Abstract
“Stimulated actin polymerization” has been proposed to be involved in force augmentation, in which prior submaximal activation of vascular smooth muscle increases the force of a subsequent maximal contraction by ∼15%. In this study, we altered stimulated actin polymerization by adjusting tissue length and then measured the effect on force augmentation. At optimal tissue length (1.0 Lo), force augmentation was observed and was associated with increased prior stimulated actin polymerization, as evidenced by increased prior Y118 paxillin phosphorylation without changes in prior S3 cofilin or cross-bridge phosphorylation. Tissue length, per se, regulated Y118 paxillin, but not S3 cofilin, phosphorylation. At short tissue length (0.6 Lo), force augmentation was observed and was associated with increased prior stimulated actin polymerization, as evidenced by reduced prior S3 cofilin phosphorylation without changes in Y118 paxillin or cross-bridge phosphorylation. At long tissue length (1.4 Lo), force augmentation was not observed, and there were no prior changes in Y118 paxillin, S3 cofilin, or cross-bridge phosphorylation. There were no significant differences in the cross-bridge phosphorylation transients before and after the force augmentation protocol at all three lengths tested. Tissues contracted faster at longer tissue lengths; contractile rate correlated with prior Y118 paxillin phosphorylation. Total stress, per se, predicted Y118 paxillin phosphorylation. These data suggest that force augmentation is regulated by stimulated actin polymerization and that stimulated actin polymerization is regulated by total arterial stress. We suggest that K+ depolarization first leads to cross-bridge phosphorylation and contraction, and the contraction-induced increase in mechanical strain increases Y118 paxillin phosphorylation, leading to stimulated actin polymerization, which further increases force, i.e., force augmentation and, possibly, latch.
Keywords: cofilin, latch phenomenon, paxillin, rheology
arterial smooth muscle contraction is primarily activated by stimulus-induced increases in myoplasmic Ca2+ concentration, which in turn activates myosin light chain (MLC) kinase (MLCK) to phosphorylate cross bridges on S19 of the myosin regulatory light chain (12). Stimulus-dependent inhibition of MLC phosphatase also increases cross-bridge phosphorylation (6, 27). Increases in cross-bridge phosphorylation are considered to be the primary regulator of contraction (14).
Maximal stimulation of arterial smooth muscle typically produces a large increase in cross-bridge phosphorylation and a rapid contraction. During the sustained phase of a maximal contraction, cross-bridge phosphorylation typically falls to intermediate levels, while force is maintained at peak levels, a process termed the “latch phenomenon” (4, 17). Murphy's latch-bridge hypothesis sought to explain the latch phenomenon by postulating the existence of latch bridges, i.e., attached, force-maintaining cross bridges formed by dephosphorylation of attached, phosphorylated cross bridges. However, it has been difficult to experimentally prove the existence of latch bridges, and several properties of the latch phenomenon cannot be explained by Murphy's latch-bridge hypothesis alone.
We recently suggested an alternate hypothesis that could explain the latch phenomenon; we found that contractile stimulation increased actin polymerization over the first several minutes of contraction, a process we termed “stimulated actin polymerization” (22). We proposed that this increased actin polymerization could be partially responsible for maintaining force during sustained contractions as cross-bridge phosphorylation falls to intermediate levels. Stimulated actin polymerization appears to be regulated by multiple proteins, including the scaffolding proteins paxillin, which is tyrosine-phosphorylated upon stimulation, and cofilin, an actin-capping and -severing protein, which is serine-phosphorylated and inactive in the unstimulated tissue but becomes dephosphorylated and activated upon stimulation (32). Dephosphorylation of cofilin allows it to sever actin filaments and provide new barbed ends for nucleation of new actin filaments, overcoming the rate-limiting step of actin polymerization (13).
We also recently uncovered a new phenomenon that we termed “force augmentation,” in which submaximal K+ depolarization increases the amount of force generated by the initial phase of a subsequent maximal K+ depolarization by ∼15% (29). We found that force augmentation was associated with prior increases in measures of stimulated actin polymerization, but not with increases in cross-bridge phosphorylation or shortening velocity, suggesting that stimulated actin polymerization determines force augmentation (29).
Length, possibly via determining resting stress, has been reported to alter the actin polymerization state of arterial smooth muscle (3, 10, 28). The goal of this study was to set the level of stimulated actin polymerization prior to stimulation by altering tissue length and then to evaluate whether force augmentation was observed. Specifically, we evaluated whether 1) long tissue lengths inhibited stimulated actin polymerization and force augmentation and 2) short tissue lengths promoted simulated actin polymerization and force augmentation.
MATERIALS AND METHODS
Tissues.
Physiological saline solution (PSS) contained (mM) 140 NaCl, 4.7 KCl, 2 MOPS, 1.2 Na2HPO4, 1.6 CaCl2, 1.2 MgSO4, 5.6 d-glucose, and 0.02 EDTA, with pH adjusted to 7.4 at 37°C. Swine common carotid arteries were obtained and dissected, and the endothelium was removed, mounted, bathed in PSS at 37°C, and set at Lo, the optimal length for force development (18). Approximately 2-mm-wide rings were mounted on two posts: one attached to a micrometer and the other to a force transducer (model FT 03, Grass Instruments). Setting length at Lo involved two contractions with 109 mM extracellular K+ PSS, where KCl was substituted stoichiometrically for NaCl. The second high extracellular K+ concentration was used to normalize all succeeding contractions (18). Isometric stress was calculated as force per cross-sectional area, estimated from measured length, weight, and density of 1.050 g/cm3. Artery preparation was as consistent as experimentally possible throughout the experiments, and there was no substantial difference in results obtained between days. All protocols were approved by the University of Virginia Animal Care and Use Committee.
Cross-bridge phosphorylation.
