Abstract
Previous studies have shown that exposure to a hypoxic in vitro environment increases the secretion of pro-angiogenic growth factors by human adipose-derived stromal cells (hASCs) [Cao Y, et al., Biochem Biophys Res Commun 332: 370–379, 2005; Kokai LE, et al., Plast Reconstr Surg 116: 1453–1460, 2005; Park BS, et al., Biomed Res (Tokyo) 31: 27–34, 2010; Rasmussen JG, et al., Cytotherapy 13: 318–328, 2010; Rehman J, et al., Circulation 109: 1292–1298, 2004]. Previously, it has been demonstrated that hASCs can differentiate into pericytes and promote microvascular stability and maintenance during angiogenesis in vivo (Amos PJ, et al., Stem Cells 26: 2682–2690, 2008; Traktuev DO, et al., Circ Res 102: 77–85, 2008). In this study, we tested the hypotheses that angiogenic induction can be increased and pericyte differentiation decreased by pretreatment of hASCs with hypoxic culture and that hASCs are similar to human bone marrow-derived stromal cells (hBMSCs) in these regards. Our data confirms previous studies showing that hASCs: 1) secrete pro-angiogenic proteins, which are upregulated following culture in hypoxia, and 2) migrate up gradients of PDGF-BB in vitro, while showing for the first time that a rat mesenteric model of angiogenesis induced by 48/80 increases the propensity of both hASCs and hBMSCs to assume perivascular phenotypes following injection. Moreover, culture of both cell types in hypoxia before injection results in a biphasic vascular length density response in this model of inflammation-induced angiogenesis. The effects of hypoxia and inflammation on the phenotype of adult progenitor cells impacts both the therapeutic and the basic science applications of the cell types, as hypoxia and inflammation are common features of natural and pathological vascular compartments in vivo.
Keywords: adipose-derived stromal cells, inflammation, hypoxia, microvasculature, adult stem cells
the ability of tissues to grow and stabilize new blood vessels in the presence of inflammation, ischemia, and hypoxia (as is desirable in wound healing, traumatic brain injury, stroke, and both coronary and peripheral vascular disease) depends on the maintenance and support of new and existing microvessels. Moreover, if engineered three-dimensional tissue equivalents (e.g., for bone, skin, nervous tissue, and cardiac tissue replacement) are to be realized as a viable therapeutic option, microvascular perfusion of these constructs must be facilitated and maintained in vivo. The overwhelming majority of approaches to engineer microvessels, both ex vivo and in vivo, have focused on the construction of new endothelialized tubes. Yet, these approaches have largely failed because the engineered microvessels are only transiently functional and quickly regress. Thus promoting long-term and stable revascularization remains a critical and challenging aspect of regenerative medicine.
Considering that every vessel in the body is covered with perivascular cells (e.g., pericytes and smooth muscle cells) and that microvessels cannot subsist and function without these cells, it is not surprising that approaches focusing exclusively on reendothelialization fail. The recruitment of stabilizing perivascular cells to the ablumenal surface of microvascular endothelium is essential to the persistence of vascular networks in vivo. Therefore, identification of therapeutically relevant approaches for revascularizing tissues with perivascular support cells that will enable long-term maintenance and perfusion is central to most regenerative strategies (14).
Pericytes, in particular, are known to stabilize and maintain endothelial cells, preserving otherwise transient increases in vascularity and preparing microvessels for investment of vascular smooth muscle cells via contact signaling in the form of ablumenal “wrapping” of small vessels (3, 4, 43, 47). The identifying characteristics of pericytes continue to be debated, but a consensus seems to be forming that pericytes are cells that physically align with the ablumenal surface of microvascular endothelium, extend pseudopods that enwrap microvessels, and express a panel of differentiation markers, such as neuron glial antigen-2 (NG2), smooth muscle α-actin (SMA), platelet-derived growth factor β-receptor (PDGFβR), and desmin (3, 4, 6, 17, 31, 32, 47). Pericytes are known to exhibit directed migration and proliferate in response to the homodimer PDGF-BB and vascular endothelial growth factor (VEGF) (3, 18). It has also been suggested that pericytes represent a multipotent cell population capable of supporting angiogenesis with the potential for adipogenesis, chondrogenesis, osteogenesis, and myogenesis (44).
The stromal vascular fraction harvested from adipose tissue contains a subpopulation of adipose-derived stromal cells (ASCs) that are the subject of a growing body of research. Studies suggest that human ASCs (hASCs), or a subpopulation thereof, contain progenitor cells that are an attractive source of pericytes for therapeutic use. ASCs have been shown to possess many of the same regenerative qualities as bone marrow-derived stem cells (BMSCs) while offering more attractive harvest and yield parameters. In animal hindlimb ischemia models, it has been shown that ASCs and BMSCs are able to rescue ischemic hindlimbs from autoamputation, increase capillary density, and improve blood flow downstream of the ligation (28, 33, 39, 41). However, the mechanism(s) responsible for these functional benefits is still in question. Data has been furnished by several groups, including ours, suggesting that hASCs are able to increase vascularity by differentiation into pericytes, which are capable of stabilizing the microvasculature, preventing vascular regression, and promoting long-term microvessel maintenance (3, 9, 46). Specifically, we have previously shown that hASCs injected intraperitoneally incorporate and assume pericyte-like phenotypes following a well-characterized inflammatory response elicited in the rat mesentery by intraperitoneal injection of compound 48/80, a mast cell degranulation agent (3). Additional experimental evidence points to indirect paracrine-mediated mechanisms, whereby the production and secretion of pro-angiogenic factors by hASCs may serve to enhance and promote angiogenesis (11, 41, 48, 49).
A number of recent studies report interesting effects of hypoxic culture on stem cell phenotype and behavior that are immediately relevant to the goals of tissue regeneration (1, 21, 22, 24, 26, 27, 42). hASCs, in particular, have been shown to increase their secretion of angiogenic and anti-apoptotic factors following exposure to hypoxic culture conditions (5, 21, 37, 40, 41). Moreover, it has been hypothesized that hypoxia may limit or delay the differentiation of progenitor cells, promoting a more plastic phenotype. For these reasons, it is important to determine the impact of hypoxic culture on the abilities of hASCs to assume a pericyte-like phenotype and/or contribute to vascular growth and stability following injection in vivo.
