Abstract
Roux-en-Y gastric bypass surgery results in sustained decreases in food intake and weight loss. A key component is likely the direct delivery of nutrients to the jejunum and resulting changes in levels of gut peptide secretion. Prior work modeling this aspect of the surgery has shown that small-volume, prolonged jejunal infusions of linoleic acid (LA) produce sustained decreases in food intake and weight loss. LA infusions also significantly elevate plasma glucagon-like peptide-1 (GLP-1) levels. To assess a role for the increased circulating GLP-1 in the feeding suppression, we examined the effect of prolonged peripheral minipump administration of the GLP-1 receptor antagonist exendin 9–39 (Ex 9) on the feeding suppression produced by jejunal LA. Using a 2 × 2 design, we infused either saline or LA in the jejunum (7 h/day, 11.4 kcal) for 5 days with a subset of animals from each group receiving either saline or Ex 9 (25 pmol·kg−1·min−1) continuously via a minipump. The antagonist alone had no effect on food intake. LA reduced daily food intake greatly in excess of the kilocalories infused. Ex 9 completely blocked the feeding suppression produced by the jejunal LA infusion. Ex 9 also attenuated the increase in plasma GLP-1 induced by jejunal LA infusions. These data demonstrate that endogenous GLP-1 receptor signaling is necessary for the reduction in food intake produced by jejunal LA infusions. Whether increased secretion of additional gut peptides is also necessary for such suppressions remains to be determined.
Keywords: glucagon-like peptide-1, intrajejunal infusions, body weight
the increasing prevalence of obesity worldwide has resulted in a significant increase in secondary pathological disorders such as cardiovascular disease, stroke, and type 2 diabetes. Bariatric surgery approaches have the greatest success in decreasing body weight and alleviating many of the secondary consequences of obesity, and Roux-en-Y gastric bypass (RYGB) surgery has the greatest long-term efficacy among bariatric surgeries (25, 31). RYGB patients can also experience a remission of type 2 diabetes mellitus within days postsurgery before significant loss of body weight has occurred, suggesting a direct effect of the surgery on type 2 diabetes that is independent of weight loss (25, 28). Therefore, understanding the underlying mechanisms that are specific to the RYGB procedure to produce such effective decreases in body weight and the amelioration of obesity-related conditions may help to identify potential pharmacological targets to combat obesity.
RYGB is a complex surgical procedure involving both gastric restriction and upper intestinal bypass. Surgical manipulation of the gastrointestinal tract is performed so that a small pouch is formed in the cardiac region of the stomach and joined directly to the jejunum. When food is ingested, nutrients bypass most of the stomach and the duodenum to enter directly into the jejunum. The bypassed intestine is then joined to the midjejunum creating a common limb that is sufficient to prevent significant malabsorption (22). The independent contributions of the gastric restriction and the upper intestinal bypass to the efficacy of the procedure are not fully known. Gastric restriction alone, which occurs with gastric banding, is also efficacious for the treatment of obesity. However, the rate of weight loss is less than after RYGB, and the rapid effect of RYGB on insulin sensitivity does not occur with gastric banding (31). Together these findings suggest an independent contribution of the distal nutrient delivery that occurs with upper intestinal bypass.
Greater nutrient delivery to the lower intestine may accentuate the secretion of lower intestinal peptides, such as glucagon-like peptide-1 (GLP-1). GLP-1 has been shown to decrease food intake (6) and is considered an antidiabetic agent because it stimulates insulin secretion, inhibits glucagon secretion, and delays gastric emptying (13). Modeling the jejunal delivery of nutrients in laboratory rats with direct infusions in the jejunum decreases food intake and body weight and increases plasma GLP-1. Specifically, intrajejunal infusions of linoleic acid (LA) or glucose (23), two infusions that decrease food intake beyond their caloric content and result in significant decreases in body weight, elevate plasma GLP-1 beyond levels produced by saline control infusions. In contrast, plasma GLP-1 is not altered after casein hydrolysate infusions that do not change food intake or body weight. A role for the increase in GLP-1 after jejunal LA or glucose infusions in reducing food intake is consistent with the known ability of exogenous administration of GLP-1 or the long-lasting agonist exendin 4 (Ex 4) to decrease food intake (6, 27, 29, 35). Antagonists to the GLP-1 receptor are known to increase food intake under certain conditions (35) or alter insulin sensitivity (33). To assess the role of increased circulating GLP-1 in the feeding suppression of intrajejunal infusion of nutrients, we examined the effect of prolonged minipump administration of the GLP-1 antagonist exendin 9–39 (Ex 9) on the feeding suppression produced by LA infusions. Studying the physiological effects of lower intestinal infusions may provide a useful model for understanding the mechanisms underlying the propensity of RYGB to ameliorate many obesity-related conditions, including type 2 diabetes.
