Abstract
Intestinal homeostasis is regulated in part by the single cell layer of the mucosal epithelium. This physical barrier is a prominent part of the innate immune system and possesses an intrinsic ability to heal damage and limit infection. The restitutive epithelial migration phase of healing requires dynamic integrin adhesion to the extracellular matrix. Previously, we have shown that the homeostatic chemokine CXCL12 utilizes intracellular calcium to increase enterocyte migration on laminin. The aim of these studies was to investigate integrin specificity and, in turn, functional responses elicited by CXCL12 stimulation. Analysis of cellular adhesion and spreading revealed CXCL12 preferentially activated laminin-specific integrins compared with collagen IV-binding integrins. Laminin-specific cell adhesion and spreading elicited by CXCL12 was dependent on intracellular calcium. CXCL12 increased activated β1-integrins on the surface of epithelial cells compared with untreated cells. RT-PCR confirmed expression of the laminin-binding integrins-α3β1, -α6β1, and -α6β4. Interestingly, shRNA-mediated depletion of laminin-specific α3- or α6-integrin subunits revealed differential functions. α3-Integrin knockdown reduced basal as well as inducible restitution. Depletion of α6-integrin specifically abolished CXCL12-stimulated, but not TGF-β1 or basal, migration. Depletion with either shα3-integrin or shα6-integrin prevented CXCL12-evoked cell spreading. Our data indicate that CXCL12 stimulates the inside-out activation of laminin-specific integrins to promote cell migratory functions. Together, our findings support the notion that extracellular mediators within the gastrointestinal mucosa coordinate cell-matrix interactions during epithelial restitution.
Keywords: restitution, adhesion, CXCR4, epithelial remodeling
the single layer of rapidly renewing intestinal epithelial cells lining the gastrointestinal tract provides a physical barrier between potentially harmful digestive contents and the body's interior (47, 48). Damage to this barrier may result from normal digestive processes or injury during inflammation. The intestinal epithelium possesses an innate ability to reseal wounds. This process consists of 1) restitution, identified by stages of cell spreading and migration into the wound, 2) proliferation to repopulate the denuded surface, and 3) differentiation of epithelial cells into a polarized monolayer (32, 37, 40, 45). Restitution occurs within minutes to hours after injury and is therefore minimally influenced by proliferation (32, 42). This inherent ability to migrate across the injured mucosa, a process we classify as basal restitution, can be increased in vitro by multiple growth factors including transforming growth factor (TGF)-β1, epidermal growth factor (EGF), trefoil factor (4, 12, 15, 41, 45) and, as we have shown, the chemokines CXCL12 and CCL20 (1, 43, 56, 61). Restitution occurs along the exposed and newly deposited basement membrane (32, 45) and requires dynamic cellular adhesion and cytoskeletal remodeling within the moving enterocyte (10, 39, 44, 50).
Epithelial cells are anchored to the basement membrane via a unique, highly specialized meshwork primarily composed of the extracellular matrix (ECM) proteins laminin, collagen IV, nidogen, and perlecan (67). Stromal ECM proteins are recognized and bound by integrin proteins localized within the epithelial cell membrane. Integrin functions reflect spatial and temporal regulation between active and inactive protein conformations. Integrin receptor specificity for ECM ligand is determined by the α- and β-subunit composition of the heterodimeric protein (26, 27). Integrin expression within the intestinal epithelium has been shown to vary, depending on cell maturity, differentiation, and position along the crypt-villus axis (2, 3, 6, 8, 14, 19, 36). Mirroring the differential integrin expression by epithelial cells, the underlying basement membrane components deposited by the epithelium and stromal fibroblasts show distinct gradients along the crypt-villus axis (6, 7, 36, 46, 55, 57). The coordinated and parallel expression pattern of ECM-ligand with integrin-receptor suggests the ordered use of different ligand-receptor pairs as epithelial cells migrate along from the crypt to villus apex. In vitro, basal restitution and cell adhesion are enhanced by the presence of ECM proteins, with collagen isoforms promoting the highest migration and adhesion followed by laminin and fibronectin (4). Furthermore, the intrinsic migration potential inherent to restitution is increased when intestinal epithelial cells are cultured on laminin (1). Together, these data support a model in which ECM proteins regulate migration through outside-in integrin signaling. Although an abundance of data indicate that extracellular stimulants enhance epithelial migration (1, 4, 12, 15, 41, 43, 45, 56, 61), roles for those effectors in inside-out regulation of integrins and cell-matrix interactions during restitution remain poorly characterized.
Chemokines have established roles in immune cell trafficking during inflammation and directing circulating immune cells to tissue sites of infection. Chemokines accumulate on glycosaminoglycans along the endothelial surface, resulting in activation of lymphocyte integrins, leading to stable adhesion, cell spreading, and eventually diapedesis of these cells (65). Intracellular signal transduction elicited by nonintegrin extracellular mediators can result in integrin activation. This type of integrin activation is termed inside-out activation (21). Stimulation with CXCL12, as well as other chemokines, has been shown to activate multiple diverse integrin heterodimers (25, 29, 53, 54). Inside-out integrin activation uses the potent second messenger calcium and the downstream small GTPase effector Rap1 to transduce the intracellular signal into a conformational change of the receptor to a high-affinity state (9, 13, 24, 29, 54). Chemokine receptors are not restricted to hematopoietic cells and are expressed by intestinal epithelial cells (18). We have previously verified that restitution of model intestinal epithelia is increased on laminin, a migration pattern that was further enhanced following stimulation with CXCL12, but not TGF-β1 (1). In addition, CXCL12-induced migration was dependent on intracellular calcium (1). CXCL12 stimulation enhances basal restitution and therefore could be a potential therapeutic for intestinal wound repair. However, the mechanism detailing how laminin and CXCL12 work together during restitution remains undefined. We hypothesized that laminin-specific integrins on the intestinal epithelial cell surface are activated by CXCL12 during restitution. We found that CXCL12 activated epithelial cell surface β1-integrins and evoked laminin-specific integrin-mediated adhesion and spreading. Chelation of intracellular calcium blocked CXCL12-laminin-specific cell adhesion and spreading. Lastly, depletion of laminin integrins-α3 and -α6 differentially regulated chemokine-evoked cell spreading and migration. These data establish for the first time that the extracellular ligand CXCL12 orchestrates enhanced enterocyte restitution through inside-out activation of defined laminin-specific integrins.
MATERIALS AND METHODS
Materials.
Purified laminin and collagen IV were obtained from Sigma (St. Louis, MO) and R&D Systems (Minneapolis, MN), respectively. Recombinant human CXCL12 was produced and purified from Escherichia coli, as previously defined (60). TGF-β1 was from R&D Systems, whereas EGF was purchased from Calbiochem (San Diego, CA). The cell-permeant dye calcein-AM was obtained from Invitrogen (Carlsbad, CA). BAPTA-AM was purchased from Sigma. Mouse antibodies specific for α3-integrin (CD49c) or β1-integrin (CD29), as well as rat IgG2a, were obtained from BD Transduction Laboratories (Bedford, MA). Antibodies against α6-integrin (CD49f), TGF-β receptor 1 (TGFβR1) and GAPDH were purchased from Cell Signaling (Danvers, MA). A polyclonal antibody specific for CXCR4 (PC390) was obtained from Calbiochem. Antibodies specific for α1- and β4-integrins, CD49a and CD104, respectively, were acquired from Abcam (Cambridge, MA) and Bioworld Technology (St. Louis Park, MN). Purified hamster anti-rat β1-integrin and hamster IgM, as well as phycoerythrin-conjugated anti human active-β1-integrin (clone HUTS-21) (35) and mouse IgG2a isotype, were purchased from BD Pharmingen (San Diego, CA). The rat anti human α6-integrin functional blocking antibody GoH3 was purchased from Millipore (Temecula, CA).
