Abstract
We previously reported that isolated endothelium-removed bovine pulmonary arteries (BPAs) contract to hypoxia associated with removal of peroxide- and cGMP-mediated relaxation. In contrast, bovine coronary arteries (BCAs) relax to hypoxia associated with cytosolic NADPH oxidation coordinating multiple relaxing mechanisms. Since we recently found that H2O2 relaxes BPAs through PKG activation by both soluble guanylate cyclase (sGC)/cGMP-dependent and cGMP-independent thiol oxidation/subunit dimerization mechanisms, we investigated if these mechanisms participate in BPA contraction and BCA relaxation to hypoxia. The contraction of BPA (precontracted with 20 mM KCl) to hypoxia was associated with decreased PKG dimerization and PKG-mediated vasodilator-stimulated phosphoprotein (VASP) phosphorylation. In contrast, exposure of 20 mM KCl-precontracted endothelium-removed BCAs to hypoxia caused relaxation and increased dimerization and VASP phosphorylation. Depletion of sGC by organoid culture of BPAs with an oxidant of the sGC heme (10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) increased aerobic force generation, decreased VASP phosphorylation, and inhibited further contraction to hypoxia and changes in VASP phosphorylation. Thiol reduction with dithiothreitol increased aerobic force in BPAs and decreased PKG dimerization, VASP phosphorylation, and the contraction to hypoxia. Furthermore, PKG-1α and sGC β1-subunit small interfering RNA-transfected BPAs demonstrated increased aerobic K+ force and inhibition of further contraction to hypoxia, associated with an attenuation of H2O2-elicited relaxation and VASP phosphorylation. Thus, decreases in both a sGC/cGMP-dependent and a dimerization-dependent activation of PKG by H2O2 appear to contribute to the contraction of BPAs elicited by hypoxia. In addition, stimulation of PKG activation by dimerization may be important in the relaxation of coronary arteries to hypoxia.
Keywords: cGMP, guanylate cyclase, oxygen sensor
previous studies from our laboratory in endothelium-removed bovine pulmonary arteries (BPAs) detected evidence that hypoxia inhibits a basal peroxide-mediated relaxation maintained under aerobic conditions associated with a hypoxic pulmonary vasoconstriction response (1, 2, 5, 6, 19). A contributing factor to the hypoxic pulmonary vasoconstriction response in BPAs appears to be removal of a cGMP relaxing mechanism associated with the metabolism of peroxide by catalase maintaining the activation of soluble guanylate cyclase (sGC) (5, 6). Recently, a new mechanism for vasodilation of the coronary circulation of rat hearts and relaxation of the isolated rat aorta by H2O2 been described associated with peroxide causing a thiol oxidation-mediated subunit dimerization of the 1α-form of PKG, associated with a cGMP-independent activation of this kinase, which is normally stimulated by cGMP (3). Our laboratory subsequently documented that both sGC/cGMP- and thiol oxidation-mediated subunit dimerization of activation of PKG appear to be involved in H2O2-mediated relaxation of BPAs, with sGC seeming to play a more dominant role (21). Thus, it could be hypothesized that a suppression of both sGC/cGMP and thiol oxidation/dimerization mechanisms of PKG activation could be regulators of the response of BPAs to hypoxia.
Our study (12) with bovine coronary arteries (BCAs) detected evidence for hypoxia eliciting cytosolic NADPH oxidation, which appears to function as a coordinator of the relaxation that is observed. Since the oxidation of cytosolic NADPH is potentially a regulator of PKG dimerization through its role in maintaining control of thiol redox by NADPH-dependent enzymes such as glutathione reductase and thioredoxin reductase (20), it could be hypothesized that PKG activation is a factor in the relaxation of BCAs to hypoxia. This hypothesis is supported by evidence for multiple mechanisms promoting the relaxation of vascular tissue through lowering intracellular Ca2+, which appear to be associated with both PKG activation (14) and the oxidation of cytosolic NADPH (10–12).
The objective of the present study was to examine the relationships and potential roles of cGMP-dependent and cGMP-independent mechanisms of PKG activation in the contraction of BPAs and relaxation of BCAs to hypoxia. Changes in both cGMP-dependent and cGMP-independent mechanisms of PKG activation were evaluated based on changes in PKG-mediated phosphorylation of Ser239 of vasodilator-stimulated phosphoprotein (VASP) (23). The role of thiol oxidation-mediated subunit dimerization of PKG in the mechanism of contraction to hypoxia was examined by detecting subunit dimerization by Western blot analysis. Observations that oxidation of the heme of sGC by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) promotes ubiquitination and proteosomal degradation depletion of this enzyme (15) were recently developed into an organoid culture method for the depletion of sGC from BPAs (21), for a subsequent examination of the role of sGC/cGMP-dependent vasodilator mechanisms in the response to peroxide. This method of sGC depletion was used in conjunction with sGC β1-subunit and PKG-1α small interfering (si)RNA-transfected BPAs to define the role of sGC/cGMP-dependent vasodilator mechanisms in the response of BPAs to hypoxia.
MATERIALS AND METHODS
Materials.
All physiological buffers were prepared using analytic grade reagent salts purchased from J. T. Baker Chemical, and all other chemicals were obtained from Sigma Chemical unless otherwise mentioned. PKG-1α and VASP antibodies for Western blot analysis were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Cell Signaling (Beverly, MA), respectively. Spermine NONOate was purchased from Cayman Chemical (Ann Arbor, MI). Transfection reagents and siRNAs were purchased from Qiagen (Valencia, CA) and Ambion (Austin, TX). All gases were obtained from Tech Air (White Plains, NY).
Tissue preparations.