Cross-bridge (S19-myosin regulatory light chain) phosphorylation was determined in swine common carotid artery rings mounted isometrically at 1.0 Lo and then treated to obtain forces similar to those observed in the stiffness experiments (22) described above. At goal force, rings were frozen in acetone-dry ice and homogenized, and the level of cross-bridge phosphorylation was determined by isoelectric focusing and immunoblotting, as described elsewhere (21). Three dilutions of homogenates were loaded to ensure that the enhanced chemiluminescence detection system was in the linear range (20). Phosphorylation is reported as moles of Pi per mole of protein.
Y118 paxillin phosphorylation.
The level of Y118 paxillin phosphorylation was determined by immunoblotting, as described elsewhere (22). Briefly, homogenates were loaded on two 10% SDS electrophoresis gels and then immunoblotted for total paxillin [1:500 dilution of primary antibody (catalog no. 03-6100, Zymed)] and Y118 phosphorylated paxillin [1:1,000 dilution of primary antibody (catalog no. 44-722G, Biosource)]. To minimize blotting and detection errors, the homogenates from all the rings dissected from each artery were loaded on the same gel. Phosphorylation for each ring was calculated as the ratio of Y118 phosphorylated paxillin to total paxillin immunostaining. Phosphorylation for each ring was then normalized to the level of Y118 phosphorylation in the unstimulated control with the control value = 1.0.
S3 cofilin phosphorylation.
Homogenates were subjected to SDS-PAGE (1.5-mm-thick mini-gels, with 12.5% acrylamide in the running gel and a 5% acrylamide stacking gel) at 200 V for 45 min in a Mini-PROTEAN 3 cell (Bio-Rad). After electrophoresis, gels were equilibrated at room temperature for 1 h with Trans-Blot buffer (25 mM Tris·HCl, pH 7.5, 192 mM glycine, and 20% methanol), and proteins were transferred to nitrocellulose (0.2 μm) at 100 V for 1 h at 4°C in a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) + Tween 20 (TBST: 20 mM Tris·HCl, pH 7.5, 137 mM NaCl, 3 mM KCl, and 0.05% Tween 20) for 1 h and incubated with 1% nonfat dry milk in TBST containing primary antibody (1:1,000 dilution of rabbit polyclonal anti-phosphoserine-3-cofilin; Cell Signaling Technology) for 1 h. Membranes were then washed (4 times for 5 min each) with TBST, incubated with anti-rabbit IgG-horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution in 1% dry milk in TBST; Chemicon) for 1 h, and washed 4 times for 5 min each with TBST and once for 5 min with TBS before chemiluminescence signal detection using the SuperSignal West Femto reagent (Pierce). The emitted light was detected and quantified with a chemiluminescence imaging analyzer (LAS3000mini, Fujifilm), and images were analyzed with MultiGauge version 3.0 software (Fujifilm). Protein loading levels were normalized to calponin detected with a rabbit polyclonal antibody raised against the full-length protein (31).
Statistical analysis.
Unless otherwise noted, values are means ± SE. Statistical significance was tested by ANOVA followed by Fisher's least significant difference test (SigmaStat, Systat Software, Richmond, CA), with P < 0.05 as the criterion for significance.
RESULTS
Cofilin phosphorylation during force augmentation.
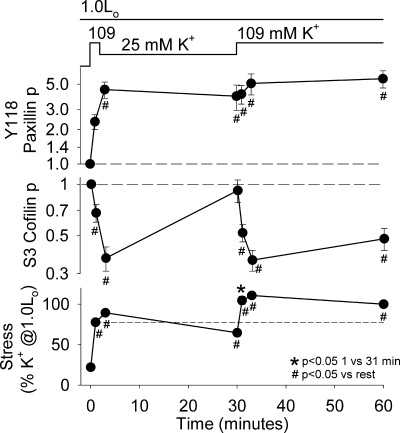
We previously showed that prior partial activation of arterial smooth muscle increased the magnitude of the initial force response to a maximal K+-depolarizing stimulus by ∼15%, a process that we termed force augmentation (29). We also previously reported that force augmentation correlated with prior stimulated actin polymerization, as measured by increases in Y118 paxillin phosphorylation, F-actin content, and a change to a more solid rheology, as measured by a decline in noise temperature. In Fig. 1, we evaluated whether prior S3 cofilin phosphorylation was associated with force augmentation at 1.0 Lo by analyzing the tissue homogenates that we previously studied for Y118 paxillin phosphorylation (29). Swine arterial smooth muscle was maximally depolarized with 109 mM K+ for 2 min, partially activated with 25 mM K+ for 28 min (with 3 changes of the solution to ensure full washout of the 109 mM K+), and finally maximally depolarized a second time with 109 mM K+ for 30 min. Stress was significantly higher 1 min after the second (force-augmented) 109 mM K+ contraction (31 min) than 1 min after the first (nonaugmented) 109 mM K+ contraction (1 min). As we previously reported, Y118 paxillin phosphorylation was significantly increased by 2 min after the first maximal contraction and remained significantly elevated throughout the augmentation protocol. Since Y118 paxillin phosphorylation was significantly higher prior to the second (augmented) contraction (30 min) than prior to the first (nonaugmented) contraction (0 min), we proposed a potential role for Y118 paxillin phosphorylation in force augmentation (29). The first and second maximal 109 mM K+ contractions caused significant decreases in S3 cofilin phosphorylation. Interestingly, S3 cofilin phosphorylation returned to levels not significantly different from rest during the 28-min 25 mM K+ partial activation step; therefore, there was no significant difference in S3 cofilin phosphorylation prior to the first, nonaugmented contraction (0 min) compared with prior to the second, augmented contraction (30 min). These data suggest that prior S3 cofilin phosphorylation was not responsible for the force augmentation observed with the second 109 mM K+ contraction at 1.0 Lo.
Fig. 1.