Thus the main goal of this study was to compare the ability of hASCs to assume pericyte-like phenotypes in an angiogenic setting after having been cultured under normal and hypoxic conditions. Based on previous studies, hypoxic culture was expected to limit the differentiation of hASCs into pericytes and enhance angiogenesis through elevated secretion of pro-angiogenic factors (11, 21, 41). Furthermore, it was hypothesized that hASCs and human BMSCs (hBMSCs) would perform similarly with respect to perivascular cell incorporation following injection regardless of culture condition, since the literature shows a great deal of phenotypic and functional similarities between the two cell types (25, 30, 38, 39).
METHODS
Cell culture.
hASCs for in vivo studies were obtained from a 38-year-old female patient undergoing elective intraoperative suction lipectomy in the Department of Plastic Surgery at the University of Virginia. hASCs for in vitro studies were obtained from the same donor (labeled as Expt 3 in graphs) as well as from a 34-year-old female patient (labeled as Expt 1) and a 52-year-old male patient (labeled as Expt 2). hASCs were isolated as previously described, and tissue harvest protocols were approved by the University of Virginia's Human Investigation Committee (3). Briefly, harvested tissue was washed several times in Hanks' buffered saline and enzymatically dissociated. The dissociated tissue was filtered to remove debris, and the resulting cell suspension was centrifuged. Pelleted stromal cells were then recovered and washed twice, filtered twice (250 μm mesh followed by 105 μm mesh), centrifuged, and decanted. Contaminating erythrocytes were lysed with an osmotic buffer, and the stromal cells were plated onto tissue culture plastic (Nunc) at a density of ∼2,000 cells/cm2 (P = 0). hBMSCs from two separate male donors were obtained from STEMCELL Technologies (Vancouver, BC, Canada) and were cultured in the same manner as hASCs. All cells were used between passages 2 and 4 except for Boyden chamber assays where cells were used between passages 3 and 5.
Cells were fed every 2 to 3 days and passaged using Accutase (Innovative Cell Technologies, San Diego, CA) upon reaching confluence. hASC culture media consisted of DMEM/F12 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Invitrogen) and 1% penicillin-streptomycin (Invitrogen). hBMSC culture media consisted of DMEM/F12 supplemented with 20% FBS and 1% penicillin-streptomycin.
Creation and validation of hypoxic vs. normal culture conditions.
hASCs and hBMSCs to be preconditioned in hypoxic culture conditions (HCC) were placed in a Modular Incubator Chamber (Billups-Rothenberg, Del Mar, CA), which was then perfused with 5% CO2 with balance N2 for 20 min to purge the chamber of oxygen. The chamber was then sealed and incubated at 37°C for 48 h. This method was previously shown to create hypoxic conditions to induce positive staining for pimonidazole adducts in hASCs using Hypoxyprobe-1, indicating induction of Po2 less than or equal to 15 mmHg (<2% O2) (1), and to increase the intracellular concentration of hypoxia-inducible-factor-1α (HIF-1α) in rat pulmonary endothelial cells (35). hASCs and hBMSCs that were cultured in normal culture conditions (NCC) were maintained at 37°C with 5% CO2 in a standard cell culture incubator.
Cell viability assay.
To determine whether adult stem cells were viable following 48 h of hypoxia, hASCs and hBMSCs were assayed with LIVE/DEAD Viability Kit for Mammalian Cells (Invitrogen). Parallel cultures of both hASCs and hBMSCs were cultured in either NCC or HCC for 48 h. At the end of the 48-h period, culture medium was aspirated, and a solution of 2 mM calcein AM and 4 mM ethidium homodimer-1 (EthD-1) was applied directly to all four cell groups. Images were obtained using a ×20 Nikon air objective, an Olympus Microfire digital camera (Olympus, Tokyo, Japan), and a Nikon TE2000-E2 microscope equipped with fluorescent confocal accessories.
Analysis of secreted proteins.
ASCs were given fresh culture medium and cultured in either NCC or HCC for 48 h. At the conclusion of the 48-h time course, HCC ASCs were removed from hypoxia and supernatant was collected from each group immediately (0 h time point). Each experimental group was given fresh culture medium and was cultured under NCC for an additional 48 h. At the end of this 48-h time period, supernatant was once again collected from both groups (48 h time point). BMSCs were cultured in parallel to the NCC ASC group, with simultaneous supernatant collection (0 and 48 h time points). All samples were submitted to Searchlight Sample Testing Service (Pierce Biotechnology, Woburn, MA) for analysis of human hepatocyte growth factor (HGF), VEGF, matrix metalloproteinase-2 (MMP2), and tissue inhibitor of metalloproteinases-1 (TIMP-1) content. Unconditioned hASC and hBMSC media samples were collected as negative controls. Conditioned media and negative controls were shipped to Pierce Biotechnology overnight on dry ice after collection. All samples were analyzed in duplicate, and each condition is reported as the average of three repeated experiments, less the average of the applicable negative control samples.
In vitro migration assays.
Scratch tests were performed as previously described (3). Briefly, confluent NCC hASCs and hBMSCs were serum starved for 48 h before experimentation using DMEM/F12 media with 0% FBS and 1% penicillin-streptomycin to limit the effects of proliferation on migration test results. A separate group of confluent hASCs, cultured in the same media, was placed in HCC for 48 h while being serum starved. Then a 1,000-μl pipette tip was used to scratch five wounds in each dish of cultured cells before the dishes were washed with Dulbecco's phosphate-buffered saline. The cells were then subjected to culture in DMEM/F12 containing no serum, 1% antibiotic-antimycotic, and 10 ng/ml recombinant human PDGF-BB (R&D Systems, Minneapolis, MN). Individual untreated controls containing only DMEM/F12 with 1% antibiotic-antimycotic were run in parallel. Scratch wounds were imaged at 0, 12, 20, and 30 h after introduction of scratches, and a minimum of 75 fields of view (FOVs) were quantified for each cell type, treatment group, and time point. In all cases, scratch healing was determined by measuring the shortest distance between scratch edges in each field of view. HCC hASCs were removed from hypoxia and immediately scratched, washed, and either imaged in an identical manner as NCC hASCs and hBMSCs.
