Abstract
Maternal overnutrition prior to and during gestation causes pronounced metabolic dysfunction in the adult offspring. However, less is known about metabolic adaptations in the offspring that occur independently of postnatal growth and nutrition. Therefore, we evaluated the impact of excess maternal dietary lipid intake on the in utero programming of body composition, hepatic function, and hypothalamic development in newborn (P0) offspring. Female mice were fed a low-fat (LF) or high-fat (HF) diet and were mated after 4, 12, and 23 wk. A subset of the obese HF dams was switched to the LF diet during the second (DR2) or third (DR3) pregnancies. The HF offspring accrued more fat mass than the LF pups, regardless of duration of maternal HF diet consumption or prepregnancy maternal adiposity. Increased neonatal adiposity was not observed in the DR3 pups. Liver weights were reduced in the HF offspring but not in the DR2 or DR3 pups. Offspring hepatic triglyceride content was reduced in the HF pups, but hepatic inflammation and expression of lipid metabolism genes were largely unaffected by maternal diet. Maternal diet did not alter the hypothalamic expression of orexigenic and anorexigenic neuropeptides in the offspring. Thus, the intrauterine programming of increased neonatal adiposity and reduced liver size by maternal overnutrition is evident in mice at birth and occurs prior to the development of maternal obesity. These observations demonstrate that dietary intervention during pregnancy minimizes the deleterious effects of maternal obesity on offspring body composition, potentially reducing the offsprings' risk of developing obesity and related diseases later in life.
Keywords: metabolic programming, liver, hypothalamus, inflammation, obesity
the global prevalence of obesity has reached epidemic proportions in recent years, not only threatening the health and well-being of affected individuals but also imposing a considerable burden on the economic and health care systems of developed and developing nations (33). In the US, the prevalence of obesity has more than doubled in adults and more than tripled in children and adolescents since the 1970s. According to estimates from the 2007–2008 National Health and Nutrition Examination Survey, 33.8% of adults and 16.9% of children aged 2–19 are obese, and 9.5% of infants and toddlers are at or above the 95th percentile of the weight-for-length growth charts (23, 24, 45, 46). Obese individuals have an increased risk of developing type 2 diabetes mellitus, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. Once restricted to adults, these metabolic diseases and conditions are now being diagnosed in children and adolescents at alarming rates, mirroring the rising rates of juvenile obesity (34, 39, 47, 52, 55).
The etiology of obesity is multifactorial, involving complex interactions between genes and the environment. In addition to the numerous lifestyle factors that contribute to the development of obesity (e.g., increased consumption of foods that are high in fat and calories, physical inactivity, etc.), a growing body of evidence suggests that many individuals are programmed to become obese while still in the womb. Exposure to an adverse intrauterine environment elicits profound and permanent adaptations in the offspring that increase their susceptibility to developing various diseases later in life (3). Fetal metabolic programming was initially studied in the context of maternal undernutrition and/or reduced intrauterine growth, both of which increase the offsprings' risk of developing obesity, hypertension, cardiovascular disease, and diabetes in adulthood (4–6, 32, 40, 48–50). In humans, the offspring of obese and/or diabetic mothers are also at greater risk of developing obesity and diabetes as children and adults (16, 18, 59, 65). Given current estimates that nearly one-half of women of childbearing age are overweight or obese (64), there is an emerging need to evaluate the impact of maternal overnutrition on offspring health and disease risk.
In rodents, exposure to an increased nutrient supply and/or maternal obesity during critical periods of development promotes obesity and impaired glucose homeostasis in the weanling or adult offspring (19, 30, 53, 62). The programming effects of increased maternal fat consumption during pregnancy and lactation are typically exacerbated when the offspring are also fed an energy-rich diet postweaning, demonstrating that metabolic adaptations that are programmed during prenatal development can be modified by postnatal growth and nutrition (11, 20, 36, 56). In contrast, other investigators have restricted their nutritional insult to the intrauterine environment by studying the offspring of high-fat (HF) diet-fed females during late fetal development or immediately after birth (25, 31, 37, 44, 56). For example, our laboratory and others have demonstrated that early third-trimester, nonhuman primate fetuses exhibit systemic inflammation, hepatic steatosis, increased hepatic oxidative stress with concurrent apoptotic cell death, and altered hypothalamic development in response to maternal consumption of a HF diet (26, 27, 42).
In the current study, we used a murine model of maternal overnutrition to study the impact of excess maternal dietary fat intake on the in utero programming of body composition, hepatic function, and hypothalamic neuropeptide expression in the offspring. Because we were interested in evaluating the neonatal consequences of metabolic programming, we examined the pups on the day of birth, thereby circumventing the influence of postnatal growth and nutrition. We followed the dams through three successive pregnancies to determine whether metabolic programming becomes more pronounced with increasing duration and severity of maternal obesity and/or impaired glucose homeostasis. Finally, in an attempt to differentiate between the relative contributions of maternal diet vs. maternal obesity, we investigated whether switching a subset of obese HF diet-fed dams to a low-fat (LF) diet during pregnancy would reverse any adverse programming events. We report that maternal HF diet consumption resulted in an altered body composition phenotype in the newborn offspring. This deleterious effect of excess maternal lipid intake occurred prior to the onset of maternal obesity and was largely normalized by switching obese dams to a LF diet during gestation.
MATERIALS AND METHODS
Animals
C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed in a temperature-controlled room on a 12:12-h light-dark cycle (lights on at 0700), with ad libitum access to food and water. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Oregon Health and Science University.
Experimental Design
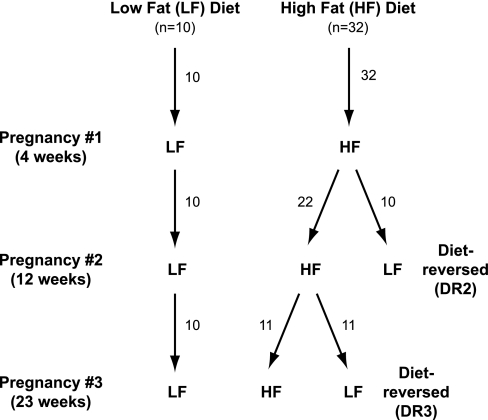
Five-week-old female mice were group housed and placed on either a LF (n = 10) or HF (n = 32) diet (Fig. 1). The LF diet (12450D; Research Diets, New Brunswick, NJ) contains 70% carbohydrate, 20% protein, and 10% fat (3.85 kcal/g). The HF diet (D12492; Research Diets) contains 20% carbohydrate, 20% protein, and 60% fat (5.24 kcal/g). After 12 wk on their diets, 10 of the HF females were switched to the LF diet [2nd pregnancy diet reversed (DR2); n = 10]. After 23 wk on their diets, an additional 11 HF females were switched to the LF diet [3rd pregnancy diet reversed (DR3); n = 11].
Fig. 1.
Schematic of experimental design. LF, low fat; HF, high fat.
To examine the influence of maternal diet on three successive pregnancies, females were paired with 7-wk-old male mice after 4, 12, and 23 wk on their respective diets. Immediately prior to each round of mating, the females were weighed, body composition was measured with an Echo MRI 4-in-1 system (Echo MRI, Houston, TX), and nonfasted blood glucose levels were measured. The females were inspected for the presence of copulatory plugs each morning. The morning that a plug was observed was designated as gestational day 0.5. Body weight and nonfasted blood glucose were measured on gestational day 17.5 (G17.5) of each pregnancy.
