Abstract
Sepsis is associated with defects in renal tubule function, but the underlying mechanisms are incompletely understood. Recently, we demonstrated that Gram-negative bacterial lipopolysaccharide (LPS) inhibits HCO3− absorption in the medullary thick ascending limb (MTAL) through activation of Toll-like receptor 4 (TLR4). Here, we examined the mechanisms responsible for inhibition of HCO3− absorption by basolateral LPS. Adding LPS to the bath decreased HCO3− absorption by 30% in rat and mouse MTALs perfused in vitro. The inhibition of HCO3− absorption was eliminated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK)/ERK inhibitors U0126 and PD98059. LPS induced a rapid (<15 min) and sustained (up to 60 min) increase in ERK phosphorylation in microdissected MTALs that was blocked by PD98059. The effects of basolateral LPS to activate ERK and inhibit HCO3− absorption were eliminated in MTALs from TLR4−/− and myeloid differentiation factor 88 (MyD88)−/− mice but were preserved in MTALs from TIR (Toll/interleukin-1 receptor) domain-containing adapter-inducing interferon-β (Trif)−/− mice. Basolateral LPS decreased apical Na+/H+ exchanger 3 NHE3 activity through a decrease in maximal velocity (Vmax). The inhibition of NHE3 by LPS was eliminated by MEK/ERK inhibitors. LPS inhibited HCO3− absorption despite the presence of physiological stimuli that activate ERK in the MTAL. We conclude that basolateral LPS inhibits HCO3− absorption in the MTAL through activation of a TLR4/MyD88/MEK/ERK pathway coupled to inhibition of NHE3. These studies identify NHE3 as a target of TLR4 signaling in the MTAL and show that bacterial molecules can impair the absorptive functions of renal tubules through inhibition of this exchanger. The ERK pathway links TLR4 to downstream modulation of ion transport proteins and represents a potential target for treatment of sepsis-induced renal tubule dysfunction.
Keywords: sepsis, Na+/H+ exchange, acid-base balance, kidney, Toll-like receptor 4 signaling
renal insufficiency is a common and severe complication of sepsis.1 The development of kidney dysfunction predicts a poor outcome, leads to prolonged hospitalization, and doubles the risk for mortality in septic patients (14, 45, 49, 59, 66, 70). Sepsis and endotoxemia induce a variety of defects in renal tubule function in association with alterations in fluid and electrolyte homeostasis that contribute to sepsis pathogenesis (19, 45, 70). These include a urinary concentrating defect (35, 57), increased fractional excretion of sodium and glucose (57, 68, 69, 75), and hypotension (11, 69, 70), as well as the development of systemic metabolic acidosis that contributes to multiple organ dysfunction (6, 11, 46, 70) and is an independent risk factor for mortality in septic patients (24, 48). The immunopathological mechanisms that underlie renal tubule dysfunction during sepsis are poorly understood.
Recently, we demonstrated that Gram-negative bacterial lipopolysaccharide (LPS) and Gram-positive bacterial molecules (lipoteichoic acid; peptidoglycan) can act directly through Toll-like receptors (TLR) to impair the transport function of renal tubules, identifying a new pathophysiological mechanism that can contribute to renal tubule dysfunction during sepsis (31, 32). In particular, absorption of HCO3− by the medullary thick ascending limb (MTAL) is inhibited by LPS, the dominant cell wall molecule of Gram-negative bacteria (31). The inhibition by LPS is mediated through activation of its cell-surface receptor TLR4. Our studies also revealed a novel sidedness to LPS receptor signaling, whereby LPS inhibits HCO3− absorption in the MTAL through the activation of different TLR4-dependent signaling pathways in the basolateral and apical membranes (31). The direct action of LPS to inhibit renal tubule HCO3− absorption may exacerbate and/or impair the ability of the kidneys to correct systemic metabolic acidosis during sepsis (31, 32). Understanding the mechanisms that underlie LPS-induced transport inhibition in the MTAL may lead to the identification of potential therapeutic targets to treat or prevent renal tubule dysfunction during sepsis. At present, the specific intracellular signals that are triggered by LPS in the MTAL and the transport proteins that are targeted downstream of LPS signaling to result in inhibition of HCO3− absorption are undefined.
The MTAL participates in acid-base homeostasis by reabsorbing most of the filtered HCO3− not reabsorbed by the proximal tubule (2, 26). Absorption of HCO3− by the MTAL depends on H+ secretion mediated by the apical membrane Na+/H+ exchanger NHE3 (2, 3, 7, 33, 82). The regulation of MTAL HCO3− absorption by a variety of physiological factors is achieved through regulation of this exchanger (26, 29, 33, 47, 81, 82). We have shown that the basolateral Na+/H+ exchanger NHE1 also is an important determinant of the HCO3− absorption rate in the MTAL. Inhibition of NHE1 with amiloride or nerve growth factor, or by NHE1 knockout, results secondarily in inhibition of apical NHE3, thereby decreasing HCO3− absorption (28, 34, 77, 78). The rate of HCO3− absorption in the MTAL thus depends on a regulatory interaction between the basolateral and apical membrane Na+/H+ exchangers, whereby basolateral NHE1 enhances the activity of apical NHE3 (28, 30, 34, 77, 78). Studies in a variety of cell systems, including immune cells, endothelial cells, and intestinal epithelial cell lines, have shown that Na+/H+ exchange activity attributable to NHE1 plays a role in LPS-induced inflammatory responses (22, 40, 52, 55, 56, 64, 65) and that NHE1 is a target for LPS-induced regulation (9, 40, 52, 56, 64, 65). These findings raise the possibility that LPS could inhibit HCO3− absorption in the MTAL through a primary effect on basolateral NHE1. Whether NHE3, the apical exchanger responsible for absorption of NaCl and/or NaHCO3 by epithelia of the kidney and intestinal tract, is modulated directly by LPS or is a downstream target of TLR signaling in renal cells has not been determined. Infection with enteropathogenic Eschericia coli for several hours decreased NHE3 activity in an intestinal epithelial cell line (38), indicating that bacterial-epithelial cell interactions can influence the activity of this exchanger.
The signal transduction pathways activated through TLRs in renal tubules are poorly defined. Activation of extracellular signal-regulated kinase (ERK) is a prominent intracellular signaling event involved in LPS-induced immune responses. Stimulation of ERK by LPS through TLR4 is important for a variety of responses in immune cells, including activation of transcription factors that promote the expression of genes encoding cytokines and other inflammatory mediators, regulation of cell proliferation and differentiation, and protection against apoptosis (4, 5, 15, 17, 21, 36, 42, 51, 53, 63). With respect to the kidney, LPS was reported to increase ERK phosphorylation and activity in a mouse renal epithelial cell line (72), and activation of ERK through TLR4 played a role in mediating the effect of uropathogenic E. coli to stimulate chemokine secretion in cultured collecting duct cells (10). We have shown that the ERK pathway plays an important role in the regulation of Na+/H+ exchange activity and HCO3− absorption in the MTAL. Activation of ERK mediates inhibition of HCO3− absorption by aldosterone and nerve growth factor (79, 83). Moreover, ERK-dependent inhibition of HCO3− absorption in the MTAL involves specific targeting of the ERK pathway to inhibit either basolateral NHE1 or apical NHE3 depending on the physiological stimulus (29, 34, 79, 83). Recent studies using selective kinase inhibitors suggest that ERK may be involved in mediating inhibition of HCO3− absorption by basolateral LPS in the MTAL (31). However, whether LPS directly activates ERK in renal tubules and whether the ERK pathway functions as a signaling intermediate to couple TLR4 to inhibition of ion transport in the MTAL remains to be determined.
