycemia.
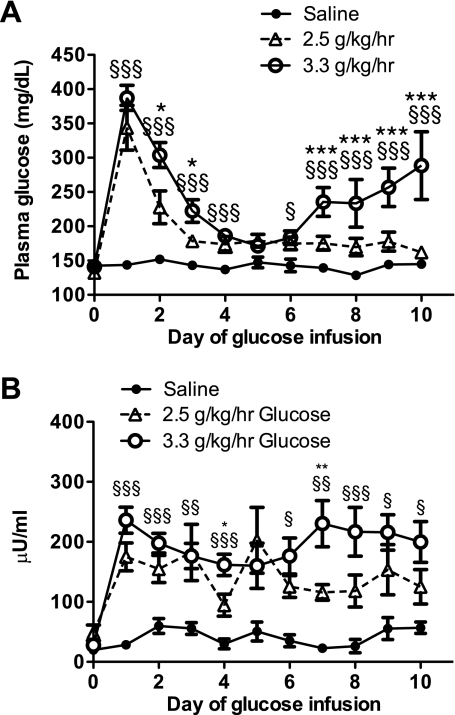
Throughout the duration of the saline infusion, there was no significant change in the fed plasma glucose or insulin concentrations (Fig. 2, A and B). In contrast, on day 1 of the infusion, both CIGI groups experienced significant hyperglycemia. By day 4, both glucose-infused groups had established a new steady state with glucose levels <200 mg/dl and a nadir by day 5 (CIGI-low 179 ± 9 mg/dl vs. CIGI-high 171 ± 4 mg/dl, P = 0.53), which was only moderately elevated compared with saline-infused rats (147 ± 8 mg/dl, P < 0.05) (Fig. 2A). The improvement in glucose homeostasis was accompanied by a steady reduction in insulin (Fig. 2B) but no change in food intake. The CIGI-low rats maintained their “adapted” plasma glucose levels throughout the duration of the 10-day infusion, with a gradual reduction in plasma insulin concentrations. In contrast, despite having the same adapted plasma glucose concentrations as the CIGI-low rats, beginning on day 7 the CIGI-high rats became progressively more hyperglycemic, with increasing plasma insulin levels. By day 7 the average plasma insulin level of CIGI-high rats was twice that of CIGI-low rats (230 ± 39 vs. 116 ± 13 μU/ml, P < 0.001) and 10 times that of the saline group (22 ± 7 μU/ml, P < 0.001). Following the adaptive period (i.e., after day 4), by the end of the study none of the control rats became hyperglycemic (i.e., glucose ≥200 mg/dl), whereas 14% of the CIGI-low and 89% of the CIGI-high rats were hyperglycemic.
Fig. 2.
Daily, nonfasting glucose and insulin in control and CIGI rats. A: daily plasma glucose concentrations. B: daily plasma insulin concentrations. ○, CIGI-high; ▵, CIGI-low; ●, saline. *P < 0.05, CIGI-high vs. CIGI-low; **P < 0.01, CIGI-high vs. CIGI-low; ***P < 0.001, CIGI-high vs. CIGI-low; §P < 0.05, CIGI-high vs. saline; §§P < 0.01, CIGI-high vs. saline; §§§P < 0.001, CIGI-high vs. saline. One-way ANOVA with Bonferroni's multiple comparison test was performed to determine significance.
CIGI-high rats at the transition to hyperglycemia.
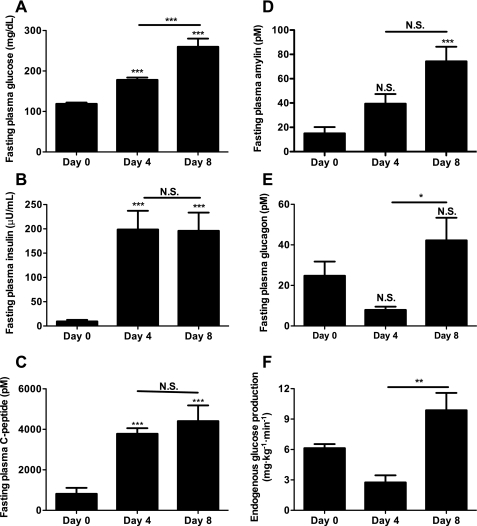
To target the transition between adaptation and hyperglycemia for closer investigation, infusion days 4 and 8 were selected for closer study. These days represent important points during the progression to worsening hyperglycemia since all CIGI-high rats were adapted on day 4 and 69% had developed frank hyperglycemia by day 8, with all but 11% becoming hyperglycemic over the following 2 days. Compared with baseline and saline-infused controls, fasting plasma electrolytes and corticosterone were not significantly elevated on day 8 of glucose infusion (Table 1). Plasma albumin dropped between day 0 and day 8 of glucose infusion, consistent with reduced protein intake, as shown in Fig. 1A. Consistent with normal liver function and insulin action, reductions were also measured in plasma, alanine aminotransferase, ketones, triglycerides, and nonesterified fatty acids. On days 0, 4, and 8 following an overnight fast, fasting plasma glucose and insulin concentrations in the CIGI-high rats were similar to the fed values (Figs. 2A and 3A). Despite the 80 mg/dl increase in fasting plasma glucose concentrations between days 4 and 8 (Fig. 3A), there was no decrement in insulin, C-peptide, or the insulin/C-peptide ratio to account for the worsening hyperglycemia (Fig. 3, B and C). Like insulin, plasma amylin was also increased in the CIGI-high rats (Fig. 3D).
