Abstract
TM4SF10 [transmembrane tetra(4)-span family 10] is a claudin-like cell junction protein that is transiently expressed during podocyte development where its expression is downregulated in differentiating podocytes coincident with the appearance of nephrin at the slit diaphragm. In a yeast two-hybrid screen, we identified adhesion and degranulation-promoting adaptor protein (ADAP), a well-known Fyn substrate and Fyn binding partner, as a TM4SF10 interacting protein in mouse kidney. Using coimmunoprecipitation and immunohistochemistry experiments in cultured human podocytes, we show that TM4SF10 colocalizes with Fyn and ADAP but does not form a stable complex with Fyn. Cytoskeletal changes and phosphorylation events mediated by Fyn activity were reversed by TM4SF10 overexpression, including a decrease in the activating tyrosine phosphorylation of Fyn (Y421), suggesting TM4SF10 may have a regulatory role in suppressing Fyn activity. In addition, TM4SF10 was reexpressed following podocyte injury by puromycin aminonucleoside treatment, and its expression enhanced the abundance of high-molecular-weight forms of nephrin indicating it may participate in a mechanism controlling nephrin's appearance at the plasma membrane. Therefore, these studies have identified ADAP as another Fyn adapter protein expressed in podocytes, and that TM4SF10, possibly through ADAP, may regulate Fyn activity. Since TM4SF10 expression is temporally regulated during kidney development, these studies may help define a mechanism by which the slit diaphragm matures as a highly specialized cell junction during podocyte differentiation.
Keywords: kidney, cell junctions, cytoskeleton, posttranslational modifications
the slit diaphragm is a unique cell junction between foot processes of podocytes responsible for the structure and function of glomerular filtration barrier (31). It is a major component of the protein barrier that separates the circulation from the urinary space, and disruption of this structure due to various inherited or acquired diseases is associated with proteinuria as part of nephrotic syndrome and progressive renal failure. This highly specialized cell junction contains proteins of both adherens and tight junctions such as P-cadherin, Zona occludens-1 (ZO-1), and junctional adhesion molecule A (JAM-A) (7, 39, 42) as well as specialized junctional proteins such as nephrin, Neph1, Podocin, CD2AP, and the protocadherin FAT1 (reviewed in Ref. 8). Developmentally, podocyte cell-cell junctions begin as typical epithelial lateral junctions at the comma and s-shaped body stage and then transit to a more basolateral position with the incorporation of the slit diaphragm-specific proteins at the capillary loop stage when foot processes begin to appear (38). The developmental process of assembling the slit diaphragm and its restructuring in diseases characterized by foot process effacement are not fully understood.
In animal models and in human diseases, the absence of nephrin leads to effacement of foot processes, indicating the inability to form a slit diaphragm that results in severe nephrotic syndrome with massive proteinuria (16, 35, 37). Nephrin mRNA is initially detected in the kidney at the late s-shaped body stage with the protein being detectable in the basolateral plasma membrane between the columnar epithelial cells (putative podocyte precursors) adjacent to the vascular cleft (12, 41). Nephrin is initially synthesized as a 155-kDa protein that undergoes posttranslational glycosylation events increasing its molecular mass to 175- or 185-kDa forms, which are required for its appearance in the plasma membrane (6, 27, 52). Structurally, nephrin is a transmembrane protein of the immunoglobulin superfamily with multiple tyrosine phosphorylation sites in its intracellular domain (29). Experimental deletion of the intracellular domain containing these phosphorylation sites results in changes in nephrin's subcellular location, concomitant with foot process effacement, and proteinuria. Therefore, it has been suggested that besides a structural role in the slit diaphragm, nephrin likely participates in signal transduction events that may mediate podocyte interactions with environmental events or injury responses to adverse stimuli (13).
The phosphorylation of nephrin is primarily through the Src family kinase Fyn, which binds nephrin directly via an SH2 domain and phosphorylates nephrin at multiple tyrosine residues (18, 21, 51). Fyn is a myristoylated protein that localizes to lipid rafts and is involved in a variety of signal transduction pathways through its association with intracellular signaling molecules (14, 44, 50). In Fyn knockout mice, foot process effacement with marked attenuation of nephrin phosphorylation was observed in detergent-resistant membrane fractions (51). Fyn activity also appears to mediate interaction of nephrin with the cytoskeleton, another factor that determines the structural integrity and proper function of the slit diaphragm (2, 49, 50, 53, 55). In a recent study, the phosphorylation of nephrin was shown to direct nephrin into raft-mediated endocytic recycling vesicles, thereby coupling nephrin phosphorylation with its removal from the plasma membrane (36).
TM4SF10 (also known as brain cell membrane protein-1, human gene name: TMEM47) is a 20-kDa transmembrane junctional protein in the PMP22/EMP/Claudin family of proteins. This cell junction protein is the vertebrate orthologue of VAB-9 in Caenorhabditis elegans, a cell membrane protein we have previously shown to be required for organization of contractile actin filaments with redundant functions in cell adhesion (45). Recently, we have established that TM4SF10 is expressed in podocytes but is temporally restricted to a window of expression in the early stages of glomerular development before the capillary loop stage (3). In subconfluent cultured podocytes TM4SF10 localizes to the perinuclear region but translocates to the cell membrane following cadherin appearance at nascent cell-cell contacts. As podocytes mature by extension of synaptopodin-rich lamellapodia, TM4SF10 expression is downregulated and is no longer present in cell contacts, suggesting a transient role for TM4SF10 in the formation of cell junctions.