Boyden chamber migration assays were also used to assess hASC and hBMSC migration in response to angiogenic growth factors. The lower chamber of a Transwell was filled with 3 ml of either hASC or hBMSC media containing one of the following: human PDGF-BB (50 ng/ml, R&D Systems), human VEGF165 (100 ng/ml, Biovision), or no additives. hASCs and hBMSCs cultured under NCC were lifted from cell culture dishes using Accutase, stained with 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI), resuspended in the corresponding media at a concentration of 50,000 cells/cm2 of culture surface in 1 ml/well aliquots, and plated on the upper surface of Transwell membrane inserts (Corning). After 30 h of culture in NCC, inserts were removed and placed on glass coverslips. The upper surfaces of the inserts were scraped using cell scrapers, and the remaining cells were fixed in 4% paraformaldehyde for 5 min. Inserts were washed three times and imaged using fluorescence microscopy. Migrated cells were counted in 10 FOVs per group (cell type and chemotactic additive), and three repeats were performed per group. The analyst was blinded to group and treatment during data analysis.
Stimulation of microvascular remodeling with compound 48/80 and cell injections.
Experiments were performed using sterile techniques according to the guidelines of the University of Virginia Animal Care and Use Committee. Eighty-four male nude rats (NCI) weighing between 125 and 225 g were divided into eight study groups: 1) NCC ASCs, 2) NCC ASCs with 48/80 stimulation, 3) HCC ASCs, 4) HCC ASCs with 48/80 stimulation, 5) NCC BMSCs, 6) NCC BMSCs with 48/80 stimulation, 7) sterile PBS vehicle control, and 8) vehicle control with 48/80 stimulation.
Cell injection and Compound 48/80 treatments were performed as previously described (3). Briefly, Compound 48/80 was injected intraperitoneally (1 ml/100 g animal weight) in 0.9% sterile NaCl twice daily on days 1 (100 μg/ml), 2 (200 μg/ml), and 3 (300 μg/ml) of the study and once daily on days 4 (400 μg/ml) and 5 (500 μg/ml). On day 4, 1 × 106 DiI-labeled hASCs or hBMSCs were injected in 0.5 ml sterile PBS ip using syringes with 25 3/8-gauge needles.
To rule out any direct effects of Compound 48/80 on injected hBMSC viability, cultured hBMSCs were exposed to media containing 5 μg/ml of Compound 48/80 for 6 days. hBMSCs were then stained using a LIVE/DEAD viability kit and imaged under confocal microscopy. A similar study to investigate the impact of Compound 48/80 on hASCs viability was previously performed (3).
Mesenteric window harvest and immunohistochemistry.
Rats were anesthetized with intramuscular injections of ketamine (80 mg/kg body wt), atropine (0.08 mg/kg body wt), and xylazine (8 mg/kg body wt). Ten mesenteric windows were harvested from each animal 10, 30, or 60 days after cell injection. Tissues were whole mounted on gelatin-coated slides before immunostaining.
To determine whether injected cells expressed markers consistent with a perivascular cell phenotype, tissues were immunostained for an array of markers known to be expressed by smooth muscle cells and pericytes, including: NG2 (1:150, rabbit polyclonal, Chemicon), SMA (1:500, FITC-conjugated clone 1A4 mouse monoclonal anti-SMA, Sigma), and PDGFβR (1:100, rabbit polyclonal, Santa Cruz Biotechnology). Tissues were washed in PBS with 0.1% saponin for 10 min three times and immunolabeled with primary antibody as well as lectin from Bandeiraea simplicifolia (BSI-lectin) preconjugated with Alexa Fluor 647 (1:200; Invitrogen) diluted in PBS buffer containing 0.1% saponin and 2% bovine albumin (Fisher Scientific) at pH 7.4 (incubation for 1 h at room temperature). Goat anti-rabbit 488-conjugated secondary antibodies were applied for 1 h at room temperature to NG2 and PDGF-βR samples for 1 h following three washes of 0.1% saponin in PBS for 10 min each. Goat anti-rabbit IgG negative staining controls as well as secondary stain only negative controls were used to stain at least one extra window per animal. All samples were washed three more times with PBS with saponin after the final round of staining before being mounted with a glass coverslip.
Image acquisition and quantification of harvested mesenteric windows.
Mesenteric tissues were examined with a Nikon Eclipse TE2000-E microscope equipped with confocal accessories (Nikon D-Eclipse C1) using ×20 Nikon water/oil and ×60 Nikon oil immersion objectives. FOVs were selected and images were taken by an analyst blinded to the treatment group and animal identity according to a conditional FOV selection hierarchy (supplemental Fig. 10). An average of 95 images was analyzed for each injection group at each time point. Three images of different FOVs were taken of each of 10 windows harvested from each rat. At least 3 rats were analyzed for each injection group at each time point, with the exception of animals receiving Compound 48/80 stimulation along with HCC hASCs harvested at day 30 as well as animals receiving no inflammatory stimulation and either PBS or HCC hASCs harvested at day 10 (each of these 3 groups represent at least 51 images obtained from 2 animals). The number of DiI-positive cells per tissue area and total microvessel length were quantified. To compliment and expand upon immunophenotypic findings, detected cells were examined for pericyte-like morphology, defined here as hASCs whose bodies were no greater than 5 μm from the abluminal surface of the endothelium. Pericyte-like phenotype was calculated by determining the percentage of cells associating closely with vessels (within 5 μm) that also expressed each perivascular marker. Perivascular marker expression was quantified relative to the number of DiI-positive cells. Morphometrists were blinded to the treatment group at the time of analysis.
Statistical analyses.
Results are presented in the form of means ± SE, unless otherwise noted. Comparisons for all data were made using the statistical analysis tools provided by SigmaPlot 5.0 (Systat Software, Chicago, IL). Data were analyzed by unpaired t-test or Mann-Whitney rank sum test when normality tests failed. Significance was asserted at P < 0.05.
RESULTS
hASCs and hBMSCs survive hypoxic culture conditions.
hASCs were positive for calcein after both NCC and HCC, indicating esterase activity within the cells, a characteristic of live cells (supplemental Fig. 1). Very few instances of EthD-1 fluorescence were observed in NCC or HCC hASCs, indicating that EthD-1 was successfully prevented access to the cells' nucleic acids, another characteristic of living cells with intact and functional plasma membranes. Similarly, hBMSCs fluoresced brightly for calcein and rarely showed positive staining for EthD-1 after either NCC or HCC. However, HCC hBMSCs were observed exhibiting inhibited cell spreading and decreased cell-cell contact relative to NCC controls.