Table 1.
Physiological fasting parameters of saline-infused and CIGI-high rats
|
Day 8 |
|||
|---|---|---|---|
| Day 0 | Saline | 3.3 g•kg−1•h−1 glucose | |
| Sodium, mmol/l | 134 ± 2 | 123 ± 4 | 123 ± 6 |
| Potassium, mmol/l | 4.1 ± 0.3 | 3.9 ± 0.4 | 4.7 ± 0.2 |
| Chloride, mmol/l | 101 ± 2 | 99 ± 3 | 95 ± 3 |
| Calcium, mmol/l | 2.56 ± 0.16 | 2.35 ± 0.24 | 1.95 ± 0.12 |
| Alanine aminotransferase, U/l | 32.3 ± 5.6 | 31.9 ± 2.4 | 17.9 ± 2‡‡ |
| Albumin, g/l | 33 ± 1 | 29 ± 1* | 24 ± 1***,‡ |
| Ketones, mmol/l | 0.92 ± 0.29 | 0.40 ± 0.05 | 0.11 ± 0.03‡ |
| Triglycerides, mmol/l | 0.68 ± 0.09 | 0.25 ± 0.02** | 0.32 ± 0.05* |
| Nonesterified fatty acids, mmol/l | 1.21 ± 0.19 | 0.50 ± 0.23 | 0.26 ± 0.08** |
| Corticosterone, nmol/l | 648 ± 141 | 322 ± 115 | 363 ± 61 |
Data are means ± SE of plasma concentrations of each analyte; n = 8–12 experiments for day 0, 3–4 experiments for day 8 saline-infused rats, and 8–11 experiments for day 8 glucose-infused rats. CIGI, chronic, intravenous glucose infused.
P < 0.05 vs. day 0;
P < 0.01 vs. day 0;
P < 0.001 vs. day 0;
P < 0.05 vs. day 8 saline;
P < 0.01 vs. day 8 saline. Significance was determined using mixed-model analysis with Bonferroni's multiple comparison test.
Fig. 3.
Fasting parameters of glucose homeostasis in CIGI-high rats. A: fasting plasma glucose; n = 51 (day 0), 26 (day 4), and 42 (day 8). B: fasting plasma insulin; n = 22 (day 0), 19 (day 4), and 27 (day 8). C: fasting plasma C-peptide; n = 16 (day 0), 15 (day 4), and 14 (day 8). D: fasting plasma amylin; n = 12 (day 0), 19 (day 4), and 30 (day 8). E: fasting plasma glucagon; n = 9 (day 0), 9 (day 4), and 32 (day 8). F: endogenous glucose production; n = 10 (day 0), 8 (day 4), and 21 (day 8). *P < 0.05; **P < 0.01; ***P < 0.001. Mixed-model analysis with Bonferroni's multiple comparison test was used in A–D, and the 2-tailed unpaired Student t-test was used in E and F.
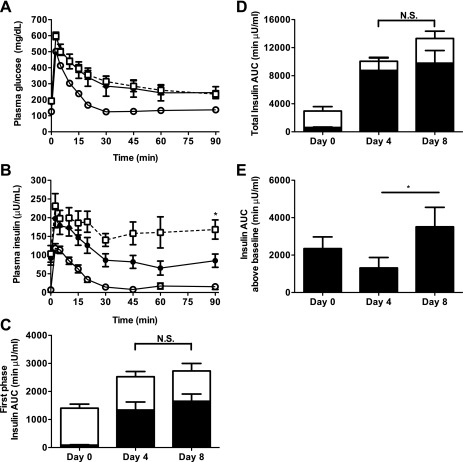
The incremental response in plasma glucose concentrations during an IVGTT (1 g/kg) was indistinguishable between days 4 and 8, although glucose clearance was slightly delayed compared with day 0 (Fig. 4A). Surprisingly, despite worsening hyperglycemia, on day 8 the CIGI-high rats had the highest plasma insulin levels throughout the IVGTT (Fig. 4B), not suggesting a decline in glucose-stimulated β-cell performance compared with day 4. Total first-phase insulin was increased significantly on both day 4 and day 8 in an IVGTT, but after accounting for the already high baseline insulin secretion, there was no difference in the acute response to glucose between days 0, 4, and 8 (Fig. 4C). The majority of plasma insulin measured during the IVGTT in glucose-infused animals was the result of increased basal secretion. Basal insulin accounted for 87% of the total insulin AUC on day 4 and 77% on day 8 compared with 21% on day 0 (Fig. 4D). The total AUC insulin secretion above baseline trended higher in the day 8 CIGI-high rats (Fig. 4, D and E). Thus, after 8–10 days of glucose infusion, the CIGI-high rats developed worsening hyperglycemia that was not associated with any demonstrable decrement in either basal or stimulated insulin secretion.
