Abstract
The ubiquitin-proteasome system (UPS) is responsible for the degradation of most cellular proteins. Alterations in cardiac UPS, including changes in the degradation of regulatory proteins and proteasome functional insufficiency, are observed in many forms of heart disease and have been shown to play an important role in cardiac pathogenesis. In the past several years, remarkable progress in understanding the mechanisms that regulate UPS-mediated protein degradation has been achieved. A transgenic mouse model of benign enhancement of cardiac proteasome proteolytic function has been created. This has led to the first demonstration of the necessity of proteasome functional insufficiency in the genesis of important pathological processes. Cardiomyocyte-restricted enhancement of proteasome proteolytic function by overexpression of proteasome activator 28α protects against cardiac proteinopathy and myocardial ischemia-reperfusion injury. Additionally, exciting advances have recently been achieved in the search for a pharmacological agent to activate the proteasome. These breakthroughs are expected to serve as an impetus to further investigation into the involvement of UPS dysfunction in molecular pathogenesis and to the development of new therapeutic strategies for combating heart disease. An interplay between the UPS and macroautophagy is increasingly suggested in noncardiac systems but is not well understood in the cardiac system. Further investigations into the interplay are expected to provide a more comprehensive picture of cardiac protein quality control and degradation.
Keywords: proteasome activator, proteinopathy, ischemia-reperfusion injury, ubiquitin ligase, congestive heart failure
this article is part of a collection on Protein Handling. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
In eukaryotic cells, the degradation of most proteins, both native and misfolded, is done by the ubiquitin-proteasome system (UPS). In general, UPS-mediated proteolysis involves tagging the target protein molecule with a polyubiquitin chain and degrading the ubiquitinated protein by the proteasome. By targeted and timely degradation of normal proteins that are no longer needed, the UPS regulates virtually all cellular processes and functions. Through the removal of misfolded proteins, the UPS plays a pivotal role in protein quality control (PQC) (132). Hence, an adequately functional UPS is vital to proteostasis in the cell, especially in the terminally differentiated cells such as neurons and cardiomyocytes. Not surprisingly, UPS dysfunction has been implicated in the progression of at least a large subset, if not all, of heart diseases to congestive heart failure (CHF) (81). A major pathogenic role of proteasome functional insufficiency (PFI) has been recently demonstrated in not only cardiac proteinopathy but also in myocardial ischemia-reperfusion (I/R) injury in mice (77). With the recent description of a pharmacological activator of the 26S proteasome (73) and a genetic means to benignly enhance proteasome-mediated degradation of misfolded/abnormal proteins in cardiomyocytes (77, 78), it is highly anticipated that developing more effective measures to treat heart diseases through mitigating cardiac UPS function will soon become a new paradigm of cardiac research. In this review article, we will highlight the most exciting major discoveries in UPS-mediated protein degradation in general as well as the new advances in the area of cardiac UPS research reported in the past several years, with an emphasis on discussing an overarching hypothesis that PFI plays a major pathogenic role in the development of most heart diseases to CHF.
Ubiquitin, Ubiquitination, and Deubiquitination
Ubiquitin is the earliest described small modifier protein that can be covalently attached to the side chain of a lysine residue of the target protein via a process known as ubiquitination. Other examples of the small modifier proteins include SUMO1, NEDD8 (Rub1 in S. cerevisiae), and ISG15. Ubiquitination is carried out by a cascade of biochemical reactions catalyzed by the ubiquitin activating enzyme (E1), conjugating enzyme (E2), and ligases (E3). Substrate specificity of ubiquitination is conferred by the E3. Ubiquitination is increasingly recognized as an important posttranslational modification that can not only target a protein for degradation, as it was first discovered, but also change the activity or function of a protein without affecting its fate. Ubiquitination can occur to another ubiquitin that is either free or already attached to a target protein, thereby generating a polyubiquitin chain. Polyubiquitination of a target protein can be achieved by multiple rounds of ubiquitination to attach one ubiquitin at a time to the preceding ubiquitin or by attaching a preassembled polyubiquitin chain to the target protein. Each of the seven lysine residues of ubiquitin (K6, K11, K27, K29, K33, K48, and K63) can be potentially used for forming the ubiquitin chain. The different linkages give rise to different topology of a polyubiquitin chain and thereby render different effects on the substrate proteins. K48 polyubiquitin chains are the most common type and predominantly signal for degradation by the proteasome. On the other hand, K63-linked polyubiquitination cannot target the substrate protein for degradation but rather alter the activity and trafficking of the modified protein. It has been shown in yeast that all non-K63 linkages may target the protein for degradation (145).
The rate-limiting step and substrate specificity of ubiquitination are conferred by the E3. E3 binds directly to the specific target protein. A ubiquitin E3 can be a single protein but more often consists of multiple proteins. A E3 enzyme contains one of the following three domains: the homologous to the E6-AP carboxyl terminus (HECT) domain, the really interesting new gene (RING) domain, or the U-box domain, which is closely related to the RING domain. The largest family of ubiquitin E3 ligases is the cullin-RING ubiquitin ligases (CRLs). During the activation of CRLs, the neddylation of the scaffold protein cullin is triggered by the binding of the F-box protein to the substrate and brings the substrate to a closer proximity to the E2 enzyme (11, 118). Hence, cullin neddylation and deneddylation regulate the catalytic dynamics of CRLs. Since the degradation of many cell cycle regulators are mediated by CRLs, drugs (e.g., MLN4924) inhibiting neddylation have been developed and are in clinical trials to treat cancer (129). Notably, perturbation of cullin deneddylation via perinatal cardiomyocyte-restricted disruption of the COP9 signalosome, a major cullin deneddylation enzyme, causes massive cardiomyocyte necrosis, dilated cardiomyopathy, and premature death in mice, illustrating an indispensable role of CRLs in postnatal cardiac development and function (131).
Muscle atrophy F-box (MAFbx, also known as atrogin-1) and the muscle-specific RING finger proteins (MuRFs) are among the most investigated ubiquitin ligases in the heart. Both MAFbx and MuRFs are involved in the degradation of sarcomeric and nonsarcomeric regulatory proteins in the heart. MuRF1 expression is upregulated in hypertrophic and failing hearts. This upregulation may contribute to pathological remodeling because a high level of transgenic MuRF1 overexpression in mouse hearts increases the propensity to heart failure during pressure overload (144). On the other hand, MuRF1 is essential to the reverse remodeling of a hypertrophic heart. MuRF1 expression was found to be significantly increased in the unloaded hearts of patients who had received a left ventricular assist device. The regression of cardiac hypertrophy (i.e., cardiac atrophy), after pressure overload is relieved, was delayed remarkably in MuRF1 knockout mice compared with the wild-type controls (143). MuRF1 can bind and ubiquitinate the muscle creatine kinase (CK) (67), especially the oxidized CK (147). Increased muscle CK protein levels and decreased CK activities were observed in MuRF1 knockout mice and MuRF1-overexpressing mouse hearts, respectively (67, 144). It appears that MuRF1 may control both protein quality and activity of CK and thereby impacts on energy metabolism in cardiomyocytes.
As an F-Box protein in muscle cells, MAFbx interacts with Skp1 and cullin1 to form the Skp1-cullin1-F-box (SCF) type of ubiquitin ligases in which the F-box protein recruits specific substrate proteins for ubiquitination (18). MAFbx can target calcineurin for degradation and suppress calcineurin-nuclear factor of activated T cell (NFAT) signaling in cultured cells (75). MAFbx was recently shown to degrade a familial hypertrophic cardiomyopathy-associated truncated cardiac myosin binding protein C (91). MAFbx was upregulated in pressure overload hypertrophied hearts and hypoxic hearts (112, 139). A similar upregulation was also observed in myocardial infarction-induced CHF in rats (1), but more recently, both MuRF1 and MAFbx were found decreased in the myocardium remote of recent infarction in human hearts (21). Transgenic overexpression of MAFbx in the heart was shown to suppress the thoracic aortic constriction-induced increase of interventricular septal thickness in systole but exacerbate left ventricular dilatation and reduced cardiac function (75). Using MAFbx knockout mice, Sadoshima and colleagues (139) have recently demonstrated that the endogenous MAFbx mediates pathological hypertrophy and cardiac failure induced by thoracic aortic constriction or β-adrenergic stimulation, most likely through enhancing proteasome-mediated degradation of IκB and thereby activating NF-κB. Activation of the forkhead box O (FOXO) family of transcription factors was known to mediate the transcription of MAFbx upon phosphatidylinositol 3-kinase/Akt inactivation during muscle atrophy (122, 130). Very interestingly, MAFbx was recently found to suppress exercise-induced Akt-dependent cardiac hypertrophy in mice through coactivating FOXO transcription factors in a polyubiquitination-dependent manner (76), suggesting that MAFbx exerts a feed-forward regulation over the activation of atrophic genes including itself in physiological hypertrophy. These seemingly conflicting reports suggest that MAFbx suppress physiological hypertrophy while mediating pathological hypertrophy and failure, making it an attractive therapeutic target.
