Abstract
Increased right atrial (RA) and ventricular (RV) chamber volumes are a late maladaptive response to chronic pulmonary hypertension. The purpose of the current investigation was to characterize the early compensatory changes that occur in the right heart during chronic RV pressure overload before the development of chamber dilation. Magnetic resonance imaging with radiofrequency tissue tagging was performed on dogs at baseline and after 10 wk of pulmonary artery banding to yield either mild RV pressure overload (36% rise in RV pressure; n = 5) or severe overload (250% rise in RV pressure; n = 4). The RV free wall was divided into three segments within a midventricular plane, and circumferential myocardial strain was calculated for each segment, the septum, and the left ventricle. Chamber volumes were calculated from stacked MRI images, and RA mechanics were characterized by calculating the RA reservoir, conduit, and pump contribution to RV filling. With mild RV overload, there were no changes in RV strain or RA function. With severe RV overload, RV circumferential strain diminished by 62% anterior (P = 0.04), 42% inferior (P = 0.03), and 50% in the septum (P = 0.02), with no change in the left ventricle (P = 0.12). RV filling became more dependent on RA conduit function, which increased from 30 ± 9 to 43 ± 13% (P = 0.01), than on RA reservoir function, which decreased from 47 ± 6 to 33 ± 4% (P = 0.04), with no change in RA pump function (P = 0.94). RA and RV volumes and RV ejection fraction were unchanged from baseline during either mild (P > 0.10) or severe RV pressure overload (P > 0.53). In response to severe RV pressure overload, RV myocardial strain is segmentally diminished and RV filling becomes more dependent on RA conduit rather than reservoir function. These compensatory mechanisms of the right heart occur early in chronic RV pressure overload before chamber dilation develops.
Keywords: pulmonary hypertension, hypertrophy, magnetic resonance imaging
chronic pulmonary hypertension (CPH) ultimately leads to maladaptive right atrial (RA) and ventricular (RV) chamber dilation and right heart failure in most patients. However, the physiologic mechanisms responsible for the progression from compensated hypertrophy to decompensated end-stage disease are poorly understood (21, 23, 38). Previous studies (11, 15, 17, 29, 31, 34) have identified alterations in RV strain patterns and systolic dynamics in patients with severe CPH and significant RV enlargement using tissue Doppler imaging, but little is known about the early adaptive response of the right heart before the development of myocardial hypertrophy and chamber dilation. Two recent clinical studies (16, 20) have shown that even mild elevation in pulmonary arterial (PA) pressures, without the presence of gross RV dilation, can affect the mechanical properties of the ventricle and result in contractile dyssynchrony.
In a canine model of chronic RV pressure overload, our laboratory (7) has previously demonstrated that RV diastolic mechanics are impaired and that the RA plays a compensatory role to maintain RV filling by augmenting contractility and increasing chamber compliance. Through ventricular interdependence, RV dyssynchrony and diminished RV contractile force can also have a negative impact on left ventricular (LV) performance (11, 12, 14, 31, 32, 37). The purpose of the current investigation was to characterize early compensatory changes in RA mechanics and differential RV and LV strain patterns in mild vs. severe RV pressure overload before the development of myocardial hypertrophy and chamber dilation, using magnetic resonance imaging (MRI)-derived instantaneous chamber volumes (3, 39) and myocardial strain analysis with tagged MRI (6, 10, 18, 26, 27).
METHODS
All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. This study was approved by the Washington University School of Medicine Animal Studies Committee and conducted according to Washington University policy.
Initial surgical preparation.
The canine model of chronic RV pressure overload previously described by our laboratory was used (7, 43, 44). Ten adult dogs (20–25 kg) were anesthetized with propofol (5–7 mg/kg) and intubated and ventilated using isoflurane (1.5–2.5% and tidal volume of 10 ml/kg). Of these, nine animals survived through the terminal study and were used for the final statistical analysis, including five with mild and four with severe RV pressure overload (described below). Median sternotomy was performed, leaving the pericardium intact to maintain normal RA and RV restraint, except for small incisions to permit instrumentation of the heart (19). A 1-cm incision was made in the pericardium over the anterior RV free wall, and a 5-Fr fluid-filled pressure catheter (Access Technologies, Skokie, IL) was introduced into the RV free wall through a purse-string suture. An inflatable silastic band (16-mm diameter; Access Technologies) was secured around the main pulmonary artery. The PA band and RV pressure (RVP) catheter were tunneled through the chest wall, connected to reservoirs, and buried in a subcutaneous pocket. The sternum was closed.
Creation of chronic RV pressure overload.
A baseline MRI was performed as described below. Approximately 1 wk after the initial operation, RV pressure overload was initiated in a stepwise manner with progressive inflation of the PA band to create either mild or severe RV pressure overload. To generate severe RV pressure overload, inflation of the PA band was performed weekly (0.3 to 0.5 ml), increasing RVP by 10 to 20 mmHg until systolic RVP reached 60 to 90 mmHg during the first 4 wk as tolerated. Pressure overload was then maintained for an additional 6 wk at goal pressure. To create mild RV pressure overload, PA band inflation was performed to match the pressure elevation curves from a previous study (1, 9) using monocrotaline infusion in canines. The target RVP was elevation to 35 mmHg by week 2 and 40 mmHg by week 4 and then maintaining inflation for an additional 6 wk at goal pressure. In two animals, intermittent or permanent catheter thrombosis precluded adequate RVP measurement before reaching goal pressure at week 4. In these cases, two-dimensional echocardiography was performed to quantify RVP. At 10 wk, a terminal MRI study was performed in all animals.
Cardiac MRI preparation.
Animals were anesthetized with propofol (5–7 mg/kg iv), intubated, and mechanically ventilated and maintained under anesthesia with propofol (0.5 mg·kg−1·min−1 continuous infusion) while in the scanner. Imaging was performed supine in a 1.5-Tesla MR scanner (Philips Medical Systems, Andover, MA). During image acquisition, the ventilator was paused for 20 to 30 s to minimize motion artifacts. A series of scout images was obtained to locate the heart and the true long-axis and short-axis planes. Subsequently, a set of sequential short-axis images were acquired in parallel planes with 6-mm intervals between slices. Image acquisition was synchronized with real-time electrocardiogram, and 30 images were obtained during the cardiac cycle. The pulse sequence used to acquire the cine images was balanced turbo field echo (sBTFE), a variation of steady-state process (SSFP). Imaging parameters included number of excitations of 4, echo time of 2.9 ms, repetition time 30.3 ms, flip angle 13°, field of view 306 × 350 mm, and image matrix 168 × 256. Tagged images were obtained with complementary spatial modulation of magnetization (CSPAMM) and acquired with a voxel dimension of 1.82/2.30/6 mm and then zero padded to a reconstructed voxel of 1.37/1.38/6 mm.
