Abstract
Adenosine plays a role in physiological and pathological conditions, and A2 adenosine receptor (AR) expression is modified in many cardiovascular disorders. In this study, we elucidated the role of the A2BAR and its relationship to the A2AAR in coronary flow (CF) changes using A2B single-knockout (KO) and A2A/2B double-KO (DKO) mice in a Langendorff setup. We used two approaches: 1) selective and nonselective AR agonists and antagonists and 2) A2AKO and A2BKO and A2A/2BDKO mice. BAY 60-6583 (a selective A2B agonist) had no effect on CF in A2BKO mice, whereas it significantly increased CF in wild-type (WT) mice (maximum of 23.3 ± 9 ml·min−1·g−1). 5′-N-ethylcarboxamido adenosine (NECA; a nonselective AR agonist) increased CF in A2BKO mice (maximum of 34.6 ± 4.7 ml·min−1·g−1) to a significantly higher degree compared with WT mice (maximum of 23.1 ± 2.1 ml·min−1·g−1). Also, CGS-21680 (a selective A2A agonist) increased CF in A2BKO mice (maximum of 29 ± 1.9 ml·min−1·g−1) to a significantly higher degree compared with WT mice (maximum of 25.1 ± 2.3 ml·min−1·g−1). SCH-58261 (an A2A-selective antagonist) inhibited the NECA-induced increase in CF to a significantly higher degree in A2BKO mice (19.3 ± 1.6 vs. 0.5 ± 0.4 ml·min−1·g−1) compared with WT mice (19 ± 3.5 vs. 3.6 ± 0.5 ml·min−1·g−1). NECA did not induce any increase in CF in A2A/2BDKO mice, whereas a significant increase was observed in WT mice (maximum of 23.1 ± 2.1 ml·min−1·g−1). Furthermore, the mitochondrial ATP-sensitive K+ (KATP) channel blocker 5-hydroxydecanoate had no effect on the NECA-induced increase in CF in WT mice, whereas the NECA-induced increase in CF in WT (17.6 ± 2 ml·min−1·g−1), A2AKO (12.5 ± 2.3 ml·min−1·g−1), and A2BKO (16.2 ± 0.8 ml·min−1·g−1) mice was significantly blunted by the KATP channel blocker glibenclamide (to 0.7 ± 0.7, 2.3 ± 1.1, and 0.9 ± 0.4 ml·min−1·g−1, respectively). Also, the CGS-21680-induced (22 ± 2.3 ml·min−1·g−1) and BAY 60-6583-induced (16.4 ± 1.60 ml·min−1·g−1) increase in CF in WT mice was significantly blunted by glibenclamide (to 1.2 ± 0.4 and 1.8 ± 1.2 ml·min−1·g−1, respectively). In conclusion, this is the first evidence supporting the compensatory upregulation of A2AARs in A2BKO mice and demonstrates that both A2AARs and A2BARs induce CF changes through KATP channels. These results identify AR-mediated CF responses that may lead to better therapeutic approaches for the treatment of cardiovascular disorders.
Keywords: isolated mouse heart, A2B knockout mice, ATP-sensitive K+ channel
adenosine is an endogenous nucleoside that is released through the breakdown of adenine nucleotides. The cardiovascular effects of adenosine are mediated through the activation of its four subtypes of receptors (ARs), namely, A1, A2A, A2B, and A3. The activation of A1ARs results in negative chronotropic and ionotropic effects and a decrease in coronary flow (CF) (68), whereas other studies have suggested that the activation of both A1ARs or A3ARs before ischemia is cardioprotective (6, 30). However, adenosine has been shown to play a vasoregulatory role in human coronary arteries (16, 17, 62, 63). It is well established that the activation of A2AARs induces positive inotropic effects and that A2AARs play a major role in the regulation of CF in ex vivo models (67, 69). Additionally, in vivo studies in dogs have shown the involvement of A2AARs in CF (reactive hyperemia) regulation (5, 77) and, thus, support a physiological role for adenosine. Adenosine-induced effects are species dependent (20, 23). For example, it has been reported that guinea pig coronary vasodilatation is more sensitive to adenosine compared with rats (72). Furthermore, a few studies have shown that the A2BAR acts as a vasoconstricting as well as a vasodilating factor in different vascular beds (13, 57), in addition to playing a cardioprotective role during ischemia-reperfusion (14, 15, 30). Nevertheless, there are no reports fully elucidating the individual and relative roles of A2BARs in relation to A2AARs in coronary vasodilatation. Our group and others (48, 67) have previously suggested a role for A2BARs in coronary artery vasodilatation by means of indirect measures such as using nonselective agonist and antagonists and A2A knockout (KO) mice, which were in accordance with previous reports (39, 63) showing A2AR-mediated vasodilatation in isolated human coronary arteries. Furthermore, this laboratory (53, 71) has shown the presence of A2BARs and A2AARs in human and porcine coronary artery endothelial and vascular smooth muscle cells (SMCs) that may further support the involvement of A2AARs and A2BARs in the regulation of CF and a further possible interaction between these two receptor subtypes. Coexpression of more than one AR subtype has also been reported from other laboratories (36, 53) on human endothelial cells; however, there are no reports studying whether the coexpressed receptors interact. We (69) have recently shown an upregulation of A2BARs in the A2AKO mouse coronary artery, suggesting an interrelationship between A2AAR and A2BAR subtypes since A2BARs compensated for the downregulation/deletion of A2AARs. Additionally, since the involvement of A2AARs and A2BARs and their down- or upregulation has been reported in many pathophysiological conditions (2, 40) and since differential expression of ARs contributes to the functional heterogeneity of human endothelial cells (21), elucidating the presence of such compensatory mechanisms and signaling pathways for ARs is essential for better understanding of the heterogeneity of vascular responses and the regulation of CF.
Whereas the A2BAR has been reported to induce its cardiovascular effects through the activation of the arachidonic acid pathway in the vasculature (13), A2AAR-induced vascular effects have been demonstrated to partly involve cytochrome P-450 expoxygenases (50). We (69) have previously demonstrated that A2AAR-induced vasodilatation is partly due to the release of nitric oxide in the mouse aorta and coronary arteries. However, A2BAR-induced vasodilatation is both nitric oxide dependent and independent in the mouse aortic conduit artery and coronary arteries, respectively (1, 69). Although ATP-sensitive K+ (KATP) channels have been shown to be present in coronary artery cells (28, 65) and K+ channels, in general, are known to be involved in the A2AAR and A2BAR-mediated hyperpolarization of coronary arteries (31, 39, 52), the involvement of KATP channels in adenosine-induced coronary artery responses remains poorly understood.
The purpose of the present study was to directly and more fully elucidate the individual contribution of the A2BAR and its relationship to the A2AAR in the A2AR-mediated induction of coronary artery vasodilatation in light of KATP channel involvement. The hypotheses tested were 1) the A2BAR contributes to coronary vasodilatation, 2) A2AARs and A2BARs interact to induce coronary artery vasodilatation and they each compensate for the deletion/downregulation of the other, and 3) the A2AAR- and A2BAR-induced increase in CF is the result of the activation of KATP channels. Since there are inconsistencies between the results obtained from transgenic models compared with those obtained using pharmacological approaches (55), in this study we used both pharmacological and molecular (targeted gene-deleted mouse model) approaches. Furthermore, we used A2A/2B double-KO (DKO) mice to remove any possible compensatory upregulation of either A2AARs or A2BARs in CF responses.
METHODS
All experimental protocols were performed according to the West Virginia University guidelines and with approval of the Animal Care and Use Committee.
Generation of DKO mice.
