Abstract
Four patients with overhydrated cation leak stomatocytosis (OHSt) exhibited the heterozygous RhAG missense mutation F65S. OHSt erythrocytes were osmotically fragile, with elevated Na and decreased K contents and increased cation channel-like activity. Xenopus oocytes expressing wild-type RhAG and RhAG F65S exhibited increased ouabain and bumetanide-resistant uptake of Li+ and 86Rb+, with secondarily increased 86Rb+ influx sensitive to ouabain and to bumetanide. Increased RhAG-associated 14C-methylammonium (MA) influx was severely reduced in RhAG F65S-expressing oocytes. RhAG-associated influxes of Li+, 86Rb+, and 14C-MA were pharmacologically distinct, and Li+ uptakes associated with RhAG and RhAG F65S were differentially inhibited by NH4+ and Gd3+. RhAG-expressing oocytes were acidified and depolarized by 5 mM bath NH3/NH4+, but alkalinized and depolarized by subsequent bath exposure to 5 mM methylammonium chloride (MA/MA+). RhAG F65S-expressing oocytes exhibited near-wild-type responses to NH4Cl, but MA/MA+ elicited attenuated alkalinization and strong hyperpolarization. Expression of RhAG or RhAG F65S increased steady-state cation currents unaltered by bath Li+ substitution or bath addition of 5 mM NH4Cl or MA/MA+. These oocyte studies suggest that 1) RhAG expression increases oocyte transport of NH3/NH4+ and MA/MA+; 2) RhAG F65S exhibits gain-of-function phenotypes of increased cation conductance/permeability, and loss-of-function phenotypes of decreased and modified MA/MA+ transport, and decreased NH3/NH4+-associated depolarization; and 3) RhAG transports NH3/NH4+ and MA/MA+ by distinct mechanisms, and/or the substrates elicit distinct cellular responses. Thus, RhAG F65S is a loss-of-function mutation for amine transport. The altered oocyte intracellular pH, membrane potential, and currents associated with RhAG or RhAG F65S expression may reflect distinct transport mechanisms.
Keywords: hemolytic anemia; Rh antigen, ammonium, lithium; rubidium; methylammonium; Xenopus oocyte
hereditary, overhydrated, cation-leak stomatocytosis (OHSt) is an autosomal dominant, variably compensated, macrocytic hemolytic anemia of fluctuating severity, characterized by circulating erythrocytes with slit-like lucencies (stomata) evident on fixed, stained peripheral blood smears. OHSt red cells exhibit cation leak, resulting in elevated cell Na+ content with reduced K+ content, with increased ouabain-resistant cation leak fluxes in the presence of presumably compensatory increases in ouabain-sensitive Na+-K+-ATPase activity, and red cell age-dependent loss from the erythroid membrane of stomatin/EBP7.2 (18, 69).
OHSt has been clinically characterized by the criteria of erythrocyte hydration state and of temperature dependence of the red cell cation leak (69). Familial dehydrated stomatocytosis maps to two loci at chromosomes 16q23–24 (27) and 2q25–26 (10), but the identity of neither mutant gene has been reported. OHSt mutations have been defined in genes encoding two abundant erythrocyte membrane transport proteins, SLC4A1/AE1/Band 3 (8) and RhAG (7). At least eight AE1 missense mutations have been identified in families with dominant OHSt (8, 33, 67, 68). Dominant OHSt was linked to heterozygous RhAG mutation F65S in thirteen patients from six pedigrees, and with RhAG mutation I61R in a single case. Both disease-associated amino acid residues are highly conserved among mammalian Rh proteins and the related plant Amt ammonia transporter proteins, and moderately well conserved among the Mep ammonia transporters of yeast (7). Human RhAG models based on crystal structures of human RhCG (28), Nitrosomonas europaea Rh50 (42), and Escherichia coli amtB (35, 84) situate both of these mutant residues in the juxta-cytoplasmic portion of the second transmembrane domain.
Overexpression in Xenopus oocytes of these RhAG stomatocytosis mutants elevated oocyte Na content, reduced K content, and increased Li+ influx, but NH4+ transport was not studied (7). Modeling of RhAG on the RhCG crystal structure attributed the increased nonspecific cation permeability of the RhAG F65S mutant to a widening of the juxta-cytoplasmic constriction formed by residues F65 and H334 to dimensions exceeding the hydrated ionic radii of Na+, K+, and NH4+ (7).
The mechanisms of NH3/NH4+ transport by Rh, Amt, and Mep polypeptides remain controversial. Crystal structures of Amt and Rh proteins have been interpreted to require permeation of neutral NH3, supplied to the vestibule or mouth of the translocation pathway by external electrostatic concentration and binding of charged NH4+ (28, 35, 42). Functional investigations of native human RhAG and of other native and recombinant Rh-associated glyco-proteins and Amt transporters have led to proposals of electroneutral NH3 transport in red cells and proteoliposomes (47, 58, 85) and in Xenopus oocytes (48, 49), electroneutral NH4+/H+ exchange in Xenopus oocytes (40, 43, 78) and epithelial cells (30, 31), electrogenic transport of NH4+ in Xenopus oocytes (41, 45, 52), and transport of both NH3 and NH4+ in HeLa cells (4). More recent studies of mouse Rhbg in Xenopus oocytes have suggested combined electrogenic and electroneutral transport of amines, with distinct transport mechanisms for NH3/NH4+ and for its commonly studied surrogate substrate, methylammonium (MA/MA+) (50, 51).
The study of disease-associated mutants is important to decipher the mechanism and substrate specificity of RhAG-mediated transport. We now report four cases of OHSt associated with the F65S mutation of RhAG, and compare the ion selectivity, pharmacology, and transport properties of this mutant polypeptide with those of wild-type RhAG. We find that RhAG mutant F65S expressed in Xenopus oocytes exhibits a loss-of-function phenotype with respect to uptake of 14C-methylammonium (MA/MA+), accompanied by a novel MA/MA+-associated hyperpolarization. Oocytes expressing either wild-type or mutant RhAG display distinct responses to added NH3/NH4+ or to added MA/MA+, and the two substrates elicit divergent changes in oocyte membrane potential (Vm) and intracellular pH (pHi). Increased cation transport associated with expression of wild-type and of mutant RhAG exhibits distinct, cation substrate-specific profiles of pharmacologic inhibition.
The results provide new insight into RhAG stomatocytosis mutant F65S as a combined loss-of-function/gain-of-function mutation for MA/MA+ transport, and suggest that the changes in solute transport, pHi, Vm, and current associated with expression of wild-type RhAG or its F65S mutant reflect distinct transport mechanisms.
METHODS
Reagents.
14C-methylammonium chloride (MA/MA+) was from ICN (Irvine, CA). 86RbCl was from PerkinElmer (Waltham, MA). 4,4′-Di-isothiocyanato-2,2′-disulfonatostilbene (DIDS) was from Calbiochem (St. Louis, MO). Grammastola spatulata mechanotoxin-4 (GsMTx-4) was from Peptides International (Osaka, Japan). All other reagents were from Sigma-Aldrich (St. Louis, MO).
Clinical histories.
Patient 1 is an infant female born at full term after an uncomplicated pregnancy by Caesarian section for failure to progress during labor. Discharged after phototherapy for mild jaundice, she was readmitted at age 12 days with pallor, and transfused for a marked anemia with mean corpuscular volume (MCV) of 144 fl and mean corpuscular hemoglobin concentration (MCHC) of 28 g/dl. The peripheral blood smear showed numerous stomatocytes with interspersed microspherocytes. A blood sample revealed both osmotically sensitive and resistant red cells, the latter proportionately increased by overnight incubation at 20°C, without cold-induced hemolysis. Additional transfusions at age 1 and 2 mo marked the end of the patient's transfusion requirement. During her first year of life, hemoglobin (Hb) averaged 9 g/dl, with 13–17% reticulocytes, MCV of 141–148 fl, and MCHC of 22 g/dl. At age 14 mo, Hb was 10.3 g/dl with hematocrit (Hct) 38%, 4.3% reticulocytes, MCV 120 fl, and MCHC of 27 g/dl. Subsequent growth and development were normal, and Hb remained above 7 g/dl despite intermittent jaundice and pallor in association with viral illnesses. At age 17 mo in the setting of splenomegaly and slight jaundice, the peripheral smear revealed continued spherostomatocytosis (Fig. 1A). Red cell membrane protein composition revealed mild stomatin deficiency, but was otherwise unremarkable (Fig. 1D). Neither parent had splenomegaly, and there was no family history of hemolytic anemia, transfusion requirement, or splenectomy. Peripubertal anemia in the father's sister later resolved.
Fig. 1.
Stomatocytosis red cell properties and RHAG mutation. A: peripheral Wright-Giemsa smear from patient 1. B: peripheral smear from patient 2. C: segments of RHAG exon 2 genomic DNA sequence from a normal control (top) and from patient 4 (bottom). WT, wild type. D: red cell membrane protein profile from a normal control (Nl) and from patient 1 (Pt).
Patient 2, a 7-yr-old female, was delivered by Caesarian section at 30 wk gestational age in the setting of limited prenatal care, intrauterine cocaine exposure, and oligohydramnios. Perinatal Hb was 5.3 g/dl, and persistent pulmonary hypertension of the newborn was treated with several transfusions. A transfusion at age 5 yr was in the setting of a febrile illness with Hb 4.7 g/dl. Subsequent normal growth and development was accompanied by fatigue, mild jaundice, and chronic hemolytic anemia. Lab values at age 7 yr revealed Hb 8.0 g/dl, Hct 31.2%, 43% reticulocytes, MCV 136 fl, MCHC 25.5 g/dl, LDH 514 U/l, total bilirubin 7.8 mg/dl, and mild hypochromia, stomatocytes, and echinocytes on smear (Fig. 1B).
Fresh and postincubation red cells exhibited increased osmotic fragility. Several months after these tests were performed, patient 2 died in the setting of gram negative meningitis and sepsis. The contribution of her OHSt to the genesis or progression of this fatal infection remains unclear.
Patient 3 is a Caucasian male of North European ancestry. Severe neonatal hemolytic anemia required erythrocyte transfusions, and jaundice required phototherapy. Infancy and early childhood were marked by frequent transfusions and splenectomy at age 4. Anemia and transfusion-dependence decreased until late adolescence. Pulmonary embolism at age 19 was followed by persistent deep vein thromboses. The patient had a myocardial infarction at age 29, at which time pulmonary hypertension was noted. Treatment with hypertransfusion was complicated by iron overload requiring chelation therapy. Typical erythrocyte indices prior to hypertransfusion therapy included Hb 12 g/dl, MCV 130 fl, MCHC 28 g/dl, and reticulocyte count 28%. Numerous stomatocytes were seen on peripheral smear, incubated erythrocyte osmotic fragility was increased, and erythrocyte membranes demonstrated decreased stomatin immunoreactivity (not shown). Paternal erythrocytes also demonstrated stomatocytic morphology and increased incubated osmotic fragility. Maternal erythrocytes were of normal morphology and osmotic fragility.
Patient 4 is a Caucasian male whose clinical data have been previously described (36, 46, 70). Following neonatal jaundice requiring exchange transfusion, ongoing hemolytic anemia during infancy and early childhood required continued erythrocyte transfusions culminating in splenectomy at age 4. His anemia and transfusion requirement decreased during adolescence. At age 21 he presented with pulmonary embolus with persistent superficial and deep vein thromboses, leading to anticoagulation and placement of an inferior vena cava filter. He has received chelation therapy for iron overload. His peripheral blood smear demonstrated numerous stomatocytes, a component of Band 7.2 was absent from his SDS-PAGE profile (36) and stomatin immunoreactivity was undetectable in red cell membranes (not shown). Erythrocyte morphology of both parents was normal.
Mutation detection.