ASCs secrete angiogenic proteins.
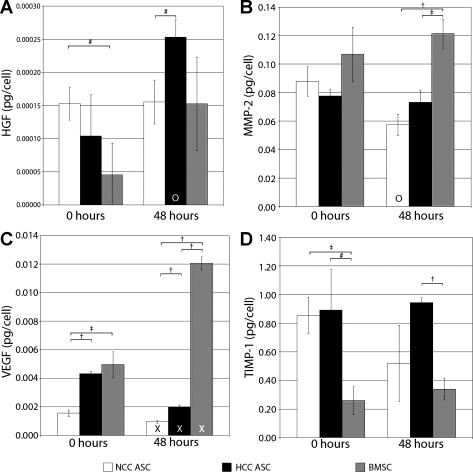
Supernatant collected from hASC and hBMSC cultures showed that both cell types secrete the growth factors HGF and VEGF165 (Fig. 1). At the 0-h time point, HGF secretion was shown to be significantly higher in NCC hASCs (1.05 × 10−4 pg/cell) than in hBMSCs (0.045 × 10−4 pg/cell), but HGF secretion by HCC hASCs were not statistically different from either group (0.066 × 10−4 pg/cell) (Fig. 1A). At the 48-h time point, HCC hASCs were found to have secreted significantly more HGF (2.53 × 10−4 pg/cell) than NCC hASCs (1.55 × 10−4 pg/cell). HCC hASCs also secreted significantly more HGF between the 0-h and 48 h time points than during the 2 days before the 0-h time point.
Fig. 1.
Secretion of growth factors and matrix enzymes by progenitor cells after 48 h (0-h time point) of normal culture conditions (NCC) or hypoxic culture conditions (HCC) and 48 h (48-h time point) of subsequent NCC culture. A: hepatocyte growth factor (HGF) secretion was highest for NCC human adipose-derived stromal cell (hASCs) at the 0-h time point but highest for HCC hASCs at the 48-h time point. B: all three cell types secrete matrix metalloproteinase-2 (MMP-2). C: among HCC hASCs, normally cultured NCC hASCs, and human bone marrow-derived stromal cells (hBMSCs), hBMSCs secrete the most vascular endothelial growth factor (VEGF) per cell. HCC hASCs secrete a statistically similar amount of HGF as NCC hASCs and a similar amount of VEGF as hBMSCs. D: hASCs produce more tissue inhibitor of metalloproteinases-1 (TIMP-1) than hBMSCs, regardless of culture condition. Values are means ± SD; *P < 0.001; †P < 0.01; #P < 0.05; XP < 0.001 vs. 0-h time point; OP < 0.05 vs. 0-h time point.
VEGF165 secretion was highest for hBMSCs (4.98 × 10−3 pg/cell) and HCC hASCs (3.59 × 10−3 pg/cell) at the 0-h time point, with both groups producing significantly more VEGF165 than NCC hASCs (1.69 × 10−3 pg/cell) (Fig. 1C). There was no significant difference in VEGF165 secretion between HCC hASCs and hBMSCs at this time point. At the 48-h time point, hBMSCs secreted significantly more VEGF (0.012 pg/cell) than either NCC hASCs (9.85 × 10−4 pg/cell) or HCC hASCs (1.99 × 10−3 pg/cell). Also, the amount of VEGF secreted by hBMSCs was significantly higher at the 48-h time point than at the 0-h time point, while the amount of VEGF secreted by each hASC group decreased significantly over the same interval. At the 48-h time point, significantly more VEGF was produced by HCC hASCs than by NCC hASCs.
ELISA was also used to assay the impact of HCC and NCC on the ability of hASCs and hBMSCs to secrete proteins related to extracellular matrix modification. NCC hASCs, HCC hASCs, and hBMSCs all secreted statistically similar amounts of MMP-2 (0.084, 0.072, and 0.107 pg/cell, respectively) at the 0-h time point, but hBMSCs secreted significantly more MMP-2 at the 48-h time point (0.121 pg/cell) than either NCC hASCs (0.057 pg/cell) or HCC hASCs (0.073 pg/cell) (Fig. 1B).
TIMP-1 secretion was higher for HCC hASCs (0.868 pg/cell) and NCC hASCs (0.784 pg/cell) than for hBMSCs (0.260 pg/cell) at the 0-h time point (Fig. 1D). NCC hASCs continued to produce significantly more TIMP-1 (0.520 pg/cell) than hBMSCs (0.340 pg/cell) at the 48-h time point.
ASCs functionally respond to chemotactic growth factors.
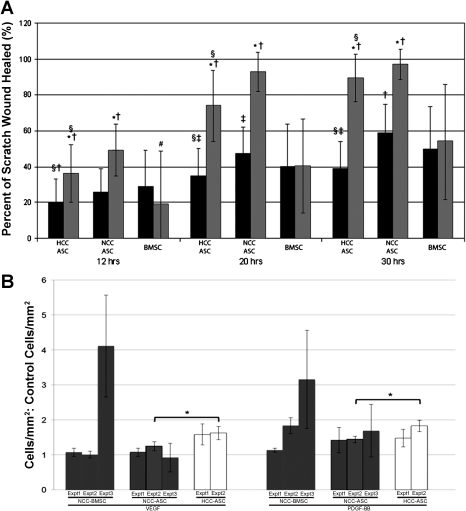
PDGF-BB significantly increased hASC migration in the scratch test assay over untreated vehicle controls (i.e., no chemokine) at 12, 20, and 30 h, regardless of whether the hASCs had been cultured in HCC or NCC (Fig. 2A). hBMSCs, on the other hand, did not show a significant increase in migration as assayed by scratch wound closure at any time point over untreated vehicle controls. Moreover, hBMSCs showed less scratch wound closure at each time point in the presence of PDGF-BB than NCC hASCs. hBMSCs showed more baseline migration than HCC hASCs under untreated conditions at all time points but less migration in the presence of PDGF-BB at all time points. At all times of data collection, NCC hASCs closed scratch wounds to a greater extent than HCC hASCs.
Fig. 2.