A2A−/− mice (backcrossed 12 generations to the C57BL/6 background) were bred with A2B−/− mice (backcrossed 12 generations to the C57BL/6 background) to generate A2A+/−-A2B+/− double heterozygotes. These double heterozygotes were then intercrossed, and 1/16 of the resultant offspring were A2A−/−-A2B−/− DKO mice. Male and female DKO breeding pairs were established from these animals to produce DKO mice. Since both A2A−/− and A2B−/− mice were inbred on a C57BL/6 backgroup, DKO mice were also inbred.
Wild-type, A2AKO, A2BKO, and A2A/2BDKO mice.
Hearts were isolated from age-matched 10- to 14-wk-old mice. Wild-type (WT) mice of a mixed C57BL/6 genetic background were purchased from The Jackson Laboratory (Bar Harbor, ME). A2BKO and A2A/2BDKO mice of the same background were generously provided by S. Tilley. A2AKO mice of the same background were generously provided by C. Ledent. All four strains were bred at the West Virginia University animal facility as a subcolony of the original strain. Mice were kept in cages with 12:12-h light-dark cycles and maintained on a standard laboratory diet with access to water ad libitum. All animal care and experimentation were in accordance with the Institutional Animal Care and Use Committee of West Virginia University and the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals.
All chemicals were prepared as 10 mM stock using DMSO (Sigma, St. Louis, MO) followed by a serial dilution with 50% DMSO and distilled water, and a further dilution to the desired concentration was achieved with distilled water (final DMSO concentration of <1%). 5′-N-ethylcarboxamido adenosine (NECA), CG-S21680, 5-hydroxydecanoate (5-HD), and glibenclamide were from Sigma, and SCH-58261 was a gift from Schering-Plough. The A2B agonist BAY 60-6583 was a gift from Bayer. All stated concentrations are the actual drug concentration delivered as 1% of CF.
Langendorff-perfused mouse heart preparation.
Isolated heart experiments were performed in accordance with the methods published earlier from our laboratory (48). In brief, mice were anesthetized with pentobarbital sodium (50 mg/kg ip). A thoracotomy was performed, and hearts were removed into heparinized (5 U/ml) ice-cold Krebs-Hensleit (KH) buffer. Hearts were retrogradely perfused rapidly through the aorta cannulated with a 20-gauge, blunt-ended needle at a constant pressure of 80 mmHg and continuously gassed with 95% O2-5% CO2 KH buffer containing (in mM) 119 NaCl, 11 glucose, 22 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 2 pyruvate, and 0.5 EDTA at 37°C in a standard Langendorff fashion and allowed to beat spontaneously. The left atrium was removed, and the left ventricle (LV) was vented with a small polyethylene apical drain. A water-filled balloon made of plastic wrap was inserted into the LV across the mitral valve, which was connected to a fluid-filled pressure transducer by polyethylene tubing for the continuous measurement of LV developed pressure (LVDP). LV diastolic pressure was adjusted to a pressure of 2–5 mmHg by filling up the LV balloon. CF was measured via a Transonic flow probe (Transonic Systems, Ithaca, NY) in the aortic perfusion line. Baseline coronary flow, LVDP, and heart rate (HR; derived from the ventricular pressure trace) were monitored for 30 min during the equilibration period and recorded on a Power Lab data-acquisition system (AD Instruments, Colorado Springs, CO). Hearts with persistent arrhythmias or poor LVDP (<80 mmHg) during the equilibration period were excluded from the study. To rule out tachyphylaxis or desensitization, at the end of each antagonist experiment, after another washout period, a repeat infusion of the agonist was performed.
Experimental protocols.
After 30 min of equilibrium, baseline CF, HR, and LVDP were measured in WT, A2BKO, and A2A/2BDKO isolated hearts. Concentration-response curve (CRCs; 10−10–10−6 M) were performed for BAY 60-6583 (a selective A2B agonist), NECA (a nonselective AR agonist), and CGS-21680 (a selective A2A agonist) by infusing the drugs into the coronary perfusate through an injection port directly proximal to the aortic cannula using a microinjection infusion pump (Harvard Apparatus, Holliston, MA). After an equilibration period, each heart was perfused with increasing concentrations of the selected drug to develop a CRC. Each drug concentration was given for 5 min with 10-min intervals to allow complete drug washout (or using recovery to baseline parameters). All concentrations are the actual concentration in the perfusate. Data were collected at the end of each infusion period and washout time, which was used as a reference for normalizing responses to the subsequent drug concentration. In the results, actual values from CRCs (Figs. 1, 2, 4, and 6) are presented as the maximum response and values from the use of a single drug concentration in the presence and absence of an antagonist (Figs. 5 and 7–9) are presented as the actual drug-induced effect (maximal response minus the baseline).
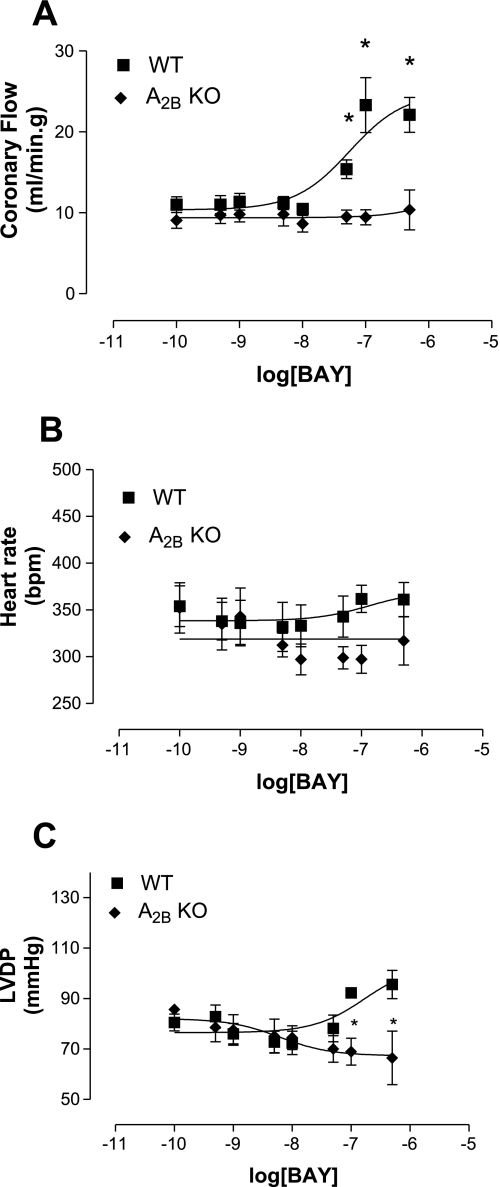
Fig. 1.
Effect of BAY 60-6583 (BAY) in A2B adenosine receptor (AR) knockout (A2BKO; n = 4) and wild-type (WT; n = 7) mice on coronary flow (CF; A), heart rate [HR; in beats/min (bpm); B] and left ventricular developed pressure (LVDP; C). Values are means ± SE. *Significant difference compared with WT mice (P < 0.05).
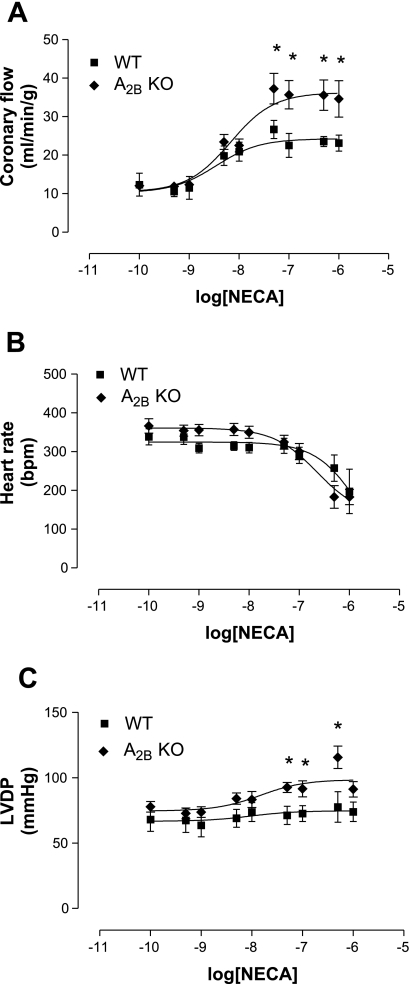
Fig. 2.