Blood samples were obtained from patients and family members under protocols approved by the Committees on Clinical Investigation and the Institutional Review Boards of New York University School of Medicine, Children's Hospital Boston, and Yale University School of Medicine. Whole blood total RNA from patients 1 and 2 was isolated with the RNeasy Mini-kit (Qiagen) and was reverse-transcribed into cDNA with the Retroscript Kit (Ambion). cDNAs encoding eAE1 polypeptide from patients 1 and 2 were amplified as two overlapping fragments by polymerase chain reaction (PCR), and the full-length cDNA coding regions were sequenced on both strands (67). The 19 coding exons of the AE1 gene amplified from genomic DNA of patients 3 and 4 were PCR-amplified and subjected to denaturing HPLC as described (68). No abnormal denaturation profiles were noted.
RhAG cDNAs from patients 1 and 2 were amplified by RT-PCR from whole blood total RNA as two overlapping fragments, purified from agarose gel, and sequenced on both strands using oligonucleotide primers presented in Supplemental Table S1 (Supplemental Material for this article is available online at the Journal website). The presence of the F65S mutation was verified in the genomic DNA of patient 1. cDNA and genomic DNA from both parents of patient 1 was also sequenced in the region of the F65S mutation. Genomic DNA was prepared from whole blood of patients 3 and 4, and the 10 coding exons of RHAG were PCR-amplified. The exon-flanking primer pairs designed with the program Primer 3 were typically ∼40 nucleotides away from the intron-exon boundary to facilitate detection of possible splice junction mutations (Supplemental Table S2). PCR amplification products were subjected to agarose gel electrophoresis and visualized by ethidium bromide staining to ensure robust amplification, and sequenced on both strands.
Hematological analysis.
Peripheral smears were stained with Wright-Giemsa. Complete blood counts were performed and whole blood hematological indices were measured with an automated hematology analyzer (Advia 120, Siemens). Osmotic fragility testing (25) was performed at room temperature before and after overnight incubation at 37°C as heparinized whole blood. Osmotic ektacytometry (13) was performed as previously described. Ektacytometry yielded values for Omin (the osmolality at which 50% of cells hemolyze), DImax (the maximal cell deformability), and Ohyp (the osmolality at which cell deformability equals half the maximum value). Patient samples were compared with a range of normal control cells.
Preparation of erythrocytes and measurement of ion content.
Whole blood collected in EDTA was passed through cotton to preadsorb white cells and was centrifuged in a Sorvall RC 28S at 2,500 rpm for 4 min at 4°C as previously described (59). The RBC pellets were washed four times with ice-cold Mg2+-free choline wash solution (CWS) containing (in mM): 150 choline Cl, 20 sucrose, and 10 Tris MOPS [3-(N-morpholino)propanesulfonic acid], pH 7.4 (4°C). Manual hematocrits were measured with a 50% (vol/vol) cell suspension prepared in Mg2+-free CWS. Aliquots of this suspension were diluted with 0.02% Acationox in double-distilled water for measurement of intracellular Na, K, and Mg contents by atomic absorption spectrometry (PerkinElmer 800, Wellesley, MA) as previously described (60). Ion content was measured before and after overnight incubation as whole blood in EDTA.
Red cell Na-K pump, KCC, NKCC, and NHE activities.
These transporter activities were measured in freshly isolated red cells without or with nystatin pretreatment as previously described (17), with modifications. Cation equilibration was performed in the presence of 40 μg/ml nystatin in a solution containing (in mM) 77 NaCl, 77 KCl, and 55 sucrose, with a final intracellular Na concentration of ∼50 mmol/l cells. Nystatin was then rapidly removed by addition of 1% bovine serum albumin. Red cell Na-K pump activity measured in a solution containing 155 mM choline chloride and 10 mM KCl was estimated as the fraction of Na+ efflux sensitive to 0.1 mM ouabain. Red cell Na-K-2Cl cotransport (NKCC) activity measured in a solution containing 154 mM choline chloride and 0.1 mM ouabain was estimated as the fraction of Na+ and K+ efflux sensitive to 10 μM bumetanide. Red cell K-Cl cotransport (KCC) activity measured in 154 mM NaCl or Na sulfamate in the presence of 0.1 mM ouabain plus 10 μM bumetanide was defined as Cl−-dependent K+ efflux. Red cell Na/H exchange (NHE) activity stimulated by hypertonic shrinkage in a solution containing (in mM) 165 choline chloride, 1 MgCl2, 10 glucose, 0.1 ouabain, 0.01 bumetanide, 10 Tris-MOPS (pH 7.4 at 37°C) was estimated as Na+ efflux sensitive to 10 μM 3-amino-6-chloro-5-(1-homopiperidyl)-N-(diaminomethylene)pyrazinecarboxamide (HMA). Leak flux was measured in red cells not previously exposed to nystatin, and was defined as ouabain- and bumetanide-insensitive Na+ efflux and K+ efflux.
Red cell ghost preparation and SDS-PAGE.
Red cells were washed three times with removal of buffy coat. Ghost membranes were prepared by hypotonic lysis (19) with added protease inhibitors. Patient and control membrane protein (30 μg) was loaded onto 4% acrylamide slab gels, and electrophoresed according to Fairbanks et al. (22) as modified by Steck (65). Gels were stained with Coomassie Blue G250, destained, and scanned wet.
On-cell patch recording on erythrocytes.
Red cells washed as above were resuspended in storage solution containing (in mM) 145 KCl, 15 NaCl, and 10 HEPES, pH 7.4, then kept at 4°C until used. Human red cells allowed to settle on coverslips were mounted on an inverted microscope in a 200 μl open chamber (WPI, Sarasota, FL) and superfused 15 min at room temperature. Borosilicate pipettes (Corning 8250) pulled with a Sutter P97 puller and fire-polished to resistances of 10–20 MΩ were front-filled and then backfilled. Symmetric bath and pipette solutions contained 150 mM Na methanesulfonate, 10 mM Na EDTA, and 10 mM Na HEPES, pH 7.4. These conditions yielded tight seals in 26% of patch attempts on patient red cells and 38% of attempts on control red cells. On-cell patch currents were recorded with the Axopatch 1-D amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA) as previously described (74). Holding potential was −VP = −50 mV [expressed as the negative of the pipette potential (e.g., equivalent to the intracellular potential with respect to the pipette)]. To determine current-voltage relationships (I-V curves) in Clampex (PCLAMP 10, Axon Instruments), the real-time control window in gap-free mode was used to record current traces of 10–30 s duration at holding potentials ranging from −100 to +100 mV, in 25 mV increments. The bath reference electrode was a silver chlorided wire with a 3 M KCl agar bridge. Data were filtered at 500 Hz, digitized at 2 kHz by Clampex, and analyzed online by Clampfit subroutines of PCLAMP. Holding potentials were expressed as −VP, the negative of the pipette potential.
cRNA transcription and protein expression in Xenopus oocytes.
cDNA encoding RhAG mutant F65V (used as a control missense substitution for comparison to disease mutant F65S) was generated by oligonucleotide mutagenesis as previously described (67, 68); sequences are available upon request. RhAG cDNA and its mutant F65S were cloned by RT-PCR from total RNA of patient 1, verified by full-length sequencing, and subcloned into oocyte expression vector pXT7. Human RhAG and its mutant F65S, both COOH-terminally tagged with the HA antigen epitope sequence, as well as RhAG F65V and glycophorin B (GPB) were subcloned into oocyte expression vector pBF. Human AE1/SLC4A1, AE1 mutants E758K and S731P, and glycophorin A (GPA) cDNAs were previously described (68). All mutant constructs were validated by DNA sequencing. cRNAs were transcribed from linearized pXT7 templates using T7 RNA polymerase, and from linearized pBF templates with SP6 RNA polymerase.
Segments of ovarian lobes were excised from female Xenopus laevis (Department of Systems Biology, Harvard Medical School, Boston, MA) anesthetized with 0.17% tricaine according to protocols approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Ovarian fragments were incubated overnight at 17°C in 1.3 mg/ml collagenase Type A (Boehringer Mannheim) and gentamycin (50 ng/ml) in MBS solution [containing (in mM) 85 NaCl, 1 KCl, 2.4 NaHCO3−, 0.41 CaCl2, 0.33 CaNO3−, 0.82 MgSO42−, 10 HEPES, pH 7.40].
Individual, manually defolliculated Stage V-VI oocytes were injected with 50 nl of H2O or with 1–20 ng human RhAG cRNA as indicated, in the absence or presence of 1–10 ng cRNA encoding human GPB, human SLC4A1/AE1 cRNA. Injected oocytes were then maintained 3–4 days at 17°C in MBS plus gentamycin.
Immunoblot.
Crude particulate fractions were prepared as described (78) from oocytes previously injected with water or with cRNA encoding wild-type or mutant RhAG bearing the COOH-terminal HA epitope. Lysates were solubilized without heating in SDS load buffer, subjected to SDS-PAGE (12%), transferred to PVDF membrane, incubated with mouse monoclonal (mAb) anti-HA (Sigma, dilution 1:250), and developed with rabbit secondary anti-Ig (1:10,000).
Confocal immunofluorescence microscopy.
Unfixed oocytes injected 3 days earlier with water or with cRNA encoding wild-type or mutant RhAG bearing the COOH-terminal HA epitope (n = 10–12 per group) were blocked in phosphate-buffered saline plus 1% bovine serum albumin (PBS-BSA) for 1 h at 4°C, incubated 1 h at 4°C with anti-HA mAb (Sigma, dilution 1:100), then washed three times for 10 min in PBS at 4°C. Antibody-exposed oocytes were then fixed at 25°C for 30 min in PBS containing 3% paraformaldehyde, washed three times in PBS supplemented with 0.002% sodium azide, and again blocked in PBS-BSA containing 0.05% saponin for 1 h at 4°C. Antibody-labeled oocytes were then incubated 1 h at 4°C with Cy3-conjugated goat anti-rabbit Ig (Jackson Immunochemicals, dilution 1:200), and again thoroughly washed in PBS-BSA with saponin. Labeled oocytes were aligned in uniform orientation along a plexiglass groove and sequentially imaged through the ×10 objective of a Zeiss LSM-510 laser scanning confocal microscope, using the 543 nm laser line at 512 × 512 resolution at a uniform setting of 85% intensity, pinhole 160 (<2.0 Airy units), detector gain 685, Amp gain 1, zero amp offset. Polypeptide abundance at each oocyte surface was estimated (ImageJ version 1.38, National Institutes of Health, Bethesda, MD) from specific fluorescence intensity (FI) at the circumference of one quadrant of an equatorial focal plane (Fig. 4). Mean background-corrected FI for quadrants of oocytes previously injected with water was subtracted from the background-corrected FI for quadrants of individual cRNA-injected oocytes to yield intensity values for surface-associated specific FI for each oocyte.
Fig. 4.
Surface expression of human RhAG-HA or RhAG-HA F65S in Xenopus oocytes is not increased by coexpression of glycophorin B (GPB). A: anti-HA immunoblot of lysates from oocytes expressing HA-tagged wild-type, F65S, or F65V mutant polypeptides. Each lane was loaded with lysate of particulate fraction prepared from 10 oocytes. One of two blots with similar results is shown. B–F: confocal immunofluorescence images of median intensity HA antigen surface expression by Xenopus oocytes expressing COOH-terminally HA-tagged wild-type RhAG (B and C) or RhAG mutant F65S (10 ng cRNA; D and E) in the presence (C and E) or absence (B and D) of coexpressed GPB (10 ng cRNA). A representative water-injected oocyte us shown in F. G: surface expression of wild-type RhAG-HA and RhAG-HA F65S measured as mean relative fluorescence intensities. Values are means ± SE for (n) oocytes, and did not differ one from another (Tukey-Kramer analysis and Dunnett's t-test).
