Abstract
Angiotensin II (ANG II) has been implicated in the pathogenesis of diabetic micro- and macrovascular disease. In vascular smooth muscle cells (VSMCs), ANG II phosphorylates and degrades insulin receptor substrate-1 (IRS-1). While the pathway responsible for IRS-1 degradation in this system is unknown, c-Jun NH2-terminal kinase (JNK) has been linked with serine phosphorylation of IRS-1 and insulin resistance. We investigated the role of JNK in ANG II-induced IRS-1 phosphorylation, degradation, Akt activation, glucose uptake, and hypertrophic signaling, focusing on three IRS-1 phosphorylation sites: Ser302, Ser307, and Ser632. Maximal IRS-1 phosphorylation on Ser632 occurred at 5 min, on Ser307 at 30 min, and on Ser302 at 60 min. The JNK inhibitor SP600125 reduced ANG II-induced IRS-1 Ser307 phosphorylation (by 80%), IRS-1 Ser302 phosphorylation (by 70%), and IRS-1 Ser632 phosphorylation (by 50%). However, JNK inhibition had no effect on ANG II-mediated IRS-1 degradation, nor did it reverse the ANG II-induced decrease in Akt phosphorylation or glucose uptake. Transfection of VSMCs with mutants S307A, S302A, or S632A of IRS-1 did not block ANG II-mediated IRS-1 degradation. In contrast, JNK inhibition attenuated insulin-induced upregulation of collagen and smooth muscle α-actin in ANG II-pretreated cells. We conclude that phosphorylation of Ser307, Ser302, and Ser632 of IRS-1 is not involved in ANG II-mediated IRS-1 degradation, and that JNK alone does not mediate ANG II-stimulated IRS-1 degradation, but rather is responsible for the hypertrophic effects of insulin on smooth muscle.
Keywords: insulin receptor substrate degradation, serine phosphorylation in insulin signaling, insulin receptor substrate-1, c-Jun NH2-terminal kinase
diabetes is at the front line of systemic diseases. Recent estimates on this epidemic project that by the year 2030, there will be 30 million diabetics in the United States alone, and 366 million worldwide (57). Vascular disease is the ultimate cause of morbidity and mortality in 80% of diabetics. Insulin resistance in the vasculature impairs vasodilation (30, 43), enhances inflammation (39), promotes medial hypertrophy (46), and alters matrix metalloproteinase activity (12, 51), which all culminate to form atherosclerotic lesions. Angiotensin II (ANG II) has been implicated in insulin resistance; indeed, several randomized, clinical trials have shown that micro- and macrovascular complications associated with diabetes improve with angiotensin-converting enzyme inhibitors (ACE-I) and ANG II type 1 receptor blockers (ARBs) (7, 52, 58).
Cross talk between the renin angiotensin system (RAS) and insulin signaling is implicated in insulin resistance (55). We have previously shown that pretreatment of vascular smooth muscle cells (VSMCs) with ANG II over 12 h causes a downregulation of insulin receptor substrate (IRS-1) in a redox-dependent manner (47). ANG II-induced IRS-1 degradation results in a decrease in insulin-induced phosphorylation of Akt and glucose uptake (15), suggesting that ANG II treatment in VSMCs impairs insulin signaling. Our previous work showed that phosphorylation of IRS-1 on Ser307 and IRS-1 degradation is significantly inhibited by tyrosine phosphorylation-deficient mutants of 3-phosphoinositide-dependent kinase-1 (PDK1), a phosphoinositol-3-kinase (PI3K)-dependent serine/threonine kinase that activates Akt and glucose transporter (GLUT-4) translocation to the cell membrane. The identity of the kinase that directly phosphorylates IRS-1 on serine, however, has not been determined, nor has the relationship between phosphorylation of IRS-1 on specific serines and subsequent IRS-1 degradation and downstream signaling in VSMCs.
Among kinases known to phosphorylate IRS-1, we chose to focus on c-Jun NH2-terminal kinase (JNK) as a potential serine/threonine kinase involved in ANG II-mediated insulin resistance. JNK, a member of mitogen-activated protein kinases, is activated by several stimuli (5), including ANG II, and has been implicated in insulin resistance (42). JNK associates with IRS-1 and phosphorylates it in Chinese hamster ovary (CHO) cells (1). Furthermore, the carboxyl terminus of IRS-1 contains JNK-binding sites. Inhibition of JNK reverses the inhibitory effects of ANG II on insulin-induced nitric oxide production in human umbilical vein endothelial cells (4). In the present study, we tested the role of JNK in ANG II-mediated IRS-1 degradation and downstream signaling in VSMCs, specifically focusing on Ser302, Ser307, and Ser632 phosphorylation of IRS-1, because all have been implicated in insulin resistance (1, 6, 32, 54). We report that ANG II induces phosphorylation of these three sites on IRS-1; furthermore, ANG II-mediated phosphorylation of Ser632, Ser302, and Ser307 is impaired by JNK inhibition. We also report that mutants of these three serine phosphorylation sites on IRS-1 do not inhibit ANG II-mediated IRS-1 degradation, but rather, JNK inhibition blocks insulin-induced upregulation of collagen and α-smooth muscle actin (α-SMA) in ANG II-pretreated cells, suggesting that insulin resistance may be cell or tissue specific and that JNK is involved in the pathogenic actions of insulin.
MATERIALS AND METHODS
Materials.