To determine a mechanistic role for TM4SF10 in podocyte differentiation and slit diaphragm development, we screened for TM4SF10-binding proteins using a yeast two-hybrid system and identified the Fyn binding protein adhesion and degranulation-promoting adaptor protein (ADAP) also known as Fyb (Fyn binding protein) or SLAP-130, which we shall refer to as ADAP (11). Since Fyn has been shown to be the primary kinase that phosphorylates nephrin, we explored possible connections between TM4SF10 with ADAP and Fyn in association with nephrin. In cultured podocytes, TM4SF10 expression appeared to change Fyn and nephrin phosphorylation, suggesting that TM4SF10 may have a regulatory function on Fyn and nephrin activity in podocytes.
MATERIALS AND METHODS
Cell lines, plasmids, and cell transduction.
Human and mouse podocyte cell lines have been previously reported (17, 26) and were cultivated as described in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin, 0.25 U/ml amphothericin B, and 2 mM l-glutamine (all from Invitrogen, Carlsbad, CA) at 37°C. Madin Darby canine kidney (MDCK), U937, and RAW264.7 cells were obtained from American Type Culture Collection and propagated with recommended conditions. For studies that required phosphatase blockade, cells were treated with 50 μm pervanadate before cell lysis as previously described (51). For in vitro studies using puromycin aminonucleoside (PAN, Sigma Aldrich, St. Louis, MO), podocytes were treated with 25 μg/ml for 24 h.
The COOH-terminus of mouse TM4SF10 was tagged with green fluorescent protein (GFP) as previously reported (3). This TM4SF10-GFP expression cassette was also subcloned into a retroviral expression system (28) for infection and stable expression per standard methods (available from Addgene, addgene.com). RNA interference (RNAi) mediated gene silencing used a small hairpin RNA (shRNA) for mouse TM4SF10 (also with activity toward human TM4SF10) and a nontarget shRNA control (MISSION, TRCN 0000126230, and SHC002, respectively, Sigma-Aldrich), and both were contained within the pLKO retroviral expression system. Virus was produced using standard amphotrophic packaging with the vesicular stomatitis virus G envelope (available from Addgene). Podocyte cultures were infected at multiplicity of infection ratio of 3:1 at 37°C, with ∼85% transduction efficiency.
A full-length nephrin expression plasmid was kindly provided by Larry Holzman and has been previously described (12). ADAP and Fyn expression plasmids were created from Image clones (Invitrogen) using standard PCR methods. The resulting PCR fragments also were cloned into pEGFPN2 or pCMV-Myc (both from Clontech, Mountain View, CA) generating GFP-tagged or Myc-tagged fusion proteins. All plasmids were sequenced to verify absence of PCR and cloning artifacts. MDCK cell were transiently transfected with Lipofectamine 2000 by standard methods and podocytes were transfected with FuGene6 (Roche Applied Science, Indianapolis, IN) as previously described (24). Cells were harvested 48 h after transfection for immunoprecipitation or Western blotting.
Yeast two-hybrid screen.
The 22 amino acid cytoplasmic NH2-terminal domain of mouse TM4SF10 was cloned into the DNA binding domain yeast two hybrid clone (Matchmaker, Clontech). The DNA binding domain-TM4SF10 fusion protein was coexpressed in yeast with activation domain clones from 8- to 12-day-old mouse kidney cDNA library (Clontech). One million clones were screened and 20 candidates were identified that grew on selective media (-ADE, -HIS, -TRP, -LEU, +3AT) and were positive for β-galactosidase activity. These putative binding partners were identified by nucleotide sequencing and were validated for interaction in mammalian cells by coimmunoprecipitation.
Cell morphology.
MDCK cells were transfected with GFP, Fyn-GFP, and TM4SF10-GFP, and stable lines were established following dilution plating and clonal selection using rings. Coexpression with TM4SF10-GFP was achieved by infection with a TM4SF10-GFP expressing lentivirus as previously described (3). These stably expressing MDCK cell lines were plated at low density (105 cells in 6-well dishes), and after 24 h six random fields for each well were photographed using phase-contrast microscopy to visualize individual cells and were photographed again after 72 h to visualize colony formation. To determine total cell numbers and cells with extensions, the images from the 24-h time point were scored by manual counting, in which cells with extension were scored positive if at least one membrane protrusion exceeded the width of the cell body. Data are presented as an average percentage, and statistical significance was determined by t-test.
Immunoprecipitation and Western blotting.
Immunoprecipitation was performed as previously reported (47) on cells lysed in a buffer containing PBS with 0.1% SDS, 1% Nonidet P-40, 0.5% deoxycholate with protease (Complete, Roche Applied Science), and phosphatase inhibitors (Phosphatase Inhibitor Cocktail Set II, EMD Biosciences, Gibbstown, NJ). Proteins were resolved on 4–20% Tris-glycine gels under denaturing conditions, except 6% denaturing gels were used to resolve the high-molecular-weight nephrin proteins. Antibodies and dilutions used are as follows: Fyn polyclonal (H-80, sc-28791, Santa Cruz Biotechnology, Santa Cruz, CA), 1:500 dilution; activated Fyn (anti-Src pY418, Invitrogen), inactive Fyn (anti-Src pY527, Cell Signaling Technology, Danvers, MA), both 1:1,000 dilution; nephrin (N2028–50, US Biological, Marblehead, MA), 1:500 dilution; phospho-nephrin pY1217 (Epitomics, Burlingame, CA), 1:10,000 dilution; ADAP (Epitomics), 1:500; GFP (JL-8, Clontech), 1:1,000 dilution; and α-tubulin (clone B-5–1-2, Sigma-Aldrich), 1:5,000 dilution. Secondary antibodies for Western blotting were goat IgG TrueBlot horseradish peroxidase (HRP)-conjugated (eBioscience, San Diego, CA) at 1:2,000 dilution, goat anti-mouse HRP, 1:2,000 dilution; mouse anti-rabbit light chain specific, 1:10,000 dilution; and goat anti-mouse light chain specific, 1:10,000 dilution (all from Jackson ImmunoResearch, West Grove, PA). Detection was by chemilluminescence (ECL, Pierce, Rockford, IL) according to manufacturer's instructions except for the incubation time was reduced to 1 min. All studies were repeated three times with independently infected or transfected cell preparations.