“Stimulated actin polymerization” and force augmentation were observed at optimal tissue length (1.0 Lo). Tissues were maximally depolarized with 109 mM K+ for 2 min, partially activated with 25 mM K+ for 28 min, and then maximally depolarized with 109 mM K+ for 30 min. Measurements were taken at rest, during the initial 109 mM K+ depolarization (at 1 min), during partial 25 mM K+ activation (at 3 and 30 min), and during the second 109 mM K+ depolarization (at 31, 33, and 60 min). Values are means ± SE (n = 4–6). Top and middle: Y118 paxillin and S3 cofilin phosphorylation [expressed as fraction of phosphorylation (p) in unstimulated tissue on a logarithmic scale]. Long-dashed lines represent normalized values at rest. Bottom: stress (expressed as percentage of prior 109 mM K+ depolarization-induced force). Short-dashed line represents value at 1 min and shows force augmentation, i.e., significantly higher force with the second 109 mM K+ contraction (at 31 min) than the first 109 mM K+ contraction (at 1 min). Differences were tested by Fisher's least significant difference test following ANOVA. Y118 paxillin phosphorylation and stress data have been published elsewhere (Fig. 1 in Ref. 29) and are presented for comparison with new data for S3 cofilin phosphorylation.
Long tissue length and force augmentation.
We studied long tissue length to determine whether force augmentation occurred in the absence of stimulated actin polymerization. Swine carotid arterial tissues were contracted at 1.0 Lo, stretched to 1.4 Lo, and then subjected to a force augmentation protocol. Briefly, tissues were allowed to equilibrate at 1.4 Lo, depolarized with 109 mM K+ for 10 min, partially activated with 25 mM K+ for 30 min, and finally maximally depolarized a second time with 109 mM K+ for 10 min. After equilibration at 1.4 Lo, swine carotid tissues did not exhibit force augmentation; specifically, there was no significant increase in stress with the second maximal contraction that was preceded by 25 mM K+ (Fig. 2, 61 min) compared with the first maximal contraction at 1.4 Lo (21 min). Increasing tissue length to 1.4 Lo alone significantly increased Y118 paxillin phosphorylation (unstimulated values were 1.00 at 1.0 Lo vs. 2.14 at 1.4 Lo). At 1.4 Lo, depolarization with 109 or 25 mM K+ did not significantly further change Y118 paxillin phosphorylation. S3 cofilin phosphorylation was not altered by increasing tissue length to 1.4 Lo (Fig. 2, 20 min). S3 cofilin phosphorylation was decreased by maximal 109 mM K+ depolarization (at 30 and 70 min) and was not altered by 25 mM K+ (at 60 min), similar to our observation at 1.0 Lo. The decrease in S3 cofilin phosphorylation did not differ between the two contractions at 1.4 Lo. Cross-bridge phosphorylation was not altered by increasing tissue length, increased initially upon 109 mM K+ depolarization, returned to intermediate values by 10 min of maximal depolarization, and fell to intermediate levels in 25 mM K+. The cross-bridge phosphorylation response also did not differ between the two contractions at 1.4 Lo.
Fig. 2.
Neither stimulated actin polymerization nor force augmentation was observed at long tissue length (1.4 Lo). Tissues were maximally depolarized with 109 mM K+ for 10 min at 1.0 Lo as a control, lengthened to 1.4 Lo gradually over the course of 60 min (not shown), and then subjected to a force augmentation protocol as follows: maximal depolarization with 109 mM K+ for 10 min, partial activation in 25 mM K+ for 30 min, and maximal depolarization with 109 mM K+ for 10 min. At 1.0 Lo, measurements were taken at rest and during the control contraction at 10 min. After tissue was stretched to 1.4 Lo, measurements were taken at rest (20 min), during the first contraction (21 and 30 min), after partial activation (60 min), and during the second contraction (61 and 70 min). Values are means ± SE (n = 5–7). Differences were tested by Fisher's least significant difference test following ANOVA.
These data show that when there was no force augmentation at 1.4 Lo, there was no difference in prior Y118 paxillin phosphorylation, prior S3 cofilin phosphorylation, or prior cross-bridge phosphorylation. It appears that the increase in Y118 paxillin phosphorylation induced by longer tissue length, per se, prevented subsequent further increases in Y118 paxillin phosphorylation and force augmentation.
Short tissue length and force augmentation.
We studied short tissue length to determine whether force augmentation was enhanced by promoting stimulated actin polymerization. Swine carotid arterial tissues were first contracted at 1.0 Lo, released to 0.6 Lo, and then subjected to the force augmentation protocol used in the long-tissue-length experiments. Similar to our observation at 1.0 Lo, tissues at 0.6 Lo exhibited force augmentation; there was a significant increase in stress 1 min after the second maximal K+ depolarization (Fig. 3, 61 min) compared with 1 min after the first maximal K+ depolarization (Fig. 3, 21 min). Stress continued to increase in the 10 min of the second maximal K+ depolarization. Cross-bridge phosphorylation levels did not significantly differ between the first and second 109 mM K+ contraction (20 to 30 min vs. 60 to 70 min). Y118 paxillin phosphorylation levels were significantly decreased by shorter lengths alone and were not significantly increased until 10 min after the second maximal 109 mM K+ depolarization, suggesting that the force augmentation observed in the second maximal 109 mM K+ depolarization was not caused by prior Y118 paxillin phosphorylation (at 60 min). S3 cofilin phosphorylation was not altered by the shortened length. Interestingly, S3 cofilin phosphorylation was significantly decreased by the first 109 mM K+ depolarization (30 min) and remained low with partial activation (25 mM K+, 60 min), consistent with a potential role for low prior S3 cofilin phosphorylation in the force augmentation at 0.6 Lo observed during the second 109 mM K+ depolarization.
Fig. 3.