RA and RV function analysis.
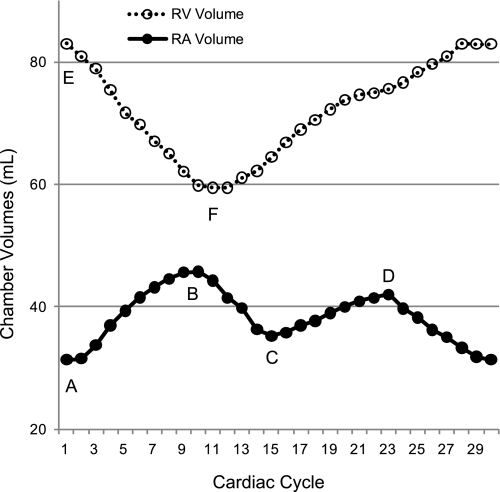
To quantify degree of ventricular hypertrophy, LV and RV mass were calculated using MRI, assuming myocardial tissue density of 1.05 gm/ml and normalized to body weight (2, 28, 33). Instantaneous RA and RV volumes were calculated using the short-axis stack images as previously described for the left heart (3, 39). Figure 1 demonstrates typical RA and RV volume curves during a full cardiac cycle in one animal. Point A represents the minimal RA volume (RAmin) at end-ventricular diastole. At end-ventricular systole, the RA reaches point B, RA maximal volume (RAmax). Point C refers to the mid-diastolic relative RA minimum volume (RArel min) at the end of rapid passive RA emptying. Point D is the relative maximal RA volume (RArel max) immediately before atrial systole. The RV end-diastolic volume (RV EDV, point E) and end-systolic volume (RV ESV, point F) were used to calculate RV stroke volume (RV SV) and ejection fraction (RV EF). End-diastolic wall thickness was measured at the midventricular level for the septum and RV and LV free walls as was RV and LV chamber diameter.
Fig. 1.
Right ventricular (RV: dotted line, ○) and right atrial (RA: solid line, ●) volume throughout the cardiac cycle in one animal. Point A, minimum RA volume; point B, maximum RA volume; point C, relative minimum RA volume; point D, relative maximum RA volume; point E, RV end-diastolic volume; point F, RV end-systolic volume. RV stroke volume = point E − point F; RA reservoir volume = point B − point C; RA booster pump volume = point D − point A; RA conduit volume = RV stroke volume − RA reservoir volume − RA pump volume.
Global RA function was characterized by calculating the reservoir, conduit, and booster pump volumes of the RA and their percentage contribution to RV filling as previously described for the left heart (3, 8, 39). RA reservoir volume was defined as RAmax − RArel min (point B − point C), RA booster pump volume was defined as RArel max − RAmin (point D − point A), and RA conduit volume was calculated by subtracting RA reservoir and pump volume from total RA inflow volume (RV SV). Volume changes during each RA emptying phase were divided by total inflow volume to yield reservoir, conduit, and pump function as a percentage of total RA inflow.
RV and LV finite element model construction.
Finite element modeling was performed using a previously described and validated method that has been employed in our laboratory since 1996 (26, 27). With spatial modulation of magnetization, radiofrequency tissue tags were created in an orthogonal grid pattern of presaturation in the myocardium, followed by a two-dimensional balanced steady-state free precession cine image acquisition as previously described (6, 18, 26, 27).
Tagged images were analyzed with custom software developed in our laboratory (26, 27), running on Silicon Graphics workstations (Silicon Graphics, Mountain View, CA). Endocardial and epicardial boundaries were manually identified for each short-axis image, and an initial B-spline representation of the tag lines on the end-diastolic images was constructed based on the known spacing between adjacent tissue tag lines. Tag lines were then located on successive images using a semiautomatic algorithm. The algorithm that was used was based on a method reported by McVeigh and Zerhouni (22). With this method, an initial guess for each tag line was adjusted based on the local pixel density function. B-spline curves from corresponding tag lines were used to construct a B-spline surface representation of the tag surfaces. Two-dimensional systolic displacements were computed at each tag line intersection within the myocardium (Fig. 2) (26). (A complete cine sequence of tagging images is included in the Supplemental Data; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website.)
Fig. 2.
Short-axis magnetic resonance imaging of right and left ventricular walls at end-diastole (A) and end-systole (B) in dog 5 (baseline study). B-splines are overlaid on tissue tags created in myocardial surface. Displacement of points within the myocardium are calculated throughout systole to determine strain.
A finite element model of the ventricles was constructed, consisting of three regional RV elements corresponding to the anterior, lateral, and inferior RV walls; four regional LV elements corresponding to the anterior, anterolateral, inferolateral, and inferior walls; one regional element corresponding to the interventricular septum; and two regional “junction-point” elements, corresponding to the anterior and posterior junction points where the RV free wall meets the LV. With a least squares fitting model, predicted displacement information was calculated for any point within the myocardium from known measured displacements using a custom-modified finite element software package (StressCheck; ESRD, St. Louis, MO).
RV and LV strain analysis.
Myocardial strain calculations were performed using a previously described and validated method (26, 27). Briefly, strain is defined as the deformation of an object, normalized to its original shape. Strain is a dimensionless quantity, and the resulting deformation of the material is expressed as fractional change from the original dimension. The formula for strain (ε) is as follows: ε = (ι − ι0)/ι0 = Δι/ι0, where ι is instantaneous length, ι0 is original length, and Δι is change in length. Langrangian strain determines change from the unstressed state, which is approximated by end-diastolic length in a beating heart. In the RV and LV walls, there are multiple components of strain, including circumferential, longitudinal, and radial strain and minimum principal strain, which represents the minimum strain among all directions emanating from a particular point. Negative strain represents segment shortening, and during systole, minimum principal strain is associated with the maximum contraction. Two-dimensional circumferential strain and minimum principal strain were measured at the midventricular level and basal level in the current study (it was not possible to measure longitudinal strain due to limited long-axis imaging). The short-axis slices selected for analysis included the midventricular slice (defined as the slice midway between the LV apex and the aortic valve) and the basal slice (midway between the midventricular slice and the base of the heart). Strain was not measured in the apical region because RV tag lines were often insufficient in this region.