Effect of 5′-N-ethylcarboxamido adenosine (NECA) in A2BKO (n = 8) and WT (n = 9) mice on CF (A), HR (B), and LVDP (C). Values are means ± SE. *Significant difference compared with WT mice (P < 0.05).
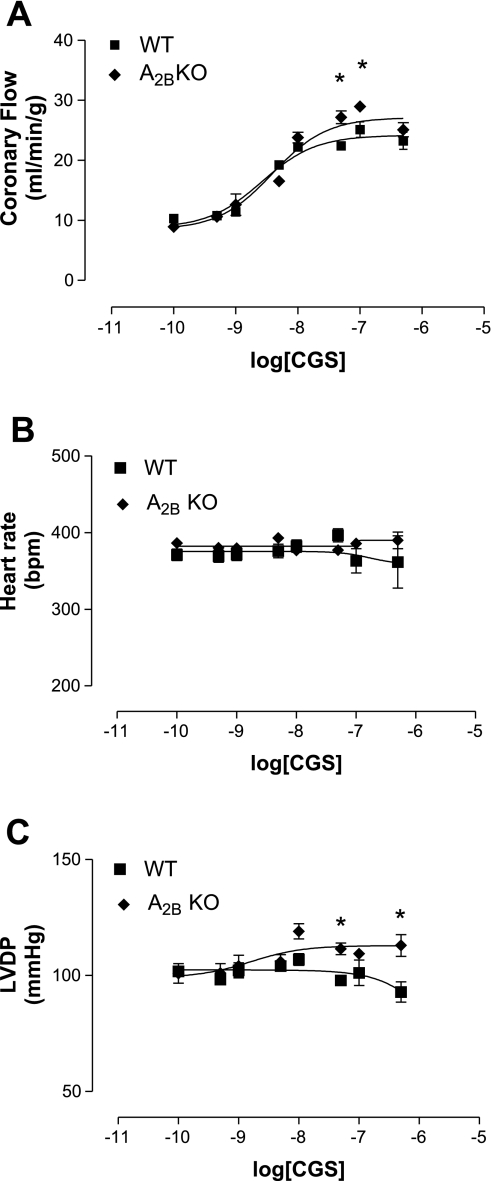
Fig. 4.
Effect of CGS-21680 (CGS) in A2BKO (n = 7) and WT (n = 7) mice on CF (A), HR (B), and LVDP (C). Values are means ± SE. *Significant difference compared with WT mice (P < 0.05).
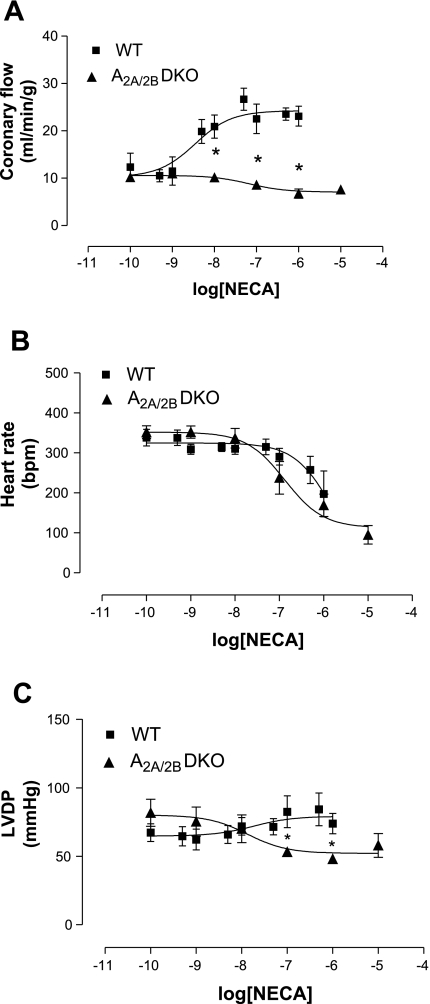
Fig. 6.
Effect of NECA in A2A/2BAR double-KO (A2A/2BDKO; n = 5) and WT (n = 9) mice on CF (A), HR (B), and LVDP (C). Values are means ± SE. *Significant difference compared with WT mice (P < 0.05).
Fig. 5.
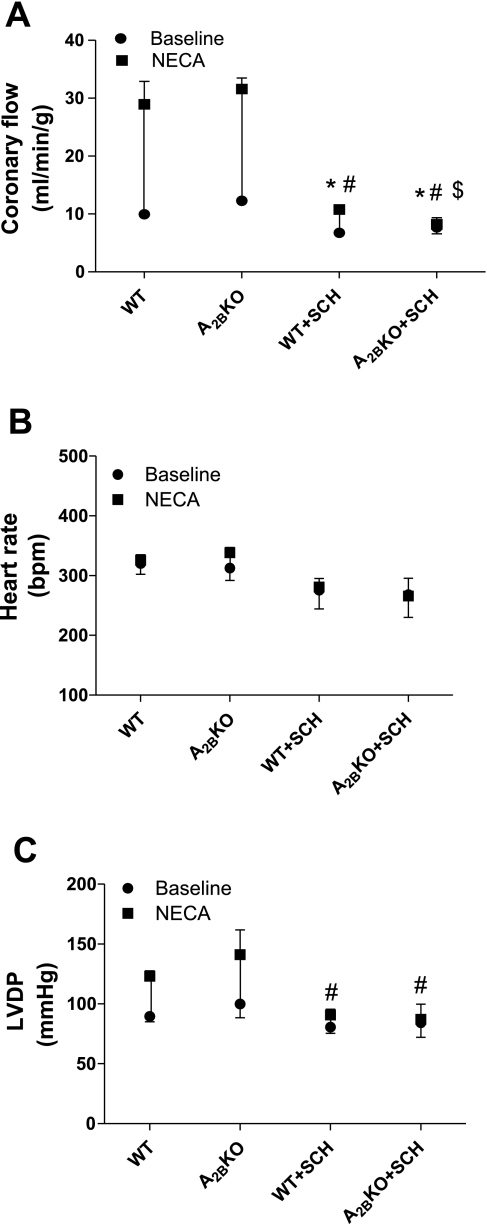
Effect of SCH-58261(SCH; 1 μM) on the NECA-induced increase in CF (A), HR (B), and LVDP (C) in WT (n = 6) and A2BKO (n = 4) mice. Values are means ± SE. *Significant difference between drug-induced effects in the presence of antagonist compared with their corresponding control; #Significant difference in baselines in the presence of antagonist compared with their corresponding control; $significant difference between WT and A2BKO antagonist-treated groups (P < 0.05).
Fig. 7.