METHODS AND PROCEDURES
Animals.
A total of 32 (n = 8 for each of the 4 experimental groups) adult male Sprague-Dawley rats (Charles River) were included in the experimental analysis. Animals with an initial weight range of 300–325 g were housed individually in stainless steel wire mesh hanging cages and maintained on a 12:12-h light-dark cycle (lights off at 1100). All rats received ad libitum standard laboratory chow (3.3 kcal/g, Global Diet-2018; Harlan Teklad) unless otherwise specified. Water was available at all times during the experiment. All procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Jejunal cannulations.
Two days before surgery, rats were switched from a standard chow diet to liquid Ensure (Abbott Laboratories, Abbott Park, IL). For cannula implantations, rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg). The animals were shaved between the shoulder blades and over the abdominal area. The exposed skin was cleaned with iodine followed by alcohol. A laparotomy incision along the ventral midline was made to expose the gastrointestinal tract. The cecum was located, and 50 cm proximal to the ileocecal junction was measured to determine the location of the cannula insertion in the jejunum. The intestines were kept moist with sterile saline throughout the surgery. A polyurethane catheter (Micro-Renathane 065; Braintree Scientific, Braintree, MA) connected at one end to a piece of Silastic sheeting (Dow Corning, Midland, MI) was inserted in the jejunum. The catheter was secured with a purse-string suture (5–0 silk) around the point of entry, and the sheeting was secured to the intestinal wall with two knots. The other end of the catheter was threaded through an opening in the abdominal wall and then passed subcutaneously to an exit on the dorsal surface of the neck. It was secured to the underlying muscles with silk sutures and bard mesh (Davol, Warwick, RI). The exposed end of the tubing was capped with a catheter obturator. The animals were allowed to recover in their home cage for 5–7 days. They received 2 days of Ensure liquid diet to facilitate weight regain after surgery followed by standard laboratory chow for the remaining days. Cannula placement and viability was assessed after death by ensuring that the cannula insertion was 50 cm from ileocecal junction and that saline infused into tubing resulted in fluid properly entering the jejunum (no leakage of fluid in the tubing or at the insertion site) and traveling toward the distal part of the intestine.
Feeding tests.
After recovery from surgery, the rats were transferred and housed in AccuDiet food intake monitoring cages (13 in. by 9 in.; Accuscan Instruments, Columbus, OH). A powdered form of the standard laboratory chow (3.3 kcal/g, Global Diet-2018C; Harlan Teklad) and water were available ad libitum throughout the feeding tests unless otherwise specified. Polyurethane tubing (∼5 ft in length, MRE-065; Braintree Scientific) was connected to syringes on a multisyringe pump (Braintree Scientific). The solution to be infused (saline or LA) was filled into each syringe and the entire length of the tubing to ensure that there was no dead space. The end of the tubing opposite the syringe was connected to the exteriorized catheter of the animal with a stainless steel 19-gauge connector. A light-weight spring was placed over the exteriorized catheter and connected to the polyurethane tubing so that it protected the tubing from twisting and damage from the animal. The polyurethane tubing was secured to the ceiling of the test chamber using putty. This allowed the rats to freely move within the chamber and access the food cups. All rats received a jejunal infusion of 0.9% saline at a rate of 0.2 ml/h for 7 h beginning at lights out for 2 days. The animals were allowed to freely move within the chamber during the delivery of the infusate. Food intake was monitored continuously by the AccuDiet system for 22 h (2 h with no food access to collect data and to prepare for the next infusion cycle). This 2-day saline infusion period allowed the rats to habituate to the test chamber and infusion cycle. After this 2-day habituation period, the animals were implanted with the osmotic minipumps (see below) and allowed to recover. One additional day of saline jejunal infusions was done for all animals before a subset of the animals in each minipump condition (saline or Ex 9) were divided into two infusate groups based on average body weight and food intake. After this last day of saline jejunal infusions, one-half of the rats in each minipump group received jejunal infusions of either saline or LA (total caloric load of 11.4 kcal; Sigma-Aldrich, St. Louis, MO) for 5 days for 7 h beginning at lights out. These parameters were chosen based on prior research showing a reduction in food intake over a multiday infusion of an equal caloric load and duration of infusion of LA and oleic acid (7) and those we have used previously (8). Food intake was monitored continuously as described above. With the use of custom-designed software, food intake data were analyzed to determine meal patterns. Initiation of a “meal” was defined as ≥200 mg food consumed. The end of each meal was registered when there was >10 min without food intake.