Immunohistochemistry.
Immunocytochemistry was performed as previously described (24) with cells grown on glass coverslips using TRITC-labeled phalloidin (Sigma Aldrich), 1:1,000 dilution or the following primary antibodies: Fyn monoclonal (clone 1S, Upstate Biotechnology Millipore, Billerica, MA), 1:50 dilution; ADAP (Epitomics), 1:250; nephrin (US Biological), 1:100; and TM4SF10 (3) and secondary antibodies were goat anti-mouse or anti-rabbit fluorescent conjugates (Jackson ImmunoResearch) at 1:400. Coverslips were mounted in a glycerol-based mounting media with DAPI or TOPRO3 to visualize nuclei and were imaged with epifluorescence or confocal microscopy.
PAN nephrosis model.
Young adult rats (∼200 g) were divided into two groups (n = 4 each group) and treated with either a single intraperitoneal injection of PAN (150 mg/kg) or an equivalent volume of saline as a control using the standard method as previously described (46). Rats were killed either 2 or 10 days posttreatment, and kidney tissue was formalin-fixed and evaluated for the induction of TM4SF10 expression by immunohistochemistry. All animal studies were approved and conducted in accordance with the animal care and use committee of Case Western Reserve University.
RESULTS
Fyn-binding protein ADAP is a TM4SF10 interacting protein.
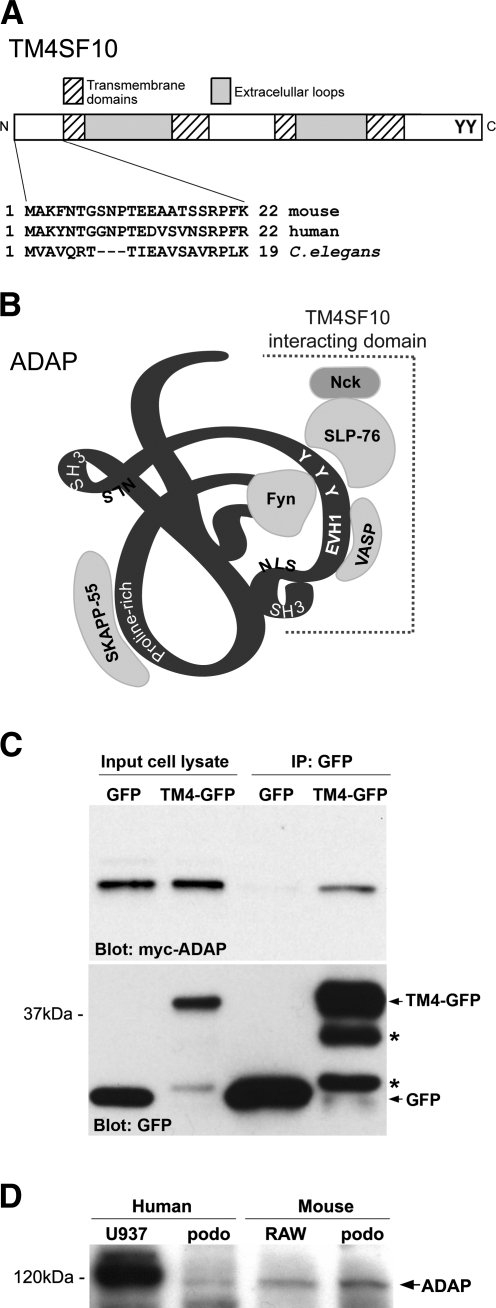
We identified ADAP as a TM4SF10 binding partner in an 8- to 12-day-old mouse kidney cDNA library using a yeast two hybrid approach. The 22 amino acid cytoplasmic NH2-terminal domain of mouse TM4SF10 was used as bait (Fig. 1A) and identified multiple, partial cDNA clones of ADAP. Based on these partial cDNAs, the TM4SF10 interaction domain with ADAP would be restricted to its COOH-terminal half (amino acids 378 to 819, Fig. 1B), which contains two SH3 domains, the Fyn binding, and phosphorylation sites and the binding sites for other interacting proteins such as VASP and SLP-76 (25, 48). As an initial confirmation of the yeast two-hybrid interaction, the ability for TM4SF10 and ADAP to interact in mammalian cells was tested by transiently expressing Myc-tagged ADAP in MDCK cells that were stably expressing either GFP or TM4SF10-GFP (Fig. 1C). Immunoprecipitating for GFP from the triton soluble fraction found that Myc-ADAP coprecipitated with TM4SF10-GFP but not GFP alone indicating that ADAP forms a stable complex with TM4SF10.
Fig. 1.