Bovine lungs and hearts were obtained from a slaughterhouse in ice-cold PBS, and second- and third-order branches of the main lobar pulmonary artery were used for BPA experiments. Left anterior descending and circumflex arteries were used for BCA experiments. BPAs and BCAs were cleaned of their connective tissue and cut into rings of 2–3 mm in diameter and width. The endothelium was removed by rubbing the lumen. Fresh and organoid cultured blood vessel rings were used in experiments for vascular reactivity and Western blot protein analysis. Organoid cultures were performed (21) with BPA rings in the absence and presence of 10 μM ODQ with DMEM media containing 10% FBS and 1% antibiotics (penicillin, streptomycin, and amphotericin B) for 48 h at 37°C with 5% CO2.
siRNA transfection.
The transfection protocol used in our previous study (1) was slightly adapted to incubate 0.3 pg siRNA for the first 24 h in DMEM at 37°C with 5% CO2 using the Qiagen transmessenger transfection reagent kit with a 1:3:2.5 ratio of enhancer R, siRNA, and transmessenger transfection reagent. Each ring was transfected in a separate well of a sterile 96-well plate with a final volume of 250 μl of the transfection media. After 24 h, rings were moved to another plate containing DMEM with 10% FBS and antibiotics (penicillin, streptomycin, and fungizone) for a further 24 h at 37°C with 5% CO2.
Sequences of the siRNAs used were as follow: sGC-β1 siRNA, sense 5′-GCAAGGUCCUCAAUCUCAAtt-3′ and antisense 3′-UUGAGAUUGAGGACCUUGCtt-′5; PKG-1αsiRNA, sense 5′-GGACAGGACUUAUCAAGCAtt-3′ and antisense 3′-UGCUUGAUAAGUCCUGUCCTC-′5; and scrambled siRNA, sense 5′-UUCUCCGAACGUGUCACGUtt-3′ and antisense 3′-ACGUGACACGUUCGGAGAAtt-5′.
Vascular reactivity.
Endothelium-removed artery rings were mounted on Grass (FT-03) or Coulborne Instruments force displacement transducers for recording isometric force development through a Powerlab data-acquisition system (AD Instruments) as previously described (1, 12, 21). Arterial rings were incubated in Krebs-bicarbonate buffer (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose) for 1 h under a resting tension of 5 g. In all experiments, arterial rings were depolarized with 123 mM KCl-containing Krebs-bicarbonate buffer, and rings were then equilibrated with Krebs-bicarbonate buffer for 30 min. In fresh BPA or BCA aerobic and hypoxia comparison experiments, arterial rings were precontracted with 20 mM KCl under aerobic conditions. Rings were subsequently exposed to hypoxia by changing the gassing from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr). Organoid cultured BPA rings in the presence or absence of the heme oxidant inhibitor 10 μM ODQ were precontracted with 25 mM KCl and then exposed to hypoxia for 20 min or continued with aerobic gassing. In reducing experiments, fresh vessels were incubated with 1 mM DTT and 5 mM deferoxamine for 20 min before precontraction with 20 mM KCl under aerobic conditions before being switched to hypoxia. In siRNA-transfected BPA experiments, arterial rings were precontracted with 25 mM KCl under aerobic conditions before being switched to hypoxia, and 0.1 mM H2O2 was added. For experiments examining the effect of 10 μM acute ODQ treatment (30 min of incubation), fresh BPAs were contracted with 30 mM KCl followed by precontraction with 25 mM KCl and then exposed to hypoxia for 20 min or continued with aerobic gassing. In experiments examining the effect of spermine NONOate on PKG dimerization during hypoxia, BPA rings were precontracted with 20 mM KCl followed by exposure to hypoxia and relaxed with 1 μM spermine NONOate. Rings were flash frozen for Western blot analysis with liquid N2 under aerobic or hypoxic conditions. Experiments were conducted by adding 10 nM serotonin to arteries already precontracted with KCl to confirm that the level of force under aerobic conditions did not alter the magnitude of the subsequent contraction to hypoxia in fresh and organoid cultured arteries.
Western blot analysis.
Frozen arterial rings were pulverized and then homogenized in lysis buffer containing protease and phosphatase inhibitors, as previously described (21). Maleimide (100 mM) was included in the lysis buffer to alkylate the thiols to avoid artifactual disulfide bond formation during homogenization. A protein quantification assay was performed using the Bradford method, and samples were prepared for gel electrophoresis. Proteins were separated using a 10% SDS-polyacrylamide gel. Thiol-reducing conditions were avoided in the samples analyzing for PKG-1α dimers. Gels were transferred to polyvinylidene difluoride membranes, and membranes were blocked with Tris-buffered saline with Tween 20 and 5% milk for 1 h. Membranes were then exposed to primary and secondary antibodies. Protein bands were visualized with an enhanced chemiluminescence kit (Pierce) on X-OMAT autoradiography paper (Kodak). Relative changes in PKG-1α monomer and dimer forms are reported as a percentage of total PKG-1α. Changes in PKG-1α monomer and dimer expression were quantified after normalization to β-actin, and phosphorylated VASP was normalized to total VASP in each individual artery studied. Protein levels were measured using densitometry analysis with UN-SCAN-IT gel software by Silk Scientific. The molecular masses of the PKG monomer and dimer are 75 and 150 kDa, respectively.
Statistical analysis.
Data values are expressed as means ± SE of the number of arterial segments (n) from different animals. Statistical analyses between two groups were performed with paired and unpaired Student's t-tests, and one-way ANOVA with the Newman Keuls correction was used for comparisons between multiple groups. P values of <0.05 were used to establish statistical significance.
RESULTS
Hypoxia decreases dimerization and PKG activity in BPAs precontracted with KCl.
