Abstract
Second-phase insulin release requires the sustained mobilization of insulin granules from internal storage pools to the cell surface for fusion with the plasma membrane. However, the detailed mechanisms underlying this process remain largely unknown. GTP-loading of the small GTPase Cdc42 is the first glucose-specific activation step in the process, although how glucose triggers Cdc42 activation is entirely unknown. In a directed candidate screen for guanine nucleotide exchange factors (GEFs), which directly activate small GTPases, Cool-1/βPix was identified in pancreatic islet beta cells. In support of its role as the beta cell Cdc42 GEF, βPix coimmunoprecipitated with Cdc42 in human islets and MIN6 beta cells in a glucose-dependent manner, peaking just prior to Cdc42 activation. Furthermore, RNAi-mediated βPix reduction by 50% corresponded to full ablation of glucose-induced Cdc42 activation and significant attenuation of basal and glucose-stimulated insulin secretion. Of the two Cdc42 guanine nucleotide dissociation inhibitor (GDI) proteins identified in beta cells, βPix competed selectively with caveolin-1 (Cav-1) but not RhoGDI in coimmunoprecipitation and GST-Cdc42-GDP interaction assays. However, a phospho-deficient Cav-1-Y14F mutant failed to compete with βPix; Cav-1Tyr14 is an established phosphorylation site for Src kinase. Taken together, these data support a new model, wherein glucose stimulates Cav-1 and induces its dissociation from Cdc42, possibly via Src kinase activation to phosphorylate Cav-1Tyr14, to promote Cdc42-βPix binding and Cdc42 activation, and to trigger downstream signaling and ultimately sustain insulin release.
Keywords: small GTPase, islet, insulin exocytosis, caveolin-1
the metabolism of glucose taken up into pancreatic β-cells initiates the signaling pathways responsible for regulated insulin secretion. Upon entry into the beta cell, glucose metabolism leads to an increase in the ratio of ATP to ADP, which subsequently causes inactivation of the ATP-dependent potassium channels and depolarization of the beta cell plasma membrane (PM). Voltage-dependent calcium channels open upon this depolarization signal, leading to the fusion of insulin granules at or juxtaposed to the PM to evoke insulin release, resulting from glucose stimulation, occurs in two distinct phases with the predominant model attributing this biphasic pattern to multiple intracellular pools of granules. First-phase secretion results from the rapid release (within 10 min) of insulin granules predocked at the PM of the beta cell, termed the “readily releasable pool”. This transient release is followed by a sustained second phase, elicited only by fuel-based secretagogues such as glucose, during which insulin is secreted for a longer duration but at a reduced rate (reviewed in Refs. 33 and 41). Secretion during this phase is thought to originate from insulin granules recruited to the PM from an intracellular storage pool, although the signaling mechanisms required to support sustained glucose-stimulated insulin secretion (GSIS), through regulation of proper timing and recruitment of such granules, are still largely unclear.
The glucose-stimulated activation of the small Rho family GTPases Cdc42 and Rac1 is key in the regulation of sustained, second-phase GSIS (reviewed in Ref. 15). Depletion of Cdc42 selectively impairs second-phase GSIS from perifused islets (39), consistent with early pharmacological findings pointing to the selective activation of Cdc42 by glucose in islet beta cells (16). Cdc42 activation occurs rapidly and is known to trigger phosphorylation and activation of its downstream effector protein p21-activated kinase (Pak1), which, in turn, activates the other downstream GTPase Rac1 (39). As a member of the Rho family of small GTPases, Cdc42 cycles through inactive GDP-bound and active GTP-bound states. In the beta cell, Cdc42 is kept inactive by association with two distinct guanine nucleotide dissociation inhibitor (GDI) proteins: Caveolin-1 (Cav-1), for the pool of Cdc42 present on the insulin secretory granules at or juxtaposed to the PM (25), and RhoGDI, for the cytosolic pool of Cdc42 (40). Depletion of either Cav-1 or RhoGDI results in inappropriate Cdc42 activation and dysfunctional insulin release. Recent efforts toward understanding Cdc42 activation point to its dependence upon upstream Arf6 activation via Arf nucleotide binding site opener (ARNO). However, because Arf6 mediates both glucose and non-glucose-stimulated insulin secretion, a role for Arf6 as direct mediator of Cdc42 activation remains unclear (11). Specific stimulus-induced activation of small GTPases is known to require a guanine nucleotide exchange factor (GEF) to destabilize the binding of GDP on the inactive GTPase and to allow for the incorporation of GTP leading to activation (31). However, GEFs involved in the glucose-stimulated cycling of Cdc42 to its active state, as well as those factors employed in the subsequent inactivation of Cdc42 to its GDP-bound state, are as of yet unknown.
In the elucidation of the glucose-specific activation mechanism for Cdc42 in the insulin secretion process, we used a candidate-based approach. There are more than 80 known GEFs for the Rho family GTPases, with variable specificity and affinities for Cdc42 (31). Eight GEFs were identified by RT-PCR in a directed screen to assess GEFs with known Cdc42 specificity. Of those eight, Cool1/βPix was detectable at the protein level in islets and beta cells. Interestingly, βPix has been shown in other cell types to facilitate signaling events at numerous points relevant to a process such as GSIS. For example, in response to an extracellular stimulus, βPix undergoes phosphorylation to activate Cdc42 in NIH3T3 cells (8, 9). In addition, βPix can interact with Pak1, relieve Pak1 autoinhibition, and facilitate autophosphorylation of Pak1 mediated by Cdc42 and Rac1 (3). In the exocytotic pathway of neuroendocrine PC12 cells, βPix can activate the GTPase Rac1 (23). Because of this multifunctional nature of βPix, we sought to determine its necessity and mechanism of action as it pertains to Cdc42 activation and GSIS in the beta cell.
In this report, we present evidence to support a role for βPix as a Cdc42 GEF in the beta cell, required for both Cdc42 activation and the release of insulin in response to glucose stimulation. Our data also imply that βPix acts upon the pool of Cdc42 bound to Cav-1, as opposed to that bound to RhoGDI. Furthermore, we demonstrate the importance of the Tyr14 phosphorylation site on Cav-1 in both the association of Cav-1 with Cdc42 and the ability of Cav-1 to compete with βPix for Cdc42. Given that Cav-1Tyr14 is an established site of phosphorylation by Src kinase (18) and that Src kinase activation is implicated in insulin secretion (5), these data support the concept of Cdc42 activation through Src-mediated phosphorylation of Cav-1 to trigger its exchange for the newly identified beta cell GEF, βPix.
MATERIALS AND METHODS
Materials.