Adhesion and degranulation-promoting adaptor protein (ADAP) is a TM4SF10 [transmembrane tetra(4)-span family 10] interacting protein and is expressed in podocytes. A: schematic diagram of TM4SF10 protein domain structure noting the highly conserved 22 amino acid NH2-terminus (N) used as bait in the yeast two hybrid screen, the four trans-membrane domains typical of the tetraspanin proteins, and potential tyrosine phosphorylation sites on the COOH-terminus (C). B: schematic ribbon diagram of the 130-kDa ADAP protein (not drawn to scale) showing protein-protein interaction motifs (proline-rich, SH3, and EVH1), putative nuclear localization signal (NLS), and known tyrosine (Y) phosphorylation sites in addition to proteins that bind ADAP in lymphocytes (Fyn, VASP, SKAPP-55, and SLP-76 which also binds Nck). The region identified in the yeast two-hybrid screen and putative binding region for TM4SF10 is shown with the dashed line and span amino acids 378 to the COOH-terminus. C: Western blot of cell lysates from MDCK cells stably expressing GFP (lanes 1, 3) or TM4SF10-green fluorescent protein (GFP) (“TM4-GFP,” lanes 2, 4), and cotransfected with myc-ADAP. Cell lysates were immunoprecipitated with anti-GFP antibodies (lanes 3, 4) and blotted for myc (top) or GFP (bottom); *translationally truncated or proteolytically cleaved TM4SF10-GFP fusion proteins. D: Western blot of whole cell lysates from human and mouse podocyte (“podo”) cell lines confirming ADAP expression. Murine monocytic cell line RAW264.7 (RAW) and human monocytic cell line U937 were used as positive controls.
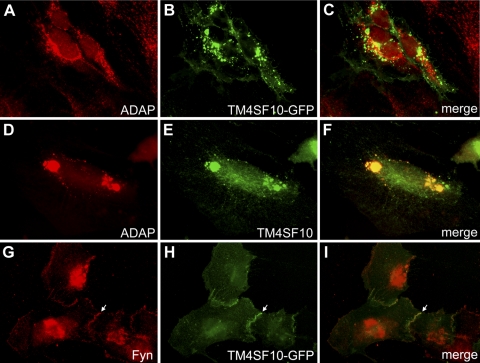
ADAP is a well-known Fyn binding protein in lymphocytes (32), but its expression outside the immune system has not been evaluated. Both human and mouse podocytes cell lines expressed ADAP by RT-PCR (verified by nucleotide sequencing of PCR products, not shown), Western blotting (Fig. 1D), and by immunohistochemistry (Fig. 2). With immunohistochemistry, ADAP expression partially colocalized with both GFP-tagged TM4SF10 (Fig. 2, A–C) and endogenous TM4SF10 (Fig. 2, D–F), predominately in cytoplasmic compartments and also the plasma membrane, consistent with previous reports on the subcellular distribution of the individual proteins (3, 4). As we have previously shown in podocytes (3), TM4SF10 concentrated at the plasma membrane in regions of cell-cell contacts (Fig. 2, G–I, arrow). Endogenous Fyn expression had a similar distribution at cell-cell contacts, and the plasma membrane pattern overlapped with TM4SF10 (Fig. 2I).
Fig. 2.
TM4SF10 colocalizes with ADAP and Fyn in podocytes. Detection by immunohistochemistry of TM4SF10-GFP (B,H) or endogenous TM4SF10 (E) in podocytes and colocalization with endogenous ADAP (A,D) or endogenous Fyn (G); respective merged images in C, F, and I. TM4SF10 was originally described as an endoplasmic reticulum (ER) and plasma membrane protein (4), and both endogenous and GFP-tagged TM4SF10 localized to intracellular compartments and the plasma membrane, with concentrations at cell-cell contacts (arrow).
Since ADAP is a Fyn-binding protein and Fyn substrate, interactions between TM4SF10 and Fyn were investigated further in podocytes. The ability of TM4SF10, ADAP, and Fyn to complex in podocytes was tested in coimmunoprecipitation experiments. Endogenous ADAP and TM4SF10-GFP coimmunoprecipitated, as well as the expected coimmunoprecipitation of ADAP and Fyn (Fig. 3A), however, a stable interaction between Fyn and TM4SF10 was not observed. With the use of podocytes cotransfected with TM4SF10-GFP and Fyn plasmids (or as a control, GFP and Fyn plasmids), Fyn-specific antibodies failed to detect Fyn in the samples of both TM4SF10-GFP and GFP expressing podocytes when immunoprecipitated with GFP antibodies (Fig. 3B). Similar findings were observed when Western blotting with GFP-specific antibodies failed to detect TM4SF10-GFP or GFP in the samples immunoprecipitated using Fyn antibodies. These results suggest that TM4SF10 and Fyn, although localized together at the plasma membrane, may not form a stable immunoprecipitable complex.
Fig. 3.
TM4SF10 coimmunoprecipitates with ADAP but not with Fyn in podocytes. A: ADAP interactions with Fyn and TM4SF10 were confirmed in podocytes stably expressing TM4SF10-GFP (“TM4-GFP”) by immunoprecipitating with Fyn, ADAP, or GFP antibodies and Western blotted for ADAP (“input”, whole cell lysates). B: Fyn interactions with TM4SF10 were tested in similar immunoprecipitation experiments, using podocytes stably expressing TM4SF10-GFP (TM4-GFP) or GFP (as a negative control), after transient transfection with Fyn plasmid. Cell lysates were immunoprecipitated with either: a control species and isotype matched IgG (IgG); an anti-Fyn antibody (Fyn); or an anti-GFP antibody (GFP), and Western blotted for either Fyn or GFP. Some of the IgG heavy chain of the immunoprecipitating antibody is observed in these blots due to cross reactivity with the Western blotting antibody.
Functional effects of TM4SF10 on Fyn activity and cell phenotype.