Minipump implantation.
After recovery from jejunal cannula implantation, rats were separated into two weight-matched groups: saline minipump (n = 16) or Ex 9 minipump (n = 16). Under isoflurane anesthesia, rats were implanted with intraperitoneal osmotic pumps (Alzet, Cupertino, CA). Before implantation, pumps were filled with either Ex 9 (25 pmol·kg−1·min−1; American Peptide, Sunnyvale, CA) or saline and primed in saline at 37°C for 6 h. The pump released 1 μl of solution/h for 7 days (Alzet model 2001). The concentration of Ex 9 was determined by performing a dose-response curve for the peptide in a separate set of animals and by selecting the lowest concentration that blocked feeding inhibition by Ex 4 (10 μg/kg) on day 4 after osmotic minipump implantation. All rats were implanted intraperitoneally with osmotic minipumps that were attached to Lynch coils delivering saline for 2 days after the surgery, allowing for recovery of food intake and body weight before the infusion experiment began.
Plasma hormone assays.
Rats were decapitated after the 7-h infusion on the fifth and final day of LA or saline infusion. Trunk blood from each rat was collected in an EDTA-coated tube containing dipeptidyl peptidase-4 and aprotinin and maintained on ice until centrifuged at 3,000 rpm for 10 min. Standard radioimmunoassay kits (Millipore, St. Charles, MO) were used to determine plasma ghrelin (active) and peptide YY (PYY) (1–36 and 3–36) and processed according to the manufacturers' protocol. Plasma GLP-1 (active) was determined using an ELISA kit (Millipore) and processed according to the manufacturers' instructions.
Data analysis.
Food intake and body weight measures were analyzed using separate three-way repeated-measures ANOVAs with infusate (saline or LA) and minipump condition (saline or Ex 9) as the between-subject factors and time as the within-subject factor using Number Crunching Statistical Software (version 2000; NCSS, Kaysville, UT). Plasma peptide levels were analyzed using two-way ANOVAs (infusate × minipump). Newman-Keuls post hoc tests were used when appropriate. Differences among groups were considered statistically significant if P < 0.05.
RESULTS
Food intake.
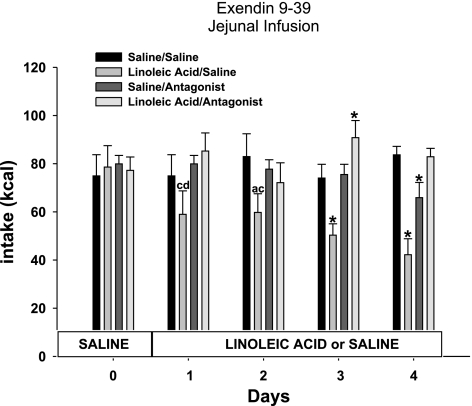
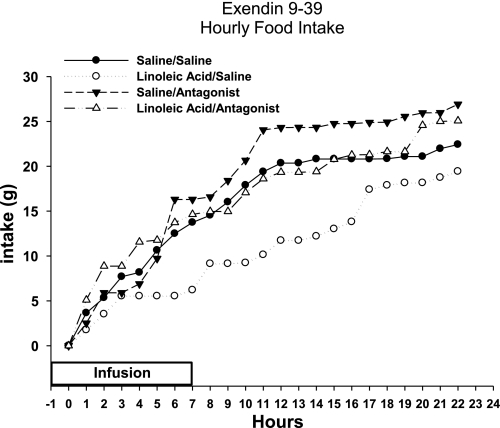
Total caloric intake was decreased significantly over the course of infusion days in animals that received jejunal infusions of LA compared with saline in the saline minipump condition [infusate F(1,28) = 8.2; minipump F(1,28) = 6.7; infusate × minipump F(1,28) = 7.8; P < 0.05; Fig. 1]. Post hoc tests revealed that infusions of LA significantly decreased the caloric intake on days 2, 3, and 4 of the infusions compared with saline infusions in the same minipump condition [infusate × time F(4,112) = 