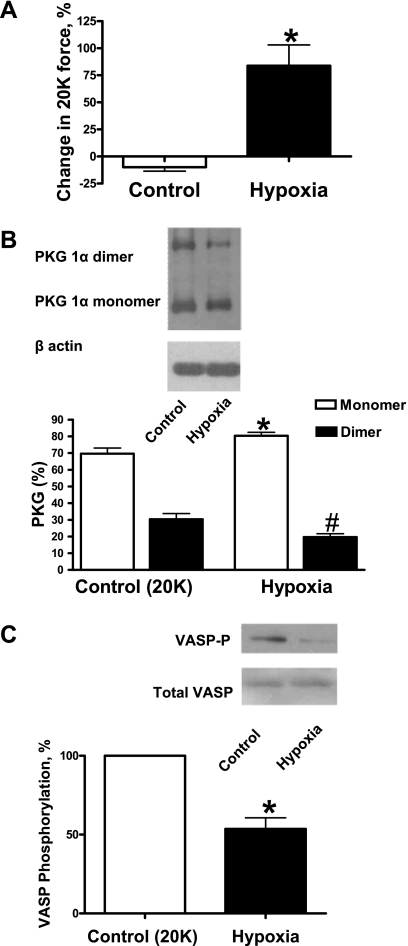
To study if hypoxia can reduce disulfide linkage between two PKG monomers, BPAs were precontracted with 20 mM K+ under aerobic conditions before exposure to hypoxia by changing the gassing in the tissue baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr). Hypoxia caused contraction of BPAs (Fig. 1A) and reduced disulfide dimerization of PKG (Fig. 1B). Phosphorylation of VASP was also decreased under hypoxia (Fig. 1C).
Fig. 1.
Effects of hypoxia in promoting the contraction of bovine pulmonary arteries (BPAs) associated with disulfide-mediated dimerization of PKG and stimulation of PKG-dependent phosphorylation of vasodilator-stimulated phosphoprotein (VASP) using 20 mM K+ (20K) as the precontracting agent. A: contractions seen under prolonged aerobic conditions [control (Ctrl)] and under hypoxia normalized to the initial 20 mM K+ force under aerobic conditions (n = 8). B: Western blot showing that hypoxia decreases the dimerization of PKG (top) and summary data showing that hypoxia decreases the disulfide-mediated dimerization of PKG (bottom). Data are reported as percentages of monomers and dimers of total PKG for each condition; n = 8. C: Western blot (top) and summary data (bottom) showing that hypoxia decreases PKG-mediated VASP phosphorylation. VASP-P, phosphorylated VASP. Data are reported as the percentage of VASP phosphorylation measured in 20 mM KCl-contracted BPAs under aerobic conditions; n = 8. *P < 0.05 vs. Ctrl; #P < 0.05 vs. Ctrl (dimer).
Hypoxia increases dimerization and PKG activity in BCAs precontracted with KCl.
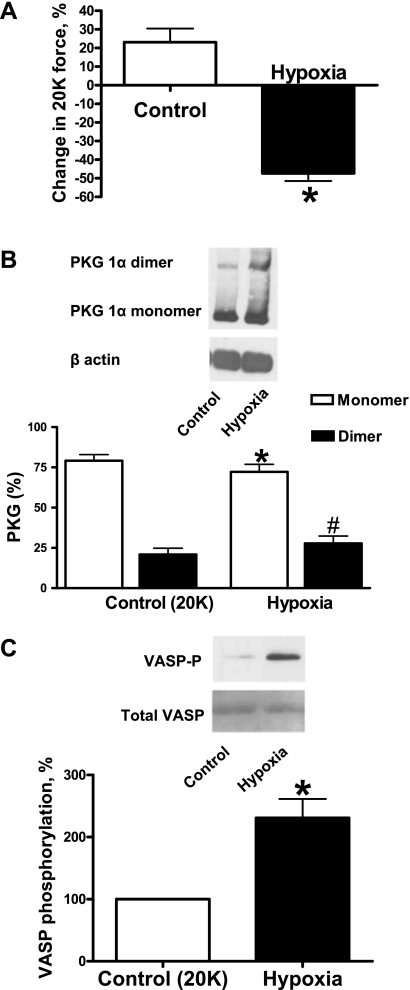
Since hypoxia was found to reduce dimerization in BPAs, we examined if there was a change in dimerization in BCAs exposed to hypoxia. Similar to BPAs, BCAs were precontracted with 20 mM K+ under aerobic conditions before exposure to hypoxia resulting from changing the gassing in the baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr). Hypoxia caused the relaxation of BCAs (Fig. 2A) and induced disulfide dimerization of PKG (Fig. 2B). Phosphorylation of VASP was also increased under hypoxia (Fig. 2C).
Fig. 2.
Effects of hypoxia in promoting the relaxation of bovine coronary arteries (BCAs) associated with disulfide-mediated dimerization of PKG and stimulation of PKG-dependent phosphorylation of VASP using 20 mM K+ as the precontracting agent. A: relaxations seen under prolonged aerobic conditions (Ctrl) and under hypoxia normalized to the initial 20 mM K+ force under aerobic conditions (n = 11). B: Western blot (top) showing that hypoxia increases the dimerization of PKG and summary data showing that hypoxia increases the disulfide-mediated dimerization of PKG (bottom; n = 10). C: Western blot (top) and summary data (bottom) showing that hypoxia increases PKG-mediated VASP phosphorylation (n = 5). *P < 0.05 vs. Ctrl; #P < 0.05 vs. Ctrl (dimer).
Effects of depleting sGC in BPAs on force generation to KCl under aerobic conditions and the contraction elicited by hypoxia.