Cell culture.
The nontransformed rat small intestinal epithelial cell line IEC-6 was purchased and maintained as previously described (1, 56). The human colonic carcinoma cell line Caco2 subclone BBe (Caco2-BBe) was a kind gift from Dr. Jerrold Turner (University of Chicago) and was maintained in DMEM (4 g/l glucose) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Omega Scientific, Tarzana, CA), 2 mM l-glutamine, and 15 mM HEPES (Invitrogen). Cells were cultured at 37°C in a 5% CO2 atmosphere.
ECMs.
Tissue culture dishes were coated with 10 μg/ml of laminin or collagen-IV. ECM proteins diluted in divalent cation containing PBS (PBS++) were added to tissue culture dishes incubated 2 h at 37°C, washed with PBS++, blocked with 1% (wt/vol) BSA for 30 min at 37°C, and washed twice with PBS++. Plates were used immediately or stored overnight at 4°C.
Cell adhesion assay.
Approximately 2 × 106 IEC-6 cells were cultured for 24 h in 10-cm dishes. The medium was removed and exchanged for serum-free media for 24 h. The cells were incubated with a calcein-AM/PBS++ solution for 20 min at 37°C to load the fluorescent dye into the cells. The calcein-AM solution was removed, and the cells were released from the tissue culture plate using 5 mM EDTA at 37°C for 10 min. Cells were collected using PBS++ and washed three times with serum-free media buffered with 25 mM HEPES (SF-HEPES media). Cells were counted using a hemocytometer, and 600,000 cells/ml were resuspended in SF-HEPES media and incubated at 37°C for 10 min. Serial dilutions of calcein-AM-loaded cells were added in parallel with samples to a matrix-coated 96-well plate. Sample wells received 30,000 cells/well plus CXCL12 (2.5 nM) or no treatment. The cells were incubated at 37°C for 1 h in a 10% CO2 incubator and then rinsed three times with PBS++. The number of adherent cells was quantified by measuring fluorescence (excitation: 485 nm; emission: 535 nm) for 1 s using Victor Wallac (Perkin-Elmer, Waltham, MA) and calculated from the standard curve. In separate assays, intracellular calcium was chelated using 10 μM BAPTA-AM loaded simultaneously with the calcein-AM. To neutralize integrin functions, 5 μg/ml of hamster anti-rat β1-integrin or, as a control, hamster IgM isotype antibodies were included during 10-min pretreatment and during the assay.
Cell-spreading assay.
IEC-6 cells were cultured and serum starved as defined in the adhesion assay. Cells were removed from the 10-cm dish using 5 mM EDTA for 10 min at 37°C and collected with PBS++. Cells were washed three times with SF-HEPES media, counted, and resuspended to 300,000 cells/ml. The cells were incubated 20 min at 37°C in the presence of 2.5 nM CXCL12, 50 ng/ml EGF, or left untreated as a control. Matrix-coated 96-well plates were prepared by placing 50 μl PBS++ containing specific treatments into the wells and incubating at 37°C in a 10% CO2 incubator to buffer the PBS++. Fifteen thousand cells/well were added to the buffered PBS++ solution and incubated 45 min at 37°C in 10% CO2. Nonadherent cells were decanted, and adherent cells were fixed with 2% (wt/vol) paraformaldehyde. Photomicrographs were taken using a Nikon Eclipse Ti (Nikon Instruments, Melville, NY) inverted microscope and a ×200 objective. The area of 50 cells/well was measured using the NIS-Elements AR 3.10 software package.
RT-PCR analysis.
Total RNA was isolated using the Trizol Reagent (Invitrogen) and precipitated before DNAse1 treatment to remove any contaminating DNA. A sample (2 μg) of total RNA was converted to cDNA using SuperScript III reverse transcriptase (Invitrogen) and random hexamers (Fermentas, Glen Burnie, MD). PCR was performed with primers listed in Table 1 to amplify RNA transcripts specific for integrin subunits. Reactions and conditions for β-actin amplification were completed as defined previously (18). Cycling conditions were as follows: single-step denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 30 s, annealing at 56–63°C for 30 s, extension at 72°C for 30 s, then final extension at 72°C for 7 min. PCR amplicons were resolved in a 1.2% agarose gel, visualized with eithidium bromide and photographed.
Table 1.
Oligonucleotide primers for RT-PCR amplification of integrin subunits
| Integrin Subunit | Sense | Antisense | Size of PCR Product, bp |
|---|---|---|---|
| α1 | 5′-CTATATTGCTGGACAGCCTCGGTAC-3′ | 5′-GGTTCCAGGCTCATTTGATATTC-3′ | 279 |
| Human-α2 | 5′-CTGGAGTGGGACCATTGTCCAG-3′ | 5′-CACTATCTGGCCGGTATAATTTG-3′ | 190 |
| Rat-α2 | 5′-GATTTAAATGGGGACTCCATCA-3′ | 5′-GGTCAGCATCAAGTGTCATGTT-3′ | 238 |
| α3 | 5′-CTGGCAGACCTGAACAATGATG-3′ | 5′-ATCCATCCTGGTTGATGTCACC-3′ | 214 |
| α5 | 5′-CCAGACTTCTTTGGCTCTGC-3′ | 5′-GTTGAACATGGAGGGGAAGA-3′ | 165 |
| α6 | 5′-GTCACCTTTGACACCCCAGATC-3′ | 5′-CAACTGTACCTCCAAAATACACC-3′ | 166 |
| α7 | 5′-GCTGTGACTGACCTCAACAGTG-3′ | 5′-CTCTTGATACCCACAGCCTCAC-3′ | 341 |
| α8 | 5′-CAGTTTGGACGAATCCACCT-3′ | 5′-TGCTGTCTGGATTGTCCTTG-3′ | 500 |
| α9 | 5′-GCAGATTCCAAATACAGC-3′ | 5′-TGTTCCTCTCCGTACTTCTT-3′ | 365 |
| α11 | 5′-GGCTCCAACAGCATCTACC-3′ | 5′-CGTGCAAATTCAATGCCAAATGC-3′ | 239 |
| αV | 5′-TCAATGAAAGGAGCYACAGA-3′ | 5′-TTGCCATCTGCCTTTAAGC-3′ | 234 |
| β1 | 5′-TACACTGGCAGTGCATGTGAC-3′ | 5′-GAAGGCTCTGCACTGAACAC-3′ | 204 |
| Human-β4 | 5′-GGCGCTGCAACACCCAGGCGGA-3′ | 5′-CTCTCCAGTGGCTCAAACACCT-3′ | 208 |
| Rat-β4 | 5′-TTTTCCAACTCCATGTCTGATG-3′ | 5′-CCCAGAATTCTTCCACATTCTC-3′ | 241 |
Flow cytometric analysis of active-β1-integrin.