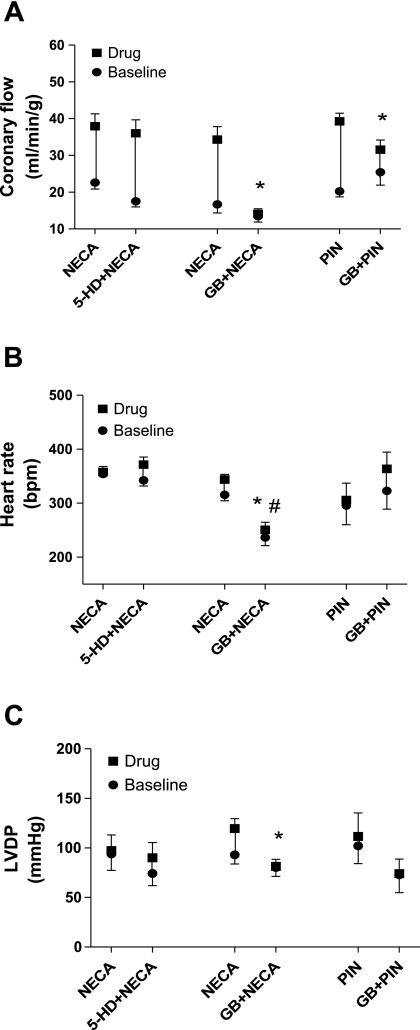
Effect of 5-hydroxydecanote (5-HD) on the NECA-induced (n = 5) increase in CF (A), HR (B), and LVDP (C) in WT mice and the effect of glibenclamide (GB) on NECA-induced (n = 4) and pinacidil (PIN)-induced (n = 6) increases in CF (A), HR (B), and LVDP (C) in WT mice. Values are means ± SE. *Significant difference in drug-induced effects in the presence of antagonist compared with their corresponding control; #Significant difference in baselines in the presence of antagonist compared with their corresponding control (P < 0.05).
Fig. 9.
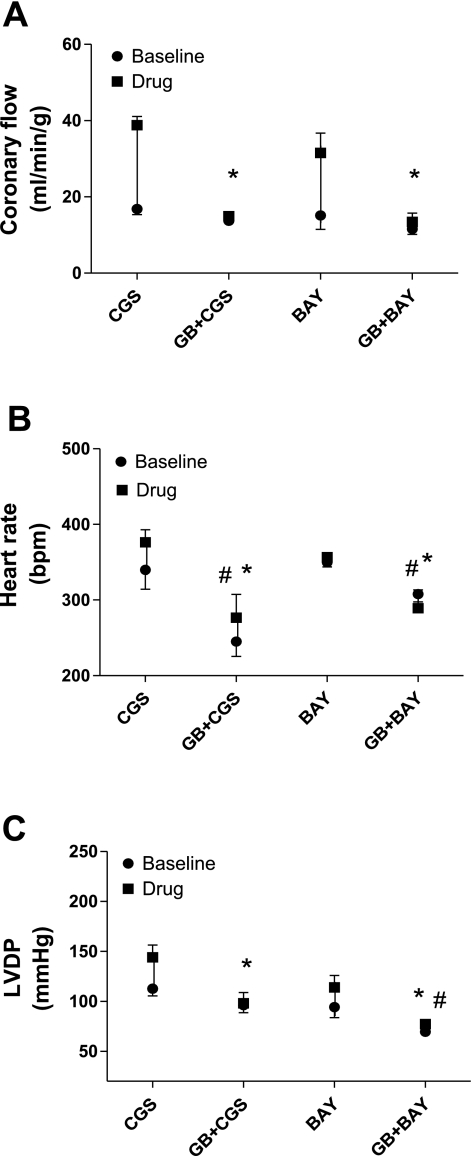
Effect of GB on CGS-induced (n = 5) and BAY-induced (n = 4) increases in CF (A), HR (B), and LVDP (C) in WT mice. Values are means ± SE. *Significant difference in drug-induced effects in the presence of antagonist compared with their corresponding control; #significant difference in baselines in the presence of antagonist compared with their corresponding control (P < 0.05).
Antagonist experiment protocol.
After a 30-min equilibration period and the measurement of baseline parameters, the agonists NECA (10−8 M in A2BKO mice or 10−8 M in A2AKO mice, the closest concentration to the EC50 obtained from previously performed experiments; see Figs. 7 and 8), BAY 60-6583 (10−7 M; see Fig. 9), and CGS-21680 (5 × 10−9 M; see Fig. 9) were infused for 5 min at a 1% rate of CF. The plateau effect of CF, HR, and LVDP were recorded, and a 10-min washout interval was allowed to reach baseline. This was followed by the infusion of the antagonists SCH-58261 (10−6 M, an A2A-selective antagonist; see Fig. 5) (71) or glibenclamide (10−5 M; see Figs. 7–9) at a 1% rate of CF for at least 10 min, after which the agonist was also added to the infusion line for an additional 5 min (for a total of 15 min). The baseline at the end of the antagonist infusion was treated as the new baseline for the subsequent agonist responses. The data at the end of the 15 min (the end of the infusion of both agonists and antagonists) were used to compare with data obtained from the first infusion of the agonist alone. At the end of the experiment, after at least 10 min of washout and reaching the baseline, the agonist was again infused to check for tachyphylaxis or desensitization.
Fig. 8.
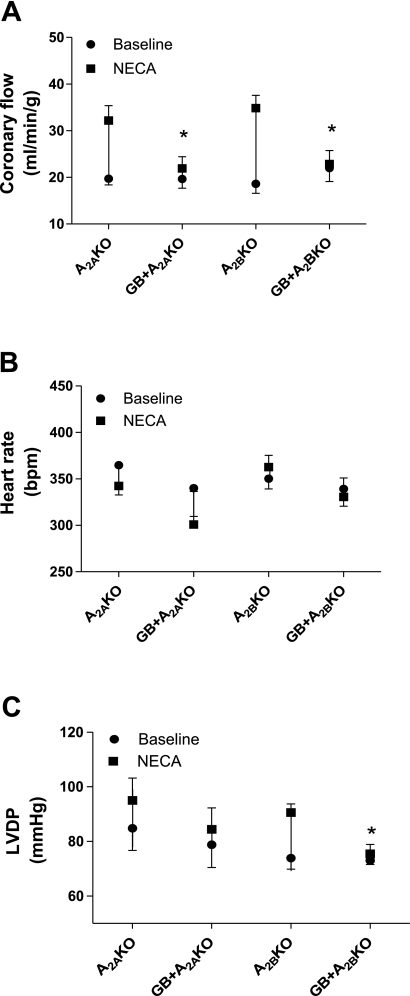
Effect of GB on the NECA-induced increase in CF (A), HR (B), and LVDP (C) in A2AKO (n = 5) and A2BKO (n = 5) mice. Values are means ± SE. *Significant difference in drug-induced effects in the presence of antagonist compared with their corresponding control (P < 0.05).
Western blot analysis.
Mouse mesenteric arteriole (up to third-branch arterioles) homogenates were obtained from isolated and cleaned tissue using ice-cold lysis buffer consisting of 0.05 M Tris-buffered saline (TBS; pH 7.4), 1% Triton X-100, 0.25% sodium deoxycholate, 150 mM sodium chloride, 1 mM EDTA, 1 mM PMSF, and Halt Protease Inhibitor Cocktail (Thermo Scientific) using a glass homogenizer. Samples were then centrifuged for 15 min at 13,000 rpm, and the supernatant was stored at −80°C. Protein extracts (30 μg protein/well) were separated on NuPAGE 4–12% bis-Tris Gels (Invitrogen) along with the Novex Sharp Protein Standard (3.5–260 kDa, Invitrogen) run in parallel. Proteins were then transferred to a polyvinylidene difluoride membrane (Millipore), blocked in 5% milk for 1 h, and then probed with anti-A2AAR rabbit polyclonal IgG antibody (45) developed in our laboratory for the detection of A2AAR protein with a dilution of 1:1,000 in TBS-Tween + 0.5% milk overnight at 4°C or with anti-β-actin (Santa Cruz Biotechnology) at a dilution of 1:5,000 at room temperature for 1 h. This was followed by an incubation with a secondary horseradish peroxidase-conjugated antibody (anti-mouse and anti-rabbit IgGs, respectively, Santa Cruz Biotechnology) for 1 h at room temperature. For the detection of bands, membranes were treated with an enhanced chemiluminescence reagent (GE Healthcare) for 1 min and subsequently exposed to ECL Hyperfilm (GE Healthcare). Relative band intensities were quantified by densitometry, and each sample was normalized to the β-actin values and calibrated to the A2AAR protein expression in WT mice.
Data and statistical analysis.