Fig. 4.
Intravenous glucose tolerance tests (GTT) in CIGI-high rats on days 0, 4, and 8 of infusion. A: plasma glucose concentrations. B: plasma insulin concentrations. In A and B, ○ = day 0, ● = day 4, and □ = day 8. There were no differences in glucose concentrations at any time point between days 4 and 8. C: insulin area under the curve (AUC) during the first 15 min of the GTT. D: total insulin AUC. There were no significant differences between days 4 and 8. In C and D, closed bars = insulin AUC due to baseline insulin secretion, and open bars = insulin AUC above baseline. There were no significant differences in total AUC, baseline AUC, or AUC above baseline between days 4 and 8. E: total insulin AUC above baseline. *P < 0.05. Data are means ± SE of 19 experiments on day 0, 18 experiments on day 4, and 7 experiments on day 8. Significance was determined by the 2-tailed unpaired Student t-test.
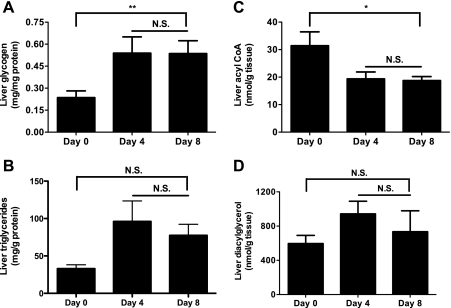
By day 4, the fasted CIGI-high rats had appropriately suppressed plasma glucagon concentrations and suppressed EGP (Fig. 3, E and F). However, by day 8, plasma glucagon concentrations increased fivefold and EGP increased threefold (Fig. 3, E and F) in CIGI-high rats. There was a trend toward increased hepatic glycogen content on both days 4 and 8 relative to day 0 (Fig. 5A); however, there were no significant differences in hepatic triglyceride, long-chain CoAs, or diacylglycerol content over the course of the infusion (Fig. 5, B–D).
Fig. 5.
Intrahepatic metabolites in CIGI-high rats. A: glycogen (n = 5 each day). There were no significant differences between days 4 and 8 by 2-tailed unpaired Student t-test. B: triglycerides [n = 6 (day 0), 7 (day 4), and 14 (day 8)]. C: acyl-CoA (n = 5 each day). D: diacylglycerol (n = 5 each day). Bars spanning between days 4 and 8 refer to P value summary from 1-way analysis of variance. *P < 0.05, **P < 0.01.
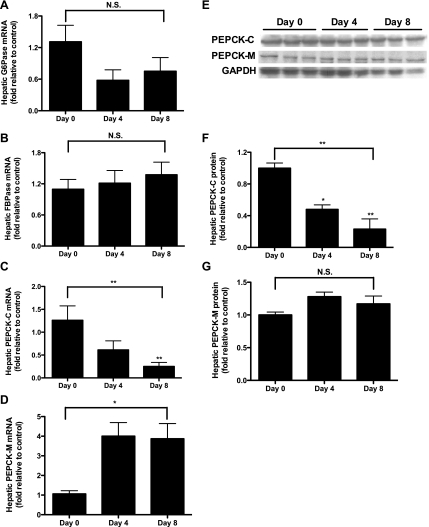
Glucose infusion is associated with variations in gluconeogenic enzymes.
The hepatic expression and protein levels of several enzymes implicated in the regulation of gluconeogenesis were studied. There was no change in mRNA for glucose-6-phosphatase or fructose-1,6-bisphosphatase over the course of the glucose infusion (Fig. 6, A and B). The mRNA and protein of PEPCK-C decreased as the infusion continued (Fig. 6, C, E, and F), whereas PEPCK-M trended up at the mRNA and, to a lesser extent, protein levels (Fig. 6, D, E, and G).
Fig. 6.
Expression and protein of hepatic gluconeogenic enzymes. A: glucose-6-phosphatase (G-6-Pase) expression [n = 7 (day 0), 7 (day 4), and 9 (day 8)]. B: fructose-1,6-bisphosphatase (FBPase) expression [n = 6 (day 0), 6 (day 4), and 10 (day 8)]. C: hepatic cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) mRNA [n = 7 (day 0), 7 (day 4), and 13 (day 8)]. D: hepatic mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) mRNA [n = 6 (day 0), 6 (day 4), and 13 (day 8)]. E: Western blots used to measure PEPCK protein, with GAPDH as a loading control. F: hepatic PEPCK-C protein (n = 3 each day). G: hepatic PEPCK-M protein (n = 3 each day). In A–F, 1-way analysis of variance was calculated (bar spanning from days 0 to 8), with individual comparison of day 0 vs. day 4 and day 0 vs. day 8 by 1-way ANOVA with Bonferroni's multiple comparison test. There were no significant differences between days 4 and 8 by 2-tailed unpaired Student t-test. Statistics over individual bars refer to comparisons vs. day 0 by 1-way ANOVA with Bonferroni's multiple comparison test. *P < 0.05; **P < 0.01.
Glucagon is an essential cause of hyperglycemia in the CIGI-high rat.