Pups were separated from the dams on the day of birth (P0). Pups were weighed, and their body composition was measured. After rapid decapitation, trunk blood was collected and pooled from each litter (3rd pregnancies only). The livers were removed and weighed. Livers from a subset of the pups (2–3 pups/litter) were flash-frozen for triglyceride analysis or terminal deoxynucleotidyl-mediated dUTP nick-end labeling (TUNEL) staining. Another subset of livers (1–2 pups/litter) was stabilized in RNAlater (Ambion, Austin, TX) for 24 h at 4°C prior to being stored at −80°C. Brains from a subset of the pups (1–2 pups/litter) were removed and cut to form hypothalamic blocks. Hypothalami were immersed in RNAlater at 4°C for 24 h and then stored at −80°C.
The dams were euthanized after 28–31 wk on their diets. Mice were weighed, body composition was measured, and blood was obtained to measure nonfasted glucose and Hb A1c. The mice were then anesthetized with a ketamine (30 mg/ml)-xylazine (2.9 mg/ml)-acepromazine (0.6 mg/ml) cocktail, and blood was obtained by cardiac puncture for measuring serum insulin and triglyceride concentrations. Livers were removed and then stabilized in RNAlater for 24 h at 4°C prior to being stored at −80°C.
Blood and Liver Chemistry
Nonfasted blood glucose was always measured at 1200 using a One Touch Ultra glucose meter (LifeScan, Milpitas, CA). Hb A1c was measured using an A1cNow+ monitor (Bayer Healthcare, Tarrytown, NY). Serum insulin concentrations were measured in duplicate with a Sensitive Rat Insulin RIA kit (no. SRI-13K; Millipore, Billerica, MA) according to the manufacturer's instructions. The intra-assay coefficient of variation was 4.2%. Serum and hepatic triglyceride concentrations were measured using a Triglyceride (GPO) Reagent Set (Pointe Scientific, Canton, MI) according to the manufacturer's instructions. The interassay coefficient of variation was 7.3%. For hepatic triglyceride analysis, frozen tissue was thawed and then homogenized in a buffer containing 150 mM NaCl, 0.1% Triton X-100, and 10 mM Tris, pH 8.0.
RNA Extraction, Reverse Transcription, and Real-Time PCR
Total RNA was extracted from RNAlater-stabilized livers and hypothalami using RNeasy kits (Qiagen, Valencia, CA). Reverse transcription reactions were performed with Taqman reverse transcription reagents (Applied Biosystems, Carlsbad, CA). For each reaction, 500 ng of RNA was combined with 5 μl 10× RT buffer, 11 μl of 25 mM MgCl2, 10 μl of dNTP mix (2.5 mM each), 2.5 μl of 50 μM random hexamers, 1 μl of RNase inhibitor, 1.25 μl of Multiscribe reverse transcriptase, and enough nuclease-free water to bring the final reaction volume to 50 μl. Reverse transcription was performed with an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) set to the following program: 25°C for 10 min, 37°C for 60 min, and 95°C for 5 min. cDNA samples were diluted with nuclease-free water to a final concentration of 1 ng/μl.
Real-time PCR was performed with Taqman reagents and an ABI 7300 system (Applied Biosystems). Each reaction contained 5 μl of Taqman Universal PCR master mix, 0.5 μl of mouse-specific primer/probe, and 5 ng of cDNA. The primer/probes are listed in Table 1. Each sample was run in triplicate, with β-actin as an endogenous control. To rule out the possibility of pituitary contamination of hypothalamic samples, individual hypothalami were excluded from data analyses if the growth hormone CT was <35. Gene expression is expressed as fold change relative to the LF group using the 2ΔΔCT method (41). Statistical analyses were performed on the ΔCT values for each gene.
Table 1.
Real-time PCR TaqMan primer/probes
| Gene Symbol | Applied Biosystems Assay ID |
|---|---|
| Actb | 4352933E |
| Il1b | Mm01336189_m1 |
| Il6 | Mm00446190_m1 |
| Tnf | Mm00443258_m1 |
| Il1r1 | Mm00434237_m1 |
| Il10 | Mm99999062_m1 |
| Ccl2 | Mm00441242_m1 |
| Crp | Mm00432680_g1 |
| Fasn | Mm00662319_m1 |
| Srebf1 | Mm00550338_m1 |
| Ppara | Mm00440939_m1 |
| Pck1 | Mm00440636_m1 |
| Mttp | Mm00435015_m1 |
| Fas | Mm00433237_m1 |
| Bax | Mm00432050_m1 |
| Bcl2 | Mm00477631_m1 |
| Pomc | Mm00435874_m1 |
| Gh | Mm00433590_g1 |
| Agrp | Mm00475829_g1 |
| Npy | Mm00445771_m1 |
| Hcrt | Mm01964030_s1 |
| Pmch | Mm01242886_g1 |
Actb, β-actin; Il1b, interleukin-1β; Il6, interleukin-6; Tnf, tumor necrosis factor-α; Il1r1, interleukin-1 type I receptor; Il10, interleukin-10; Ccl2, monocyte chemotactic protein-1; Crp, C-reactive protein; Fasn, fatty acid synthase; Srebf1, sterol regulatory element-binding protein-1; Ppara, peroxisome proliferator-activated receptor-α; Pck1, phosphoenolpyruvate carboxykinase 1; Mttp, microsomal triglyceride transport protein; Pomc, proopiomelanocortin; Gh, growth hormone; Agrp, agouti-related protein; Npy, neuropeptide Y; Hcrt, orexin; Pmch, pro-melanin-concentrating hormone.
TUNEL Staining
Frozen livers from a subset of the third pregnancy LF (n = 5 pups from 5 different litters) and HF pups (n = 5 pups from 4 different litters) were embedded in OCT compound (Sakura Finetek USA, Torrance, CA). Fourteen-micrometer sections were cut on a cryostat and mounted onto Superfrost Plus slides (VWR International, West Chester, PA). TUNEL staining was performed with a TACS 2 TdT-diaminobenzidine (DAB) In Situ Apoptosis Detection Kit (R & D Systems, Minneapolis, MN) according to the manufacturer's instructions. Briefly, liver sections were rehydrated in a graded ethanol series, fixed in 3.7% formaldehyde, incubated with Cytonin for 30 min, labeled with TdT labeling reaction mix for 1 h at 37°C, incubated with streptavidin-horseradish peroxidase, and immersed in DAB until adequate staining was detected. Sections were then counterstained with 1% methyl green, dehydrated in a graded ethanol series, cleared in xylene, and coverslipped. Specific staining of apoptotic nuclei was confirmed by omitting TdT enzyme from the negative control slide. For each animal, a blinded observer counted the number of TUNEL-positive nuclei in nine randomly-selected ×400 microscopic fields. Data are expressed as the total number of apopotic cells per animal.
Statistics
Data are expressed as means ± SE for each group. Statistical analyses were performed using Prism Software (Version 5.0b; Prism Software, Irvine, CA). Data were analyzed initially by two-way ANOVA (sex × diet) followed by Bonferroni corrected t-test if the overall ANOVA revealed a significant effect of sex, diet, or an interaction between sex and diet. Because there were very few statistically significant interactions between sex and diet by two-way ANOVA (which are mentioned in results), data from the male and female pups were then combined for further statistical analysis and data presentation. For the first pregnancy, differences between the two groups were assessed by t-test. For the second and third pregnancies, differences between groups were assessed by one-way ANOVA followed by Bonferroni corrected t-test if the overall ANOVA was significant. Nonparametric analyses (Mann-Whitney U-test or KruskalWallis one-way analysis of variance followed by Dunn's multiple comparison test) were used to analyze pup hepatic triglyceride concentrations. Pup mortality was analyzed with a Fisher's exact test (1st pregnancies) or chi-square tests (2nd and 3rd pregnancies). Chi-square tests were also used to determine whether offspring sex ratios differed from the expected 1:1 ratio. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Maternal Body Composition and Glucose Homeostasis
Pregnancy no. 1.