MDCK cells expressing Fyn-GFP exhibited a markedly different morphology from their typical cobblestone appearance (Fig. 4A). The majority of cells expressing Fyn-GFP extended protrusions (Fig. 4B), whereas MDCK cells expressing GFP or TM4SF10-GFP had no differences in cell morphology compared with untransfected controls. Coexpression of TM4SF10-GFP with Fyn-GFP, however, suppressed this morphological change, and the cells resumed more typical individual cell morphology and colony formation (Fig. 4, A and B), suggesting TM4SF10 inhibited this effect induced by Fyn. In addition, Fyn overexpression in MDCK cells caused total cellular increases in phosphotyrosine detectable by Western blotting, including the autophosphorylation of Fyn and phosphorylation of ADAP, a known substrate of Fyn. Similar to the cell morphology observations, coexpressing TM4SF10-GFP blocked Fyn activity resulting in an overall reduced level of tyrosine phosphorylation, including the phosphorylation of both ADAP and Fyn (Fig. 4C). Fyn, like the other Src family kinases, has nine potential tyrosine phosphorylation sites with two of them being well characterized regarding their function (Fig. 4D). Tyrosine (Y)532 mediates an intramolecular interaction with the SH2 domain, blocking its ability to bind phosphotyrosine-containing proteins, thus rendering the kinase inactive when phosphorylated. However, phosphorylation of Y421 in the kinase domain leads to stimulation of kinase activity (23). Western blotting with antibodies specific for these individual tyrosine phosphorylation sites found TM4SF10-GFP overexpression reduced the level of the activating Y421 phosphorylation, with no change in the Y532 phosphorylation (Fig. 4E). Together, these studies suggest that TM4SF10 expression levels was inversely related to an activation event for Fyn (Y421 phosphorylation) and total cellular phosphotyrosine levels, which were concurrent with changes in cell morphology.
Fig. 4.
TM4SF10 functionally alters cell shape and Fyn activity in MDCK cells. A: MDCK cells stably expressing GFP, TM4SF10-GFP, or Fyn-GFP, and coexpression of TM4SF10-GFP in Fyn-GFP expressing cells were examined by phase-contrast microscopy to monitor changes in colony formation behavior. B: MDCK cells expressing the same GFP fusion proteins as shown in A were plated at low density to visual morphology of individual cells. Representative cell images are shown and percentage of cells with extensions are graphed below (*P < 0.001 compared with all other samples, **P < 0.001 compared with Fyn-GFP). C: MDCK cells stably expressing Fyn-GFP were cotransfected with TM4SF10-GFP or ADAP-GFP, followed by immunoblotting with an anti-phospho-tyrosine antibody (P-Tyr) to show total cellular tyrosine kinase activity. D: schematic diagram of Fyn kinase and two major phosphorylation sites: the activating phospho-tyrosine421 (pY421) of the kinase domain and the inactivating phospho-tyrosine532 (pY532) that induces conformation changes that block the SH2 domain. E: effect of TM4SF10-GFP on Fyn phosphorylation using Western blotting for total Fyn and phospho-specific antibodies. The additional band on the Src pY421 blot (which is not detected by the Fyn-specific antibody) is likely another Src family kinase since these antibodies will detect the same phospho-epitope on other Src kinases; however, the degree of phosphorylation of this other band does not appear to change with TM4SF10-GFP expression.
In podocytes, TM4SF10-GFP overexpression also had an effect on cytoskeletal structure, inducing predominately peripheral and circumferential F-actin bundling as opposed to the predominant stress fiber cross striations in the absence of TM4SF10-GFP (Fig. 5, A–C).
Fig. 5.
TM4SF10 colocalizes with nephrin and is associated with changes in nephrin abundance and phosphorylation in podocytes. A–C: immunohistochemistry for F-actin (Phalloidin) comparing a podocyte with and without TM4SF10-GFP expression. Cells expressing TM4SF10-GFP cells had more circumferential bands (B, arrows) of F-actin, whereas cells without TM4SF10-GFP had a more typical stress fiber appearance of F-actin cross striations. D–G: colocalization of TM4SF10-GFP and nephrin in podocytes. E–G: higher magnification of boxed region in D showing partial colocalization of nephrin and TM4SF10-GFP in fine, distal cell extension. H: Western blot of podocyte nephrin expression following overexpression or knockdown of TM4SF10-GFP using a TM4SF10-specific short hairpin RNA (shRNA). The high-molecular-weight forms of nephrin at 175 and 185 kDa are noted with arrows. Efficiency of shRNA knockdown of TM4SF10 was confirmed by Western blot with anti-GFP antibodies and Tubulin was used as a loading control. I: Western blot of total nephrin abundance and presence of nephrin tyrosine1217 phosphorylation (pY1217) with and without TM4SF10-GFP overexpression and with and without preincubation with pervanadate to block tyrosine phosphatase activity.
TM4SF10 and nephrin interactions.