Deubiquitination, the counter process of ubiquitination, is driven by deubiquitination enzymes (DUBs) and represents another important layer of regulation on ubiquitination and proteasome-mediated proteolysis. Very few reports have touched on DUBs in the heart. A significant increase in the protein levels of ubiquitin carboxyl-terminal hydrolase (UCH) was detected in explanted failing human hearts with dilated cardiomyopathy (142). Abro1 (also known as KIAA0157), a subunit of the BRCC36-containing isopeptidase complex (BRISC) DUB enzyme predominantly expressed in the heart, was significantly upregulated in mouse hearts with myocardial I/R. Abro1 protein expression was found to be significantly increased in the infarct area but not the remote area of explanted human hearts with ischemic heart disease. This study further demonstrates that the upregulation of Abro1 leads to K63-specific deubiquitination of specific protein targets (20), consistent with the known DUB property of BRISC (22). The (patho)physiological significance of DUBs in the heart remains to be investigated.
To date, examinations of human hearts with end-stage heart failure of virtually all etiology, as well as the animal hearts of experimental cardiomyopathies, have consistently shown marked increases in ubiquitinated proteins, but the cause underlying the increase remains elusive (132). PFI and/or deregulation in ubiquitination and/or deubiquitination are all potential causes.
Proteasomes
In general, the degradation of polyubiquitinated proteins is performed by the 26S proteasome, a protease complex composed of a barrel-shaped 20S proteasome and the regulatory particle or activation complex (often the 19S proteasome) at one or both ends of the 20S proteasome. The 20S proteasome is formed by an axial stack of four heptameric rings: two opposing β-rings sandwiched by two outer α-rings. Each α- or β-ring contains seven unique subunits (α1 through α7, and β1 through β7). The 19S proteasome, whose constituents are less defined, recognizes and removes the ubiquitin chain on the target protein molecules, unfolds them, and channels the unfolded polypeptides into the proteolytic chamber of the 20S proteasome. Proteasome proteolytic activities reside in the interior face of the β-rings of the 20S. The β5-, β2-, and β1-subunits are respectively responsible for the chymotrypsin-like, trypsin-like, and caspase-like (also known as peptidylglutamyl-peptide hydrolase) activities, the three peptidase activities identified in the 20S proteasome. The α-rings serve as the gate to control the entry of polypeptides into the proteolytic chamber and regulate the exit of proteolytic products.
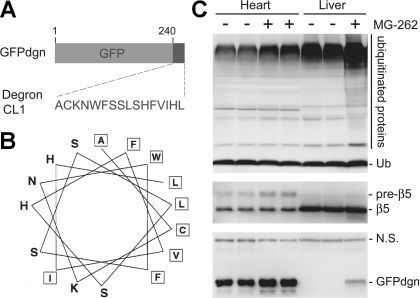
Currently, the assessment of proteasome proteolytic function mainly uses two types of substrates: the synthetic small fluorogenic compounds and the full-length protein substrates. Furthermore, two kinds of small fluorogenic substrates are currently available: the first kind is the most commonly used and emits quantifiable fluorescent signals when being cleaved selectively by a proteasome peptidase (116), whereas the other becomes fluorescent when irreversibly bound to the active peptidase subunits of the proteasome (140). Assays employing these fluorogenic compounds can only measure the peptidase activities of the proteasome. They cannot discern whether the proteasome proteolytic function in the cell is adequate to meet cellular needs to maintain protein homeostasis and PQC under a given circumstance. Proteasome peptidase activities can substantially increase in response to inadequate UPS proteolytic function, but the increased activity does not always sufficiently meet an increased demand. Hence, PFI and increased proteasome peptidase activities may coexist in a pathological setting. To detect PFI, changes in the protein levels of known proteasome substrates are monitored as a measure to evaluate alterations in proteasome function. Increased protein levels of endogenous bona fide substrates of the proteasome (e.g., IκB or β-catenin) are often used as an indicator of decreased proteasome function. However, a major limitation is associated with this method: the synthesis of endogenous proteins is highly regulated and may be increased by the condition being studied, which can confound the data interpretation. To circumvent the limitation, surrogate full-length protein substrates for the UPS have been developed by modifying fluorescent proteins. For instance, one such modified green fluorescent protein (GFP) was created by carboxyl fusion of degron CL1 to GFP and known as GFPu or GFPdgn. GFPu/GFPdgn is considered a surrogate of misfolded proteins because degron CL1 bears a stretch of surface-exposed hydrophobic amino acid residues (37), a signature conformation of misfolded polypeptides (132). When moderately expressed, these reporter GFPs are biologically inert, well tolerated by the cell, and easy to be detected. Driven by a constitutive promoter, their synthesis is much less influenced by a pathological condition, compared with the endogenous proteins. A transgenic mouse model of ubiquitous and constitutive expression of GFPdgn has been created and validated as an inverse reporter of proteasome functional status (Fig. 1). The use of GFPdgn mice has remarkably promoted experimental investigations into the involvement of UPS dysfunction in cardiac pathogenesis (8, 15, 29, 70, 77, 83).
Fig. 1.
A transgenic mouse model expressing carboxyl fusion of degron CL1 to green fluorescent protein (GFPdgn) as a surrogate misfolded protein. A: an illustration of the design of GFPdgn transgene and the sequence of degron CL1. GFPdgn is a fusion protein derived from carboxyl fusion of an enhanced GFP with degron CL1, which contains the depicted 16 amino acid residues. B: sequence and helical wheel representation of degron CL1 [adopted from Gilon et al. with permission (37)]. The hydrophobic amino acid residues are boxed. Note that in its helical structure, degron CL1 contains surface exposure of a long patch of hydrophobic amino acid residues. C: representative images of Western blot analysis of the indicated proteins in cardiac and hepatic tissues from adult GFPdgn transgenic mice (line 3) 20 h after an intraperitoneal injection of proteasome inhibitor MG-262 (5 μmol/kg) or the vehicle control. At the baseline, GFPdgn is expressed at a higher level in the heart than in the liver. MG-262 treatment increases ubiquitinated proteins, the precursor of the β5-subunit of the 20S proteasome (pre-β5) and GFPdgn proteins in both organs shown here. Ub, ubiquitin; NS, not significant.
Regulations on the Proteasome
It becomes apparent that the assembly and activities of the proteasome are well adjusted by the cell to harmonize with its energy metabolism and to accommodate the needs of various cellular responses to internal and external stresses (44, 71). As revealed by functional proteomics, proteasome composition and activities among different organs of mice show striking variations, perhaps to meet the substrate diversity (125). This is echoed by a recent study by Kloss et al. (61), which showed that different subpopulations of proteasomes enriched from rat myocardium responded differentially to proteasome inhibitors in vitro. Hence, a better understanding of the regulation on proteasome proteolytic function is a prerequisite for a productive search for new measures to manipulate the proteasome to treat disease, especially PFI-associated diseases.
ATP and energy metabolism.
UPS-mediated degradation of target proteins requires ATP. The intracellular ATP level is normally in the low millimolar range, depending on cell types (40). Interestingly, the optimal ATP concentration for in vitro proteasome peptidase activity assays is lower than the physiological level of ATP in the cell by a factor of ∼10 (105). Huang et al. (48) have recently shown that pharmacologically reducing ATP production in cultured cells yields bidirectional effects on the UPS-mediated degradation of bona fide endogenous substrates and GFPu with a significant initial increase of proteasomal degradation when ATP is moderately decreased, followed by a decrease of proteasomal degradation upon ATP depletion. It is therefore proposed that under normal baseline conditions, the physiological levels of intracellular ATP may gear the proteasome activity at a relatively low level, allowing a rapid increase in proteasome function upon a moderate ATP reduction during an acute stress (48). This proposition remains to be tested in intact animals but is consistent with the fact that myocardial proteasome peptidase activities are often increased during the initial phase of pressure overload cardiac hypertrophy and acute myocardial infarction (27). This moderate ATP reduction-mediated proteasome activation may potentially be important for the cell to timely remove misfolded proteins under an acute stress as well as during the early phase of a long-lasting stress. In the latter case, this regulation conceivably helps the cell to maintain protein homeostasis before the more sustainable compensatory mechanisms, such as increasing proteasome synthesis and/or altered proteasome composition, come into effect.
Posttranslational modifications of proteasome subunits.