The purpose of the present study was to determine the signal transduction and transport mechanisms responsible for inhibition of HCO3− absorption by basolateral LPS in the MTAL. We focus on mechanisms underlying inhibition by basolateral LPS because this pathway is upregulated during sepsis (80). The results show that basolateral LPS inhibits HCO3− absorption through the activation of a TLR4/myeloid differentiation factor 88 (MyD88)/ERK pathway that is coupled to inhibition of NHE3. These studies identify NHE3 as a downstream target of LPS-induced TLR4 signaling in the MTAL and show that the ERK pathway plays a role in linking TLRs to the inhibition of renal tubule ion transport.
METHODS
Animals.
Male Sprague-Dawley rats (50–100 g body wt) were purchased from Taconic (Germantown, NY). Mice deficient in TLR4 (C57BL/10ScNJ; TLR4−/−), MyD88 [B6.129P2(SJL)-Myd88tm1.1Defr/J; MyD88−/−], and TIR (Toll/interleukin-1 receptor) domain-containing adapter-inducing interferon-β (Trif) (C57BL/6J-Ticam1Lps2/J; Trif−/−), and wild-type control mice (C57BL/10SnJ for TLR4−/−; C57BL/6J for MyD88−/− and Trif−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were 6 to 8 wk old. The animals were maintained under pathogen-free conditions in microisolator cages and received standard rodent chow (NIH 31 diet, Ziegler) and water up to the time of experiments. All protocols in this study were approved by the IACUC of The University of Texas Medical Branch.
Tubule perfusion and measurement of net HCO3− absorption.
MTALs were isolated and perfused in vitro as previously described (25, 31, 79). Tubules were dissected from the inner stripe of the outer medulla at 10°C in control bath solution (see below), transferred to a bath chamber on the stage of an inverted microscope, and mounted on concentric glass pipets for perfusion at 37°C. The tubules were perfused and bathed in control solution that contained (in mM) 146 Na+, 4 K+, 122 Cl−, 25 HCO3−, 2.0 Ca2+, 1.5 Mg2+, 2.0 phosphate, 1.2 SO42−, 1.0 citrate, 2.0 lactate, and 5.5 glucose (equilibrated with 95% O2-5% CO2, pH 7.45 at 37°C). Solutions containing LPS (ultra pure E. coli K12, InvivoGen) and other experimental agents were prepared as described (31, 34, 77, 83). Experimental agents were added to the bath solution as described in results. In one series of HCO3− transport experiments (see Fig. 5B), Na+ in the bath solution was replaced with N-methyl-d-glucammonium (NMDG+).
Fig. 5.
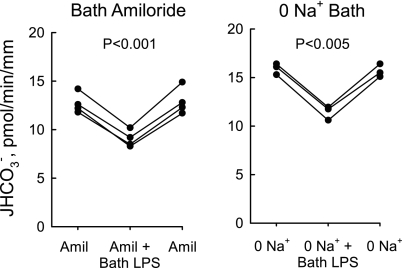
Inhibition of HCO3− absorption by bath LPS does not involve basolateral Na+/H+ exchange. MTALs from rats were studied with 10 μM amiloride in the bath (A) or in a Na+-free bath (B), conditions that inhibit basolateral Na+/H+ exchange (28, 34, 77, 78). LPS (500 ng/ml) was then added to and removed from the bath solution. In B, Na+ in the bath was replaced with NMDG+; the lumen was perfused with control solution containing 146 mM Na+ (28, 77). JHCO3−, data points, lines, and P values are as in Fig. 1. Mean values are given in results.
The protocol for study of transepithelial HCO3− absorption was as described (25, 34, 83). Tubules were equilibrated for 20–30 min at 37°C in the initial perfusion, and bath solutions and the luminal flow rate (normalized per unit tubule length) was adjusted to 1.5–1.9 nl·min−1·mm−1. One to three 10-min tubule fluid samples were then collected for each period (initial, experimental, and recovery). The tubules were allowed to reequilibrate for 5–10 min after an experimental agent was added to or removed from the bath solution. The absolute rate of HCO3− absorption (JHCO3−, pmol·min−1·mm−1) was calculated from the luminal flow rate and the difference between total CO2 concentrations measured in perfused and collected fluids (25). An average HCO3− absorption rate was calculated for each period studied in a given tubule. When repeat measurements were made at the beginning and end of an experiment (initial and recovery periods), the values were averaged. Single tubule values are presented in Figs. 1, 4, 5, and 7. Mean values ± SE (n = number of tubules) are presented in the text.
Fig. 1.
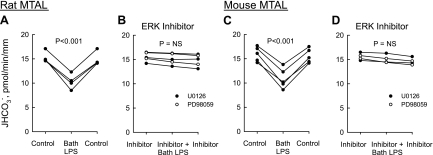
Inhibitors of extracellular signal-regulated kinase (ERK) activation eliminate inhibition of HCO3− absorption by bath lipopolysaccharide (LPS). Medullary thick ascending limbs (MTAL) from Sprague-Dawley rats and C57BL/6J mice were isolated and perfused in vitro. Tubules were studied in control solution (A and C) or bathed with 15 μM U0126 or 15 μM PD98059 (B and D), and then LPS (500 ng/ml) was added to and removed from the bath solution. Absolute rates of HCO3− absorption (JHCO3−) were measured as described in methods. Data points are average values for single tubules. Lines connect paired measurements made in the same tubule. P values are for paired t-test. NS, not significant. Mean values are given in results.
Fig. 4.
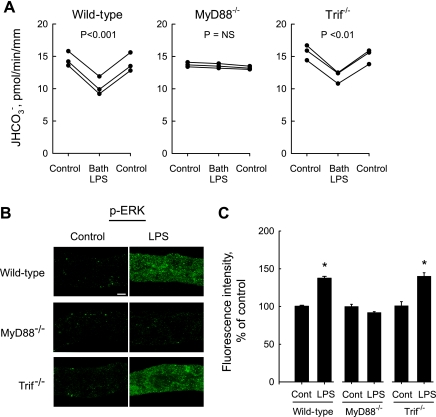
Role of myeloid differentiation factor 88 (MyD88) and TIR (Toll/interleukin-1 receptor) domain-containing adapter-inducing interferon-β (Trif) in responses to LPS. A: MTALs from wild-type, MyD88−/−, and Trif−/− mice were perfused in vitro under control conditions, and then LPS (500 ng/ml) was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 1. NS, not significant. B: MTALs from wild-type, MyD88−/−, and Trif−/− mice were incubated for 15 min at 37°C in vitro in the absence (control) and presence of LPS. The tubules were stained with p-ERK antibody and analyzed by confocal immunofluorescence as in Fig. 2A. The effect of LPS to increase ERK phosphorylation was eliminated in MTALs from MyD88−/− mice. Images are representative of at least six tubules of each type. C: intensity of p-ERK staining was quantified for experiments in B as described in methods and is presented as a percentage of control level measured in the same experiment. *P < 0.05 vs. control. Control fluorescence intensity did not differ in the three groups.
Fig. 7.
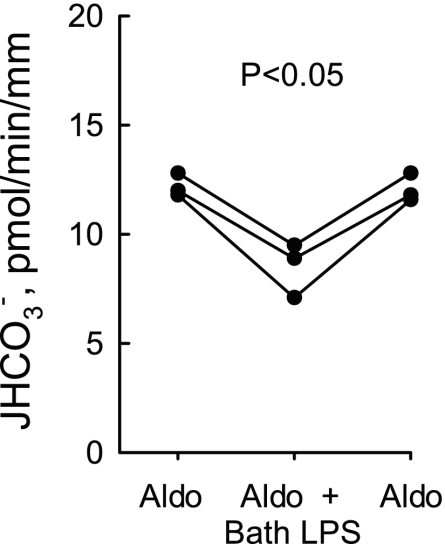
Inhibition of HCO3− absorption by bath LPS is additive to inhibition by aldosterone. MTALs from rats were bathed with aldosterone (1 nM) and then LPS (500 ng/ml) was added to and removed from the bath solution. JHCO3−, data points, lines, and P value are as in Fig. 1.