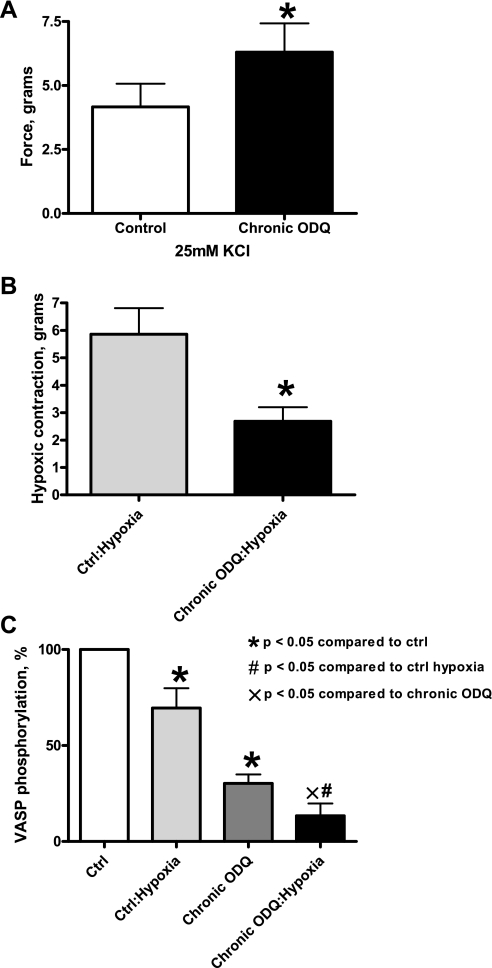
Treatment of BPAs with an oxidant of sGC heme (10 μM ODQ) for 48 h was found to promote a marked (∼85%) depletion of sGC without altering the expression of the contractile apparatus protein α-actin or the effects of peroxide on PKG dimerization (21). When sGC was depleted by organoid culture with ODQ, force development to 25 mM was increased compared with BPA organoid cultured in the absence of ODQ (Fig. 3A), presumably by eliminating the cGMP-mediated relaxation to endogenous peroxide seen under aerobic conditions (4, 5). The contraction to hypoxia was observed to be attenuated by sGC depletion via organoid culture treatment with ODQ (Fig. 3B). In addition, ODQ-elicited depletion of sGC attenuated VASP phosphorylation under aerobic conditions, with hypoxia decreasing VASP phosphorylation even further (Fig. 3C).
Fig. 3.
Effects of chronic 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) treatment on K+ contraction, hypoxic contraction, and VASP phosphorylation. A: contraction to 25 mM K+ was enhanced by the presence of 10 μM ODQ during 48 h of organoid culture in BPAs under aerobic conditions (n = 6). B: contraction to hypoxia was attenuated in the presence of 10 μM ODQ during 48 h of organoid culture in BPAs (n = 7). C: Western blot analysis showing decreased PKG-mediated VASP phosphorylation in ODQ-mediated depletion of soluble guanylate cyclase (sGC) under aerobic conditions, with hypoxia lowering it even further (n = 5–8). *P < 0.05 vs. Ctrl; #P < 0.05 vs. Ctrl (hypoxia); ×P < 0.05 vs. chronic ODQ.
Thiol-reducing conditions increase BPA force generation to KCl under aerobic conditions and decrease PKG dimerization, VASP phosphorylation, and the contraction elicited by hypoxia.
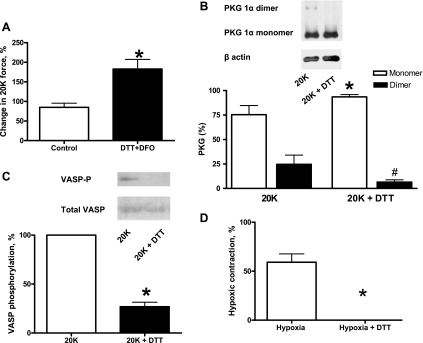
Exposure of BPAs to 1 mM DTT (with 5 mM deferoxamine) 20 min before hypoxia and subsequent contraction with 20 mM K+ was used to enhance thiol-reducing conditions. Deferoxamine was included to chelate the traces of iron that are present in physiological buffers to attenuate iron-mediated oxidation of DTT and H2O2. DTT-treated BPAs showed increased contraction to 20 mM K+ under aerobic conditions (Fig. 4A). The presence of DTT also attenuated disulfide-mediated dimerization (Fig. 4B) and PKG activity based on detection of lower levels of VASP phosphorylation under aerobic conditions (Fig. 4C). Hypoxic contraction was abolished in DTT-treated BPAs (Fig. 4D). To rule out the possibility that increased aerobic force directly attenuated force development to hypoxia, the effects of hypoxia were also studied in BPAs precontracted with 20 mM KCl and subsequently further contracted with 10 nM serotonin. Under conditions of an ∼60% increase in force, the contraction to hypoxia was 5.08 ± 1.16 g, and this was not significantly different from the contraction of 4.95 ± 0.80 g observed in the presence of only 20 mM KCl (n = 4).
Fig. 4.
Effects of the thiol reductant DTT on K+ force, disulfide-mediated dimerization of PKG, and VASP phosphorylation under aerobic conditions and hypoxic contraction. A: contraction to 20 mM K+ normalized to 20 mM K+ (before treatment) was increased in the presence of 1 mM DTT (n = 7). B: typical Western blot (top) and summary data (bottom) showing that 1 mM DTT reduces the dimerization of PKG (n = 8). C: Western blot (top) and summary data (bottom) showing that 1 mM DTT decreases VASP phosphorylation by PKG (n = 7). D: contraction to hypoxia normalized to 20 mM K+ (before treatment) was abolished in the presence of 1 mM DTT (n = 7). Deferoxamine (DFO; 5 mM) was included in experiments with DTT to suppress its oxidation by iron impurities in buffers. *P < 0.05 vs. Ctrl; #P < 0.05 vs. Ctrl (dimer).