Western blot data and baseline functional data of A2AKO, A2BKO, and A2A/2BDKO groups were compared with the WT group and analyzed using t-test. Differences in dose-response curves and responses to each drug at the same concentration between WT, A2BKO, A2AKO, and A2A/2BDKO hearts were analyzed using two-way ANOVA for repeated measures. Antagonist-induced responses (HR, LVDP, and CF) for WT, A2AKO, and A2BKO hearts were compared using a t-test. Statistical comparisons were done on percent changes. Results were considered significant when P < 0.05. Values are means ± SE. EC50 values were calculated using Prism (Graphpad Software, La Jolla, CA).
RESULTS
Baseline function in WT, A2AKO, A2BKO, and A2A/2BDKO isolated hearts.
Table 1 shows the baseline parameters for CF, HR, and LVDP for A2AKO, A2BKO, A2A/2BDKO, and WT mice after 30 min of equilibration of the isolated hearts, as described in methods. Average body weights of A2BKO mice were significantly lower compared with WT mice, whereas heart weight-to-body weight ratios were not significantly different in any KO mice compared with WT mice. Furthermore, no significant baseline differences were observed in CF and HR in any of the KO mice compared with WT mice, whereas LVDP was significantly higher in A2A/2BDKO hearts compared with WT hearts (Table 1).
Table 1.
Baseline data for WT, A2AKO, A2BKO, and A2A/2BDKO mouse hearts
| WT | A2AKO | A2BKO | A2A/2BDKO | |
|---|---|---|---|---|
| No. of mice | 30 | 12 | 29 | 15 |
| Body weight, g | 25 ± 0.6 | 25 ± 1.2 | 23.8 ± 0.6* | 24.7 ± 1.1 |
| Heart weight, g | 0.1 | 0.1 | 0.1 | 0.1 |
| Heart weight-to-body weight ratio, % | 0.5 ± 0.4 | 0.4 | 0.4 ± 0.1 | 0.4 |
| Coronary flow, ml•min−1•g−1 | 15.2 ± 0.8 | 17.2 ± 0.9 | 14.5 ± 0.7 | 15.6 ± 1.8 |
| Heart rate, beats/min | 341.6 ± 10.3 | 356.8 ± 14.5 | 345.1 ± 7.8 | 330.4 ± 17.8 |
| Developed pressure, mmHg | 80.3 ± 4.2 | 81.2 ± 4.8 | 83.3 ± 3.3 | 97.1 ± 6.2* |
Values are means ± SE. WT, wild type; KO, knockout; DKO, double KO. All parameters were collected after 30 min of equilibration in a Langendorff preparation.
Significantly different compared with WT hearts.
Effect of A2BAR activation on CF.
BAY 60-6583 (an A2B-selective agonist) caused a concentration-dependent increase in CF in WT mice, whereas no effect was observed in A2BKO mice (Fig. 1A). BAY 60-6583 increased CF in WT mice (with a maximum of 23.3 ± 9 ml·min−1·g−1, n = 7) to a significantly higher degree compared with A2BKO mice (no response; Fig. 1A). BAY 60-6583 had no effect on HR in either WT or A2BKO animals (Fig. 1B), whereas it induced a significant increase in LVDP from baseline in WT animals to a maximum of 95.6 ± 14.9 mmHg (n = 7; Fig. 1C). BAY 60-6583 had no effect on LVDP in A2BKO hearts (Fig. 1C).
Contribution of A2AARs in AR-mediated coronary vasodilatation of A2BKO mice.
Since we wanted to test if the A2AAR compensates for the A2BAR in the production of coronary vasodilatation in A2BKO mice, we used NECA (a nonselective AR agonist) in A2BKO and WT mice. NECA increased CF in A2BKO mice (with a maximum of 34.6 ± 4.7 ml·min−1·g−1, n = 8, EC50: 6.8 × 10−9 ± 0.8 M) to a significantly higher degree compared with WT mice (with a maximum of 23.1 ± 2.1 ml·min−1·g−1, n = 9, EC50: 3.5 × 10−9 ± 0.6 M; Fig. 2A). Furthermore, NECA induced a significant increase in LVDP of A2BKO hearts compared with WT hearts with a maximum increase of 115.5 ± 8.5 and 77.5 ± 11.6 mmHg, respectively (Fig. 2C), whereas a decrease in HR was noted in both WT and A2BKO mice with no significant difference between the two groups (Fig. 2B).
Upregulation of A2AAR expression in A2BKO isolated mesenteric arterioles.
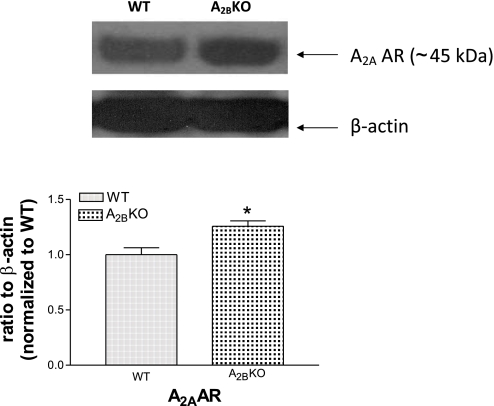
We used mesenteric arterioles as resistance vessels to analyze A2AAR expression levels in A2BKO mice. A2AAR protein was significantly increased in A2BKO isolated mesenteric arterioles compared with WT isolated mesenteric arterioles (25.7% higher expression, n = 4, P < 0.05; Fig. 3).
Fig. 3.
A2AAR expression levels in WT (n = 4) and A2BKO (n = 4) isolated mesenteric arterioles. Results are representative of n = 4 for each group. Values are means ± SE. *Significant difference compared with WT mesenteric arterioles (P < 0.05).
Upregulation of A2AARs in A2BKO mice.
CGS-21680 increased CF in A2BKO mice (27.2 ± 2.6 ml·min−1·g−1 at 5 × 10−8 M and 29 ± 1.9 ml·min−1·g−1 at 10−7 M, EC50: 3.9 × 10−9 ± 0.7 M, n = 7) to a significantly higher degree compared with WT mice (22.4 ± 1.5 ml·min−1·g−1 at 5 × 10−8 M and 25.1 ± 2.3 ml·min−1·g−1 at 10−7 M, EC50: 2.6 × 10−9 ± 0.7 M, n = 7; Fig. 4A). Additionally, CGS-21680 caused a significant increase in LVDP in A2BKO hearts (111.5 ± 6.7 mmHg at 5 × 10−8 M and 113 ± 8.1 mmHg at 5 × 10−7 M) compared with WT hearts (97.9 ± 4.9 mmHg at 5 × 10−8 M and 93 ± 6.1 mmHg at 5 × 10−7 M), whereas there were no differences between the HR induced by CGS-21680 when WT and A2BKO mice were compared (Fig. 4, B and C).
Upregulation of A2AARs in A2BKO mice.
To further confirm the upregulation of A2AARs in A2BKO mice as a compensatory response for the regulation of CF, we used SCH-58261 (an A2A-selective antagonist) and tested its effect on the NECA (10 nM)-induced increase in CF in WT and A2BKO mice. SCH-58261 (1 μM) significantly decreased the baseline CF of WT (from 9.9 ± 0.7 to 6.8 ± 0.7 ml·min−1·g−1) and A2BKO (from 12.3 ± 0.6 to 7.7 ± 1.1 ml·min−1·g−1) mice (Fig. 5A). SCH-58261 also caused a significantly higher inhibition of the NECA-induced increase in CF in A2BKO mice (19.3 ± 1.6 vs. 0.5 ± 0.4 ml·min−1·g−1, n = 4) compared with WT mice (19 ± 3.5 vs. 3.6 ± 0.5 ml·min−1·g−1, n = 6; Fig. 5A). NECA (10 nM) and SCH-58261 had no significant effect on HR in both WT and A2BKO animals (Fig. 5B). However, NECA significantly increased LVDP in both A2BKO (from 99.9 ± 11.5 to 141 ± 20.8 mmHg, n = 4) and WT (from 89.5 ± 4.4 to 123.1 ± 4.5 mmHg, n = 6) hearts (Fig. 5C). SCH-58261 significantly reduced the baseline LVDP in both A2BKO (from 99.9 ± 11.6 to 84.2 ± 12.2 mmHg) and WT (from 89.5 ± 4.4 to 80.6 ± 5.2 mmHg) hearts. Additionally, SCH-58261 significantly reduced the NECA-induced increase in LVDP in both A2BKO and WT groups (34.9 ± 5.9 vs. 2.8 ± 1.5 mmHg, n = 4, and 33.6 ± 5.7 vs. 6.7 ± 4 mmHg, n = 4, respectively) compared with their respective controls (Fig. 5C).