Measurement of pHi and apical Na+/H+ exchange activity.
pHi was measured in isolated, perfused MTALs by use of the pH-sensitive dye BCECF and a computer-controlled spectrofluorometer (CM-X, SPEX Industries) coupled to the perfusion apparatus, as previously described (77, 81). MTALs were perfused and bathed in Na+-free, HEPES-buffered solution that contained (in mM) 145 NMDG+, 4 K+, 147 Cl−, 2.0 Ca2+, 1.5 Mg2+, 1.0 phosphate, 1.0 SO42−, 1.0 citrate, 2.0 lactate, 5.5 glucose, and 5 HEPES (equilibrated with 100% O2; titrated to pH 7.4). The lumen solution also contained furosemide to block Na+-K+-2Cl− cotransport activity, and the bath contained ethylisopropyl amiloride (50 μM) to eliminate any contribution of basolateral Na+/H+ exchange to the Na+-induced changes in pHi. Apical Na+/H+ exchange activity was determined by measurement of the initial rate of pHi increase after addition of 145 mM Na+ to the lumen solution (Na+ replaced NMDG+) (81, 82). Interruption of pHi recovery at various points along the recovery curve permits determination of the Na+/H+ exchange rate over a range of pHi values, with appropriate corrections for a variable background acid loading rate (81). The Na+-dependent pHi recovery was inhibited ≥90% by lumen ethylisopropyl amiloride (50 μM) under all experimental conditions. Apical Na+/H+ exchange rates (JNa+/H+, pmol·min−1·mm−1) were calculated as (dpHi/dt) × βi × V, where dpHi/dt (pH units/min) is the initial slope of the record of pHi vs time, βi is the intrinsic intracellular buffering power (mM/pH unit), and V is cell volume per unit tubule length (nl/mm), measured as previously described (77, 81, 82). Experimental agents were added to the bath solution as described in results.
Immunoblotting.
Immunoblot analysis of phosphorylated ERK1/2 was carried out using a previously described inner stripe tissue preparation (30, 83). Thin strips of tissue were microdissected at 10°C from the inner stripe of the outer medulla of rat kidney. This preparation is highly enriched in MTALs and exhibits regulatory changes in signaling proteins that accurately reflect changes observed in the MTAL (27, 30, 79, 83). After dissection, the tissue strips were divided into samples of equal amount and incubated for various times at 37°C in the absence and presence of LPS using the same control solution as in HCO3− transport experiments. After incubation, the tissue samples were homogenized in ice-cold PBS and solubilized for 2 h at 4°C in RIPA buffer with protease inhibitors. Samples of equal protein content (50 μg/lane) were separated by SDS-PAGE using 9% gels and transferred to PVDF membranes as described (30, 34). Membranes were blocked with 5% BSA in Tris-buffered saline (TBS)/Tween and incubated overnight at 4°C with antiphospho-ERK1/2-Thr202/Tyr204 (1:2,500) or anti-ERK1/2 (1:5,000) antibody (Cell Signaling Technology). After washing in TBS, horseradish peroxidase-conjugated anti-rabbit secondary antibody was applied, and immunoreactive bands were detected by chemiluminescence (Luminol Reagent, Santa Cruz, CA). Protein bands were quantified by densitometry (MetaMorph).
Confocal immunofluorescence microscopy.
MTALs were studied by confocal microscopy as previously described (30, 78). Rat and mouse MTALs were microdissected and mounted on Cell-Tak-coated coverslips at 10°C. The tubules were then incubated in the absence and presence of LPS for 15 min at 37°C in a flowing bath using the same control solution as in HCO3− transport experiments. After incubation, the tubules were washed with PBS and fixed and permeabilized in acetone at −20°C for 10 min. The tubules were incubated in Image-iT FX signal enhancer (Invitrogen) for 30 min at room temperature, washed, and blocked in 10% goat serum in PBS for 1 h at room temperature. The tubules were then incubated overnight at 4°C with a 1:200 dilution of antiphospho-ERK1/2 antibody, washed, and then incubated for 1 h at room temperature in Alexa 488-conjugated goat anti-rabbit IgG antibody (1:100; Invitrogen) in blocking buffer. Fluorescence staining was examined using a Zeiss laser-scanning confocal microscope (LSM510 UV META) as described (30, 31, 78). Tubules were imaged longitudinally, and Z-axis optical sections (0.4 μm) were obtained through a plane at the center of the tubule, which provides a cross-sectional view of cells in the lateral tubule walls. For individual experiments, two to four tubules from the same kidney for each experimental condition, or from wild-type and null-mutant mice, were fixed and stained identically and imaged in a single session at identical settings of illumination, gain, and exposure time. Fluorescence intensity of antiphospho-ERK1/2 (p-ERK1/2) staining was quantified as previously described (30). Two-dimensional image analysis was performed using MetaMorph software in which a box (4.2 × 1.4 μm) was positioned on the cytoplasm in the midregion of the cell, and pixel intensity per unit area was determined after background subtraction. Three different cells were analyzed per optical section, and three optical sections were analyzed per tubule, one section at the center of the tubule and two sections positioned 0.12 μm above and below the center section. The measurements were averaged to obtain a value for each tubule. Fluorescence intensity for experimental groups was expressed as a percentage of the control value measured in the same experiment. Mean values (n = number of tubules) were used for statistical analysis.
Analysis.
Results are presented as means ± SE. Differences between means were evaluated using Student's t-test for paired or unpaired data, or analysis of variance with Newman-Keuls multiple range test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Inhibitors of ERK activation eliminate inhibition of HCO3− absorption by bath LPS.
Under control conditions, adding LPS (500 ng/ml) to the bath decreased HCO3− absorption by 32% (from 15.1 ± 0.6 to 10.3 ± 0.7 pmol·min−1·mm−1) in MTALs from rats (Fig. 1A) and by 29% (from 15.7 ± 0.6 to 11.1 ± 0.8 pmol·min−1·mm−1) in MTALs from mice (Fig. 1C). The inhibition by LPS is rapid (<15 min), sustained for up to 60 min, and reversible. To determine whether the ERK pathway is involved in mediating the inhibition by LPS, we examined the effects of U0126 and PD98059, two selective inhibitors of MEK1/2 that block activation of ERK1/2 in the MTAL (30, 79, 83). The inhibition of HCO3− absorption by bath LPS was eliminated completely in rat and mouse MTALs bathed with U0126 or PD98059 (Fig. 1, B and D). These results extend our previous observations (31) and support an essential role for the ERK pathway in mediating the inhibition of HCO3− absorption by basolateral LPS.
LPS activates ERK in the MTAL.
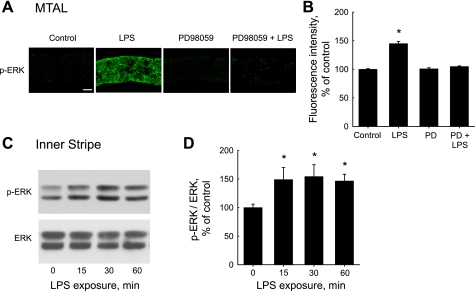
To determine whether LPS activates ERK in the MTAL, we examined the effects of LPS on ERK phosphorylation. MTALs dissected from rats were incubated in the absence and presence of LPS for 15 min, stained with p-ERK antibody, and then analyzed by confocal immunofluorescence (Fig. 2, A and B). Stimulation with LPS increased p-ERK staining 1.5 ± 0.1-fold. The LPS-induced increase in ERK phosphorylation was eliminated by PD98059. Treatment with LPS for 15 min caused a similar increase in ERK phosphorylation in MTALs from mice (see below).
Fig. 2.