A: in scratch test migration assays, NCC hASCs consistently showed higher baseline migration and higher migration in the presence of platelet-derived growth factor BB (PDGF-BB) than HCC hASCs. hBMSCs did not exhibit an increase in migration in the scratch test assay in the presence of PDGF-BB (shaded bars) compared with untreated controls (solid bars). Values are means ± SD; *P < 0.001 vs. untreated control same cell group/time point; #P < 0.05 vs. untreated control same cell group/time point; †P < 0.001 vs. hBMSC at the same time point; ‡P < 0.05 vs. hBMSC at the same time point; §P < 0.01 vs. NCC hASC at the same time point. B: NCC ASCs were able to migrate significantly more toward a gradient of PDGF-BB, but not VEGF, than untreated negative controls in Boyden chamber migration assays. BMSCs were able to migrate more in the presence of both VEGF and PDGF-BB than negative controls, but baseline hASC migration was higher than that of BMSCs. Values are means ± SD; *P < 0.001 vs. untreated control same cell group/time point; #P < 0.01 vs. untreated control same cell group/time point; †P < 0.001 vs. hBMSC at the same time point; ‡P < 0.05 vs. hBMSC at the same time point; @P < 0.01 vs. hASCs treated with VEGF.
In Boyden chamber migration assays, NCC hASCs, HCC hASCs, and NCC hBMSCs exhibited a directional chemotactic migration response in the presence of PDGF-BB compared with untreated controls (Fig. 2B). An increase in migration was observed when HCC hASCs were placed in a VEGF165 gradient, but changes in migration for NCC hASCs and NCC hBMSCs (compared to untreated controls) were inconsistent and, for the most part, negligible. Significant increases in hASC migration were observed for only one donor between HCC hASCs and NCC hASCs in the presence of both VEGF165 and PDGF-BB gradients, but experiments using a second donor showed similar trends. NCC hBMSC migration for a second donor (Expt 3) did not agree well with the two experiments performed with the first donor (Expts 1 and 2, Fig. 2B); however, the absolute value of migrated cells was also found to be much lower with the second donor than the first (supplemental Fig. 4). No mitogenic effect from PDGF-BB stimulation was observed for either cell type during nonquantitative assessment of cell number.
hASCs and hBMSCs assume pericyte-like phenotypes in response to inflammation in vivo.
hBMSCs treated with 5 mM Compound 48/80 in culture for 6 days maintained cell viability (supplemental Fig. 2) based on EthD-1 exclusion and positive enzymatic activity on calcein AM media additive. We have previously confirmed a similar result for hASCs (3). SMA expression was negligible for NCC hASCs (0.0%), HCC hASCs (0.0%), and hBMSCs (4.17%) when treated with Compound 48/80 in culture for 48 h following 48 h of either HCC or NCC, depending on the group.
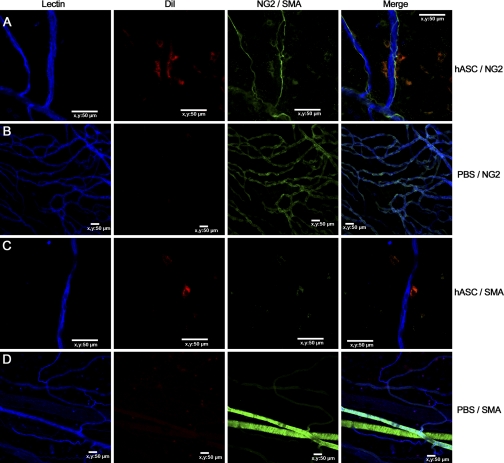
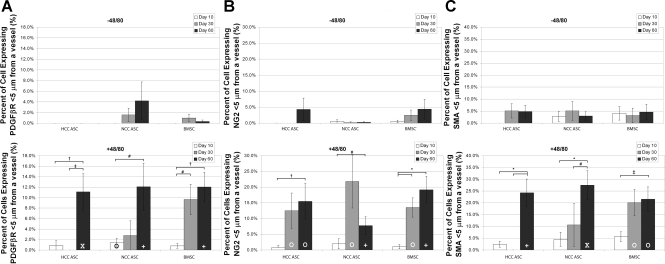
When rat mesenteric tissues were imaged following intraperitoneal injection of HCC hASCs, NCC hASCs, or NCC hBMSCs, each of these injected cell populations were identifiable in tissue sections harvested 10, 30, and 60 days postinjection, confirming their ability to incorporate into tissue. Moreover, each group of cells exhibited some expression of the pericyte markers PDGFβR, NG2, and SMA (Fig. 3, day 60 shown). The percentages of cells expressing these markers, however, were almost all <50% of the total number of observed cells, even at day 60 (supplemental Figs. 4–6). Expression of all markers by cells injected into mesenteries stimulated with Compound 48/80 increased significantly over the 60-day time course.
Fig. 3.
1,1′-Dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled hASCs (red) exhibit perivascular positioning with respect to blood vessels (blue) in inflamed and angiogenic rat mesenteric windows as well as pericyte markers like neuron glial antigen-2 (NG2) and smooth muscle α-actin (SMA) (A and C, respectively). SMA and NG2 staining of inflamed rat mesenteries injected with vehicle PBS instead of cells showed positive staining of native pericytes (B) and smooth muscle cells (D). Nerves in the vascular bed also stained positively for NG2 (A).
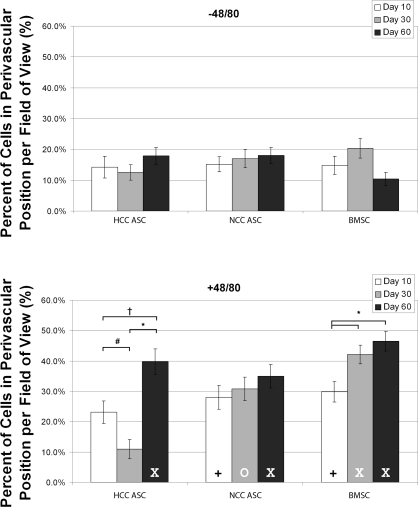
Each of the three cell types showed increased perivascular association by day 60 when introduced into an inflamed environment compared with when they were introduced into a quiescent environment (i.e., an environment where Compound 48/80 was not introduced to stimulate inflammation) (Fig. 4). Perivascular association of NCC hBMSCs increased from day 10 to day 60 in mesenteries treated with Compound 48/80, but there was no difference between the percentages of perivascular association by NCC hASCs at any of the three time points measured. Perivascular association of HCC hASCs was biphasic during the study, dropping significantly at day 30 before rising again at day 60.