Anti-IRS-1 monoclonal antibody was from BD Biosciences (Franklin Lakes, NJ), and polyclonal antibody was from Upstate Biotechnology (Billerica, MA). Phospho-JNK, JNK, phosphospecific antibodies against IRS-1 Ser632, Ser302, Ser307, phospho-Akt (Ser473), total Akt, and hemagglutinin (HA)-tag antibodies were from Cell Signaling Technology (Beverly, MA). SP600125 was from Invitrogen (Carlsbad, CA). All other chemicals and reagents, including DMEM (with 25 mM HEPES and 4.5 g glucose), were from Sigma (St. Louis, MO).
Cell culture.
VSMCs from rat thoracic aorta were isolated by enzymatic digestion and grown in DMEM with 10% calf serum as described previously (19). All experimental procedures were approved by the Emory University Institutional Animal Care and Use Committee in accordance with the guidelines established by the University. Cells from passages 2-12 grown at 70–80% confluence were made quiescent in DMEM with 0.1% calf serum for 24–48 h.
Immunoprecipitation and Western analysis.
To test the effect of JNK inhibition on ANG II-mediated serine phosphorylation of IRS-1, cells were pretreated with 30 nM of the specific JNK inhibitor, SP600125 (an anthrapyrazole inhibitor), for 30 min, followed by incubation with 100 nM ANG II for 5 min (Ser632), 30 min (Ser307), or 60 min (Ser302). Cells were harvested in lysis buffer, using standard techniques as described previously (48). Total IRS-1 was immunoprecipitated using A/G agarose beads, followed by Western blotting with IRS-1 phosphospecific antibodies. After incubation with horseradish peroxidase-conjugated secondary antibodies, proteins were detected by enhanced chemiluminescence. Band intensity was quantified by film densitometry using ImageJ software (National Institutes of Health, Bethesda, MD).
Creation of IRS-1 mutants and transfection.
A pcDNA3 plasmid construct encoding wild-type rat IRS-1 cDNA with a COOH-terminal HA tag was generously provided by Dr. Yasushi Kaburagi (27). Site-directed mutagenesis was performed using the QuikChange II XL kit from Stratagene (Santa Clara, CA) to replace selected serine residues with alanine. A single serine residue was targeted to create mutants S307A and S302A. Ser307 corresponds to Ser312 in humans, and Ser 302 corresponds to Ser307 in humans. To create mutant S632/635A, alanine residues were substituted for both serines 632 and 635. These residues correspond to serines 636 and 639 in the human sequence. Clones of mutants were screened by PCR followed by restriction fragment length polymorphism analysis and confirmed by sequencing. VSMCs (2 × 106 cells) were transiently transfected with 5 μg wild-type or mutant IRS-1 plasmid using Lipofectamine 2000 (Invitrogen) or electroporation (Amaxa, program P-024) and reagents for primary smooth muscle cells (VPI-1004).
Measurement of glucose uptake.
VSMCs made quiescent in serum-free media for 24 h were stimulated with or without ANG II (100 nM) for 18 h in the presence or absence of the JNK inhibitor SP600125 (30 nM). Subsequently, cells were incubated in Krebs-Ringer-HEPES buffer (15 mM HEPES, pH 7.4, 105 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM KH2PO4, 1.4 mM MgSO4, and 10 mM NaHCO3) for 2 h. Then, cells were treated with bovine insulin (100 nM) for 30 min, and 0.2 mM 2-deoxy-d-glucose (with 1 μCi/ml 2-deoxy-d-[3H]glucose) was added for 30 additional minutes. After incubation with glucose, cells were washed three times with ice-cold PBS. Cells were lysed with 0.4 M NaOH and neutralized with HCl, and the amount of radiolabeled glucose uptake was determined by scintillation counting.
Real-time reverse transcription-polymerase chain reaction and α-SMA expression.
mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and type I collagen was analyzed by real-time PCR, as described previously (11). Real-time PCR was performed using collagen type I [forward (F): 5′-TCACCTACAGCACGCTTG-3′ and reverse (R): 5′-GGTCTGTTTCCAGGGTTG-3′], and GAPDH primers (F: 5′-TGAACGGGAAGCTCACTGG-3′ and R: 5′-TCCACCACCCTGTTGCTGTA-3′). All data were normalized against GAPDH mRNA expression. Expression of α-SMA was determined using immunofluorescence, as previously reported (11).
Statistical analysis.
All statistical analysis was performed using GraphPad Prism 4. Results are expressed as means ± SE. One-way ANOVA followed by Bonferroni's test was used to determine statistical significance. P < 0.05 was considered significant.
RESULTS
ANG II induces phosphorylation of c-Jun NH2-terminal kinase.
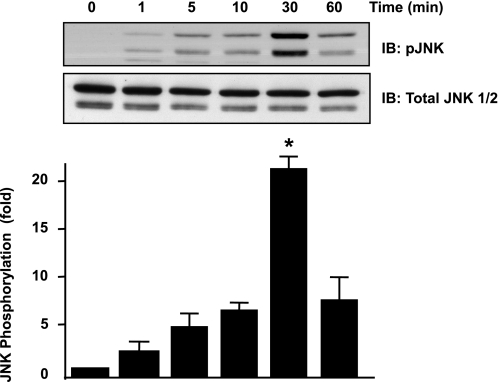
To study the role of JNK in ANG II-mediated insulin resistance, we first performed a time course experiment, stimulating VSMCs with 100 nM of ANG II for 0, 1, 5, 10, 30, and 60 min. We determined that ANG II phosphorylates JNK maximally at 30 min in VSMCs (Fig. 1).