LPS increases ERK phosphorylation in the MTAL. A: MTALs dissected from rats were incubated in vitro at 37°C in control solution, LPS (500 ng/ml), PD98059 (15 μM), or PD98059 + LPS for 15 min and then fixed and stained with antiphospho-ERK1/2-Thr202/Tyr204 (p-ERK) antibody. The tubules were analyzed by confocal immunofluorescence as described in methods. Images are Z-axis sections (0.4 μm) taken through a plane at the center of the tubule showing a cross-sectional view of cells in the lateral tubule walls (30, 31, 78). LPS increased p-ERK labeling, and this increase was eliminated by PD98059. Images are representative of at least eight tubules of each type. Scale bar = 5 μm. B: intensity of p-ERK staining for experiments in A was quantified as described in methods and is presented as a percentage of the control level. Bars are means ± SE. *P < 0.05 vs. control (ANOVA). C: time course of ERK activation. Inner stripe tissue from rats was incubated in vitro at 37°C in the absence and presence of LPS for the indicated times, and cell lysates were then immunoblotted with p-ERK antibody for analysis of ERK phosphorylation and anti-ERK antibody for total ERK level. Blots are representative of 3 independent experiments. D: p-ERK levels normalized for total ERK were determined for experiments in C by densitometry. p-ERK/ERK ratios are presented as a percentage of the control value (0 min) measured in the same experiment. Bars are means ± SE. *P < 0.05 vs. 0 min.
To determine the time course of LPS-induced ERK activation, inner stripe tissue was incubated in vitro in the absence and presence of LPS for various times, and then ERK phosphorylation was analyzed by immunoblotting (Fig. 2, C and D). Phosphorylation of ERK was increased within 15 min of exposure to LPS and remained elevated for at least 60 min. ERK phosphorylation was increased 1.5 ± 0.1-fold at 15 min, 1.6 ± 0.1-fold at 30 min, and 1.5 ± 0.1-fold at 60 min relative to control tissue not treated with LPS (n = 3; P < 0.05). Taken together with the preceding immunofluorescence experiments, these results indicate that LPS induces rapid and sustained activation of ERK in the MTAL and support a role for the ERK pathway in mediating inhibition of HCO3− absorption.
LPS-induced ERK activation is mediated through TLR4.
Previously we demonstrated that basolateral LPS inhibits HCO3− absorption in the MTAL through activation of its cell-surface receptor TLR4 (31). To determine whether TLR4 mediates the activation of ERK, the effects of LPS on ERK phosphorylation were examined in MTALs from wild-type and TLR4−/− mice (Fig. 3). Similar to results in MTALs from rats, treatment with LPS for 15 min increased ERK phosphorylation 1.6 ± 0.1-fold in MTALs from wild-type mice. In contrast, LPS had no effect on phosphorylation of ERK in MTALs from TLR4−/− mice. These results indicate that activation of ERK by LPS in the MTAL is mediated through TLR4. The inability of LPS to activate ERK in MTALs from the TLR4−/− mice can explain our previous finding that basolateral LPS fails to inhibit HCO3− absorption in the TLR4−/− MTALs (31).
Fig. 3.
LPS-induced ERK activation is mediated through Toll-like recptor 4 (TLR4). A: MTALs dissected from wild-type and TLR4−/− mice were incubated in vitro for 15 min at 37°C in the absence (control) and presence of LPS. The tubules were then fixed and stained with p-ERK antibody and analyzed by confocal immunofluorescence as in Fig. 2A. LPS increased ERK phosphorylation in MTALs from wild-type mice but had no effect in MTALs from TLR4−/− mice. Images are representative of at least six tubules of each type. B: intensity of p-ERK staining was quantified for experiments in A as described in methods and is presented as a percentage of control level measured in the same experiment. *P < 0.05 vs. wild-type control.
Role of MyD88 and Trif in responses to LPS.
Binding of LPS to the TLR4 complex generates intracellular signals through two distinct pathways mediated by recruitment of the adaptor molecules MyD88 and Trif (42, 43, 58). To determine the role of these adaptors in the inhibition of HCO3− absorption by bath LPS, MTALs from wild-type, MyD88−/−, and Trif−/− mice were perfused in vitro. As shown in Fig. 4A, adding LPS to the bath decreased HCO3− absorption by 28% (from 14.3 ± 0.7 to 10.3 ± 0.8 pmol·min−1·mm−1) in MTALs from wild-type mice and by 23% (from 15.3 ± 0.6 to 11.8 ± 0.5 pmol·min−1·mm−1) in MTALs from Trif−/− mice. In contrast, bath LPS had no effect on HCO3− absorption in MTALs from MyD88−/− mice. These results demonstrate that inhibition of HCO3− absorption by basolateral LPS is dependent on MyD88.
Further experiments were carried out to determine the role of MyD88 and Trif in LPS-induced ERK activation. The effects of LPS on ERK phosphorylation were studied in MTALs from wild-type, MyD88−/−, and Trif−/− mice by confocal immunofluorescence. As shown in Fig. 4, B and C, LPS increased ERK phosphorylation 1.5 ± 0.1-fold in MTALs from wild-type mice and 1.6 ± 0.1-fold in MTALs from Trif−/− mice but had no effect on phosphorylation of ERK in MTALs from MyD88−/− mice. Taken together, these results demonstrate that the TLR4-dependent effects of basolateral LPS to stimulate ERK and inhibit HCO3− absorption are mediated through MyD88.
Inhibition by bath LPS does not involve basolateral Na+/H+ exchange.
We have shown previously that activation of ERK inhibits HCO3− absorption in the MTAL through two distinct mechanisms: 1) primary inhibition of basolateral NHE1, which results secondarily in inhibition of apical NHE3 (77, 83); and 2) direct coupling of the ERK pathway to inhibition of NHE3 (29, 79). To test whether NHE1 is involved in LPS-induced inhibition of HCO3− absorption, the effects of bath LPS were examined in the presence of 10 μM bath amiloride and in the absence of bath Na+, two conditions that inhibit basolateral Na+/H+ exchange and prevent inhibition of HCO3− absorption mediated through NHE1 (28, 34, 77, 78). The results in Fig. 5 show that bath LPS reduced HCO3− absorption by 30 ± 1% in tubules bathed with amiloride and by 29 ± 2% in tubules studied in a Na+-free bath, decreases similar to those observed in MTALs studied in control conditions (Fig. 1). Thus the inhibition of HCO3− absorption by basolateral LPS is not mediated through basolateral Na+/H+ exchange.
Bath LPS inhibits apical Na +/H + exchange through an ERK-dependent pathway.
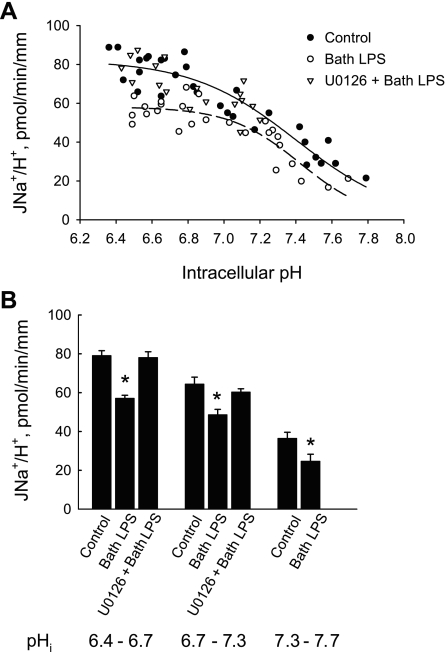
Further studies were carried out to determine whether bath LPS decreases HCO3− absorption through primary inhibition of the apical Na+/H+ exchanger NHE3. MTALs were studied under control conditions and with LPS or U0126 + LPS in the bath solution for 15–20 min. As shown in Fig. 6, bath LPS decreased apical Na+/H+ exchange activity over the range of pHi values studied. The exchanger exhibited a sigmoidal dependence on pHi, as reported previously (77, 81, 82). Kinetic analysis showed that the inhibition of apical Na+/H+ exchange by LPS was due to a 30% decrease in maximal velocity (Vmax, 82 ± 4 pmol·min−1·mm−1, control vs. 57 ± 3 pmol·min−1·mm−1, bath LPS; P < 0.05), with no change in apparent affinity for intracellular H+ , 7.39 pH units, control vs. 7.41 pH units, bath LPS). The effect of bath LPS to decrease apical Na+/H+ exchange activity was eliminated in tubules bathed with U0126 (Fig. 6, A and B). These results indicate that basolateral LPS inhibits apical NHE3 in the MTAL through an ERK-dependent signaling pathway.