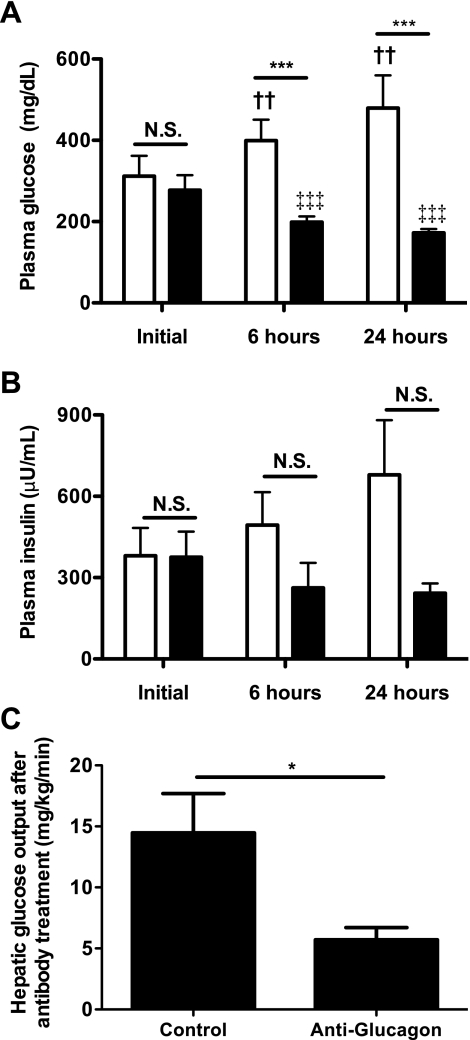
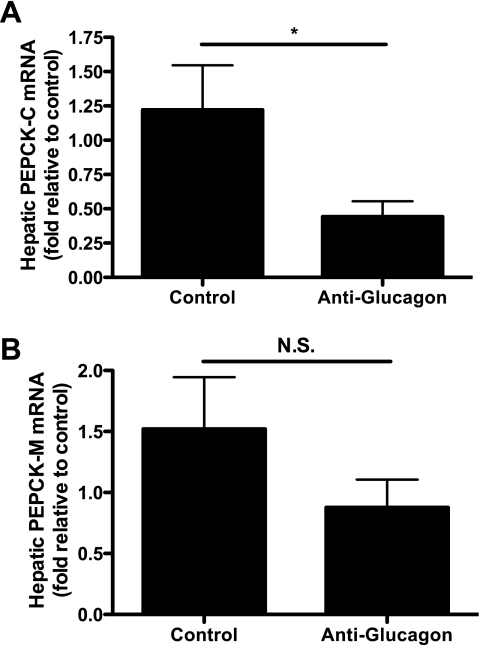
To assess whether α-cell dysfunction leading to increases in plasma glucagon concentrations was responsible for the increased EGP in the CIGI-high rat, we administered a monoclonal antibody against glucagon (36) to hyperglycemic CIGI-high rats on day 8. Fasting plasma glucose and insulin levels were identical prior to antibody administration (Fig. 7, A and B). CIGI-high rats receiving a control antibody had a progressive rise in plasma glucose and insulin concentrations following treatment. In dramatic contrast, within 6 h the anti-glucagon antibody reduced plasma glucose concentrations by an average of 70 mg/dl, and the improvement continued to a 110 mg/dl reduction at 24 h. Thus, the consequence of neutralizing glucagon was a relative 300 mg/dl difference in plasma glucose concentrations between the control and treated groups 24 h after antibody administration (479 ± 33 vs. 172 ± 4 mg/dl, respectively; P = 0.0002). In response to the neutralization of glucagon, plasma insulin also trended lower (Fig. 7B), suggesting that the beneficial effects of glucagon reduction were not mediated through enhanced β-cell activity. Plasma glucose and insulin concentrations 24 h after antibody treatment were identical to levels on day 4 (glucose after antibody 172 ± 10 vs. 178 ± 6 mg/dl on day 4, P = 0.64; insulin after antibody 242 ± 36 vs. 198 ± 39 μU/ml on day 4, P = 0.52). Since the rate of glucose infusion was unchanged, this argues against an increase in insulin resistance accounting for the hyperglycemia and implicates excessive glucagon secretion. Blocking glucagon reduced hepatic glucose production by 60% compared with control animals after 6 h (Fig. 7C), resulting in an ∼20% reduction of the total rate of endogenous glucose appearance. Anti-glucagon antibody treatment was associated with a significant reduction in PEPCK-M at the expression level, whereas PEPCK-C also trended down (Fig. 8).
Fig. 7.
Response to treatment with anti-glucagon antibodies in hyperglycemic CIGI-high rats. A: plasma glucose concentrations before and after antibody treatment. B: plasma insulin concentrations before and after treatment. C: hepatic glucose output 6 h after treatment. In A and B, open bars = control group, and closed bars = anti-glucagon antibody treatment group. In A–C, n = 9 after 6 h and 6 after 24 h in the control group, and n = 7 after 6 h and 7 after 24 h in the anti-glucagon antibody group. Significance was evaluated by 2-tailed unpaired Student t-test. *P < 0.05; ***P < 0.001; ††P < 0.01 vs. control group at baseline; ‡‡‡P < 0.001 vs. anti-glucagon antibody treated group at baseline.