Isotopic influx assays in Xenopus oocytes.
Unidirectional influx of 14C-MA was measured in Xenopus oocytes as previously described (43, 78). Six oocytes in 200 μl SOS uptake buffer [containing (in mM) 100 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, pH 7.6] with added 1 μCi/ml 14C-MA (20 μM) and 1.5 mM unlabeled MA were incubated for 60 min. Uptake was stopped by washing the oocytes six times with 1 ml of ice-cold unlabeled uptake buffer. Oocytes were then solubilized in 200 μl of 5% SDS and analyzed for radioactivity by liquid scintillation counting.
Unidirectional 86Rb+ influx studies (representing K+ influx) were carried out for 30 min periods in ND-96 containing 1 μCi 86RbCl. Oocytes were preincubated for 30 min in ND-96 containing 500 μM ouabain and 5 μM bumetanide as indicated. Some influx experiments were performed in the presence of DIDS (500 μM), amiloride (0.1 and 1.0 mM), NH4Cl (5 mM), LiCl (5 mM), RbCl (5 mM), KCl (5 mM), GsMTx-4 (1 μM), phloretin (500 μM), or Gd3+ (50 μM). High K+ bath solutions were prepared by substituting 96 mM NaCl with 96 mM KCl.
Net lithium influx measurements in Xenopus oocytes.
Oocytes previously injected with wild-type or mutant RhAG cRNA or with H2O were incubated at room temperature for 2 h in Li-MBS [containing (in mM) 85 LiNO3, 1 KNO3, 2.4 NaHCO3, 0.41 CaCl2, 0.33 Ca(NO3)2, 0.82 MgSO4, 15 Tris-HNO3, pH 7.40] with added ouabain (500 μM) and bumetanide (10 μM). Following incubation, oocytes were washed three times in ice-cold double-distilled (dd) H2O, individual oocytes were transferred to microcentrifuge tubes, and excess H2O was removed. Oocyte samples were dried at 95°C, dissolved in 50 μl 0.1 N NaOH, and diluted with an additional 250 μl dd H2O. Lithium content was measured by atomic absorption spectroscopy.
Microelectrode measurement of oocyte intracellular pH and membrane potential.
Oocyte pHi was measured as described previously (66) with pH microelectrodes during bath superfusion at 3–4 ml/min. Microelectrodes were pulled on a Kopf model 730 vertical puller (Tujunga, CA) and had resistances of 2–3 MΩ. The electrodes were calibrated at pH values of 6.0, 7.0, and 8.0, and exhibited slopes of at least 54 mV/pH unit before and after the experiments.
Oocyte two-electrode voltage clamp.
Borosilicate pipettes pulled with a Sutter P-87 micropipette puller had resistances of 2–10 MΩ when filled with 3 M KCl. Xenopus oocytes expressing wild-type or mutant RhAG polypeptides were impaled, and Vm was allowed to stabilize. Voltage pulse protocols generated with Clampex consisted of 400 ms steps from −100 mV to +40 mV. Holding potential between episodes was −30 mV. Steady-state currents were measured with measured with a Geneclamp 500 amplifier connected to a computer with a Digidata 1322A interface (Molecular Devices). Data were acquired and analyzed with Clampex routines from PCLAMP 10. In some oocytes, current was monitored over time at a fixed holding potential of −30 mV.
RESULTS
Hematological indices.
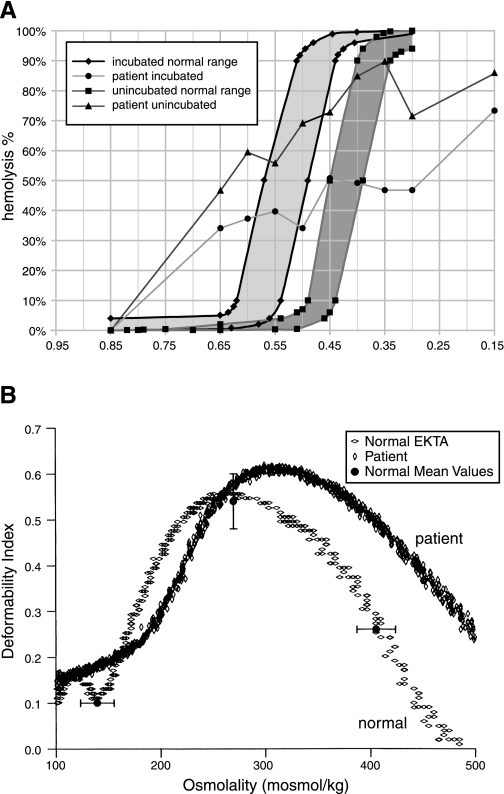
Hematological indices for the four patients are presented in Table 1. Severity of anemia varied among them, but all had macrocytosis beyond the magnitude expected for the degree of reticulocytosis. Osmotic fragility testing of patient 1 erythrocytes revealed a large subpopulation of osmotically fragile cells, as well as a second fraction of cells of anomalous osmotic resistance (Fig. 2A) despite the modest increase in cation content produced by overnight incubation at 4°C (Table 2). Ektacytometry testing (Fig. 2B) confirmed this broad range in osmotic fragility and the presence of a population of large cells, consistent with the volume distribution measured by the Advia 120 (Table 1). The cellular changes did not lead to a significantly increased deformability in isotonic conditions (290 mosmol). The shift of the curve to higher osmolality was more pronounced at the hyperosmolality side. A maximum deformability, slightly greater than 1 SD above normal, was reached between 310 and 320 mosmol. Together, these data confirm altered characteristics of these cells under different osmotic conditions.
Table 1.
Hematological indices
| Hb, g/dl | Hct, % | Retics, % | MCV, fl | MCHC, g/dl | RDW | Bili, mg/dl | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 10.6 | 40.1 | 15.4 | 127 | 26.3 | 13.3 | |
| Mother | 13.7 | 39.6 | 1.7 | 83.5 | 34.4 | 13.4 | |
| Father | 13.7 | 39.1 | 1.8 | 93.4 | 35.0 | 11.8 | |
| Patient 2 | 8.0 | 31.2 | 43.4 | 129.6 | 25.4 | 12.4 | 7.8 (total) |
| Patient 3 | 12.0 | 28 | 130 | 28 | |||
| Patient 4 | |||||||
| Pre-Splx* | 8.0 | 25.7 | 34.6 | 122 | 31.3 | 2.9 (indirect) | |
| Post-Splx* | 11.0 | 11–21 | 115 | 29.3–30 |
Fig. 2.
Fragility of stomatocytosis red cell membrane. A: osmotic lysis curves. X-axis values represent relative tonicity. Normal range of control red cells unincubated (▪) or after 24-h incubation as heparinized whole blood at 37°C (♦). Patient 1 red cells unincubated (▴) or after 24 h incubation (•). B: osmotic deformability curves measured by ektacytometry (EKTA) for red cells from patient 1 (vertical ◊) compared with a normal control (horizontal ◊). Normal mean values for three values of osmotic strength are portrayed as filled circles ± SD. Omin was 177 (normal range 140 ± 16.0); Dimax was 0.62 (normal range 0.54 ± 0.06); Ohyp was 482 (normal range 406 ± 18.3). Terms are described in methods.
Table 2.
Red cell cation content
| Na, mmol/l cells | K, mmol/l cells | Na + K, mmol/l cells | Na, mmol/kg Hb | K, mmol/kg Hb | Na + K, mmol/kg Hb | |
|---|---|---|---|---|---|---|
| Fresh red blood cells | ||||||
| Patient 1 | 87.7 | 35.1 | 122.8 | 373 | 149 | 522 |
| Mother | 8.2 | 98.0 | 106.2 | 26.4 | 317 | 343 |
| Father | 6.1 | 87.5 | 93.6 | 19.4 | 279 | 298 |
| O/N 4°C (EDTA) | ||||||
| Patient 1 | 94.6 | 38.9 | 133 | 402 | 165 | 567 |
| Mother | 9.1 | 90.0 | 99.1 | 29 | 291 | 320 |
| Father | 7.3 | 74.4 | 81.7 | 23 | 237 | 260 |
| Patient 2 | 82.5 ± 3.5 | 50.5 ± 2 | 133 ± 2.7 | 313 ± 18 | 192 ± 11 | 505 ± 15 |
| Control | 10.3 ± 0.3 | 97.9 ± 3 | 108 ± 1.7 | 28.1 ± 3.0 | 314 ± 4 | 342 ± 3.5 |
| Patient 4 | ||||||
| Pre-Splx* | 61.0 | 54.8 | 115.8 | |||
| Post-Splx* | 73.0 | 37.0 | 110.0 | |||
| Post-Splx† | 74‡ | 67‡ | 141‡ | |||
Patient 1 values are means from single triplicate determinations. Patient 2 values are means ± SE for 6 determinations. Patient 2 “control” is from an unrelated volunteer donor without known erythrocyte pathology. Data are unavailable for patient 3. Data for patient 4 are taken from Ref. 42 (*) and Ref. 33 (†).
Units of mEq/1013 cells.
O/N, overnight; Splx, splenectomy.
Red cell ion contents and ion transport activities.
Freshly isolated erythrocytes of patients 1, 2, and 4 were marked by elevated Na contents and reduced K contents, and these values increased little after overnight incubation at 4°C (Table 2; ion content data were unavailable for patient 3). Patient 1 red cells exhibited ouabain-insensitive rates of Na “leak” efflux 70- to 90-fold higher than red cells from either parent. Ouabain-insensitive K efflux leak was 10- to 15-fold higher than in red cells from either parent. Both Na and K leak efflux rates fell to near zero upon temperature reduction from 37°C to 0°C. Ouabain-sensitive fluxes were not available from patient 1. K-Cl cotransport in isotonic conditions was fourfold elevated in patient 1 red cells compared with parental red cells, and was insensitive to further stimulation by hypotonicity, in contrast to parental cells (Supplemental Table S3). NKCC and NHE activities were unchanged in red cells of patient 2 (data not shown).
Patient 1 red cells exhibited increased cation channel-like activity, as shown in the cell-attached patch record in Fig. 3A. Estimated single-channel conductance measured at −Vp = −50 mV was 22 pS (Fig. 3B). Mean NPo in four stable gigohm seals was ∼20-fold higher than observed in five stable seals on red cells from the patient's unaffected father (Fig. 3C) and in our previous recordings from normal red cells (74). There was no overlap between the groups. Similar currents were recorded from gigohm seals on three red cells of patient 2 (not shown).
Fig. 3.
Ion channel activity of stomatocytosis red cells. A: on-cell patch record from a representative red cell of patient 1. B: current-voltage relationship of currents from patch in A. C: values of NPo (number of active channels in patch × channel open probability) for on-cell patch currents from RHAG F65S stomatocytes from patient 1, compared with pooled results from two normal red cells of the patient's father and three red cells with indistinguishable NPo values from a normal volunteer (means ± SE). *P < 0.05 by Mann-Whitney rank sum test.
Mutation detection.
Since cation leak-stomatocytosis has been associated with mutations in the SLC4A1 and RhAG genes, both were examined in these patients. The eAE1/SLC4A1 cDNA sequence (NM_000342) was normal in patient 1. Patient 2 was heterozygous for the AE1 coding polymorphism V862I (rs5026, c.2733G>A, heterozygosity 0.069) and for the synonymous polymorphism at D896 (rs45497993, c.2837T>C, heterozygosity 0.187). Genomic DNA of patients 3 and 4 exhibited no mutations in exons and flanking sequences of the AE1/SLC4A1 gene.