Involvement of A2AARs and A2BARs in the production of coronary vasodilatation.
To rule out the compensatory response between A2AARs and A2BARs, NECA was used in A2A/2BDKO mice (Fig. 6). NECA did not induce any increase in CF in A2A/2BDKO mice (n = 5), whereas a concentration-dependent response was observed in WT mice, with a maximum increase up to 23.1 ± 2.1 ml·min−1·g−1 (n = 9; Fig. 6A). Additionally, the NECA-induced effect on LVDP was significantly lower in A2A/2BDKO hearts (53 ± 2 mmHg at 10−7 M, EC50: 1.3 × 10−8 ± 0.4 M) compared with WT hearts (82.5 ± 11.6 mmHg at 10−7 M, EC50: 1.8 × 10−8 ± 0.2 M; Fig. 6C). However, the decrease in HR in WT hearts was not different compared with A2A/2BDKO hearts (Fig. 6B).
Involvement of KATP channels in the A2AAR- and A2BAR-induced increase in CF.
We used 5-HD (a mitochondrial KATP channel blocker, 10−4 M) to test its effect on the NECA (10−8 M)-induced increase in CF in WT mice. NECA concentrations were chosen based on the EC50 values obtained from previously performed experiments (10−8 M in WT and A2BKO mice and 5 × 10−8 M in A2AKO mice). 5-HD had no effect on the NECA-induced increase in CF (15.3 ± 2.8 vs. 18.5 ± 3.1 ml·min−1·g−1, n = 5; Fig. 7A). Additionally, 5-HD had no effect on the NECA-induced changes in HR and LVDP (Fig. 7, B and C). Furthermore, we used glibenclamide (a KATP channel blocker) to test its effect on the NECA-induced increase in CF in WT, A2AKO, and A2BKO mice (Figs. 7A and 8A). Glibenclamide significantly reduced the NECA-induced increase in CF in WT (17.6 ± 2 vs. 0.7 ± 0.7 ml·min−1·g−1, n = 4; Fig. 7A), A2AKO (12.5 ± 2.3 vs. 2.3 ± 1.1 ml·min−1·g−1, n = 5; Fig. 8A), and A2BKO (16.2 ± 0.8 vs. 0.9 ± 0.4 ml·min−1·g−1, n = 5; Fig. 8A) mice. However, glibenclamide did not change the NECA-induced effect on HR in A2AKO and A2BKO mice, whereas it significantly reduced the NECA-induced effect on HR in WT mice compared with the control (28.5 ± 9.7 vs. −8.1 ± 7.6 beats/min), where it also significantly reduced the basal HR from 315.5 ± 11.1 to 236.6 ± 15.2 beats/min (Figs. 7B and 8B). Furthermore, glibenclamide did not change the NECA-induced effect on LVDP in A2AKO hearts, whereas it significantly reduced LVDP in WT (26.4 ± 9.9 vs. 1.2 ± 3.6 mmHg) and A2BKO (16.7 ± 3.3 vs. 2.4 ± 2.3 mmHg, n = 5) hearts (Figs. 7C and 8C). To confirm the effect of glibenclamide, we tested its effect on pinacidil (a KATP channel opener as a positive control). Glibenclamide significantly reduced the pinacidil-induced increase in CF from baseline in WT mice (19 ± 3 vs. 10.2 ± 1.8 ml·min−1·g−1, n = 6). However, glibenclamide did not change the pinacidil-induced effect on HR and LVDP in WT mice (Fig. 7).
Additionally, we tested the effect of glibenclamide on the CGS-21680 (an A2A-selective agonist)- and BAY 60-6583 (an A2B-selective agonist)-induced increases in CF in WT mice (Fig. 9A). Glibenclamide significantly reduced the effect of CGS-21680 (5 × 10−9 M, 22 ± 2.3 ml·min−1·g−1, n = 5) and BAY 60-6583 (10−7 M, 16.4 ± 1.6 ml·min−1·g−1, n = 4) on CF to 1.2 ± 0.4 (n = 5) and 1.8 ± 1.2 (n = 4) ml·min−1·g−1, respectively (Fig. 9A). In the presence of glibenclamide, both the baseline and drug-induced effects on HR were significantly decreased compared with their controls (Fig. 9B). Additionally, glibenclamide significantly reduced the CGS-21680- and BAY 60-6583-induced increases in LVDP (31.6 ± 13.1 vs. 1.9 ± 4.7 mmHg, n = 5, and 20.1 ± 1.7 vs. 7.7 ± 5.5 mmHg, n = 4, respectively; Fig. 9C).
DISCUSSION
In this study, we further elucidated the contribution of the A2BAR and its relationship to the A2AAR in A2AR-mediated coronary artery vasodilatation. With the use of A2A/2BDKO mice, we also showed, for the first time, that A2AARs and A2BARs, of the four AR subtypes, contribute to coronary artery vasodilatation and that downregulation/deletion of either of A2AARs or A2BARs leads to a compensatory upregulation of the other AR, thus suggesting an interrelationship between these two receptor subtypes. Furthermore, we showed, for the first time, the involvement of nonmitochondrial KATP channels in A2BAR-mediated coronary artery vasodilatation and finally showed the direct involvement of KATP channels in the A2AAR-mediated increase in CF using AR KO mice.
The role of adenosine in tissue protection may involve mechanisms such as increasing the tissue blood flow and protecting against ischemic damage (37). Adenosine is well known to play a vasoregulatory role in human coronary arteries (16, 17, 62, 63). We and others (5, 63, 67, 69) have also previously demonstrated the pivotal role of A2AARs in CF regulation. We also have preliminary data showing the involvement of A2ARs in coronary reactive hyperemia that indirectly supports a physiological role for adenosine in CF regulation (data not shown), which is in support of previous in vivo and ex vivo reactive hyperemia studies (5, 12, 77). Additionally, it has been suggested that A2AAR activation contributes to basal tone in the coronary circulation through the release of nitric oxide (69). Our present data (Fig. 5A) also support such a role for the A2AAR since SCH-58261 (an A2A-selective antagonist) significantly reduced the baseline CF.
Due to its low affinity for adenosine, the A2BAR is known to be activated in conditions where a significant increase in adenosine levels is observed, such as in ischemic conditions (25). Therefore, the A2BAR may be more involved in pathophysiological conditions rather than physiological situations. However, the cardiovascular effects of the A2BAR and its role in coronary vasodilatation still remain to be fully elucidated. It has been reported that the A2BAR plays a role in ischemia-reperfusion and preconditioning (15). Additionally, previous studies have used indirect measures such as nonselective agonist/antagonist (alloxazine), which is only about 10-fold more selective toward A2BARs relative to A2AARs (8), or A2AKO mice (without the use of A2BKO) to indirectly study the role of A2BARs in CF regulation (48, 67). In this study, we demonstrated the contribution of both A2AARs and A2BARs in coronary artery vasodilatation, where we further clarified the individual role of A2BARs with the use of the selective A2BAR agonist BAY 60-6583 and A2BKO mice (Fig. 1). We also demonstrated that A2AARs and A2BARs, out of all ARs, are involved in inducing coronary artery vasodilatation with the use of A2A/2BDKO mice (Fig. 6). Reports suggesting that A1ARs and A3ARs negatively modulate the role of A2AARs and A2BARs in CF regulation (66, 68) and the presence of only A2AARs and A2BARs on human and porcine coronary endothelial cells (53) may also indirectly support their involvement in coronary artery vasodilatation.