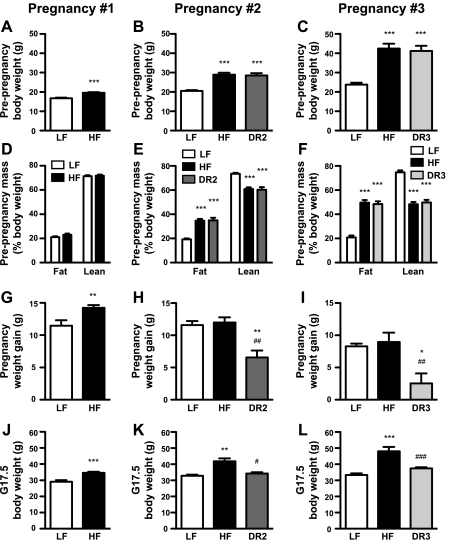
To ascertain whether long-term HF diet consumption induced obesity and/or disturbed glucose homeostasis in the dams, we measured maternal body composition and circulating glucose levels prior to and/or at the end of each pregnancy. The HF mice were significantly heavier than the LF mice after 4 wk on their respective diets (Fig. 2A), but fat and lean body mass did not differ between the two groups (Fig. 2D). All but one mouse became pregnant during the first round of mating (Table 2). The HF mice gained significantly more weight during gestation (Fig. 2G) and thus continued to weigh more than the LF mice on G17.5 (Fig. 2J). Blood glucose did not differ between the LF and HF dams prior to pregnancy (after 4 wk on their diets) or on G17.5 (Table 2).
Fig. 2.
Maternal body composition and gestation weight gain. Prepregnancy body weight (A–C), prepregnancy body composition (D–F), pregnancy weight gain (G–I), and end-gestation body weight (J–L) of dams during their 1st, 2nd, and 3rd pregnancies. For pregnancy no. 1, n = 9–10 (LF) and n = 29–32 (HF). For pregnancy no. 2, n = 8–10 (LF), n = 16–22 (HF), and n = 6–10 [2nd pregnancy diet reversed (DR2)]. For pregnancy no. 3, n = 7–10 (LF), n = 6–11 (HF), and n = 8–11 [3rd pregnancy diet-reversed (DR3)]. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. LF dams; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. HF dams.
Table 2.
Maternal glucose and pregnancy outcomes
| LF | HF | DR2 | DR3 | |
|---|---|---|---|---|
| Pregnancy no. 1 (4 wk) | ||||
| Prepregnancy glucose, mg/dl | 148 ± 10 | 155 ± 4 | ||
| G17.5 glucose, mg/dl | 136 ± 6 | 134 ± 5 | ||
| No. of successful pregnancies | 10/10 | 31/32 | ||
| Litter size (pups/litter) | 6 ± 2 | 7 ± 2* | ||
| Pup mortality (%total pups) | 12 | 8 | ||
| Sex ratio (males/females) | 0.47: 0.53 | 0.48: 0.52 | ||
| Pregnancy no. 2 (12 wk) | ||||
| Prepregnancy glucose, mg/dl | 143 ± 5 | 170 ± 5** | 173 ± 9** | |
| G17.5 glucose, mg/dl | 134 ± 6 | 141 ± 8 | 134 ± 7 | |
| No. of successful pregnancies | 10/10 | 21/22 | 8/10 | |
| Litter size (pups/litter) | 6 ± 1 | 7 ± 2 | 6 ± 1 | |
| Pup mortality (%total pups)# | 3 | 23 | 4 | |
| Sex ratio (males/females) | 0.54: 0.46 | 0.49: 0.51 | 0.63: 0.37 | |
| Pregnancy no. 3 (23 wk) | ||||
| Prepregnancy glucose, mg/dl | 154 ± 7 | 167 ± 7 | 174 ± 8 | |
| G17.5 glucose, mg/dl | 139 ± 9 | 130 ± 9 | 120 ± 7 | |
| No. of successful pregnancies | 10/10 | 9/11 | 10/11 | |
| Litter size (pups/litter) | 6 ± 1 | 4 ± 2 | 6 ± 2 | |
| Pup mortality (%total pups)# | 4 | 40 | 43 | |
| Sex ratio (males/females) | 0.66: 0.34† | 0.64: 0.36 | 0.49: 0.51 |
Values are means ± SE. LF, low fat; HF, high fat; DR2, 2nd pregnancy diet reversed; DR3, 3rd pregnancy diet reversed; G17.5, gestational day 17.5.
P < 0.05 and
P < 0.01 vs. LF dams;
P < 0.05 by χ2 test compared with the expected 1:1 sex ratio;
P ≤ 0.0001 by χ2 test.
Pregnancy no. 2.
After 12 wk on their respective diets (immediately prior to switching the DR2 group to LF chow), the HF and DR2 females weighed significantly more than the LF females (Fig. 2B). At this time point, the HF and DR2 dams had also accumulated more fat mass and less lean body mass than their LF counterparts (Fig. 2E). During the second round of mating, three dams failed to become pregnant (Table 2). The DR2 dams gained significantly less weight during pregnancy than the other two groups (Fig. 2H). The HF dams were heavier than both the LF and DR2 dams on G17.5 (Fig. 2K). G17.5 body weight did not differ between the LF and DR2 groups. Although circulating glucose was significantly higher in the HF and DR2 dams than in the LF females prior to the second pregnancies (after 12 wk on their diets), blood glucose on G17.5 no longer differed between the three maternal diet groups (Table 2).
Pregnancy no. 3.
After 23 wk on their diets (immediately prior to switching the DR3 females to LF chow), the HF and DR3 dams weighed 78 and 73% more, respectively, than the LF dams (Fig. 2C). The HF and DR3 dams also had more than twice the amount of fat mass and significantly less lean body mass than the LF dams (Fig. 2F). All but three dams were successfully impregnated in the third and final round of mating (Table 2). The DR3 mice gained significantly less weight during gestation than the other two groups (Fig. 2I). On G17.5, the HF dams were heavier than the dams in the other two groups; body weight on G17.5 did not differ between the LF and DR3 dams (Fig. 2L). There were no differences in maternal blood glucose between the three diet groups prior to pregnancy (after 23 wk on their diets) or on G17.5 (Table 2).
End of Experiment
When the dams were euthanized after 28–31 wk on their diets, the HF dams were heavier and had more fat mass and less lean body mass than the LF or DR3 dams (Table 3). Glucose, Hb A1c, and serum triglyceride levels did not differ between the three groups (Table 3). There was a trend toward higher serum insulin levels in the HF dams compared with the LF dams, but the overall ANOVA did not reach statistical significance (P = 0.08; Table 3). Hepatic mRNA expression of the proinflammatory cytokines interleukin-1β (Il1b), interleukin-6 (Il6), and tumor necrosis factor-α (Tnf) was unaltered by maternal diet (Table 3).
Table 3.