Fig. 8.
Hepatic PEPCK expression after anti-glucagon antibody treatment. A: PEPCK-C expression (n = 6 in each group). B: hepatic PEPCK-M expression (n = 7 in each group). *P < 0.05.
DISCUSSION
The progression to T2D is generally believed to occur when the rate of glucose appearance in the blood exceeds the ability of the β-cell to mediate adequate glucose clearance. Our experiments were designed to establish whether extended periods of increased glucose turnover could impair islet α-cell and β-cell function in otherwise normal animals. Continuous intravenous glucose infusion increased the rate of appearance of glucose five to eight times fasting levels in normal, healthy adult Sprague-Dawley rats and consistently precipitated hyperglycemia following a period of adaptation. Surprisingly, the hyperglycemia was not associated with a decrement in either fasting or glucose-stimulated insulin secretion. Rather, hyperglycemia followed an inappropriate increase in plasma glucagon concentrations and a consequent increase EGP. Blocking the action of glucagon with an anti-glucagon antibody reversed these defects, demonstrating that the increased plasma glucagon concentrations were responsible for the increased EGP and hyperglycemia. These observations in chronically glucose-infused rats suggest that 1) increased basal demand on the islet simulating excessive EGP is associated with inappropriately increased plasma glucagon concentrations prior to a measurable decline in insulin secretion, 2) modest increases in plasma glucagon concentrations can increase EGP sufficiently to cause hyperglycemia, and 3) hyperglycemia can occur in the absence of a detectable decrement in insulin secretion.
Fasting hyperglycemia is a cardinal feature of diabetes (1), and it can be attributable mostly to excessive EGP (4, 10, 27, 43, 45). As EGP increases, the basal islet workload will also increase. Two weeks of clamping plasma glucose concentrations at ≥250 mg/dl in four 50% pancreatectomized dogs with multiple daily injections of glucagon were required to induce severe, persistent hyperglycemia, hypoinsulinemia, insulin resistance, and elevated plasma glucagon concentrations (17). Notably, the remaining islets of these dogs had diminished β-cell mass but normal glucagon immunoreactivity. Under these circumstances, it was not possible to determine the direct cause of the hyperglucagonemia or its contribution to the consequent diabetes. To simulate the increases in EGP associated with hepatic insulin resistance in otherwise normal, healthy rats, the CIGI model was utilized. By using two separate venous lines connected to a dual channel swivel, blood sampling could be maintained from one catheter while constant infusion was maintained through the other for ≤10 days. An advantage of this model is the lack of underlying hyperlipidemia, hypercorticosteronemia, obesity, islet deficiency (from partial pancreatectomy), isolated β-cell deficiency, impaired leptin signaling, or other genetic abnormalities present in other models used to study the early stages of T2D.
Other laboratories have demonstrated similar adaptive physiology over the first 2–4 days of chronic glucose infusion in normal rats (20, 40, 41). Hager et al. (15) showed diminished insulin sensitivity on day 3 relative to day 0, and although we could not directly compare insulin sensitivity during glucose infusion to day 0 before glucose infusion was begun, our data are consistent with these findings. Similar to our study, other groups have shown that adaptation to glucose infusion gradually diminishes plasma glucose concentrations and concomitantly decreases plasma insulin concentrations, suggesting improved insulin sensitivity between days 1 and 4, whether related to increases in the rate of glucose disposal or decreases in the rate of glucose appearance. Loss of glucose homeostasis by day 8 of the infusion was accompanied by increases in EGP, glucose, and insulin, suggesting that either increased hepatic insulin resistance or an alternative insulin-independent gluconeogenic pathway was activated. Because within 6 h of anti-glucagon antibody treatment EGP was suppressed and glucose and insulin had returned to their day 4 levels, our data suggest the latter possibility. This is further supported by suppressed ketogenesis (Table 1) and downregulation of PEPCK-C (Fig. 6, C, E, and F) indicating that at least some pathways of hepatic insulin signaling remained intact.
Modest reversible impairments in glucose-stimulated insulin secretion have also been observed in vitro during pancreatic perfusions in short-term glucose-infused rats (9, 11, 22–25). Of note, on day 4 of our study the CIGI-high rats had increased insulin secretion in vivo during an IVGTT compared with those on day 0, and no decrement was measured between days 4 and 8 (Fig. 4, B–E). Indeed, rats showed a trend toward increased insulin secretion during the IVGTT on day 8 relative to day 4. Although β-cell function is clearly inadequate to maintain euglycemia by day 8, our data imply that this is not the result of absolute failure of glucose-stimulated insulin secretion by the β-cell.