Radioimmunoassay-grade BSA was purchased from Sigma. The n-octyl glucoside was obtained from Research Products International (Mt. Prospect, IL). Glutathione-Sepharose beads and enhanced chemiluminescence reagent were purchased from GE Healthcare (Piscataway, NJ). SuperSignal West Femto chemiluminescent substrate, the active Cdc42 pull-down and detection kit, containing the mouse anti-Cdc42 antibody, and goat anti-mouse horseradish peroxidase secondary antibody were acquired from Thermo Scientific (Rockford, IL). The hGH ELISA kit was purchased from Roche (Indianapolis, IN). The RNeasy mini kit was purchased from Qiagen (Germantown, MD). StrataScript RT was obtained from Agilent Technologies (Santa Clara, CA). BioMix Red was purchased from Bioline (Taunton, MA). TrueBlot reagents were obtained from eBioscience (Arnold, MD). Rabbit anti-GAPDH, anti-GST, and mouse anti-VAMP2 antibodies were purchased from Abcam (Cambridge, MA), Affinity BioReagents (Golden, CO), and Synaptic Systems (Göttingen, Germany), respectively. Rabbit anti-βPix and mouse anti-βPix antibodies were obtained from Millipore (Billerica, MA), Cell Signaling (Temecula, CA), and BD Biosciences (Mountain View, CA). Rabbit anti-Syntaxin 4 was produced in-house, as described previously (42). Rabbit IgG, mouse anti-Myc (9E10), and rabbit anti-RhoGDI were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-rabbit horseradish peroxidase secondary antibody and TransFectin were purchased from Bio-Rad (Hercules, CA), respectively. Lipofectamine 2000 transfection reagent and Stealth siRNA oligonucleotides were purchased from Invitrogen (Carlsbad, CA): siCon, gcgcgcuuuguaggauucgaaccuatt; siβPix1, gaggaccuaggagaguucauggaaatt; and siβPix2, gaguucagcaaacacuucauauuuatt.
Plasmids.
The pcDNA3-hGH construct was a kind gift from Dr. Philippe Halban (University of Geneva Medical School, Geneva, Switzerland). Full-length pcDNA3.1-hRhoGDIα was kindly provided by Dr. Anjan Kowluru (Wayne State University School of Medicine, Detroit, MI). The pcDNA3-Cav-1-myc-His construct was generated by subcloning the PCR-generated product into the 5′-EcoRI and 3′-BamHI sites of the pcDNA3.1-myc-His expression vector; the Y14F mutant was generated by site-directed mutagenesis from this plasmid (Quick-Change, Stratagene). All constructs were verified by DNA sequencing.
Cell culture, transient transfection, and hGH secretion assays.
MIN6 cells (a gift from Dr. John Hutton, University of Colorado Health Sciences Center, Denver, CO) were cultured in DMEM (25 mM glucose) supplemented with 15% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml l-glutamine, and 50 μM β-mercaptoethanol, as described previously (12). At 50–60% confluence, cells were transfected with siRNA oligonucleotides using Lipofectamine 2000 for 48-h incubation. A nontargeting siRNA was provided from the manufacturer. In the hGH secretion assays, cells were additionally transfected with 0.5 μg of the pcDNA3-hGH construct. Transfected cells were maintained in the supplemented DMEM for 48 h, washed twice, and incubated in freshly prepared modified Krebs-Ringer bicarbonate buffer (MKRBB) for 2 h. Cells were stimulated with 20 mM d-glucose for the times designated in the figure legends. Cells were harvested in Nonidet P-40 lysis buffer (25 mM HEPES, pH 7.4, 1% Nonidet P-40, 10% glycerol, 50 μM sodium fluoride, 10 mM sodium pyrophosphate, 137 mM sodium chloride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml pepstatin, and 5 μg/ml leupeptin), and lysates were cleared by microcentrifugation for 10 min at 4°C for subsequent use in activation assays and binding assays. Human growth hormone secreted into the MKRBB was quantified by ELISA. For the βPix-Cdc42 competition experiments, MIN6 cells at 50–60% confluence were transfected with 30 μg of the pcDNA3-Cav-1-myc-His (WT or Y14F) or pcDNA3.1-hRhoGDIα DNAs using TransFectin; 48 h posttransfection, cells were washed twice and incubated for 2 h in MKRBB. Cells were subsequently lysed in Triton X-100 lysis buffer containing n-octyl glucoside (10 mM Tris·HCl, pH 8.0, 0.25% Triton X-100, 60 mM n-octyl glucoside, 10% glycerol, 50 μM sodium fluoride, 10 mM sodium pyrophosphate, 150 mM sodium chloride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml pepstatin, and 5 μg/ml leupeptin) to generate cleared detergent homogenates for the coimmunoprecipitation and pull-down assays.
Mouse islet isolation.
Islets were obtained from C57BL6J mice housed in the Indiana University School of Medicine Laboratory Animal Research Center, following the approved Institutional Animal Care and Use Committee protocols and standards of procedure. Briefly, pancreata pooled from five ∼12-wk-old male mice were batch-digested with collagenase, purified using a Ficoll density gradient, and incubated overnight in RPMI 1640 at 37°C, 5% CO2 (34) Islets (200 per gel lane) were handpicked into tubes for lysis in SDS-PAGE loading buffer, and islet proteins resolved on SDS-PAGE. Human islets obtained from two normal-body mass index nondiabetic healthy donors through the Integrated Islet Distribution Program (IIDP) were lysed similarly for SDS-PAGE following protocols approved by the Indiana University Biosafety Committee.
Directed screen for candidate GEFs in MIN6 cells.
Total RNA was isolated from unstimulated MIN6 beta cells and the Titan One Tube RT-PCR kit (Roche, Indianapolis, IN) was used to generate cDNA and amplify sequences (35 cycles) following the manufacturer's instructions. Specific PCR primer sequences are shown in Table 1.
Table 1.
Candidate Dbl family Cdc42 GEFs that were screened for mRNA expression in MIN6 cells
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| βPix | ctctctgctacaaggaggatctcag | catactcagagtcttccgagaggt |
| Vav-3 | cagtgaggacctaggagagttcat | ggaagagcaggaggtatctctca |
| Vav-2 | tggctgctgtcttcatcaac | cagcaggtggtacttcagca |
| NGEF | ctgatgaactgacgctggaa | ttccctaaggccctctgaat |
| FRG | gtaatggctggcagaagctc | cctggagagtgatgtgagca |
| Fdg1 | cccagaagtctttggagctg | ttcctgacaccaggaaggtc |
| Fdg3 | agaatggcaccactcaggac | gctcctcatcctggctacag |
| Frabin (Fdg4) | gcccctacttcttgtacctcaa | ctctgggctaagagtagtctccttc |
RNA isolation and RT-PCR.