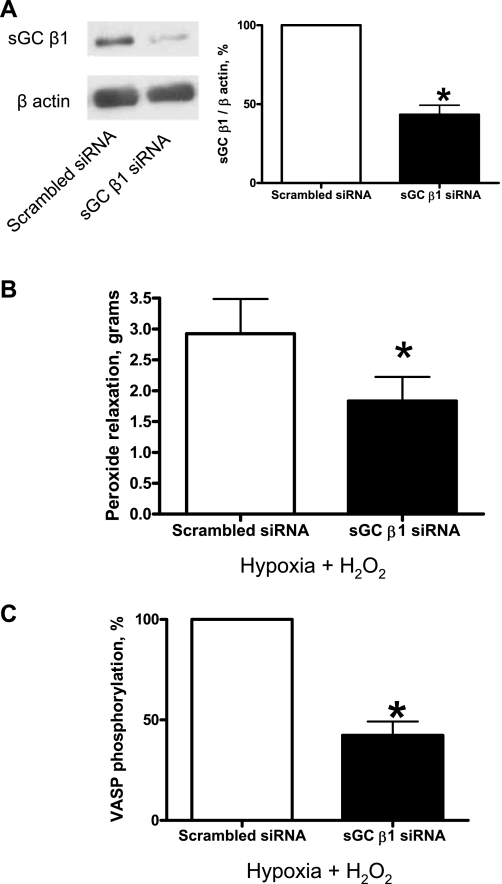
siRNA knockdown of the sGC β1-subunit in BPAs showed decreased H2O2 relaxation and decreased VASP phosphorylation.
sGC β1-subunit siRNA transfection of BPAs for 48 h resulted in decreased sGC-β1 protein expression (Fig. 5A). sGC-β1 siRNA-transfected BPAs were precontracted with 25 mM K+ under aerobic conditions before exposure to hypoxia by changing the gassing in the tissue baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr), and 0.1 mM H2O2 was added. sGC-β1 siRNA-transfected BPAs demonstrated decreased peroxide relaxation (Fig. 5B). These experiments were conducted under hypoxia to remove the effects of endogenous peroxide on PKG relaxing mechanisms. The previously described (21) elevation of VASP phosphorylation by peroxide under hypoxia was also decreased in sGC-β1 siRNA-transfected BPAs compared with the scrambled siRNA control (Fig. 5C).
Fig. 5.
Effects of small interfering (si)RNA knockdown of the sGC β1-subunit in BPAs on the response to H2O2 relaxation and VASP phosphorylation. A: Western blot analysis showing that sGC-β1 siRNA transfection for 48 h decreased expression of the sGC β1-subunit (n = 12). B: with sGC-β1 siRNA transfection, relaxation to H2O2 was attenuated (n = 12) and VASP phosphorylation (C) was decreased (n = 11) compared with the scrambled siRNA control. Decreases in force associated with the relaxation to peroxide were not reported as percent relaxation because sGC depletion caused an increase in the contraction to 25 mM KCl (see Fig. 6A). *P < 0.05 vs. Ctrl.
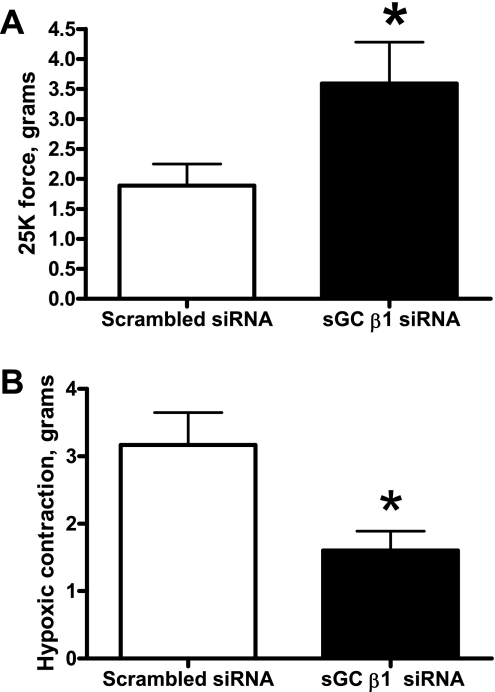
siRNA knockdown of the sGC β1-subunit in BPAs showed increased 25 mM K+ force and decreased hypoxic contraction.
sGC-β1 siRNA-transfected BPAs were precontracted with 25 mM K+ under aerobic conditions before exposure to hypoxia by changing the gassing in the tissue baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr). sGC-β1 siRNA-transfected BPAs demonstrated increased 25 mM K+ force under aerobic conditions (Fig. 6A). Hypoxic contraction was attenuated in sGC-β1 siRNA-transfected BPAs compared with the scrambled siRNA control (Fig. 6B).
Fig. 6.
Effects of siRNA knockdown of the sGC β1-subunit in BPAs on the response to K+ and hypoxia. With sGC-β1 siRNA transfection, the contraction to 25 mM K+ was enhanced (n = 11; A) and the contraction to hypoxia was attenuated (n = 12; B) compared with the scrambled siRNA control. *P < 0.05 vs. Ctrl.
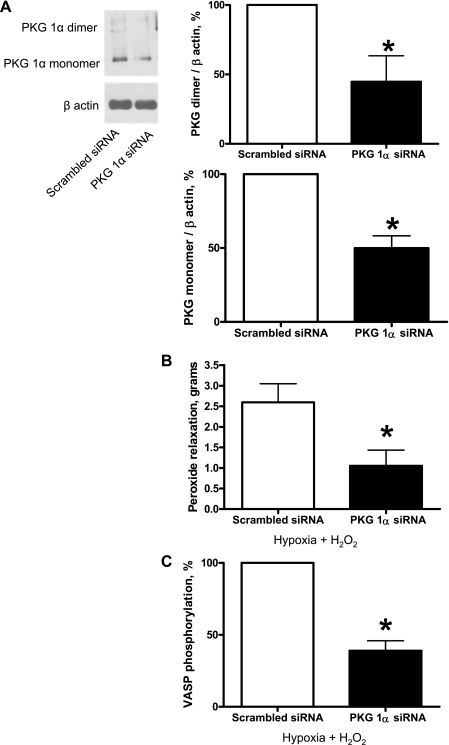
siRNA knockdown of PKG-1α in BPAs showed decreased H2O2 relaxation and decreased VASP phosphorylation.