Approximately 2 × 106 Caco2-BBe cells were grown for 24 h in 10-cm dishes followed by a 24-h serum starvation. Cells were rinsed with calcium-magnesium-free PBS and released from tissue culture dishes using 10 mM EDTA for 15 min at 37°C. Cells were collected by centrifugation and rinsed three times with PBS++ and resuspended in serum-free media. The cells were treated and incubated 1 h at 37°C with gentle agitation. Maximal β1-integrin activation was chemically induced by addition of 1 mM MnCl2. Cells were transferred to fluorescence-activated cell sorting tubes, centrifuged, and washed in PBS++/ 0.5% (wt/vol) BSA. Cells were incubated 1 h at 37°C with mouse anti-human active-β1-integrin-conjugated phycoerythrin antibody and washed three times in PBS++/BSA solution. Cells were resuspended in ice-cold PBS++ containing 1.25 μg/ml 4,6-diamidino-2-phenylindole and analyzed on a BD-LSR II flow cytometer (BD Biosciences, San Jose, CA). Cells were gated on the live cell population, and the mean fluorescence intensity was determined for each treatment.
shRNA construction.
shRNA targeting rat α3-integrin was designed, and viral particles were generated using the LentiLox 3.7 (LL3.7) system (Addgene, Cambridge, MA). Rat-specific α3-integrin shRNA oligonucleotide sequences were: 5′-tGCTACATGATTCAGCGGAAttcaagagaTTCCGCTGAATCATGTAGCtttttggaaac-3′ and 5′-tcgagtttccaaaaaGCTACATGATTCAGCGGAAtctcttgaaTTCCGCTGAATCATGTAGCa-3′. Oligonucleotides were annealed and cloned into the HpaI/XhoI digested pLL3.7-green fluorescent protein (LL3.7-GFP) expression vector. Plasmid orientation was verified by PCR and DNA sequencing. A plasmid stock containing a puromycin selectable shRNA specific for rat and human α6-integrin (RHS3979–9624958) in the pLKO.1 backbone was obtained from Open Biosystems (Huntsville, AL).
Lentiviral production.
Control LL3.7 or LKO.1 plasmids separately encoded an empty or scrambled sequence. Control and rat-specific shα3-integrin LL3.7 vectors or shα6-integrin pLKO.1 vectors were individually transfected with the accessory plasmids, pVSVG, pREV, and pRRE, into HEK293T cells using TransIT-293 transfection reagent (Mirus, Madison, WI) as previously described (1).
Cell migration and live cell imaging.
Wild-type or stably transduced IEC-6 cells were cultured in 60-mm dishes until confluent and then serum starved for 24 h. Monolayers were wounded with a sterile razor blade and incubated in serum-free medium alone or with the indicated stimuli for 18 h at 37°C, as described previously (1, 43, 56). Six photomicrographs per wound were obtained using ×100 magnification, and migration was determined by counting the cells that crossed the wound edge. Caco2-BBe cell migration was assessed similar to that described for IEC-6 cells with the following modifications: functional blocking or isotype control antibodies were included at 10 μg/ml with varying treatments, photomicrographs were captured after 20 h, and quantified by measuring the total area covered by the epithelial sheet from the wound origin. Live cell imaging of wild-type and shα3-integrin-depleted IEC-6 cells was completed by placing wounded monolayers immediately into a stage-mounted incubation system with flowmeter INU-NI-FI (Tokai Hit) atop a Nikon Eclipse Ti microscope. The environment achieved with this system is 37°C, 5% CO2, 21% O2, and 74% N2. Photomicrographs were obtained every 30 min for 18 h, creating an image sequence that was converted to .avi format for analysis in Image J (NIH) software running the MtrackJ macro to track cell migration of 10 leading-edge cells per sequence. Linear distance was determined from cell origin. Linear persistence was determined by calculating the ratio of linear migration to total cell migration (38).
Immunoblot analysis.
Wild-type and lentiviral-transduced IEC-6 cells used as the unstimulated controls during the migration assays were solubilized in modified RIPA buffer [50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 0.25% (vol/vol) sodium deoxycholate, 1.0% (vol/vol) NP-40, 0.1% (vol/vol) SDS, and 1 mM EDTA] supplemented with Protease Inhibitor Cocktail Set III (EMD Biosciences, San Diego, CA) and 10 mM sodium orthovanadate, 40 mM β-glycerolphosphate, and 20 mM sodium fluoride phosphatase inhibitors as defined previously (1). Densitometric analysis was performed using a FluorChem HD2 (Cell Biosciences, Santa Clara, CA) and AlphaView 3.2.4.0 software to quantify protein levels.
Statistical analysis.
Differences between wild-type, transfection controls, and shRNA integrin-depleted cells as well as multiple stimulations were analyzed using a one-way ANOVA with a Dunnet's post hoc analysis (GraphPad Prism 4, La Jolla, CA). Multiple comparisons between groups were analyzed using a two-way ANOVA with a Bonferroni post hoc analysis used to identify pairwise differences (GraphPad Prism 4). Statistically significant differences between control and α3 knockdown cell migration rates were determined using an unpaired t-test. Statistical significance was set at P ≤ 0.05.
RESULTS
CXCL12 stimulation increases adhesion and spreading on laminin.
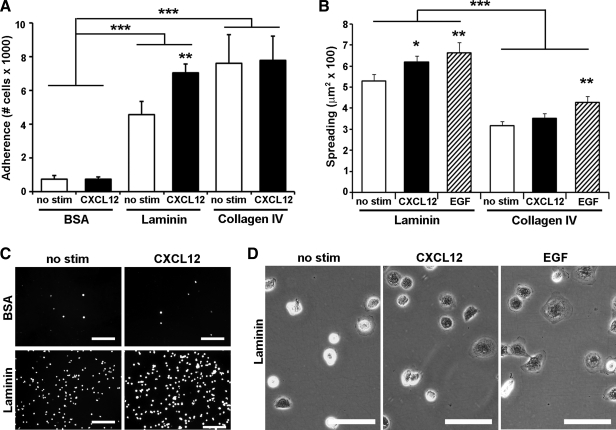
Our previous data suggest a preferential activation of laminin-specific integrins by CXCL12 (1). Integrin activation can be indirectly assessed by measuring cellular functions such as adhesion and spreading. Using these assays, we tested the effect of CXCL12 stimulation on cells cultured on laminin and collagen IV, the two predominant ECM proteins of the intestinal basement membrane (6, 7, 36, 46, 57). Cell adhesion was significantly increased on both laminin and collagen IV compared with BSA-treated control wells (Fig. 1A). CXCL12 significantly enhanced the observed increase in adhesion to laminin while having little, to no, effect on adhesion to collagen (Fig. 1, A and C). After initial adhesion, cells flatten or spread by extension of lamellipodia. Cell spreading, analyzed as a complementary functional test of integrin signaling, revealed that IEC-6 cells seeded to laminin were significantly larger than cells on collagen IV (Fig. 1B). In addition to the increase in adhesion, CXCL12 preferentially promoted cell spreading on laminin compared with collagen IV (Fig. 1, B and D). As shown in Fig. 1B, collagen IV did not prevent or otherwise limit cell spreading, as EGF was still able to promote increased cell spreading, as noted previously (4). These data demonstrate that CXCL12 stimulation leads to functional activation of laminin-specific integrins.
Fig. 1.
Enterocyte adhesion and spreading on laminin is increased by CXCL12 stimulation. An intestinal epithelial cell line, IEC-6, cells were seeded to laminin, collagen IV, or BSA-coated wells. A: IEC-6 cell adhesion was increased on laminin and collagen IV compared with control wells. Stimulation with CXCL12 (2.5 nM) (solid bars) increased adhesion compared with unstimulated control cells (open bars). CXCL12 increases adhesion on laminin but had little to no effect on adhesion to BSA or collagen IV. B: adherent IEC-6 cells spread more on laminin coating compared with collagen IV. CXCL12 stimulation (2.5 nM) (solid bars) increased the area of IEC-6 cells spreading on laminin similar to the positive control epidermal growth factor (EGF) (50 ng/ml) (hatched bars). CXCL12-induced spreading was specific for laminin and was not seen on collagen IV. C: representative immunofluorescence images of calcein-AM-loaded IEC-6 cells adhering to laminin or BSA. D: representative photomicrographs of spreading IEC-6 cells on laminin dishes. Data are mean number of adherent cells or cell area ± SE from 3–5 experiments. Asterisk denotes statistically significant difference from untreated cells or BSA (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) measured by two-way ANOVA. Scale bar = 100 μm in C and 50 μm in D.