Total RNA from MIN6 cells and mouse brain lysates was obtained using the RNeasy mini kit. RNA (10 μg) was reverse-transcribed with StrataScript reverse transcriptase, and 1% of the product was used for RT-PCR. The primers used for detection of βPix isoforms (A and B/C) were as follows: βPixA/B/C, forward 5′-ggttatcgaagcttattgcaca; βPixA reverse 5′-ggtgagagatatatgagcagca , βPixB/C, reverse 5′-aacgcatacaccgtatccac. GAPDH was used a positive control for the integrity of both lysate sets. The primers used for GAPDH detection were forward 5′-atggtgaaggtcggtgtgaacg and reverse 5′-gttgtcatggatgaccttggcc. RT-PCR was performed with BioMix Red for 30 cycles: 94°C for 1 min, 54°C for 2 min, and 71°C for 3 min, with a final 10-min elongation at 71°C. PCR products were visualized on 2% agarose gels.
Subcellular fractionation.
Subcellular fractions were prepared as previously described (26). All fractionation steps were performed at 4°C. Briefly, MIN6 cells at 70–80% confluence were harvested into 1 ml of homogenization buffer (20 mM Tris·HCL, pH 7.4, 0.5 mM EDTA, 0.5 mM EGTA, 250 mM sucrose, 1 mM dithiothreitol, 100 μM phenylmethylsulfonyl fluoride, 4 μg/ml aprotinin, 2 μg/ml pepstatin, and 10 μg/ml leupeptin). Cells were homogenized by 10 strokes through a 27-gauge needle, and then centrifuged at 900 g for 10 min. Postnuclear supernatants were centrifuged for 5,500 g for 15 min. The resulting supernatant was centrifuged at 25,000 g for 20 min to obtain the secretory granule fraction in the pellet. This supernatant was centrifuged further at 100,000 g for 1 h to acquire the cytosolic fraction. The PM fraction was prepared by mixing the postnuclear pellet with 1 volume of buffer A (0.25 M sucrose, 1 mM MgCl2, and 10 mM Tris·HCl, pH 7.4) and 2 volumes of buffer B (2 M sucrose, 1 mM MgCl2, 10 mM Tris·HCl, pH 7.4). This mixture was overlaid with buffer A and centrifuged at 113,000 g for 1 h to obtain an interface containing the PM. The interface was collected, diluted to 1.5 ml with homogenization buffer, and centrifuged at 6,000 g for 10 min. The resulting pellet contained the PM fraction. All collected pellets were resuspended in 1% Nonidet P-40 lysis buffer.
Pancreas section immunostaining.
Immunofluorescence staining of pancreatic sections proceeded, as described previously (7). Briefly, pancreata from wild-type (C57BL/6J strain, 5 mo old) mice were fixed with 4% paraformaldehyde, paraffin-embedded, and longitudinally sectioned at 5-μm thickness and 100-μm intervals. The sectioned tissues were deparaffinized, rehydrated, and blocked in 5% donkey serum (Sigma, St. Louis, MO), followed by an overnight incubation at 4°C with rabbit anti-βPix (Millipore) and mouse anti-insulin (Santa Cruz) or guinea pig anti-glucagon (Millipore) antibodies. The sectioned tissues were then washed with PBS at room temperature, followed by a 1-h incubation at room temperature with Alexa Fluor 488, Alexa Fluor 555 (Invitrogen), or Cy5-conjugated (Jackson Immunoresearch, Bar Harbor, ME) secondary antibodies. The slides were then washed three times in PBS, overlayed with antifading mounting medium (Biomeda, Foster City, CA), and mounted with coverslips for imaging analysis using the Olympus FV1000 confocal microscope. Images were prepared for presentation using ImageJ (National Institutes of Health) with minimal processing.
Coimmunoprecipitation and immunoblotting.
For βPix immunoprecipitation, 2 mg of cleared MIN6 detergent lysates were combined with 2 μg of rabbit anti-βPix antibody (Millipore) for 2 h at 4°C, followed by the addition of Trueblot beads for an additional 2 h. Human islet lysates were prepared similarly, following a 2-h preincubation followed by a 2-min glucose stimulation (16.7 mM), as previously described (42), using 400- to 500-μg islet protein per reaction. After three washes with lysis buffer, the resulting immunoprecipitates were subjected to 12% SDS-PAGE, followed by transfer to PVDF membranes for immunoblotting with rabbit anti-βPix (Cell Signaling), mouse anti-Cdc42 (Thermo), mouse anti-Myc (Santa Cruz Biotechnology), and rabbit anti-RhoGDI (Santa Cruz Biotechnology) primary antibodies, followed by incubation with Trueblot secondary antibodies for 1 h at RT. Proteins were visualized by enhanced chemiluminescence.
Cdc42 activation assays.
The EZ-Detect Cdc42 activation kit from Pierce (Rockford, IL) was used to detect the GTP-loaded forms of Cdc42, as described previously (39, 40). Freshly prepared whole cell lysates (500 μg) were combined with 20 μg of GST-Pak1-PBD-agarose and rotated for 1 h at 4°C. Proteins were eluted from the beads after 3 washes with the kit lysis/binding/wash buffer and subjected to 12% SDS-PAGE followed by transfer to PVDF membranes for immunoblotting with mouse anti-Cdc42 (Thermo) antibody. The relative abundance of eluted Cdc42 was determined by Western blot analysis and densitometry.
GST-Cdc42-GDP interaction assay.
The GST-Cdc42 fusion protein expression and GDP loading was performed as previously described (26). Briefly, 10 μg of GST-Cdc42 linked to Sepharose beads was incubated in buffer (0.1 M Tris, pH 7.4, 1 mM EDTA, 2 mM DTT, 0.2 M NaCl ) at a final concentration of 0.1 mM GDP for 10 min at 30°C, combined with 50 mM MgCl2. Freshly GDP-loaded GST-Cdc42 Sepharose was immediately incubated with cleared detergent lysates (2 mg, Triton X-100 and n-octylglucoside buffer) prepared from transfected cells for 2 h at 4°C. Following three washes with PBS supplemented with 2.5 mM MgCl2, proteins were eluted from the Sepharose beads and proteins resolved on 12% SDS-PAGE followed by transfer to PVDF membrane for immunoblotting with mouse anti-βPix (BD Biosciences), mouse anti-Myc (Santa Cruz), and rabbit anti-RhoGDI (Santa Cruz) antibodies.
Statistical analysis.
All data were evaluated for statistical significance using a two-tailed Student's t-test. Data are expressed as the average ± SE.
RESULTS
βPix isoform expression and localization in islet beta cells.