Using immunohistochemistry, nephrin colocalized with TM4SF10-GFP in intracellular compartments and also in distal cell extensions in podocytes (Fig. 5, D–G), suggesting there may be spatiotemporal events where TM4SF10 and nephrin may interact. A functional effect of TM4SF10 on nephrin was examined using TM4SF10-GFP overexpression or inhibition by RNAi-mediated gene silencing. Normal podocytes and podocytes stably expressing TM4SF10-GFP with and without coexpression of a TM4SF10-specific shRNA, were transiently transfected with a full-length nephrin plasmid and examined by Western blotting (Fig. 5H). The presence of both the 175- and 185-kDa forms of nephrin were observed in greater abundance in cells expressing TM4SF10-GFP; however, when TM4SF10 expression was reduced by RNAi, the abundance of both 175- and 185-kDa forms was reduced. To examine nephrin phosphorylation, several prior studies have shown the phosphorylated forms of nephrin can only be observed by Western blotting using a pervanadate pretreatment to block the action of phosphatases (18, 21, 50, 51). Podocytes with and without TM4SF10-GFP overexpression were pretreated with pervanadate and blotted for total nephrin and nephrin phosphorylated at tyrosine1217 (Fig. 5I). Similar to Fig. 5H, overexpression of TM4SF10-GFP in the absence of pervanadate was associated with a greater abundance of the high molecular weight forms of nephrin (Fig. 5I, compare lanes 1 and 3). Pervanadate pretreatment in the absence of TM4SF10-GFP (Fig. 5I, lane 2) resulted in almost undetectable total nephrin but a strong signal for phosphorylated nephrin, suggesting the small amount of total nephrin in lane 2 represented only phosphorylated nephrin. Pervanadate pretreatment in the presence of TM4SF10-GFP (Fig. 5I, lane 4) was associated with a greater abundance of total nephrin compared with treated cells in the absence of TM4SF10-GFP (Fig. 5I, lane 2); however, there was no difference in the level of phosphorylated nephrin. This shows that a greater proportion of nephrin was not phosphorylated in the presence of TM4SF10-GFP, suggesting that TM4SF10-GFP overexpression inhibited nephrin phosphorylation. This is consistent with the decreased Fyn-mediated tyrosine phosphorylation by TM4SF10-GFP observed in MDCK cells (Fig. 4C) and also reenforces the importance of phosphorylation event in regulating the abundance or stability of high-molecular-weight forms of nephrin as previously reported (18, 21, 50, 51).
TM4SF10 reexpression during podocyte injury.
Our previous observation that TM4SF10 is expressed only during podocyte development (3) would suggest that TM4SF10 might be reexpressed during podocyte injury repair process where reestablishment of cell junctions is required. The expression of TM4SF10 was examined in differentiated mouse podocytes using an in vitro model of podocyte injury using PAN (40), an agent that causes foot process effacement in vivo. Compared with untreated cells, PAN treatment resulted in new expression of TM4SF10 with concentrations at cell-cell contacts that colocalized with cadherin (Fig. 6A). A similar induction of TM4SF10 was observed in vivo using the standard PAN nephrosis model in rats, a model that replicates foot process effacement typical of minimal change disease (46). With PAN treatment, we observed a peak in proteinuria 7–10 days postinjection as is typical for the model (not shown); however, TM4SF10 expression at an earlier time (2 days postinjection) was already elevated compared with untreated (Fig. 6B). By day 10 at the peak of proteinuria, there was strong TM4SF10 expression in a typical podocyte distribution pattern. This study indicated that TM4SF10 induction began at a time before the detection of massive proteinuria, and its expression continued to increase paralleling the peak of glomerular damage and proteinuria. These studies indicate that TM4SF10 expression can reoccur during an injury repair process and may have a role in the dynamics of foot process retraction and/or formation.
Fig. 6.
Puromycin aminonucleoside (PAN) treatment induces reexpression of TM4SF10. A: cultured podocytes were treated with PAN or phosphate-buffered saline as a control and were immunostained for native expression of TM4SF10 (green) and cadherin (red), along with the nuclear stain DAPI (blue). Note the simplification of interdigitations of cell-cell contacts (cadherin-positive cell junctions) with PAN treatment compared with the control treated cells. B: immunohistochemistry for TM4SF10 (green) in kidney sections from rats treated with PAN or saline as a control using the standard PAN nephrosis model of podocyte injury. In this model, postinjection day 2 is a time point before the onset of severe proteinuria, whereas day 10 is a time point where rats exhibit nephrotic range proteinuria. Panels are matched exposures; ×40 magnification.
DISCUSSION
This is the first report of ADAP expression in podocytes. ADAP is now the second protein, along with CD2AP (43), which were originally described in T cells with functional roles in formation of the T cell receptor immunological synapse. In T cells, ADAP is a critical scaffolding center for many phosphotyrosine-mediated interactions in T cell receptor signaling and is known to interact and cooperate with Nck to regulate actin reorganization (20, 30, 48). It is required for T cell receptor-induced integrin activity and is itself phosphorylated and activated by Fyn upon T-cell activation (10, 11). In ADAP null (fyb−/−) mice, inside-out signaling via integrins is disrupted during interactions with antigen presenting cells, indicating that ADAP is required in the immunological synapse for signaling pathways through integrins (33). Although a renal phenotype was not described in the original publication of fyb−/− mice (33), our preliminary observations found adult fyb−/− mice have abnormal glomerular histology (L. A. Bruggeman and J. S. Simske, unpublished observations). Further evaluation of these null mice will help determine possible functional effects of ADAP in podocyte signaling events mediated by Fyn and TM4SF10. In addition, ADAP immunoprecipitated both Fyn and TM4SF10 in podocytes, however, we did not observe TM4SF10 and Fyn were both present in an immunoprecipitable complex. One explanation is that ADAP cannot simultaneously bind Fyn and TM4SF10 so that TM4SF10-ADAP and Fyn-ADAP complexes are mutually exclusive. Alternatively, the complex containing ADAP, Fyn, and TM4SF10 may be transient. Therefore, future studies evaluating ADAP as a potential regulatory hub in podocytes will likely be important in establishing the mechanism of TM4SF10 in modifying Fyn activity.