PKG-1α siRNA transfection of BPA for 48 h resulted in decreased PKG-1α monomer and dimer protein expression (Fig. 7A). PKG-1α siRNA-transfected BPA were precontracted with 25 mM K+ under aerobic conditions before exposure to hypoxia by changing the gassing in the tissue baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr), and 0.1 mM H2O2 was added. PKG-1α siRNA-transfected BPAs demonstrated decreased peroxide relaxation (Fig. 7B). VASP phosphorylation was also decreased in PKG-1α siRNA-transfected BPAs compared with the scrambled siRNA control (Fig. 7C).
Fig. 7.
Effects of siRNA knockdown of PKG-1α in BPAs on the response to H2O2 relaxation and VASP phosphorylation. A: Western blot analysis showing that PKG-1α siRNA transfection for 48 h decreased expression of PKG-1α monomer and dimer subunits (n = 7). B: with PKG-1α siRNA transfection, relaxation to H2O2 was attenuated (n = 7) and VASP phosphorylation (C) was decreased (n = 7) compared with the scrambled siRNA control. Decreases in force associated with the relaxation to peroxide were not reported as percent relaxation because PKG depletion caused an increase in the contraction to 25 mM KCl (see Fig. 8A). *P < 0.05 vs. Ctrl.
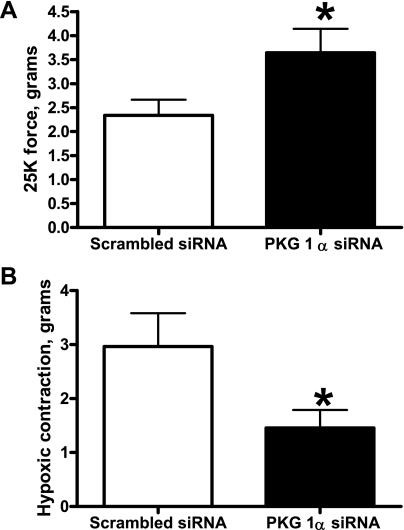
siRNA knockdown of PKG-1α in BPAs showed increased 25 mm K+ force and decreased hypoxic contraction.
PKG-1α siRNA-transfected BPAs were precontracted with 25 mM K+ under aerobic conditions before exposure to hypoxia by changing the gassing in the tissue baths from 21% O2-5% CO2-74% N2 to 5% CO2-95% N2 (Po2: ∼8–10 Torr). PKG-1α siRNA-transfected BPAs demonstrated increased 25 mM K+ force under aerobic conditions (Fig. 8A). Hypoxic contraction was attenuated in PKG-1α siRNA-transfected BPAs compared with the scrambled siRNA control (Fig. 8B).
Fig. 8.
Effects of siRNA knockdown of PKG-1α in BPAs on the response to K+ and hypoxia. With PKG-1α siRNA transfection, the contraction to 25 mM K+ was enhanced (n = 7; A) and the contraction to hypoxia was attenuated (n = 9; B) compared with the scrambled siRNA control. *P < 0.05 vs. Ctrl.
Nitric oxide stimulation of sGC is not involved in hypoxia-mediated contraction and dimerization in fresh BPAs.
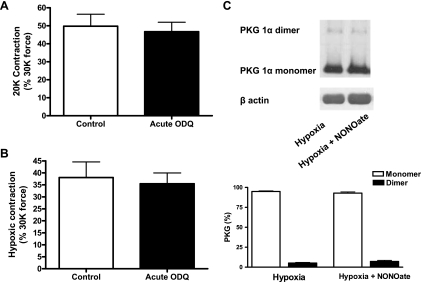
To rule out the involvement of nitric oxide in hypoxia-mediated contraction in fresh BPAs, vessels were incubated with 10 μM ODQ for 30 min before being contracted with K+. This acute treatment with ODQ did not alter the force generation to K+ under aerobic conditions (Fig. 9A) or the contraction to hypoxia (Fig. 9B). Since ODQ inhibits increases in BPA cGMP levels elicited by nitric oxide under hypoxic conditions (16), nitric oxide-mediated activation of sGC does not have a role in hypoxia-mediated contraction.
Fig. 9.
Effects of acute treatment of freshly isolated BPAs with the heme oxidant/sGC inhibitor 10 μM ODQ on force generation to K+ and on the contraction to hypoxia and effects of spermine NONOate on PKG dimerization under hypoxia. A and B: summary data showing that 10 μM ODQ did not alter the force generation to K+ under normoxia normalized to 30 mM K+ (30K) force (n = 8; A) or the contraction to hypoxia normalized to 30K force (n = 8; B). C: Western blot analysis showing that disulfide-mediated dimerization in the presence of 1 μM spermine NONOate was not altered (top) and summary data showing that spermine NONOate had no effects on the disulfide-mediated dimerization of PKG (bottom; n = 8).
The involvement of hypoxia promoting contraction in fresh BPAs through a suppression of nitric oxide regulating PKG dimerization was further examined because nitric oxide can produce relaxation under hypoxia through mechanisms not involving increases in cGMP or subject to inhibition by acute treatment with ODQ (16). When BPAs precontracted with 20 mM K+ were exposed to relaxation with 1 μM spermine NONOate, the exposure to nitric oxide did not alter the relationship between PKG monomer and dimer formation under hypoxia (Fig. 9C). Thus, disulfide-mediated dimerization of PKG does not participate in the cGMP-independent relaxation of BPAs to nitric oxide under hypoxia.