Fig. 6.
Bath LPS inhibits apical Na+/H+ exchange through an ERK-dependent pathway. A: MTALs from rats were studied under control conditions and with 500 ng/ml LPS or 15 μM U0126 + LPS in the bath for 15–20 min. Apical Na+/H+ exchange rates (JNa+/H+) were determined from initial rates of pHi increase measured after addition of Na+ to the tubule lumen (see methods). Data points are from 16 control tubules, 19 tubules with LPS, and 10 tubules with U0126 + LPS. Solid (control) and dashed (bath LPS) lines are from least-squares fits to the Hill equation (81, 82); kinetic parameters (Vmax and apparent affinity for intracellular H+) are given in results. B: data from A were grouped over the indicated pHi intervals to obtain mean exchange rates (± SE). *P < 0.05 vs. other conditions in each interval. U0126 alone has no effect on apical Na+/H+ exchange activity (79).
Inhibition of HCO3− absorption by bath LPS is additive to inhibition by aldosterone.
In previous studies, we demonstrated that aldosterone (1 nM) decreases HCO3− absorption by 29% in the MTAL through ERK-dependent inhibition of NHE3 (29, 79). These findings suggest that aldosterone and bath LPS inhibit HCO3− absorption through a common mechanism and raise the question of whether LPS can influence MTAL function in the presence of physiological stimuli that increase ERK activity. To address this, we asked whether bath LPS would inhibit HCO3− absorption in the presence of aldosterone. In tubules bathed with 1 nM aldosterone, adding LPS to the bath decreased HCO3− absorption by 30%, from 12.1 ± 0.3 to 8.5 ± 0.7 pmol·min−1·mm−1 (Fig. 7). Thus LPS inhibits HCO3− absorption by a similar amount in the absence or presence of aldosterone. These results suggest that the mechanisms by which LPS and aldosterone inhibit NHE3 through ERK are different and show that the ability of basolateral LPS to impair MTAL function is maintained despite the presence of other, physiological stimuli that activate the ERK pathway.
DISCUSSION
Impaired renal function accentuates the pathogenesis and lethality of sepsis through the loss of metabolic, fluid, and electrolyte homeostasis (19, 45, 70). Recently, we demonstrated that Gram-negative LPS inhibits HCO3− absorption in the MTAL through activation of TLR4, establishing that bacterial molecules can act directly to alter the transport function of renal tubules by interacting with cell-surface receptors and activating intracellular signaling pathways (31). Understanding the mechanisms involved in the inhibition of HCO3− absorption by LPS will provide insights into cellular pathways and signaling molecules that link innate immune receptors to renal tubule transport processes and that can be activated to impair renal tubule function during sepsis and other inflammatory conditions. In the present study, we show that basolateral LPS decreases HCO3− absorption in the MTAL by inhibiting apical NHE3 through activation of the MEK/ERK signaling pathway (Fig. 8). Activation of ERK by LPS is mediated through TLR4 and the adaptor protein MyD88 and decreases NHE3 activity through a decrease in Vmax. These results identify NHE3 as a downstream target of TLR4 signaling in renal epithelial cells and show that bacterial molecules can impair the absorptive functions of renal tubules through inhibition of this exchanger.
Fig. 8.
Model for inhibition of HCO3− absorption by basolateral LPS in the MTAL. Basolateral LPS decreases HCO3− absorption by inhibiting Na+/H+ exchanger 3 NHE3 through activation of the MEK/ERK signaling pathway. The LPS-induced ERK activation is mediated through TLR4 and the adaptor molecule MyD88. Arrows do not necessarily imply direct relationships; regulatory steps may involve additional signaling components.
Although TLR4 on renal cells plays a role in mediating inflammatory kidney injury in a variety of infectious and noninfectious conditions (12, 18, 20, 44, 50, 61, 67, 74, 84–87), mechanisms of TLR4 signaling in segments of the nephron are poorly understood. In response to LPS, the intracellular adaptor molecules MyD88 and Trif are recruited to the TLR4 complex and mediate the induction of distinct intracellular signaling pathways and inflammatory responses (39, 42, 43, 58). A prominent feature of TLR signaling is the induced expression of immune and inflammatory mediators through the activation of mitogen-activated protein kinases (4, 15, 36, 43). Activation of ERK by LPS in a variety of cell types leads to the activation of transcription factors that regulate the induction of genes encoding cytokines and other proteins involved in the inflammatory response, including tumor necrosis factor-α, IL-1β, IL-10, and Cox-2 (4, 5, 17, 21, 36, 43, 63). LPS stimulates ERK1/2 through TLR4-mediated phosphorylation and activation of its upstream activator MEK1/2 (4, 5, 17, 21, 76). In macrophages, LPS activates ERK through both MyD88-dependent and MyD88-independent (Trif-dependent) pathways (39, 41, 53, 63). The results of the present study show that LPS increases ERK1/2 phosphorylation in the MTAL. Both the activation of ERK and the inhibition of HCO3− absorption by basolateral LPS are mediated through TLR4, are blocked by selective MEK1/2 inhibitors, and are eliminated in MyD88-deficient MTALs but preserved in MTALs that lack Trif.2 Taken together, these results support the conclusion that basolateral LPS inhibits HCO3− absorption in the MTAL through the activation of a TLR4/MyD88/MEK1/2/ERK1/2 signaling pathway and identify MEK/ERK as a key downstream pathway that links TLR4 to inhibition of MTAL transport. In addition, we reported recently that the ability of basolateral LPS to inhibit HCO3− absorption through ERK is upregulated in MTALs from septic mice (80). These findings suggest that the ERK pathway may represent a potential therapeutic target to treat or prevent sepsis-induced renal tubule dysfunction. Important areas for future work will be to identify the signaling components that connect the TLR4/MyD88 receptor complex to activation of MEK1/2 in the MTAL and to understand how the LPS-induced ERK pathway may be upregulated during sepsis.
The Na+/H+ exchanger isoform NHE3 is expressed selectively in the apical membrane of renal and intestinal epithelial cells, where it mediates the reabsorption of NaCl and/or NaHCO3 (2, 8, 16, 26, 60, 71). In the MTAL, NHE3 mediates the luminal H+ secretion required for HCO3− absorption, as evidenced by functional studies (26, 33), immunocytochemical localization (3, 7), inhibitor sensitivity (28, 77, 82), acute regulatory responses (27, 81, 82), and chronic adaptations (33, 47). The present study shows that LPS acts via ERK to inhibit NHE3, resulting in a decrease in HCO3− absorption. LPS inhibits NHE3 when exchanger activity is studied independently of the activity of other transporters and in the absence of a change in the driving force for the exchanger, indicating that the LPS-induced ERK pathway is coupled directly to inhibition of NHE3. LPS decreases NHE3 activity through a decrease in Vmax, with no change in the intracellular H+ affinity. Thus LPS-induced ERK activation leads to a decrease in the intrinsic activity of individual transporters within the membrane and/or alters the subcellular distribution of NHE3 to decrease the number of transporters in active apical membrane domains. The molecular mechanisms that underlie acute regulation of NHE3 by ERK are undefined. NHE3 is a component of macromolecular complexes in the plasma membrane, where its activity is regulated through phosphorylation of its carboxy-terminal cytoplasmic domain by protein kinases such as PKA, and through interactions with a variety of accessory proteins and signaling molecules, including calcineurin homologous protein, megalin, the cytoskeleton-binding protein ezrin, and sodium-hydrogen exchanger regulatory factor (NHERF)-1/2 (1, 8, 16, 60). Whether these function as targets for mediating inhibition of NHE3 by ERK, or a downstream effector of ERK, is not known. Whether apical NHE3 activity is regulated in the MTAL by membrane trafficking, and whether this process may be influenced by ERK, also remains to be determined.