Stimulated actin polymerization and force augmentation were observed at short tissue length (0.6 Lo). Tissues were maximally depolarized with 109 mM K+ for 10 min at 1.0 Lo as a control, shortened to 0.6 Lo over the course of 60 min (not shown), and then subjected to a force augmentation protocol as follows: maximal depolarization with 109 mM K+ for 10 min, partial activation in 25 mM K+ for 30 min, and maximal depolarization with 109 mM K+ for 10 min. At 1.0 Lo, measurements were taken at rest and during the control contraction at 10 min. After release to 0.6 Lo, measurements were taken at rest (20 min), during the first contraction (21 and 30 min), after partial activation (60 min), and during the second contraction (61 and 70 min). Values are means ± SE (n = 5–7). Differences were tested by Fisher's least significant difference test following ANOVA.
In summary, force augmentation was associated with significantly low prior S3 cofilin phosphorylation at 0.6 Lo (Fig. 3) and with significantly high prior Y118 paxillin phosphorylation at 1.0 Lo (Fig. 1). Force augmentation was not observed at 1.4 Lo, a length where there was no significant change in prior Y118 paxillin or S3 cofilin phosphorylation (Fig. 2).
The effect of length alone on the unstimulated tissues and tissues stimulated with a single maximal 109 mM K+-induced contraction is shown in Fig. 4. Altering tissue length in unstimulated tissues significantly altered Y118 paxillin phosphorylation and resting stress but did not significantly alter cross-bridge phosphorylation or S3 cofilin phosphorylation. These data suggest that only Y118 paxillin phosphorylation is regulated by changes in length (or changes in resting stress induced by changes in length).
Fig. 4.
Mean biochemical and mechanical characteristics prior to and 10 min into a 109 mM K+ contraction at 0.6, 0.8, 1.0, 1.2, and 1.4 Lo. Mean data from Figs. 1–3 are replotted to emphasize effect of tissue length on resting and 109 mM K+-depolarized tissues. Values are means ± SE (n = 5–7). Differences were tested by Fisher's least significant difference test following ANOVA.
Length did not change the increase in cross-bridge phosphorylation observed with 10 min of 109 mM K+ depolarization (Fig. 4). A 10-min depolarization with 109 mM K+ increased Y118 paxillin phosphorylation significantly at 1.0 Lo, but not at 0.6 or 1.4 Lo (Fig. 4). A 10-min depolarization with 109 mM K+ decreased S3 cofilin phosphorylation at all lengths and increased force at all lengths (the increase was maximal at 1.0 Lo).
The biochemical measures prior to a nonaugmented (first) vs. augmented (second) contraction at 0.6, 1.0, and 1.4 Lo are shown in Fig. 5. Prior cross-bridge phosphorylation did not differ at any length, suggesting that prior cross-bridge phosphorylation was not responsible for the force augmentation observed at 0.6 and 1.0 Lo. Prior Y118 paxillin phosphorylation was significantly elevated prior to the force-augmented contraction at 1.0 Lo, but not at 0.6 or 1.4 Lo, suggesting that Y118 paxillin phosphorylation may be involved in force augmentation at 1.0 Lo. S3 cofilin phosphorylation (Fig. 5) remained significantly decreased prior to the force augmentation step at 0.6 Lo, but not at 1.0 or 1.4 Lo, suggesting that S3 cofilin phosphorylation may be involved in force augmentation at 0.6 Lo. Force augmentation was not observed at 1.4 Lo, a length where Y118 paxillin and S3 cofilin phosphorylation did not differ between the first and second contractions. These data suggest that force augmentation occurred only when Y118 paxillin phosphorylation was increased or S3 cofilin phosphorylation was decreased.
Fig. 5.
Force augmentation is associated with increased Y118 paxillin phosphorylation at 1.0 Lo and with decreased S3 cofilin phosphorylation at 0.6 Lo. Mean data from Figs. 1–3 are replotted to emphasize biochemical characteristics prior to a nonaugmented (first) contraction and an augmented (second) contraction (following partial activation). Force augmentation in the second contraction is shown as percentage above the first contraction. Values are means ± SE (n = 5–7). Differences were tested by Fisher's least significant difference test following ANOVA: *P < 0.05, first contraction vs. second contraction (for biochemical measurements) and percent increase vs. baseline (for force augmentation).
Length-tension relation and the time course of contraction.
Since longer lengths, per se, increased Y118 paxillin phosphorylation and since prior increased Y118 paxillin phosphorylation at 1.0 Lo was associated with a larger contraction (force augmentation), we hypothesized that increases in Y118 paxillin phosphorylation prior to stimulation may be associated with more rapid contractions. Swine carotid arterial rings were equilibrated at 0.6, 0.8, 1.0, 1.2, and 1.4 Lo and contracted with 109 mM K+ for 10 min. Figure 6A shows the classic length-tension relation with higher passive forces at longer lengths and maximal active force with 10 min of K+ depolarization at 1.0 Lo. At longer lengths, the active force 1 min after K+ depolarization (Fig. 6B) was maximal at 1.4 Lo, not at 1.0 Lo. Figure 6C shows the time course of these contractions: tissues at 1.4 Lo contracted faster (as measured by 1 min force) than tissues kept at 1.0 Lo, which contracted faster than tissues kept at 0.6 Lo.
Fig. 6.