A variety of posttranslational modifications in many subunits of cardiac proteasomes are revealed by proteomic analyses (125). Moreover, the molecular events exerting the modifications and the functional consequences of the modifications have begun to be unraveled. cAMP-dependent protein kinase A (PKA) and protein phosphatase 2A (PP2A) were copurified with intact murine cardiac 20S proteasomes (151). The inhibition of PP2A or the addition of PKA clearly altered the phosphorylation profiles of the proteasome. It appears that hyperphosphorylated 20S proteasomes have a higher peptidase activity than the hypophosphorylated ones (32, 151). In a nonmyocyte system, PKA was shown to enhance proteasome functions through phosphorylating Ser120 of regulatory particle ATPase 6 (Rpt6) (146), a required subunit of the 19S proteasome. The stimulatory effect of PKA and the phosphorylation of Rpt6 were reversible by PP1γ (146). More recently, it was reported that PKA activation enhances myocardial 26S proteasome assembly and activity in dogs and that PKA mediates the enhancement of proteasome assembly and activity in the heart by ischemic preconditioning (IPC) (5). Failure to activate PKA appears to be responsible for the depressed caspase-like and trypsin-like peptidase activities of 20S proteasomes in the heart with β-adrenergic stimulation-induced cardiac hypertrophy (30). Protein kinase N (PKN), a stress-activated protein kinase, can also increase cardiac proteasome chymotrypsin-like activity, and this increase seems to be essential to the protection of PKN against myocardial I/R injury (133). It remains to be elucidated how PKN increases proteasome activities.
Proteasome-associated partners.
DUBs associated with the 19S proteasome have important effects on proteasome proteolytic function. Regulatory particle non-ATPase 11 (RPN11) is a stoichiometric DUB subunit of the 19S proteasome. During degradation of polyubiquitinated proteins, RPN11 removes the ubiquitin chain en bloc by cutting at the base of the ubiquitin chain, frees the substrate, and promotes substrate translocation into the 20S, while recycling the ubiquitin. The association of two other DUBs, UCH37 and ubiquitin-specific protease 14 (USP14), with 19S proteasomes is reversible. In contrast to RPN11, USP14 and UCH37 reduce the length of the ubiquitin chain from its tip distal to the substrate, thereby shortening the chain rather than removing the chain en bloc, which slows down the proteasome-mediated degradation of the ubiquitinated substrates. Recently, an exciting breakthrough was achieved by screening for small-molecule compounds that inhibit USP14 deubiquitination. A chemical inhibitor of USP14 was discovered and shown to enhance proteasome-mediated degradation of the tested proteasome substrates, including some of the proteins linked to neural degenerative disease (73). This may represent the invention of the first small-molecule proteasome activator, although proteasome inhibitors have been clinically used to treat disease (2). It will be interesting and important to determine whether the proteasome activator can enhance proteasome function in vivo and whether it is safe and efficacious to be used to treat diseases with PFI.
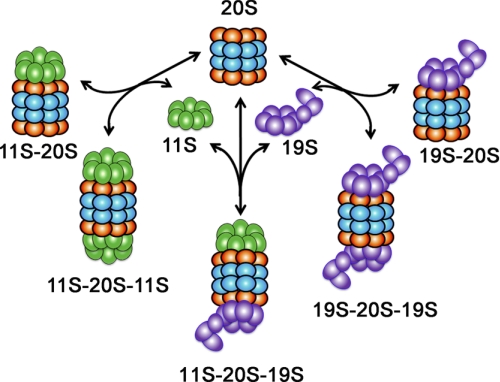
As in many other cell types, some of the 20S proteasome in cardiomyocytes is also associated with the 11S proteasome (Fig. 2), also known as proteasome activator 28 (PA28) or 11S regulator (113, 135). The 11S proteasome consists of either a heteroheptamer of PA28α and PA28β (α3β4 or α4β3) or a homoheptamer of PA28γ molecules (113). A 20S proteasome can be simultaneously capped by a 19S at one end and by an 11S at the other, forming a hybrid proteasome. The association of the 11S with the 20S proteasome was generally reported to enhance antigen processing during viral infection (97), but recent studies show that PA28γ can mediate the degradation of several endogenous regulatory proteins by the 20S proteasome (17, 79, 80). Myocardial PA28α and PA28β protein levels were increased in rats with experimental diabetes (107). Overexpression of PA28α stabilized PA28β and thereby upregulates 11S proteasomes in cultured cardiomyocytes and nonmyocytes. More importantly, proteasomal degradation of GFPu, but not endogenous or overexpressed normal proteins that are UPS substrates, was markedly enhanced by PA28α overexpression. Furthermore, hydrogen peroxide treatment induced increases in protein carbonyls, and cell death was significantly attenuated by PA28α overexpression in cultured cardiomyocytes (78). These exciting findings suggest that the upregulation of 11S proteasomes by PA28α overexpression can enhance proteasome-mediated removal of misfolded/damaged proteins, promote PQC, and thereby protect against oxidative stress. More excitingly, these in vitro findings have been confirmed in mice in vivo (see Establishing PFI as major pathogenic factor in the heart).
Fig. 2.
A schematic depiction of proteasome complexes expressed in mammalian cells. The 20S proteasome harbors all the proteolytic activities of the proteasome. To degrade proteins, the 20S needs to team up with the 19S and/or the 11S proteasome activator.
Proteasome assembly and composition.
Quite a few subunits of mammalian proteasomes have different isoforms (39). The activities of cardiac proteasomes assembled from different isoforms of the subunits show discernible variations (31). Each of the three peptidase subunits (β1, β2, and β5) of the 20S proteasome can be replaced by an inducible isoform (β1i, β2i, and β5i, respectively) upon viral infection or interferon treatment. Proteasomes containing the inducible subunit are referred to as immunoproteasomes. Immunoproteasomes show higher peptidase activities and may enhance antigen processing during viral infection. It turns out that interferons also increase the production of reactive oxygen species, thereby increasing the production of oxidized damaged proteins. The enhanced proteolytic activity of immunoproteasomes facilitates the clearance of damaged proteins and protects against the oxidative stress (49, 104, 126). When the assembly of immunoproteasome is impaired by the ablation of the β5i (PSMB8, LMP7) gene, interferon treatment or inflammation causes an accumulation of polyubiquitinated proteins and protein aggregates in the cell (126). The synthesis of immunoproteasomes, PA28α and PA28β, as well as 20S proteasomes, can be induced by oxidative stress (104). These recent research findings unveil a previously unrecognized role of immunoproteasomes in PQC under stress conditions.
A concerted increase in the synthesis of proteasomal subunits in response to proteasome inhibition or PFI was observed in cultured cells and implicated in the heart (15, 92). Nuclear factor erythroid-derived 2-related factor 1 (NRF1), a transcription factor of the cap “n” collar basic leucine zipper family, was recently shown to play an essential role in this feedback loop (74, 111). The proteasome inhibitor-induced proteasome bounce-back response in mouse embryonic fibroblasts was abolished by Nrf1 knockout, whereas overexpression of Nrf1 facilitated the proteasome recovery of the proteasome inhibitor-treated cells. Promoter-reporter assays revealed that Nrf1 activates the transcription of proteasome genes via the antioxidant response elements in the proteasome subunit genes. NRF1 knockdown in human cancer cells exacerbated the cell killing effect of a proteasome inhibitor (111). Neuronal deletion of Nrf1 in mice decreased proteasome gene expression, impaired proteasome function, and led to neurodegeneration (74). The role of NRF1 in cardiac PQC remains to be determined.
Proteasome Dysfunction in Cardiac Diseases
Perhaps with the exception of viral myocarditis and anthracycline-induced cardiomyopathy (70, 85, 86), the majority of the studies using animal models have suggested that cardiac proteasome function is impaired/insufficient during the progression of a large subset of heart diseases to CHF. This is best illustrated by cardiac proteinopathy and myocardial I/R injury, although proteasome malfunction has also been observed in other types of cardiomyopathy (6, 81, 110). Indeed, the establishment of a mouse model of cardiomyocyte-restricted proteasome functional enhancement has recently enabled experimentation that establishes the necessity of PFI in the genesis of cardiac proteinopathy and I/R injury in mice (77), which marks the first direct demonstration of the pathogenic role of PFI in any organs or diseases.
PFI in cardiac proteinopathy.