Fig. 4.
For all injected cell groups, the number of cells associating closely with blood vessels was significantly lower by day 60 in unstimulated mesenteries compared with inflamed mesenteries. hBMSCs showed steady increase in perivascular association in these tissues, and HCC hASCs showed a biphasic response in stimulated tissues. Values are means ± SE, *P < 0.001; †P < 0.01; #P < 0.05; XP < 0.001 vs. unstimulated; +P < 0.01 vs. unstimulated; OP < 0.05 vs. unstimulated.
By day 60, the percentages of perivascular-positioned hASCs or hBMSCs that also expressed the three pericyte markers (PDGFβR, NG2, and SMA) were significantly higher for each cell type when the cells were injected into animals that had received Compound 48/80 than in unstimulated animals (Fig. 5, A–C). NG2 was the first marker to show a significant increase in expression by all cells delivered to inflamed (Compound 48/80-treated) tissues over unstimulated tissues (Fig. 7, day 30).
Fig. 5.
The number of cells exhibiting pericyte-like phenotypes (A: concurrent PDGFβR expression and perivascular positioning; B: concurrent NG2 expression and perivascular positioning; C: concurrent SMA expression and perivascular positioning) in each group was low at all time points in unstimulated mesenteries, but all groups showed increases in pericyte-like phenotype by day 60 when injected into inflamed mesenteries. hBMSCs showed an increase in pericyte-like phenotype slightly earlier than either hASC group in the Compound 48/80-treated tissues. Values are means ± SE, *P < 0.001; †P < 0.01; #P < 0.05; XP < 0.001 vs. unstimulated; +P < 0.01 vs. unstimulated; OP < 0.05 vs. unstimulated.
Impact of hASCs and hBMSCs on microvascular length density.
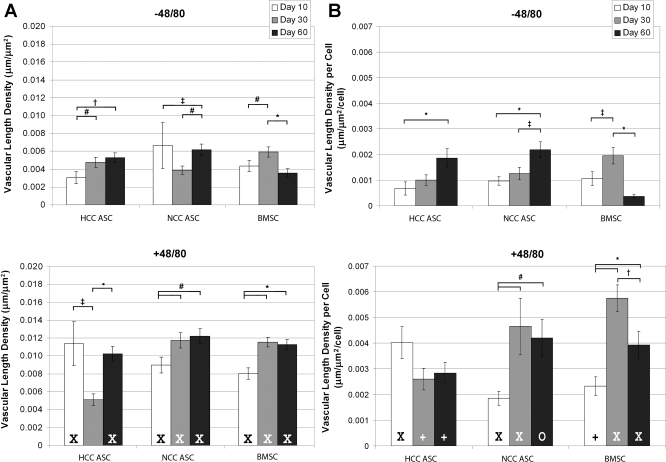
Vascular length density measurements showed that animals treated with Compound 48/80 had higher blood vessel densities than animals that did not receive inflammatory stimulation (Fig. 6A). The one exception to this result was at day 30 in animals receiving HCC hASCs; for this time point and cell group, animals showed similar vascular length densities regardless of Compound 48/80 treatment. In contrast to this dip in length density in animals treated with HCC hASCs, the vascular length density of animals receiving Compound 48/80 and either NCC hASCs or hBMSCs was found to increase from day 10 to day 30, with significant increases holding through day 60.
Fig. 6.
A: hBMSCs and NCC hASCs showed a general increase in vascular length density over time in inflamed mesenteries, but HCC hASCs produced a biphasic length density profile over time. B: angiogenic efficiency (length density per cell) was found to increase over 60 days for NCC hASCs, but hBMSCs seemed to peak in angiogenic efficiency at day 30. HCC hASCs showed an increase in angiogenic efficiency from day 10 to day 60 in unstimulated mesenteries but no change in angiogenic efficiency when injected into stimulated mesenteries. Values are means ± SE, *P < 0.001; †P < 0.01; #P < 0.05; XP < 0.001 vs. unstimulated; +P < 0.01 vs. unstimulated; OP < 0.05 vs. unstimulated.
When the angiogenic efficiency of the injected cells (as calculated by dividing the length density by the number of cells per FOV) was examined, more vascular length density per cell was observed in animals receiving Compound 48/80 compared with animals receiving vehicle injections (Fig. 6B). Interestingly, these differences were significant (for all time points and cell groups) despite the fact that a higher number of cells were observed per FOV at day 10 in animals receiving HCC hASCs and vehicle injections instead of Compound 48/80 (supplemental Fig. 3). Angiogenic efficiency increased over the 60-day time course for NCC hASCs, but hBMSCs seemed to show a peak in angiogenic efficiency around day 30. HCC hASCs increased vascular length density per cell from day 10 to day 60 in the absence of Compound 48/80 but did not show any significant change when injected into animals treated with Compound 48/80.
Animals receiving PBS instead of any cellular injection showed the highest vascular length densities at all time points compared with animals receiving cellular injections (data not shown). These data have been omitted because it was found that the FOV selection protocol (as outlined in supplemental Fig. 10) artificially inflated vascular length density values for PBS controls. Briefly, FOV selection was based first on the highest densities of cells and then the highest apparent vascular density. Since PBS samples do not have any cells, the FOVs selected always had the highest vessel density in each window. This inherently increases the calculated vascular length density per FOV compared with groups receiving cell treatment in which the absolute highest vessel densities were only analyzed if cells were also present in the same FOV. It is worth noting that vascular length density values in animals treated with Compound 48/80 were significantly higher at all time points than animals not receiving Compound 48/80.
DISCUSSION
Adipose tissue is an appealing source of stem cells because of its abundance in most individuals, relative ease of harvest, robust culture qualities, utility in either autologous or allogeneic therapies, broad differentiation potential both in vitro and in vivo, and regenerative capabilities in vivo (16, 20, 36, 50, 52). Along with other groups, we have previously demonstrated that a subpopulation of hASCs possesses the potential to contribute to angiogenesis and vascular stability in vivo via pericyte-like mechanisms (3, 46) and rescue ischemic tissue in preclinical models (28, 39, 41). Clinical trials are now investigating the ability of hASCs to offer therapeutic advantages in a range of disease applications, including myocardial infarction, Crohn's fistulas, and soft tissue reconstruction (2, 13, 19, 29). Consequently, direct and indirect evidence is mounting that hASCs are an appealing tool for regulating angiogenesis and microvessel stabilization.