In contrast, all four patients uniformly exhibited the single, heterozygous RhAG mutation F65S. Patients 1 and 2 were heterozygous for the c.256T>C substitution in codon 65 of the RhAG cDNA (NM_000324), encoding missense substitution F65S in the RhAG polypeptide. The same heterozygous mutation was also demonstrated in PCR-amplified exon 2 of patient 1's RhAG gene (Fig. 1C). The heterozygous RhAG F65S mutation in patients 3 and 4 was detected by SSCP screening and directed exon sequencing.
The RhAG cDNA sequences and the genomic exon 2 sequences of patient 1's parents were normal. Thus, patient 1's RhAG F65S mutation appears to be a de novo mutation. Parents of patients 2, 3, and 4 were unavailable for RhAG sequence analysis. Previously reported RhAG F65S mutations were autosomal dominant in five kindreds and de novo in one patient (7).
RhAG and RhAG F65S mutant HA-tagged polypeptides are expressed at the Xenopus oocyte surface.
Wild-type and mutant RhAG cDNAs were COOH-terminally tagged with HA-epitope, and transcribed cRNA was injected into Xenopus oocytes. Total RhAG-HA F65S polypeptide accumulation was moderately decreased compared with wild-type RhAG, as detected by anti-HA (Fig. 4A) and by anti-RhAG mAb 2D10 (not shown, and ref. 78), consistent with reduced abundance of RhAG F65S in OHSt patient red cells (7). However, confocal immunofluorescence microscopy indicated that wild-type and mutant RhAG proteins were present at or near the oocyte surface at indistinguishable levels (Fig. 4, B–G). Coexpression of GPB, an erythroid RhAG-binding protein (3, 57, 73), did not alter abundance of either wild-type or mutant RhAG polypeptide at the oocyte periphery under the conditions studied.
Cation transport in oocytes expressing RhAG and RhAG mutant F65S.
The first report of stomatocytosis associated with RhAG mutant F65S documented modest Li+ uptake by oocytes expressing wild-type RhAG (10 ng cRNA), and sixfold higher Li+ influx into oocytes expressing the F65S mutant (7). Those experiments were performed with coexpressed GPB, a component of the human erythrocyte membrane complex that includes RhAG, RhD, and RhCE, and perhaps also AE1 (6, 57). We found (Fig. 5A) that oocytes expressing wild-type RhAG alone exhibited greatly increased Li+ influx, with only modest incremental Li+ influx evident in oocytes expressing the F65S mutant at cRNA levels equivalent to those reported previously (7). The minimal stimulation of Li+ influx in the setting of 1 ng injected RhAG cRNA was substantially increased by coexpression of GPB (Fig. 5B). Although RhAG surface expression in K562 cells was enhanced by coexpression of AE1 (3), RhAG-associated Li+ influx into oocytes was not enhanced by AE1 coexpression (Fig. 5C).
Fig. 5.
Human RhAG F65S mediates increased lithium uptake compared with wild-type RhAG. A: lithium uptake by oocytes previously injected with water or with cRNA encoding wild-type RhAG or RhAG mutant F65S (10 ng cRNA). Values are means ± SE for (n) oocytes. *P < 0.05 (gray bar) compared with wild-type RhAG (Dunnett's t-test). B: lithium uptake by oocytes expressing wild-type RhAG was increased in the presence of coexpressed GPB (1 ng injected cRNA of each type). Values are means ± SE (n) oocytes. *P < 0.05 (gray bar) compared with wild-type RhAG (Dunnett's t-test). C: coexpression of wild-type AE1 with wild-type RhAG did not increase RhAG-mediated lithium uptake (1 ng cRNA). Ouabain (0.5 mM) and bumetanide (5 μM) were present in all experiments. P > 0.05 compared with wild-type RhAG (Dunnett's t-test).
If RhAG expression is indeed associated with nonspecific cation channel activity, it might be expected also to increase K+ “leak” permeability. Figure 6A shows that ouabain- and bumetanide-resistant 86Rb+ influx increased more than twofold with RhAG expression, and more than threefold with expression of RhAG mutant F65S. These rates of 86Rb+ influx from 2 mM bath K+ were nearly 100-fold lower than Li+ influx rates (Fig. 5), perhaps in part reflecting the 50-fold difference in substrate concentrations for bath K+ and Li+. GPB coexpression modestly enhanced RhAG-associated 86Rb+ uptake, but coexpression of AE1 was without stimulatory effect (Fig. 6, B and C).
Fig. 6.
Human RhAG F65S mediates increased ouabain-resistant 86Rb+ uptake compared with wild-type RhAG. A: 86Rb+ uptake by oocytes previously injected with water or with cRNA encoding wild-type RhAG or RhAG F65S (10 ng cRNA). *P < 0.05 (gray bar) compared with wild-type RhAG (Dunnett's t-test). B: 86Rb+ uptake by oocytes expressing wild-type RhAG was enhanced by coexpressed GPB (*P < 0.05, Dunnett's t-test). Rates were water-subtracted. C: coexpression of wild-type RhAG with wild-type AE1 did not increase RhAG-mediated 86Rb+ uptake at low (1 ng) or higher cRNA concentrations (10 ng cRNA). Rates were water-subtracted. Ouabain (0.5 mM) and bumetanide (5 μM) bumetanide were present in all experiments. *P < 0.05 compared with wild-type RhAG (Dunnett's t-test).
Ouabain-sensitive 86Rb+ flux associated with expression of RhAG and RhAG mutant F65S.
The elevated Na content of stomatocytes heterozygous for RhAG F65S likely reflects the balance between elevated cation leak and (presumably secondary) elevated ouabain-sensitive transport of Na+ and K+, reflecting increased activity of erythroid Na+-K+-ATPase. Endogenous, oocyte NKCC also contributes to intracellular cation balance. We therefore examined ouabain- and bumetanide-sensitive 86Rb+ influx into Xenopus oocytes expressing various stomatocytosis-associated mutants. 86Rb+ influx was stimulated in RhAG-expressing oocytes, and to greater degree in oocytes expressing RhAG mutant F65S. Wild-type AE1 produced no elevation of activity, but oocytes expressing AE1 cation leak stomatocytosis mutants S731P or E758K activated ouabain-sensitive 86Rb+ uptake to levels lower than those of RhAG polypeptides (Fig. 7A). Rates of ouabain and bumetanide-resistant 86Rb+ influx into the same groups of oocytes were ∼40-fold slower, but with similar rank order (Fig. 7B). The increased 86Rb+ influx accompanying RhAG expression was mediated by increased activities of both endogenous Na+-K+-ATPase and endogenous Na-K-2Cl cotransport (Fig. 7C).
Fig. 7.
Expression of wild-type RhAG or of RhAG F65S upregulates Na+/K+ ATPase activity in Xenopus oocytes. A: 86Rb+ influx into Xenopus oocytes expressing wild-type RhAG, RhAG F65S, AE1 E758K, or AE1 S731P was sensitive to inhibition by ouabain (500 μM) and bumetanide (5 μM). Wild-type AE1 served as a negative control for 86Rb+ influx (67, 68). 10 ng cRNA was injected for all constructs. H2O-corrected values are means ± SE for (n) oocytes. *P < 0.05 compared with wild-type RhAG (Student's unpaired t-test). B: reproduction of data in A, with magnified y-axis scale showing 86Rb+ influx insensitive to ouabain + bumetanide, emphasizing that wild-type RhAG and its F65S mutant increase 86Rb+ “leak” flux insensitive to ouabain and bumetanide. *P < 0.05 compared with wild-type RhAG. C: inhibition of RhAG-associated 86Rb+ influx by 0.5 mM ouabain alone, by 5 μM bumetanide alone, and by the combination. D: 3 days′ incubation of oocytes coexpressing RhAG and GPB in high K+ bath (gray bars) significantly reduced 86Rb+ influx sensitive to inhibition by ouabain + bumetanide (compare y-axis values to A and C). Black bars indicate 3 days' incubation in ND-96.
To the degree that elevated ouabain-sensitive 86Rb+ influx in RhAG-expressing oocytes reflects oocyte Na+ loading secondary to RhAG-associated increased cation permeability, then substitution of bath Na+ with K+ during the 72 h period preceding assay might suppress increased pump activity. Indeed, incubation of oocytes coexpressing RhAG and GPB in high-K medium reduced ouabain- and bumetanide-sensitive 86Rb+ influx by 94% (compare y-axes of Fig. 7, D and A), while the component of 86Rb+ influx insensitive to ouabain and bumetanide was unchanged (Fig. 7D). This result is consistent with activation of Na+-K+-ATPase secondary to oocyte Na+ loading by the RhAG expression-associated cation leak. Activation of endogenous oocyte NKCC has been noted previously in the setting of overexpression of trout AE1 Cl−/HCO3− exchanger (29).
Pharmacological properties of RhAG-associated cation fluxes.
If increased ouabain-insensitive uptakes of Li+ and 86Rb+ associated with expression of RhAG or RhAG mutant F65S polypeptides reflect the same cation permeability pathway, they should exhibit similar pharmacological profiles. Figure 8A shows that RhAG-associated ouabain-insensitive 86Rb+ influx was DIDS-insensitive, but completely inhibited by 1 mM amiloride and by 500 μM phloretin, and nearly completely by 5 mM NH4Cl and by 50 μM Gd3+. 86Rb+ uptake into uninjected oocytes was insensitive to 1 mM amiloride or to 500 μM phloretin, but was inhibited 65% by either 5 mM NH4Cl or 5 mM MA (n = 9–12, not shown). This inhibition of endogenous ouabain- and bumetanide-insensitive 86Rb+ influx accounted for 35% of the inhibition by NH4Cl or MA of 86Rb+ influx into RhAG-expressing oocytes (Fig. 8, A and B). RhAG-associated ouabain-insensitive Li+ uptake was also DIDS-insensitive, and showed substantial inhibition by amiloride, phloretin, and Gd3+. However, neither 5 mM NH4Cl (Fig. 8C) nor 5 mM MA (not shown P = 0.2, n = 10) inhibited Li+ influx. The ouabain- and bumetanide-insensitive Li+ uptake into uninjected oocytes (only 5–10% of that into RhAG-expressing oocytes) was insensitive to DIDS and to phloretin, but inhibited 35% by amiloride, 20% by Gd3+, 22% by NH4Cl, and completely by MA (P < 0.05; n = 10, not shown).
Fig. 8.
Pharmacological profiles of Rb+ influx and lithium uptake into oocytes expressing wild-type RhAG or RhAG F65S. A: effects of inhibitors on 86Rb+ influx into Xenopus oocytes expressing wild-type RhAG. B: effects of inhibitors on 86Rb+ influx into oocytes expressing RhAG F65S. C: effects of inhibitors on Li+ influx into oocytes expressing wild-type RhAG. D: effects of inhibitors on Li+ influx into oocytes expressing RhAG F65S. Values are means ± SE for (n) oocytes. Inhibitor concentrations were DIDS (500 μM), amiloride (1 mM), NH4Cl (5 mM), methylammonium (MA; 5 mM), phloretin (500 μM), and Gd3+ (50 μM). Ouabain (0.5 mM) and bumetanide (5 μM) were present in all experiments. *P < 0.05 (gray bar) compared with absence of inhibitor (Dunnett's t-test). Inhibitor effects on uninjected oocytes are summarized in results.
Ouabain-insensitive 86Rb+ influx associated with RhAG F65S was only slightly less sensitive to inhibition by amiloride, phloretin, Gd3+, and NH4Cl than that associated with wild-type RhAG. MA (5 mM) inhibited RhAG F65S-associated 86Rb+ influx to the same degree as 5 mM NH4Cl (Fig. 8B). Li+ influx associated with RhAG F65S shared with RhAG sensitivity to amiloride and phloretin, but unlike RhAG was insensitive to inhibition by 50 μM Gd3+. Thus, wild-type and F65S mutant RhAG differed in their pharmacological inhibition profiles, and each exhibited distinct inhibition profiles for uptake of 86Rb+ and for Li+. Influx of 86Rb+ (n = 8) and of Li+ (n = 21) into RhAG-expressing oocytes was insensitive to inhibition by 1 μM GsMTx-4 (not shown), an inhibitor of stretch-activated cation channels.