Intracellular calcium regulates CXCL12-stimulated cell adhesion and spreading.
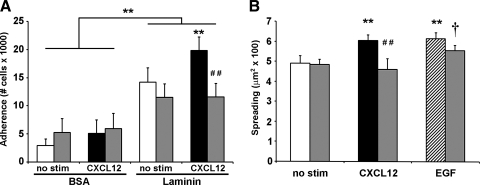
We have established that CXCL12-increased migration on laminin is dependent on intracellular calcium (1). On the basis of those findings, we postulated that CXCL12-stimulated adhesion and spreading were likewise regulated by receptor-mediated mobilization of intracellular calcium. To test this notion, we employed the intracellular calcium chelator BAPTA-AM, at a concentration we have previously shown to block CXCL12-induced migration (1). Chelation of intracellular calcium abrogated CXCL12-stimulated cell adhesion to laminin while having little impact on basal adhesion (Fig. 2A). CXCL12-increased cell spreading on laminin was similarly blocked by BAPTA-AM (Fig. 2B). Moreover, in contrast with CXCL12 and as demonstrated in Fig. 2B, EGF-stimulated cell spreading was minimally affected by calcium chelation. Combined, these data show that CXCL12-evoked inside-out integrin activation is dependent on intracellular calcium.
Fig. 2.
CXCL12-induced adhesion and spreading are dependent on intracellular calcium. IEC-6 cells were pretreated with 10 μM BAPTA-AM (shaded bars) or PBS (open, solid, and hatched bars) for 30 min. A: cell adhesion to laminin was increased by stimulation with 2.5 nM CXCL12 (solid bars) compared with untreated cells (open bars). Adhesion elicited by CXCL12 stimulation was blocked by BAPTA-AM pretreatment. B: CXCL12 (2.5 nM) (solid bar) and EGF (50 ng/ml) (hatched bar) increased spreading of IEC-6 cells cultured on laminin for 45 min. Chelation of intracellular calcium specifically blocked CXCL12-stimulated increase in cell area while having little effect on EGF-induced spreading. Values are means ± SE of from 3–4 separate experiments. Asterisk denotes statistically significant difference from untreated cells or BSA (**P ≤ 0.01). Pound symbol indicates statistically significant difference between CXCL12-stimulated cells pretreated with PBS or BAPTA-AM (##P ≤ 0.01). Dagger indicates a statistically significant difference (†P ≤ 0.05) between unstimulated and EGF-treated BAPTA-AM pretreated cells.
CXCL12 stimulation activates β1-integrins.
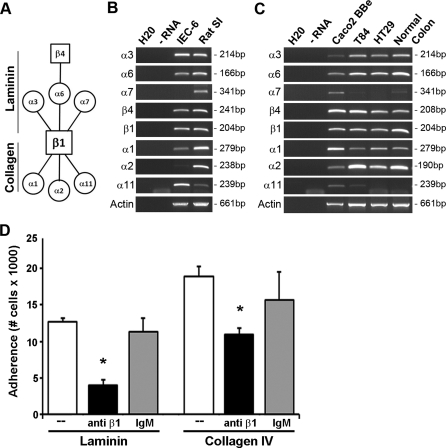
CXCL12 stimulation has been shown to activate multiple integrin heterodimers during immune cell trafficking (29, 53, 54). Within the gastrointestinal mucosa, the predominant integrins contain the β1- or β4-subunit. The β1-subunit complexes with multiple α-subunits, conferring different ligand specificities (Fig. 3A). Using RT-PCR, we confirmed transcript expression within model rat IEC-6, as well as human Caco2-BBe, T84, and HT-29 intestinal epithelial cell lines. In particular, mRNA transcripts for laminin binding integrin subunits-α3 and -α6 were expressed by each of the cell lines analyzed (Fig. 3, B and C). The laminin-binding integrin-α7 was detected solely in Caco2-BBe cells (3). Transcripts for integrin subunits-α1, -α2, and -α11, which primarily bind collagen isoforms (66) but which may also engage laminin, albeit, with lower affinity (17), were also detected. Expression of fibronectin-binding subunits-α5 and -αV were detected in each of the cell lines, whereas the α8-subunit was not expressed (data not shown).
Fig. 3.
Epithelial-expressed β1-integrin regulates IEC-6 adhesion. A: schematic diagram of laminin and collagen integrin receptor heterodimer partners. Squares indicate β-subunits, and circles represent α-subunit binding partners. B: integrin transcript was detected in rat intestinal epithelial cell line IEC-6. C: integrin transcript expressed in human intestinal cell lines, Caco2-BBe, T84, and HT29. H2O (negative control), RNA (RT negative control), and RNA isolated from rat small intestine (ratSI) or normal human colon tissue (positive control) were included in the analysis. D: functional β1-integrin blocking antibody (solid bars) decreases IEC-6 cell adhesion to laminin and collagen IV compared with untreated (open bars) or isotype (shaded bar) controls. Data are means ± SE from 3 experiments. Asterisk denotes statistically significant difference from untreated cells (*P ≤ 0.05).
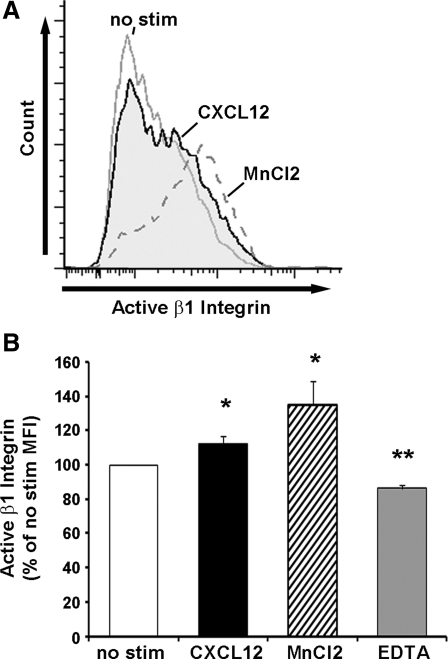
Given the consistent expression of the β1- and β4-integrin heterodimeric binding partners, we next tested whether β1-integrin functional blockade modulated cell adhesion. IEC-6 cell adhesion to both laminin and collagen IV was significantly decreased when cells were pretreated with 5 μg/ml anti-rat β1-integrin, whereas the same concentration of isotype matched irrelevant antibody had little effect compared with untreated cells (Fig. 3D). Using a previously characterized monoclonal antibody specific for the active confirmation of the human integrin (33, 35), we demonstrated that CXCL12 stimulation increased surface levels of active β1-integrin (Fig. 4). These data indicate that CXCL12-induced integrin activation is not limited to cells of hematopoietic lineage.
Fig. 4.
CXCL12 stimulation increases active-β1-integrin. A: representative histogram showing CXCL12 stimulation (2.5 nM) (shaded region) for 1 h at 37° increased active-β1-integrin on the cell surface of Caco2-BBe cells compared with unstimulated cells (gray line). MnCl2 (1 mM) (gray dashed line) synthetically produced maximal β1-integrin activation. B: quantification of active β1-integrin on Caco2-BBe cells that were untreated (no stim, open bars) stimulated with 2.5 nM CXCL12 (solid) or incubated with synthetic controls MnCl2 (hatched bar) or EDTA (5 mM) (shaded bar). Data are expressed as the mean percentage of unstimulated mean fluorescence intensity ± SE of 5–6 experiments. Asterisk denotes statistically significant difference from untreated cells (*P ≤ 0.05, **P ≤ 0.01).