Maternal body composition and blood analyses at the end of the experiment
| LF (n = 5–10) | HF (n = 5–11) | DR3 (n = 6–11) | |
|---|---|---|---|
| Body weight, g | 25.23 ± 0.69 | 44.81 ± 2.67* | 29.13 ± 0.72# |
| Fat mass, %body weight | 18.79 ± 1.08 | 48.05 ± 2.41* | 21.98 ± 1.10# |
| Lean mass, %body weight | 77.16 ± 0.99 | 50.23 ± 2.30* | 75.04 ± 1.10# |
| Glucose, mg/dl | 136 ± 3 | 150 ± 6 | 148 ± 3 |
| Hb A1c, % | 4.1 ± 0.04 | 4.2 ± 0.09 | 4.2 ± 0.06 |
| Insulin, ng/ml | 0.30 ± 0.04 | 0.50 ± 0.05 | 0.43 ± 0.10 |
| Serum TG, mg/dl | 37.55 ± 4.02 | 37.14 ± 3.16 | 29.89 ± 2.50 |
| Hepatic Il1b (relative expression) | 1.000 ± 0.142 | 0.912 ± 0.186 | 1.668 ± 0.337 |
| Hepatic Il6 (relative expression) | 1.000 ± 0.187 | 1.355 ± 0.280 | 2.009 ± 0.363 |
| Hepatic Tnf (relative expression) | 1.000 ± 0.090 | 0.764 ± 0.113 | 1.305 ± 0.359 |
Values are means ± SE. TG, triglycerides. Dams were euthanized after 28–31 wk on their respective diets. Hepatic gene expression was normalized to β-actin.
P < 0.001 vs. LF dams;
P < 0.001 vs. HF dams.
Offspring Litter Characteristics
The HF dams had larger first litters than the LF dams (Table 2). Average litter size did not differ between the groups for the second and third pregnancies. Offspring sex ratios did not depart from the expected 1:1 ratio in any of the groups except for the LF offspring from the third pregnancies (Table 2). There were significant differences in pup mortality between the maternal diet groups in the second (P = 0.0001) and third pregnancies (P < 0.0001), with considerably more dead (and often cannibalized) pups in the HF dam cages (Table 2). In the third pregnancies, pup mortality was also high in the DR3 group.
Offspring Body Composition
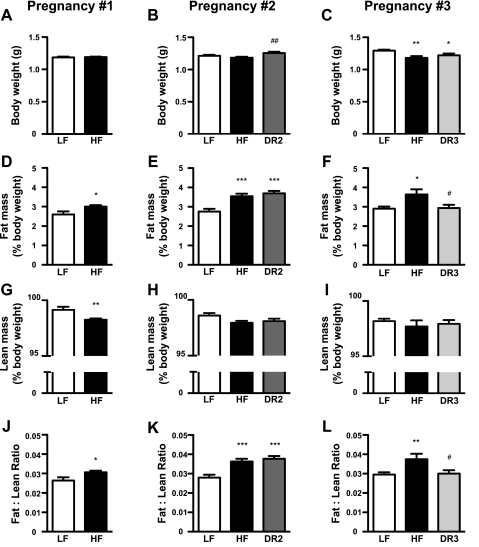
In the first and second pregnancies, body weights of the P0 offspring did not differ between the LF and HF groups (Fig. 3, A and B). In the third pregnancies, the HF and DR3 pups weighed significantly less than the LF offspring (Fig. 3C). Lean body mass in the HF pups was reduced compared with the LF pups in all three pregnancies, but the difference reached statistical significance only for the first pregnancies (Fig. 3, G–I). In the first pregnancy litters, lean body mass was reduced significantly in the HF males (LF males: 99.4 ± 1.6% vs. HF males: 97.9 ± 1.6%, P < 0.01) but not in the HF females (LF females: 99.0 ± 2.0% vs. HF females: 98.6 ± 1.9%, P > 0.05). In each of the three pregnancies, pups from the HF dams had significantly more fat mass than their LF counterparts (Figs. 3, D–F), as well as higher fat/lean ratios (Fig. 3, J–L). In the first pregnancy litters, only the HF males exhibited increased fat mass (LF males: 2.1 ± 0.9% vs. HF males: 3.1 ± 1.1%, P < 0.005; LF females: 3.0 ± 1.1% vs. HF females: 2.9 ± 1.1%, P > 0.05) and fat/lean ratios (LF males: 0.022 ± 0.009% vs. HF males: 0.031 ± 0.011%, P < 0.01; LF females: 0.031 ± 0.012% vs. HF females: 0.030 ± 0.012%, P > 0.05). There were no significant interactions between diet and sex with respect to fat mass or fat/lean ratios in the second or third pregnancies. The DR2 offspring had more fat mass and a higher fat/lean ratio than the LF pups during the second pregnancies (Fig. 3, E and K) despite the fact that the DR2 dams gained approximately one-half as much body weight as the LF dams during gestation. However, fat mass and the fat/lean ratio in the DR3 offspring did not differ from those of the LF group during the third pregnancies (Fig. 3, F and L).
Fig. 3.
Offspring body composition. Body weight (A–C), fat mass (D–F), lean body mass (G–I), and fat/lean ratios (J–L) of newborn (P0) pups from the 1st, 2nd, and 3rd pregnancies. For pregnancy no. 1, n = 47 (LF) and n = 199 (HF). For pregnancy no. 2, n = 58 (LF), n = 98 (HF), and n = 47 (DR2). For pregnancy no. 3, n = 49–50 (LF), n = 20 (HF), and n = 37 (DR3). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. LF dams; #P < 0.05 and ##P < 0.01 vs. HF dams.
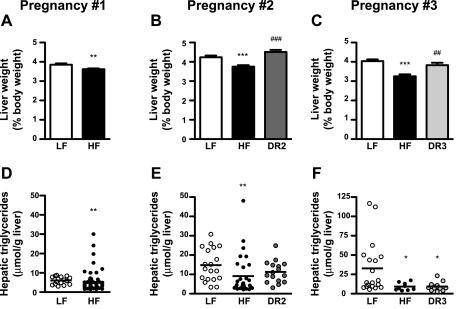
Offspring Liver Size and Triglyceride Content
Given that we consistently observed that the neonatal HF pups accrued more fat mass than the LF offspring, we investigated whether the liver was a site of increased triglyceride storage in the HF pups. In all three pregnancies, the HF pups had significantly smaller livers than the LF pups (Fig. 4, A–C). In the second and third pregnancies, maternal diet reversal normalized pup liver weights in the DR2 and DR3 pups such that they did not differ from the offspring of the LF dams. Hepatic triglyceride concentrations were significantly lower in the HF pups than in the LF pups in all three pregnancies (Fig. 4, D–F). In the third pregnancies, hepatic triglyceride concentrations were also reduced significantly in the DR3 pups compared with the LF offspring (Fig. 4F). Pup serum triglyceride concentrations were also significantly lower in the DR3 pups compared with the LF pups (LF: 79.6 ± 11 mg/dl, HF: 51.3 ± 6 mg/dl, DR3: 45.03 ± 3 mg/dl; P < 0.05).
Fig. 4.
Offspring liver weights and triglyceride content. Liver weights (A–C) and triglyceride content (D–F) of P0 pups from the 1st, 2nd, and 3rd pregnancies. For pregnancy no. 1: LF, n = 16 (triglycerides) or n = 45 (liver weights); and HF, n = 64 (triglycerides) or n = 164 (liver weights). For pregnancy no. 2: LF, n = 19 (triglycerides) or n = 56 (liver weights); HF, n = 16 (triglycerides) or n = 85 (liver weights); and DR2, n = 30 (triglycerides) or n = 43 (liver weights). For pregnancy no. 3: LF, n = 19 (triglycerides) or n = 50 (liver weights); HF, n = 9 (triglycerides) or n = 50 (liver weights); and DR3, n = 13 (triglycerides) or n = 36 (liver weights). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. LF dams; ##P < 0.01 and ###P < 0.001 vs. HF dams.