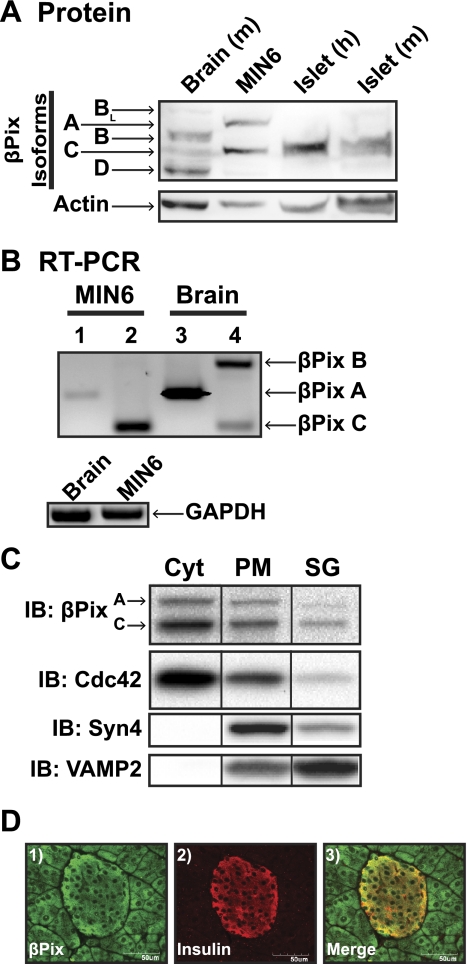
A candidate PCR screen for known Cdc42 GEFs using specific primer sets detected the presence of eight putative targets in MIN6 beta cell lysates: βPix, Vav-2, Vav-3, NGEF, Frg, Fgd1, Fgd3 (Table 1). Of these eight, only βPix could be confirmed by Western blot analysis (using commercially available antibodies), which consisted of two bands corresponding to the A and C isoforms of βPix (Fig. 1A). Human and mouse islets also expressed the C isoform, and akin to the MIN6 beta cells, failed to express the isoforms BL, B, and D present in the brain lysates. Specific RT-PCR screening of cDNA libraries generated from MIN6 cells and mouse brain was used to confirm the preferential expression of isoforms βPixA and βPixC in beta cells (Fig. 1B). Identification of βPixC in all of these systems pointed to the possibility that βPix served as a Cdc42 GEF in islet beta cells and validated the MIN6 cell as a suitable model system for further mechanistic investigations.
Fig. 1.
βPix isoforms A and C are expressed in MIN6 beta cells and pancreatic islets and colocalize with Cdc42 in MIN6 subcellular fractions. A: βPix protein was detectable by immunoblotting in MIN6 lysates, human islets, and mouse islets. Mouse brain lysates were used as a positive control for the many isoforms of βPix expressed. Anti-actin immunoblot (IB) detection was evaluated for protein loading. B: RT-PCR was used to detect isoforms of βPix expressed in MIN6 β-cells (A isoform primers used in lanes 1 and 3; B and C primer sets used together in lanes 2 and 4). Mouse brain, which contains all three isoforms, was used as a positive control for the primer sets, and GAPDH was used as a positive control for the integrity of both lysate sets. C: clarified MIN6 cell lysates were partitioned into subcellular fractions: PM, plasma membrane; SG, storage granule pool; Cyt, remaining soluble fraction. βPix content in each fraction was assessed by immunoblot (IB). Cdc42, Syntaxin 4 (Syn4) and VAMP2 were used to validate the integrity of the Cyt, PM, and SG fractions, respectively. Reassembly of noncontiguous lanes from within the same gel is demarcated by black lines. Data are representative of at least three independent experiments. D: mouse pancreas sections were costained for βPix and insulin as described in materials and methods; representative of three pancreatic sections.
Cdc42 activation and cycling events occur at distinct locations within the cell. To determine whether βPix colocalized with Cdc42, as would be appropriate for a Cdc42 GEF, we evaluated subcellular fractions prepared from MIN6 beta cells, as described previously (39). Under unstimulated conditions, Cdc42 is known to localize to plasma membrane (PM) and cytosolic compartments, as has been described in other cell types, but also has been localized to the insulin storage granules (SG), which are distributed throughout the beta cell. βPixA- and C-isoforms were present in the same fractions and similarly distributed (Fig. 1C). Fraction integrity was validated by immunoblotting for known plasma membrane and secretory granule marker proteins Syntaxin 4 and VAMP2, respectively. Thus, βPixA and βPixC isoforms localized with Cdc42 in beta cells, consistent with their expected roles as Cdc42 GEFs. Furthermore, βPix staining of mouse pancreas sections in Fig. 1D showed the protein to be expressed in exocrine cells, as well as insulin-containing endocrine cells (and glucagon-containing cells, data not shown), consistent with its ubiquitous expression in many diverse cell types.
βPix rapidly interacts with Cdc42 in a glucose-sensitive manner.
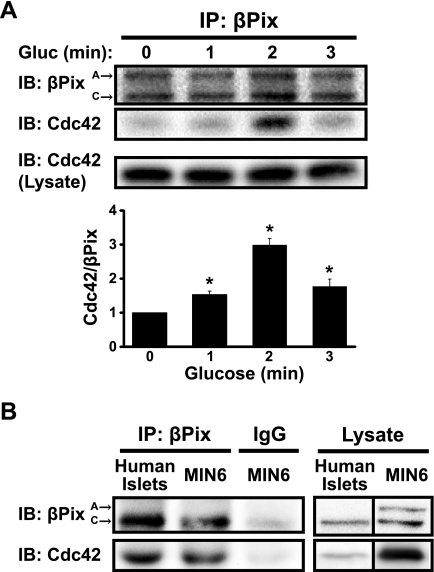
Cdc42 is maximally activated within 3 min after glucose stimulation of MIN6 beta cells (27), prior to the onset of second-phase insulin release. Therefore, to trigger activation appropriately, association of Cdc42 with its corresponding GEF would be predicted to occur within this time frame. To determine the time course of βPix-Cdc42 interaction, MIN6 cells were subjected to glucose stimulation, and the cleared detergent cell lysates used for coimmunoprecipitation of the complex at early time points. As shown in Fig. 2A, a maximal ∼3-fold increase in coimmunoprecipitation of Cdc42 with anti-βPix antibody occurred within 2 min of glucose stimulation, relative to that in unstimulated cell lysates. Importantly, the association of Cdc42-bPix with 2-min glucose stimulation was reproducible using human islet lysates (Fig. 2B). The timing of this complex formation within 3 min of glucose stimulation would be consistent with the binding of a GEF to its GTPase immediately prior to GTPase activation; replication in human islets provides substantial physiological relevance to this binding event in the process of insulin release.
Fig. 2.
βPix associates rapidly with Cdc42 in response to glucose stimulation in human islets and MIN6 beta cells. βPix was immunoprecipitated (IP) from cleared detergent lysates prepared from either MIN6 cells preincubated in MKRBB for 2 h and then acutely stimulated with 20 mM glucose for 1, 2, or 3 min (A), or human islets preincubated 2 h in KRBH buffer (B) and then acutely stimulated with 16.7 mM glucose for 2 min. Proteins were resolved by 12% SDS-PAGE and immunoblotted (IB) with anti-βPix and anti-Cdc42 antibodies. Input lysates validated equal Cdc42 expression under all conditions, and IgG immunoprecipitation validated the specificity of the Cdc42/βPix association. Data are shown as the average ± SE of three independent experiments, quantified for the ratio of Cdc42/βPix; *P < 0.05 vs. basal for MIN6 cells. Human islet blots are representative of two independent coimmunoprecipitation experiments using two human donor batches of islets. Reassembly of noncontiguous lanes from within the same gel is demarcated by black lines.