Basal cell migration is decreased after α3-integrin depletion.
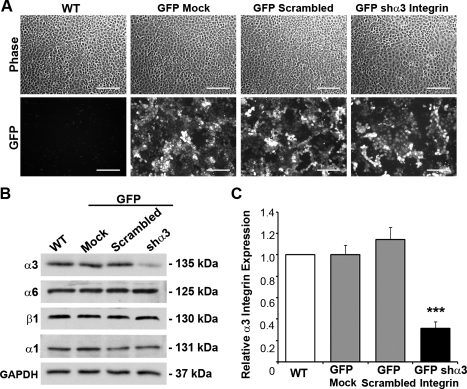
Given that β1-integrin neutralization globally reduced cell adhesion, we next sought to alter α-integrin function to specifically address the role for CXCL12-mediated cellular functions. We first chose to deplete the endogenous level of the α3-integrin subunit in IEC-6 cells to specifically dissect the role of CXCL12-mediated cellular functions. For this we used lentiviral-mediated delivery of shRNA specific for the α3-integrin subunit. As demonstrated using a GFP-shRNA vector, IEC-6 cells were equally transduced with lentiviral particles expressing shα3-integrin, a scrambled sequence, or empty vector (Fig. 5A). Immunoblot analysis showed significant 60–70% depletion of endogenous α3-integrin protein (Fig. 5, B and C). Levels of the heterodimeric binding partner, β1-integrin, and the collagen IV-specific α1-integrin were unchanged following α3-integrin knockdown.
Fig. 5.
α3-Integrin depletion within enterocytes. A: IEC-6 cells were equally transduced using LL3.7 green fluorescence protein (GFP) vectors, which were empty (GFP-Mock), contained a scrambled sequence (GFP-Scrambled), or shRNA specific for rat α3-integrin (GFP-shα3 Integrin). WT, wild-type. B: representative immunoblot detecting α3 and other epithelial integrins within WT, transduction controls, and GFP-shα3 integrin IEC-6 cells. C: densitometric analysis showing significant depletion of α3-integrin in GFP-shα3 cells (solid bar) compared with WT (open bar) and transduction controls (shaded bars). Data expressed as relative α3-integrin level normalized to GAPDH loading control from 9 different analyses. Asterisk denotes statistically significant difference from WT cells (***P ≤ 0.001) measured by ANOVA. Scale bar = 200 μm.
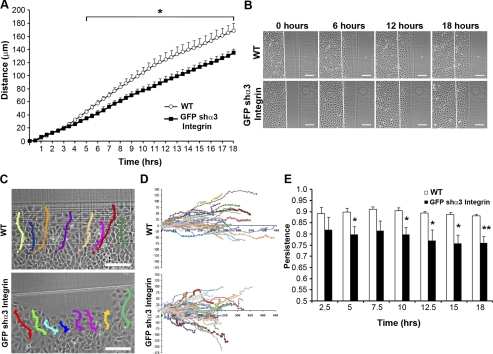
Using live-cell imaging, we found that basal migration in cells depleted of α3-integrin was significantly reduced compared with wild-type cells starting 5 h after wounding, whereas the epithelial sheet integrity was little, if at all, altered (Fig. 6, A and B). To further characterize the difference in migration, we next analyzed directional movement of cells within the epithelial sheet. Consistent with the changes in migration, knockdown of α3-integrin perturbed the directional migration of those cells compared with wild-type IEC-6 cells, which were more linear (Fig. 6, C and D). Calculation of migration linear persistence revealed that wild-type cells migrated with significantly greater directional persistence than shα3-integrin IEC-6 cells (Fig. 6E). In particular, we noted that, although migration distances were statistically different after 5 h, marked alterations in linear persistence was detectable as early as 2.5 h. Thus the laminin receptor provides a specific cue for directional movement in collective cell migration.
Fig. 6.
Depletion of α3-integrin decreased basal cell restitution through altered linear migration persistence. A: live cell imaging analysis reveals that the basal migration rate of GFP-shα3 integrin (■) knockdown IEC-6 cells is diminished compared with WT cells (○). Data are means ± SE distance from start from 4 individual experiments with 8–10 cell tracks measured per experiment. B: representative time-lapsed images from 0, 6, 12 and 18 h. C: representative migration track overlays of WT and GFP-shα3 integrin IEC-6 cell migration. D: cell trajectories for all cells measured within the 4 individual experiments (WT, n = 32; GFP-shα3 integrin, n = 40). E: persistence of cell migration for WT (open bars) and GFP-shα3 integrin (solid bars) IEC-6 cells. Data are the means ± SE of 4 individual experiments with 8–10 cell tracks measured per experiment. Scale bar = 125 μm. Asterisk denotes statistically significant difference from untreated cells (*P ≤ 0.05, **P ≤ 0.01).
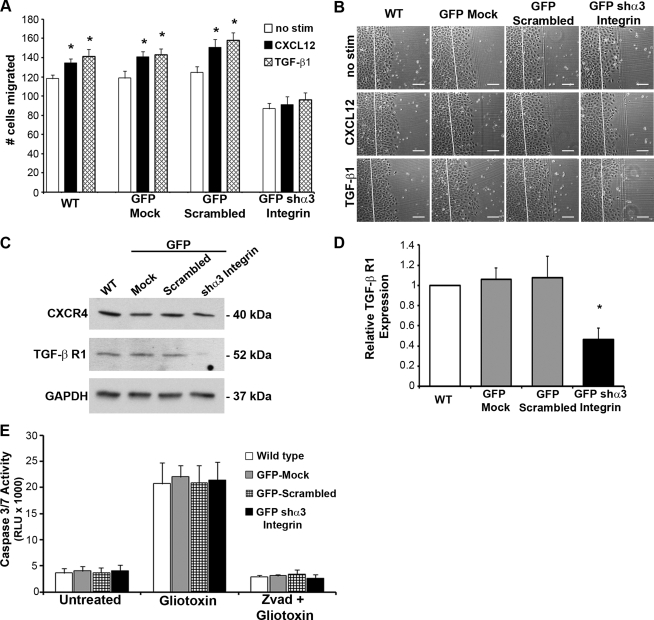
Using a complimentary approach, we found that decreased basal migration could not be recovered by either of the restitution-inducing stimulants CXCL12 or TGF-β1 (Fig. 7, A and B). Immunoblot analysis revealed that the lack of migration from CXCL12 stimulation was not due to reduction of its cognate receptor CXCR4 (Fig. 7C). However, TGFβR1 protein levels were significantly reduced in the α3-integrin-depleted cells (Fig. 7, C and D). Analysis of apoptosis in α3-subunit-depleted cells indicated that the reduction in basal cell migration was not attributable to alterations in cell death in knockdown and wild-type cells (Fig. 7E). Together these data demonstrate that α3-integrin is utilized for basal and inducible cell migration and also implicate α3-integrin in the regulation of TGFβR1.
Fig. 7.