Offspring Expression of Metabolic and Inflammatory Genes
To determine whether excess maternal lipid intake altered hepatic glucose and/or lipid metabolism in the offspring, we measured the hepatic expression of metabolic genes involved in lipogenesis [sterol regulatory element-binding protein 1 (Srebf1) and fatty acid synthase (Fasn)], fatty acid oxidation [peroxisome proliferator-activated receptor-α (Ppara)], lipoprotein assembly [microsomal triglyceride transfer protein (Mttp)], and gluconeogenesis [phosphoenolpyruvate carboxykinase 1 (Pck1)] (Tables 4, 5, and 6). Hepatic Srebf1 was slightly but significantly elevated in the HF pups compared with their LF counterparts in the second pregnancies (Table 5). In the second pregnancies, Fasn expression was significantly higher in both the HF (1.8-fold) and DR2 (2.6-fold) pup livers compared with the LF pups (Table 5). We did not detect significant differential expression of Ppara, Pck1, or Mttp between the LF and HF pups in any pregnancy (Tables 4, 5, and 6).
Table 4.
Hepatic and hypothalamic gene expression in 1st-pregnancy offspring
| Pregnancy no. 1 | LF | HF |
|---|---|---|
| Liver | (n = 9) | (n = 30) |
| Il1b | 1.000 ± 0.285 | 0.585 ± 0.126* |
| Il6 | 1.000 ± 0.091 | 0.720 ± 0.084* |
| Tnf | 1.000 ± 0.062 | 1.054 ± 0.047 |
| Il1r1 | 1.000 ± 0.081 | 1.107 ± 0.099 |
| Il10 | 1.000 ± 0.639 | 0.457 ± 0.149 |
| Ccl2 | 1.000 ± 0.174 | 0.936 ± 0.092 |
| Crp | 1.000 ± 0.060 | 1.042 ± 0.055 |
| Fasn | 1.000 ± 0.153 | 1.122 ± 0.141 |
| Srebf1 | 1.000 ± 0.099 | 0.902 ± 0.027 |
| Ppara | 1.000 ± 0.115 | 0.892 ± 0.064 |
| Pck1 | 1.000 ± 0.154 | 0.862 ± 0.065 |
| Mttp | 1.000 ± 0.111 | 1.033 ± 0.064 |
| Fas | 1.000 ± 0.174 | 0.927 ± 0.071 |
| Bax | 1.000 ± 0.052 | 0.951 ± 0.046 |
| Bcl2 | 1.000 ± 0.097 | 0.934 ± 0.028 |
| Hypothalamus | (n = 8) | (n = 26–28) |
| Hcrt | 1.000 ± 0.052 | 1.106 ± 0.050 |
| Pmch | 1.000 ± 0.105 | 0.996 ± 0.053 |
| Il1b | 1.000 ± 0.076 | 1.314 ± 0.092 |
| Il6 | 1.000 ± 0.100 | 1.160 ± 0.151 |
Values are means ± SE. Gene expression was normalized to β-actin.
P < 0.05 vs. LF pups.
Table 5.
Hepatic and hypothalamic gene expression in 2nd-pregnancy offspring
| Pregnancy no. 2 | LF | HF | DR2 |
|---|---|---|---|
| Liver | (n = 17–18) | (n = 28–32) | (n = 14) |
| Il1b | 1.000 ± 0.229 | 2.583 ± 0.592* | 1.395 ± 0.295 |
| Il6 | 1.000 ± 0.138 | 1.538 ± 0.212 | 1.233 ± 0.280 |
| Tnf | 1.000 ± 0.124 | 0.924 ± 0.061 | 0.938 ± 0.081 |
| Il1r1 | 1.000 ± 0.090 | 1.006 ± 0.093 | 0.638 ± 0.045*,# |
| Il10 | 1.000 ± 0.121 | 3.335 ± 0.674* | 1.726 ± 0.402 |
| Ccl2 | 1.000 ± 0.170 | 0.620 ± 0.083* | 0.854 ± 0.134 |
| Crp | 1.000 ± 0.078 | 1.142 ± 0.095 | 1.224 ± 0.140 |
| Fasn | 1.000 ± 0.118 | 1.813 ± 0.190* | 2.644 ± 0.525** |
| Srebf1 | 1.000 ± 0.062 | 1.254 ± 0.068* | 1.061 ± 0.085 |
| Ppara | 1.000 ± 0.109 | 1.036 ± 0.090 | 0.638 ± 0.099*,## |
| Pck1 | |||
| Mttp | 1.000 ± 0.073 | 0.911 ± 0.060 | 0.892 ± 0.087 |
| Fas | 1.000 ± 0.107 | 0.847 ± 0.057 | 0.985 ± 0.133 |
| Bax | 1.000 ± 0.069 | 1.006 ± 0.050 | 0.861 ± 0.045 |
| Bcl2 | 1.000 ± 0.046 | 1.102 ± 0.046 | 0.978 ± 0.055 |
| Hypothalamus | (n = 14–15) | (n = 8–10) | (n = 14) |
| Hcrt | 1.000 ± 0.071 | 1.173 ± 0.077 | 1.010 ± 0.078 |
| Pmch | 1.000 ± 0.042 | 1.065 ± 0.061 | 0.923 ± 0.075 |
| Il1b | 1.000 ± 0.142 | 1.603 ± 0.289 | 1.125 ± 0.188 |
| Il6 | 1.000 ± 0.090 | 1.337 ± 0.137 | 0.994 ± 0.164 |
Values are means ± SE. Gene expression was normalized to β-actin.
P < 0.05,
P < 0.01 vs. LF pups;
P < 0.05,
P < 0.01 vs. HF pups.
Table 6.
Hepatic and hypothalamic gene expression in 3rd pregnancy offspring
| Pregnancy no. 3 | LF | HF | DR3 |
|---|---|---|---|
| Liver | (n = 15) | (n = 10) | (n = 14) |
| Il1b | 1.000 ± 0.153 | 5.959 ± 2.478 | 3.719 ± 1.384 |
| Il6 | 1.000 ± 0.231 | 3.072 ± 1.248 | 3.036 ± 1.052 |
| Tnf | 1.000 ± 0.050 | 1.048 ± 0.146 | 1.247 ± 0.116 |
| Il1r1 | 1.000 ± 0.097 | 1.409 ± 0.183 | 1.539 ± 0.204 |
| Il10 | 1.000 ± 0.242 | 3.066 ± 1.244 | 3.755 ± 1.024 |
| Ccl2 | 1.000 ± 0.221 | 3.564 ± 1.957 | 2.039 ± 0.725 |
| Crp | 1.000 ± 0.072 | 1.013 ± 0.169 | 0.895 ± 0.077 |
| Fasn | 1.000 ± 0.139 | 0.985 ± 0.090 | 1.171 ± 0.163 |
| Srebf1 | 1.000 ± 0.036 | 1.111 ± 0.045 | 1.184 ± 0.061* |
| Ppara | 1.000 ± 0.112 | 1.140 ± 0.167 | 1.050 ± 0.107 |
| Pck1 | 1.000 ± 0.112 | 1.237 ± 0.239 | 1.044 ± 0.131 |
| Mttp | 1.000 ± 0.152 | 0.675 ± 0.068 | 0.677 ± 0.037 |
| Fas | 1.000 ± 0.137 | 0.670 ± 0.106 | 0.662 ± 0.087 |
| Bax | 1.000 ± 0.053 | 1.141 ± 0.106 | 1.061 ± 0.076 |
| Bcl2 | 1.000 ± 0.036 | 1.153 ± 0.124 | 1.112 ± 0.052 |
| Hypothalamus | (n = 14–15) | (n = 8–10) | (n = 14) |
| Hcrt | 1.000 ± 0.080 | 0.872 ± 0.111 | 0.905 ± 0.087 |
| Pmch | 1.000 ± 0.068 | 0.968 ± 0.103 | 0.872 ± 0.064 |
| Il1b | 1.000 ± 0.111 | 2.261 ± 0.689 | 2.866 ± 1.080 |
| Il6 | 1.000 ± 0.215 | 0.808 ± 0.067 | 0.626 ± 0.085 |
Values are means ± SE. Gene expression was normalized to β-actin.