DISCUSSION
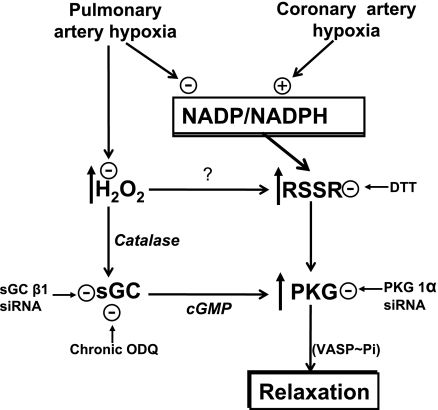
This study provides evidence that removal of both cGMP-dependent and cGMP-independent mechanisms of PKG activation (as shown in Fig. 10) appear to participate in the contraction of BPAs to hypoxia. The depletion of sGC increased aerobic force generation by KCl and attenuated further contraction to hypoxia and decreases in VASP phosphorylation. This, together with results from our previous studies (5, 6), suggests that a contributing factor to hypoxia-induced BPA contraction is hypoxia removing an O2-dependent tonic depression of KCl-induced force by peroxide stimulating sGC- and cGMP-dependent activation of PKG. Hypoxia also promoted reduced dimerization of PKG associated with decreased VASP phosphorylation, suggesting that removal of this cGMP-independent mechanism of PKG activation (3, 21) could also be participating in the contraction of BPAs elicited by hypoxia. These observations are in marked contrast to hypoxia eliciting relaxation of BCAs in a manner associated with increased PKG dimerization and increased VASP phosphorylation, suggesting that mechanisms stimulating PKG can also participate in the relaxation of coronary arteries to hypoxia.
Fig. 10.
Model showing the how peroxide metabolism and cytosolic NADPH redox potentially control both cGMP-dependent and cGMP-independent mechanisms of PKG activation that appear to be contributing to the mechanism of BPA contraction and BCA relaxation to hypoxia. RSSR represents the process controlling the dimerization of PKG subunits.
Our previous work provided evidence for hypoxia suppressing a peroxide-mediated relaxation that is normally present in pulmonary arteries under aerobic conditions (1, 2, 5, 6, 19). When sGC has been depleted, aerobic force generation is increased and VASP phosphorylation is decreased in a manner consistent with removing a tonic cGMP-stimulated PKG-mediated relaxation (as shown in Figs. 3 and 6). In addition, the contraction and magnitude of the decrease in VASP phosphorylation in response to hypoxia were attenuated by depletion of sGC. Since the depletion of sGC does not alter PKG subunit dimerization (21) (data not shown), it appears that the depletion of sGC does not alter this cGMP-independent mechanism of PKG activation. The depletion of PKG-1α also increased aerobic force generation and inhibited the contraction to hypoxia. These data further support the role of decreases in PKG activity in mediating the contraction of BPAs to hypoxia. The absence of an inhibitory effect of an acute addition of ODQ on the contraction to hypoxia confirms previous reports (3, 13) showing that the stimulation of sGC and PKG activation by dimerization by peroxide are not altered by this inhibitor of sGC activation. In addition, the absence of an effect of acute exposure to ODQ on the contraction to hypoxia also provides evidence that nitric oxide regulation of sGC is not involved in the response that is observed. Moreover, since nitric oxide can relax BPAs through a mechanism not inhibited by acute ODQ or associated with increases in cGMP (16), the data showing that spermine NONOate caused relaxation without increasing PKG dimerization further suggests that changes in endogenous nitric oxide do not influence changes in PKG dimerization elicited by hypoxia. The detection of thiol reduction with DTT decreasing dimerization of PKG and PKG activation under conditions where it increased aerobic force and attenuated the contraction to hypoxia suggests that removal of this cGMP-independent mechanism of PKG activation may also contribute to the contraction that is observed.
Our studies in BPAs contracted with KCl suggest hypoxia decreases peroxide (1, 2, 5) and increases cytosolic NADPH (11), which could be two factors coordinating the decrease in PKG dimerization that is observed. Decreasing peroxide could promote reversal of PKG dimerization by removing the conditions promoting thiol oxidation, whereas cytosolic NADPH redox is likely to also control thiol redox mechanisms influencing PKG dimerization through its influence of NADPH-dependent glutathione and thioredoxin reductases (20, 22). These systems control glutathione and thioredoxin redox, which are known local regulators of the thiol redox status on proteins (8). For example, our recent study (22) suggests that promoting oxidation of cytosolic NADPH by inhibiting glucose-6-phosphate dehydrogenase can increase PKG dimerization and VASP phosphorylation under hypoxic conditions where relaxation was observed in BPAs precontracted with 20 mM KCl. Thus, the control of thiol redox by both peroxide and cytosolic NADPH levels are potential key factors in the regulation of processes influencing the dimerization-mediated activation of PKG (see Fig. 10).
The relaxation of BCAs to hypoxia seems to begin with a peroxide-independent (9, 18) metabolic stress promoting the oxidation of cytosolic NADPH (12), which appears to coordinate multiple mechanisms lowering intracellular Ca2+ (10). Since the data in the present study detected dimerization of PKG under conditions where hypoxia promoted the relaxation of BCAs precontracted with 20 mM KCl, it appears that PKG activation could be a contributing factor to coordinating mechanisms through which hypoxia and cytosolic NADPH oxidation promote relaxation. When we tried to deplete sGC by organoid culture of BCAs with the sGC heme oxidant 10 μM ODQ, we found that organoid culture itself converted the response of BCAs to hypoxia from relaxation to a contraction resembling the response seen in BPAs. This adaptation of BCAs to organoid culture made it difficult to access the role of both cGMP-dependent and cGMP-independent mechanisms of PKG activation using either chronic ODQ or siRNA treatments. It has previously been observed that prolonged incubation of the rabbit aorta caused an adaptation that converted the response to hypoxia from relaxation to contraction (7). In addition, organoid culture of porcine coronary arteries was reported to attenuate the detection of relaxation to hypoxia (25).