Length-tension relation and speed of contraction in swine carotid arterial smooth muscle showing higher 1-min active force and more rapid contractions at 1.4 Lo than at shorter lengths. A: active and passive force (F) at 0.6, 0.8, 1.0, 1.2, and 1.4 Lo was measured and normalized as percentage of 10-min active force maximal value at 1.0 Lo. Maximal active force was observed at 1.0 Lo, with force generation capacity decreasing above and below 1.0 Lo, while maximal passive force was observed at 1.4 Lo. B: active force at 0.6, 0.8, 1.0, 1.2, and 1.4 Lo was measured at 1 and 10 min after depolarization (with all values normalized to 10-min maximal value at 1.0 Lo). Maximal active force at 10 min was observed at 1.0 Lo, while maximal active force at 1 min was observed at 1.4 Lo. C: force, as percentage of 10-min maximal value, at rest and after 109 mM K+ depolarization for 1, 3, and 10 min in tissues held at 0.6, 0.8, 1.0, 1.2, and 1.4 Lo. Tissues shortened at 0.6 Lo achieved ∼49% maximal stress after 1 min of depolarization. Tissues held at 1.0 Lo achieved ∼53% maximal stress after 1 min of depolarization. Tissues lengthened to 1.4 Lo achieved ∼67% maximal stress after 1 min of depolarization, suggesting that longer tissue length increased speed of contraction. Values are means ± SE (n = 5).
In Fig. 7, data from Fig. 6 were replotted to demonstrate that Y118 paxillin phosphorylation measured prior to 109 mM K+ depolarization significantly correlated with the speed of 109 mM K+-induced contraction (r2 = 0.96), but cross-bridge phosphorylation did not. These data suggest that higher prior Y118 paxillin phosphorylation could be the cause of the more rapid contraction at longer lengths.
Fig. 7.
Prior stimulated actin polymerization was associated with initial stress development rates. Prior Y118 paxillin phosphorylation (a measure of prior stimulated actin polymerization) strongly and positively correlated with rate of contraction (normalized total stress observed at 1 min of contraction as percentage of stress at 10 min). Prior cross-bridge phosphorylation did not correlate with rate of contraction. Values are means ± SE (n = 5–7).
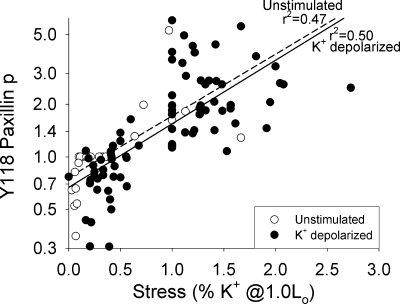
The experiments in Figs. 2 and 3 showed higher Y118 paxillin phosphorylation with longer length (which increased passive stress), with K+ depolarization (which increased active stress), and with longer length and K+ depolarization (which increased passive and active stress). We therefore hypothesized that total stress, per se, could regulate Y118 paxillin phosphorylation. We found a single dependence of Y118 paxillin phosphorylation as a function of total stress in tissues that were not stimulated at various lengths or subjected to K+ depolarization at various lengths (Fig. 8). Unstimulated and K+-depolarized tissues show a significant correlation between total stress and Y118 paxillin phosphorylation (r2 = 0.47 for unstimulated, r2 = 0.50 for K+-depolarized, and r2 = 0.52 for combined data, regression line for both not shown). These data suggest that Y118 paxillin phosphorylation could be regulated by total smooth muscle stress.
Fig. 8.
Measured total stress, induced by length change and/or K+ depolarization, quantitatively predicted Y118 paxillin phosphorylation. Y118 paxillin phosphorylation strongly correlated with total stress in unstimulated (dashed regression line) and K+-depolarized (solid regression line) tissues at various lengths. Data from Figs. 2 and 3 at 0.6, 1.0, and 1.4 Lo are combined.
DISCUSSION
Mediators of actin polymerization.
We previously reported that actin polymerization increased during the sustained phase of swine carotid artery contraction (22); this increase in actin polymerization is a process we termed stimulated actin polymerization. We also found that when prior submaximal stimuli induced stimulated actin polymerization, a subsequent maximal stimulation induced a larger initial contraction, a process we termed force augmentation (Fig. 1) (29). We demonstrated stimulated actin polymerization by measuring 1) regulation of actin polymerization via Y118 paxillin phosphorylation, 2) actin polymerization directly with the F-actin assay, and 3) a change to a more solid rheology as measured by decreased noise temperature, hysteresivity, and phase angle. Figure 9 provides a schema of some of the pathways regulating cross-bridge cycling and some of the pathways regulating actin polymerization in vascular smooth muscle.
Fig. 9.
Schema of events leading to contraction in vascular smooth muscle. Classic contractile pathways involving regulation of phosphorylated cross bridges (MLCp) are shown in double lines at left. Proposed stimulated actin polymerization-based pathways are shown in solid lines at right. Abl, Abelson murine leukemia viral oncogene (tyrosine kinase); Arp2/3, actin-related proteins 2 and 3; cdc42, cell division control protein 42 homolog; CPI-17, PKC phosphorylation-dependent inhibitor of myosin phosphatase; CrkII, cell division control protein 2-related kinase 2; DAG, diacylglycerol; FAK, focal adhesion kinase; Gα, G protein α-subunit; GPCR, G protein-coupled receptor; HSP27, heat shock protein 27; IP3, inositol 1,4,5-trisphosphate; LIMK, LIM kinase; mDia, murine diaphanous homolog of Drosophila; MK2, mitogen-activated protein kinase 2; MLC, myosin light chain; MLCp, phosphorylated myosin regulatory light chain; MLCK, MLC kinase; MLCP, MLC phosphatase; nWASp, neuronal Wiskott-Aldrich syndrome protein; p130CAS, Crk-associated substrate of p130; p38MAPK, mitogen-activated protein kinase; PAK, p21-activated kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PYK2, protein tyrosine kinase 2; Rac, Ras-related C3 botulinum toxin substrate; RhoGEF, Rho guanine nucleotide exchange factor; ROCK, Rho-associated protein kinase; Src, sarcoma-related protooncogenic tyrosine kinase.