8.9; minipump × time F(4,112) = 5.6; P < 0.05; Fig. 1]. There was no interaction of infusate × minipump condition × time [F(4,112) = 2.1]. The suppression of food intake was significantly greater than the 11.4 kcal of LA infused, since the decrease in food intake remains significant even when the 11.4 kcal were added to the total caloric intake for the LA group (P < 0.05; Fig. 1). Ex 9 significantly blocked the LA infusion-induced decrease in food intake (P < 0.05; Fig. 1). A significant attenuation of the decrease in food intake by Ex 9 was seen on days 3 and 4 of jejunal LA infusions (P < 0.05; Fig. 1). When hourly intakes were analyzed on a representative infusion day (day 3) for each of the infusion conditions, food intake was suppressed during the infusion in the LA/saline minipump condition, and this suppression of intake persisted through the remainder of the dark cycle following infusion and throughout the subsequent light cycle (P < 0.05; Fig. 2). Ex 9 blocked this suppression in food intake throughout the infusion and noninfusion period in the LA/antagonist condition (P < 0.05; Fig. 2).
Fig. 1.
Daily caloric intake of animals with jejunal infusions of saline or linoleic acid combined with minipump administration of saline or exendin 9–39 (Ex 9). Values are means ± SE. The letters represent significant differences from other specific groups (P < 0.05). a, Saline/saline; b, linoleic acid/saline; c, saline/antagonist; d, linoleic acid/antagonist. *Significant difference from all other groups (P < 0.05).
Fig. 2.
Cumulative hourly food intake for animals with jejunal infusions of saline or linoleic acid combined with minipump administration of saline or Ex 9.
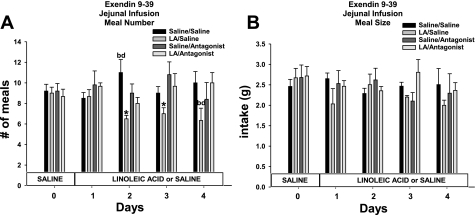
The decrease in total daily caloric intake in the LA/saline minipump condition was accounted for by a significant decrease in meal number across infusion days compared with animals infused with saline/saline minipump [infusate F(1,28) = 7.8; P < 0.05; Figs. 3A]. Ex 9 significantly blocked the decrease in meal number [minipump F(1,28) = 9.2; infusate × minipump F(1,28) = 5.6; P < 0.05; Fig. 3A]. There was no difference in meal size across the groups [infusate F(1,28) = 1.6; minipump F(1,28) = 1.2; infusate × minipump F(1,28) = 0.6; infusate × time F(4,112) = 1.2; minipump × time F(4,112) = 1.8; infusate × minipump × time F(4,112) = 0.6].
Fig. 3.
Daily no. of meals (A) and average meal size (B) for animals infused with saline or LA combined with minipump administration of saline or Ex 9. Values are means ± SE. The letters represent significant differences from other specific groups (P < 0.05). a, Saline/saline; b, linoleic acid (LA)/saline; c, saline/antagonist; d, LA/antagonist. *Significant difference from all other groups (P < 0.05).
Body weight.