Several lines of experimentation in other model organisms indicate ADAP is associated with regions of dynamic actin activity (5, 54). Our observations in MDCK cells and podocytes also indicated TM4SF10 expression altered Fyn-dependent F-actin distribution and blocked cell protrusions. This corroborates data from a published morphological screen that identified TM4SF10 as a protein that, when overexpressed, potently blocks neurite outgrowth in nerve growth factor-stimulated PC12 cells (19). Part of the well-known transformation effects of Src-family proteins is the induction of lamellipodia/filopodia through the reorganization of actin filaments (1, 34). Our observations that TM4SF10 overexpression prevented cell extensions induced by Fyn, and the prior studies in PC12 cells mentioned above may suggest a general role for TM4SF10 in suppressing lamellipodia/filopodia formation mediated by src kinases. With the known functions of ADAP in both integrin-based cell adhesion and actin dynamics, further study of Fyn-ADAP-TM4SF10 interactions in podocytes may help identify a unifying mechanism in integrin function and actin dynamics during both foot process formation in development and effacement in disease.
We have previously shown a restricted window of TM4SF10 expression early during glomerular development (3). It is not present in adult glomeruli, however, it was reexpressed during podocyte injury repair processes induced by PAN treatment. This temporal pattern is similar to changes in nephrin phosphorylation observed by Verma et al. (50) during development and similar injury repair processes. Our observations that TM4SF10 reduces the active form of Fyn and enhances the appearance of the high-molecular-weight forms of nephrin suggests that TM4SF10 function may have a role in regulating Fyn activity with an effect on the nephrin protein.
Maturation of nephrin by various posttranslational modifications appear to be critical for its function including transport of nephrin to the plasma membrane, stable nephrin-NEPH1 interactions, and that the fully glycosylated 185-kDa plasma membrane form may be the only form susceptible to Fyn phosphorylation (9, 21, 44, 52). Upon appearance at the plasma membrane, nephrin ectodomain engagement and subsequent Fyn phosphorylation of its cytoplasmic domain results in recruitment of Nck, phosphoinositide 3-kinase, and their associated proteins and may initiate directed actin polymerization and elongation at this site (15, 22, 49, 50, 55). Although it is not fully understood how the nephrin molecule is regulated by these posttranslational modifications, the status of nephrin phosphorylation appears to effect its subcellular localization, stability, and signaling to actin. Our combined observations that TM4SF10 decreased Fyn activity and preserved the high molecular weight but hypophosphorylated forms of nephrin is similar to the observation by Lahdenpera et al. (18) where nephrin mutants lacking the cytoplasmic domain containing the Fyn phosphorylation sites were associated with higher nephrin protein abundance. It had been speculated that this may reflect a possible instability of the nephrin protein when hyperphosphorylated (21). In support of this concept, recent studies have implicated hyperphosphorylation of nephrin with its removal from the plasma membrane by raft-mediated endocytosis (36). Although not fully understood, these studies are beginning to reveal the mechanism by which nephrin phosphorylation ultimately controls both the formation and remodeling of the slit diaphragm and foot processes during development and in disease.
In conclusion, the mechanism of slit diaphragm assembly is a highly orchestrated series of developmentally coordinated sequential events, which may be a much more dynamic process in podocyte response to injury and disease than previously thought. In C. elegans, the TM4SF10 orthologue VAB-9 regulates actin organization at the adherens junction complex, resulting in changes in cell morphology. We speculate that TM4SF10 may have a similar role during the formation of the precursor cadherin-based cell junction early in podocyte development and also may modulate signaling by nephrin to the actin cytoskeleton or integrins during slit diaphragm and foot process formation. Our basic model is that TM4SF10 expression correlates with a phenotype in which extension of cellular processes is not supported; in podocytes this may reflect a dedifferentiated state during glomerular development or with foot process effacement in disease. In addition, the novel identification of ADAP emphasizes the growing molecular similarity of podocyte signaling mechanisms to synaptic structures such as the immunological synapse or the neurological synapse, and theories modeling the slit diaphragm with properties of both cell junctions and synapses may be a productive direction for future investigations.
GRANTS
This work was supported in part by National Institutes of Health Grant DK061395 and MetroHealth Medical Center institutional funds. A. Padiyar was supported by NIH Training Grant DK007470.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A.A., L.A.B., and J.S.S. conception and design of research; T.A.A., Z.W., A.P., L.A.B., and J.S.S. performed experiments; T.A.A., L.A.B., and J.S.S. analyzed data; T.A.A., L.A.B., and J.S.S. interpreted results of experiments; T.A.A. and L.A.B. prepared figures; T.A.A. drafted manuscript; T.A.A., Z.W., A.P., L.A.B., and J.S.S. approved final version of manuscript; L.A.B. and J.S.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. John Sedor for critical review of the manuscript.
Present address of A. Padiyar: Dept. of Medicine, University Hospitals Case Medical Center, Cleveland, OH 44106.