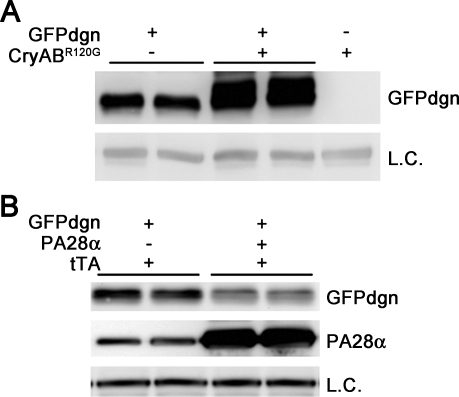
Proteinopathies are a family of diseases caused by misfolded proteins. The presence of aberrant protein aggregates in the cell is the hallmark of proteinopathy. The most studied cardiac proteinopathy is desmin-related cardiomyopathy [DRC; for recent review, see McLendon and Robbins (90) in this series]. By crossbreeding with the GFPdgn mice (Fig. 3A), severe PFI has been revealed in two transgenic mouse models of DRC (16, 83). The insufficiency mainly resides in the delivery of ubiquitinated proteins into the 20S proteasome as decreased protein abundance of critical 19S proteasome subunits, namely Rpt3 and Rpt5, are concurrently associated with the PFI, whereas the peptidase activities of the 20S proteasome are not decreased. Further studies have determined that aberrant protein aggregation is in part responsible for PFI in DRC hearts (16, 84). These are important findings, because although DRC per se is by no means a common disease, the DRC mice represent valuable models for pathogenic elucidation and therapeutic exploration of cardiac proteinopathies. Increasing reports suggest that a large subset of common heart diseases likely share pathogenic mechanisms with cardiac proteinopathy. Aberrant protein aggregates are also observed and considered the trigger of autophagy activation in the pressure-overloaded mouse heart (137). Significant increases in myocardial ubiquitinated proteins have been invariably reported in studies using this experimental method, although some of the reports dispute the direction of changes in the proteasome peptidase activities as well as the effect of systemic proteasome inhibition on the hypertrophic responses and function of pressure-overloaded hearts (47, 136). Both the accumulation of aberrant protein aggregates and elevated levels of ubiquitinated proteins are consistent with PFI, but the sufficiency of proteasome function in a pressure-overloaded heart has never yet been directly assessed. Recent studies on human hearts with end-stage heart failure of common etiologies have unveiled a very high prevalence of aberrant protein aggregates in the form of preamyloid oligomers in the diseased hearts (35, 119). When coexisted with increased ubiquitinated proteins, aberrant protein aggregation can be both a sign of PFI and a potential cause of proteasome impairment. Hence, these observations place a large subset of CHF into the category of cardiac proteinopathy.
Fig. 3.
Use of GFPdgn reporter mice reveals proteasome functional insufficiency (PFI, A) and proteasome functional enhancement (B) in the heart. A: representative image of Western blot analyses for GFPdgn in the GFPdgn/CryABR120G double transgenic mouse heart, compared with littermate GFPdgn single transgenic mouse hearts. The soluble fraction of myocardial protein extracts from 1-mo-old mice of the indicated genotypes (top labels) were used for the analyses. PFI in the CryABR120G-based desmin-related cardiomyopathy mouse heart is revealed by the increased GFPdgn protein levels in the double transgenic mouse hearts. B: representative image of Western blot analyses for GFPdgn and proteasome activator 28α (PA28α) in the heart. Total proteins were extracted from ventricular myocardium of littermate mice of the indicated genotypes (labels at the top of the panel) at 8 wk of age and used for Western blot analyses for the indicated proteins. Note that GFPdgn protein levels were markedly decreased in PA28α overexpression hearts, indicating that proteasome proteolytic function in the heart is significantly enhanced by cardiomyocyte-restricted overexpression of PA28α. LC, loading control; tTA, tetracycline-controlled transactivator.
PFI in myocardial I/R injury.
Myocardial I/R injury represents a major pathological process in both the development and therapeutic intervention of ischemic heart disease. The earliest indications that the proteasome may become dysfunctional following ischemia were from studies of the brain, which reported increases in mitochondrial insoluble ubiquitin-conjugates and decreased 26S proteasome activity in gerbil cortex and hippocampus following transient forebrain ischemia (45, 57). Subsequent studies of brain have confirmed and extended these studies and suggested that the proteasome dysfunction may be secondary to oxidative stress (4, 59).
In 2001, Bulteau et al. (12) first reported decreased proteasome chymotryptic, caspase- and trypsin-like activity following 30 min of in vivo left anterior descending coronary artery occlusion. This was accompanied by increased ubiquitinated proteins, yet following purification, only the decrease in trypsin-like activity was discernible. Powell and coworkers (105, 108) subsequently confirmed this observation in the isolated perfused heart model and demonstrated that ATP-dependent proteasome activity is preferentially affected, suggesting defects in 26S proteasome function, consistent with increases in myocardial ubiquitinated proteins. Various mechanisms have been proposed to explain the dysfunction, but recent experimental findings support two potential explanations: 1) ATP depletion during ischemia and 2) oxidation of proteasome and/or regulatory subunits.
ATP is required for docking and activation of the proteasome and for proper function of the ubiquitination machinery (128). A recent study (48) has provided evidence that intracellular ATP levels regulate proteasome activity. It stands to reason that ATP depletion during ischemia (56) could be partially responsible for decreased proteasome activity in the ischemic heart. Two recent studies (5, 19) have invoked this mechanism to explain decreased postischemic proteasome activity observed in their models. However, if a lack of ATP was the underlying mechanism for the dysfunction, it would be expected that adding ATP back would reverse the dysfunction. Both studies measured proteasome activity in the presence of ATP, yet the dysfunction was still present. We have shown that even when measured in the presence of optimal concentrations of ATP, postischemic proteasome activity is still diminished (105). While this does not necessarily mean that ATP depletion is not a contributing factor to proteasome dysfunction during the ischemic period, it suggests the presence of a more persistent defect in the postischemic proteasome.
Oxidative modification of proteins affects their secondary and tertiary structures, resulting in an unfolding and exposure of hydrophobic patches and leading to a loss of function and enhanced degradation (24, 25). During myocardial I/R, many cytosolic, myofibrillar, and mitochondrial proteins are subject to various oxidative modifications (60, 106), and it stands to reason that since the proteasome is composed of multiple protein subunits, it could be a potential target for oxidative attack. Many studies have evaluated the vulnerability of the 20S and 26S proteasomes and have shown that exposure of purified proteasome to oxidants, including peroxynitrite (3, 102), hypochlorite and hydrogen peroxide (114), and 4-hydroxynonenal (34, 101) inactivates the proteasome with the 26S configuration ∼10-fold more sensitive (114). Indeed, Bulteau et al. (12) reported 4-hydroxynonelation of several α-type subunits of the 20S proteasome following I/R, though the relationship to the decreased proteasome activity was not clear. In fact, proteasomes purified from rat heart seem to be somewhat resistant to inactivation by 4-hydroxynonenal, requiring concentrations in excess of 100 μM (33). Other studies have shown that pretreatment of isolated hearts with α-tocotrienol (a vitamin E analog) preserves postischemic proteasome function (23). The higher vulnerability of the 26S proteasome to oxidative inactivation (115) and the observations that the ATP-dependent activity of the proteasome is preferentially affected by I/R (19, 105) suggest that subunits of the 19S regulatory particle may be more sensitive to oxidative damage than those in the 20S. A recent study by Divald et al. (28) has identified Rpt3/Rpt5 as the only 26S proteasome subunits that are significantly carbonylated during myocardial I/R. This observation is consistent with a previous report showing that the Rpt3 subunit is sensitive to carbonylation reactions in SH-SY5Y cells exposed to an oxidative environment (54). It is of interest that Predmore et al. (110) have also observed that the same subunits appear to be oxidized in the ventricular tissue of explanted human hearts with end-stage heart failure.
IPC uses preexposure to short episodes of ischemia to decrease vulnerability of the myocardium to subsequent longer durations of ischemia, resulting in improved postischemic hemodynamic function and reduced markers of myocardial injury (98). Many mechanisms have been proposed, but current thinking is that IPC involves preischemic signaling changes that open the inward mitochondrial ATP-sensitive K+ channels (41) and prevent opening of the mitochondrial permeability transition pore (65). Three recent studies (5, 19, 28) have suggested that perhaps preserving proteasome function plays a role in IPC by facilitating some of the associated pre- or postischemic signaling changes. As discussed above, the UPS may become dysfunctional during ischemia; therefore, by necessity IPC must in some way preserve postischemic proteasome function. In fact, this had already been established by studies of IPC protocols tested to protect the brain from I/R injury, which showed less accumulation of proapoptotic proteins and protein aggregates (82, 93, 109). At about the same time of the brain IPC studies, Powell et al. demonstrated that pharmacological preconditioning of the myocardium with nicorandil (108), an agent thought to open the inward mitochondrial ATP-sensitive K+ channels (124), affords some protection of postischemic function of the proteasome. Nonetheless, all three of these recent studies do present convincing evidence that IPC does diminish postischemic dysfunction of the UPS, including improved peptidase activity and less accumulation of ubiquitinated or misfolded proteins (5, 19, 28). Where they differ is in the proposed mechanism as described below.