Azakura et al. (2) suggested that the impairment of adenosine-related signaling contributes to the pathophysiology of congestive heart failure. In addition, we have previously demonstrated that the A2AAR is upregulated in high-salt diet-fed mice compared with normal-salt diet-fed mice (51) and that failure to upregulate the A2AAR-induced pathway contributes to the development of hypertension (44). Furthermore, a few studies have shown that the A2BAR is selectively upregulated in ischemic mouse hearts and by hypoxic conditions (3, 18) and that differential expression of ARs contributes to the functional heterogeneity of human endothelial cells (21). We (69) have recently shown the upregulation of A2BARs in A2AKO mice, and in the present study, we demonstrated the upregulation of A2AARs in A2BKO mice (Figs. 2–5), suggesting the presence of a relationship between these two ARs. Therefore, further understanding of the relationship between A2AARs and A2BARs may help us toward the development of newer therapeutic approaches. Also, the observation that the downregulation/deletion of A2AARs or A2BARs leads to a compensatory upregulation of A2BARs and A2AARs, respectively, itself supports an important regulatory function for each of these AR subtypes. It is interesting to mention that this trend has been observed in hypoxia of human umbilical vein endothelial cells and bronchial SMCs, where hypoxia modulates the expression of ARs by decreasing A2AAR mRNA levels but increasing A2BAR mRNA levels (22). It is also noteworthy to mention that adenosine acts as a feedback mediator in the heart during hypertrophy and hypoxia (34), during which conditions other studies (2, 3, 18, 22) have shown the modification of AR expression. These kinds of interactions have also been observed between other receptor subtypes, such as adrenoceptors (ADRs), where the α1ADR compensates for the downregulation of the βADR in pathological conditions such as cardiac remodeling (75).
Adenosine and its agonist are currently being used in clinical settings. The vasoregulatory properties of ARs are used clinically in humans for the diagnosis of coronary artery disease through the use of adenosine (Adenoscan) as a substitute for exercise stress testing in myocardial perfusion imaging (7). The use of Adenoscan has its own limitations due to it blocking atrioventricular conduction [due to the activation of A1ARs (4, 7)] and inducing bronchospasm [probably due to the activation of A1ARs, A3ARs, or A2BARs (24, 59)]. However, these side effects may be alleviated by the development of more A2AR subtype-selective agonists. A new A2AAR-selective agonist, called regadenoson (Lexiscan), has been developed and approved by the Federal Drug Administration, which is being used clinically in myocardial perfusion imaging. During this use of adenosine and its agonist in determining coronary reserve, the assessment of the true maximal dilatation of coronary arteries is an important factor (34). However, it has been reported that maximal dilatation of coronary arteries with vasodilators such as adenosine does not eliminate all coronary vasomotor tone and that the role of hemodynamic changes should also be considered (34, 73). Nevertheless, the knowledge of the presence of a compensatory interaction between A2AARs and A2BARs may be helpful since most cardiac imagings are done in patients with vascular disease, where AR expression may have been already modified due to the disease condition. Furthermore, adenosine is being investigated as a treatment during percutaneous coronary intervention to reduce the myocardial reperfusion injury in patients with myocardial infarction (which is usually associated with worsening of cardiac injury and function). In these patients, the intracoronary injection of adenosine is followed by a better myocardial salvage and thus prevention of LV remodeling, ejection fraction, decreased infarct size, and ST segment elevation resolution (10, 26, 46). However, Desmet et al. (11) demonstrated that adenosine significantly ameliorates ST segment resolution, whereas it does not improve myocardial salvage or coronary blood flow. Additionally, in another study, Ross et al. (61) showed a relationship between infarct size and the primary clinical end point (death or heart failure) where adenosine significantly reduced the infarct size, whereas it did not improve the clinical outcome.
It is well known that arterioles, compared with coronary conduit arteries, are the major players in flow regulation, and, as previously reported, the A2AAR may be the major coronary artery vasodilator compared with A2BARs. Furthermore, large coronary arterioles have been shown to be less responsive to adenosine than small ones, and Hein et al. (32) have reported that mainly A2AARs are expressed in coronary arterioles (42). The same disparity of receptor subtype distribution has also been seen with ADRs, with adrenergic constriction of large arterioles being mediated by α1ADRs, whereas in terminal arterioles, α2ADRs exert this function (56). Additionally, in coronary arterioles, a greater α2ADR response has been shown compared with larger coronary arteries (70). Thus, showing the upregulation of A2AARs in A2BKO mice would most likely not be reflected on larger coronary conduit arteries such as the left anterior descending coronary artery. Isolation of the mouse left anterior descending coronary artery and other large coronary conduit arteries is technically difficult but possible; however, isolation of coronary resistance arterioles from the mouse heart is almost impossible, and, to our knowledge, no one has been able to isolate coronary arterioles so far, which is a limitation in our study. However, A2AAR protein expression in mouse mesenteric artery resistance vessels (up to the third branch of arterioles) showed the upregulation of A2AARs in A2BKO mice, which confirms the compensatory upregulation of A2AARs in A2BKO resistance vessels (Fig. 3).
The occurrence of the upregulatory mechanism phenomenon in our study may be TNF-α induced and posttranscriptionally regulated (41) or due to DNA methylation, since this may regulate AR cell surface expression levels (9). Furthermore, HIF-1 has also been suggested to upregulate the expression of A2BARs under hypoxic conditions (76). Although the underlying mechanism may not be understood at this time, it seems that this relationship only exists between A2AARs and A2BARs and not between A1ARs or A3ARs, since there was no significant difference between the CRCs for 2-chloro-N6-cyclopentyladenosine (an A1-selective agonist) and 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide (an A3AR-selective agonist) in A2A/2BDKO mice compared with WT mice (data not shown).
K+ channels have been suggested to be involved in the regulation of blood flow and blood pressure and in AR signaling pathways (29, 49, 78). For example, inward rectifier K+ channels may play a role, since their presence has been reported on coronary artery SMCs (54, 60, 65) in addition to their role as the dominant K+ conductance in resting membrane potential (38). Moreover, adenosine has been reported to activate KATP channels in rabbit mesenteric arteries and isolated coronary artery SMCs (58, 65). Others have demonstrated the presence of KATP channels on coronary arteries and coronary artery SMCs (28, 65) and their role in A2A-induced effects in reactive hyperemia and retinal microvessel dilation (5, 33, 77). However, there are no reports, to our knowledge, suggesting the involvement of KATP channels in A2AAR- and A2BAR-induced changes in mouse CF.
In this study, we used the mitochondrial KATP channel blocker 5-HD and glibenclamide to test the involvement of KATP channels in A2AAR- and A2BAR-induced CF changes. Glibenclamide blocked the NECA-induced increase in CF in A2AKO and A2BKO mice and the BAY 60-6583- and CGS21680-induced increases in CF in WT mice. However, 5-HD had no effect on the NECA-induced increase in CF, which may suggest a role for nonmitochondrial KATP channels in both A2AAR and A2BAR signaling pathways (Figs. 7, 8, and 9A). However, this study could not show if the KATP channels were located on endothelial cells or SMCs. This is important since endothelial dysfunction is an early risk factor for cardiovascular diseases, where a reduced adenosine response has been reported (19). Furthermore, Wang et al. (74) suggested that the activation of endothelial KATP channels might result in protection against endothelial dysfunction. We think that the adenosine-induced activation of KATP channels may be indirectly through the release of some other mediators, such as hydrogen peroxide (27), which has previously been suggested to be the endothelium-derived hyperpolarizing factor (47) and which may be involved in cardiovascular dysfunction (64). Elucidations of these signaling pathways will help us better understand the underlying cause of the reduced adenosine response in cardiovascular diseases.