REFERENCES
- 1. Behrens J, Vakaet L, Friis R, Winterhager E, Van RF, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, Quaggin SE, Li SS, Gruenheid S, Jones N, Pawson T. Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol Cell Biol 28: 2035–2046, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruggeman LA, Martinka S, Simske JS. Expression of TM4SF10, a Claudin/EMP/PMP22 family cell junction protein, during mouse kidney development and podocyte differentiation. Dev Dyn 236: 596–605, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Christophe-Hobertus C, Szpirer C, Guyon R, Christophe D. Identification of the gene encoding Brain Cell Membrane Protein 1 (BCMP1), a putative four-transmembrane protein distantly related to the peripheral myelin protein 22/epithelial membrane proteins and the claudins. BMC Genomics 2: 3, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci 114: 4307–4318, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Fujii Y, Khoshnoodi J, Takenaka H, Hosoyamada M, Nakajo A, Bessho F, Kudo A, Takahashi S, Arimura Y, Yamada A, Nagasawa T, Ruotsalainen V, Tryggvason K, Lee AS, Yan K. The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int 69: 1350–1359, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG. Slit diaphragms contain tight junction proteins. J Am Soc Nephrol 20:1491–1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg P, Verma R, Holzman LB. Slit diaphragm junctional complex and regulation of the cytoskeleton. Nephron Exp Nephrol 106: e67–e72, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 14: 918–926, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Griffiths EK, Krawczyk C, Kong YY, Raab M, Hyduk SJ, Bouchard D, Chan VS, Kozieradzki I, Oliveira-Dos-Santos AJ, Wakeham A, Ohashi PS, Cybulsky MI, Rudd CE, Penninger JM. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293: 2260–2263, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Griffiths EK, Penninger JM. Communication between the TCR and integrins: role of the molecular adapter ADAP/Fyb/Slap. Curr Opin Immunol 14: 317–322, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Holzman LB, St, John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56: 1481–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, Saleem MA, Walz G, Benzing T. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet 12: 3397–3405, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, Konieczkowski M, Mukherjee A, Schechtman S, Khan S, Schelling JR, Ross MD, Bruggeman LA, Sedor JR. Podocyte Injury Induces Nuclear Translocation of WTIP via Microtubule-dependent Transport. J Biol Chem 285: 9995–10004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 64: 404–413, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Laketa V, Simpson JC, Bechtel S, Wiemann S, Pepperkok R. High-content microscopy identifies new neurite outgrowth regulators. Mol Biol Cell 18: 242–252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lettau M, Pieper J, Gerneth A, Lengl-Janssen B, Voss M, Linkermann A, Schmidt H, Gelhaus C, Leippe M, Kabelitz D, Janssen O. The adapter protein Nck: role of individual SH3 and SH2 binding modules for protein interactions in T lymphocytes. Protein Sci 19: 658–669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Li H, Zhu J, Aoudjit L, Latreille M, Kawachi H, Larose L, Takano T. Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem Biophys Res Commun 349: 310–316, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell 102: 635–646, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Martinka S, Bruggeman LA. Persistent NF-κB activation in renal epithelial cells in mouse modelof HIV-associated nephropathy. Am J Physiol Renal Physiol 290: F657–F665, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menasche G, Kliche S, Bezman N, Schraven B. Regulation of T-cell antigen receptor-mediated inside-out signaling by cytosolic adapter proteins and Rap1 effector molecules. Immunol Rev 218: 82–91, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Mundel P, Reiser J, Zuniga Mejia BA, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Nakajo A, Khoshnoodi J, Takenaka H, Hagiwara E, Watanabe T, Kawakami H, Kurayama R, Sekine Y, Bessho F, Takahashi S, Swiatecka-Urban A, Tryggvason K, Yan K. Mizoribine corrects defective nephrin biogenesis by restoring intracellular energy balance. J Am Soc Nephrol 18: 2554–2564, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272: 263–267, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Patrakka J, Tryggvason K. Nephrin–a unique structural and signaling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Pauker MH, Reicher B, Fried S, Perl O, Barda-Saad M. Functional cooperativity between the proteins Nck and ADAP is fundamental for actin reorganization. Mol Cell Biol 13: 2653–2666, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Peterson EJ. The TCR ADAPts to integrin-mediated cell adhesion. Immunol Rev 192: 113–121, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu QH, Pribila JT, Freedman BD, Shimizu Y, Koretzky GA. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293: 2263–2265, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Platek A, Mettlen M, Camby I, Kiss R, Amyere M, Courtoy PJ. v-Src accelerates spontaneous motility via phosphoinositide 3-kinase, phospholipase C and phospholipase D, but abrogates chemotaxis in Rat-1 and MDCK cells. J Cell Sci 117: 4849–4861, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rantanen M, Palmen T, Patari A, Ahola H, Lehtonen S, Astrom E, Floss T, Vauti F, Wurst W, Ruiz P, Kerjaschki D, Holthofer H. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol 13: 1586–1594, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978 [PubMed] [Google Scholar]
- 39. Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, Schelling JR, Sedor JR. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol 289: F431–F441, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestila M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H. Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 157: 1905–1916, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111: 1255–1263, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthofer H. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol 159: 1069–1077, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simske JS, Koppen M, Sims P, Hodgkin J, Yonkof A, Hardin J. The cell junction protein VAB-9 regulates adhesion and epidermal morphology in C. elegans. Nat Cell Biol 5: 619–625, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Smoyer WE, Mundel P, Gupta A, Welsh MJ. Podocyte alpha-actinin induction precedes foot process effacement in experimental nephrotic syndrome. Am J Physiol Renal Physiol 273: F150–F157, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Srichai MB, Konieczkowski M, Padiyar A, Konieczkowski DJ, Mukherjee A, Hayden PS, Kamat S, El-Meanawy MA, Khan S, Mundel P, Lee SB, Bruggeman LA, Schelling JR, Sedor JR. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem 279: 14398–14408, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Sylvester M, Kliche S, Lange S, Geithner S, Klemm C, Schlosser A, Grossmann A, Stelzl U, Schraven B, Krause E, Freund C. Adhesion and degranulation promoting adapter protein (ADAP) is a central hub for phosphotyrosine-mediated interactions in T cells. PLos One 5: e11708, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tryggvason K, Pikkarainen T, Patrakka J. Nck links nephrin to actin in kidney podocytes. Cell 125: 221–224, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K. N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol 13: 1385–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Yuan H, Takeuchi E, Salant DJ. Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton. Am J Physiol Renal Physiol 282: F585–F591, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Yuan M, Mogemark L, Fallman M. Fyn binding protein, Fyb, interacts with mammalian actin binding protein, mAbp1. FEBS Lett 579: 2339–2347, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]