P < 0.05 vs. LF pups.
Both obesity and pregnancy are states of low-grade systemic inflammation. Newborn humans born to obese mothers have elevated inflammatory cytokines in their cord blood (14), and the early third-trimester fetuses of HF diet-fed macaques have increased circulating cytokines (42). Because the liver is a major immune organ, we evaluated whether maternal HF diet consumption altered the expression of cytokines and acute phase reactants in the offspring livers. In the first litters of pups, hepatic mRNA expression of the proinflammatory cytokines Il1b and Il6 was reduced significantly in the offspring from the HF dams (Table 4). In the second pregnancies, hepatic mRNA expression of Il1b and the anti-inflammatory cytokine interleukin-10 (Il10) was elevated 2.6- and 3.3-fold, respectively, in the HF pups compared with the LF pups (Table 5). We did not detect any significant differences in Tnf, interleukin-1 type I receptor (Il1r1), monocyte chemotactic protein-1 (Ccl2), or C-reactive protein (Crp) gene expression between the LF and HF pups in any of the three pregnancies (Tables 4, 5, and 6).
We also assessed whether maternal HF diet consumption resulted in local inflammation in the hypothalami of the offspring. Hypothalamic expression of Il1b and Il6 mRNAs did not differ between the groups in any of the pregnancies (Tables 4, 5, and 6).
Offspring Hepatic Apoptosis
We next determined whether increased hepatic apoptosis contributed to the reduced liver weights that were consistently observed in the HF pups. We did not detect any significant differences in the hepatic expression of proapoptotic (Fas and Bax) or antiapoptotic (Bcl2) genes in any pregnancy (Tables 4, 5, and 6). Furthermore, in the third pregnancy pup livers, the number of TUNEL-positive cells did not differ between the offspring of LF and HF dams (LF: 66 ± 15 cells vs. HF: 100 ± 24 cells; P > 0.05).
Offspring Hypothalamic Neuropeptide Gene Expression
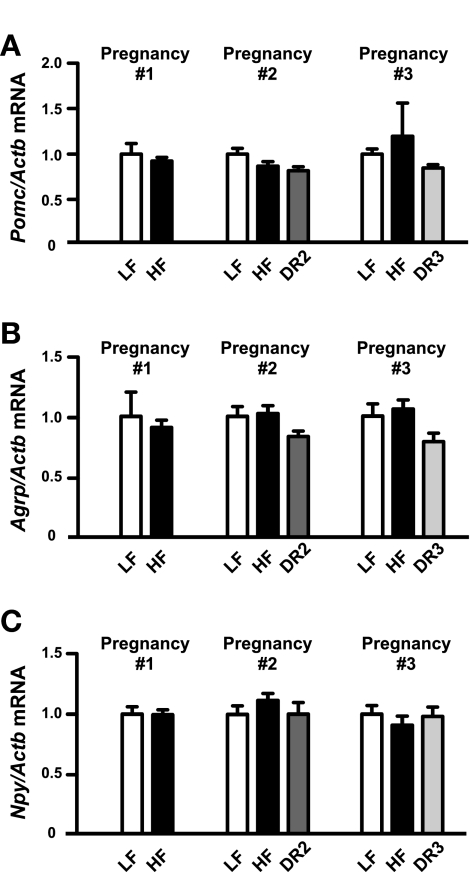
Finally, we assessed whether altered body composition in the HF pups was associated with differential expression of hypothalamic neuropeptides that regulate feeding and metabolism. We did not observe any differences in hypothalamic mRNA expression of proopiomelanocortin (Pomc; the precursor of the anorexigenic neuropeptide α-MSH) or the orexigenic neuropeptides agouti-related protein (Agrp), neuropeptide Y (Npy), or orexin (Hcrt) in the offspring from any of the three pregnancies (Fig. 5, A–C, and Tables 4, 5, and 6). When data from the male and female pups were combined, we were unable to detect any differences in hypothalamic mRNA expression of the orexigenic neuropeptide melanin-concentrating hormone (Pmch) in the offspring from any gestation (Tables 4, 5, and 6). However, when the data were segregated by sex, Pmch was slightly but significantly elevated in the DR3 females compared with either the LF females or HF females from the third pregnancies (data not shown).
Fig. 5.
Offspring hypothalamic neuropeptide expression. Hypothalamic mRNA expression of proopiomelanocortin (Pomc; A), agouti-related peptide (Agrp; B), and neuropeptide Y (Npy; C) in P0 pups from the 1st, 2nd, and 3rd pregnancies. Gene expression was normalized to β-actin. For pregnancy no. 1, n = 8 (LF) and n = 28 (HF). For pregnancy no. 2, n = 17 (LF), n = 32 (HF), and n = 13 (DR2). For pregnancy no. 3, n = 15 (LF), n = 8–10 (HF), and n = 14 (DR3).
DISCUSSION
In humans, the offspring of mothers who were obese and/or diabetic during pregnancy are at increased risk of developing obesity, insulin resistance, and metabolic syndrome as infants (14, 15), children (10, 65), and adults (17). In the current experiment, the HF dams were obese during their second and third pregnancies but never developed overt diabetes, as indicated by normal nonfasting blood glucose levels at all but one measured time point (12 wk) and normal Hb A1c values at the end of the experiment. Serum insulin levels were ∼70% higher in the HF dams than in the LF dams after 28–31 wk on their diets, suggesting that the HF dams developed some degree of insulin resistance over the course of the experiment. This is consistent with a previous report demonstrating that female C57BL/6 mice became progressively hyperinsulinemic but developed only mild hyperglycemia during long-term (1 yr) HF diet consumption (67). Hepatic proinflammatory cytokine expression was not elevated in the HF dams at the end of the experiment. Given the sexually dimorphic inflammatory response to HF diet consumption in male vs. female rodents [i.e., HF diet-induced inflammation is more pronounced in males (29)], it is not surprising that we did not detect any evidence of maternal hepatic inflammation in our experiment. However, because we were only able to assess maternal inflammation at the end of the experiment, it is unknown whether the HF dams exhibited an exaggerated inflammatory response during pregnancy.
In all three pregnancies, the newborn HF pups accumulated more fat mass (and less lean body mass in the 1st pregnancy) than their LF counterparts. Because the HF dams were not yet obese during their first pregnancies, this implicates a role for excess maternal dietary lipid intake (rather than maternal obesity) in the programming of increased neonatal adiposity. This is consistent with previous findings in rodent models, in which the weanling and adult offspring of HF diet-fed mothers exhibit increased adiposity, adipocyte hypertrophy, and increased hepatic and white adipose tissue expression of lipogenic and adipogenic genes (8, 11, 12, 30, 36, 53, 66). Previous studies have revealed sex differences in the developmental programming of obesity, insulin resistance, and hypertension, with males often being more sensitive to early-life programming events than females (22, 38, 60, 68). In the present experiment, body composition was altered only in the male HF offspring from the first pregnancies; in subsequent pregnancies, this sexually dimorphic response was no longer observed. Because the HF dams became more obese over the course of the experiment, the exaggerated metabolic insult to the developing offspring was sufficient to elicit changes in body composition in both the male and female HF offspring.