Following a pattern similar to that demonstrated by other laboratories (22, 23, 40, 41), after the initial adaptive phase, both CIGI-high and -low rats reached a new steady-state plasma glucose that was indistinguishable from each other (Fig. 2A). The CIGI-low rats maintained this new steady state throughout the duration of the glucose infusion, whereas the CIGI-high group became increasingly hyperglycemic despite identical static and dynamic insulin secretion. Although it is clear that hyperglycemia is injurious to many tissues, in principle, since hyperglycemia is the hallmark that diabetes has already occurred, some prior insult or deficiency is necessary to beget hyperglycemia. Comparison between days 4 and 8 of the CIGI-high rats was instructive. Day 4 represents a time of gradual improvement in glucose homeostasis, whereas day 8 marks the point of worsening for the majority of the rats. Surprisingly, there was no decrement in fasting plasma insulin concentrations between days 4 and 8. The identical insulin response (above baseline) to the same glucose challenge during the IVGTT on days 0, 4, and 8 suggests that dynamic insulin secretion by the β-cells remained intact. It is not well understood whether the inability to normalize the plasma glucose concentration in the setting of increased appearance of glucose in the early stages of T2D represents true β-cell dysfunction or rather the presence of a different dose-response relationship. In our studies, whereas there was insufficient circulating insulin to maintain normal plasma glucose levels, insulin secretion did not decline when plasma glucose increased as the infusion continued (Fig. 3, A and B) nor increase when plasma glucose decreased after anti-glucagon antibody treatment (Fig. 7, A and B). Consequently, some level of responsiveness to changes in glucose was maintained in both stimulated and static insulin secretion. As anticipated because amylin is cosecreted with insulin, amylin levels also increased on days 4 and 8. Although there was a trend toward increased plasma amylin concentrations on day 8 of infusion, there was no significant difference, and the amylin/insulin ratios did not vary significantly. The lack of a change in these data between days 4 and 8 supports the notion that β-cell secretion remained robust at the time of glycemic decompensation.
Despite unchanged static and dynamic insulin secretion on day 8, rats became progressively more hyperglycemic, with striking increases in plasma glucagon concentrations and EGP. Glucagon dysregulation has been best studied in patients and animal models of type 1 diabetes, in which hyperglucagonemia is believed at least in part to result from loss of inhibition by endogenous insulin (34). In the setting of low insulin, hyperglycemia paradoxically stimulates glucagon secretion (5, 21). However, in our studies, hyperglycemia and hyperglucagonemia developed progressively in the setting of unchanged plasma insulin concentrations. We have now demonstrated that chronic glucose infusion can lead to hyperglucagonemia, but the mechanism of this in our model remains unknown. Although loss of insulin secretion does not appear to be the cause, other possible etiologies include altered intraislet signaling (e.g., GABA or zinc from the β-cell, glutamate from the α-cell, somatostatin from the δ-cell), altered central or peripheral autonomic input (6, 8, 29), or potentially α-cell insulin resistance. Although inappropriate control of glucagon release has been better characterized in type 1 diabetes, type 2 diabetic patients also have reduced suppression of glucagon secretion by hyperglycemia (42) and by a high carbohydrate load (31) via an unclear mechanism. In our studies, there was no difference in plasma corticosterone concentrations between days 4 and 8 (Table 1), and both were lower than at the beginning of the study. Circulating catecholamines were not measured, but the reduced corticosterone argues against a systemic stress response. Because such hyperglucagonemia may be a key feature in the development of human diabetes, additional studies will be needed to clarify the mechanism of increased glucagon secretion.
Interfering with glucagon function prevents or corrects hyperglycemia in animals. Glucagon receptor knockout mice have lower blood glucose levels and improved glucose tolerance (12, 32), and mice with impaired glucagon secretion are protected from high-fat diet-induced hyperglycemia (14). Blocking the action of glucagon with an anti-glucagon antibody (13, 36) or an antisense oligonucleotide against the glucagon receptor (26) corrects hyperglycemia in diet-induced and genetic rodent models of diabetes. Similarly, in our studies, treatment with an anti-glucagon antibody restored plasma glucose to adaptive levels and reduced EGP by 60%. There was a reduction in plasma insulin levels as the plasma glucose concentrations fell; thus augmented insulin secretion cannot account for the improvement in glycemia. Glucagon can potentiate insulin release in different experimental settings, but the physiological relevance of this to our model is unclear. In principle, a reduction in glucagon-stimulated insulin secretion could potentially explain the reduced insulin levels following anti-glucagon antibody administration; however, a more likely explanation is an appropriate response of the β-cell to reduced EGP and plasma glucose.
Twenty-four hours after anti-glucagon antibody treatment, plasma glucose and insulin levels were identical to those on day 4. Since glucose infusion rate and plasma glucose and insulin were the same at each of these times, systemic insulin resistance cannot account for the development of hyperglycemia. Our data corroborate studies by Laury et al. (20), who showed that an increased glucose infusion rate was necessary to continually lamp glucose at 170 mg/dl during a 4-day chronic glucose clamp in rats, implying improving whole body insulin sensitivity in these animals during the early days of infusion. The lower plasma transaminase levels in our animals rule out hepatic inflammation, and the reduction in plasma β-hydroxybutyrate between days 0 and 8 indicates that insulin-mediated suppression of ketogenesis remains intact in the CIGI rats. Similarly, the reduction in plasma triglycerides and free fatty acids suggests intact insulin-mediated suppression of lipogenesis and argues against a role for lipotoxicity in islet dysfunction.