RhAG-mediated 14C-MA transport is greatly reduced by the F65S mutation.
MA has long been considered a surrogate substrate for NH3/NH4+ in the study of yeast and plant ammonium transport (37), and MA uptake into RhAG-expressing Xenopus oocytes exhibited affinity similar to that of NH3/NH4+ (43, 78). RhAG-mediated uptake of 14C-MA into Xenopus oocytes (78) was unchanged by addition of a COOH-terminal HA tag, and not further stimulated under these conditions by coexpression of GPB (Fig. 9A), RhCE, GPB + RhCE in combination, or AE1 (not shown). 14C-MA uptake by RhAG was inhibited by bath addition of 5 mM NH4Cl, as shown previously (78), but not by bath addition of 50 μM Gd3+, 500 μM phloretin, 5 mM Li+, 5 mM Rb+, or 5 mM K+ (Fig. 9B). 14C-MA uptake was also unaffected by 500 μM DIDS, by 100 μM ouabain, and by bath substitution of 96 mM bath NaCl with 96 mM LiCl, LiNO3 (not shown), KCl, or N-methyl-d-glucamine (NMDG) chloride (78). Remarkably, the mutant RhAG F65S exhibited severely reduced 14C-MA uptake. 14C-MA uptake by oocytes expressing engineered mutant F65V resembled that of oocytes expressing wild-type RhAG (Fig. 9C), demonstrating the importance of the substituted amino acid side chain at position 65. RhAG F65S-associated 14C-MA uptake was not further stimulated by coexpression with GPB (not shown).
Fig. 9.
14C-MA uptake by wild-type RhAG and mutant F65S. A: rates of 14C-MA uptake by oocytes previously injected with water or with cRNA encoding RhAG or RhAG (6 ng) coexpressed with GPB (6 ng). Values are means ± SE for 20 oocytes. B: pharmacological profile of 14C-MA influx by oocytes expressing RhAG without or with coexpressed GPB. Values are means ± SE for 20 oocytes. *P < 0.05 vs. no added drug; ANOVA. C: time course of 14C-MA uptake (1.5 mM MA) by oocytes previously injected with water (n = 18) or with 23 ng cRNA encoding RhAG-HA (n = 16), RhAG-F65S-HA (n = 18), or RhAG-F65V-HA (n = 18). All time points represent 3 experiments, each with 5–6 oocytes. Results were indistinguishable in the absence of the HA tag (not shown).
Differential effects of NH4Cl and MA on Vm and pH of oocytes expressing RhAG and RhAG mutant polypeptides.
Comparison of MA/MA+ transport with that of NH3/NH4+ in oocytes expressing the RhAG-related epithelial Rhbg polypeptide from mouse revealed substantial differences in transport properties (50) and/or metabolic consequences of oocyte accumulation of NH3/NH4+ and of MA/MA+. We therefore compared the effects of MA/MA+ transport with those of NH3/NH4+ transport in oocytes expressing RHAG wild-type and RhAG mutant polypeptides.
Figure 10A shows that a representative oocyte expressing wild-type RhAG responded to 5 mM NH4Cl with enhanced reversible depolarization (top trace) and pHi acidification (bottom trace), suggesting predominant transport of NH4+, as reported previously for Rhbg (50). In contrast, the reversible depolarization produced by subsequent bath addition of 5 mM MA was accompanied by reversible alkalinization, consistent with predominant transport of the uncharged species, together with slower transport of MA+ [and/or of other cation(s)]. Similar changes in Vm and pHi were exhibited by a representative oocyte expressing the 14C-MA transport-competent RhAG mutant F65V in response to transient, sequential bath addition of NH4Cl and MA (Fig. 10B). An oocyte expressing the 14C-MA transport-defective RhAG stomatocytosis mutant F65S resembled oocytes expressing wild-type RhAG and mutant F65V in its reversible NH4Cl-induced depolarization and acidification. However, this F65S-expressing oocyte responded to MA exposure with attenuated alkalinization and, remarkably, a reversible hyperpolarization (Fig. 10C) that contrasted dramatically with the MA-induced reversible depolarization exhibited by wild-type and F65V oocytes.
Fig. 10.
Effects of NH4Cl and MA on intracellular pH and membrane potential in RhAG expressing oocytes. A, top: trace of membrane potential (Vm) recorded from representative oocytes previously injected with 20 ng cRNA encoding wild-type RhAG, during sequential bath application and removal of 5 mM NH4Cl followed by 5 mM methylammonium Cl (MA). A, bottom: simultaneously recorded trace of intracellular pH (pHi) from the same oocyte. B, top: Vm trace recorded from representative oocyte expressing wild-type RhAG-F65V (20 ng cRNA) during sequential bath application and removal of 5 mM NH4Cl followed by 5 mM MA. B, bottom: simultaneous pHi trace from the same oocyte. C, top: Vm trace from representative oocyte expressing wild-type RhAG-F65S (20 ng cRNA) during sequential bath application and removal of 5 mM NH4Cl followed by 5 mM MA. C, bottom: simultaneous pHi trace from the same oocyte.
Figure 11 summarizes the results from multiple experiments in the format of those shown in Fig. 10. Resting oocyte Vm recorded in ND-96 was depolarized 15–20 mV in oocytes expressing RhAG or mutant RhAG polypeptides (P < 0.05, Fig. 11A). Oocyte expression of RhAG or RhAG mutants F65V or F65S did not produce a statistically significant change in oocyte basal pHi (Fig. 11C). Resting pHi in water-injected oocytes was also unaltered by sequential exposure first to 5 mM NH4Cl and then to 5 mM MA (Fig. 11D). The same water-injected oocytes were depolarized 9 mV by 5 mM NH4Cl, but after removal of NH4Cl, subsequent exposure to 5 mM MA had no effect on Vm (Fig. 11B). Oocytes expressing wild-type RhAG or 14C-MA transport-competent RhAG mutant F65V were reversibly depolarized 15 or 26 mV, respectively, by 5 mM NH4Cl. Subsequent exposure to 5 mM MA reversibly depolarized the same oocytes by 9 or 21 mV, respectively. Vm of oocytes expressing 14C-MA transport-defective RhAG mutant F65S was depolarized only 4 mV by bath addition of 5 mM NH4Cl (P < 0.01 vs. wild-type RhAG and vs. RhAG F65V, Tukey-Kramer test). In marked contrast, Vm of RhAG F65S-expressing oocytes was hyperpolarized −12 mV by subsequent bath exposure to 5 mM MA (Fig. 11B, P < 0.01 vs. water, wild-type RhAG, and RhAG F65V). Consistent with this hyperpolarization, bath addition of 5 mM MA to oocytes expressing RhAG mutant F65S and clamped at −30 mV elicited a peak outward (hyperpolarizing) current of +11 ± 6 nA (n = 10) in contrast to the inward (depolarizing) current of −36 ± 7 nA elicited in oocytes expressing wild-type RhAG at the same clamp potential (n = 10, P < 0.001, not shown).
Fig. 11.
Summarized effects of NH4Cl and MA on Vm and pHi in oocytes expressing wild-type RhAG and mutants F65V and F65S. A: resting Vm in oocytes expressing wild-type and mutant RhAG, compared with Vm of water-injected oocytes (*P < 0.05 vs. water, Dunnett's t-test). B: change in membrane potential (ΔVm) elicited by bath addition of 5 mM NH4Cl (black bars) or by 5 mM MA (gray bars) in oocytes previously injected with water or with cRNA encoding wild-type RhAG, RhAG mutant F65V, or RhAG stomatocytosis mutant F65S. *P < 0.05 vs. water-injected oocytes for NH4+ exposure, and vs. water-injected oocytes for MA exposure; Dunnett's t-test. [Tukey-Kramer analysis indicated that NH4Cl-induced ΔVm of F65V and F65S both differed from wild-type RhAG (P < 0.01), and that MA-induced ΔVm of F65V and F65S also both differed from wild-type RhAG (P < 0.01)]. C: resting pHi was statistically indistinguishable among oocytes expressing wild-type and mutant RhAG and water-injected oocytes. D: change in pHi elicited by bath addition of 5 mM NH4Cl (black bars) or by 5 mM MA (gray bars) in oocytes previously injected with water or with cRNA encoding wild-type RhAG or mutants F65V or F65S. *P < 0.05 vs. water-injected oocytes for NH4+ exposure, and vs. water-injected oocytes for MA exposure; Dunnett's t-test. Values are means ± SE for (n) oocytes.
The depolarizations induced by NH4Cl and by MA in oocytes expressing wild-type RhAG were accompanied by contrasting pHi responses of NH4Cl-induced acidification and MA-induced alkalinization (Fig. 11D), as recently reported for Rhbg (50). NH4Cl acidified oocytes expressing wild-type RhAG (P < 0.05), but intracellular acidification in oocytes expressing RhAG mutants F65S or F65V fell short of statistical significance (vs. water-injected or RhAG-expressing oocytes). Subsequent exposure to MA alkalinized oocytes expressing wild-type RhAG or mutant F65V (P < 0.01), but alkalinization of oocytes expressing RhAG F65S was not statistically significant (Fig. 11D).
Steady-state oocyte currents associated with expression of RhAG and RhAG mutant polypeptides.
The above amine-induced changes in Vm prompted examination of steady-state two-electrode voltage-clamp currents in oocytes previously injected with water or with cRNA encoding RhAG or RhAG mutant F65S. As shown in Fig. 12, A–C, and summarized in Fig. 12D, RhAG expression increased the magnitude of inward linear currents measured in NaCl bath by more than threefold, and depolarized reversal potential from −44 to −20 mV. Expression of RhAG F65S additionally increased steady-state inward currents measured in NaCl bath to a value fivefold greater than in water-injected oocytes, without further change in reversal potential. Sequential bath substitution of ND-96 first with 96 mM LiCl and then with ND-96 containing 5 mM NH4Cl (at constant [K+], [Ca2+], and Mg2+]), produced no significant changes in mean steady-state currents associated with expression of RhAG (Fig. 12B) or of RhAG mutant F65S (Fig. 12C and Supplemental Fig. S1). Addition to NaCl bath of 5 mM MA also failed to change steady-state currents to oocytes expressing RHAG (n = 10), OHSt mutant F65S (n = 10) or engineered mutant F65V (n = 9; not shown). Oocyte currents associated with expression of either RhAG or RhAG F65S were partially inhibited by 1 mM amiloride (29–33%) and 500 μM phloretin (26–34%), and marginally inhibited by 50 μm Gd3+ (13%), as summarized in Supplemental Table S4.
Fig. 12.
Steady-state current-voltage (I-V) relationships in oocytes previously injected with water or with 20 ng cRNA encoding wild-type RhAG or mutant F65S. A: I-V curves recorded from oocytes previously injected with water and exposed sequentially to baths of ND-96, 96 mM LiCl, and ND-96 containing 5 mM NH4Cl. B: I-V curves recorded from oocytes previously injected with 20 ng cRNA encoding wild-type RHAG and exposed sequentially to baths of ND-96, 96 mM LiCl, and ND-96 containing 5 mM NH4Cl. C: I-V curves recorded from oocytes previously injected with 20 ng cRNA encoding RHAG stomatocytosis mutant F65S and exposed sequentially to baths of ND-96, 96 mM LiCl, and ND-96 containing 5 mM NH4Cl. Results qualitatively similar to those of B and C were observed in oocytes injected with 10 ng cRNA (n = 10–12 each group, not shown). D: mean current magnitude at holding potential of −100 mV in oocytes previously injected with water, wild-type RHAG, and RHAG F65S. Values are means ± SE for (n) oocytes from 2 frogs in A–C, and from 3 frogs in D. *P < 0.05 compared with wild-type RhAG; **P < 0.005 compared with water (ANOVA with Bonferroni correction).