Fig. 1.
ANG II induces JNK phosphorylation maximally at 30 min. Vascular smooth muscles cells (VSMCs) were stimulated with 100 nM ANG II for the indicated times, and Western blotting with anti-phospho (p)-JNK antibody or total JNK was performed. A representative blot is shown at top (n = 3). IB, immunoblotting. Bar graph represents means ± SE. *P < 0.05 compared with no stimulation.
ANG II induces phosphorylation of IRS-1 Ser632, Ser307, and Ser302.
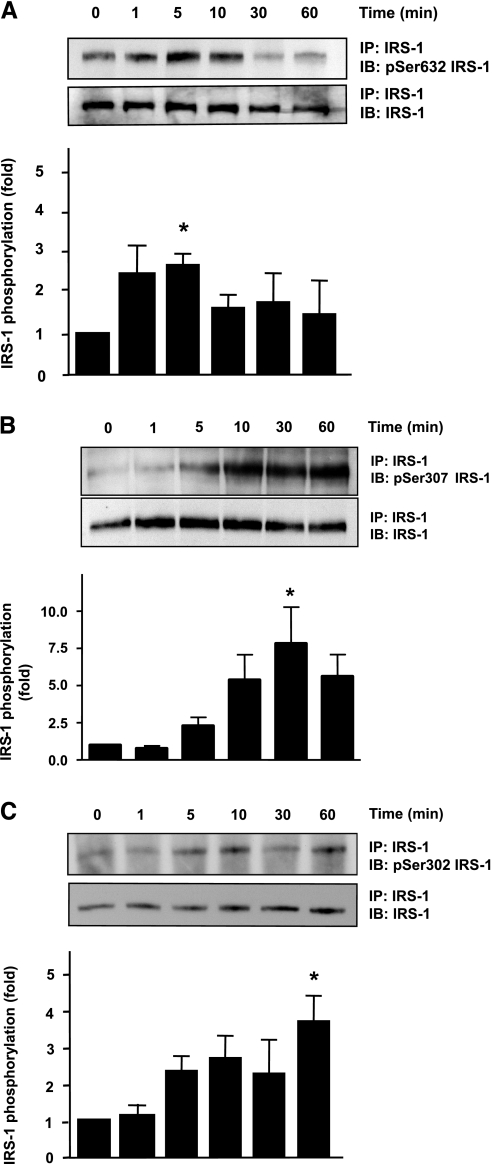
Increased serine phosphorylation of IRS-1 has been implicated in disrupting the insulin-induced metabolic pathway involving PI3K/Akt (6, 14, 49). Numerous serine/threonine phosphorylation sites exist on IRS-1, and specific sites that may be associated with ANG II-mediated IRS-1 degradation are unknown. IRS-1 Ser632, Ser307, and Ser302 have all been implicated in insulin resistance, and IRS-1 serine phosphorylation on 307 has been linked to degradation in rat skeletal muscle (20). Thus, we first determined whether ANG II induces phosphorylation of any of these sites. As shown in Fig. 2, A–C, ANG II phosphorylates IRS-1 Ser632 maximally at 5 min, Ser307 at 30 min, and Ser302 at 60 min.
Fig. 2.
ANG II induces phosphorylation of insulin receptor substrate-1 (IRS-1) at multiple serine residues. VSMCs were stimulated with 100 nM ANG II for the indicated times. Immunoprecipitation (IP) of IRS-1 followed by Western blotting with phosphospecific antibodies was performed. A: ANG II induced IRS-1 Ser632 phosphorylation maximally at 5 min (n = 3). B: ANG II induced IRS-1 Ser307 phosphorylation maximally at 30 min (n = 6). C: ANG II induced IRS-1 Ser302 phosphorylation maximally at 60 min (n = 3). Bar graphs represent means ± SE. *P < 0.05 compared with no stimulation.
JNK inhibition blocks ANG II-induced IRS-1 Ser632, Ser307, and Ser302 phosphorylation.