It is well-established that most mesenchymal stem cells, including hBMSCs and hASCs, reside within specific microenvironments that are found in regionally localized niches (8, 9, 44) and actively respond to changes in their microenvironments by altering gene expression, production of growth factors, proliferating, differentiating, and undergoing morphological changes (10, 12, 23, 34, 51). The amount of oxygen in a given microenvironment has been shown to be an important environmental cue for cell behavior, and both hASCs and hBMSCs have been shown to be functionally responsive to changes in oxygen levels (1, 5, 40–42, 45). Therefore, the aim of this research was to investigate the impact of culturing cells in different oxygenation environments on promoting the in vivo pericyte-like behaviors of hASCs in an established rat model of angiogenesis and microvascular remodeling. This work also directly compared the functional effects of hASCs on a remodeling vascular bed to those of the more thoroughly studied adult stem cell hBMSCs.
A very interesting result in this work arose from the choice of angiogenic stimulation. By using an inflammatory agent to induce angiogenesis in the rat mesentery, new blood vessels were formed without decreasing natural oxygenation or nutrient delivery to the tissue. When each of the three tested cell groups were placed into the inflamed environment created by Compound 48/80 stimulation, cells showed marked increases in perivascular association, pericyte marker expression, angiogenic efficiency and induced vascular length density. This result indicates that inflammation causes stromal progenitor cells to acquire a pericyte-like phenotype. As it has been asserted that pericytes function to counteract the effects of inflammation (15), the assumption of pericyte-like phenotypes by stromal progenitor cells may be a mechanism by which inflamed environments are brought back to homeostasis. It is important to note that PBS controls also showed an increase in length density for all time points when injected into stimulated mesenteries compared with unstimulated mesenteries (data not shown). Therefore, it can be concluded that injection of NCC hASCs and hBMSCs into inflamed tissues increases vascular length density over time, but the absolute increase in vascular length density may not be significantly higher than what is seen in unstimulated tissues.
It was hypothesized at the outset of this study that culturing hASCs under HCC would increase the angiogenic paracrine activity of hASCs, leading to increased vascularity in inflamed tissues. Indeed, the secretion of VEGF and HGF were elevated in cells that had been exposed to hypoxic conditions, but proteins associated with migration, MMP-2, and TIMP-1 were largely unchanged. When HCC hASCs and NCC hASCs were subjected to scratch migration assays, it was found that baseline migration for NCC hASCs was higher than that of HCC hASCs. It was also observed that the addition of PDGF-BB increased the migration rate for hASCs compared with untreated controls (values > 1), regardless of culture oxygenation, indicating that hASCs increase their motility when in the presence PDGF-BB. During in vivo testing, it was observed that HCC hASCs showed a biphasic response with respect to perivascular association and vascular length density in mesenteric tissues stimulated with the inflammatory agent Compound 48/80 while NCC hASCs showed no such changes with respect to either metric. Taken together, these results could indicate a phenotypic switch in hASCs based on oxygen sensation. Hypoxic preconditioning was not used for hBMSCs during in vivo experimentation since hBMSCs appeared to be detrimentally affected bythe hypoxic culture conditions used in this study (as assessed by morphology and decreased baseline migration; supplemental Figs. 1 and 4); however, it has been shown that hBMSCs exhibit increased migration after hypoxia by other groups (42).
As HCC hASCs showed increased secretion of VEGF and HGF and decreased migration relative to NCC hASCs in scratch tests, it can be hypothesized that hypoxic conditions induce a functional phenotype in hASCs primarily characterized by secretion in which migration pathways are less pronounced. Boyden chamber results, however, suggest that migration does not decrease when hASCs are subjected to hypoxia. It is important to note that these results do not constitute definitive proof of differentiation or dedifferentiation, and injected cells may be reverting back to a phenotype exhibited before harvest. Such a change in phenotype may also help to explain vascular length density readouts in which animals treated with Compound 48/80 and HCC hASCs showed decreased length density briefly at the day 30 time point, but NCC hASCs increased over the 60-day time course. Specifically, HCC hASCs, originally in an indirect paracrine vascular support role resulting from low-oxygen culture, may switch to a direct support role (as perivascular cells) after injection into the normally oxygenated peritoneal cavity of rat hosts. Furthermore, in vitro experiments measuring SMA expression support the assertion that HCC culture may delay the expression of SMA in hASCs (supplementary Fig. 3). This hypothetical oxygen-sensing mechanism is physiologically interesting because it would allow cells within a tissue to sense ischemic events, induce initial angiogenesis in the ischemic region through indirect paracrine mechanisms, and then to stabilize newly formed vessels through a direct perivascular investment mechanism before they lose cohesion (7). It is likely that any oxygen-sensitive cells collected from hASC isolation represent a relatively small subpopulation of the cultured hASC cells. In the in vivo setting, it may be the case that native pericytes from the host animal were recruited to the vessels in response to paracrine secretions of the injected cells as well, since the number of native pericytes, while unquantified was observed to be far greater than the number of injected cells exhibiting pericyte-like phenotypes. Thus the delayed expression of mature pericyte markers by injected HCC hASCs may be caused in part by the initial recruitment of native pericytes through paracrine mechanisms. Cells acting as paracrine agents may or may not be the same cells that directly invest as pericytes, but their identification and purification hold value for both the understanding of the biology of remodeling blood vessel beds and the application of that biology in therapeutic approaches.
Some interesting similarities and differences were also observed in this research between NCC hASCs and NCC hBMSCs. hBMSCs showed a similar propensity to express pericyte markers, associate directly with blood vessels, and support vascular density as hASCs in vivo. hBMSCs were able to associate with blood vessels and express pericyte markers slightly earlier than NCC hASCs despite less baseline migration than hASCs in scratch tests. Additionally, Boyden chamber migration tests showed similar baseline migration between NCC hASCs and NCC hBMSCs, but the impact of HCC on migration was drastically different between the two cell types (supplementary Fig. 4). The observed differences may be a result of the relatively purity hBMSC population (cells were subjected to additional sorting by vendor based on surface marker expression and adherence to culture plastic) compared with hASCs, which were purified through adherence to culture plastic alone.