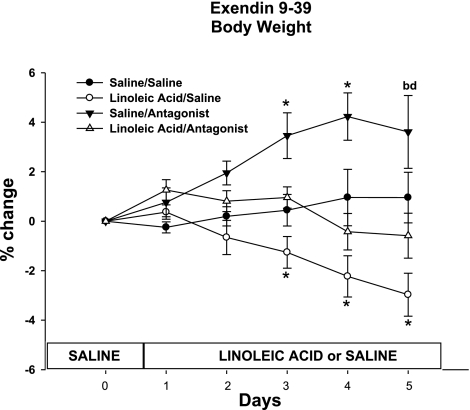
The average body weight for each group was similar before the start of the LA or saline infusions began on day 0 (saline/saline 397.6 ± 6.2, LA/saline 396.5 ± 8.3, saline/antagonist 401.8 ± 7.2, LA/antagonist 393.3 ± 6.95). There was a significant decrease in body weight in the LA/saline minipump condition compared with the saline/saline condition [infusate F(1,28) = 22.7; P < 0.05; Fig. 4]. Specifically, significant decreases in body weight on days 3, 4, and 5 were seen for the LA/saline minipump-treated animals compared with saline/saline-infused animals [infusate × time F(5,140) = 32.6; P < 0.05; Fig. 4]. This body weight difference was attenuated significantly by the LA/antagonist [minipump F(1,28) = 18.9; infusate × minipump F(1,28) = 26.8]. There was a significant increase in body weight for the saline/antagonist animals. Specifically, body weight was increased on days 3, 4, and 5 compared with all other groups [minipump × time F(5,140) = 21.8; infusate × minipump × time F(5,140) = 4.2].
Fig. 4.
Percent change of body weight from day 0 of animals infused with saline or LA combined with minipump administration of saline or Ex 9 (saline/saline 397.6 ± 6.2, LA/saline 396.5 ± 8.3, saline/antagonist 401.8 ± 7.2, LA/antagonist 393.3 ± 6.95). Values are means ± SE. The letters represent significant differences from other specific groups (P < 0.05). a, Saline/saline; b, LA/saline; c, saline/antagonist; d, LA/antagonist.*Significant difference from all other groups (P < 0.05).
Plasma hormones.
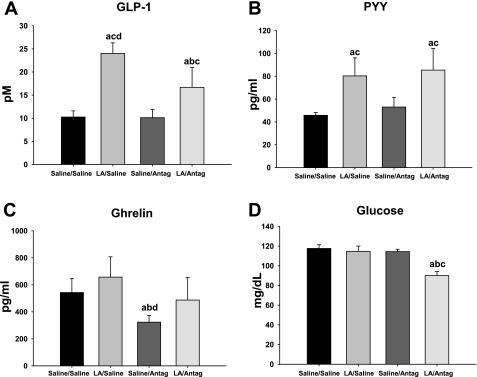
Plasma GLP-1 levels were increased significantly in the LA/saline minipump condition compared with all other groups [infusate F(1,28) = 6.2; minipump F(1,28) = 8.3; infusate × minipump F(1,28) = 7.8; P < 0.05; Fig. 5A]. Although the plasma GLP-1 levels were also increased in the LA/antagonist condition, Ex 9 attenuated this increase compared with LA/saline (P < 0.05; Fig. 5A). Plasma PYY levels were increased significantly in the LA-infused animals compared with the saline-infused controls in each minipump condition [infusate F(1,28) = 7.2; P < 0.05; Fig. 5B]. There was no significant difference in the antagonist compared with the saline minipump condition in either infusion condition [minipump F(1,28) = 3.6; Fig. 5B]. Plasma ghrelin levels were decreased significantly in the saline/antagonist animals compared with all other groups [infusate F(1,28) = 8.2; minipump F(1,28) = 6.6; infusate × minipump F(1,28) = 5.2; P < 0.05; Fig. 5C]. Plasma glucose levels were decreased significantly in the LA/antagonist condition compared with all other groups [infusate F(1,28) = 5.6; minipump F(1,28) = 6.7; infusate × minipump F(1,28) = 6.1; P < 0.05; Fig. 5D].
Fig. 5.
Plasma glucagon like peptide-1 (GLP-1; A), peptide YY [PYY-(3–36); B], ghrelin (active; C), and glucose (D) for animals infused with saline or LA combined with minipump administration of saline or Ex 9. Values are means ± SE. The letters represent significant differences from other specific groups (P < 0.05). a, Saline/saline; b, LA/saline; c, saline/antagonist; d, LA/antagonist.
DISCUSSION
To assess a role for increased circulating GLP-1 in the feeding suppression produced by jejunal infusions of LA, we tested if chronic blockade of the GLP-1 receptor using prolonged minipump administration of the GLP-1 antagonist Ex 9 would block the decrease in food intake after LA infusion in laboratory rats. As we have seen in our previous study (8), LA reduced daily food intake greatly in excess of the kilocalories infused. Ex 9 completely blocked the feeding suppression produced by the jejunal LA infusion. The antagonist had no effect on food intake in the saline infusion condition. These data demonstrate that endogenous GLP-1 receptor signaling is necessary for the reduction in food intake produced by jejunal LA infusions.