βPix knockdown inhibits GSIS and Cdc42 activation.
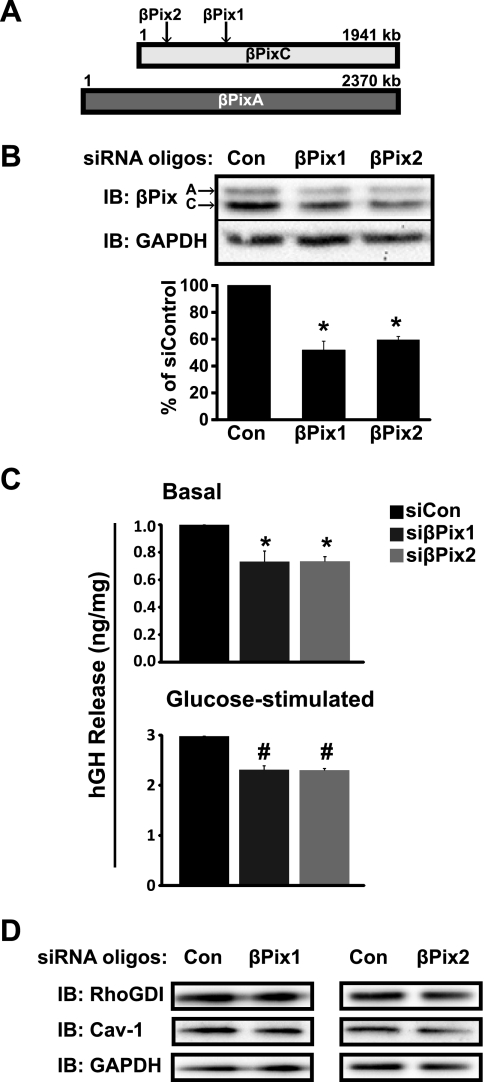
Upon association with their cognate GTPase, GEF proteins facilitate GTP loading, which in turn, evokes effector interaction and downstream signaling. In the islet beta cell, glucose-stimulated Cdc42 activation induces insulin release (25, 39, 40). To determine the requirement for βPix to facilitate insulin release via Cdc42 activation, we used siRNA-mediated knockdown of βPixA- and C-isoforms. MIN6 cells were transiently transfected with two commercially available siRNA oligonucleotides targeting different regions of βPixA and C isoforms (Fig. 3A); A and C isoforms migrated on SDS-PAGE at ∼85 and 78 kDa, respectively. Both siRNA oligonucleotides reduced endogenous βPixA- and C-isoform protein expression to ∼50–60% of the levels of control cells transfected with nontargeting siRNA control oligonucleotides (Fig. 3B); the combination of both A- and C- isoform siRNA oligonucleotides showed similar knockdown efficiency (data not shown). Neither of the βPix siRNA oligonucleotides exerted off-target effects upon GAPDH, which was used for normalization.
Fig. 3.
Knockdown of βPix in MIN6 beta cells attenuates both basal and glucose-stimulated insulin secretion. A: schematic of βPix isoform organization and targeting location of the siRNA oligonucleotides. B: two siRNA oligonucleotides sets, designated βPix1 and βPix2, or control (Con) siRNA were transfected into MIN6 cells and knockdown efficiency determined by anti-βPix immunoblotting (IB). GAPDH was used for normalization of loading. Data represent the average ± SE of five independent experiments; *P < 0.05 vs. siCon. C: glucose-stimulated insulin secretion was assessed using an hGH reporter-based assay. MIN6 cells were cotransfected with hGH and either siCon or siβPix2; 48 h later, cells were preincubated in glucose-free MKRBB for 2 h and then left unstimulated or stimulated with 20 mM glucose for 60 min. Secretion of hGH into the MKRBB was quantified by ELISA and normalized to hGH content in the corresponding cell lysates. Insulin levels were adjusted for corresponding total cell protein content and normalized to unstimulated siCon set equal to 1.0 in each assay, and data are presented as the average ± SE of three independent experiments; *P < 0.05 vs. unstimulated siCon; #P < 0.05 vs. glucose-stimulated siCon. D: protein expression of Cav-1 and RhoGDI in cells transfected with siRNA oligonucleotides targeted for βPix was validated from lysates in B.
Using these βPix siRNA oligonucleotides, we examined the effects of βPixA/C knockdown upon basal and glucose-stimulated insulin secretion from MIN6 beta cells, using a reporter-based assay to sample secretion more selectively from transfectable cells. Human growth hormone (hGH) suffices as such a reporter of insulin secretion from transfected cells, since hGH is packaged into secretory granules similarly to insulin and is secreted with insulin upon glucose stimulation (37). MIN6 cells were cotransfected with hGH cDNA plus siβPix1, siβPix2, or the nontargeting siCon siRNA oligonucleotides, and hGH secreted in the absence and presence of glucose stimulation was assessed. Compared with siCon, cells transfected with either siβPix1 or siβPix2 exhibited a significant ∼25–30% reduction in both basal and glucose-stimulated hGH secretion (Fig. 3C). Changes in secretion were not attributable to alterations in expression of the Cdc42 GDI proteins, Cav-1, and RhoGDI, in response to siβPix1 or siβPix2 oligonucleotide-mediated depletion of βPix (Fig. 3D). These data further support a role of βPix as an important Cdc42 GEF in glucose-stimulated insulin secretion.
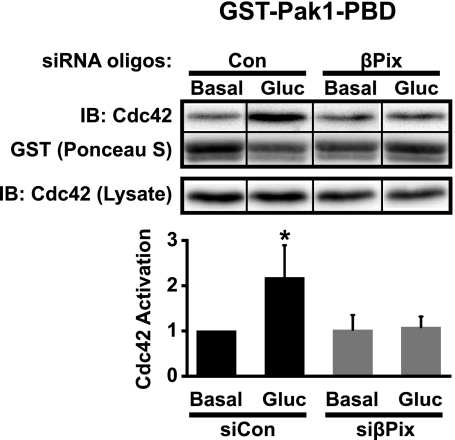
We next determined whether the effects of βPix silencing upon insulin release were linked directly to Cdc42 activation. Cdc42 activation was measured in cells treated with siCon and one of the two siRNAs (βPix2), due to the vast number of cells required for a single activation measurement and the similarity of knockdown between each βPix siRNA. Cdc42 activation was determined using the GST-Pak1-PBD interaction assay of Cdc42 from unstimulated and glucose-stimulated cleared whole cell detergent lysates. Compared with the more than twofold glucose-stimulated (3 min) increase in Cdc42 activation in control cells, knockdown of βPix resulted in full ablation of Cdc42 activation (Fig. 4). No statistically significant differences in basal Cdc42 activation or Cdc42 protein expression under any conditions were detected. These data would be consistent with the designation of βPix as a glucose-responsive GEF for Cdc42 in MIN6 beta cells.