Depletion of α3-integrin prevents inducible cell restitution. A: migration-inducing stimulants CXCL12 (2.5 nM) (solid bars) or transforming growth factor (TGF)-β1 (5 ng/ml) (hatched bars) were unable to increase migration of GFP-shα3 integrin-depleted IEC-6 cells. Control WT, GFP-Mock, and GFP-Scrambled control transduced cells had significant induction of migration by both CXCL12 and TGF-β1 compared with unstimulated (open bars) controls. Data are means ± SE of 9 individual experiments. B: representative photomicrographs of WT, GFP-Mock, GFP-Scrambled, and GFP-shα3 integrin IEC-6 cells stimulated with CXCL12, TGF-β1, or left untreated (no stim). Scale bar = 125 μm. C: representative immunoblots show depletion of TGF-β receptor 1 (TGFβR1), whereas CXCR4 levels were unaffected by α3-integrin depletion. D: densitometric quantification of TGFβR1 in WT (open bar), GFP-Mock, GFP-scrambled (shaded bars), and GFP-shα3 integrin (solid bar) IEC-6 cells confirmed significant depletion of TGFβR1 in GFP-shα3 integrin cells. Data are expressed as relative TGFβR1 levels normalized to the GAPDH loading control from 7 different immunoblot analyses. Asterisk denotes statistically significant difference from WT cells (*P ≤ 0.05). E: caspase 3/7 activity in WT, GFP-Mock, GFP-scrambled, and GFP-shα3 integrin IEC-6 cells was assessed using a luciferase-based assay. Gliotoxin (2 μg/ml) treatment was used to induce cell death, whereas zVAD (10 μg/ml), a pan-caspase inhibitor, confirmed that gliotoxin-induced cell death was the result of caspase activity. Values are means ± SE of 3 individual experiments.
CXCL12-stimulated migration is diminished in α6-integrin-depleted or functionally blocked cells.
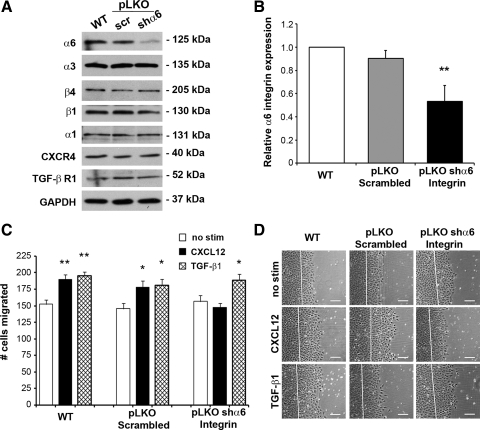
In agreement with the consistent expression of a second laminin binding receptor (Fig. 3), a puromycin-selectable shRNA vector was used to significantly deplete α6-integrin in IEC-6 cells (Fig. 8, A and B). Expression of the α6-integrin-binding partners, β4 and β1, as well as the collagen IV-binding α1-subunit, were unaffected by lentiviral transduction or α6-integrin knockdown (Fig. 8, A and B). In contrast to α3-depleted cells, knockdown of α6-integrin did not demonstrably effect TGFβR1 expression (Fig. 8A). Basal migration of α6-integrin-depleted IEC-6 cells was minimally, if at all, altered. However, CXCL12 was unable to elicit migration in α6-integrin knockdown cells (Fig. 8, C and D). This effect of α6-integrin depletion revealed that CXCL12 stimulation specifically utilizes this laminin-binding integrin to promote enhanced migration because TGF-β1-stimulated migration was unaffected. To complement these findings, we extended our investigation to investigate laminin-receptor signaling in human Caco2-BBe cells. As shown in Table 2, we determined that a neutralizing monoclonal antibody to α6-integrin significantly blocked CXCL12-induced migration. Consistent with IEC-6 knockdown cells, neutralization of α6-integrin specifically blocked chemokine-induced migration, with little, if any, change in basal or TGF-β1-stimulated restitution (Table 2). These data suggest that CXCL12 activates integrins containing the α6-subunit, resulting in the augmentation of cell migration on laminin (1).
Fig. 8.
α6-Integrin is required for CXCL12-stimulated migration. A: representative immunoblot analyses of WT, pLKO-scrambled and pLKO-shα6 integrin IEC-6 cells showed decreased α6, whereas level of other integrins was unaffected. B: quantification of α6 reduction in WT, pLKO-scrambled, pLKO-shα6 IEC-6 cells. Values are relative α6 expression normalized to GAPDH loading control compared with WT cells from 6 different immunoblot analyses. C: CXCL12 stimulation (2.5 nM) (solid bars) was unable to stimulate increased migration in α6-depleted IEC-6 cells, whereas 5 ng/ml TGF-β1 (hatched bars) stimulated significantly more migration than untreated cells (open bars). Inducible migration within WT and pLKO-scrambled control cells remained intact. Values are means ± SE of 6 individual experiments. Asterisk denotes statistically significant difference from WT or untreated cells (*P ≤ 0.05, **P ≤ 0.01). Scale bar = 125 μm.
Table 2.
Functional blockade of α6-integrin function blocks CXCL12-stimulated migration of Caco2-BBe cells
| Cell Migration, μM × 1,000 |
|||
|---|---|---|---|
| None | Anti-α6 | Rat IgG | |
| No stimulation | 64.605 ± 5.451 | 59.682 ± 6.019 | 60.542 ± 1.947 |
| CXCL12 | 94.886 ± 8.361* | 50.063 ± 2.736† | 80.931 ± 2.565* |
| TGF-β1 | 91.886 ± 5.078* | 80.214 ± 9.602* | 80.879 ± 2.460* |
Wounded Caco2-BBe monolayers were stimulated with 3.75 nM CXCL12, 5 ng/ml transforming growth factor (TGF)-β1, or left unstimulated in the presence of 10 μg/ml α6-integrin functional blocking antibody (anti-α6) or isotype control (rat IgG). Data are expressed as area of sheet migration from wound origin after 20 h of migration ± SE from 4 individual experiments.
Statistically significant difference (P ≤ 0.05) between no stimulation and motogen-treated monolayers within control, neutralizing antibody, or isotype antibody groups.
Statistically significant difference (P ≤ 0.05) between CXCL12-stimulated monolayers treated with integrin-neutralizing monoclonal antibody (anti-α6) compared to cells without antibody (none).
Depletion of α3- or α6-integrins blocks CXCL12-induced cell spreading.
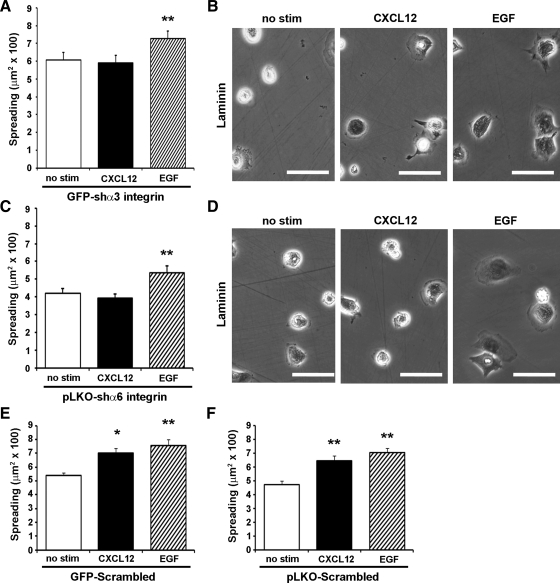
Returning to the cell-spreading assay, we next sought to determine whether laminin-specific integrin depletion would affect integrin-mediated functions. The ability of cells to spread following attachment to laminin matrix was unchanged following depletion of either the α3-integrin or α6-integrin. In agreement with the marked inhibition in CXCL12-stimulated migration, spreading of chemokine-treated cells was abolished in α3-integrin or α6-integrin knockdown cells (Fig. 9). This response was specific for CXCL12, as addition of EGF significantly increased cell area. Both CXCL12 and EGF significantly enhanced cell spreading of GFP-scrambled or pLKO-scrambled control cells, indicating that gene delivery did not affect inducible cell spreading (Fig. 9, E and F). Together these data suggest that CXCL12 activates both α3- and α6-containing integrin heterodimers to promote cell spreading.
