Abstract
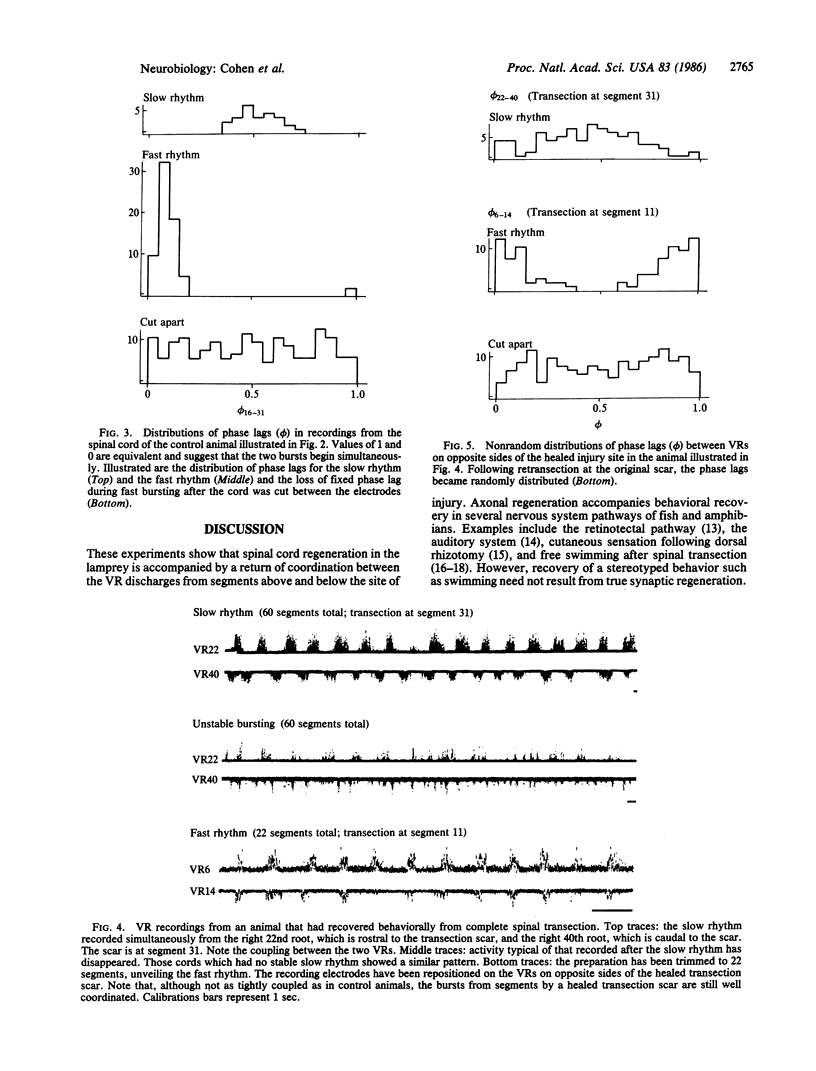
Axons in the larval sea lamprey can regenerate across the site of a spinal cord transection and form functioning synapses with some of their normal target neurons. The animals recover normal-appearing locomotion, but whether the regenerating axons and their synaptic connections are capable of playing a functional role during this behavior is unknown. To test this, "fictive" swimming was induced in the isolated spinal cord by the addition of D-glutamate to the bathing solution. Ventral root discharges of segments above and below a healed transection showed a high degree of phase-locking. This strongly suggests that the behavioral recovery is mediated by regenerated functional synaptic connections subserving intersegmental coordination of the central pattern generator for locomotion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers J., Carpenter G. A., Currie S., Kinch J. Which behavior does the lamprey central motor program mediate? Science. 1983 Sep 23;221(4617):1312–1314. doi: 10.1126/science.6137060. [DOI] [PubMed] [Google Scholar]
- Bernstein J. J., Bernstein M. E. Effect of glial-ependymal scar and teflon arrest on the regenerative capacity of goldfish spinal cord. Exp Neurol. 1967 Sep;19(1):25–32. doi: 10.1016/0014-4886(67)90004-0. [DOI] [PubMed] [Google Scholar]
- Cohen A. H., Holmes P. J., Rand R. H. The nature of the coupling between segmental oscillators of the lamprey spinal generator for locomotion: a mathematical model. J Math Biol. 1982;13(3):345–369. doi: 10.1007/BF00276069. [DOI] [PubMed] [Google Scholar]
- Cohen A. H., Wallén P. The neuronal correlate of locomotion in fish. "Fictive swimming" induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41(1):11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Currie S. N., Ayers J. Regeneration of locomotor command systems in the sea lamprey. Brain Res. 1983 Nov 21;279(1-2):238–240. doi: 10.1016/0006-8993(83)90183-x. [DOI] [PubMed] [Google Scholar]
- David S., Aguayo A. J. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981 Nov 20;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Grillner S., McClellan A., Sigvardt K. Mechanosensitive neurons in the spinal cord of the lamprey. Brain Res. 1982 Mar 4;235(1):169–173. doi: 10.1016/0006-8993(82)90208-6. [DOI] [PubMed] [Google Scholar]
- Lee M. T. Regeneration and functional reconnection of an identified vertebrate central neuron. J Neurosci. 1982 Dec;2(12):1793–1811. doi: 10.1523/JNEUROSCI.02-12-01793.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler S. A., Selzer M. E. Regeneration of functional synapses between individual recognizable neurons in the lamprey spinal cord. Science. 1985 Aug 23;229(4715):774–776. doi: 10.1126/science.2992085. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Regeneration of Müller and Mauthner axons after spinal transection in larval lampreys. J Comp Neurol. 1976 Aug 15;168(4):545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Sah D. W., Frank E. Regeneration of sensory-motor synapses in the spinal cord of the bullfrog. J Neurosci. 1984 Nov;4(11):2784–2791. doi: 10.1523/JNEUROSCI.04-11-02784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer M. E. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol. 1978 Apr;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenevi U., Björklund A., Svendgaard N. A. Transplantation of central and peripheral monoamine neurons to the adult rat brain: techniques and conditions for survival. Brain Res. 1976 Sep 10;114(1):1–20. doi: 10.1016/0006-8993(76)91003-9. [DOI] [PubMed] [Google Scholar]
- Wallén P., Williams T. L. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J Physiol. 1984 Feb;347:225–239. doi: 10.1113/jphysiol.1984.sp015063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. R., Cohen M. J. Synaptic regeneration and glial reactions in the transected spinal cord of the lamprey. J Neurocytol. 1981 Feb;10(1):57–79. doi: 10.1007/BF01181745. [DOI] [PubMed] [Google Scholar]
- Wood M. R., Cohen M. J. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science. 1979 Oct 19;206(4416):344–347. doi: 10.1126/science.482943. [DOI] [PubMed] [Google Scholar]
- Yin H. S., Selzer M. E. Axonal regeneration in lamprey spinal cord. J Neurosci. 1983 Jun;3(6):1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon H., Capranica R. R. Reformation of organized connections in the auditory system after generation of the eighth nerve. Science. 1981 Jul 10;213(4504):242–244. doi: 10.1126/science.6972599. [DOI] [PubMed] [Google Scholar]