Fig. 4.
Knockdown of βPix in MIN6 beta cells abolishes glucose-stimulated Cdc42 activation. Cells transfected with Control (Con) or βPix (βPix2) siRNA oligonucleotides were preincubated in glucose-free MKRBB for 2 h and then left unstimulated or acutely stimulated (3 min) with 20 mM glucose. Cdc42-GTP captured on GST-Pak1-PBD beads was resolved by 12% SDS-PAGE and detected by anti-Cdc42 immunoblotting (IB). Reassembly of noncontiguous lanes from within the same gel is demarcated by black lines. Activation levels were adjusted for GST loading and normalized to unstimulated siCon set equal to 1.0 in each assay, and data are presented as the average ± SE of three independent experiments; *P < 0.05 vs. unstimulated siCon.
βPix functions as Cdc42 GEF for exchange with Cav-1 but not with RhoGDI.
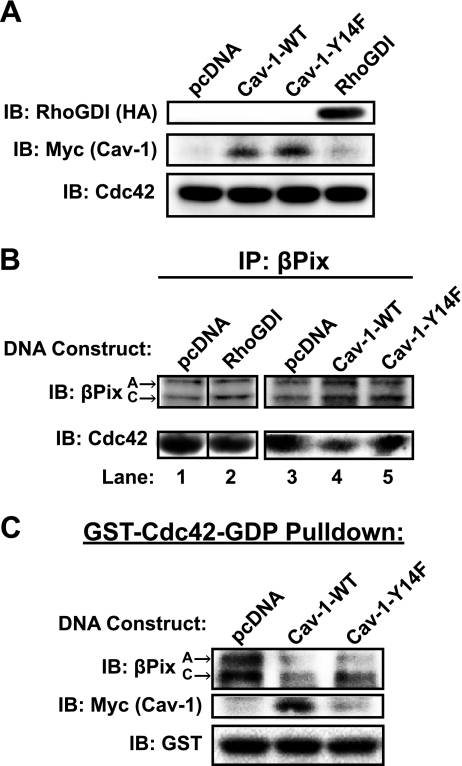
The association of a GEF and a GDI with their cognate GTPase should be mutually exclusive, as the GEF-catalyzed exchange of GDP for GTP requires the prior dissociation of the GDI from the GTPase. Given that Cdc42 in beta cells associates with two different GDIs, Cav-1 and RhoGDI, in the SG/PM and cytosolic fractions, respectively (25, 40), we sought to determine which of these GDI-GTPase complexes βPix preferentially opposed. To accomplish this, we transfected MIN6 cells to express HA-tagged RhoGDI or myc-tagged Cav-1 (Fig. 5A), to disrupt endogenous βPix-Cdc42 complexes relative to complexes formed in cells transfected with vector alone. A phospho-defective form of Cav-1 (Cav-1-Y14F) was also tested, on the basis of the putative importance of this site in Cav-1 interactions. While RhoGDI expression failed to disrupt Cdc42 binding to βPix, Cav-1 (wild-type, WT) effectively displaced a substantial portion of βPix from Cdc42 binding (Fig. 5B, lanes 1–4). By contrast, Cav-1-Y14F expression failed to disrupt βPix-Cdc42 complexes (Fig. 5B, lane 5). These data suggest that Cav-1-Cdc42 complexes are the target of βPix in MIN6 beta cells.
Fig. 5.
Cav-1 Tyr14 is required for Cdc42-Cav-1 and βPix-Cdc42 complex formation. A: MIN6 cells were transiently transfected with RhoGDI-HA or Myc-tagged Cav-1 DNAs (WT or Cav-Y14F). Cells were preincubated in MKRBB for 2 h, glucose-stimulated for 2 min, and the resultant cleared detergent cell lysates were immunoblotted to validate expression of recombinant proteins, relative to endogenous Cdc42. Lysates were otherwise subjected to anti-βPix immunoprecipitation (IP) reactions (B) or GST-Cdc42-GDP interaction assays (C), as described in materials and methods. Precipitates were resolved on 12% SDS-PAGE, transferred to PVDF, and immunoblotted (IB) for βPix, Cdc42, Myc (Cav-1), and GST. Reassembly of noncontiguous lanes from within the same gel is demarcated by black lines. Data shown are representative of 3–5 independent experiments.
In a second approach, cell lysates were subjected to interaction with exogenous GST-Cdc42-GDP (GDP loading of the GST-Cdc42 fusion protein occurred immediately prior to use in the pull-down assay). The ability of Cav-1-WT or Cav-1-Y14F to compete with endogenous βPix for Cdc42 binding was assessed by βPix immunoblotting. In the absence of a competitor GDI, βPixA-, and C-isoforms associated with the GST-Cdc42-GDP protein (Fig. 5C). Cav-1-WT was able to displace a considerable amount of βPix from GDP-loaded GST-Cdc42. Cav-1-WT was able to interact with the GDP-loaded GST-Cdc42, consistent with its known function as a Cdc42 GDI. Compared with Cav-1-WT, the Cav-1 Y14F mutant competed less well with βPix for association with Cdc42 and bound poorly to the GST-Cdc42-GDP protein, implicating this site for normal GDI-GTPase complex formation. This second approach also ruled out the possibility that Cav-1 had merely sequestered Cdc42 to a location in the cell where βPix could not access it.
DISCUSSION
Cdc42 activation is crucial for the process of sustained insulin release from the pancreatic islet beta cell. While two distinct GDIs for Cdc42, namely Cav-1 and RhoGDI, have been identified in islet beta cells, with each GDI associating with distinct pools of Cdc42 that have unique usage in the process of insulin release, the GEF proteins participating in displacement of Cdc42 from these GDIs have remained elusive. In this report, we have identified βPix as the first known Cdc42 GEF protein in human islets and MIN6 beta cells and have linked it to displacement with one particular GDI, Cav-1. βPix sufficed in meeting the criteria expected of a Cdc42 GEF in islet beta cells: 1) the binding of βPix to Cdc42 rapidly increased within 2 min of glucose treatment, just prior to the previously established peak time of Cdc42 activation in beta cells; 2) RNAi-mediated reduction of βPix protein resulted in ablation of Cdc42 activation; 3) reduction of βPix protein attenuated both basal and glucose-stimulated insulin secretion; and 4) βPix competed with Cav-1 for binding to Cdc42-GDP. Furthermore, our studies indicate that in the islet beta cell, βPix acts upon the Cav-1-Cdc42, rather than the RhoGDI-Cdc42, GDI-GTPase complex, which localizes βPix function to that pool of Cdc42 present on insulin secretory granules, which are at or juxtaposed to the PM (26). Taken together, these criteria place βPix as the first direct upstream activator of Cdc42 in the process of insulin release.