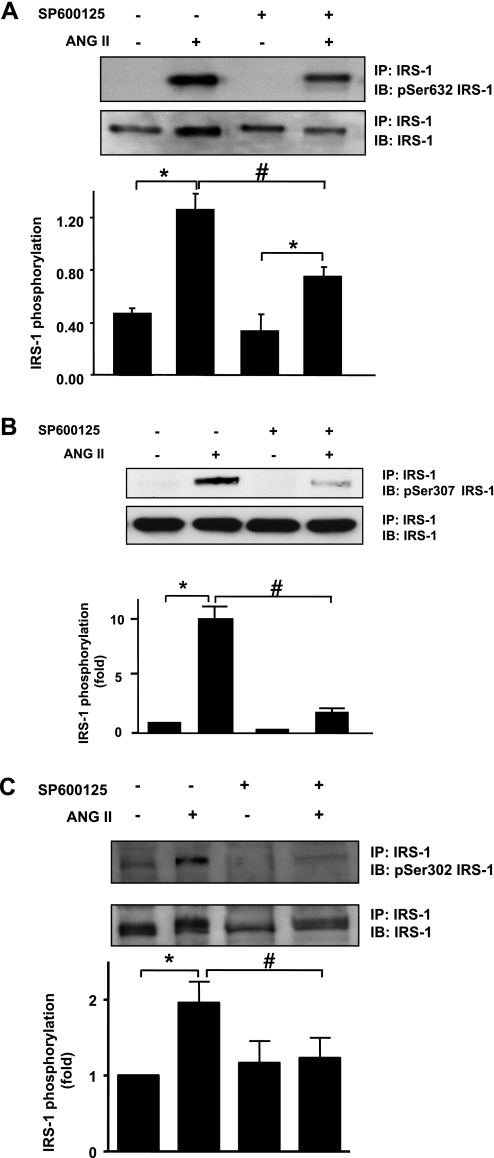
To determine whether these serines are phosphorylated by JNK, we pretreated VSMCs with 30 nM of the JNK inhibitor SP600125 for 30 min. The concentration of SP600125 used inhibits JNK phosphorylation by ANG II in our cells (data not shown) and has also been used by others (17). After pretreatment with SP600125, VSMCs were stimulated with 100 nM ANG II for the time required for maximal phosphorylation (5 min for Ser632, 30 min for Ser307, and 60 min for Ser302). ANG II-mediated IRS-1 Ser632 phosphorylation was affected significantly by JNK inhibition (Fig. 3A), ANG II-induced IRS-1 Ser307 phosphorylation was reduced approximately by 80% (Fig. 3B), and Ser302 phosphorylation was abolished (Fig. 3C) after JNK inhibition.
Fig. 3.
JNK inhibition decreases ANG II-induced IRS-1 Ser632, Ser307, and Ser302 phosphorylation. A: VSMCs were pretreated with SP600125 (30 nM, 30 min) followed by stimulation with ANG II (100 nM) for 5 min. Western blotting with an antiphosphospecific antibody for Ser632 IRS-1 was performed (n = 7). B: VSMCs were pretreated with SP600125 (30 nM, 30 min) followed by stimulation with ANG II (100 nM) for 30 min. Total IRS-1 was immunoprecipitated, followed by Western blotting with an antiphosphospecific antibody for Ser307 IRS-1 (n = 3). C: VSMCs pretreated with JNK inhibitor SP600125 at 30 nM for 30 min were stimulated with ANG II for 60 min, immunoprecipitated, and then immunoblotted with Ser302 phosphospecific IRS-1 antibody. Bar graphs represent means ± SE (n = 3). *P < 0.05 compared with no stimulation; #P < 0.05 compared with stimulation without JNK inhibitor.
JNK inhibition does not affect ANG II-induced insulin resistance.
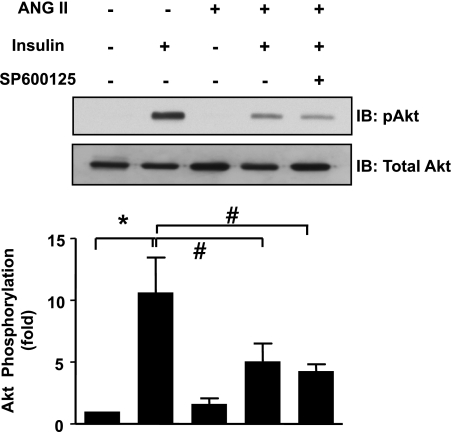
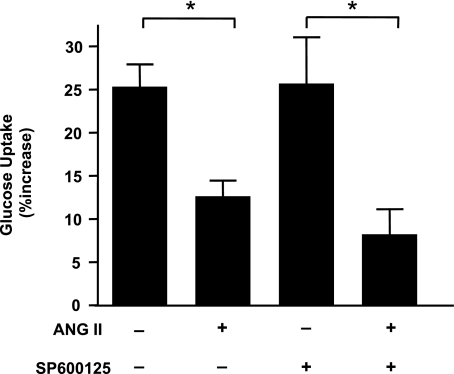
Previously published data from our laboratory show that ANG II mediates IRS-1 degradation and decreases phosphorylation of Akt and subsequent glucose uptake (47). To determine whether JNK-mediated serine phosphorylation contributes to ANG II-induced insulin resistance, we tested the sensitivity of the ANG II-induced decrease in insulin-dependent phosphorylation of Akt, glucose uptake, and IRS-1 degradation to JNK inhibition. Pretreatment of VSMCs with SP600125 did not affect phosphorylation of Akt (Fig. 4), nor did it influence glucose uptake (Fig. 5) induced by insulin (100 nM). ANG II induced a decrease in glucose uptake by 50% compared with baseline, which was not affected by SP600125. Moreover, JNK inhibition did not block IRS-1 degradation after 18 h of ANG II exposure, indicating that JNK does not play a significant role in ANG II-mediated IRS-1 degradation in VSMCs (Fig. 6).
Fig. 4.
JNK inhibition does not reverse ANG II-induced impairment of Akt phosphorylation. VSMCs were pretreated with SP600125 (30 nM, 30 min) followed by stimulation with ANG II (100 nM, 18 h). Then, cells were stimulated with insulin (100 nM, 5 min), followed by Western blotting with an antibody against phosphorylated Akt and total Akt (n = 4). There was no significant difference in insulin-mediated Akt phosphorylation with or without JNK inhibitor, with ANG II pretreatment (bar 4 vs. 5). *P < 0.05 compared with no insulin; #P < 0.05 compared with no stimulation with ANG II.
Fig. 5.