GLP-1 and Ex 4 have been shown to decrease food intake, but studies investigating the blockade of the GLP-1 receptor using Ex 9 have shown mixed results. In one study, Ex 9 increased food intake at 1 and 2 h after injection when administered during the light phase or at 1 h after injection when administered 1 h after the onset of the dark phase, but it had no effect on food intake when administered immediately before the onset of the dark phase (35). Ex 9 also attenuates the decrease in food intake after a glucose preload when intake was measured 2 h after intraperitoneal injection of the antagonist (35). When cumulative food intake is measured after a single dose of Ex 9, there is no effect on food intake 24 or 48 h after intraperitoneal injection (24). In the present study, the dose of antagonist employed did not affect food intake when animals were infused with saline in the jejunum. Only when additional nutrients from the LA infusion were given did we see an attenuation of the LA-induced decrease in food intake in the animals treated with Ex 9. In both the present and our past study (8), plasma GLP-1 levels are increased significantly in the LA infusion animals compared with the saline-infused animals. Ex 9 may only be inhibiting the effect of this additional nutrient-driven release of GLP-1. This increase in circulating GLP-1 is attenuated in the LA/antagonist condition compared with LA/saline-treated animals. The idea that Ex 9 plays a greater role in inhibiting GLP-1-induced decreases in food intake is supported by Ruttimann et al. (26). They found that intrameal intraperitoneal infusions of Ex 9 prevented the satiating effect of exogenous GLP-1 administration but did not affect the intake of the first meal when infused alone (26). Thus, it appears that the effectiveness of Ex 9 to block the inhibition of food intake occurs only under conditions when GLP-1 levels are known to be high.
Ex 9 significantly blocked the LA infusion-induced decrease in meal number and increased meal size in the present study. The effect of jejunal infusions of LA to alter meal number is a replication of our previous finding (8). Typically, though, the decreases in food intake elicited by exogenous GLP-1 or Ex 4 are attributed to decreases in meal size, not meal number (6, 27, 29, 35). In these other reports, GLP-1 or Ex 4 were given as an exogenous single bolus (35), 2.5–5 min intraperitoneally or hepatic portal vein infusions (27), 3-h intravenous infusion cycles (6), or multiday injections (29), whereas the present 7-h intrajejunal infusion may trigger an endogenous response to infusates that may not be mimicked by these exogenous administration cycles. The reported effects of RYGB in rats on meal patterns have been inconsistent. In a Sprague-Dawley rat model of RYGB fed a chow diet that are known to have increases in plasma GLP-1 levels over multiple days, there were no significant differences in meal size or meal frequency (10). Obese Zucker rats with RYGB show a decrease in meal size with no change in meal frequency 5–10 days after surgery but no change in either parameter 11–19 days after RYGB (36). Animals that had undergone RYGB also have a reduced stomach size combined with the intestinal bypass. The immediate reduction in stomach size could be contributing to the short-term reduction in meal size after surgery. Nonetheless, an investigation of the timing of elevation in physiological GLP-1 levels could lead to a better understanding of how GLP-1 could potentially trigger a differential decrease in meal number and/or meal size. It could also be the site of action that determines the GLP-1 effect on meal patterns because activation of central GLP-1 receptors decreases food intake by a reduction in meal number, not through an alteration in meal size (11). Even though the infusion of LA in the present study is triggering a myriad of responses that together may produce the present meal pattern, it appears that altering the timing of effect of GLP-1 and/or the site of action may also determine differential feeding patterns.