DISCUSSION
We describe here four North American patients with overhydrated stomatocytosis (OHSt) heterozygous for RhAG missense mutation F65S. Xenopus oocytes expressing RhAG exhibited ouabain- and bumetanide-insensitive uptake of 14C-methylammonium (MA), 86Rb+, and Li+. Oocytes expressing RhAG F65S polypeptide exhibited severely reduced influx of 14C-MA, accompanied by moderately increased ouabain-insensitive uptake of 86Rb+ and Li+. Cation influxes associated with expression of wild-type and mutant RhAG polypeptides exhibited distinct pharmacological profiles. RhAG-expressing oocytes were slowly depolarized by NH4Cl and by MA, whereas MA exposure rapidly hyperpolarized oocytes expressing RhAG F65S. NH4Cl acidified and MA alkalinized pHi in oocytes expressing wild-type RhAG, but neither pHi response was statistically significant in oocytes expressing RhAG F65S. We conclude that RhAG F65S expressed in Xenopus oocytes exhibits both the gain-of-function phenotypes of enhanced currents and enhanced Rb+ and Li+ permeabilities (likely endogenous), and the loss-of-function phenotype of severely decreased MA/MA+ transport with altered properties. We further show that transport of NH3/NH4+ or of MA/MA+ by oocytes expressing wild-type RhAG produces opposing changes in pHi, and that these changes are attenuated by the OHSt mutation F65S. Thus, although structural modeling, mammalian cell expression, and proteoliposome reconstitution experiments are consistent with electroneutral transport of NH3 by RhAG, RhAG expression in Xenopus oocytes appears to activate additional electrogenic cation transport pathways differentially utilized and/or regulated by NH3/NH4+ and MA/MA+, and altered by the F65S mutation.
Clinical genetics.
Seventeen of the 18 OHSt patients with defined RhAG mutations reported to date, representing 10 unrelated cohorts and including the four presented in this paper, harbor a uniform, heterozygous mutation encoding the missense substitution F65S. Patient 1 reported here appears to harbor a de novo mutation, as does one previously reported OHSt patient with the F65S mutation. Only one other RhAG mutation, I61R, has been reported in a single OHSt patient (7). Thus, the F65S mutation in a highly conserved RhAG residue represents a mutational hot-spot for RhAG-associated familial and de novo OHSt.
RhAG variants also encode the RHAG blood group system comprising blood group antigens Duclos-negative (homozygous RhAG Q106E), DSLK-negative (homozygous K164Q), and Ol(a+) (heterozygous S227L) located in putative extracellular loops of RhAG (72). Rh(mod) red cells homozygous for Ol(a+) S227L exhibited decreased red cell abundance of RhAG and Rh polypeptides, accompanied by decreased RhAG macrocomplex components CD47, LW, and U antigen (72). At least 10 other RhAG missense mutations have been associated with Rh(mod) or with Rh(null) phenotypes (7, 72). Thus, OHSt RhAG mutation F65S, associated with decreased RhAG (Fig. 4A), Rh polypeptides, and stomatin (7), might be considered a clinical subset within the larger immunological group of Rh(mod) mutations.
RhAG F65S-associated OHSt red cell properties.
Only some OHSt patient red cells exhibit stomatocytic morphology (Fig. 1A), and the elevated MCV was not accompanied by remarkable anisocytosis (Table 1). The relationships between this morphological heterogeneity and the phenotypes of partial stomatin deficiency (Fig. 1D), heterogeneity of osmotic fragility and altered deformability (Fig. 2), variable NPo of the increased leak current (Fig. 3), and constitutively elevated K-Cl cotransport activity (Supplemental Table S3) remain unknown. The increased K-Cl cotransport activity in patient red cells might be attributed in part to the younger mean cell age evidenced by the increased reticulocyte count (Table 2). Elevated K-Cl cotransport activity was also noted in OHSt red cells with the heterozygous AE1 H734R mutation, accompanied by altered KCC regulatory properties (5). The on-cell patch current measured in the absence of ouabain at 22°C (Fig. 3) is ∼ 50- to 500-fold higher than that expected (without temperature correction) from the ouabain-sensitive Na efflux leak measured at 37°C (Supplemental Table S3). This discrepancy and a similarly large discrepancy between net conductive anion flux in human red cells and red cell anion currents recorded by on-cell patch (1) likely reflect conductance activation by mechanical stresses within the patch.
RhAG F65S surface expression in Xenopus oocytes.
Although RhAG F65S accumulates to lower levels than the wild-type protein when expressed in red cells (7, 78) and in Xenopus oocytes (Fig. 4A), oocyte surface expression of the RhAG F65S mutant polypeptide in oocytes was not statistically reduced, whether in the absence or presence of glycophorin B (Fig. 4, B–G). These findings suggest that mutant polypeptide stability was reduced relative to wild-type in intracellular compartments, but not at the surface membrane. Glycophorin B coexpression enhanced wild-type RhAG-associated Li+ uptake only in the setting of RhAG cRNA injection in low ng quantities (Fig. 5B), as previously reported for enhancement of AE1 surface expression by glycophorin A (GPA). Oocyte surface expression of RhAG was not increased by coexpression of RhCE (78) or of AE1 (as judged by Li+ influx), in contrast to erythroid K562 cells (3), and likely reflecting the absence in oocytes of K562 cell erythroid scaffolding or other proteins of the erythroid membrane cytoskeletal macrocomplex1 (see p. C16).
Increased Li+ uptake associated with RhAG F65S expression in Xenopus oocytes.
The first report of the RhAG F65S mutation in cation-leak HSt attributed reversed Na and K contents in HSt red cells and Xenopus oocytes and increased oocyte Li+ transport to a novel nonspecific cation permeability reflecting steric and electrostatic alteration of the putative NH3/NH4+ permeation pathway through the mutant RhAG polypeptide (7). However, we found that oocyte Li+ influx was greatly elevated by wild-type RhAG expression, with only modest further increase conferred by the F65S mutation (Fig. 5). In this context, at least, the RhAG-expressing oocyte may not model with fidelity the normal red cell. Li+ bath substitution did not alter either increased 14C-MA uptake or elevated steady-state currents in oocytes expressing wild-type or F65S mutant RhAG (Fig. 12), consistent with distinct transport pathways for Li+ and MA. Among the still poorly defined Li+ transport pathways in red cells and/or oocytes are plasmalemmal Na+/H+ (9, 20) and Na+/Li+ exchange (54), vacuolar cation/H+ exchange (56, 80), Li+-Cl− cotransport (39), volume-activated Cl− conductance (2), and the Gd3+- and redox-sensitive cation conductance activated by amino acid transporter 4F2hc/LAT1 (75), which in red cells (61) binds and regulates OHSt gene product GLUT1 (53). Any of these transporters might also be modulated by Li+ inhibition of glycogen synthase-β or inositol-1-phosphatase.
Upregulation of oocyte Na+-K+-ATPase and NKCC activities by wild-type and F65S mutant RhAG.
The reversed Na and K contents in HSt red cells (Table 2 and Ref. 7) suggest substantial K+ permeability of the proposed nonspecific cation conductance of HSt red cells and RhAG F65S-expressing oocytes.
Indeed, ouabain- and bumetanide-insensitive 86Rb+ influx into oocytes expressing either wild-type RhAG or HSt mutant F65S was modestly increased (Fig. 6). The cation leak in red cells and oocytes was accompanied by 200-fold activation of ouabain- and bumetanide-sensitive 86Rb+ influx in oocytes expressing RhAG or RgAG F65S (Fig. 7), representing upregulation of both Na-K-ATPase and NKCC activities (pharmacologically defined). This endogenous K+ transport activity is 60% of rates achieved by heterologous overexpression of X. laevis α1 Na+-K+-ATPase subunit alone, or 30% of rates with coexpressed β1 (34). Elevated pump activity was shared by HSt red cells (46) and by oocytes expressing HSt mutants of AE1, but not wild-type AE1 (Fig. 7A). Moreover, ouabain- and bumetanide-sensitive 86Rb+ influx was reduced nearly 95% by maintenance of oocytes in 96 mM K+ bath during the 3-day period of RhAG protein synthesis (Fig. 7C), suggesting an important role for elevated intracellular Na content and reduced intracellular K content in upregulation of endogenous oocyte pump and cotransporter activities. Prolonged oocyte exposure to high ouabain concentrations (7) may have activated additional oocyte signaling pathways such as Src (82, 83), caveolin-1 (71), adducin (23), ERK/MAPK (63), and others (14, 16, 38, 71, 77, 81), further modulating ion transport activities.
Pharmacology of RhAG-associated cation leak, and a novel loss-of-function phenotype of RhAG mutant F65S.
If a uniform, nonspecific cation conductance were responsible for all observed RhAG-associated increased cation fluxes, the distinct ion fluxes might well exhibit identical pharmacological properties. However, while amiloride, phloretin, and Gd3+ inhibited both 86Rb+ and Li+ uptake associated with oocyte expression of wild-type RhAG, NH4Cl and MA inhibited only pump-independent 86Rb+ uptake, but not Li+ uptake. RhAG HSt mutant F65S-associated Li+ uptake was inhibited by NH4Cl and by MA, but was insensitive to inhibition by Gd3+. Notably, RhAG F65S-associated uptakes of both 86Rb+ and Li+ were comparably inhibited by 5 mM MA (Fig. 8).
If RhAG-associated uptake of Rb+ and Li+ represents permeation through the RhAG polypeptide, then 14C-MA uptake might display identical pharmacological properties. 14C-MA uptake associated with wild-type RhAG expression was reduced by 5 mM NH4Cl (Fig. 9B) (78), but was insensitive to inhibition by Rb+ and Li+ (Fig. 9B), ions whose uptake was inhibited by NH4+ (Fig. 8). RhAG-associated 14C-MA uptake was also insensitive to inhibition by Gd3+ and phloretin (Fig. 9B), both effective inhibitors of Rb+ and Li+ uptake (Fig. 8).
14C-MA uptake was nearly abolished in oocytes expressing RhAG F65S (Fig. 9C), accompanied by undiminished polypeptide surface expression (Fig. 4) and by increased, pharmacologically distinct, ouabain-insensitive uptakes of 86Rb+ and Li+. <!---->These data together suggest that overexpression of either RhAG or its F65S mutant likely increases endogenous oocyte cation permeabilities, rather than (or in addition to) cation conductance(s) intrinsic to the wild-type or mutant RhAG polypeptide. Evidence supporting activation of endogenous cation permeabilities has been presented for AE1 HSt mutants E758K (68) and H734R (5) and for NHE8 of Anopheles aegyptii (56). Rh polypeptide-associated NH4+ secretion by medaka skin ionocytes is similarly accompanied by elevated activity of Na+/H+ exchange and/or other H+ secretory mechanisms (79).
Electrophysiological properties of amine uptake by oocytes expressing wild-type and mutant RhAG.
The mechanism of ammonia transport by Rh family proteins remains controversial, with a range of evidence favoring either the uncharged or the charged species. In Xenopus oocytes, Rhbg may transport NH3/NH4+ and MA/MA+ by different mechanisms, or with distinct physiological consequences that differ at different amine concentrations (50, 51) (48, 49). Musa-Aziz et al. (49) used extracellular surface pH (pHs) electrodes to document extracellular surface acidification of RhAG-expressing oocytes in response to bath addition of 0.5 mM NH4Cl, representing predominance of RhAG-associated NH3 transport. We observed with intracellular pH electrodes (Figs. 10 and 11) that oocytes expressing wild-type RhAG acidified and depolarized in response to 5 mM NH4Cl, results similar to those exhibited by mouse Rhbg in oocytes studied with pHi microelectrode (50), and consistent with predominant inward transport of NH4+ (with or without other cations). The different findings likely reflect both the different NH4Cl concentrations applied to the oocytes and the different techniques used. The extracellular pHs electrode detects pHs excursions produced by the lower NH4Cl concentration of 0.5 mM, whereas pHi microelectrodes do not detect any pHi change upon bath addition of 0.5 mM NH4Cl.