The apparent selectivity of βPix for the membrane-localized Cdc42-Cav-1 complex is intriguing, especially so when considering the specificity of usage of granule pools during the distinct phases of insulin secretion. Using two distinct assays to evaluate preference of βPix for the Cdc42-Cav-1 or Cdc42-RhoGDI complexes for competitive exchange, we showed that Cav-1 has the ability to effectively compete with βPix for Cdc42 binding, whereas RhoGDI did not. Although it has been previously demonstrated that βPix is a GEF for Cdc42, this is the first report of βPix action exclusively upon a membrane-bound pool of Cdc42. In this particular pool, Cdc42 utilizes Cav-1 as the GDI (25); such Cdc42-Cav-1 GTPase-GDI complexes have since been reported in multiple systems (6, 10, 24). One possible explanation for this preference of βPix for the membrane-localized pool of Cdc42 in the beta cell could be accounted for through caveolar scaffolding to couple βPix to Cav-1, or perhaps subcellular relocalization of βPix upon glucose stimulation. In support of the first possibility, βPix association with Cav-1 has been noted in regulating endothelin signaling through Gα (4). In consideration of the second possibility, GEF proteins are indeed commonly localized to cytosol under resting conditions, and then can undergo activation and translocate to the PM to catalyze exchange on the GTPase (2, 19, 22). As Cav-1 is not found in the cytosol, either βPix must translocate to the PM to interact with this pool of Cdc42 or only the βPix localized at the PM participates in the Cdc42 GDP-GTP exchange. Investigations exploring these two theories in beta cells are currently under way in our laboratory. Evidence of both, however, is reported in the literature. In epithelial cells and fibroblasts, βPix localized at the PM via interactions with GIT and paxillin participates in the activation of both Cdc42 and Rac1 following cell adhesion-induced integrin engagement, ultimately resulting in actin polymerization and cell migration (36). On the other hand, K+ stimulates recruitment of βPix from the cytosol to the PM in response to the tumor suppressor Scribble in neuroendocrine cells (1, 23). The mechanisms underlying βPix recruitment are not clearly understood and require further analysis. One possibility is that stimulus-induced phosphorylation triggers translocation of βPix to the PM. Feng et al. (8, 9) demonstrated that βPix is phosphorylated on Tyr442 in response to growth factors and/or Src-kinase signaling, and loss of Src activity resulted in a significant reduction in βPix phosphorylation.
Notably, Src kinase activity was also implicated by our data showing the inability of the Cav-1-Y14F mutant protein to compete with βPix for Cdc42 association, despite equivalent expression, since Cav-1Tyr14 is a known substrate for Src kinase (18). Indeed, Src kinase activity has previously been implicated in beta cell insulin release, and these data further suggest its action may be relayed through Cav-1 in the mechanism of Cdc42 cycling in the beta cell. In other cell types, the stimulus-induced tyrosine phosphorylation of Cav-1Tyr14 by Src kinase alters Cav-1 interaction with and action upon other molecules (13, 18, 28). Importantly, we found that the Cav-1-Y14F mutant bound poorly to Cdc42, suggestive of the importance of this site. Thus, future studies will test the model that Src kinase mediates tyrosine phosphorylation of βPix and/or Cav-1 in response to an upstream signal generated by glucose stimulation, which would induce the switch from Cdc42-Cav-1 to Cdc42-βPix binding to trigger Cdc42 activation. Given the pivotal importance of Cav-1 to the switch with βPix to evoke Cdc42 activation, it is highly relevant to note that the human Cav-1 gene is located in an obesity-related chromosomal region (29) and that aberrations in protein/mRNA expression of Cav-1 might serve as an early biomarker of beta cell dysfunction in obese and at-risk prediabetic individuals.
Recent progress has been made toward identification of upstream signals generated by glucose stimulation. Kowluru and colleagues (11) clearly demonstrated a requirement for early (1 min) activation of Arf6 (a GTPase of the Arf family of small G proteins) through ARNO (the GEF for Arf6) in islet beta cells to trigger the glucose-induced activation of Cdc42 and Rac1. Upon activation, Arf6 triggers phospholipase-D, which generates fusogenic lipid mediators, such as phosphatidic acid and phosphatidylinositol-4,5-bisphosphate. These lipid mediators promote the activation and translocation of Rac1 from the cytosol to the PM (21). Whether or not the fusogenic lipid mediators are involved in activation of βPix and/or Cdc42 is an attractive question for future investigations.
While, at first glance, it might seem odd that full loss of Cdc42 activation with βPix knockdown coincided with only a partial loss of GSIS, we speculate that this might be due to the limited role of its cognate GTPase Cdc42 only in second-phase secretion. This would also be the case were βPix found to participate in Rac1 activation or in scaffolding for Pak1, since both of these events are downstream of Cdc42 in GSIS (17, 39). However, to definitively place βPix in the pathway of second-phase secretion, islet perifusion studies of βPix-depleted islets are required. At present, this is limited by insufficient knockdown efficiency and the absence of a knockout mouse model of βPix. It is also important to note that while knockdown of βPix prevented an increase in glucose-stimulated Cdc42 activation, activated Cdc42 was still present. Given these data and that the islet beta cell utilizes at least two distinct GDIs to regulate Cdc42, it would be characteristic for more than one GEF protein to participate in Cdc42 regulation as well. In light of this, we are examining each additional GEF candidate identified as being expressed in MIN6 cells from our initial screen, as well as GEFs implicated in exocytotic mechanisms elsewhere. That βPix knockdown should exert a small yet significant reduction in basal secretion is fitting for its role as a Cdc42 activator, as this is the opposite functional effect of knockdown of the Cdc42 GDI, Cav-1 (25). Unlike the effects of Cav-1 knockdown, βPix knockdown and depression of basal secretion were not accompanied by a similar change in Cdc42 activation. This is likely a technical limitation of the assay itself, given the already minimalistic basal Cdc42 activation levels, quantitated at only ∼7% of the total Cdc42 population in MIN6 cells (27). However, since βPix reportedly functions in scaffolding and as a Rac1 GEF in other cell types (23, 32, 35), it remains a possibility that βPix may play an additional role beyond its Cdc42 GEF function in islet beta cells.