Statistical analysis.
Hemodynamic data, RA and RV function parameters, and RV and LV strain values are reported as means ± SD. Data obtained during baseline and at 10 wk were compared using paired Student's t-test or two-way repeated-measures ANOVA as appropriate. Linear regression analysis was used to correlate degree of hypertrophy with myocardial strain. Statistical analyses were performed using SigmaStat (2.03; SPSS, Chicago, IL).
RESULTS
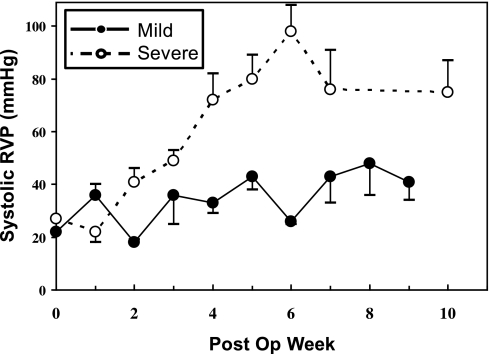
Figure 3 summarizes mean weekly systolic RVP for the mild and severe CPH animals, demonstrating a more substantial rise in RVP beyond week 1 with severe CPH. At 4 wk, RVP increased from 24.0 ± 8.7 mmHg at baseline to 33.3 ± 8.5 mmHg with mild (P = 0.29) and 71.8 ± 15.6 mmHg with severe RV pressure overload (P = 0.004). At 10 wk, there was no change in LV mass or LV mass normalized to body weight with either mild or severe RV pressure overload (Table 1; P > 0.17 for all). With severe RV overload, there was an average 53% increase in RV mass and RV mass normalized to body weight (P = 0.04). With mild RV overload, the average 20% increase in RV mass did not reach statistical significance (P = 0.21).
Fig. 3.
Average weekly systolic RV pressure measurements with severe RV pressure overload (solid line, ●) and mild RV pressure overload (dotted line, ○). Error bars depict SE at each time point.
Table 1.
Effects of mild and severe right ventricular overload on ventricular mass and right atrial and ventricular volumes
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| RV mass, g | 28.8 ± 4.1 | 34.7 ± 10.8 | 0.21 | 24.5 ± 8.1 | 36.3 ± 13.0 | 0.04 |
| n-RV mass, g/kg | 1.4 ± 0.2 | 1.7 ± 0.5 | 0.22 | 1.2 ± 0.4 | 1.8 ± 0.7 | 0.04 |
| LV mass, g | 124.9 ± 9.7 | 110.4 ± 15.0 | 0.18 | 94.3 ± 30.5 | 97.5 ± 14.0 | 0.81 |
| n-LV mass, g/kg | 6.2 ± 0.5 | 5.4 ± 0.6 | 0.17 | 4.7 ± 1.6 | 4.9 ± 0.7 | 0.81 |
| RV EDV, ml | 80.9 ± 8.5 | 85.2 ± 12.4 | 0.25 | 81.2 ± 11.7 | 83.1 ± 12.1 | 0.65 |
| RV ESV, ml | 58.2 ± 9.9 | 59.3 ± 16.1 | 0.87 | 59.3 ± 10.5 | 59.3 ± 13.1 | 0.99 |
| RV SV, ml | 22.7 ± 3.6 | 25.9 ± 7.8 | 0.48 | 21.9 ± 3.4 | 23.9 ± 8.5 | 0.53 |
| RV EF | 0.28 ± 0.06 | 0.31 ± 0.11 | 0.61 | 0.27 ± 0.05 | 0.29 ± 0.09 | 0.65 |
| RAVMAX, ml | 42.2 ± 2.1 | 46.8 ± 5.5 | 0.20 | 41.8 ± 6.6 | 42.5 ± 3.0 | 0.76 |
| RAVMIN, ml | 32.1 ± 2.9 | 38.1 ± 5.2 | 0.10 | 31.1 ± 4.1 | 32.4 ± 1.4 | 0.59 |
Data are means ± SD. PA, pulmonary arterial; RV, right ventricular; LV, left ventricular; n, normalized to body weight; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; RA, right atrial; RAVMAX, maximum RA volume; RAVMIN, minimum RA volume. P values were determined using repeated-measures ANOVA.
RA and RV volume, function, and geometry.
At 10 wk, there was no significant change in end-diastolic or end-systolic RA or RV volumes or RV ejection fraction with either mild or severe RV pressure overload (Table 1; P > 0.10 for all). With mild RV overload, end-diastolic wall thickness did not change in the ventricular free walls or septum, but severe overload was associated with 20% septal thickening (P = 0.04; Table 2). There was no change in RV chamber cross-sectional diameter with mild or severe overload, but LV chamber diameter fell by 5% with mild (P = 0.05) and 15% with severe (P < 0.001) overload, consistent with a leftward septal shift. With mild RV overload, there was no change in the contribution of reservoir, conduit, or pump function to RV filling, but with severe RV pressure overload, RV filling became more dependent on RA conduit than reservoir function (Table 3). With severe RV pressure overload, conduit function increased from 30 to 43% (P = 0.01), reservoir function decreased from 47 to 33% (P = 0.04), but booster pump function did not change, at 23 vs. 24% (P = 0.70).
Table 2.
Effects of mild and severe right ventricular overload on end-diastolic biventricular geometry
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| Wall thickness, mm | ||||||
| RV free wall | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.39 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.19 |
| Septum | 0.9 ± 0.1 | 0.9 ± 0.3 | 0.78 | 0.8 ± 0.2 | 1.0 ± 0.2 | 0.04 |
| LV free wall | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.67 | 0.8 ± 0.3 | 1.1 ± 0.1 | 0.12 |
| Chamber diameter, mm | ||||||
| RV | 2.4 ± 0.2 | 2.4 ± 0.4 | 0.53 | 2.3 ± 0.4 | 2.3 ± 0.3 | 0.82 |
| LV | 4.2 ± 0.3 | 4.0 ± 0.3 | 0.05 | 3.9 ± 0.3 | 3.3 ± 0.3 | 0.001 |
Data are means ± SD. P values were determined using repeated-measures ANOVA.
Table 3.