The ubiquitously expressed Na+/H+ exchanger isoform NHE1 has been identified as a target for modulation by LPS in several cell types (9, 40, 52, 56, 65). Moreover, we have shown that ERK can inhibit HCO3− absorption in the MTAL through inhibition of basolateral NHE1 (34, 77, 83). The results of the present study show, however, that NHE1 plays no role in mediating the inhibition of HCO3− absorption by basolateral LPS. Thus either NHE1 activity is not affected by basolateral LPS signaling in the MTAL or changes in NHE1 activity induced by basolateral LPS are not involved in mediating inhibition of HCO3− absorption.
In previous studies we demonstrated that ERK-dependent regulation of HCO3− absorption in the MTAL involves ERK signal specificity. Nerve growth factor (NGF) and aldosterone both inhibit HCO3− absorption through activation of ERK, but the inhibition occurs through different mechanisms. NGF decreases HCO3− absorption through direct coupling of the ERK pathway to inhibition of basolateral NHE1, which results secondarily in inhibition of apical NHE3 (34, 77, 83). In contrast, aldosterone decreases HCO3− absorption through direct coupling of the ERK pathway to inhibition of NHE3, independent of NHE1 (29, 79). Thus, in the MTAL, the ERK pathway can be targeted primarily to inhibit basolateral NHE1 or apical NHE3, depending on the physiological stimulus. The results of the present study suggest an additional level of specificity in ERK-dependent regulation of Na+/H+ exchange in the MTAL. Basolateral LPS inhibits HCO3− absorption through mechanisms that appear to be identical to those observed with aldosterone, namely, direct coupling of the ERK pathway to inhibition of NHE3 through a decrease in Vmax. Our results show, however, that the inhibition of HCO3− absorption by basolateral LPS is fully maintained in the presence of a maximal inhibitory concentration of aldosterone. The additivity of the LPS and aldosterone inhibitory effects suggests that the molecular mechanisms mediating ERK-dependent inhibition of NHE3 by the two stimuli may differ. The potential mechanisms by which ERK signals arising from different stimuli may differentially regulate NHE3 are undefined and may be complex (54). Possible contributing factors could include the targeting of different downstream effectors of ERK to different regulatory components of the NHE3 macromolecular complex and the regulation of different ERK pools through the activation of different upstream kinases. The latter mechanism is of interest in view of recent findings that TLR-dependent activation of ERK is mediated through a specific a mitogen-activated protein kinase kinase kinase (MAP3K) Tpl2, which activates ERK1/2 through phosphorylation and activation of MEK1/2 (4, 5, 17, 21, 76). Tpl2 is required for LPS-induced ERK activation in various cell types and functions as a specific downstream mediator of ERK activation in TLR signaling (4, 17, 21, 23, 42, 76). Thus Tpl2 enables TLR-induced ERK activation that is distinct from ERK activation mediated through other members of the MAP3K family, such as the mitogen-responsive MAP3K Raf-1 (5, 13, 23, 37, 76). Whether Tpl2 mediates TLR-dependent activation of ERK in renal tubules and may play a role in the specificity of ERK signaling in the MTAL will be important areas for future research. Our finding that LPS and aldosterone appear to inhibit NHE3 through different ERK-dependent mechanisms has important implications for the pathogenesis of sepsis because it reveals mechanisms of TLR signal specificity that enable bacterial molecules to impair renal tubule transport despite the presence of other, physiological stimuli that converge on the same signaling pathways. Understanding the molecular events that underlie this TLR signal specificity will be important for selective therapeutic targeting of pathogen-induced inflammatory pathways in renal tubules. The Tpl2-ERK pathway recently has been identified as a potential therapeutic target for treatment of a number of autoimmune and inflammatory diseases (23, 37).
In addition to the acute effect of the ERK pathway to inhibit NHE3 in the MTAL, ERK has been shown to play a role in the long-term (24 h) stimulation of NHE3 by acid media in renal proximal tubule cells (73). This chronic stimulation likely involves an effect of acid-induced ERK activation to mediate increased transcription of endothelin-1, which then functions as an autocrine or paracrine factor to upregulate NHE3 (62). These findings illustrate further the complexity of ERK-dependent regulation of NHE3, which may involve both direct and indirect mechanisms. As noted earlier, the acute effect of LPS to inhibit HCO3− absorption through ERK is enhanced in MTALs from septic mice that exhibit systemic metabolic acidosis (80). These findings underscore the importance of understanding the distinct molecular components responsible for specificity of ERK signaling in response to different stimuli, and how these pathways interact, to permit effective therapeutic targeting of ERK signals leading to pathogen-induced renal tubule dysfunction.
Fine control of NHE3 in renal tubules is essential for regulation of sodium and volume balance, acid-base balance, and blood pressure (2, 8, 26, 60, 71). In the present study, we demonstrate that interaction of LPS with TLR4 induces cell signals that inhibit NHE3 in the MTAL. The decrease in NHE3 activity results in a decrease in HCO3− absorptive capacity that could impair the ability of the kidneys to correct systemic metabolic acidosis that contributes to sepsis pathogenesis (6, 11, 24, 46, 48, 70). These studies identify NHE3 as a target of TLR4 signaling in the MTAL and suggest that activation of TLRs by bacterial molecules and other microbial pathogens can disrupt renal tubule absorptive processes important for volume and acid-base homeostasis through inhibition of this exchanger. TLR4 is expressed constitutively throughout the nephron, including segments of the proximal tubule, thick ascending limb, and collecting duct (10, 18, 20, 31, 44, 50, 84–87), and TLR4 has been shown to play a role in mediating inflammatory kidney injury in a variety of conditions, including sepsis, ischemia-reperfusion, diabetes, and nephrotoxic injury (12, 18, 44, 50, 67, 74, 84–87). Our studies raise the possibility that effects of LPS or endogenous ligands to activate TLR4 signaling and impair NHE3 function could contribute to renal transport defects observed in these pathological conditions.
In addition to our finding that LPS decreases NHE3 activity acutely in the MTAL through TLR4-ERK signaling, LPS may induce long-term NHE3 regulation. Intraperitoneal injection of LPS to induce endotoxemia decreased NHE3 protein expression after 6–12 h in whole kidneys of mice (75) and in inner stripe of outer medulla of kidneys from rats (57). The precise systemic factors responsible for downregulation of NHE3 in the LPS-treated animals and whether renal NHE3 expression is decreased in more clinically relevant models of sepsis (14) remain to be determined. However, we found that the capacity of the MTAL to absorb HCO3− is reduced in a mouse model of sepsis induced by cecal ligation and puncture (80), an effect that could be explained by decreased NHE3 expression. Thus, during Gram-negative sepsis, LPS may act through a combination of short-term and long-term mechanisms to reduce NHE3 activity in renal tubules: acute inhibition through the induction of TLR4 signaling pathways coupled directly to inhibition of NHE3, and chronic inhibition through the induction of undefined molecular signals that reduce NHE3 protein abundance.