This study provides evidence that inhibition of PKG activation through both cGMP-dependent and cGMP-independent mechanisms appear to be prominent factors in the mechanism of BPA contraction to hypoxia, and increased PKG dimerization could be a contributing factor to the relaxation of BCAs to hypoxia. Since hypoxia elicits increases in NADPH in BPAs and decreases in NADPH in BCAs, changes in cytosolic NADPH redox could be a factor in controlling the direction of changes in the balance between various systems influencing thiol redox that are likely to regulate PKG in the vascular O2-sensing mechanisms studied. Overall, based on our previous work on the involvement of decreased peroxide in the contractile response to BPA to hypoxia (1, 2, 5, 6) and the effects of sGC depletion examined in the present study, suppression of the peroxide regulation of sGC appears to be a dominant factor in the hypoxic pulmonary vasoconstriction response seen in BPAs. While much controversy exists regarding the redox changes and effector systems contributing to the responses of pulmonary and systemic arteries to hypoxia (17, 24, 26, 27), the multiple redox mechanisms influencing the activity of PKG and systems controlled by PKG regulating vascular force (14) make it an attractive system for coordinating responses to hypoxia.
GRANTS
This study was support by National Heart, Lung, and Blood Institute Grants HL-31069, HL-43023, and HL-66331.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Portions of this study were presented at the 2010 Experimental Biology Meeting in Anaheim, CA (21a).
REFERENCES
- 1. Ahmad M, Kelly MR, Zhao X, Kandhi S, Wolin MS. Roles for Nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol 298: H1879–H1888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad M, Zhao X, Kelly MR, Kandhi S, Perez O, Abraham NG, Wolin MS. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecSOD. Am J Physiol Heart Circ Physiol 297: H1453–H1461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIα enables oxidant-induced activation. Science 317: 1393–1397, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Burke-Wolin TM, Wolin MS. H2O2 and cGMP may function as an O2 sensor in the pulmonary artery. J Appl Physiol 66: 167–170, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Burke-Wolin TM, Wolin MS. Inhibition of cGMP-associated pulmonary arterial relaxation to H2O2 and O2 by ethanol. Am J Physiol Heart Circ Physiol 258: H1267–H1273, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Detar R, Bohr DF. Contractile responses of isolated vascular smooth muscle during prolonged exposure to anoxia. Am J Physiol 222: 1269–1277, 1972 [DOI] [PubMed] [Google Scholar]
- 8. Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287: C246–C256, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Gao Q, Zhao X, Ahmad M, Wolin MS. Mitochondrial-derived hydrogen peroxide inhibits relaxation of bovine coronary arterial smooth muscle to hypoxia through stimulation of ERK MAP kinase. Am J Physiol Heart Circ Physiol 297: H2262–H2269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupte SA, Arshad M, Viola S, Kaminski PM, Ungvari Z, Rabbani G, Koller A, Wolin MS. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol Heart Circ Physiol 285: H2316–H2326, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Gupte RS, Rawat DK, Chettimada S, Cioffi DL, Wolin MS, Gerthoffer WT, McMurtry IF, Gupte SA. Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction. J Biol Chem 285: 19561–19571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupte SA, Wolin MS. Hypoxia promotes relaxation of bovine coronary arteries through lowering cytosolic NADPH. Am J Physiol Heart Circ Physiol 290: H2228–H2238, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Iesaki T, Gupte SA, Kaminski PM, Wolin MS. Inhibition of guanylate cyclase stimulation by NO and bovine arterial relaxation to peroxynitrite and H2O2. Am J Physiol Heart Circ Physiol 277: H978–H985, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91: 1421–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch JP, Schmidt HH, Müller-Esterl W. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 105: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Mingone CJ, Gupte SA, Iesaki T, Wolin MS. Hypoxia enhances a cGMP-independent nitric oxide relaxing mechanism in pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 285: L296–L304, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 98: 390–403, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Mohazzab-H KM, Agarwal R, Wolin MS. Influence of glutathione peroxidase on coronary artery responses to alterations in Po2 and H2O2. Am J Physiol Heart Circ Physiol 276: H235–H241, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Mohazzab-H KM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery Po2 sensor. Am J Physiol Lung Cell Mol Physiol 267: L823–L831, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Neo BH, Kandhi S, Ahmad M, Wolin MS. Redox regulation of guanylate cyclase and protein kinase G in vascular responses to hypoxia. Respir Physiol Neurobiol 174: 259–264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neo BH, Kandhi S, Wolin MS. Roles for soluble guanylate cyclase and a thiol oxidation-elicited subunit dimerization of protein kinase G in pulmonary artery relaxation to hydrogen peroxide. Am J Physiol Heart Circ Physiol 299: H1235–H1241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a. Neo BH, Kandhi S, Wolin MS. Decreased protein kinase G activation by disulfide-mediated subunit dimerization is observed when hypoxia promotes contraction of bovine pulmonary arteries. FASEB J 24: 795.–4., 2010 [Google Scholar]
- 22. Neo BH, Wolin M. Potential role of NADPH redox in regulating thiol oxidation-elicited subunit dimerization activation of protein kinase G in the relaxation of bovine pulmonary arteries to pentose phosphate pathway inhibitors. FASEB J 24: 795.–5., 2010 [Google Scholar]
- 23. Smolenski A, Bachmann C, Reinhard K, Hönig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem 273: 20029–20035, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Thorne GD, Ishida Y, Paul RJ. Hypoxic vasorelaxation: Ca2+-dependent Ca2+-independent mechanisms. Cell Calcium 36: 201–208, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Thorne GD, Shimizu S, Paul RJ. Hypoxic vasodilation in porcine coronary artery is preferentially inhibited by organ culture. Am J Physiol Cell Physiol 281: C24–C32, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Weir EK, Olschewski A. Role of ion channels in acute and chronic responses of the pulmonary vasculature to hypoxia. Cardiovasc Res 71: 630–641, 2006 [DOI] [PubMed] [Google Scholar]