Effects of mild and severe right ventricular overload on right atrial reservoir, conduit, and pump function
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| RA reservoir volume, ml | 8.9 ± 1.4 | 8.3 ± 1.5 | 0.40 | 10.3 ± 1.3 | 8.1 ± 3.5 | 0.18 |
| RV CV, ml | 7.7 ± 4.0 | 12.2 ± 7.2 | 0.34 | 6.6 ± 2.3 | 10.2 ± 4.2 | 0.07 |
| RV PV, ml | 6.2 ± 1.3 | 5.5 ± 0.5 | 0.32 | 5.1 ± 1.3 | 5.5 ± 3.6 | 0.74 |
| RA reservoir function | 39 ± 5% | 34 ± 8% | 0.33 | 47 ± 6% | 33 ± 4% | 0.04 |
| RA conduit function | 33 ± 11% | 42 ± 20% | 0.44 | 30 ± 9% | 43 ± 13% | 0.01 |
| RA booster pump function | 28 ± 9% | 24 ± 12% | 0.55 | 23 ± 5% | 24 ± 14% | 0.94 |
Data are means ± SD. CV, conduit volume; PV, booster pump volume. P values were determined using repeated-measures ANOVA.
Circumferential myocardial strain.
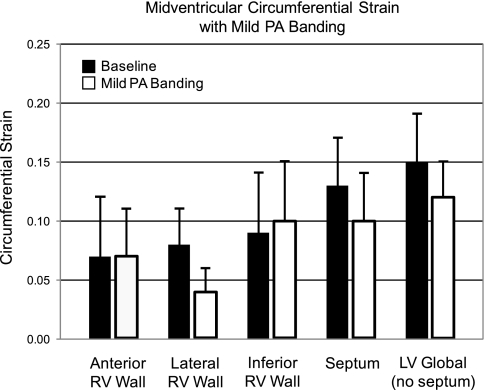
With mild RV pressure overload, circumferential strain did not change in any wall at either the midventricular (Table 4) or basal level (Table 5). Figure 4 demonstrates no significant change in RV or LV circumferential strain with mild RV overload at the midventricular level. With severe RV pressure overload, circumferential strain was perturbed in the anterior and inferior RV walls and the septum at the midventricular level (Table 4) but only the anterior RV wall at the basal level (Table 5). Figure 5 demonstrates a fall in circumferential strain with severe RV overload at the midventricular level in the anterior RV wall (62% decline), inferior RV wall (42%), and septum (50%) but no change in global LV strain or in the lateral RV wall. At the midventricular RV-LV junction regions, circumferential strain fell by 78% anteriorly but did not change posteriorly (Table 4). At the basal level, there was a 57% fall in anterior RV wall circumferential strain and a tendency for a fall in the anterior RV-LV junction region (P = 0.08), but circumferential strain did not change in any other RV or LV wall (P > 0.31; Table 5).
Table 4.
Effects of mild and severe right ventricular overload on circumferential strain at the midventricular level
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| Anterior RV | 0.07 ± 0.05 | 0.07 ± 0.04 | 0.85 | 0.10 ± 0.07 | 0.04 ± 0.04 | 0.04 |
| Lateral RV | 0.08 ± 0.03 | 0.04 ± 0.02 | 0.10 | 0.10 ± 0.05 | 0.08 ± 0.03 | 0.59 |
| Posterior RV | 0.09 ± 0.05 | 0.10 ± 0.05 | 0.77 | 0.15 ± 0.04 | 0.09 ± 0.03 | 0.03 |
| Septum | 0.13 ± 0.04 | 0.10 ± 0.04 | 0.37 | 0.18 ± 0.05 | 0.09 ± 0.05 | 0.02 |
| Global LV | 0.15 ± 0.04 | 0.12 ± 0.03 | 0.29 | 0.17 ± 0.03 | 0.12 ± 0.04 | 0.12 |
| Anterior RV-LV junction | 0.04 ± 0.01 | 0.05 ± 0.04 | 0.91 | 0.09 ± 0.05 | 0.02 ± 0.02 | 0.02 |
| Posterior RV-LV junction | 0.11 ± 0.03 | 0.11 ± 0.04 | 0.85 | 0.18 ± 0.05 | 0.14 ± 0.03 | 0.31 |
Data are means ± SD. P values were determined using repeated-measures ANOVA.
Table 5.
Effects of mild and severe right ventricular overload on circumferential strain at the basal level
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| Anterior RV | 0.11 ± 0.03 | 0.09 ± 0.02 | 0.18 | 0.12 ± 0.04 | 0.05 ± 0.03 | 0.02 |
| Lateral RV | 0.05 ± 0.05 | 0.08 ± 0.03 | 0.51 | 0.08 ± 0.06 | 0.08 ± 0.04 | 0.95 |
| Posterior RV | 0.06 ± 0.01 | 0.07 ± 0.03 | 0.40 | 0.11 ± 0.03 | 0.09 ± 0.04 | 0.48 |
| Septum | 0.09 ± 0.04 | 0.14 ± 0.04 | 0.20 | 0.14 ± 0.06 | 0.12 ± 0.08 | 0.70 |
| Global LV | 0.13 ± 0.03 | 0.13 ± 0.03 | 0.99 | 0.15 ± 0.03 | 0.12 ± 0.05 | 0.31 |
| Anterior RV-LV junction | 0.11 ± 0.05 | 0.08 ± 0.04 | 0.14 | 0.08 ± 0.05 | 0.01 ± 0.03 | 0.08 |
| Posterior RV-LV junction | 0.10 ± 0.06 | 0.07 ± 0.06 | 0.33 | 0.09 ± 0.09 | 0.09 ± 0.12 | 0.82 |
Data are means ± SD. P values were determined using repeated-measures ANOVA.
Fig. 4.
Effects of mild pulmonary artery (PA) banding on midventricular circumferential strain in the RV anterior, lateral, and inferiors walls, the interventricular septum, and the left ventricular (LV) free wall (excluding the septum). Black bars are baseline, and white bars are with mild PA banding (means ± 1SD).
Fig. 5.
Effects of severe PA banding on midventricular circumferential strain in the RV anterior, lateral, and inferiors walls, the interventricular septum, and the LV free wall (excluding the septum). Black bars are baseline, and white bars are with severe PA banding (means ± 1SD; *P < 0.05).
Minimum principal strain.