In summary, the results of the present study demonstrate that basolateral LPS inhibits apical NHE3 in the MTAL through the activation of a TLR4/MyD88/MEK/ERK signaling pathway. The LPS-induced inhibition of NHE3 results in a decrease in transepithelial HCO3− absorption and is due to a decrease in Vmax of the exchanger. The ability of LPS to inhibit HCO3− absorption through ERK is maintained despite the presence of other, physiological stimuli that activate the ERK pathway in MTAL cells. These studies identify NHE3 as a direct downstream target of TLR4 signaling in the MTAL and suggest that bacterial molecules can impair renal absorptive functions important for extracellular fluid volume and acid-base regulation through the induction of TLR-mediated intracellular signals that modulate NHE3 activity. Our results identify ERK as a key signaling intermediate that links the TLR4/MyD88 receptor complex to downstream modulation of ion transport proteins and raise the possibility that molecular components of the TLR4-ERK signaling pathway may be therapeutic targets for treatment of sepsis-induced renal tubule dysfunction.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-038217.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B. A. Watts, III, E. R. Sherwood, and D. W. Good conception and design of research; B. A. Watts, III and T. George performed experiments; B. A. Watts, III, T. George, and D. W. Good analyzed data; B. A. Watts, III, E. R. Sherwood, and D. W. Good interpreted results of experiments; B. A. Watts, III and D. W. Good prepared figures; B. A. Watts, III and D. W. Good edited and revised manuscript; B. A. Watts, III and D. W. Good approved final version of manuscript; D. W. Good drafted manuscript.
Footnotes
This article is the topic of an Editorial Focus by Di Sole and Girardi (13a).
Treatment with basolateral LPS for up to 40 min failed to inhibit HCO3− absorption in MTALs from MyD88−/− mice. Whether longer-term exposure of the tubules to LPS may result in inhibition of HCO3− absorption due to delayed activation of ERK via an MyD88-independent pathway (39, 41) was not investigated.
REFERENCES
- 1. Alexander RT, Grinstein S. Tethering, recycling and activation of the epithelial sodium-proton exchanger, NHE-3. J Exp Biol 212: 1630–1637, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Alpern RJ. Renal acidification mechanisms. In: The Kidney, edited by Brenner BM, Rector FC., Jr Philadelphia, PA: Saunders, 2000, Vol. I, p. 455–519 [Google Scholar]
- 3. Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol Cell Biol 85: 420–424, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA 103: 3274–3279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgart K, Radermacher P, Calzia E, Hauser B. Pathophysiology of tissue acidosis in septic shock: Blocked microcirculation or impaired cellular respiration. Crit Care Med 36: 640–642, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Renal Physiol 273: F289–F299, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Bobulescu IA, Moe OW. Luminal Na+/H+ exchange in the proximal tubule. Pflügers Arch 458: 5–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cetin S, Dunkelbarger J, Li J, Boyle P, Ergun O, Qureshi F, Ford H, Upperman J, Watkins S, Hackam DJ. Endotoxin differentially modulates the basolateral and apical sodium/proton exchangers in enterocytes. Surgery 136: 375–383, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Chassin C, Goujon JM, Darche S, duMerle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, LeBouguenec C, Buzoni-Gatel D, Vandewalle A. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol 177: 4773–4784, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cohen J. The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol 172: 2629–2635, 2004 [DOI] [PubMed] [Google Scholar]
- 13. David MD, Cochrane CL, Duncan SK, Schrader JW. Pure lipopolysaccharide or synthetic lipid A induces activation of p21Ras in primary macrophages through a pathway dependent on Src family kinases and PI3K. J Immunol 175: 8236–8241, 2005 [DOI] [PubMed] [Google Scholar]
- 13a. Di Sole F, Girardi ACC. Uncovering the pathway of sepsis-induced renal tubular dysfunction. Focus on “Basolateral LPS inhibits NHE3 and HCO3− absorption through TLR4/MyD88-dependent ERK activation in medullary thick ascending limb.” Am J Physiol Cell Physiol (September 21, 2011). doi:10.1152/ajpcell.00350.2011 [DOI] [PubMed] [Google Scholar]
- 14. Doi K, Leelahavanichkul A, Yuen PST, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 20: 55–72, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103: 1071–1083, 2000 [DOI] [PubMed] [Google Scholar]
- 18. El-Achkar TM, Dagher PC. Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol 2: 568–581, 2006 [DOI] [PubMed] [Google Scholar]
- 19. El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gram-negative sepsis. Eur J Clin Invest 38: 39–44, 2008 [DOI] [PubMed] [Google Scholar]
- 20. El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes G, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290: F1034–F1043, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J 21: 4831–4840, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaidano G, Ghigo D, Schena M, Bergui L, Treves S, Turrini F, Cappio FC, Bosia A. Na+/H+ exchange activation mediates the lipopolysaccharide-induced proliferation of human B lymphocytes and is impaired in malignant B-chronic lymphocytic leukemia lymphocytes. J Immunol 142: 913–918, 1989 [PubMed] [Google Scholar]
- 23. George D, Salmeron A. Cot/Tpl-2 protein kinase as a target for the treatment of inflammatory disease. Curr Topics Med Chem 9: 611–622, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Gomez J, Garcia-Vazquez E, Banos R, Canteras M, Ruiz J, Banos V, Herrero JA, Valdes M. Predictors of mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia: the role of empiric antibiotic therapy. Eur J Clin Microbiol Infect Dis 26: 239–245, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Good DW. Inhibition of bicarbonate absorption by peptide hormones and cyclic adenosine monophosphate in rat medullary thick ascending limb. J Clin Invest 85: 1006–1013, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Good DW. The thick ascending limb as a site of renal bicarbonate reabsorption. Sem Nephrol 13: 225–235, 1993 [PubMed] [Google Scholar]
- 27. Good DW, Di Mari JF, Watts BA., III Hyposmolality stimulates Na+/H+ exchange and HCO3− absorption in thick ascending limb via PI3-kinase. Am J Physiol Cell Physiol 279: C1443–C1454, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Good DW, George T, Watts BA., III Basolateral membrane Na+/H+ exchange enhances HCO3− absorption in rat medullary thick ascending limb: Evidence for functional coupling between basolateral and apical membrane Na+/H+ exchangers. Proc Natl Acad Sci USA 92: 12525–12529, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Good DW, George T, Watts BA., III Nongenomic regulation by aldosterone of the epithelial NHE3 Na+/H+ exchanger. Am J Physiol Cell Physiol 290: C757–C763, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Good DW, George T, Watts BA., III Nerve growth factor inhibits Na+/H+ exchange and HCO3− absorption through parallel phosphatidylinositol 3-kinase-mTOR and ERK pathways in thick ascending limb. J Biol Chem 283: 26602–26611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Good DW, George T, Watts BA., III Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol 297: F866–F874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Good DW, George T, Watts BA., III Toll-like receptor 2 mediates inhibition of HCO3− absorption by bacterial lipoprotein in medullary thick ascending limb. Am J Physiol Renal Physiol 299: F536–F544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Good DW, Watts BA., III Functional roles of apical membrane Na+/H+ exchange in rat medullary thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 270: F691–F699, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Good DW, Watts BA, III, George T, Meyer J, Shull GE. Transepithelial HCO3− absorption is defective in renal thick ascending limbs from NHE1 Na+/H+ exchanger null mutant mice. Am J Physiol Renal Physiol 287: F1244–F1249, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Grinevich V, Knepper M, Verbalis J, Reyes I, Aguilera G. Acute endotoxemia in rats induces down-regulation of V2 vasopressin receptors and aquaporin-2 content in the kidney medulla. Kidney Int 65: 54–62, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal 13: 85–94, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Hall JP, Kurdi Y, Hsu S, Cuzzo