After oocyte exposure to NH3/NH4+, subsequent exposure to 5 mM MA/MA+ alkalinized oocytes while also depolarizing them, suggesting transport of both MA and MA+. MA/MA+ addition to oocytes clamped at constant −30 mV holding potential detectably elicited small currents consistent with the Vm changes observed in Figs. 10 and 11. Oocyte steady-state currents were increased by expression of RhAG, and increased to higher levels by RhAG F65S. However, these steady-state currents were unaltered by Li+ replacement or by addition of 5 mM NH4+ (Fig. 12) or MA+. It is possible that inter-oocyte variability of native currents may have obscured low-amplitude amine-induced currents.
The Vm and pHi responses to NH3/NH4+ and MA/MA+ exhibited by oocytes expressing human RhAG thus resembled those reported for oocytes expressing mouse Rhbg (50). The depolarizations produced by both NH4Cl and MA, though not supporting electroneutral NH4+/H+ exchange (30, 31, 43, 78), might represent additional, secondary activities.
RhAG-expressing oocyte responses to amines might reflect 1) MA+ transport by RhAG, with parallel activation of Na+/H+ exchange or another alkalinizing pathway; 2) uncharged MA transport by RhAG, with activation of endogenous cation conductance; and 3) NH3/NH4+ signaling by pathways that elevate intracellular [Ca2+] (12, 32, 44, 64), inhibit H1 histamine and M3 muscarinic receptor signaling (32), inhibit amantadine transport (26), activate basal autophagy, protect against TNF-α-induced cell death (21), and regulate glutamine metabolism. Any of these mechanisms are compatible with the observed RhAG-associated uptake of 14C-MA (Fig. 9), but the effect of MA on NH3/NH4+ signaling remains unknown. Endogenous oocyte conductances affected could include NH4+-permeable nonspecific cation conductances, K+ channels, Na+-K+-ATPase (15), and (as yet undetected in oocytes) NH4+- and MA+-permeable T-type Ca2+ channels (11) or acid-activated cation channel ASIC 1a which, in the presence of low mM NH4+, is active at pH 7.0 (55).
The NH4Cl-induced pHi acidification of oocytes expressing RhAG HSt mutant F65S was similar to or less than that of oocytes expressing wild-type RhAG. However, oocytes expressing mutant F65S exhibited a rapid hyperpolarization in response to MA/MA+ in contrast to the slower, NH4Cl-induced depolarization, and accompanied by little or no reduction in intracellular alkalinization. In the context of loss of 14C-MA uptake, the rapid hyperpolarization may reflect activation of an endogenous K+ channel, or block of an endogenous cation channel, rather than slower (and as yet undescribed) activation of vacuolar H+-ATPase or Na+-K+-ATPase (76). The similarly depolarized resting Vm of oocytes expressing RhAG and RhAG F65S suggests little or no contribution from the dominant endogenous Cl− conductance.
Conclusion.
We report four new cases of HSt associated with heterozygous RhAG F65S mutations. Mutant red cells exhibited elevated red cell cation leak and channel activity and increased K-Cl cotransport activity. RhAG F65S expression in oocytes was associated with elevated ouabain- and bumetanide-resistant influx of Li+ and 86Rb+, accompanied by secondary activation of endogenous Na+-K+-ATPase activity. RhAG F65S exhibited a loss-of-function for 14C-MA influx. As shown for mouse Rhbg, oocytes expressing human wild-type RhAG transported NH3/NH4+ and MA/MA+ by different mechanisms, consistent with a combination of electroneutral and electrogenic pathways, and these pathways were differentially altered in the setting of OHSt mutant F65S. In addition, RhAG-associated, ouabain- and bumetanide-resistant transport of Rb+, Li+, and MA differed in their pharmacological properties. These findings, together with the MA-induced hyperpolarization exhibited by oocytes expressing RhAG F65S and previous data and molecular modeling consistent with electroneutral NH3 transport, suggest that the increased cation permeabilities accompanying overexpression of wild-type and mutant RhAG polypeptides represent secondarily altered regulation of endogenous permeability pathways. This conclusion does not rule out increased cation permeability intrinsic to the RhAG F65S polypeptide, but does suggest novel functional properties arising from modulation of plasmalemmal protein macrocomplexes in oocytes and in red cells.
GRANTS
This work was funded by National Institutes of Health Grants HL077765 (to S. L. Alper and C. Brugnara), HL090632 (to A. Rivera), HL092535 (to F. A. Kuypers), DK62039 (to P. G. Gallagher), and HL081608 (to S. E. Lux). C. M. Westhoff was supported by the American Red Cross Holland Laboratory (Bethesda, MD).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
NOTE ADDED IN PROOF
While this paper was in revision, Flatt et al. reported (Blood 2011, in press) association of missense mutations in erythroid glucose transporter GLUT1 with familial stomatin-deficient cryohydrocytosis, a rare form of cation-leak stomatocytosis.
Footnotes
GPA coexpression in oocytes unexpectedly enhanced RhAG-associated Li+ influx about as well as GPB coexpression (n = 25, not shown). Thus, GPB domains common to both GPA and GPB may contribute predominantly to the interaction of GPB with RhAG in oocytes. In the absence of GPB and AE1, GPA interaction with RhAG in oocytes may increase RhAG abundance. The observed functional interaction between GPA and RhAG recalls previously reported examples of their coordinated trafficking. Thus, in enucleating nb/nb Ank1-deficient mouse spherocyte precursors, in which AE1 remained predominantly in the membrane surrounding the nucleus, GPA accompanied RhAG predominantly to the reticulocyte membrane (62). This apparent plasticity of GPA association contrasted with that of 4.1R-binding protein GPC, which in 4.1R−/− enucleating elliptocyte precursors remained exclusively in the perinuclear membrane. The functional association of GPA with RhAG in the absence of AE1 is consistent with the temporal pattern of RhAG and GPA expression preceding expression of AE1 in CD34+ erythroid precursors, and with coexpression of GPA and RhAG in the absence of AE1 in intact and detergent-extracted K562 cells (24). The association of GPA with RhAG in the absence of AE1 is also consistent with the sustained presence of GPA and GPB at 30–100% and 56% of respective wild-type abundance in membranes of human AE1-null (Band 3 Coimbra) red cells, which exhibit up to 15% of normal RhAG abundance. It also supports the hypothesized presence of heterotetramers of GPA and GPB homodimers among the closely associated RhAG and AE1 macrocomplexes of normal red cell membranes (6). However, GPA coexpression with RhAG (1 ng cRNA each) did not increase ouabain- and bumetanide-sensitive 86Rb+ influx.
REFERENCES
- 1. Alper SL, Vandorpe DH, Peters LL, Brugnara C. Reduced DIDS-sensitive chloride conductance in Ae1−/− mouse erythrocytes. Blood Cells Mol Dis 41: 22–34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai Z, Zhang H, Zhu L, Zuo W, Ye J, Feng J, Wang L, Chen L. Lithium inhibits cell volume regulation by acting on chloride channels and modifies ultrastructures of the cell membrane in nasopharyngeal carcinoma cells. Eur J Pharmacol 641: 88–95, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Beckmann R, Smythe JS, Anstee DJ, Tanner MJ. Coexpression of band 3 mutants and Rh polypeptides: differential effects of band 3 on the expression of the Rh complex containing D polypeptide and the Rh complex containing CcEe polypeptide. Blood 97: 2496–2505, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Benjelloun F, Bakouh N, Fritsch J, Hulin P, Lipecka J, Edelman A, Planelles G, Thomas SR, Cherif-Zahar B. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4+ transport in HeLa cells. Pflügers Arch 450: 155–167, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bogdanova A, Goede JS, Weiss E, Bogdanov N, Bennekou P, Bernhardt I, Lutz HU. Cryohydrocytosis: increased activity of cation carriers in red cells from a patient with a band 3 mutation. Haematologica 95: 189–198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101: 4180–4188, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bruce LJ, Guizouarn H, Burton NM, Gabillat N, Poole J, Flatt JF, Brady RL, Borgese F, Delaunay J, Stewart GW. The monovalent cation leak in overhydrated stomatocytic red blood cells results from amino acid substitutions in the Rh-associated glycoprotein. Blood 113: 1350–1357, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Bruce LJ, Robinson HC, Guizouarn H, Borgese F, Harrison P, King MJ, Goede JS, Coles SE, Gore DM, Lutz HU, Ficarella R, Layton DM, Iolascon A, Ellory JC, Stewart GW. Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride-bicarbonate exchanger, AE1. Nat Genet 37: 1258–1263, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Busch S, Burckhardt BC, Siffert W. Expression of the human sodium/proton exchanger NHE-1 in Xenopus laevis oocytes enhances sodium/proton exchange activity and establishes sodium/lithium countertransport. Pflügers Arch 429: 859–869, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Carella M, d'Adamo AP, Grootenboer-Mignot S, Vantyghem MC, Esposito L, D'Eustacchio A, Ficarella R, Stewart GW, Gasparini P, Delaunay J, Iolascon A. A second locus mapping to 2q35–36 for familial pseudohyperkalaemia. Eur J Hum Genet 12: 1073–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Cataldi M, Perez-Reyes E, Tsien RW. Differences in apparent pore sizes of low and high voltage-activated Ca2+ channels. J Biol Chem 277: 45969–45976, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Chernova MN, Stewart AK, Jiang L, Friedman DJ, Kunes YZ, Alper SL. Structure-function relationships of AE2 regulation by Cai2+-sensitive stimulators NH4+ and hypertonicity. Am J Physiol Cell Physiol 284: C1235–C1246, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Clark MR, Mohandas N, Shohet SB. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood 61: 899–910, 1983 [PubMed] [Google Scholar]
- 14. Correa Gde R, Cunha KC, dos Santos AA, de Araujo EG. The trophic effect of ouabain on retinal ganglion cell is mediated by EGF receptor and PKC delta activation. Neurochem Res 35: 1343–1352, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Cougnon M, Bouyer P, Hulin P, Anagnostopoulos T, Planelles G. Further investigation of ionic diffusive properties and of NH4+ pathways in Xenopus laevis oocyte cell membrane. Pflügers Arch 431: 658–667, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Daniel L, Etkovitz N, Weiss SR, Rubinstein S, Ickowicz D, Breitbart H. Regulation of the sperm EGF receptor by ouabain leads to initiation of the acrosome reaction. Dev Biol 344: 650–657, 2010 [DOI] [PubMed] [Google Scholar]
- 17. De Franceschi L, Rivera A, Fleming MD, Honczarenko M, Peters LL, Gascard P, Mohandas N, Brugnara C. Evidence for a protective role of the Gardos channel against hemolysis in murine spherocytosis. Blood 106: 1454–1459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delaunay J. The hereditary stomatocytoses: genetic disorders of the red cell membrane permeability to monovalent cations. Semin Hematol 41: 165–172, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100: 119–130, 1963 [DOI] [PubMed] [Google Scholar]
- 20. Dunham PB, Kelley SJ, Logue PJ, Mutolo MJ, Milanick MA. Na+-inhibitory sites of the Na+/H+ exchanger are Li+ substrate sites. Am J Physiol Cell Physiol 289: C277–C282, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal 3: ra31, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Fairbanks G, Steck TL, Wallach DF. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10: 2606–2617, 1971 [DOI] [PubMed] [Google Scholar]
- 23. Ferrandi M, Molinari I, Torielli L, Padoani G, Salardi S, Rastaldi MP, Ferrari P, Bianchi G. Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: experimental studies. Sci Transl Med 2: 59ra86, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Gane P, Le Van Kim C, Bony V, El Nemer W, Mouro I, Nicolas V, Colin Y, Cartron JP. Flow cytometric analysis of the association between blood group-related proteins and the detergent-insoluble material of K562 cells and erythroid precursors. Br J Haematol 113: 680–688, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Glader BE, Fortier N, Albala MM, Nathan DG. Congenital hemolytic anemia associated with dehydrated erythrocytes and increased potassium loss. N Engl J Med 291: 491–496, 1974 [DOI] [PubMed] [Google Scholar]