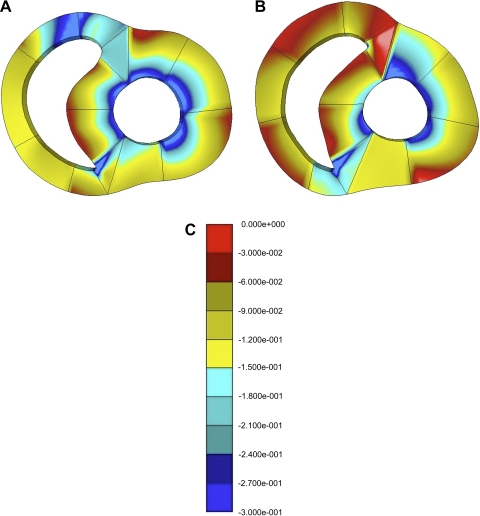
With mild RV overload, minimum principal strain did not fall in any RV or LV wall at the midventricular level (P > 0.13; Table 6). However, at the basal level, there was a significant 35% fall in minimum principal strain in the anterior RV-LV junction region (P = 0.01) and a tendency to fall in the RV anterior wall (p = 0.08; Table 7). With severe RV overload, minimum principal strain tended to fall in most walls at the midventricular level (Table 6) but only in the anterior RV wall (P = 0.04) and anterior RV-LV junction region (p = 0.06) at the basal level (Table 7). Figure 6 demonstrates a fall in midventricular minimum principal strain in the RV wall and septum, with no substantial change in the LV wall.
Table 6.
Effects of mild and severe right ventricular overload on minimum principal strain at the midventricular level
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| Anterior RV | 0.14 ± 0.06 | 0.11 ± 0.05 | 0.33 | 0.20 ± 0.04 | 0.11 ± 0.05 | 0.08 |
| Lateral RV | 0.12 ± 0.05 | 0.08 ± 0.03 | 0.26 | 0.14 ± 0.02 | 0.10 ± 0.03 | 0.05 |
| Posterior RV | 0.19 ± 0.10 | 0.12 ± 0.06 | 0.30 | 0.19 ± 0.09 | 0.11 ± 0.04 | 0.07 |
| Septum | 0.16 ± 0.04 | 0.12 ± 0.04 | 0.13 | 0.19 ± 0.05 | 0.12 ± 0.02 | 0.05 |
| Global LV | 0.17 ± 0.04 | 0.14 ± 0.03 | 0.28 | 0.20 ± 0.04 | 0.13 ± 0.04 | 0.07 |
| Anterior RV-LV junction | 0.15 ± 0.02 | 0.12 ± 0.06 | 0.36 | 0.19 ± 0.05 | 0.08 ± 0.03 | 0.01 |
| Posterior RV-LV junction | 0.16 ± 0.04 | 0.17 ± 0.04 | 0.58 | 0.26 ± 0.09 | 0.19 ± 0.04 | 0.31 |
Data are means ± SD. P values were determined using repeated-measures ANOVA.
Table 7.
Effects of mild and severe right ventricular overload on minimum principal strain at the basal level
| Baseline | Mild PA Band | P Value | Baseline | Severe PA Band | P Value | |
|---|---|---|---|---|---|---|
| Anterior RV | 0.15 ± 0.05 | 0.11 ± 0.03 | 0.08 | 0.24 ± 0.11 | 0.08 ± 0.03 | 0.04 |
| Lateral RV | 0.11 ± 0.07 | 0.10 ± 0.03 | 0.78 | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.40 |
| Posterior RV | 0.12 ± 0.06 | 0.09 ± 0.04 | 0.35 | 0.15 ± 0.06 | 0.10 ± 0.04 | 0.26 |
| Septum | 0.14 ± 0.03 | 0.16 ± 0.04 | 0.26 | 0.17 ± 0.06 | 0.16 ± 0.08 | 0.74 |
| Global LV | 0.16 ± 0.02 | 0.15 ± 0.04 | 0.83 | 0.18 ± 0.03 | 0.16 ± 0.06 | 0.52 |
| Anterior RV-LV junction | 0.21 ± 0.05 | 0.14 ± 0.05 | 0.01 | 0.16 ± 0.04 | 0.08 ± 0.03 | 0.06 |
| Posterior RV-LV junction | 0.19 ± 0.09 | 0.16 ± 0.06 | 0.66 | 0.18 ± 0.12 | 0.19 ± 0.12 | 0.79 |
Data are means ± SD. P values were determined using repeated-measures ANOVA.
Fig. 6.
Midventricular minimum principal strain contour maps for dog 7 at baseline (A) and after 10 wk of severe pulmonary artery banding (B). Color scale (C) quantifies the degree of minimum principal strain throughout the ventricular walls. Red represents low strain (i.e., strain near zero), blue represents high strain (i.e., −0.27 to −0.30), and yellow represents medium strain (i.e., −0.12 to −0.15). Note the decrease in strain in the RV anterior and inferior walls and the septum (increased red and decreased blue regions at 10 wk compared with baseline) but no substantial change in the LV walls.
To examine the relationship between degree of hypertrophy and myocardial strain, the percentage change in RV mass for each animal was plotted against circumferential and minimum principal strain in the RV-LV junction regions at the midventricular level. There was a significant correlation between the degree of hypertrophy and myocardial strain in both the anterior RV-LV junction region (r = 0.49, r2 = 0.24, P = 0.04) and the posterior RV-LV junction region (r = 0.78, r2 = 0.61, P < 0.001). Figure 7 demonstrates the correlation between degree of hypertrophy and strain in the RV-LV junction regions at the midventricular level; as RV mass increased, myocardial strain increased.
Fig. 7.
Relationship between degree of hypertrophy, quantified by percentage change in RV mass, and circumferential (●) and minimum principal (○) strain in the anterior (A) and posterior (B) RV-LV junction regions at the midventricular level. There was a significant correlation between hypertrophy and strain in the RV-LV junction regions; as RV mass increased, myocardial strain increased.
DISCUSSION
The current study sought to investigate early compensatory mechanisms of the RA and RV response to RV pressure overload before the development of fixed right heart chamber dilation in an experimental model. In contrast to previous studies in humans that have evaluated RV mechanics in CPH using echocardiography (11, 15, 17, 29, 31), MRI with tissue tagging was used to measure circumferential and minimum principle strain rather than longitudinal strain. The resulting data revealed that early RV pressure overload, without chamber enlargement, has a measurable effect on RA function and RV strain patterns when overload is severe. With a 2.5-fold rise in RV afterload, RV filling became more dependent on RA conduit than reservoir function, which likely reflects loss of RV diastolic compliance and consequent stiffening of the RA and RV walls. The RV myocardial strain results from this study were similar to those reported by Lopez-Candales et al. (16), in that reduction in myocardial strain was seen at several RV segments in the severe RV pressure overload group.