One potential mechanism is that IPC increases protein kinase A (PKA)-mediated activation of proteasome. As indicated earlier, PKA can phosphorylate several subunits of the 20S proteasome (151) as well as Rpt6 (146), which results in activation of proteasome peptidase activities. Asai et al. (5) present convincing evidence that PKA can enhance assembly of intact 26S proteasomes. They suggest that IPC results in transient preischemic increases in PKA which enhances assembly of intact 26S proteasome. Presumably, the increased assembled 26S proteasome then carries over to the postischemic period and explains the improved function of the UPS. While these investigators did indeed show higher peptidase activity in the immediate postischemic period, the assembly of intact proteasome at this time point was not assessed; thus it is not clear that they are related.
The second suggested mechanism is that IPC enhances proteasome-mediated degradation of protein kinase C-δ (PKC-δ) during reperfusion. Churchill et al. (19) have proposed that the UPS regulates the ratio between the prodeath kinase PKC-δ and the prosurvival kinase PKC-ε. This theory is based on reports that the inhibition of PKC-δ protects the heart from ischemic injury (52) and that IPC results in the activation and translocation of PKC-ε to the cardiac mitochondria (7). Churchill et al. (19) show that IPC does improve postischemic UPS function, which presumably leads to the observed altered ratios of PKC-δ to PKC-ε, since a proteasome inhibitor prevented this and blocked the protective effects. While this is a novel mechanism, it should be noted that several studies have shown that IPC can alter levels of both prosurvival and prodeath proteins, including phosphatase and tensin homolog on chromosome 10 (PTEN) (13, 14) and IκB (42) and have invoked regulation by the UPS. Indeed, Divald et al. (28) have recently shown that IPC decreases postischemic Bax levels through a UPS-mediated pathway. Given the many diverse pathways regulated by the UPS, that while important it is unlikely that only proteasome-mediated changes in PKC-δ account for the protective effects of IPC.
Finally, decreased postischemic oxidation of 19S regulatory particle subunits is the third proposed mechanism. As explained above, Rpt5 is carbonylated during myocardial ischemia (28). Since ischemia appears to have a preferential effect on the ATP-dependent activity of the proteasome and IPC appears to preferentially improve the ATP-dependent activity of the proteasome, Divald et al. examined whether IPC also decreased carbonylation of Rpt5 and did observe this to happen. The Rpt5 subunit plays pivotal roles in the attachment of the “base” of the 19S regulatory particle to 20S proteasome α-rings (36) and the binding of the 19S particle “lid” to the base (26). It is possible that by decreasing oxidation of this subunit, IPC improves docking of the 19S regulatory particle with the 20S proteasome. However, it is unclear that the improved peptidase activity by IPC is related to the decreased oxidation of just this one subunit.
Establishing PFI as major pathogenic factor in the heart.
As described earlier, PFI is observed in a variety of diseases, such as neural degenerative diseases, cardiac proteinopathy, and more recently a large subset of CHF. However, it has been impossible to directly test whether PFI plays a role in the development of any of these diseases because a proteasome activator was unavailable until very recently. With our recent discovery of a benign measure to enhance proteasome function, this situation is rapidly changing. Using cultured cardiomyocytes and HEK cells, we found that overexpressing PA28α (PA28αOE) stabilizes PA28β and thereby upregulates 11S proteasomes, which destabilizes GFPu, a surrogate misfolded protein substrate for the UPS (141), without affecting the protein levels of endogenous and ectopically overexpressed native proteins (78). Hence, this establishes the first benign measure to enhance proteasome proteolytic function in cultured cells. To demonstrate a biological relevance of this exciting new measure, we have further tested the effect of PA28αOE on oxidative stress. Enhancing proteasome function by PA28αOE was able to decrease the abundance of oxidized proteins and the prevalence of apoptosis in cultured cardiomyocytes triggered by hydrogen peroxide treatment (78). These findings suggest an important role of PFI in the injury by oxidative stress, which is a major cause of myocardial I/R.
To test whether PA28αOE enhances proteasome function in intact animals, we have created transgenic mouse lines to achieve temporally controllable cardiomyocyte-restricted (CR-) PA28αOE (77). Mice with CR-PA28αOE initiated at the perinatal stage do not show discernible changes in the expression of the fetal gene program, heart function, or cardiac morphology by at least one year of age, the longest time observed. Introducing GFPdgn into the CR-PA28αOE mice via crossbreeding, we were able to validate that CR-PA28αOE facilitates the degradation of GFPdgn, a surrogate misfolded protein (Fig. 3B). The effect on GFPdgn protein stability disappears when CR-PA28αOE is turned off (77). These experiments mark the establishment of the first genetic method and animal model to benignly enhance proteasome function in the heart or any organs. Taking advantage of this model, we have further demonstrated that an overexpression of PA28α significantly attenuates aberrant protein aggregation and cardiac hypertrophy and delays the premature death of a mouse model of cardiac proteinopathy (77). Moreover, we showed that CR-PA28αOE protects against myocardial I/R injury in intact mice (77). These studies prove for the first time an important pathogenic role of PFI in myocardial I/R injury and cardiac proteinopathy in both cell culture and intact animals. It is anticipated that these exciting discoveries, including the invent of new ways to benignly enhance proteasome function in a cell and an organ, will serve as an impetus to a better understanding of the pathogenic roles of UPS dysfunction and to developing new therapeutic strategies to treat a large subset of diseases, including heart diseases.
Potential mechanisms underlying the pathogenic role of PFI.
It appears that PFI can compromise the heart through a number of mechanisms, such as impairing PQC and disturbing signaling pathways. The sarcomere is the fundamental unit of mechanical function in the striated muscle to which cardiac muscle belongs. An extremely vigorous stoichiometry among different sarcomeric proteins is maintained for proper function of the sarcomere. Replacing a damaged protein in the sarcomere with a new one must be accurately coupled with the removal of the damaged protein. The degradation of virtually all myofibrillar proteins is carried out by the UPS. Hence, cardiac PFI will conceivably leave some of the defective proteins in the sarcomere not timely replaced, which will undoubtedly reduce the contractility of the sarcomere and ultimately the mechanical performance of the heart. In this regard, PFI may have a similar effect as the genetic mutations of the sarcomeric proteins, which are known to cause inherited cardiomyopathies (89), a major cause of CHF.
PFI inevitably accumulates misfolded proteins and leads to aberrant protein aggregation. The expression of misfolded proteins and resultant aberrant protein aggregation have proven to be sufficient to cause cardiomyopathy and CHF in both humans and mice (87, 103, 120). In addition to physical disruption to the cell, aberrant protein aggregates can be rather detrimental to multiple cellular processes. Aggregation-prone proteins can directly interact and inhibit proteasome proteolytic subunits (38, 68). In both cultured cardiomyocytes and intact mouse hearts, aberrant protein aggregation can impair proteasome proteolytic function (9, 15, 83, 84, 121, 123), thereby forming a vicious cycle.
Mitochondrial dysfunction and programmed cell death are often associated with CHF. Mitochondrial malfunction and increased cell death have been observed in the hearts of a transgenic mouse model of cardiac proteinopathy that displays severe PFI before other cardiac pathology becomes discernible (15, 88). Pharmacological proteasomal inhibition has been shown to induce cell death in cultured cardiomyocytes (29, 138).
The calcineurin-NFAT pathway is a well-established signaling pathway mediating pathological remodeling of the heart (96). Interestingly, both PFI and the loss of an important chaperone [e.g., heat shock protein B2 (HSPB2)/HSPB5 double knockout] can activate the calcineurin-NFAT pathway in the heart (69, 136), suggesting that PQC inadequacy may play a role in the activation of this pathway during pathological cardiac remodeling.
Interplay Between the UPS and Macroautophagy in Target Protein Degradation
Macroautophagy (commonly known as autophagy) sequesters a portion of cytoplasm (often including organelles, such as mitochondria) into a double-membrane vesicle (known as an autophagosome) for fusion with and degradation by lysosomes, representing another major catabolic pathway in the cell essential for cellular homeostasis in the heart (99). Nonselective autophagy during starvation helps the cell to temporarily survive an energy crisis by self-eating a portion of its cytoplasm (127, 134). Selective autophagy, on the other hand, serves as a major executor of quality control by removing aged/damaged organelles and perhaps aggregated proteins (94, 148, 150). Altered autophagic activities were observed in animal models of human disease including heart disease, implicated in failing human hearts (58), and are emerging as a potentially important pathogenic factor and therapeutic target (43, 100, 117, 127). The UPS and autophagy were considered two parallel pathways that do not interact, but emerging evidence is unveiling an intricate interplay between the two pathways (Fig. 4).
Fig. 4.