To investigate why EGP was elevated despite some evidence of normal hepatic insulin sensitivity, the hepatic expression of regulatory enzymes of gluconeogenesis was assessed. Neither glucose-6-phosphatase nor fructose-1,6-bisphosphatase mRNA (Fig. 6, A and B) changed significantly between days 4 and 8. In contrast and in keeping with preserved hepatic insulin signaling, PEPCK-C levels were progressively suppressed on days 4 and 8 as insulin levels climbed. The day 8 increase in EGP posed an apparent paradox since the enzymatic reaction catalyzed by PEPCK is essential for gluconeogenesis from the key gluconeogenic substrates such as lactate, alanine, glutamine, and pyruvate. Anti-glucagon antibody treatment did lead to a small but significant reduction in the already low PEPCK-C mRNA, but there was no change in protein expression. A potential explanation of the metabolic source of the increased gluconeogenic flux is the correlation of PEPCK-M expression with glucagon levels and with EGP. PEPCK-M is generally considered to be constitutively expressed in rodents and not under the influence of either glucagon or insulin. Whether the combination of insulin and glucagon or some other signal is able to alter PEPCK-M expression remains to be determined. Further studies will also be required to ascertain the relevance of PEPCK-M in glucose production, but the changes in the expression of this isoform suggest that it may play a previously unappreciated role in glucose homeostasis, just as it has been shown recently to play a key role in glucose sensing in the pancreatic β-cell (37).
In summary, the CIGI-high rat provides a model system to study the effect of increased glucose load on islet function and glycemic control in the absence of other underlying abnormalities. A crucial insight from studying these otherwise healthy young rats is that neither loss of insulin secretion nor apparent increases in systemic insulin resistance were associated with the development of fasting and postprandial hyperglycemia. Importantly, although there were trends toward differences in liver glycogen, acyl-CoA, and triglycerides in glucose-infused rats compared with baseline (Fig. 5, A–C), there were no differences between days 4 and 8, when the rats were progressing to worsening hyperglycemia. Simply an increased rate of glucose appearance from the continuous infusion of glucose was a sufficient insult to lead to loss of glucose homeostasis. Surprisingly, the increased workload on the β-cell was not associated with an absolute reduction in insulin secretion. Arguably, there may be a relative deficit in β-cell function, because the animals became increasingly hyperglycemic with unchanged insulin levels. The onset of hyperglycemia was associated with inappropriately elevated plasma glucagon concentrations, which were sufficient to increase EGP, leading to hyperglycemia. Blocking the action of glucagon in this model reversed the hyperglycemia, restrained EGP, and lowered plasma insulin concentrations. Further studies are warranted to investigate whether the increased EGP that is observed in humans as they progress to T2D is related to inappropriate glucagon release independent of insulin release and/or action.
GRANTS
These studies were supported by grants from the US Public Health Service (R01-DK-40936, R01-DK-71071, K08-DK-80142, P30-DK-45735, P30-DK-34989, T32-GM-07205, and UL1-RR-024139) and the American Diabetes Association (1-09-RA-86).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to T. May, S. Skowronek, Y. Kosover, and A. Groszmann of Yale University for expert technical assistance.
REFERENCES
- 1. No authors listed Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 120: 1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Amer J Med Sci 257: 415–419, 1969 [DOI] [PubMed] [Google Scholar]
- 3. Bier DM, Arnold KJ, Sherman WR, Holland WH, Holmes WF, Kipnis DM. In vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes 26: 1005–1015, 1977 [DOI] [PubMed] [Google Scholar]
- 4. Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest 74: 1238–1246, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braaten JT, Faloona GR, Unger RH. The effect of insulin on the alpha-cell response to hyperglycemia in alloxan diabetes. J Clin Invest 53: 1017–1021, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burcelin R, Eddouks M, Kande J, Assan R, Girard J. Evidence that GLUT-2 mRNA and protein concentrations are decreased by hyperinsulinemia and increased by hyperglycaemia in liver of diabetic rats. Biochem J 288: 675–679, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burcelin R, Eddouks M, Maury J, Kande J, Assan R, Girard J. Excessive glucose production, rather than insulin resistance, accounts for hyperglycaemia in recent-onset streptozotocin-diabetic rats. Diabetologia 38: 283–290, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Burcelin R, Thorens B. Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Diabetes 50: 1282–1289, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Colella RM, May JM, Bonner-Weir S, Leahy JL, Weir GC. Glucose utilization in islets of hyperglycemic rat models with impaired glucose-induced insulin secretion. Metabolism 36: 335–337, 1987 [DOI] [PubMed] [Google Scholar]
- 10. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37: 667–687, 1988 [DOI] [PubMed] [Google Scholar]