Inhibition of JNK does not improve glucose uptake reduction after ANG II. Uptake of radioactive glucose was measured in VSMCs pretreated with SP600125 (30 nM, 30 min) followed by stimulation with ANG II (100 nM) for 18 h. JNK inhibitor has no significant effect on glucose uptake (bar 2 vs. 4). Bar graphs represent means ± SE (n = 4). *P < 0.05 compared with no stimulation in the presence or absence of inhibitor.
Fig. 6.
Inhibition of JNK does not prevent ANG II-induced IRS-1 degradation. VSMCs were treated with ANG II (100 nM) for 18 h with or without SP600125 (30 nM), and samples were immunoblotted for IRS-1. Bar graph represents loss of IRS-1 protein following ANG II treatment, expressed as means ± SE of percent decrease from control (n = 3).
IRS-1 Ser632, Ser307, and Ser302 do not mediate ANG II-induced IRS-1 degradation.
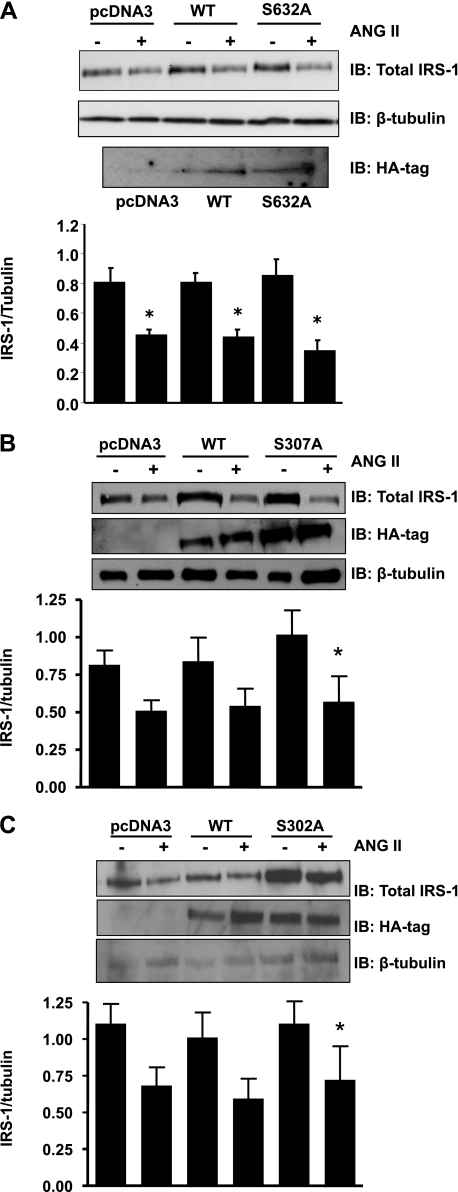
The ineffectiveness of JNK inhibition to block ANG II-induced insulin resistance suggests that those Ser sites on IRS-1 sensitive to JNK inhibition are not related to the ability of ANG II to degrade IRS-1. To test this concept, we created point mutations of serine to alanine at the three JNK-sensitive sites (S632A, S307A, and S302A). VSMC transfection with IRS-1 mutants S632A, S307A, and S302A failed to block ANG II-mediated IRS-1 degradation (Fig. 7, A–C).
Fig. 7.
Mutation of IRS-1 does not prevent its degradation by ANG II. VSMCs were transfected with IRS-1 mutants S632A (A), S307A (B), and S302A (C), as described in materials and methods, and incubated with ANG II (100 nM) for 18 h. Western blotting with antibodies against total IRS-1, hemagglutinin (HA)-tag, or β-tubulin was performed. There was no significant difference in IRS-1 levels between wild type (WT) and mutants in the presence of ANG II (bar 4 vs. 6 in A, B, and C). Bar graphs represent means ± SE (in A and B, n = 4; in C, n = 3). *P < 0.05 compared with no stimulation for the corresponding transfection.
JNK inhibition attenuates insulin-induced collagen synthesis and α-SMA expression in ANG II pretreated VSMCs.
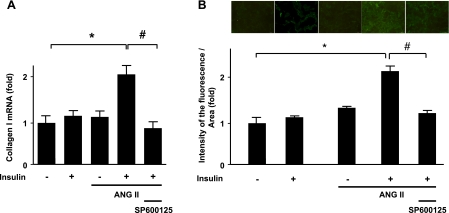
ANG II is known to cause VSMC hypertrophy (9, 25). In addition to regulation of glucose homeostasis, insulin also induces VSMC hypertrophy (21). To evaluate the potential involvement of JNK in ANG II-enhanced, insulin-mediated hypertrophic signaling, we measured collagen synthesis using RT-PCR and α-SMA expression using immunofluorescence staining. Pretreatment with ANG II significantly increased the mRNA levels of collagen type I induced by insulin, as shown in Fig. 8A. Preincubation with SP600125 (30 nM for 30 min) markedly attenuated ANG II/insulin-induced increases in collagen type I mRNA expression. Similarly, ANG II increased insulin-induced α-SMA expression, which was diminished by pretreatment with SP600125 (Fig. 8B), suggesting that the hypertrophic responses of increased α-SMA and collagen type I expression induced by insulin are augmented by pretreatment with ANG II in a JNK-dependent manner.
Fig. 8.