To our knowledge, this is the first report in rodents demonstrating that long-term maternal HF diet consumption results in the deposition of excess fat mass in offspring in utero. Catalano et al. (14) reported that children of obese mothers have a greater percent body fat at birth and that neonatal adiposity is strongly correlated with neonatal insulin resistance. Despite the increased accrual of fat mass in the HF pups, they still had considerably less body fat than human infants at birth [∼3.5% in the P0 mouse pups vs. 13% in humans (54)]. It is unknown whether increased neonatal fat mass is a consequence of increased maternal transfer of fatty acids across the placenta, increased de novo lipogenesis, or both. Switching obese dams to a LF diet just during gestation prevented the DR3 pups from accumulating excess fat mass during the third pregnancies. Because the DR3 dams gained less weight than the LF dams during gestation, we cannot ascertain whether reduced maternal weight gain during pregnancy or removal of the HF diet per se was directly responsible for normalizing fat mass in the DR3 pups. Surprisingly, maternal diet reversal did not normalize adiposity in the DR2 offspring. This observation is counterintuitive, since one would predict that diet reversal would be more effective during the earlier pregnancy, when the maternal prepregnancy metabolic phenotype was less severe. The normalization of offspring adiposity only in the DR3 pups might be attributable to the fact that the DR3 dams gained less than one-half as much body weight during gestation as the DR2 dams.
The liver was not a source of increased lipid storage in the neonatal HF offspring. Unexpectedly, the HF pup livers were smaller and had lower triglyceride contents than the LF pup livers. Interestingly, hepatic triglyceride content increased with parity in the LF offspring but remained relatively constant across the three pregnancies in the HF pups. Although liver size was normalized in the pups from the diet-reversed dams, hepatic triglyceride content was still reduced in the DR3 offspring. Thus, decreased hepatic lipid storage in the HF and diet-reversed offspring occurred independently of maternal fat consumption during pregnancy or gestational weight gain. The observation that hepatic mRNA expression of a lipogenic enzyme (Fasn) and transcription factor (Srebf1) was elevated and the expression of a transcription factor that promotes β-oxidation (Ppara) was unaffected by maternal HF diet consumption suggests that the reduced liver triglyceride content in the HF pups did not result from decreased hepatic lipogenesis or increased fatty acid oxidation.
The lack of hepatic triglyceride accumulation in the HF pups is in direct contrast to observations made in adult rodents and fetal nonhuman primates, in which maternal HF diet consumption leads to pronounced steatosis, oxidative stress, and altered expression of enzymes and transcription factors that are involved in glucose and lipid metabolism in the livers of the offspring (2, 7, 11, 28, 42, 69). In the study by McCurdy et al. (42), the nonhuman primate fetuses were studied at a developmental stage prior to the development of white adipose tissue (57, 61). Whereas newborn mice have the capacity to store triglycerides in their adipose tissue, early third-trimester nonhuman primate fetuses must divert excess triglycerides to the liver and other organs, which could explain why hepatic steatosis was observed in fetal macaques but not in newborn mouse pups. Alternatively, it is conceivable that there were differences in hepatic triglyceride accumulation in the mouse pups in utero. Birth marks the first time that a mammal must contend with thermogenesis and a discontinuous nutrient supply. Whereas mammals metabolize glucose primarily in utero, lipid metabolism predominates after birth (9, 35). Because of this rapid shift in lipid utilization immediately after birth, triglyceride concentrations in the livers of the newborn offspring might not be reflective of hepatic lipid storage at earlier developmental stages.
We observed minimal evidence of hepatic inflammation and no changes in the hypothalamic expression of proinflammatory cytokine mRNAs in the HF pups. Hepatic inflammation was not detected in the fetal offspring of HF diet-fed macaques (26), but hypothalamic Il1b and Il1r1 mRNAs were elevated, most likely in response to increased peripheral inflammation in these animals (27, 42). Also, we did not observe any evidence of increased hepatic apoptosis in the HF pups because we were unable to detect differences in the expression of proapoptotic (Fas and Bax) or antiapoptotic (Bcl2) genes, nor did we detect a significant increase in TUNEL staining. Increased apoptosis was apparent in the livers of macaque fetuses in response to maternal HF diet consumption (26). The relative lack of hepatic apoptosis, lipid accumulation, or inflammation in the HF mouse pups is consistent with the positive correlation between severity of nonalcoholic steatohepatitis and hepatocyte apoptosis in humans (21). Collectively, our findings demonstrate that, in the setting of maternal obesity/HF diet consumption, changes in offspring body composition precede the onset of additional features of metabolic syndrome and nonalcoholic fatty liver disease.
We did not observe an effect of maternal diet or obesity on the expression of hypothalamic neuropeptides that regulate feeding and metabolism. Maternal HF diet consumption alters development of the hypothalamic melanocortin system in early third-trimester nonhuman primate fetuses, as evidenced by increased expression of Pomc and melanocortin-4 receptor mRNAs as well as reduced Agrp mRNA and peptide content (27). Although insufficient blood volume prevented us from measuring hormone levels in the P0 mouse pups, we predict that circulating leptin was elevated in the HF pups as a consequence of their increased fat mass. This is true for human infants of obese mothers, who have increased body fat and cord blood leptin compared with the infants of lean mothers (14). Although leptin increases hypothalamic Pomc mRNA and reduces Agrp mRNA in adult rodents (43, 63), the expression of these genes is not altered in neonatal mice that are chronically treated with leptin (1), a finding that is consistent with the results of the current experiment. In other studies in mice and rats, maternal HF diet consumption has been linked to decreased (44), increased (25, 31), or unchanged (25) hypothalamic Pomc and Agrp gene expression in late embryonic (E19.5–E21) or neonatal (P1) offspring. Differences in the timing, duration, and fatty acid composition of the HF diets in the different studies might explain these disparate outcomes. Although we did not detect any differences in hypothalamic gene expression, we cannot exclude the possibility that maternal overnutrition caused epigenetic modifications in hypothalamic appetite-regulating genes, as has been reported previously for hypothalamic Pomc in the fetal offspring of undernourished ewes (58).
Collectively, our assessment of the pup livers and hypothalami demonstrates that newborn offspring of HF diet-fed dams are largely resistant to hepatic steatosis, apoptosis, inflammation, and altered hypothalamic development. Despite the fact that newborn mice are developmentally similar to early third-trimester fetal macaques, the two experimental models are not equivalent with respect to metabolic programming by maternal overnutrition. Therefore, caution must be exercised when using murine models as surrogates for studying the impact of maternal nutrition on hepatic function or hypothalamic development in primates.
Long-term maternal consumption of a HF diet resulted in a marked increase in offspring mortality in the second and third pregnancies, as has been documented previously in HF diet-fed rats (13, 30, 51). Maternal diet reversal reduced pup mortality in the second pregnancies but not in the third pregnancies. Increased offspring mortality in the DR3 group might have been attributable to the more severe metabolic phenotype of the DR3 dams as they entered their third pregnancies (compared with when they entered their 2nd pregnancies 11 wk prior). The fact that increased pup mortality occurred in the HF and DR3 groups indicates that the maternal and/or fetal defects that caused perinatal death arose independently of maternal diet consumption during gestation. It is unknown whether the pups died before or after birth. By studying the pups on P0, we effectively restricted our analyses to the most viable (and presumably healthiest) offspring. If we had instead evaluated the offspring during late fetal development, we might have unknowingly included less healthy (i.e., perimortem) animals in our analyses, which might have resulted in a more severe offspring phenotype.
In summary, the in utero programming of increased offspring adiposity and reduced liver size by maternal overnutrition is already evident in mice at the time of birth. These malprogramming events occur prior to the development of obesity in dams consuming a HF diet and are prevented by switching obese dams to a LF diet during gestation alone. These observations reveal that dietary intervention during pregnancy minimizes the detrimental impact of maternal obesity on offspring body composition, which would in turn be expected to reduce the offsprings' risk of developing obesity and related disorders later in life.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-078590 and T32-DK-07680 (Multidisciplinary Training in Neuroendocrinology).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Ayesha Batra, Autumn Fletcher, Kevin Joines, Peter Levasseur, Erin McEvoy, Dr. Swetha Reddy, and Xinxia Zhu for their technical assistance with this series of experiments. We are also grateful to Theodore Braun for providing critical feedback on this article.