- 11. De Souza CJ, Caportorto JV, Cornell-Kennon S, Wu YJ, Steil GM, Trivedi N, Weir GC. Beta-cell dysfunction in 48-hour glucose-infused rats is not a consequence of elevated plasma lipid or islet triglyceride levels. Metabolism 49: 755–759, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 100: 1438–1443, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu W, Yan H, Winters KA, Komorowski R, Vonderfecht S, Atangan L, Sivits G, Hill D, Yang J, Bi V, Shen Y, Hu S, Boone T, Lindberg RA, Véniant MM. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther 331: 871–881, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Gustavsson N, Seah T, Lao Y, Radda GK, Südhof TC, Han W. Delayed onset of hyperglycemia in a mouse model with impaired glucagon secretion demonstrates that dysregulated glucagon secretion promotes hyperglycaemia and type 2 diabetes. Diabetologia 54: 415–422, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Hager SR, Jochen AL, Kalkhoff RK. Insulin resistance in normal rats infused with glucose for 72 h. Am J Physiol Endocrinol Metab 260: E353–E362, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi S, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49: 2063–2069, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imamura T, Koffler M, Helderman JH, Prince D, Thirlby R, Inman L, Unger RH. Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes 37: 600–609, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Kadish AH, Little RL, Sternberg JC. A new and rapid method for the determination of glucose by measurement of rate of oxygen consumption. Clin Chem 14: 116–131, 1968 [Google Scholar]
- 19. Laube H, Fussgänger RD, Maier V, Pfeiffer EF. Hyperglucagonemia of the isolated perfused pancreas of diabetic mice (db-db). Diabetologia 9: 841–847, 1973 [DOI] [PubMed] [Google Scholar]
- 20. Laury MC, Penicaud L, Ktorza A, Benhalem H, Bihoreau MT, Picon L. In vivo insulin secretion and action in hyperglycemic rat. Am J Physiol Endocrinol Metab 257: E180–E184, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Le Marchand SJ, Piston DW. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J Biol Chem 285: 14389–14398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leahy JL, Cooper HE, Deal DA, Weir GC. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest 77: 908–915, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leahy JL, Cooper HE, Weir GC. Impaired insulin secretion associated with near normoglycemia. Study in normal rats with 96-h in vivo glucose infusions. Diabetes 36: 459–464, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Leahy JL, Weir GC. Beta-cell dysfunction in hyperglycemic rat models: recovery of glucose-induced insulin secretion with lowering of the ambient glucose level. Diabetologia 34: 640–647, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Leahy JL, Weir GC. Evolution of abnormal insulin secretory responses during 48-h in vivo hyperglycemia. Diabetes 37: 217–222, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Liang Y, Osborne MC, Monia BP, Bhanot S, Watts LM, She P, DeCarlo SO, Chen X, Demarest K. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53: 410–417, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Maggs DG, Buchanan TA, Burant CF, Cline G, Gumbiner B, Hsueh WA, Inzucchi S, Kelley D, Nolan J, Olefsky JM, Polonsky KS, Silver D, Valiquett TR, Shulman GI. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 128: 176–185, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90: 1323–1327, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bay I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest 115: 3545–3553, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meier JJ, Ueberberg S, Korbas S, Schneider S. Diminished glucagon suppression after β-cell reduction is due to impaired α-cell function rather than an expansion of α-cell mass. Am J Physiol Endocrinol Metab 300: E717–E723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. N Engl J Med 283: 109–115, 1970 [DOI] [PubMed] [Google Scholar]
- 32. Parker JC, Andrews KM, Allen MR, Stock JL, McNeish JD. Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun 290: 839–843, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64: 106–110, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Sakurai H, Dobbs RE, Unger RH. The role of glucagon in the pathogenesis of the endogenous hyperglycemia of diabetes mellitus. Metabolism 24: 1287–1297, 1975 [DOI] [PubMed] [Google Scholar]
- 35. Singh V, Grötzinger C, Nowak KW, Zacharias S, Göncz E, Pless G, Sauer IM, Eichhorn I, Pfeiffer-Guglielmi B, Hamprecht B, Wiedenmann B, Plöckinger U, Strowski MZ. Somatostatin receptor subtype-2-deficient mice with diet-induced obesity have hyperglycemia, nonfasting hyperglucagonemia, and decreased hepatic glycogen deposition. Endocrinology 148: 3887–3899, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Sørensen H, Brand CL, Neschen S, Holst JJ, Fosgerau K, Nishimura E, Shulman GI. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes 55: 2843–2848, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, Shulman GI, Kibbey RG. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem 284: 26578–26590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stearns SB, Benzo CA. Glucagon and insulin relationships in genetically diabetic (db/db) and in streptozotocin-induced diabetic mice. Horm Metab Res 10: 20–23, 1978 [DOI] [PubMed] [Google Scholar]
- 39. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 40. Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC. Adaptation of β-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab 280: E788–E796, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Topp BG, McArthur MD, Finegood DT. Metabolic adaptations to chronic glucose infusion in rats. Diabetologia 47: 1602–1610, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D. Diminished cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 74: 1318–1328, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 48: 2197–2203, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Yu XX, Lewin DA, Forrest W, Adams SH. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J 16: 155–168, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Zargar AH, Masoodi SR, Khan AK, Bashir MI, Laway BA, Wani AI, Dar FA. Impaired fasting glucose and impaired glucose tolerance - lack of agreement between the two categories in a North Indian population. Diabetes Res Clin Pract 51: 145–149, 2001 [DOI] [PubMed] [Google Scholar]