The chronic antagonist preparation used in the present study would likely affect GLP-1 receptors systemically, but little is known about the exact mode of action of the antagonist to effect food intake and body weight. Ex 9 is a truncated form of Ex 4 and is similar to Ex 4 in that it is not quickly degraded. Although Ex 9 blocks the GLP-1-induced decreases in food intake when both receptor agonist and antagonist are delivered centrally or peripherally, third ventricular administration of Ex 9 does not block peripheral GLP-1-induced decreases in food intake (35). This limitation of Ex 9 to only block locally administered GLP-1 may have more to do with the quick degradation of GLP-1 and not explain the mechanism of Ex 9 to induce changes in food intake and body weight over a longer term. Centrally administered Ex 9 does attenuate the inhibitory effect on food intake of two longer-lasting agonists, Ex 4 and liraglutide, after intraperitoneal injection in rats (15). There is evidence that both of these GLP-1 agonists are able to cross the blood-brain barrier to have a direct effect on central GLP-1 receptors, but the lack of a complete blockade of the inhibitory effect of Ex 4 or liraglutide suggests that mechanisms in addition to central receptors play a role (16, 20). The site of action of continuous intraperitoneal infusion of Ex 9 to block the inhibitory effect of jejunal infusion of LA is not known. The results from multiple studies show that Ex 9 does not appear to cross the blood-brain barrier. Intravenous injection of radioactivity-labeled Ex 9 did not result in any uptake by the brain in mice (3). Peripheral injection of Ex 9 also did not antagonize the effect of central GLP-1 to decrease food intake or alter cardiovascular parameters (4, 35). Thus, the action of Ex 9 may solely be on peripheral GLP-1 receptors. The administration of Ex 9 in the intraperitoneal cavity allows for access to many organ-specific GLP-1 receptors, but it is not clear from our results the specific site of action (see below for further detail on the physiological site of action of endogenous GLP-1). Alterations in GLP-1 receptor function (i.e., internalization of receptor or alterations in receptor expression) after acute or chronic antagonism have not been studied, but these changes may also contribute to the effect of Ex 9 in the present study. The functional significance of Ex 9 binding to GLP-1 receptors is further complicated by a report stating that Ex 9 is capable of acting as an inverse agonist on GLP-1 receptors (30).
There is evidence in both rodents and humans to suggest that GLP-1 or other receptor agonists decrease energy expenditure (2, 9, 12, 18). We have found that multiday injections of Ex 4 in rats decrease food intake and body weight, but the decreases in body weight are not as pronounced as pair-fed animals (unpublished data). GLP-1-mediated decreases in body mass may thus be separate from those mediating the decreases in food intake. In the present study, Ex 9 is producing greater increases in body mass in animals infused with saline compared with animals infused with LA. This suggests that Ex 9 may be blocking the effect of endogenous GLP-1 to decrease body mass. It is not clear if this is a response that is specific to our model because there is no effect of a single intraperitoneal injection of Ex 9 on body weight when measured 24 and 48 h after administration (24). In contrast, repeated central injections or chronic central minipump administration of Ex 9 in rodents also increases body mass (5, 21). Yamamoto et al. (37) have suggested that peripheral GLP-1 may mediate changes in the autonomic nervous system through central nuclei. It may be the case that alterations in energy expenditure, independent of food intake, are mediated centrally and require longer-term modulation of the GLP-1 system.
It is not clear how endogenous GLP-1 may be mediating decreases in food intake and body weight. It is thought that GLP-1 is released in response to nutrients lower in the intestine [a non-nutrient-stimulated release has also been postulated (35)]. Because GLP-1 is degraded rapidly by dipeptidyl peptidase IV after its release, the mode of action is probably not hormonal. Evidence supports the notion that endogenously secreted GLP-1 from the gastrointestinal tract suppresses intake by acting in a local-humoral and/or paracrine-like fashion on adjacent GLP-1 receptors expressed on vagal afferents innervating that hepatoportal bed and gastrointestinal tract, respectively (1, 27, 36). A direct effect of GLP-1 on vagal afferents in the lamina propria of the intestinal tract has been postulated by Lu et al. (19) because GLP-1 receptors are colocalized with these fibers. Vagotomy or vagal afferent damage with capsaicin also blocks the feeding inhibition triggered by peripheral GLP-1 or Ex 4 (1, 14, 32). Although our intraperitoneal route of administration of Ex 9 may represent a more physiological profile of action for the GLP-1 receptor-mediated effects on food intake, it is not clear if the multiday use of the antagonist has effects that extend beyond the proposed paracrine mode of action of GLP-1.