REFERENCES
- 1. Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept 92: 1–7, 2000. . [DOI] [PubMed] [Google Scholar]
- 2. Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA, Torsoni AS, Torsoni MA. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. In press . [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ. The fetal and infant origins of adult disease. BMJ 301: 1111, 1990. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 301: 259–262, 1990. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67, 1993. . [DOI] [PubMed] [Google Scholar]
- 6. Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 306: 422–426, 1993. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bayol SA, Simbi BH, Fowkes RC, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology 151: 1451–1461, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567: 951–961, 2005. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackburn ST. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective. St. Louis, MO: Saunders Elsevier, 2007. . [Google Scholar]
- 10. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005. . [DOI] [PubMed] [Google Scholar]
- 11. Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50: 1796–1808, 2009. . [DOI] [PubMed] [Google Scholar]
- 12. Buckley AJ, Keseru B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism 54: 500–507, 2005. . [DOI] [PubMed] [Google Scholar]
- 13. Bue JM, Hausman DB, Berdanier CD. Gestational diabetes in the BHE rat: influence of dietary fat. Am J Obstet Gynecol 161: 234–240, 1989. . [DOI] [PubMed] [Google Scholar]
- 14. Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32: 1076–1080, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 189: 1698–1704, 2003. . [DOI] [PubMed] [Google Scholar]
- 16. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31: 340–346, 2008. . [DOI] [PubMed] [Google Scholar]
- 17. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49: 2208–2211, 2000. . [DOI] [PubMed] [Google Scholar]
- 18. Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Jr, Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 31: 1422–1426, 2008. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150: 4999–5009, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr 102: 514–519, 2009. . [DOI] [PubMed] [Google Scholar]
- 21. Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003. . [DOI] [PubMed] [Google Scholar]
- 22. Flanagan DE, Moore VM, Godsland IF, Cockington RA, Robinson JS, Phillips DI. Fetal growth and the physiological control of glucose tolerance in adults: a minimal model analysis. Am J Physiol Endocrinol Metab 278: E700–E706, 2000. . [DOI] [PubMed] [Google Scholar]
- 23. Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 22: 39–47, 1998. . [DOI] [PubMed] [Google Scholar]
- 24. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241, 2010. . [DOI] [PubMed] [Google Scholar]
- 25. Gout J, Sarafian D, Mutel E, Vigier M, Rajas F, Mithieux G, Begeot M, Naville D. Metabolic and melanocortin gene expression alterations in male offspring of obese mice. Mol Cell Endocrinol 319: 99–108, 2010. . [DOI] [PubMed] [Google Scholar]
- 26. Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, Braun TP, Grove KL, Friedman JE, Marks DL. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One 6: e17261, 2011. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151: 1622–1632, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gregorio BM, Souza-Mello V, Carvalho JJ, Mandarim-de-Lacerda CA, Aguila MB. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am J Obstet Gynecol 203: 495.e1–495.e8, 2010. . [DOI] [PubMed] [Google Scholar]
- 29. Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 34: 989–1000, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav 57: 681–686, 1995. . [DOI] [PubMed] [Google Scholar]
- 31. Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol 200: 293–300, 2009. . [DOI] [PubMed] [Google Scholar]
- 32. Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022, 1991. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes 3: 285–295, 2010. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics 116: 473–480, 2005. . [DOI] [PubMed] [Google Scholar]
- 35. Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 16: 202–210, 2000. . [DOI] [PubMed] [Google Scholar]
- 36. Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 587: 905–915, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23: 271–278, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knight BS, Pennell CE, Adamson SL, Lye SJ. The impact of murine strain and sex on postnatal development after maternal dietary restriction during pregnancy. J Physiol 581: 873–881, 2007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwiterovich PO., Jr Recognition and management of dyslipidemia in children and adolescents. J Clin Endocrinol Metab 93: 4200–4209, 2008. . [DOI] [PubMed] [Google Scholar]
- 40. Leon DA, Lithell HO, Vâgerö D, Koupilová I, Mohsen R, Berglund L, Lithell UB, McKeigue PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915–29. BMJ 317: 241–245, 1998. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] Method. Methods 25: 402–408, 2001. . [DOI] [PubMed] [Google Scholar]
- 42. McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119: 323–335, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140: 814–817, 1999. . [DOI] [PubMed] [Google Scholar]
- 44. Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond) 33: 115–122, 2009. . [DOI] [PubMed] [Google Scholar]
- 45. Ogden C, Carroll M. Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2007–2008 [Online]. Centers for Disease Control and Prevention. www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.htm [June 2010] .
- 46. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 303: 242–249, 2010. . [DOI] [PubMed] [Google Scholar]
- 47. Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 146: 693–700, 2005. . [DOI] [PubMed] [Google Scholar]
- 48. Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351: 173–177, 1998. . [DOI] [PubMed] [Google Scholar]
- 49. Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70: 811–816, 1999. . [DOI] [PubMed] [Google Scholar]
- 50. Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 315: 396–400, 1997. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Richardson LR, Godwin J, Wilkes S, Cannon M. Reproductive performance of rats receiving various levels of dietary protein and fat. J Nutr 82: 257–262, 1964. . [DOI] [PubMed] [Google Scholar]
- 52. Roberts EA. Pediatric nonalcoholic fatty liver disease (NAFLD): a “growing” problem? J Hepatol 46: 1133–1142, 2007. . [DOI] [PubMed] [Google Scholar]
- 53. Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383–392, 2008. . [DOI] [PubMed] [Google Scholar]
- 54. Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr 76: 1096–1100, 2002. . [DOI] [PubMed] [Google Scholar]
- 55. Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension 40: 441–447, 2002. . [DOI] [PubMed] [Google Scholar]
- 56. Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab 291: E792–E799, 2006. . [DOI] [PubMed] [Google Scholar]
- 57. Stephenson T, Budge H, Mostyn A, Pearce S, Webb R, Symonds ME. Fetal and neonatal adipose maturation: a primary site of cytokine and cytokine-receptor action. Biochem Soc Trans 29: 80–85, 2001. . [DOI] [PubMed] [Google Scholar]
- 58. Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 151: 3652–3664, 2010. . [DOI] [PubMed] [Google Scholar]
- 59. Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes (Lond) 33: 743–752, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol 175: 757–767, 2002. . [DOI] [PubMed] [Google Scholar]
- 61. Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 179: 293–299, 2003. . [DOI] [PubMed] [Google Scholar]
- 62. Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA, Poston L. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol 288: R134–R139, 2005. . [DOI] [PubMed] [Google Scholar]
- 63. Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138: 5063–5066, 1997. . [DOI] [PubMed] [Google Scholar]
- 64. Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J 13: 268–273, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114: e29–e36, 2004. . [DOI] [PubMed] [Google Scholar]
- 66. White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol 296: R1464–R1472, 2009. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. . [DOI] [PubMed] [Google Scholar]
- 68. Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005. . [DOI] [PubMed] [Google Scholar]
- 69. Zhang J, Wang C, Terroni PL, Cagampang FR, Hanson M, Byrne CD. High-unsaturated-fat, high-protein, and low-carbohydrate diet during pregnancy and lactation modulates hepatic lipid metabolism in female adult offspring. Am J Physiol Regul Integr Comp Physiol 288: R112–R118, 2005. . [DOI] [PubMed] [Google Scholar]