In this study, we showed that cofilin, a known biochemical mediator of actin polymerization (Fig. 9), is also dynamically regulated in swine carotid arterial smooth muscle. At optimal tissue length (1.0 Lo), S3 cofilin phosphorylation significantly decreased with maximal stimulation; i.e., it fell during the sustained phase of contraction, suggesting that it could be partly involved in regulation of stimulated actin polymerization (Figs. 1 and 9). Interestingly, S3 cofilin phosphorylation increased back to resting levels with submaximal stimulation at 1.0 Lo (Fig. 1), suggesting that S3 cofilin phosphorylation is activated by maximal, but not submaximal, stimulation (a finding different from that observed with Y118 paxillin phosphorylation). This result suggests that S3 cofilin dephosphorylation is not involved in regulation of the latch phenomenon, because latch is observed with maximal and submaximal stimulation at 1.0 Lo (4, 17). Unlike Y118 paxillin phosphorylation, in which elevated prior values correlated with force augmentation, the near-resting values of S3 cofilin phosphorylation with submaximal stimulation did not correlate with force augmentation at 1.0 Lo (Figs. 1 and 5), suggesting that S3 cofilin dephosphorylation is not responsible for force augmentation at 1.0 Lo.
Force augmentation at varying lengths.
We also previously reported that stimulated actin polymerization, but not cross-bridge phosphorylation, predicted subsequent force augmentation at 1.0 Lo (29). By using the resting length of the tissue to adjust the resting actin polymerization state, we were able to test whether stimulated actin polymerization is always observed with force augmentation.
The force augmentation protocol at short tissue length (0.6 Lo) showed a significant increase in stress and a significant decrease in S3 cofilin phosphorylation prior to the second maximal contraction (following partial activation with 25 mM K+), demonstrating force augmentation associated with reduced S3 cofilin phosphorylation at 0.6 Lo (Figs. 3 and 5). Y118 paxillin and cross-bridge phosphorylation did not differ between the first and second contractions at 0.6 Lo. This result differed from that at 1.0 Lo, where significantly higher Y118 paxillin phosphorylation without changes in S3 cofilin phosphorylation was associated with force augmentation (Figs. 1 and 5). The force generation apparatus may also be slower at shorter lengths, since we see a partial inhibition of the initial cross-bridge phosphorylation transient and lags in Y118 paxillin phosphorylation and stress in the second, force-augmented contraction (Figs. 3, 6, and 7).
The force augmentation protocol at long tissue length (1.4 Lo) resulted in no increase in stress (i.e., no force augmentation) and no significant change in Y118 paxillin or S3 cofilin phosphorylation prior to the second maximal contraction (following partial activation with 25 mM K+). Therefore, there was no force augmentation when there was no prior change in Y118 paxillin or S3 cofilin phosphorylation at 1.4 Lo (Figs. 2 and 5). By increasing the resting length of the tissue to 1.4 Lo, we also increase the resting stress of the tissue, which may in turn activate the mechanical sensors that regulate actin polymerization via integrins, focal adhesion kinase (FAK)/sarcoma-related protooncogenic tyrosine kinase (Src)/Abelson murine leukemia viral oncogene (tyrosine kinase) (Abl), to Y118 paxillin phosphorylation. Since the actin polymerization pathways were already preactivated and near maximal, we observed no further force augmentation and no further increase in Y118 paxillin phosphorylation, as predicted (Fig. 2, no significance for 21 vs. 61 min). This result differed from that at 0.6 and 1.0 Lo.
Latch phenomenon at varying lengths.
Upon maximal stimulation of arterial smooth muscle, there is a transient increase in cross-bridge phosphorylation to high levels followed by a rapid contraction. After the initial transient, cross-bridge phosphorylation falls to submaximal levels while contractile force is maintained at high levels; this was the first definition of the latch phenomenon (5). Latch is better defined as higher contractile force than expected from the level of cross-bridge phosphorylation: this results in a sigmoid relation between cross-bridge phosphorylation and force, such that resting force is present at ∼0.15 mol Pi/mol and maximal force is present at ≥0.3 mol Pi/mol (5, 14, 23). Without latch, this relation would be linear. This relation suggests that a high cross-bridge phosphorylation transient is not needed for latch. Submaximal stimulation of arterial smooth muscle results in a gradual increase in cross-bridge phosphorylation quickly followed by a gradual contraction.
Performing a careful evaluation of the latch state, we previously reported that latch was observed at 0.6 and 1.0 Lo, as evidenced by a curvilinear relation between steady-state stress and steady-state cross-bridge phosphorylation (16). At 1.4 Lo, we found a linear relation between steady-state stress and steady-state cross-bridge phosphorylation, leading us to conclude that latch was not present at 1.4 Lo (16). In the present study, at 0.6 Lo, we observed significant increases in contractile force from 1 to 10 min, with no significant changes in cross-bridge phosphorylation, indicating that latch was present at 0.6 Lo (Fig. 3, 20 to 30 min and 60 to 70 min). At 0.6 Lo, we observed a reduction in S3 cofilin phosphorylation but no change in Y118 paxillin phosphorylation, suggesting that S3 cofilin dephosphorylation could be involved in latch at 0.6 Lo. At 1.4 Lo, we also observed a reduction in S3 cofilin phosphorylation with no significant change in Y118 paxillin phosphorylation. If stimulated actin polymerization is responsible for latch, we should see changes in regulators of stimulated actin polymerization only when latch is observed. The decrease in S3 cofilin phosphorylation at 1.4 Lo, where there is no latch, suggests that S3 cofilin dephosphorylation, per se, is not solely responsible for latch.
Regulation of stimulated actin polymerization by tissue length and by K+ depolarization.