RYGB impacts a variety of neuroendocrine controls of ingestion (e.g., amylin, PYY), but GLP-1 has been implicated as one of the mediators of the long-term decreases in food intake and body weight. Our data speak to the necessity of the increase in GLP-1 in mediating the feeding inhibitory effect of jejunal LA. It is very possible that increases in other peptides are also contributing to this response and that only in their combination are they effective. Future experiments could directly assess the roles of other gut peptides using specific antagonists.
RYGB permanently disrupts the normal passage of nutrients with more undigested nutrients reaching lower in the intestine. The L cells of the lower intestine are directly responsive to intraluminal nutrients and thus may secrete specific neuropeptides in proportion to the amount of nutrients present. Greater postprandial increases in plasma GLP-1 occur in patients postsurgery compared with levels before RYGB surgery. Previous studies have shown that obese individuals who have undergone RYGB show a rapid and sustained increase in postprandial GLP-1 secretion that is greater in magnitude than that seen in either obese patients who have undergone gastric banding or obese controls with no surgical intervention (17). The underlying mechanism that accounts for such an altered postprandial GLP-1 response following RYGB is still not known, but our results would suggest that increased nutrient-driven increases in GLP-1 seen after RYGB gastric bypass could be a primary mediator decreasing food intake and body weight. Identification of the mechanisms underlying the effectiveness of RYGB could eventually lead to the development of pharmacological or behavioral tools that do not require surgery or at least less invasive surgery.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-19302.
DISCLOSURES
There are no conflicts of interest to declare.
REFERENCES
- 1. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 127: 546–558, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther 309: 469–475, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol Endocrinol Metab 277: E784–E791, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288: R1695–R1706, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cox JE, Tyler WJ, Randich A, Kelm GR, Bharaj SS, Jandacek RJ, Meller ST. Suppression of food intake, body weight, and body fat by jejunal fatty acid infusions. Am J Physiol Regul Integr Comp Physiol 278: R604–R610, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Dailey MJ, Tamashiro KL, Terrillion CE, Moran TH. Nutrient specific feeding and endocrine effects of jejunal infusions. Obesity (Silver Spring) 18: 904–910, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes Relat Metab Disord 24: 288–298, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Furnes MW, Stenstrom B, Tommeras K, Skoglund T, Dickson SL, Kulseng B, Zhao CM, Chen D. Feeding behavior in rats subjected to gastrectomy or gastric bypass surgery. Eur Surg Res 40: 279–288, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holst JJ. Enteroglucagon. Annu Rev Physiol 59: 257–271, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol 273: G920–G927, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27: 313–318, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3: 597–601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50: 2530–2539, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol 293: G963–G971, 2007 [DOI] [PubMed] [Google Scholar]
- 20. McClean P, Fung K, McCurtin R, Gault V, Holscher C. Novel GLP-1 analogues cross the blood brain barrier: a link between diabetes and Alzheimer's disease. Soc Neurosci Meeting, Chicago, IL 2009, p. 528–529 [Google Scholar]
- 21. Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140: 244–250, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Naslund I. Gastric bypass versus gastroplasty. A prospective study of differences in two surgical procedures for morbid obesity. Acta Chir Scand Suppl 536: 1–60, 1987 [PubMed] [Google Scholar]
- 23. Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 27: 2464–2469, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Nowak A, Bojanowska E. Effects of peripheral or central GLP-1 receptor blockade on leptin-induced suppression of appetite. J Physiol Pharmacol 59: 501–510, 2008 [PubMed] [Google Scholar]
- 25. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, de Ramon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222: 339–350, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav 100: 291–296, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238: 467–484, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293: R983–R987, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Serre V, Dolci W, Schaerer E, Scrocchi L, Drucker D, Efrat S, Thorens B. Exendin-(9–39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′,5′-monophosphate levels and beta-cell glucose competence. Endocrinology 139: 4448–4454, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Tseng CC, Zhang XY, Wolfe MM. Effect of GIP and GLP-1 antagonists on insulin release in the rat. Am J Physiol Endocrinol Metab 276: E1049–E1054, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Williams DL. Expecting to eat: glucagon-like peptide-1 and the anticipation of meals. Endocrinology 151: 445–447, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu Y, Ohinata K, Meguid MM, Marx W, Tada T, Chen C, Quinn R, Inui A. Gastric bypass model in the obese rat to study metabolic mechanisms of weight loss. J Surg Res 107: 56–63, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23: 2939–2946, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]