Fig. 9.
Depletion of laminin-specific α3 and α6 blocks CXCL12-stimulated cell spreading. A and C: CXCL12-stimulated (2.5 nM) (solid bar) cell spreading was attenuated in GFP-shα3 integrin and pLKO-shα6 integrin expressing IEC-6 cells, whereas EGF stimulation (50 ng/ml) (hatched bar) was still able to evoke increased cell spreading. B and D: representative images of GFP-shα3 integrin and pLKO-shα6 integrin IEC-6 cells spreading on laminin. E and F: cell spreading was little affected by lentiviral gene transduction, as CXCL12- and EGF-induced functions remained intact in GFP-scrambled and pLKO-scrambled IEC-6 cells. Values are means ± SE of 3–4 individual experiments. Asterisk denotes statistically significant difference from untreated cells (*P ≤ 0.05, **P ≤ 0.01). Scale bar = 50 μm.
DISCUSSION
Directed collective cell migration is required for proper organogenesis, wound repair, and disease progression (50). Extra- and intracellular signals are thought to cooperatively organize cellular sheet migration. In leukocytes, outside-in and inside-out signaling can be evoked by various stimuli, including ECM proteins, cytokines, or chemokines, and results in altered affinity states of cell surface integrins. Epithelial cells also require the ability to modulate the affinity of integrins based on spatial and temporal cues to elicit adhesion, flattening, and migration. However, thus far, intestinal restitution studies have focused largely on migration-inducing effectors (1, 4, 12, 15, 41, 43, 45, 56, 61) or matrix proteins (4, 51). With this report, we have shown that the restitution-inducing stimulant CXCL12 enhances restitution by orchestrating inside-out activation of laminin-specific integrins and demonstrated the importance of these integrins during cellular adhesion, spreading, and migration.
Dissection of integrin-ECM interactions within intestinal epithelial cells has shown differential spreading and migration on individual ECM proteins (4, 5). Furthermore, EGF stimulation can modulate those functions through collagen-specific α1- and α2-integrins linking an extracellular cytokine with cellular adhesion responses (4, 51). Here we have expanded our understanding to include evidence that chemokine stimulation modulates integrin activation and functions. We previously demonstrated that the chemokine CXCL12 significantly increased migration of multiple model intestinal epithelia and that this stimulation could be further enhanced on laminin (1, 43, 56). CXCL12 has been shown within other cell types to activate hematopoietic-restricted β2-integrins as well as several β1-integrins (25, 29, 53, 54). In nonhematopoietic intestinal epithelial cells, we built upon that repertoire to establish that CXCL12 regulates adhesion and spreading on laminin receptors. Cell adhesion and spreading on collagen IV, which facilitated Caco2-BBe spreading after EGF stimulation (51), was unchanged by CXCL12. The differential responses mediated by growth factors and chemokines support a model in which different restitution-inducing motogens activate distinct subsets of integrin-matrix receptors.
In hematopoietic cell lineages, the intracellular signaling cascade of integrin activation has been linked with calcium as a key regulator (13, 24). Release of intracellular calcium stores leads to activation of the small GTPase Rap1, which is necessary and sufficient for the resulting integrin activation (9, 29, 54). We have previously shown that CXCL12, as well as the inducible chemokine CCL20, both elicit restitutive cell migration dependent on intracellular calcium (1, 61). CXCL12 evokes biological responses following binding to its cognate receptors CXCR4 and CXCR7, both members of the G protein-coupled receptor superfamily. G protein-coupled receptor signaling activates integrins through specific interactions between the cytoplasmic domains of the β-subunit and the intracellular scaffolding protein talin (11, 30, 64). Consistent with our prior report, we demonstrated in this work that chelation of intracellular calcium ablated CXCL12-stimulated cell adhesion and spreading on laminin. However, cell spreading evoked by EGF, which has been shown to use α1- and α2-integrins (4), was unaffected by chelation of intracellular calcium, suggesting that an alternative, calcium-independent pathway was triggered. Our data suggest that chemokine-driven intracellular calcium mobilization elicits a similar Rap1 signaling pathway as a central regulator of intestinal epithelial cell activation of laminin-specific integrins.
To facilitate cell migration, integrins must be dynamic, cycling between active and inactive conformations. Direct detection of active integrins on hematopoietic cells by flow cytometry has proven to be a powerful method to determine the effects of various stimuli (24, 63). Using a flow cytometric approach, we showed that CXCL12 stimulation led to increased active β1-integrins on the intestinal epithelial cell surface. Given the restricted specificity of the antibody, those studies utilized the human Caco2-BBe cell line, a model previously used to define differential responses for cell adhesion, spreading, and migration during restitution (4, 5, 51). Increased levels of active β1-integrin suggest that CXCL12 promotes epithelial restitution though inside-out integrin activation, akin to stable adhesion and diapedesis of leukocytes. Using neutralizing antibodies, we also demonstrated that blockade of β1-integrin has significant global effects, decreasing enterocyte adhesion to both laminin and collagen IV. This finding is consistent with previous reports in β1-null keratinocytes (22) and perinatal lethality evidenced from mice with targeted epithelial knockout of β1-integrin (28). Our data suggest that chemokine-induced activation of β1-integrins in epithelial cells preferentially activates laminin-binding integrins.
Intestinal epithelial cells express integrin α-subunits that bind multiple ECM proteins including laminin, collagen, and fibronectin when heterodimerized with the β1-subunit. The α3-subunit pairs with the β1-subunit, whereas α6-integrin heterodimerizes with β1- or β4-subunits. Each integrin combination binds similar laminin isoforms, albeit with differing affinities, suggesting both functional redundancy and divergence (23, 34, 52, 58). α3-Integrin knockout mice suffer neonatal death attributed to kidney and lung developmental malformations (31), whereas α6- or β4-integrin knockout mice survive to birth, dying shortly thereafter from a skin detachment syndrome mimicking human epidermolysis bullosa (20, 59). Because of the lack of animal models or functional blocking antibodies for rat integrins, lentiviral-mediated depletion of α3- and α6-integrins was used. The individual depletion of specific α-integrin subunits allowed us to decipher the laminin-binding subunit(s) activated by CXCL12. Baseline migration of unstimulated cells was dramatically inhibited in α3-integrin-depleted cells. Interestingly, the difference in cell migration reflected decreased linear persistence during migration. Although α3-integrins were shown to be involved in basal cell migration, restitution was not completely abolished, suggesting that a certain level of migration is facilitated by additional integrin-ECM interactions. Consistent with our findings, α3-integrin-null keratinocytes adhere to laminin but are unable to send out lamellipodia and spread (16). Furthermore, stimulation with CXCL12 or TGF-β1 did not rescue migration in α3-integrin knockdown cells, suggesting that subunit plays a more universal role in enterocyte migration. In contrast, genetic depletion or functional blockade of α6-integrin resulted in little, if any, change in basal sheet migration. Interestingly, these alterations to α6-integrin specifically blocked CXCL12-, and not TGF-β1, stimulated migration. The inability of CXCL12 to elicit migration in either α3- or α6-integrin-depleted cells may reflect the inability of those cells to spread when stimulated with CXCL12 following adherence to laminin. Cellular spreading machinery remained intact as EGF effectively increased spreading. Intriguingly, these data are the first to describe CXCL12 activation of laminin-specific integrins and suggest that α3- and α6-integrins elicit both overlapping and distinct functions on laminin.