Tissue length can regulate actin polymerization through a number of pathways (Fig. 9), including activation of integrins (1, 25, 30) or activation of stress-dependent receptor tyrosine kinases (8, 26). Reducing resting tissue length to 0.6 Lo decreased the resting stress of the tissue (Figs. 3 and 6) and reduced Y118 paxillin phosphorylation (Figs. 3 and 4). Increasing resting tissue length to 1.4 Lo increased the resting stress of the tissue (Figs. 2 and 6) and increased Y118 paxillin phosphorylation (Figs. 2 and 4). We found a significant dependence of Y118 paxillin phosphorylation on total stress in unstimulated tissues (Fig. 8). These are the expected results given the proposed mechanism for regulation of Y118 paxillin phosphorylation through changes in length, which alter mechanical strain via integrins, which in turn activate FAK/Src/Abl and then Y118 paxillin phosphorylation, as proposed in Fig. 9.
We also found a significant dependence of Y118 paxillin phosphorylation on stress in K+-depolarized tissues at all three lengths (Fig. 8). Intriguingly, the dependence of Y118 paxillin phosphorylation on stress in K+-depolarized tissues was similar to the dependence in unstimulated tissues (Fig. 8), suggesting that total stress alone regulates Y118 paxillin phosphorylation. It is likely that there is feedback from K+-induced contraction to increase Y118 paxillin phosphorylation by increasing integrin signaling (Fig. 9, solid arrow at far right), which could be the mechanism responsible for the stimulated actin polymerization during the sustained phase of contraction at 1.0 Lo (Fig. 1). This mechanism would explain the finding that increases in Y118 paxillin phosphorylation lag behind contraction (Fig. 1) (29). These data suggest that K+ depolarization first leads to cross-bridge phosphorylation and contraction, and this contraction-induced increase in mechanical strain leads to activation of an integrin pathway and Y118 phosphorylation of paxillin (Fig. 9, solid arrow at far right) followed by increased (stimulated) actin polymerization.
A further increase in stimulated actin polymerization was not seen at longer lengths, where force and Y118 paxillin phosphorylation are high prior to contractile stimulation. This occurs because the increased length, per se, has already increased total stress and, thereby, Y118 paxillin phosphorylation. This sustained high, but unchanged, Y118 paxillin phosphorylation also correlates with the lack of force augmentation.
Interestingly, stress did not always predict Y118 paxillin phosphorylation: we previously found that readdition of Ca2+ to Ca2+-depleted swine carotid arterial tissues increased force without increasing Y118 paxillin phosphorylation or inducing force augmentation (29). This finding suggests that Ca2+ depletion could interfere with signaling from integrins to Y118 paxillin phosphorylation.
Length and length-induced changes in stress did not alter S3 cofilin phosphorylation (Figs. 2–4). These are the expected results given the proposed mechanism for regulation of S3 cofilin phosphorylation through Rho, Rho-associated protein kinase, and LIM kinase, rather than through mechanical strain (Fig. 9).
Tissue length-tension relation and speed of contraction.
Changes in resting tissue length altered the speed of contraction as measured by the 1-min active forces in Fig. 6C. Decreasing resting tissue length to 0.6 Lo resulted in tissues that generated ∼49% of maximal force after 1 min of depolarization. Increasing tissue length to 1.4 Lo resulted in tissues that generated ∼67% of maximal force after 1 min of depolarization. These data suggest that there is some process that increases the speed of contraction at longer lengths. Figure 7 shows that the prior level of Y118 paxillin phosphorylation correlates with the rate of 109 mM K+-induced contraction (r2 = 0.96), suggesting that the more rapid contraction could possibly be determined by prior stimulated actin polymerization (which is induced by increased tissue stress; Fig. 8). The other mechanism could be the smaller cross-bridge phosphorylation transient at 0.6 Lo (Fig. 2).
The change in the peak of the 1-min length-tension relation to longer lengths at 1 min of contraction seen in Fig. 6B is reminiscent of our data showing that the peak of the length-tension relation was between 1.2 and 1.4 Lo in forskolin-treated swine carotid artery, i.e., arteries exhibiting force suppression (Fig. 5 of Ref. 16). The most likely mechanism for the current finding that the peak of the 1-min length-tension relation is at 1.2–1.4 Lo is the more rapid contraction at longer lengths, possibly from prior stimulated actin polymerization at longer lengths. However, we cannot rule out the possibility that the mechanism(s) responsible for the rightward shift in the length-tension relation at 1 min after depolarization is similar to the mechanism responsible for the rightward shift in the length-tension relation with force suppression (16). Importantly, the longer tissue length flattened the dependence of force on cross-bridge phosphorylation; i.e., there was no latch at 1.4 Lo compared with 1.0 and 0.6 Lo (15, 16, 19).
Alternate mechanisms.
Figure 9 provides some alternate mechanisms leading to contraction in vascular smooth muscle. Our results have given credence to stimulated actin polymerization based on some of the pathways shown in Fig. 9, right. Increasing tissue length alone increased Y118 paxillin phosphorylation, likely via increased strain-activating integrins. S3 cofilin phosphorylation was not affected by this increased tissue length, an expected result that is more in line with the classical contractile pathway via G protein-coupled receptors.
Limitations.
As represented in the schematic in Fig. 9, experiments aimed at delineating the roles of stimulated actin polymerization in smooth muscle contraction are considerably limited by biology. Since one of our aims was to differentiate between the roles of stimulated actin polymerization and basal actin polymerization, long-term application of actin polymerization inhibitors is problematic, since these agents cause a global knockdown of actin polymerization. Similarly, proteins such as Rho, paxillin, and cofilin are critical to numerous cellular processes, so their long-term knockdown would result in many unexpected, unintended, and indiscernible consequences. Noncovalent modifications of some proteins in the actin polymerization pathway (Fig. 9) are more difficult to test in intact tissues, so we chose not to focus on those. We are, therefore, left with the correlative studies shown above to analyze testable hypotheses.
In the present study, using testable hypotheses, we showed that long tissue lengths inhibited stimulated actin polymerization and force augmentation. We also showed that short tissue lengths did not inhibit stimulated actin polymerization or force augmentation. We found no evidence for force augmentation without stimulated actin polymerization. Similarly, we found no evidence for stimulated actin polymerization that did not lead to force augmentation.