In conclusion, we report the identification of βPix as a sought-after GEF that mediates exchange of Cdc42 from its membrane associated Cav-1 GDI in the process of insulin secretion from pancreatic β-cells. Once activated, Cdc42 induces Pak1 phosphorylation and subsequent Rac1 activation in β-cells (11, 39). In addition to established importance of small GTPases in multiple rodent models of islet dysfunction (14), we have found that Cdc42 activation and Pak1 phosphorylation, in particular, are crucially important for sustaining insulin release from human islets (Z. Wang and D. C. Thurmond, unpublished data). Remarkably, this signaling cascade is utilized in incretin secretion (20), and incretins are well known for their ability to enhance sustained insulin release (38). More broadly, given the emerging prevalence of Cdc42-Cav-1 complexes across multiple systems (6, 10, 24), coupled with roles for Src kinase in mediating the regulation of multiple proteins within this triad, the apparent preference of βPix for this complex over other Cdc42-GDI complexes may represent a level of specificity for GEF usage not previously appreciated/recognized.
GRANTS
The following work was supported by grants from the National Institutes of Health (DK076614 and DK067912 to DCT; T32DK064466 to SMY; CTSI-KL2 RR025760 to ZW), the Showalter Trust of Indiana University School of Medicine (to EO) and the American Heart Association (10PRE3040010 to MAK).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Drs. Philippe Halban (University of Geneva) and Anjan Kowluru (Wayne State University) for their generous gifts of the pcDNA3-hGH and pcDNA3.1-hRhoGDIα plasmids, respectively. We also thank Dr. John Hutton (University of Colorado Health Sciences Center) for the MIN6 beta cell line. We thank Alexandra Ernst (Herman B Wells Center Summer Internship program), Wei Luo (now at GeneScript, Piscataway, NJ) and Alice Ke (now at the University of Washington, Seattle, WA) for technical assistance in with initial GST-Cdc42-GDP pulldown experiments, for generating the pcDNA3.1-Cav-1-Y14F-myc mutant, and with the initial GEF screens, respectively. Human islets were obtained through the Integrated Islet Distribution Program (IIDP).
This work fulfilled, in part, D. Kepner's requirements for her PhD degree, awarded posthumously from Indiana University.
REFERENCES
- 1. Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, Van Dorsselaer A, Vitale N, Borg JP. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 14: 987–995, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC. Membrane translocation of P-Rex1 is mediated by G protein betagamma subunits and phosphoinositide 3-kinase. J Biol Chem 282: 29967–29976, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bokoch GM. Biology of the P21-activated kinases. Annu Rev Biochem 72: 743–781, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Chahdi A, Sorokin A. The role of beta(1)Pix/caveolin-1 interaction in endothelin signaling through Galpha subunits. Biochem Biophys Res Commun 391: 1330–1335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H, Straub SG, Sharp GW. Inhibitory role of Src family tyrosine kinases on Ca2+-dependent insulin release. Am J Physiol Endocrinol Metab 292: E845–E852, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cheng ZJ, Singh RD, Holicky EL, Wheatley CL, Marks DL, Pagano RE. Co-regulation of caveolar and Cdc42-dependent fluid phase endocytosis by phosphocaveolin-1. J Biol Chem 285: 15119–15125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, Mirmira RG. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol 29: 2053–2067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA. Cool-1 functions as an essential regulatory node for EGFreceptor- and Src-mediated cell growth. Nat Cell Biol 8: 945–956, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Feng Q, Baird D, Yoo S, Antonyak M, Cerione RA. Phosphorylation of the cool-1/beta-Pix protein serves as a regulatory signal for the migration and invasive activity of Src-transformed cells. J Biol Chem 285: 18806–18816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grande-Garcia A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol 177: 683–694, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A. Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 beta-cells and rat islets. Biochem Pharmacol 81: 1016–1027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ke B, Oh E, Thurmond DC. Doc2beta is a novel Munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J Biol Chem 282: 21786–21797, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kimura A, Mora S, Shigematsu S, Pessin JE, Saltiel AR. The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J Biol Chem 277: 30153–30158, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kowluru A. Regulatory roles for small G proteins in the pancreatic beta-cell: lessons from models of impaired insulin secretion. Am J Physiol Endocrinol Metab 285: E669–E684, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev 31: 52–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest 98: 540–555, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose- and forskolin-induced insulin secretion in INS-1 beta-cells. Am J Physiol Endocrinol Metab 286: E818–E827, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem 271: 3863–3868, 1996 [PubMed] [Google Scholar]
- 19. Li Y, Asuri S, Rebhun JF, Castro AF, Paranavitana NC, Quilliam LA. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J Biol Chem 281: 2506–2514, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lim GE, Xu M, Sun J, Jin T, Brubaker PL. The Rho GTPase, Cdc42, is required for insulin-induced actin remodeling and glucagon-like peptide-1 secretion in the intestinal endocrine L cell. Endocrinology 150: 5249–5261, 2009 [DOI] [PubMed] [Google Scholar]
- 21. McDonald P, Veluthakal R, Kaur H, Kowluru A. Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS 832/13 cells. Am J Physiol Cell Physiol 292: C1216–C1220, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Michiels F, Stam JC, Hordijk PL, van der Kammen RA, Ruuls-Van Stalle L, Feltkamp CA, Collard JG. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and c-Jun NH2-terminal kinase activation. J Cell Biol 137: 387–398, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Momboisse F, Lonchamp E, Calco V, Ceridono M, Vitale N, Bader MF, Gasman S. βPIX-activated Rac1 stimulates the activation of phospholipase D, which is associated with exocytosis in neuroendocrine cells. J Cell Sci 122: 798–806, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Nethe M, Anthony EC, Fernandez-Borja M, Dee R, Geerts D, Hensbergen PJ, Deelder AM, Schmidt G, Hordijk PL. Focal-adhesion targeting links caveolin-1 to a Rac1-degradation pathway. J Cell Sci 123: 1948–1958, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Nevins AK, Thurmond DC. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J Biol Chem 281: 18961–18972, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Nevins AK, Thurmond DC. A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J Biol Chem 280: 1944–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol 285: C698–C710, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell 10: 975–986, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perusse L, Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Snyder EE, Bouchard C. The human obesity gene map: the 2004 update. Obes Res 13: 381–490, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. The cell physiology of biphasic insulin secretion. News Physiol Sci 15: 72–77, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Schlenker O, Rittinger K. Structures of dimeric GIT1 and trimeric beta-PIX and implications for GIT-PIX complex assembly. J Mol Biol 386: 280–289, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest 121: 2118–2125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spurlin BA, Thomas RM, Nevins AK, Kim HJ, Kim YJ, Noh HL, Shulman GI, Kim JK, Thurmond DC. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes 52: 1910–1917, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell 18: 2346–2355, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol 172: 759–769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomas A, Yermen B, Min L, Pessin JE, Halban PA. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J Cell Sci 119: 2156–2167, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Vilsboll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab 11 Suppl 3 11–18, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem 282: 9536–9546, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Z, Thurmond DC. Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion. J Biol Chem 285: 6186–6197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122: 893–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiseman DA, Kalwat MA, Thurmond DC. Stimulus-induced S-nitrosylation of syntaxin 4 impacts insulin granule exocytosis. J Biol Chem 286: 16344–16354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]