Previous work (8) from our laboratory in subjects with acute PA hypertension demonstrated that increased ventricular pressures favor a shift towards conduit over reservoir function. In a chronic canine CPH model, we previously estimated reservoir and conduit function as RA inflow with the tricuspid valve closed vs. open, respectively (7). This method, however, can be confounded by the variably unknown atrial filling component that constitutes the booster pump contribution to trans-tricuspid flow. The bichamber MRI methodology employed in the current study allowed us to measure absolute conduit and reservoir function, independent of the booster pump contribution. Suga (35) used a computer model to demonstrate the intimate relationship between atrial distensibility and cardiac performance. With chronic pressure overload, RV diastolic dysfunction is the norm, including prolonged diastolic relaxation times and increased RV diastolic stiffness (4, 5, 13), all consistent with stiffening of the atrium and a shift towards conduit function. Future studies will address the impact of compliance restriction, as would be seen with myocardial fibrosis or restrictive or constrictive limitations on the capacity of the RA to alter its conduit-to-reservoir ratio and impact RV filling.
Several studies (11, 15, 17, 29, 31) have been published regarding the maladaptive mechanical changes that occur in RV strain in response to chronic RV pressure overload. Pirat et al. (29) studied 58 patients with CPH using speckle-tracking echocardiography and found that peak RV systolic myocardial velocities, RV strain rate, and RV strain were significantly impaired. In a similar group of patients, Puwanant et al. (31) found that RV lateral wall longitudinal strain and interventricular septal longitudinal strain were both reduced with CPH. Huez et al. (11) noted diminished RV strain and strain rates along the RV free wall but with a mid-apical predominance. Lopez-Candales et al. (15, 17) identified significant differential perturbations in strain and velocity generation along the RV free wall with highest strain in the basal segments, diminishing towards the apex. Although the maladaptive RV dyssynchrony in response to CPH with RV hypertrophy is well-established, little is known about the early compensatory mechanisms of the right heart to RV pressure overload before the development of right heart chamber dilation.
Lopez-Candales et al. (16) demonstrated the presence of dyssynchronous longitudinal strain along the RV free wall with moderate CPH (mean systolic RVP of 49 mmHg) before the development of gross abnormalities in RV size and function or clinical manifestation of overt RV failure. They speculated that diminution in RV strain may be a preceding step in the progression to clinical failure, in that it was present despite the absence of hemodynamically decompensated RV function, and may be a useful parameter to follow patients serially. In the current study, there were minimal changes in RV strain when RV pressure overload was mild. However, as early as 6 wk following the development of severe RV pressure overload (mean systolic RVP of 72 mmHg), RV strain was perturbed through the RV free wall and in the interventricular septum, most substantially in the midventricular rather than basal regions. Others (11, 12, 14, 31, 32, 37) have also shown that RV dysfunction with CPH can be associated with impaired LV function through biventricular interactions. In the current report, while LV circumferential strain did not fall, there was a trend for minimum principle strain to fall in the LV midventricular level with severe RV pressure overload. Alterations in interventricular geometry were also beginning to manifest with diminution in LV cross-sectional diameter and septal thickening. Impairment of LV function likely occurs later in the progression of disease.
Segmental changes in strain have been related to anatomic differences in fiber orientation in different ventricular regions. We (24, 25) have previously demonstrated that ventricular twist and torsion (rotation) is independent of afterload but directly related to increased volume load and inotropic state. Ventricular strain, however, is load dependent and can be altered by changes in preload and afterload. An increase in regional RV wall stress (afterload), which is directly related to pressure and inversely related to wall thickness, would tend to diminish strain. We found that while there was no change in right-sided chamber volume, elevated RV afterload consistently impaired strain. This occurred before the development of measureable RV hypertrophy (no change in wall thickness), which, if present, may have reversed RV strain towards normal. Although the fall in RV strain identified in early pressure overload was not substantial enough to impact RV ejection fraction, it may play a role in diminished diastolic function.
In the current report, there was a significant correlation between hypertrophy and strain in the anterior and posterior RV-LV junction points. As RV mass increased, and as pressure overload progressed from mild to severe, myocardial strain increased. These findings are consistent with LV studies from previous investigators (40, 41, 42) who have demonstrated that the severity of LV hypertrophy is linearly related to the severity of LV molecular dysfunction. With aortic banding, abnormal high-energy phosphate metabolism and diminished creatine kinase protein levels were proportional to the degree of LV hypertrophy but were not the result of persistent abnormalities of myocardial perfusion (40, 42). Ye et al. (41) also noted an increase in LV end-diastolic pressure but no change in LV performance or oxygen consumption during stress, suggesting an adaptive atrial response to LV pressure overload.
Heterogeneous segmental changes, not only in the anterior and posterior RV-LV junction points, but in the anterior and inferior RV regions, may be due to fiber orientation being more oblique in these regions than in the lateral RA free wall. Plunkett and Buckberg (30) demonstrated differential orientation of the transverse RV free wall geometry (which allows contraction or bellows-type motion) vs. the oblique septal fiber orientation, essential for LV twisting, a critical component of LV rather than RV ejection. They speculated that distortion of this normal structure-to-function relationship underlies the pathophysiologic mechanisms of RV failure. Further studies examining differential radial wall changes would be necessary to address the relative contribution of altered myofiber architecture on impaired RV function in CPH.
Potential limitations.
There are several limitations in the interpretation of the findings of the current report. First, the relatively small number of animals used in mild and severe RV pressure overload groups could have resulted in type I error; however, this was a very challenging and expensive protocol, and we felt after analysis of the first nine animals that the statistics were strong and the data were sufficient to support the conclusions. Thus we did not feel it was necessary or cost effective to include more animals. Second, we measured circumferential RV strain and minimum principal strain in only the midventricular and basal short axis. We would have preferred to perform a complete strain analysis covering all RV regions (apex, lateral free wall, and outflow region), including longitudinal strain. However, we were not able to consistently measure RV strain at the apical level because the RV tag lines were often insufficient in this region and our protocol did not permit analysis of longitudinal strain. The RV wall is substantially thinner and more trabeculated than the LV wall, and tag lines disappear more quickly than in the LV myocardium. These issues were particularly challenging in the RV apical wall, and as such, RV strain was not measured at the apical level because RV tag lines were often insufficient in this region. Future studies of RV apical strain and longitudinal strain will be important to our understanding of the impact of chronic RV overload on RV mechanical properties. In addition, it would have also been interesting to include measurements of regional RV wall stress, but we did not measure RVP during MRI; thus it was not possible to calculate wall stress without making a significant number of assumptions. In future studies, we plan to implant MRI compatible catheters, but the present analysis includes only regional wall strain, not regional wall stress.