Wound healing is promoted by multiple stimulants; however, a link between these motogen surface receptors and integrins has not been demonstrated. We found that depletion of α3-integrin within intestinal epithelial cells resulted in reduced levels of TGFβR1, implicating integrins in the regulation of TGF-β signaling. This reduction of TGFβR1 may explain why α3-integrin-depleted cells had reduced basal migration, consistent with the notion that TGF-β plays a key role in basal and induced wound repair (12, 49). Alternatively, although in silico analyses indicated that the sequence was unique for α3-integrin, the short hairpin used may have off-target effects within the TGFβR1 transcription and translation pathway. Interestingly, other integrin subunits have been linked to TGF-β receptor expression (49, 62). Thus crosstalk between integrins and TGF-β signaling likely participate in intestinal wound repair. Intestinal wound healing occurs within a complex in vivo environment, which requires coordination of signals from various stimuli to the receptors binding the ECM. Our data reveal that chemokines promote restitution using alternative signaling pathways to TGF-β1 or EGF, signaling through calcium to lead to heightened function of specific integrins. Chemokines have long been known to activate integrin receptors during leukocyte chemotaxis and cell-cell adherence during immune responses; we have shown here that laminin-specific integrins activated by the chemokine CXCL12 coordinate injury repair of adherent intestinal epithelial cells.
GRANTS
This work was supported by the National Institutes of Health (DK062066).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Recombinant CXCL12 was provided by Dr. Brian Volkman (Medical College of Wisconsin). Erik Meijering developed and wrote the MTrackJ macro for use with NIH Image J software.
REFERENCES
- 1.Agle KA, Vongsa RA, Dwinell MB. Calcium mobilization triggered by the chemokine CXCL12 regulates migration in wounded intestinal epithelial monolayers. J Biol Chem 285: 16066–16075, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basora N, Desloges N, Chang Q, Bouatrouss Y, Gosselin J, Poisson J, Sheppard D, Beaulieu JF. Expression of the alpha9beta1 integrin in human colonic epithelial cells: resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int J Cancer 75: 738–743, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Basora N, Vachon PH, Herring-Gillam FE, Perreault N, Beaulieu JF. Relation between integrin alpha7Bbeta1 expression in human intestinal cells and enterocytic differentiation. Gastroenterology 113: 1510–1521, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest 90: 15–23, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res 225: 301–305, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci 102: 427–436, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu JF, Vachon PH. Reciprocal expression of laminin A-chain isoforms along the crypt-villus axis in the human small intestine. Gastroenterology 106: 829–839, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell 101: 695–708, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardi B, Guidetti GF, Campus F, Crittenden JR, Graybiel AM, Balduini C, Torti M. The small GTPase Rap1b regulates the cross talk between platelet integrin alpha2beta1 and integrin alphaIIbbeta3. Blood 107: 2728–2735, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Calderwood DA. Integrin activation. J Cell Sci 117: 657–666, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ciacci C, Lind SE, Podolsky DK. Transforming growth factor beta regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology 105: 93–101, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med 10: 982–986, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Desloges N, Basora N, Perreault N, Bouatrouss Y, Sheppard D, Beaulieu JF. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: relation with cell proliferation. J Cell Biochem 71: 536–545, 1998 [PubMed] [Google Scholar]
- 15.Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis 7: 68–77, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Dipersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. Alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol 137: 729–742, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dipersio CM, Shah S, Hynes RO. alpha 3A beta 1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J Cell Sci 108: 2321–2336, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 117: 359–367, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Dydensborg AB, Teller IC, Basora N, Groulx JF, Auclair J, Francoeur C, Escaffit F, Pare F, Herring E, Menard D, Beaulieu JF. Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem Cell Biol 131: 531–536, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le MM. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13: 370–373, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol 17: 509–516, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Gu J, Sumida Y, Sanzen N, Sekiguchi K. Laminin-10/11 and fibronectin differentially regulate integrin-dependent Rho and Rac activation via p130(Cas)-CrkII-DOCK180 pathway. J Biol Chem 276: 27090–27097, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol 16: 1796–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene 24: 4462–4471, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Humphries MJ. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci 97: 585–592, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol 175: 505–514, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol 4: 741–748, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301: 1720–1725, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Lacy ER. Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol 10, Suppl 1: S72–S77, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell 7: 585–595, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol 156: 985–996, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem 271: 11067–11075, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc Res Tech 51: 169–178, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med 31: S532–S537, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 135: 1771–1780, 2008 [DOI] [PubMed] [Google Scholar]
- 39.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol Gastrointest Liver Physiol 263: G426–G435, 1992 [DOI] [PubMed] [Google Scholar]
- 40.McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol Gastrointest Liver Physiol 276: G1087–G1093, 1999 [DOI] [PubMed] [Google Scholar]
- 41.McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol 162: 1355–1360, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore R, Carlson S, Madara JL. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Lab Invest 60: 237–244, 1989 [PubMed] [Google Scholar]
- 43.Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest 87: 807–817, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501–1511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci 50, Suppl 1: S34–S38, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Perreault N, Vachon PH, Beaulieu JF. Appearance and distribution of laminin A chain isoforms and integrin alpha 2, alpha 3, alpha 6, beta 1, and beta 4 subunits in the developing human small intestinal mucosa. Anat Rec 242: 242–250, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol Gastrointest Liver Physiol 277: G495–G499, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 78: 219–243, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, Hodivala-Dilke K. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat Med 11: 167–174, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via α1β1- and α2β1-integrin-dependent Src kinase activation. Am J Physiol Gastrointest Liver Physiol 286: G547–G557, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Seltana A, Basora N, Beaulieu JF. Intestinal epithelial wound healing assay in an epithelial-mesenchymal co-culture system. Wound Repair Regen 18: 114–122, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol 6: 497–506, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol 161: 417–427, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon-Assmann P, Bouziges F, Arnold C, Haffen K, Kedinger M. Epithelial-mesenchymal interactions in the production of basement membrane components in the gut. Development 102: 339–347, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol 288: G316–G326, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Teller IC, Auclair J, Herring E, Gauthier R, Menard D, Beaulieu JF. Laminins in the developing and adult human small intestine: relation with the functional absorptive unit. Dev Dyn 236: 1980–1990, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Teller IC, Beaulieu JF. Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev Mol Med 3: 1–18, 2001 [DOI] [PubMed] [Google Scholar]
- 59.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet 13: 366–369, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Veldkamp CT, Peterson FC, Hayes PL, Mattmiller JE, Haugner JC, 3rd, de la Cruz N, Volkman BF. On-column refolding of recombinant chemokines for NMR studies and biological assays. Protein Expr Purif 52: 202–209, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vongsa RA, Zimmerman NP, Dwinell MB. CCR6 regulation of the actin cytoskeleton orchestrates human beta defensin-2- and CCL20-mediated restitution of colonic epithelial cells. J Biol Chem 284: 10034–10045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, Sun L, Zborowska E, Willson JK, Gong J, Verraraghavan J, Brattain MG. Control of type II transforming growth factor-beta receptor expression by integrin ligation. J Biol Chem 274: 12840–12847, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis VA, Ginsberg MH, Shattil SJ. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol 181: 1211–1222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell 128: 171–182, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Wei J, Shaw LM, Mercurio AM. Integrin signaling in leukocytes: lessons from the alpha6beta1 integrin. J Leukoc Biol 61: 397–407, 1997 [DOI] [PubMed] [Google Scholar]
- 66.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol 36: 1405–1410, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15: 1277–1294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]