JNK inhibition attenuates insulin-induced collagen synthesis and α-smooth muscle actin (α-SMA) expression in ANG II-pretreated VSMCs. VSMCs were pretreated with ANG II (100 nM) for 18 h and then exposed to 100 nM insulin for 18 h (A) or 24 h (B). Cells were pretreated with SP600125 (30 nM, 30 min) before ANG II incubation. Collagen I mRNA was measured by quantitative RT-PCR. Expression of α-SMA was determined using immunofluorescence. Bar graphs represent means ± SE (n = 4). *P < 0.05 compared with control; #P < 0.05 compared with insulin stimulation with ANG II pretreatment.
DISCUSSION
The mechanism of insulin resistance involving cross talk between components of the RAS and insulin signaling is complex; modulation of insulin signaling by RAS occurs at multiple levels (15). Upon agonist binding, the heterotetrameric insulin receptor undergoes tyrosine autophosphorylation (28, 56), leading to activation of several docking proteins, including IRS. Insulin-stimulated tyrosine phosphorylation of IRS-1 allows it to associate with the p85 subunit of PI3K, a critical component that leads to activation of Akt. Akt activation propagates downstream signaling necessary for cell survival, including glucose transport and protein synthesis (3). Several studies have noted that manipulation of the RAS by ACE inhibitors and ARBs leads to improved insulin sensitivity (24, 26, 33, 35–36).
Several of over 70 serine residues in IRS-1 (45) play a regulatory role in mediating the actions of insulin (49, 55). Hyperglycemia, hyperinsulinemia, okadaic acid (38), mitochondrial dysfunction (31), and inflammatory cytokines such as tumor necrosis factor-α (22, 40) all stimulate serine phosphorylation of IRS-1. Increased serine/threonine phosphorylation disrupts IRS-1 association with the p85 subunit of PI3K (13), disrupts GLUT-4 translocation (8), decreases tyrosine phosphorylation (13, 56), and leads to IRS-1 degradation (38).
In searching for the molecular mechanisms by which ANG II leads to insulin resistance, previous work from our laboratory showed that ANG II phosphorylates IRS-1 on Ser307 and decreases IRS-1 levels in VSMCs (47). However, we did not determine whether these two observations were causally related nor did we identify the serine/threonine kinase that catalyzes that phosphorylation. Therefore, in this study, we investigated the mechanism of ANG II-induced IRS-1 degradation by focusing on the serine/threonine kinase, JNK. In skeletal muscle, JNK activity and IRS-1 Ser307 levels are increased before IRS-1 degradation (20). However, in contrast to other cell types, JNK inhibition does not affect IRS-1 degradation in our experiments on VSMCs, indicating that specific mechanisms of insulin resistance vary and could be cell/tissue specific. Even though JNK is seemingly a viable pharmacologic target for insulin resistance, others have also demonstrated that JNK may not be a converging point in insulin resistance via all mechanisms. Greene and colleagues (18) showed that insulin-stimulated IRS-1 degradation was partly dependent on phosphorylation of human Ser312 (rat Ser307), but that JNK was not the kinase responsible for this degradation. In contrast, in skeletal muscle cells, it has been shown that inducible nitric oxide synthase-induced IRS-1 degradation is JNK independent (44).
We chose to focus on IRS-1 serine phosphorylation sites that have been implicated in insulin resistance (50). Of these, IRS-1 Ser307 has been most extensively researched (2). JNK stimulates phosphorylation on IRS-1 Ser307 by insulin and negatively impacts insulin signaling (1, 2). Danielsson and colleagues (10) recently showed that IRS-1 Ser307 phosphorylation leads to decreased IRS-1 tyrosine phosphorylation by insulin, explaining a potential mechanism by which Ser307 disrupts insulin signaling. Interestingly, in 3T3-L1 fibroblasts and adipocytes, insulin itself stimulates IRS-1 phosphorylation at Ser307 (41), demonstrating insulin's ability to trigger pathways that regulate its own downstream effects. IRS-1 phosphorylation of Ser307 and Ser302 is increased in insulin-resistant C57B/L6 mice; furthermore, JNK1-mediated disruption of insulin receptor/IRS-1 interaction is dependent on IRS-1 phosphorylation on Ser302 and Ser307 (54). In diabetic human skeletal myotubes, there is an increased basal phosphorylation of IRS-1 Ser632, which may be associated with reduced IRS-1 tyrosine phosphorylation (6).
We find it interesting that the hormone ANG II stimulates phosphorylation of all three of these sites, with maximal phosphorylation occurring at various times, depending on the serine site. We show that ANG II phosphorylates IRS-1 Ser632 maximally at 5 min, Ser307 at 30 min, and Ser302 at 60 min. One can speculate that the differential times of IRS-1 serine phosphorylation likely modulates the spatial interaction of IRS-1 with its local environment. The sites phosphorylated earlier may induce short-term transduction events that are quite different from the stimulation of sites that require a longer exposure to ANG II.