Thirdly, the MRI-derived atrial conduit, reservoir, and booster pump functional parameters derived from instantaneous RA and RV volume changes during cardiac cycle are load dependent, and, while EDVs and ESVs were unchanged with early RV pressure overload, there could have been some impact of RV afterload elevation on the conduit-to-reservoir ratio. Fourthly, there are important technical decisions we made during MRI acquisition that could have been done differently. We have historically performed image acquisition during paused ventilation for simplification of analysis and to minimize the confounding impact of the respiratory cycle on RV function. However, MRI image acquisition could have also been performed during free breathing with multiple number of excitations, which may represent a more physiologic condition than image acquisition during paused ventilation. In addition, we performed image acquisition with slice spacing of 6 mm, with a voxel dimension of 1.82/2.30/6 mm that was zero padded to a reconstructed voxel of 1.37/1.38/6 mm. It is important to note that slice thickness greatly conditions the voxel size and the spatial resolution of the pulse sequence. The larger the voxel, the higher the partial volume effect. A higher partial volume effect could alter volume quantifications, particular in the RV because of differing myocardial wall thickness (4 to 5 mm) and increased trabeculations compared with the LV (typical wall thickness 8 to 11 mm) and RA (typical wall thickness <2 to 3 mm). Finally, we obtained a set number of images (n = 30) per cardiac cycle rather than adjusting the number of images based on the specific heart rate, which could produce different temporal resolutions if a substantial difference exists in heart rate between studies. Depending on the degree of heart rate variability, adjusting the number of images per cardiac cycle based on R-R interval might yield a more accurate approach with more consistent temporal resolution. However, all function and strain measurements were dependent on the phase of the cardiac cycle in relation to filling and/or chamber volume rather than time, limiting the potential negative impact of this methodologic choice.
In summary, the current study revealed that early compensatory changes occur in both RA function and RV strain in response to severe pressure overload before the development of myocardial hypertrophy or chamber dilation. Despite no change in RA or RV EDVs or ESVs or RV ejection fraction, the shift from RA reservoir to conduit function and changes in RV strain suggest alterations at the myofibril level, potentially in fiber orientation and loading capabilities that will impact RV filling during afterload challenge. Changes in myocardial strain manifest early in the development of chronic pressure overload and may provide a useful parameter to follow disease progression or therapeutic response in patients with CPH.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-92088 and T32-HL-07776 (to A. Aziz). N. N. Ufere was also supported by a Research Scholarship from the American Association for Thoracic Surgery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R. K. V., H. S. M., B. P. C., and M. R. M. conception and design of research; R. K. V., A. A., N. N. U., A. K. T., and N. J. B. performed experiments; R. K. V., A. A., H. S. M., N. N. U., A. K. T., N. J. B., B. P. C., and M. R. M. analyzed data; R. K. V., H. S. M., B. P. C., and M. R. M. interpreted results of experiments; R. K. V. and M. R. M. prepared figures; R. K. V. and M. R. M. drafted manuscript; R. K. V., A. A., H. S. M., B. P. C., and M. R. M. edited and revised manuscript; R. K. V., A. A., H. S. M., N. N. U., A. K. T., N. J. B., B. P. C., and M. R. M. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge P. Diane Toeniskoetter and Naomi R. Still for technical assistance and Richard B. Schuessler for assistance with study design and interpretation.
REFERENCES
- 1. Aziz A, Lee AM, Ufere NN, Damiano RJ, Jr, Townsend RR, Moon MR. Proteomic profiling of chronic pulmonary hypertension suggests development of both adaptive and maladaptive pathology. Circulation 120, Suppl II: II–852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared with echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 8: 221–228, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bowman AW, Kovacs SJ. Left atrial conduit volume is generated by deviation from the constant-volume state of the left heart: a combined MRI-echocardiographic study. Am J Physiol Heart Circ Physiol 286: H2416–H2424, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chen EP, Bittner HB, Tull F, Biswas SS, Davis RD, Van Trigt P. An adult canine model of chronic pulmonary hypertension for cardiopulmonary transplantation. J Heart Lung Transplant 16: 538- 547, 1997 [PubMed] [Google Scholar]
- 5. Chen EP, Craig DM, Bittner HB, Davis RD, Van Trigt P. Pharmacological strategies for improving diastolic dysfunction in the setting of chronic pulmonary hypertension. Circulation 97: 1606- 1612, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Cupps BP, Bree DR, Wollmuth JR, Howells AC, Voeller RK, Rogers JG, Pasque MK. Myocardial viability mapping by magnetic resonance-based multiparametric systolic strain analysis. Ann Thorac Surg 86: 1546–1553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 112: I212–218, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR. Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol 288: H2140–H2145, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Gust R, Schuster DP. Vascular remodeling in experimentally induced subacute canine pulmonary hypertension. Exp Lung Res 27: 1–12, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Hamdan A, Thouet T, Kelle S, Paetsch I, Gebker R, Wellnhofer E, Schnackenburg B, Fahmy AS, Osman NF, Fleck E. Regional right ventricular function and timing of contraction in healthy volunteers evaluated by strain-encoded MRI. J Magn Reson Imaging 28: 1379–1385, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Huez S, Vachiery JL, Unger P, Brimioulle S, Naeije R. Tissue Doppler imaging evaluation of cardiac adaptation to severe pulmonary hypertension. Am J Cardiol 100: 1473–1478, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Leeuwenburgh BP, Helbing WA, Steendijk P, Schoof PH, Baan J. Effects of acute left ventricular unloading on right ventricular function in normal and chronic right ventricular pressure-overloaded lambs. J Thorac Cardiovasc Surg 125: 481–490, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Leeuwenburgh BP, Steendijk P, Helbing WA, Baan J. Indexes of diastolic RV function. Load dependence and changes after chronic RV pressure overload in lambs. Am J Physiol Heart Circ Physiol 282: H1350–H1358, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Lopez-Candales A, Bazaz R, Edelman K, Gulyasy