J, Liu J, Telliez JP, Seidl KJ, Winkler A, Hu Y, Green N, Askew GR, Tam S, Clark JD, Lin LL. Pharmacologic inhibition of Tpl2 blocks inflammatory responses in primary human monocytes, Synoviocytes, and blood. J Biol Chem 282: 33295–33304, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 287: G370–G378, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Hoebe K, Du X, Georgl P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signaling. Nature 424: 743–748, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Kamachi F, Ban HS, Hirasawa N, Ohuchi K. Inhibition of lipopolysaccharide-induced prostaglandin E2 production and inflammation by the Na+/H+ exchanger inhibitors. J Pharmacol Exp Ther 321: 345–352, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Kawai T, Akira S. TLR signaling. Cell Death Diff 13: 816–825, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Klenzak J, Himmelfarb J. Sepsis and the kidney. Crit Care Clin 21: 211–222, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Kraut JA, Kurtz I. Use of base in the treatment of severe acidemic states. Am J Kidney Dis 38: 703–727, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Laghmani K, Borensztein P, Ambuhl P, Froissart M, Bichara M, Moe OW, Alpern RJ, Paillard M. Chronic metabolic acidosis enhances NHE-3 protein abundance and transport activity in the rat thick ascending limb by increasing NHE-3 mRNA. J Clin Invest 99: 1–7, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee SW, Hong YS, Park DW, Chio SH, Moon SW, Park JS, Kim JY, Baek KJ. Acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg Med J 25: 659–665, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 50. Lim SW, Li C, Ahn KO, Moon IS, Ahn C, Lee JR, Yang CW. Cyclosporine-induced renal injury induces Toll-like receptor and maturation of dendritic cells. Transplantation 80: 691–699, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Liu W, Ouyang X, Yang J, Liu J, Li Q, Gu Y, Fukata M, Lin T, He JC, Abreu M, Unkeless JC, Mayer L, Xiong H. AP-1 activated by Toll-like receptors regulates expression of IL-23 p19. J Biol Chem 284: 24006–24016, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Kintner DB, Chanana V, Algharabli J, Chen X, Gao Y, Chen J, Ferrazzano P, Olson JK, Sun D. Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis. J Neurosci 30: 15210–15220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-α autocrine signaling. J Immunol 178: 3731–3739, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31: 268–275, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Nemeth ZH, Deitch EA, Lu Q, Szabo C, Hasko G. NHE blockade inhibits chemokine production and NF-κB activation in immunostimulated endothelial cells. Am J Physiol Cell Physiol 283: C396–C403, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Nemeth ZH, Deitch EA, Szabo C, Mabley JG, Pacher P, Fekete Z, Hauser CJ, Hasko G. Na+/H+ exchanger blockade inhibits enterocyte inflammatory response and protects against colitis. Am J Physiol Gastrointest Liver Physiol 283: G122–G132, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Olesen ETB, de Seigneux S, Wang G, Lutken SC, Frokiaer J, Kwon TH, Nielsen S. Rapid and segmental specific dysregulation of AQP2, S256-pAQP2 and renal sodium transporters in rats with LPS-induced endotoxaemia. Nephrol Dial Transplant 24: 2338–2349, 2009 [DOI] [PubMed] [Google Scholar]
- 58. O'Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adapters in Toll-like receptor signaling. Nat Rev Immunol 7: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, Frei U, Eckardt KU, Loeffler M, John S. Acute renal failure in patients with severe sepsis and septic shock- a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant 23: 904–909, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch 447: 549–565, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Patole PS, Schubert S, Hildinger K, Khandoga S, Khandoga A, Segerer S, Henger A, Kretzler M, Werner M, Krombach F, Schlondorff D, Anders HJ. Toll-like receptor-4: Renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int 68: 2582–2587, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Preisig PA. The acid-activated signaling pathway: Starting with Pyk2 and ending with increased NHE3 activity. Kidney Int 72: 1324–1329, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Qi HY, Shelhamer JH. Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J Biol Chem 280: 38969–38975, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Rosoff PM, Cantley LC. Lipopolysaccharide and phorbol esters induce differentiation but have opposite effects on phosphatidylinositol turnover and Ca2+ mobilization in 70Z/3 pre-B lymphocytes. J Biol Chem 260: 9209–9215, 1985 [PubMed] [Google Scholar]
- 65. Rotte A, Pasham V, Eichenmuller M, Mahmud H, Xuan NT, Shumilina E, Gotz F, Lang F. Effect of bacterial lipopolysaccharide on Na+/H+ exchanger activity in dendritic cells. Cell Physiol Biochem 26: 553–562, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, Schein R, Summer W, Wright P, Walley KR. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med 28: 3405–3411, 2000 [DOI] [PubMed] [Google Scholar]
- 67. Sataranatarajan K, Feliers D, Mariappan MM, Lee MJ, Jimenez F, Chen Y, Gosh-Choudhury G, Ahuja S, Kasinath BS. Toll-like receptor 4 knockout mice are resistant to diabetic kidney injury (Abstract). J Am Soc Nephrol 19: 107A, 2008 [Google Scholar]
- 68. Schmidt C, Hocherl K, Bucher M. Regulation of renal glucose transporters during severe inflammation. Am J Physiol Renal Physiol 292: F804–F811, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorption defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 72. Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami KI, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of Toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Tsuganezawa T, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int 62: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 74. Wang JJ, Zhang SX, Mott R, Chen Y, Knapp RR, Cao W, Ma JX. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol 294: F1166–F1173, 2008 [DOI] [PubMed] [Google Scholar]
- 75. Wang W, Li C, Summer SN, Falk S, Wang W, Ljubanovic D, Schrier RJ. Role of AQP1 in endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1473–F1480, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Waterfield MR, Zhang M, Norman LP, Sun SC. NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol Cell 11: 685–694, 2003 [DOI] [PubMed] [Google Scholar]
- 77. Watts BA, III, George T, Good DW. Nerve growth factor inhibits HCO3− absorption in renal thick ascending limb through inhibition of basolateral membrane Na+/H+ exchange. J Biol Chem 274: 7841–7847, 1999 [DOI] [PubMed] [Google Scholar]
- 78. Watts BA, III, George T, Good DW. The basolateral NHE1 Na+/H+ exchanger regulates transepithelial HCO3− absorption through actin cytoskeleton remodeling in renal thick ascending limb. J Biol Chem 280: 11439–11447, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Watts BA, III, George T, Good DW. Aldosterone inhibits apical NHE3 and HCO3− absorption via a nongenomic, ERK-dependent pathway in medullary thick ascending limb. Am J Physiol Renal Physiol 291: F1005–F1013, 2006 [DOI] [PubMed] [Google Scholar]
- 80. Watts BA, III, George T, Sherwood ER, Good DW. Sepsis impairs HCO3− absorption in the medullary thick ascending limb through a two hit mechanism (Abstract). J Am Soc Nephrol 21: 253A, 2010 [Google Scholar]
- 81. Watts BA, III, Good DW. Apical membrane Na+/H+ exchange in rat medullary thick ascending limb: pHi-dependence and inhibition by hyperosmolality. J Biol Chem 269: 20250–20255, 1994 [PubMed] [Google Scholar]
- 82. Watts BA, III, Good DW. Hyposmolality stimulates apical membrane Na+/H+ exchange and HCO3− absorption in renal thick ascending limb. J Clin Invest 104: 1593–1602, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watts BA, III, Good DW. Extracellular signal-regulated kinase mediates inhibition of Na+/H+ exchange and HCO3− absorption by nerve growth factor in MTAL. Am J Physiol Renal Physiol 282: F1056–F1063, 2002 [DOI] [PubMed] [Google Scholar]
- 84. Wolfs TGAM, Buurman WA, van Schadewijk A, de Vries B, Daemen MARC, Hiemstra PS, van't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 85. Wu H, Chen G, Wybern KR, Yin J, Bertolio P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117, 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zager RA, Johnson ACM, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol 292: F304–F312, 2007 [DOI] [PubMed] [Google Scholar]
- 87. Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]