We tested the effect of inhibition of JNK in ANG II-mediated IRS-1 serine phosphorylation of the three sites targeted by ANG II. We confirmed that ANG II induces maximal phosphorylation of JNK at 30 min, which is consistent with other reports (23, 53), and we found that in VSMCs, inhibition of JNK leads to a decrease in ANG II-induced Ser 632, Ser307, and Ser302 phosphorylation. We expected that JNK inhibition would improve ANG II-mediated effects on phosphorylation of Akt, glucose uptake, and IRS-1 degradation. To our surprise, JNK inhibition did not reverse the inhibition of insulin-induced Akt phosphorylation produced by ANG II at 18 h, nor did it affect ANG II-induced decrease in glucose transport or IRS-1 degradation. These observations suggest that in VSMCs, IRS-1 serine sites Ser632, Ser307, and Ser302 are not individually involved in ANG II-mediated IRS-1 degradation and insulin resistance. This was confirmed by the ability of ANG II to induce degradation of all three mutants of IRS-1 S632A, S307A, and S302A. Whether inhibition of several sites at once blocks ANG II-mediated IRS-1 degradation remains to be tested. The possibility of sequential activation of multiple serine phosphorylation sites as a requirement for ANG II-mediated IRS-1 degradation cannot be ruled out. Werner et al. (54) report that even though JNK phosphorylates Ser307 and Ser302 and both are needed for disruption of insulin receptor interaction with IRS-1, these two sites alone or in combination are not sufficient. This is interesting because ANG II mediates phosphorylation of different serine sites at different time points.
We do not detect a causal relationship between activation of JNK by ANG II and IRS-1 degradation in VSMCs. Of course, JNK may play a role in acute insulin resistance, during a short-term exposure to ANG II. Also, even though we find that JNK is not involved in ANG II-mediated IRS-1 degradation, our data show that it regulates other aspects of insulin-induced pathology. JNK inhibition attenuated collagen synthesis and α-SMA expression induced by insulin in ANG II-pretreated VSMCs (Fig. 8), suggesting that early JNK activation may play a role in mechanisms leading to vascular remodeling. Furthermore, inhibition of JNK partially blocks ANG II-mediated serine phosphorylation of IRS-1 Ser632, Ser 307, and Ser302, which implicates ANG II and JNK in other cellular processes mediated by IRS-1. Our study suggests that a kinase other than JNK, such as PDK1, IκB (29), mammalian target of rapamycin (mTOR) (34, 37), or Rho kinase (ROCK) (16), may be responsible for ANG II-mediated insulin resistance in the vasculature. The possibility of a combined interaction of JNK and another kinase also exists, and JNK may play a role in insulin resistance via other mechanisms, such as resistance mechanisms induced by insulin itself.
Even though we have focused on the role of IRS-1 and JNK in glucose homeostasis and cellular hypertrophy, IRS-1 proteins are involved in many other cellular functions, including protein metabolism, migration, apoptosis, and amino acid transport. We recognize that unlike skeletal muscle and liver, the primary role of VSMCs is not glucose homeostasis; however, the local RAS and its influence on glucose uptake provide insight into the vascular cell's ability to be modulated by an agonist like insulin. Indeed, the ability of ANG II to stimulate the phosphorylation of the three serine sites Ser632, Ser302, and Ser307 may be significant in the overall search for mechanisms of insulin resistance. Whether phosphorylation of these sites reduces the overall efficiency of IRS-1 tyrosine phosphorylation in the presence of insulin and ANG II remains to be tested. The identification of serine/threonine kinases involved in ANG II-mediated IRS-1 degradation and the regulatory serine/threonine IRS-1 sites involved will greatly enhance our ability to understand the influence of the RAS on insulin signaling, and possibly manipulate it to improve insulin resistance and vascular remodeling.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-038206, HL-75209, and HL-07754.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.H., P.K.M., Y.T., B.L., and K.K.G. conception and design of research; H.H., P.K.M., Y.T., B.L., B.S.-R., and A.S.M. performed experiments; H.H., P.K.M., B.L., and B.S.-R. analyzed data; H.H., P.K.M., B.L., B.S.-R., A.S.M., and K.K.G. interpreted results of experiments; H.H., P.K.M., and B.S.-R. prepared figures; H.H., P.K.M., Y.T., B.L., B.S.-R., A.S.M., and K.K.G. edited and revised manuscript; H.H., P.K.M., Y.T., B.L., B.S.-R., A.S.M., and K.K.G. approved final version of manuscript; P.K.M. drafted manuscript.