B. Altered early left ventricular diastolic wall velocities in pulmonary hypertension: a tissue Doppler study. Echocardiography 26: 1159–1166, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Lopez-Candales A, Dohi K, Bazaz R, Edelman K. Relation of right ventricular free wall mechanical delay to right ventricular dysfunction as determined by tissue Doppler imaging. Am J Cardiol 96: 602–606, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Lopez-Candales A, Rajagopalan N, Dohi K, Gulyasy B, Edelman K, Bazaz R. Abnormal right ventricular myocardial strain generation in mild pulmonary hypertension. Echocardiography 24: 615–622, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Candales A, Rajagopalan N, Gulyasy B, Edelman K, Bazaz R. Differential strain and velocity generation along the right ventricular free wall in pulmonary hypertension. Can J Cardiol 25: e73–e77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maniar HS, Cupps BP, Potter DD, Moustakidis P, Camillo CJ, Chu CM, Pasque MK, Sundt TM., III Ventricular function after coronary artery bypass grafting: evaluation by magnetic resonance imaging and myocardial strain analysis. J Thorac Cardiovasc Surg 128: 76–82, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Maniar HS, Prasad SM, Gaynor SL, Chu CM, Steendijk P, Moon MR. Impact of pericardial restraint on right atrial mechanics during acute right ventricular pressure load. Am J Physiol Heart Circ Physiol 284: H350–H357, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Matias C, Isla LP, Vasconcelos M, Almeria C, Rodrigo JL, Serra V, Zamorano J. Speckle-tracking-derived strain and strain-rate analysis: a technique for the evaluation of early alterations in right ventricle systolic function in patients with systemic sclerosis and normal pulmonary artery pressure. J Cardiovasc Med (Hagerstown) 10: 129–134, 2009 [DOI] [PubMed] [Google Scholar]
- 21. McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009 [DOI] [PubMed] [Google Scholar]
- 22. McVeigh ER, Zerhouni EA. Noninvasive measurement of transmural gradients in myocardial strain with MR imaging. Radiology 180: 677–683, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation 118: 1486–1495, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Moon MR, Ingels NB, Daughters GT, Stinson EB, Hansen DE, Miller DC. Alterations in left ventricular twist mechanics with inotropic stimulation and volume loading in human subjects. Circulation 89: 142–150, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Moon MR, DeAnda A, Jr, Daughters GT, 2nd, Ingels NB, Miller DC. Effects of chordal disruption on regional left ventricular torsional deformation. Circulation 94, Suppl II: II143–151, 1996 [PubMed] [Google Scholar]
- 26. Moulton MJ, Creswell LL, Cowning SW, Actis RL, Szabo BA, Vannier MW, Pasque MK. Spline surface interpolation for calculating 3-D ventricular strains from MRI tissue tagging. Am J Physiol Heart Circ Physiol 270: H281–H297, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Moustakidis P, Cupps BP, Pomerantz BJ, Scheri RP, Maniar HS, Kates AM, Gropler RJ, Pasque MK, Sundt TM. Noninvasive, quantitative assessment of left ventricular function in ischemic cardiomyopathy. J Surg Res 116: 187–196, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Murakami Y, Zhang Y, Cho YK, Mansoor AM, Chung JK, Chu C, Francis G, Ugurbil K, Bache RJ, From AH, Jerosch-Herold M, Wilke N, Zhang J. Myocardial oxygenation during high work states in hearts with postinfarction remodeling. Circulation 99: 942–948, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol 98: 699–704, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Plunkett MD, Buckberg GD. Pathophysiologic implications of the helical ventricular myocardial band: considerations for right ventricular restoration. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann 10: 68–75, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Puwanant S, Park M, Popovic ZB, Tang WH, Farha S, George D, Sharp J, Puntawangkoon J, Loyd JE, Erzurum SC, Thomas JD. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 121: 259–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramani GV, Bazaz R, Edelman K, Lopez-Candales A. Pulmonary hypertension affects left ventricular basal twist: a novel use for speckle-tracking imaging. Echocardiography 26: 44–51, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Shors SM, Fung CW, Francois CJ, Finn JP, Fieno DS. Accurate quantification of right ventricular mass at MR imaging by using cine true fast imaging with steady-state precession: study in dogs. Radiology 230:383–388, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Simon MA, Rajagopalan N, Mathier MA, Shroff SG, Pinsky MR, Lopez-Candales A. Tissue Doppler imaging of right ventricular decompensation in pulmonary hypertension. Congest Heart Fail 15: 271–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suga H. Importance of atrial compliance in cardiac performance. Circ Res 35: 39–43, 1974 [DOI] [PubMed] [Google Scholar]
- 36. Vetter FJ, Simons SB, Mironov S, Hyatt CJ, Pertsov AM. Epicardial fiber organization in swine right ventricle and its impact on propagation. Circ Res 96: 244–251, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Visner MS, Arentzen CE, Crumbley AJ, Larson EV, III, O′Connor MJ, Anderson RW. The effects of pressure-induced right ventricular hypertrophy on left ventricular diastolic properties and dynamic geometry in the conscious dog. Circulation 74: 410–419, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Voeller RK, Zierer A, Lall SC, Sakamoto S, Chang NL, Schuessler RB, Moon MR, Damiano RJ., Jr The effects of the Cox maze procedure on atrial function. J Thorac Cardiovasc Surg 136: 1257–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing heart. A new porcine model. Circulation 103: 1570–1576, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Ye Y, Wang C, Zhang J, Cho YK, Gong G, Murakami Y, Bache RJ. Myocardial creatine kinase kinetics and isoform expression in hearts with severe LV hypertrophy. Am J Physiol Heart Circ Physiol 281: H376–H386, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Merkle H, Hendrich K, Garwood M, From AHL, Ugurbil K, Bache RJ. Bioenergetic abnormalities associated with severe left ventricular hypertrrophy. J Clin Invest 92: 993–1003, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zierer A, Melby SJ, Voeller RK, Moon MR. Interatrial shunt for chronic pulmonary hypertension: differential impact of low-flow vs. high-flow shunting. Am J Physiol Heart Circ Physiol 296: H639–H644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zierer A, Voeller RK, Melby SJ, Steendijk P, Moon MR. Impact of calcium-channel blockers on right heart function in a controlled model of chronic pulmonary hypertension. Eur J Anaesthesiol 26: 253–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]