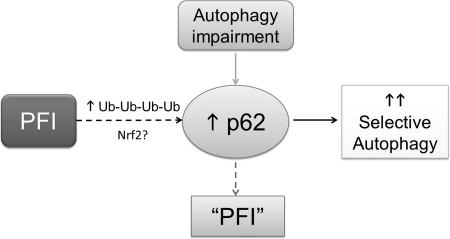
An illustration of a working hypothesis on the interplay between proteasome-mediated proteolysis and the selective autophagy. On the one hand, PFI accumulates ubiquitin conjugates which, through interaction, retain p62 proteins in the cell. PFI may also activate the nuclear factor erythroid-derived 2-related factor-1 (Nrf2)-mediated transcriptional program to activate p62 synthesis. The binding of p62 to ubiquitin conjugates in protein aggregates may recruit autophagosomes to and remove the aggregates, thereby increasing selective autophagy. On the other hand, chronic autophagy inhibition/impairment can accumulate p62, which is a substrate of autophagy. The accumulated p62 can bind to ubiquitinated proteins formed during normal ubiquitination and hinder them from being delivered to the proteasome for degradation; therefore, autophagy impairment may indirectly induce a PFI-like phenotype, although proteasome assembly and activities may not be decreased.
On the one hand, an accumulation of ubiquitinated proteins resulting from PFI not only leads to aberrant protein aggregation but also retains ubiquitin binding proteins, such as p62/SQSTM1. The increase of p62 protein levels during PFI may also result from an increase of p62 synthesis. The latter can be mediated by increased transactivation of NRF2, a major transcription factor for antioxidant genes. The p62 is a target gene of NRF2 and can further increase the activity of NRF2 in a feed-forward fashion (53, 55, 62, 72). Under the unstressed condition, the Kelch-repeat domain of Kelch-like ECH-associated protein 1 (KEAP1) functions as a NRF2-specific adaptor protein in the cullin3-based ubiquitin ligase complex and constitutively targets NRF2 for ubiquitination and proteasome-mediated degradation. Under oxidative stress, KEAP1 is modified through oxidation or adduction of one or more of its cysteine residues, resulting in a putative conformational change that decreases its ability to serve as an ubiquitin ligase substrate adaptor, thereby increasing NRF2 (46). Hence, PFI may directly stabilize NRF2. In addition, PFI may elicit oxidative stress, which in turn inactivates KEAP1 and thereby indirectly stabilizes NRF2. The increased p62, resulting from either the detainment by ubiquitinated proteins or increased synthesis, further increases NRF2 likely through preventing KEAP1 from binding NRF2 (62).
By interacting with microtubule-associated protein-1 light chain 3, a critical component of autophagosome, p62 may recruit autophagy to help remove the protein aggregates. Indeed, in both cultured cardiomyocytes and intact mice, we and others have observed activation of autophagy by pharmacological proteasome inhibition (137, 149). In cultured cardiomyocytes and intact mouse hearts overexpressing misfolded proteins, myocardial p62 protein levels and autophagic flux are markedly increased (148). The increase in p62 proteins is in part due to increased synthesis because p62 mRNA levels are also upregulated (148). Although the molecular mechanism underlying the increased p62 synthesis during PFI remains to be further delineated, it has been demonstrated in some noncardiac cell types that p62 plays a critical role in mediating selective autophagy (10, 50, 51).
On the other hand, can autophagy inhibition activate the UPS? In cardiomyocyte cultures, Tannous et al. (137) showed that chronic inhibition of autophagosome formation using 3-methyladenine increased an in vitro assayed proteasome peptidase activity, suggesting that autophagy impairment may activate the proteasome. However, by assessing the removal of both endogenous and ectopically overexpressed surrogate substrate proteins of the UPS, Korolchuk et al. (66) found in noncardiac cells that defective autophagy impaired the delivery of ubiquitinated proteins to the proteasome and thereby slowed down the degradation of UPS substrates, although neither the abundance nor the activities of the proteasome were altered. They further revealed that the accumulation of p62 was responsible for the impairment. p62 is degraded by selective autophagy (50); therefore, p62 accumulates when autophagic flux is impaired (95). Autophagic inhibition accumulates ubiquitin-positive protein aggregates in both hepatocytes and neurons and cause liver injury and neurodegeneration, respectively (63, 64). Genetic ablation of p62 attenuates the autophagic inhibition-induced aggregate accumulation, but this only ameliorates the liver injury and not neurodegeneration (64), suggesting that cellular responses to autophagic inhibition is likely cell-type specific.
Taken together, it appears that p62 is a nexus via which the proteasome-mediated proteolysis and autophagy interact with one another. Further investigation into the molecular underpinning of the interplay between the UPS and autophagy in cardiomyocytes will likely yield new insights into cardiac pathophysiology, helping identify new therapeutic targets to more effectively treat heart disease.
Conclusions and Future Directions
Although the molecular mechanisms underlying UPS dysfunction and mediating its pathogenic roles in the heart only begin to be unveiled, it has become apparent that altered proteolysis, especially PFI, may play an important role in the progression of a range of heart diseases to CHF. We believe that future research in this paradigm should be aimed at achieving a comprehensive understanding on 1) the mechanisms underlying UPS dysfunctions in various pathological conditions; 2) molecular pathways that govern the degradation of not only misfolded proteins but also key regulatory proteins or protein complexes; and 3) how different proteolytic systems including the UPS, autophagy, and other proteases in the cell are orchestrated to maintain protein homeostasis under physiological and pathological conditions. Advances in these areas will no doubt give rise to new strategies to captivate the power of targeted protein degradation for fighting heart disease.
GRANTS
This work is in part supported by National Heart, Lung, and Blood Institute Grants HL-072166 and HL-085629 (to X. Wang) and HL-0668936 (to S. R. Powell and X. Wang) and American Heart Association Grants 0740025N (to X. Wang), 0620032Z (to H. Zheng), and 11SDG6960011 (to H. Su).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.W. conception and design of research; X.W., H.Z., and S.R.P. analyzed data; X.W., J.L., H.Z., and S.R.P. interpreted results of experiments; X.W., J.L., and H.S. prepared figures; X.W. and S.R.P. drafted manuscript; X.W., H.S., and S.R.P. edited and revised manuscript; X.W., J.L., H.Z., H.S., and S.R.P. approved final version of manuscript; J.L. and H.Z. performed experiments.
ACKNOWLEDGMENTS
Dr. X. Wang is a recipient of an Established Investigator Award from the American Heart Association.
Present address of H. Zheng: Dept. of Anesthesia and Medicine, and Cardiovascular Division, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 02115.