REFERENCES
- 1.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8: 55–62, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res 94: 1211–1218, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev 70: 1061–1095, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y. Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52: 1319–1325, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Clark SF, Martin S, Carozzi AJ, Hill MM, James DE. Intracellular localization of phosphatidylinositide 3-kinase and insulin receptor substrate-1 in adipocytes: potential involvement of a membrane skeleton. J Cell Biol 140: 1211–1225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 68: 450–456, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Danielsson A, Nystrom FH, Stralfors P. Phosphorylation of IRS1 at serine 307 and serine 312 in response to insulin in human adipocytes. Biochem Biophys Res Commun 342: 1183–1187, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Diah S, Zhang GX, Nagai Y, Zhang W, Gang L, Kimura S, Hamid MR, Tamiya T, Nishiyama A, Hitomi H. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp Cell Res 314: 3654–3662, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res 77: 863–868, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes 55: 2392–2397, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Egawa K, Sharma PM, Nakashima N, Huang Y, Huver E, Boss GR, Olefsky JM. Membrane-targeted phosphatidylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. J Biol Chem 274: 14306–14314, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 100: 2158–2169, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2: 119–129, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Cazarin ML, Smith JL, Clair DK, Piascik MT. The alpha1D-adrenergic receptor induces vascular smooth muscle apoptosis via a p53-dependent mechanism. Mol Pharmacol 74: 1000–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by serine 312 phosphorylation. J Biol Chem 278: 8199–8211, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem 266: 15498–15504, 1991 [PubMed] [Google Scholar]
- 20.Hilder TL, Tou JC, Grindeland RE, Wade CE, Graves LM. Phosphorylation of insulin receptor substrate-1 serine 307 correlates with JNK activity in atrophic skeletal muscle. FEBS Lett 553: 63–67, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hitomi H, Kaifu K, Fujita Y, Sofue T, Nakano D, Moriwaki K, Hara T, Kiyomoto H, Kohno M, Kobori H, Nishiyama A. Angiotensin II shifts insulin signaling into vascular remodeling from glucose metabolism in vascular smooth muscle cells. Am J Hypertens 24: 1149–1155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665–668, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Huang XC, Deng T, Sumners C. Angiotensin II stimulates activation of Fos-regulating kinase and c-Jun NH2-terminal kinase in neuronal cultures from rat brain. Endocrinology 139: 245–251, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Iimura O, Shimamoto K, Matsuda K, Masuda A, Takizawa H, Higashiura K, Miyazaki Y, Hirata A, Ura N, Nakagawa M. Effects of angiotensin receptor antagonist and angiotensin converting enzyme inhibitor on insulin sensitivity in fructose-fed hypertensive rats and essential hypertensives. Am J Hypertens 8: 353–357, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Itoh H, Mukoyama M, Pratt RE, Gibbons GH, Dzau VJ. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest 91: 2268–2274, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer SN, Katovich MJ. Effect of acute and chronic losartan treatment on glucose tolerance and insulin sensitivity in fructose-fed rats. Am J Hypertens 9: 662–668, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Kaburagi Y, Satoh S, Yamamoto-Honda R, Ito T, Ueki K, Akanuma Y, Sekihara H, Kimura S, Kadowaki T. Insulin-independent and wortmannin-resistant targeting of IRS-3 to the plasma membrane via its pleckstrin homology domain mediates a different interaction with the insulin receptor from that of IRS-1. Diabetologia 44: 992–1004, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kasuga M, Zick Y, Blithe DL, Crettaz M, Kahn CR. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature 298: 667–669, 1982 [DOI] [PubMed] [Google Scholar]
- 29.Kim F, Tysseling KA, Rice J, Gallis B, Haji L, Giachelli CM, Raines EW, Corson MA, Schwartz MW. Activation of IKKbeta by glucose is necessary and sufficient to impair insulin signaling and nitric oxide production in endothelial cells. J Mol Cell Cardiol 39: 327–334, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes 51: 522–527, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Mothe I, Van Obberghen E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J Biol Chem 271: 11222–11227, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Munoz MC, Argentino DP, Dominici FP, Turyn D, Toblli JE. Irbesartan restores the in-vivo insulin signaling pathway leading to Akt activation in obese Zucker rats. J Hypertens 24: 1607–1617, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Mussig K, Fiedler H, Staiger H, Weigert C, Lehmann R, Schleicher ED, Haring HU. Insulin-induced stimulation of JNK and the PI 3-kinase/mTOR pathway leads to phosphorylation of serine 318 of IRS-1 in C2C12 myotubes. Biochem Biophys Res Commun 335: 819–825, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Nawano M, Anai M, Funaki M, Kobayashi H, Kanda A, Fukushima Y, Inukai K, Ogihara T, Sakoda H, Onishi Y, Kikuchi M, Yazaki Y, Oka Y, Asano T. Imidapril, an angiotensin-converting enzyme inhibitor, improves insulin sensitivity by enhancing signal transduction via insulin receptor substrate proteins and improving vascular resistance in the Zucker fatty rat. Metabolism 48: 1248–1255, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 40: 872–879, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA 98: 4640–4645, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes 50: 24–31, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40: 1286–1292, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Qi C, Pekala PH. Tumor necrosis factor-alpha-induced insulin resistance in adipocytes. Proc Soc Exp Biol Med 223: 128–135, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107: 181–189, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci USA 103: 16454–16459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn JA, Kaneki M. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem 280: 14203–14211, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352: 73–77, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M, Shinozaki K, Kanazawa A, Hara Y, Hattori Y, Tsushima M, Harano Y. Insulin resistance as an independent risk factor for carotid wall thickening. Hypertension 28: 593–598, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol 25: 1142–1147, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C494–C499, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Tanti JF, Gual P, Gremeaux T, Gonzalez T, Barres R, Le Marchand-Brustel Y. Alteration in insulin action: role of IRS-1 serine phosphorylation in the retroregulation of insulin signalling. Ann Endocrinol (Paris) 65: 43–48, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol 9: 753–762, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res 88: 1291–1298, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Vermes E, Ducharme A, Bourassa MG, Lessard M, White M, Tardif JC. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD). Circulation 107: 1291–1296, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Wen Y, Scott S, Liu Y, Gonzales N, Nadler JL. Evidence that angiotensin II and lipoxygenase products activate c-Jun NH2-terminal kinase. Circ Res 81: 651–655, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem 279: 35298–35305, 2004 [DOI] [PubMed] [Google Scholar]
- 55.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283: E413–E422, 2002 [DOI] [PubMed] [Google Scholar]
- 56.White MF, Shoelson SE, Keutmann H, Kahn CR. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem 263: 2969–2980, 1988 [PubMed] [Google Scholar]
- 57.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]