In summary, this study showed that oxygen concentration has an effect on hASC biology, normally cultured hASCs behave similarly in vitro and in vivo compared with hBMSCs, and inflammation has a dramatic effect on the assumption of pericytic phenotypes by stromal progenitor cells. These results suggest that hASCs and hBMSCs both have potential to improve vascularization when used therapeutically. In tissue engineering or regeneration applications where stabilization and support of blood vessels is critical, these cells are able to provide the necessary direct and indirect signals to maintain vascularity over significant periods of time. Based on ease of harvest and relative abundance, hASCs may offer the more practical option for commercial therapeutic strategies, but the mounting experimental evidence of similarities between hASCs and hBMSCs may mean that the two cell types, while distinct, are developmentally linked.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-082838 and HL-091312 (to S. M. Peirce) and HL-72141 (to A. J. Katz) and by a charitable gift from Dr. and Mrs. Peyton Weary (to A. J. Katz).
DISCLOSURES
P. J. Amos, C. L. Mulvey, S. A. Seaman, J. Walpole, K. E. Degen, H. Shang, and S. M. Peirce have nocompeting financial interests. A. J. Katz is named inventor on issued and pending patents related to the isolation and use of adipose-derived stem cells, matrix, and other adipose-related technologies and is engaged in efforts to commercialize such.
AUTHOR CONTRIBUTIONS
Author contributions: P. J. Amos, A. J. Katz, and S. M. Peirce: conception and design of research; P. J. Amos, C. L. Mulvey, S. A. Seaman, J. Walpole, K. E. Degen, and H. Shang performed experiments; P. J. Amos, C. L. Mulvey, S. A. Seaman, J. Walpole, and K. E. Degen analyzed data; P. J. Amos, S. A. Seaman, J. Walpole, and S. M. Peirce interpreted results of experiments; P. J. Amos, S. A. Seaman, and J. Walpole prepared figures; P. J. Amos drafted manuscript; P. J. Amos, C. L. Mulvey, S. A. Seaman, J. Walpole, and S. M. Peirce edited and revised manuscript; P. J. Amos and S. M. Peirce approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the faculty and staff of the Department of Plastic Surgery for facilitating the harvest of adipose tissue.
REFERENCES
- 1. Amos PJ, Bailey AM, Shang H, Katz AJ, Lawrence MB, Peirce SM. Functional binding of human adipose-derived stromal cells: effects of extraction method and hypoxia pretreatment. Ann Plast Surg 60: 437–444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, Rodeheaver GT, Peirce SM, Katz AJ. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng 16: 1595–1606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells 26: 2682–2690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332: 370–379, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chantrain CF, Henriet P, Jodele S, Emonard H, Feron O, Courtoy PJ, De Clerck YA, Marbaix E. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer 42: 310–318, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Peault B. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev 20: 429–434, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 30: 1104–1109, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 10. De Matteis R, Zingaretti MC, Murano I, Vitali A, Frontini A, Giannulis I, Barbatelli G, Marcucci F, Bordicchia M, Sarzani R, Raviola E, Cinti S. In vivo physiological transdifferentiation of adult adipose cells. Stem Cells 27: 2761–2768, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLos One 4: e5643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13: 1299–1312, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Olmo D, Herreros D, De-La-Quintana P, Guadalajara H, Trebol J, Georgiev-Hristov T, Garcia-Arranz M. Adipose-derived stem cells in Crohn's rectovaginal fistula. Case Report Med 2010: 961758, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, Usas A, Peault B, Huard J, Wagner WR, Vorp DA. Pericyte-based human tissue engineered vascular grafts. Biomaterials 31: 8235–8244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med 12: 171–172, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Bae YC, Jung JS, Kim JH. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci 119: 4994–5005, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kale S, Hanai J, Chan B, Karihaloo A, Grotendorst G, Cantley L, Sukhatme VP. Microarray analysis of in vitro pericyte differentiation reveals an angiogenic program of gene expression. FASEB J 19: 270–271, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kang YJ, Jeon ES, Song HY, Woo JS, Jung JS, Kim YK, Kim JH. Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 95: 1135–1145, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48: 15–24, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kokai LE, Rubin JP, Marra KG. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg 116: 1453–1460, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17: 540–547, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun 341: 882–888, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis 18: 221–227, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem 281: 30678–30683, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25: 750–760, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol 290: C1139–C1146, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng 13: 1159–1171, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110: 349–355, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12: 459–465, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Miyazaki M, Zuk PA, Zou J, Yoon SH, Wei F, Morishita Y, Sintuu C, Wang JC. Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine 33: 863–869, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation 13: 261–273, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation 12: 151–160, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol 25: 2542–2547, 2005 [DOI] [PubMed] [Google Scholar]
- 34. O'Neill TJ, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res 97: 1027–1035, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol Lung Cell Mol Physiol 274: L212–L219, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Palpant NJ, Yasuda S, MacDougald O, Metzger JM. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol 43: 362–370, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park BS, Kim WS, Choi JS, Kim HK, Won JH, Ohkubo F, Fukuoka H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res (Tokyo) 31: 27–34, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Peroni D, Scambi I, Pasini A, Lisi V, Bifari F, Krampera M, Rigotti G, Sbarbati A, Galie M. Stem molecular signature of adipose-derived stromal cells. Exp Cell Res 314: 603–615, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109: 656–663, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Rasmussen JG, Frobert O, Pilgaard L, Kastrup J, Simonsen U, Zachar V, Fink T. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy 13: 318–328, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109: 1292–1298, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26: 2173–2182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shepro D, Morel NM. Pericyte physiology. FASEB J 7: 1031–1038, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science 322: 583–586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI, Paterno J, Neofytou E, Longaker MT, Gurtner GC. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 27: 266–274, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102: 77–85, 2008 [DOI] [PubMed] [Google Scholar]
- 47. von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 312: 623–629, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 291: R880–R884, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, Tan J, Lee WH, Hampel H, Dodel R, Johnstone BH, March KL, Farlow MR, Du Y. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 27: 478–488, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Wosnitza M, Hemmrich K, Groger A, Graber S, Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation 75: 12–23, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng 12: 1891–1901, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.