Summary.
At all three lengths tested, the cross-bridge phosphorylation response was not significantly different in a comparison of the first (nonaugmented) contraction with the second (augmented) contraction, indicating that cross-bridge phosphorylation does not regulate force augmentation (Fig. 5). Force augmentation was observed at 0.6 and 1.0 Lo, and the actin polymerization pathway was significantly activated at both of these lengths by S3 cofilin dephosphorylation and Y118 paxillin phosphorylation, respectively (Fig. 5). Total stress, per se, predicted Y118 paxillin phosphorylation (Fig. 8). These data suggest that force augmentation is regulated by stimulated actin polymerization and that stimulated actin polymerization is regulated by total arterial stress. One interesting finding, which will be the focus of future work, is that force augmentation and latch may be manifestations of the same phenomenon and that both may be regulated by actin polymerization.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-71191 (to C. M. Rembold), Mid-Atlantic American Heart Association Grants 0855369E (to C. M. Rembold) and 0815232E (to A. D. Tejani), and Canadian Institutes of Health Research Grant MOP13101 (to M. P. Walsh). M. P. Walsh is an Alberta Heritage Foundation for Medical Research Scientist and recipient of a Canada Research Chair (Tier 1) in Vascular Smooth Muscle Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The technical assistance of Shaojie Han and Marcia Ripley with biochemical assays is appreciated. Arteries were donated by Harrisonburg Wholesale Meat (Harrisonburg, VA), T & E Meats (Harrisonburg, VA), and Gore's Processing (Edinburg, VA).
REFERENCES
- 1. Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J 16: 72–76, 2002 [DOI] [PubMed] [Google Scholar]
- 2. DeFeo TT, Morgan KG. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol 369: 269–282, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deng L, Bosse Y, Brown N, Chin LY, Connolly SC, Fairbank NJ, King GG, Maksym GN, Pare PD, Seow CY, Stephens NL. Stress and strain in the contractile and cytoskeletal filaments of airway smooth muscle. Pulm Pharmacol Ther 22: 407–416, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 211: 495–497, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Driska SP. High myosin light chain phosphatase activity in arterial smooth muscle: can it explain the latch phenomenon? Prog Clin Biol Res 245: 387–398, 1987 [PubMed] [Google Scholar]
- 6. Etter EF, Eto M, Wardle RL, Brautigan DL, Murphy RA. Activation of myosin light chain phosphatase in intact arterial smooth muscle during nitric oxide-induced relaxation. J Biol Chem 276: 34681–34685, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheologoy of living cells. Phys Rev Lett 87: 148102, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Gokina NI, Osol G. Actin cytoskeletal modulation of pressure-induced depolarization and Ca2+ influx in cerebral arteries. Am J Physiol Heart Circ Physiol 282: H1410–H1420, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Gunst SJ, Fredberg JJ. The first three minutes: smooth muscle contraction, cytoskeletal events, and soft glasses. J Appl Physiol 95: 413–425, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Herlihy JT, Murphy RA. Force-velocity and series elastic characteristics of smooth muscle from the hog carotid artery. Circ Res 34: 461–466, 1974 [DOI] [PubMed] [Google Scholar]
- 11. Meiss RA. Influence of intercellular tissue connections on airway muscle mechanics. J Appl Physiol 86: 5–15, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Murphy RA, Rembold CM. The latch-bridge hypothesis of smooth muscle contraction. Can J Physiol Pharmacol 83: 857–864, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science 326: 1208–1212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ratz PH, Hai CM, Murphy RA. Dependence of stress on cross-bridge phosphorylation in vascular smooth muscle. Am J Physiol Cell Physiol 256: C96–C100, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Rembold CM. Force suppression and the cross-bridge cycle in swine carotid artery. Am J Physiol Cell Physiol 293: C1003–C1009, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rembold CM, Meeks MK, Ripley ML, Han S. Longer muscle lengths recapitulate force suppression in swine carotid artery. Am J Physiol Heart Circ Physiol 292: H1065–H1070, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rembold CM, Murphy RA. Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res 58: 803–815, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Rembold CM, Murphy RA. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res 63: 593–603, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Rembold CM, Murphy RA. Muscle length, shortening, myoplasmic [Ca2+], and activation of arterial smooth muscle. Circ Res 66: 1354–1361, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Rembold CM, O'Connor MJ. Caldesmon and heat shock protein 20 in nitroglycerin- and magnesium-induced relaxation of swine carotid artery. Biochim Biophys Acta 1500: 257–264, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Rembold CM, O'Connor MJ, Clarkson M, Wardle RL, Murphy RA. HSP20 phosphorylation in nitroglycerin- and forskolin-induced sustained reductions in swine carotid media tone. J Appl Physiol 91: 1460–1466, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol 293: C993–C1002, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rembold CM, Wardle RL, Wingard CJ, Batts TW, Etter EF, Murphy RA. Cooperative attachment of cross bridges predicts regulation of smooth muscle force by myosin phosphorylation. Am J Physiol Cell Physiol 287: C594–C602, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Rhee AY, Brozovich FV. The smooth muscle cross-bridge cycle studied using sinusoidal length perturbations. Biophys J 79: 1511–1523, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93: 548–556, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 71: 435–478, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Speden RN. The effect of initial strip length on the noradrenaline-induced contraction of arterial strips. J Physiol 154: 15–25, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tejani AD, Rembold CM. Force augmentation and stimulated actin polymerization in swine carotid artery. Am J Physiol Cell Physiol 298: C182–C190, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Turner CE. Paxillin interactions. J Cell Sci 113: 4139–4140, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem 265: 10148–10155, 1990 [PubMed] [Google Scholar]
- 32. Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem 283: 36522–36531, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]