REFERENCES
- 1.Adams V, Linke A, Wisloff U, Doring C, Erbs S, Krankel N, Witt CC, Labeit S, Muller-Werdan U, Schuler G, Hambrecht R. Myocardial expression of Murf-1 and MAFbx after induction of chronic heart failure: effect on myocardial contractility. Cardiovasc Res 73: 120–129, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ahmad K. Proteasome inhibitor for treatment of multiple myeloma. Lancet Oncol 6: 546, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Amici M, Lupidi G, Angeletti M, Fioretti E, Eleuteri AM. Peroxynitrite-induced oxidation and its effects on isolated proteasomal systems. Free Radic Biol Med 34: 987–996, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Asai A, Tanahashi N, Qiu JH, Saito N, Chi S, Kawahara N, Tanaka K, Kirino T. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J Cereb Blood Flow Metab 22: 705–710, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Asai M, Tsukamoto O, Minamino T, Asanuma H, Fujita M, Asano Y, Takahama H, Sasaki H, Higo S, Asakura M, Takashima S, Hori M, Kitakaze M. PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol 46: 452–462, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bahrudin U, Morisaki H, Morisaki T, Ninomiya H, Higaki K, Nanba E, Igawa O, Takashima S, Mizuta E, Miake J, Yamamoto Y, Shirayoshi Y, Kitakaze M, Carrier L, Hisatome I. Ubiquitin-proteasome system impairment caused by a missense cardiac myosin-binding protein C mutation and associated with cardiac dysfunction in hypertrophic cardiomyopathy. J Mol Biol 384: 896–907, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res 92: 873–880, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bence NF, Bennett EJ, Kopito RR. Application and analysis of the GFPu family of ubiquitin-proteasome system reporters. Methods Enzymol 399: 481–490, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bennett EJ, Bence NF, Jayakumar R, Kopito RR. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell 17: 351–365, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bjorkoy G, Lamark T, Johansen T. p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy 2: 138–139, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bornstein G, Ganoth D, Hershko A. Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc Natl Acad Sci USA 103: 11515–11520, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem 276: 30057–30063, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res 97: 1351–1359, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Cai ZP, Shen Z, Van Kaer L, Becker LC. Ischemic preconditioning-induced cardioprotection is lost in mice with immunoproteasome subunit low molecular mass polypeptide-2 deficiency. FASEB J 22: 4248–4257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Liu JB, Horak KM, Zheng H, Kumarapeli ARK, Li J, Li F, Gerdes AM, Wawrousek EF, Wang X. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res 97: 1018–1028, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Liu JB, Horak KM, Zheng H, Kumarapeli AR, Li J, Li F, Gerdes AM, Wawrousek EF, Wang X. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res 97: 1018–1026, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell 26: 843–852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chew EH, Hagen T. Substrate-mediated regulation of cullin neddylation. J Biol Chem 282: 17032–17040, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res 85: 385–394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cilenti L, Balakrishnan MP, Wang XL, Ambivero C, Sterlicchi M, Del Monte F, Ma XL, Zervos AS. Regulation of Abro1/KIAA0157 during myocardial infarction and cell death reveals a novel cardioprotective mechanism for Lys63-specific deubiquitination. J Mol Cell Cardiol 34: 652–661, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conraads VM, Vrints CJ, Rodrigus IE, Hoymans VY, Van Craenenbroeck EM, Bosmans J, Claeys MJ, Van Herck P, Linke A, Schuler G, Adams V. Depressed expression of MuRF1 and MAFbx in areas remote of recent myocardial infarction: a mechanism contributing to myocardial remodeling? Basic Res Cardiol 105: 219–226, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Cooper EM, Cutcliffe C, Kristiansen TZ, Pandey A, Pickart CM, Cohen RE. K63-specific deubiquitination by two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J 28: 621–631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S, Powell SR, Wang P, Divald A, Nesaretnam K, Tosaki A, Cordis GA, Maulik N, Das DK. Cardioprotection with palm tocotrienol: antioxidant activity of tocotrienol is linked with its ability to stabilize proteasomes. Am J Physiol Heart Circ Physiol 289: H361–H367, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Davies KJ, Delsignore ME. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem 262: 9908–9913, 1987 [PubMed] [Google Scholar]
- 25.Davies KJ, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem 262: 9914–9920, 1987 [PubMed] [Google Scholar]
- 26.DeMartino GN. Purification of PA700, the 19S regulatory complex of the 26S proteasome. Methods Enzymol 398: 295–306, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation 114: 1821–1828, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, Gomes AV, Powell SR. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ Res 106: 1829–1838, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, Harden NR, Li F, Gerdes AM, Wang X. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol 287: H1417–H1425, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res 107: 1094–1101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drews O, Wildgruber R, Zong C, Sukop U, Nissum M, Gomes AV, Ping P. Mammalian proteasome subpopulations with distinct molecular compositions and proteolytic activities. Mol Cell Proteomics 6: 2021–2031, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Drews O, Zong C, Ping P. Exploring proteasome complexes by proteomic approaches. Proteomics 7: 1047–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Farout L, Mary J, Vinh J, Szweda LI, Friguet B. Inactivation of the proteasome by 4-hydroxy-2-nonenal is site specific and dependant on 20S proteasome subtypes. Arch Biochem Biophys 453: 135–142, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett 405: 21–25, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, Rota M, Gwathmey JK, Dec GW, Aretz T, Leri A, Semigran MJ, Anversa P, Macgillivray TE, Tanzi RE, del Monte F. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 121: 1216–1226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillette TG, Kumar B, Thompson D, Slaughter CA, DeMartino GN. Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and activation of the 26 S proteasome. J Biol Chem 283: 31813–31822, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilon T, Chomsky O, Kulka RG. Degradation signals recognized by the Ubc6p-Ubc7p ubiquitin-conjugating enzyme pair. Mol Cell Biol 20: 7214–7219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg AL. On prions, proteasomes, and mad cows. N Engl J Med 357: 1150–1152, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, Jones RC, Thyparambil S, Wang GW, Qiao X, Bardag-Gorce F, Ping P. Mapping the murine cardiac 26S proteasome complexes. Circ Res 99: 362–371, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM. A novel method for measurement of submembrane ATP concentration. J Biol Chem 275: 30046–30049, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res 84: 973–979, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Gurusamy N, Goswami S, Malik G, Das DK. Oxidative injury induces selective rather than global inhibition of proteasomal activity. J Mol Cell Cardiol 44: 419–428, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res 104: 150–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna J, Meides A, Zhang DP, Finley D. A ubiquitin stress response induces altered proteasome composition. Cell 129: 747–759, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Hayashi T, Takada K, Matsuda M. Post-transient ischemia increase in ubiquitin conjugates in the early reperfusion. Neuroreport 3: 519–520, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34: 176–188, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Hedhli N, Depre C. Proteasome inhibitors and cardiac cell growth. Cardiovasc Res 85: 321–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H, Zhang X, Li S, Liu N, Lian W, McDowell E, Zhou P, Zhao C, Guo H, Zhang C, Yang C, Wen G, Dong X, Lu L, Ma N, Dong W, Dou QP, Wang X, Liu J. Physiological levels of ATP negatively regulate proteasome function. Cell Res 20: 1372–1385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussong SA, Kapphahn RJ, Phillips SL, Maldonado M, Ferrington DA. Immunoproteasome deficiency alters retinal proteasome's response to stress. J Neurochem 113: 1481–1490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ichimura Y, Kominami E, Tanaka K, Komatsu M. Selective turnover of p62/A170/SQSTM1 by autophagy. Autophagy 4: 1063–1066, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, Yamane T, Tanaka K, Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem 283: 22847–22857, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation 108: 2304–2307, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 193: 275–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 s proteasome. Biochemistry 44: 13893–13901, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285: 22576–22591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jennings RB, Steenbergen C., Jr Nucleotide metabolism and cellular damage in myocardial ischemia. Annu Rev Physiol 47: 727–749, 1985 [DOI] [PubMed] [Google Scholar]
- 57.Kamikubo T, Hayashi T. Changes in proteasome activity following transient ischemia. Neurochem Int 28: 209–212, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, Frazier OH, Taegtmeyer H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 120: S191–S197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab 20: 1467–1473, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Khaliulin I, Schwalb H, Wang P, Houminer E, Grinberg L, Katzeff H, Borman JB, Powell SR. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radic Biol Med 37: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Kloss A, Meiners S, Ludwig A, Dahlmann B. Multiple cardiac proteasome subtypes differ in their susceptibility to proteasome inhibitors. Cardiovasc Res 85: 367–375, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12: 213–223, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441: 880–884, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131: 1149–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Korge P, Honda HM, Weiss JN. Protection of cardiac mitochondria by diazoxide and protein kinase C: implications for ischemic preconditioning. Proc Natl Acad Sci USA 99: 3312–3317, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell 33: 517–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 376: 1224–1236, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Kristiansen M, Deriziotis P, Dimcheff DE, Jackson GS, Ovaa H, Naumann H, Clarke AR, van Leeuwen FW, Menendez-Benito V, Dantuma NP, Portis JL, Collinge J, Tabrizi SJ. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell 26: 175–188, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Kumarapeli AR, Su H, Huang W, Tang M, Zheng H, Horak KM, Li M, Wang X. Alpha B-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res 103: 1473–1482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumarapeli RKA, Horak KM, Glasford JW, Li J, Chen Q, Liu J, Zheng H, Wang X. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J 19: 2051–2053, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Kusmierczyk AR, Kunjappu MJ, Funakoshi M, Hochstrasser M. A multimeric assembly factor controls the formation of alternative 20S proteasomes. Nat Struct Mol Biol 15: 237–244, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol 30: 3275–3285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467: 179–184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee CS, Lee C, Hu T, Nguyen JM, Zhang J, Martin MV, Vawter MP, Huang EJ, Chan JY. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc Natl Acad Sci USA 108: 8408–8413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest 114: 1058–1071, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest 117: 3211–3223, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest 121: 3689–3700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28alpha overexpression protects against oxidative stress. FASEB J 25: 883–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell 26: 831–842, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O'Malley BW. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell 124: 381–392, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Li YF, Wang X. The role of the proteasome in heart disease. Biochim Biophys Acta 1809: 141–149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu C, Chen S, Kamme F, Hu BR. Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience 134: 69–80, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J 20: 362–364, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Liu J, Tang M, Mestril R, Wang X. Aberrant protein aggregation is essential for a mutant desmin to impair the proteolytic function of the ubiquitin-proteasome system in cardiomyocytes. J Mol Cell Cardiol 40: 451–454, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Zheng H, Tang M, Ryu YC, Wang X. A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome. Am J Physiol Heart Circ Physiol 295: H2541–H2550, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo H, Wong J, Wong B. Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc Res 85: 347–356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maloyan A, Gulick J, Glabe CG, Kayed R, Robbins J. Exercise reverses preamyloid oligomer and prolongs survival in alphaB-crystallin-based desmin-related cardiomyopathy. Proc Natl Acad Sci USA 104: 5995–6000, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation 112: 3451–3461, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marian AJ. Hypertrophic cardiomyopathy: from genetics to treatment. Eur J Clin Invest 40: 360–369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McLendon PM, Robbins J. Desmin-related Cardiomyopathy: An Unfolding Story. Am J Physiol Heart Circ Physiol 301: H1220–H1228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T, Carrier L. Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovasc Res 85: 357–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, Kruger E. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of Mammalian proteasomes. J Biol Chem 278: 21517–21525, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, Minami M, Schindler CK, Saugstad JA, Simon RP. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem 281: 7429–7436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murata S, Udono H, Tanahashi N, Hamada N, Watanabe K, Adachi K, Yamano T, Yui K, Kobayashi N, Kasahara M, Tanaka K, Chiba T. Immunoproteasome assembly and antigen presentation in mice lacking both PA28alpha and PA28beta. EMBO J 20: 5898–5907, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murry CE, Jennings RB, Reimer KA. New insights into potential mechanisms of ischemic preconditioning. Circulation 84: 442–445, 1991 [DOI] [PubMed] [Google Scholar]
- 99.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ 16: 31–38, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem 274: 23787–23793, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Osna NA, Haorah J, Krutik VM, Donohue TM., Jr Peroxynitrite alters the catalytic activity of rodent liver proteasome in vitro and in vivo. Hepatology 40: 574–582, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation 117: 2743–2751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J 432: 585–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Powell SR, Davies KJ, Divald A. Optimal determination of heart tissue 26S-proteasome activity requires maximal stimulating ATP concentrations. J Mol Cell Cardiol 42: 265–269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Powell SR, Gurzenda EM, Wahezi SE. Actin is oxidized during myocardial ischemia. Free Radic Biol Med 30: 1171–1176, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Powell SR, Samuel SM, Wang P, Divald A, Thirunavukkarasu M, Koneru S, Wang X, Maulik N. Upregulation of myocardial 11S-activated proteasome in experimental hyperglycemia. J Mol Cell Cardiol 44: 618–621, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Powell SR, Wang P, Katzeff H, Shringarpure R, Teoh C, Khaliulin I, Das DK, Davies KJ, Schwalb H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal 7: 538–546, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, Davalos A, Castillo J, Lorenzo P, Lizasoain I. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab 25: 193–203, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 121: 997–1004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell 38: 17–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Razeghi P, Baskin KK, Sharma S, Young ME, Stepkowski S, Essop MF, Taegtmeyer H. Atrophy, hypertrophy, and hypoxemia induce transcriptional regulators of the ubiquitin proteasome system in the rat heart. Biochem Biophys Res Commun 342: 361–364, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol 15: 27–33, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J 335: 637–642, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys 377: 65–68, 2000 [DOI] [PubMed] [Google Scholar]
- 116.Rodgers KJ, Dean RT. Assessment of proteasome activity in cell lysates and tissue homogenates using peptide substrates. Int J Biochem Cell Biol 35: 716–727, 2003 [DOI] [PubMed] [Google Scholar]
- 117.Rothermel BA, Hill JA. The heart of autophagy: deconstructing cardiac proteotoxicity. Autophagy 4: 932–935, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol Cell 32: 21–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, Robbins J. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci USA 101: 10132–10136, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanbe A, Osinska H, Villa C, Gulick J, Klevitsky R, Glabe CG, Kayed R, Robbins J. Reversal of amyloid-induced heart disease in desmin-related cardiomyopathy. Proc Natl Acad Sci USA 102: 13592–13597, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanbe A, Yamauchi J, Miyamoto Y, Fujiwara Y, Murabe M, Tanoue A. Interruption of CryAB-amyloid oligomer formation by HSP22. J Biol Chem 282: 555–563, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarikas A, Carrier L, Schenke C, Doll D, Flavigny J, Lindenberg KS, Eschenhagen T, Zolk O. Impairment of the ubiquitin-proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc Res 66: 33–44, 2005 [DOI] [PubMed] [Google Scholar]
- 124.Sato T, Sasaki N, O'Rourke B, Marban E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J Am Coll Cardiol 35: 514–518, 2000 [DOI] [PubMed] [Google Scholar]
- 125.Scruggs SB, Ping P, Zong C. Heterogeneous cardiac proteasomes: mandated by diverse substrates? Physiology (Bethesda) 26: 106–114, 2011 [DOI] [PubMed] [Google Scholar]
- 126.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, Prozorovski T, Lange N, Steffen J, Rieger M, Kuckelkorn U, Aktas O, Kloetzel PM, Kruger E. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142: 613–624, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem 284: 28319–28331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith DM, Fraga H, Reis C, Kafri G, Goldberg AL. ATP binds to proteasomal ATPases in pairs with distinct functional effects, implying an ordered reaction cycle. Cell 144: 526–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458: 732–736, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 131.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res 108: 40–50, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Su H, Wang X. The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective. Cardiovasc Res 85: 253–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takagi H, Hsu CP, Kajimoto K, Shao D, Yang Y, Maejima Y, Zhai P, Yehia G, Yamada C, Zablocki D, Sadoshima J. Activation of PKN mediates survival of cardiac myocytes in the heart during ischemia/reperfusion. Circ Res 107: 642–649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takemura G, Kanamori H, Goto K, Maruyama R, Tsujimoto A, Fujiwara H, Seishima M, Minatoguchi S. Autophagy maintains cardiac function in the starved adult. Autophagy 5: 1034–1036, 2009 [DOI] [PubMed] [Google Scholar]
- 135.Tanahashi N, Murakami Y, Minami Y, Shimbara N, Hendil KB, Tanaka Hybrid proteasomes K Induction by interferon-gamma and contribution to ATP-dependent proteolysis. J Biol Chem 275: 14336–14345, 2000 [DOI] [PubMed] [Google Scholar]
- 136.Tang M, Li J, Huang W, Su H, Liang Q, Tian Z, Horak KM, Molkentin JD, Wang X. Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotes maladaptive remodelling of stressed mouse hearts. Cardiovasc Res 88: 424–433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, Miller FJ, Jr, Rothermel BA, Hill JA. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation 117: 3070–3078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsukamoto O, Minamino T, Okada K, Shintani Y, Takashima S, Kato H, Liao Y, Okazaki H, Asai M, Hirata A, Fujita M, Asano Y, Yamazaki S, Asanuma H, Hori M, Kitakaze M. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun 340: 1125–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Usui S, Maejima Y, Pain J, Hong C, Cho J, Park JY, Zablocki D, Tian B, Glass DJ, Sadoshima J. Endogenous muscle atrophy F-Box mediates pressure overload-induced cardiac hypertrophy through regulation of nuclear factor-κB. Circ Res 109: 161–171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verdoes M, Florea BI, Menendez-Benito V, Maynard CJ, Witte MD, van der Linden WA, van den Nieuwendijk AM, Hofmann T, Berkers CR, van Leeuwen FW, Groothuis TA, Leeuwenburgh MA, Ovaa H, Neefjes JJ, Filippov DV, van der Marel GA, Dantuma NP, Overkleeft HS. A fluorescent broad-spectrum proteasome inhibitor for labeling proteasomes in vitro and in vivo. Chem Biol 13: 1217–1226, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol 45: 11–27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weekes J, Morrison K, Mullen A, Wait R, Barton P, Dunn MJ. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics 3: 208–216, 2003 [DOI] [PubMed] [Google Scholar]
- 143.Willis MS, Rojas M, Li L, Selzman CH, Tang RH, Stansfield WE, Rodriguez JE, Glass DJ, Patterson C. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am J Physiol Heart Circ Physiol 296: H997–H1006, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Willis MS, Schisler JC, Li L, Rodriguez JE, Hilliard EG, Charles PC, Patterson C. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circ Res 105: 80–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137: 133–145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem 282: 22460–22471, 2007 [DOI] [PubMed] [Google Scholar]
- 147.Zhao TJ, Yan YB, Liu Y, Zhou HM. The generation of the oxidized form of creatine kinase is a negative regulation on muscle creatine kinase. J Biol Chem 282: 12022–12029, 2007 [DOI] [PubMed] [Google Scholar]
- 148.Zheng Q, Su H, Ranek MJ, Wang X. Autophagy and p62 in Cardiac Proteinopathy. Circ Res 109: 296–308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng Q, Su H, Tian Z, Wang X. Proteasome malfunction activates macroautophagy in the heart. Am J Cardiovasc Dis 1: 214–226, 2011 [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng Q, Wang X. Autophagy and the ubiquitin-proteasome system in cardiac dysfunction. Panminerva Med 52: 9–25, 2010 [PMC free article] [PubMed] [Google Scholar]
- 151.Zong C, Gomes AV, Drews O, Li X, Young GW, Berhane B, Qiao X, French SW, Bardag-Gorce F, Ping P. Regulation of murine cardiac 20S proteasomes: role of associating partners. Circ Res 99: 372–380, 2006 [DOI] [PubMed] [Google Scholar]