- 26. Goralski KB, Bose R, Sitar DS. NH4+ modulates renal tubule amantadine transport independently of intracellular pH changes. Eur J Pharmacol 541: 87–94, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Grootenboer S, Schischmanoff PO, Laurendeau I, Cynober T, Tchernia G, Dommergues JP, Dhermy D, Bost M, Varet B, Snyder M, Ballas SK, Ducot B, Babron MC, Stewart GW, Gasparini P, Iolascon A, Delaunay J. Pleiotropic syndrome of dehydrated hereditary stomatocytosis, pseudohyperkalemia, and perinatal edema maps to 16q23–q24. Blood 96: 2599–2605, 2000 [PubMed] [Google Scholar]
- 28. Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, Sali A, Westhoff CM, Stroud RM. Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci USA 107: 9638–9643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guizouarn H, Motais R. Swelling activation of transport pathways in erythrocytes: effects of Cl−, ionic strength, and volume changes. Am J Physiol Cell Physiol 276: C210–C220, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Hillmann P, Kose M, Sohl K, Muller CE. Ammonium-induced calcium mobilization in 1321N1 astrocytoma cells. Toxicol Appl Pharmacol 227: 36–47, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Iolascon A, De Falco L, Borgese F, Esposito MR, Avvisati RA, Izzo P, Piscopo C, Guizouarn H, Biondani A, Pantaleo A, De Franceschi L. A novel erythroid anion exchange variant (Gly796Arg) of hereditary stomatocytosis associated with dyserythropoiesis. Haematologica 94: 1049–1059, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaunin P, Horisberger JD, Richter K, Good PJ, Rossier BC, Geering K. Processing, intracellular transport, and functional expression of endogenous and exogenous alpha-beta 3 Na,K-ATPase complexes in Xenopus oocytes. J Biol Chem 267: 577–585, 1992 [PubMed] [Google Scholar]
- 35. Khademi S, O'Connell J, 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science 305: 1587–1594, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Lande WM, Thiemann PV, Mentzer WC., Jr Missing band 7 membrane protein in two patients with high Na, low K erythrocytes. J Clin Invest 70: 1273–1280, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lanquar V, Loque D, Hormann F, Yuan L, Bohner A, Engelsberger WR, Lalonde S, Schulze WX, von Wiren N, Frommer WB. Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell 21: 3610–3622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, Ponce A, Flores-Benitez D, Rincon-Heredia R, Padilla-Benavides T, Castillo A, Shoshani L, Cereijido M. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci USA 107: 11387–11392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lauf PK, Chimote AA, Adragna NC. Lithium fluxes indicate presence of Na-Cl cotransport (NCC) in human lens epithelial cells. Cell Physiol Biochem 21: 335–346, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J Physiol 559: 751–759, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ludewig U, von Wiren N, Frommer WB. Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J Biol Chem 277: 13548–13555, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Lupo D, Li XD, Durand A, Tomizaki T, Cherif-Zahar B, Matassi G, Merrick M, Winkler FK. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci USA 104: 19303–19308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marin M, Sellier C, Paul-Antoine AF, Cailliau K, Browaeys-Poly E, Bodart JF, Vilain JP. Calcium dynamics during physiological acidification in Xenopus oocyte. J Membr Biol 236: 233–245, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Mayer M, Dynowski M, Ludewig U. Ammonium ion transport by the AMT/Rh homologue LeAMT1;1. Biochem J 396: 431–437, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mentzer WC, Jr, Smith WB, Goldstone J, Shohet SB. Hereditary stomatocytosis: membrane and metabolism studies. Blood 46: 659–669, 1975 [PubMed] [Google Scholar]
- 47. Mouro-Chanteloup I, Cochet S, Chami M, Genetet S, Zidi-Yahiaoui N, Engel A, Colin Y, Bertrand O, Ripoche P. Functional reconstitution into liposomes of purified human RhCG ammonia channel. PLos One 5: e8921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4(+). J Membr Biol 228: 15–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakhoul NL, Abdulnour-Nakhoul SM, Boulpaep EL, Rabon E, Schmidt E, Hamm LL. Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am J Physiol Cell Physiol 299: C695–C705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakhoul NL, Abdulnour-Nakhoul SM, Schmidt E, Doetjes R, Rabon E, Hamm LL. pH sensitivity of ammonium transport by Rhbg. Am J Physiol Cell Physiol 299: C1386–C1397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakhoul NL, Dejong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Ohno H, Nakatsu Y, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Otani Y, Okubo H, Yoneda M, Fukushima T, Kamata H, Nishimura F, Kurihara H, Katagiri H, Oka Y, Asano T. 4F2hc stabilizes GLUT1 protein and increases glucose transport activity. Am J Physiol Cell Physiol 300: C1047–C1054, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Pandey GN, Sarkadi B, Haas M, Gunn RB, Davis JM, Tosteson DC. Lithium transport pathways in human red blood cells. J Gen Physiol 72: 233–247, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci USA 103: 11376–11380, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Piermarini PM, Weihrauch D, Meyer H, Huss M, Beyenbach KW. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am J Physiol Renal Physiol 296: F730–F750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ridgwell K, Eyers SA, Mawby WJ, Anstee DJ, Tanner MJ. Studies on the glycoprotein associated with Rh (rhesus) blood group antigen expression in the human red blood cell membrane. J Biol Chem 269: 6410–6416, 1994 [PubMed] [Google Scholar]
- 58. Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH(3) into red blood cells. Proc Natl Acad Sci USA 101: 17222–17227, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood 99: 357–603, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Rivera A, Rotter MA, Brugnara C. Endothelins activate Ca2+-gated K+ channels via endothelin B receptors in CD-1 mouse erythrocytes. Am J Physiol Cell Physiol 277: C746–C754, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Rotoli BM, Closs EI, Barilli A, Visigalli R, Simon A, Habermeier A, Bianchi N, Gambari R, Gazzola GC, Bussolati O, Dall'Asta V. Arginine transport in human erythroid cells: discrimination of CAT1 and 4F2hc/y+LAT2 roles. Pflügers Arch 458: 1163–1173, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Salomao M, Chen K, Villalobos J, Mohandas N, An X, Chasis JA. Hereditary spherocytosis and hereditary elliptocytosis: aberrant protein sorting during erythroblast enucleation. Blood 116: 267–269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Silva E, Soares-da-Silva P. Long-term regulation of Na(+),K(+)-ATPase in opossum kidney cells by ouabain. J Cell Physiol 226: 2391–2372, 2011 [DOI] [PubMed] [Google Scholar]
- 64. Slotki I, Schwartz JH, Alexander EA. Interrelationship between cell pH and cell calcium in rat inner medullary collecting duct cells. Am J Physiol Cell Physiol 265: C432–C438, 1993 [DOI] [PubMed] [Google Scholar]
- 65. Steck TL. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol 66: 295–305, 1972 [DOI] [PubMed] [Google Scholar]
- 66. Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH2-terminal cytoplasmic domain. Am J Physiol Cell Physiol 281: C1344–C1354, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Stewart AK, Kedar PS, Shmukler BE, Vandorpe DH, Hsu A, Glader B, Rivera A, Brugnara C, Alper SL. Functional characterization and modified rescue of novel AE1 mutation R730C associated with overhydrated cation leak stomatocytosis. Am J Physiol Cell Physiol 300: C1034–C1046, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stewart AK, Vandorpe DH, Heneghan JF, Chebib F, Stolpe K, Akhavein A, Edelman EJ, Maksimova Y, Gallagher PG, Alper SL. The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes. Am J Physiol Cell Physiol 298: C283–C297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stewart GW. Hemolytic disease due to membrane ion channel disorders. Curr Opin Hematol 11: 244–250, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Stewart GW, Amess JA, Eber SW, Kingswood C, Lane PA, Smith BD, Mentzer WC. Thrombo-embolic disease after splenectomy for hereditary stomatocytosis. Br J Haematol 93: 303–310, 1996 [DOI] [PubMed] [Google Scholar]
- 71. Tian J, Li X, Liang M, Liu L, Xie JX, Ye Q, Kometiani P, Tillekeratne M, Jin R, Xie Z. Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem 284: 14921–14929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tilley L, Green C, Poole J, Gaskell A, Ridgwell K, Burton NM, Uchikawa M, Tsuneyama H, Ogasawara K, Akkok CA, Daniels G. A new blood group system, RHAG: three antigens resulting from amino acid substitutions in the Rh-associated glycoprotein. Vox Sang 98: 151–159, 2010 [DOI] [PubMed] [Google Scholar]
- 73. Van Kim CL, Colin Y, Cartron JP. Rh proteins: key structural and functional components of the red cell membrane. Blood Rev 20: 93–110, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Vandorpe DH, Xu C, Shmukler BE, Otterbein LE, Trudel M, Sachs F, Gottlieb PA, Brugnara C, Alper SL. Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLos One 5: e8732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wagner CA, Broer A, Albers A, Gamper N, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1. J Physiol 526: 35–46, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wall SM. Ammonium transport and the role of the Na,K-ATPase. Miner Electrolyte Metab 22: 311–317, 1996 [PubMed] [Google Scholar]
- 77. Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, Southall N, Wang S, Xia M, Austin CP, Zheng W, Xie Z, Sun Y. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res 69: 6556–6564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Westhoff CM, Ferreri-Jacobia M, Mak DO, Foskett JK. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J Biol Chem 277: 12499–12502, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Wu SC, Horng JL, Liu ST, Hwang PP, Wen ZH, Lin CS, Lin LY. Ammonium-dependent sodium uptake in mitochondrion-rich cells of medaka (Oryzias latipes) larvae. Am J Physiol Cell Physiol 298: C237–C250, 2010 [DOI] [PubMed] [Google Scholar]
- 80. Xiang M, Feng M, Muend S, Rao R. A human Na+/H+ antiporter sharing evolutionary origins with bacterial NhaA may be a candidate gene for essential hypertension. Proc Natl Acad Sci USA 104: 18677–18681, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu KY, Zhu W, Xiao RP. Serine496 of beta2 subunit of L-type Ca2+ channel participates in molecular crosstalk between activation of (Na++K+)-ATPase and the channel. Biochem Biophys Res Commun 402: 319–323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xue Z, Li B, Gu L, Hu X, Li M, Butterworth RF, Peng L. Increased Na, K-ATPase alpha2 isoform gene expression by ammonia in astrocytes and in brain in vivo. Neurochem Int 57: 395–403 [DOI] [PubMed] [Google Scholar]
- 83. Ye Q, Li Z, Tian J, Xie JX, Liu L, Xie Z. Identification of a potential receptor that couples ion transport to protein kinase activity. J Biol Chem 286: 6225–6232, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci USA 101: 17090–17095, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zidi-Yahiaoui N, Callebaut I, Genetet S, Le Van Kim C, Cartron JP, Colin Y, Ripoche P, Mouro-Chanteloup I. Functional analysis of human RhCG: comparison with E. coli ammonium transporter reveals similarities in the pore and differences in the vestibule. Am J Physiol Cell Physiol 297: C537–C547, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.