The availability of gene-deleted KO mice has been an important tool to dissect the physiological and pharmacological pathways for elucidating the role of a single receptor. However, in some instances, mice lacking a specific receptor gene exhibit phenotypic differences, such as the higher blood pressure in A2AKO mice (43). Therefore, in this study, we used both pharmacological and molecular (single KO and DKO) approaches to confirm our results. We also evaluated the baseline parameters of WT and KO hearts (Table 1). The baseline LVDP of A2A/2BDKO hearts was significantly higher compared with WT hearts, which may be attributed to alterations at second messenger or translational levels. The relationship between A2AARs and A2BARs and the activation of other signaling pathways, such as an increase in the levels of cAMP and Ca2+, may compensate for the deletion of A2A/2BARs (35, 37). Even ADRs may compensate for the absence of A2AARs and A2BARs, since there are reports showing direct and indirect anti-β-adrenergic effects of adenosine, suggesting the presence of an interaction between these two different G protein-coupled receptors (49). Further experiments are required to better understand the relationship between these two receptor classes. There were no significant differences in baseline HR, LVDP, and CF of A2AKO and A2BKO mice compared with WT mice. We also tested A2BKO mice for endothelium functionality using bradykinin, where we found no significant differences between A2BKO and WT mice, thus showing the presence of a normal functional endothelium in A2BKO mice (data not shown).
CGS-21680 and NECA induced an increase in LVDP of WT and A2BKO hearts but not A2A/2BDKO hearts. BAY 60-6583 also induced an increase in LVDP of WT mice. These data support our previous report (69) showing that A2AARs and A2BARs may be involved in cardiac contractility and positive inotropic effects (Figs. 1, 2, and 4C). Changes in CF can affect contractility (Gregg effect), which is an inherent problem with the isolated perfused heart preparation. However, as shown in the present study (Figs. 2 and 6), the increase in CF comes earlier than the changes in LVDP, which may suggest that the observed effects of A2AR activation on LVDP may not be due to Gregg's effect. The opening of KATP channels may also affect contractility independent of CF changes, which may be independent of nonmitochondrial KATP channels in the present study since there were no differences observed in NECA-induced changes in LVDP in the presence of 5-HD (a mitochondrial KATP channel blocker), whereas a significant decrease in NECA-induced changes in LVDP was shown in the presence of glibenclamide (a nonselective KATP channel blocker; Fig. 7). Additional studies are needed to define the role of A2ARs on LVDP. However, our data from A2A/2BDKO mice are very important since they show that only A2AARs and A2BARs are involved in LVDP and coronary vasodilatation, which could be further exploited for therapeutic approaches such as heart failure.
The present study also confirms our previous finding (68) that the A1AR plays a role in HR, whereas the A2AAR and A2BAR do not, since BAY 60-6583 (Fig. 1B) and CGS-21680 (Fig. 4B) did not affect HR, whereas NECA (a nonselective agonist) decreased HR (Fig. 2B). Additionally, our data showed a decrease in the baseline HR in the presence of glibenclamide (Figs. 7 and 9B), which could be due to the presence of KATP channels on atria and ventricles (79) and the KATP channel-dependent K+ efflux-induced shortening of cardiac action potentials and, hence, a decrease in HR (79).
In conclusion, we showed, for the first time, the individual role of the A2BAR and the contribution of the A2AAR in A2AR-mediated coronary vasodilatation using A2BKO and A2A/2BDKO mice along with the use of A2BAR- and A2AAR-selective agonists. We found that A2AARs nd A2BARs contribute to coronary artery vasodilatation, which involves KATP channels, and that A2AARs are upregulated in A2BKO mice. These findings are another step toward a better understanding of the pharmacology of ARs in the coronary artery and the heterogeneity of CF responses by ARs, which may lead to better therapeutic approaches for the treatment of cardiovascular disorders.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-027339, HL-09444, HL-071802, and T32-HL-090610.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M. S. S. conception and design of research; M. S. S. performed experiments; M. S. S. analyzed data; M. S. S. interpreted results of experiments; M. S. S. prepared figures; M. S. S. drafted manuscript; M. S. S. and S. J. M. edited and revised manuscript; M. S. S., B. T., T. K., S. L. T., C. L., and S. J. M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Gerry Hobbs (West Virginia University) for help with the statistical analysis of the data.
REFERENCES
- 1. Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol 292: H719–H725, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Asakura M, Asanuma H, Kim J, Liao Y, Nakamaru K, Fujita M, Komamura K, Isomura T, Furukawa H, Tomoike H, Kitakaze M. Impact of adenosine receptor signaling and metabolism on pathophysiology in patients with chronic heart failure. Hypertens Res 30: 781–787, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ashton KJ, Nilsson U, Willems L, Holmgren K, Headrick JP. Effects of aging and ischemia on adenosine receptor transcription in mouse myocardium. Biochem Biophys Res Commun 312: 367–372, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bertolet BD, Belardinelli L, Franco EA, Nichols WW, Kerensky RA, Hill JA. Selective attenuation by N-0861 (N6-endonorboran-2-yl-9-methyladenine) of cardiac A1 adenosine receptor-mediated effects in humans. Circulation 93: 1871–1876, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A2A and A2B receptors to ischemic coronary dilation: role of KV and KATP channels. Microcirculation 17: 600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borea PA, Gessi S, Bar-Yehuda S, Fishman P. A3 adenosine receptor: pharmacology and role in disease. Handb Exp Pharmacol: 297–327, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Botvinick EH. Current methods of pharmacologic stress testing and the potential advantages of new agents. J Nucl Med Technol 37: 14–25, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol 47: 801–814, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Buira SP, Albasanz JL, Dentesano G, Moreno J, Martin M, Ferrer I, Barrachina M. DNA methylation regulates adenosine A2A receptor cell surface expression levels. J Neurochem 112: 1273–1285 [DOI] [PubMed] [Google Scholar]
- 10. Claeys MJ, Bosmans J, De Ceuninck M, Beunis A, Vergauwen W, Vorlat A, Vrints CJ. Effect of intracoronary adenosine infusion during coronary intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 94: 9–13, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, Ganame J, Dymarkowski S, Janssens S, Belmans A, Van de Werf F. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J 32: 867–877, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol 294: H2371–H2381, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Donoso MV, Lopez R, Miranda R, Briones R, Huidobro-Toro JP. A2B adenosine receptor mediates human chorionic vasoconstriction and signals through arachidonic acid cascade. Am J Physiol Heart Circ Physiol 288: H2439–H2449, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118: 166–175, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Edlund A, Conradsson T, Sollevi A. A role for adenosine in coronary vasoregulation in man. Effects of theophylline and enprofylline. Clin Physiol 15: 623–636, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Edlund A, Sollevi A. Theophylline increases coronary vascular tone in humans: evidence for a role of endogenous adenosine in flow regulation. Acta Physiol Scand 155: 303–311, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198: 783–796, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fahim M, Hussain T, Mustafa SJ. Role of endothelium in adenosine receptor-mediated vasorelaxation in hypertensive rats. Fundam Clin Pharmacol 15: 325–334, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev 49: 381–402, 1997 [PubMed] [Google Scholar]
- 21. Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res 90: 531–538, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 44: 649–654, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Flood A, Headrick JP. Functional characterization of coronary vascular adenosine receptors in the mouse. Br J Pharmacol 133: 1063–1072, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forsythe P, McGarvey LP, Heaney LG, MacMahon J, Ennis M. Adenosine induces histamine release from human bronchoalveolar lavage mast cells. Clin Sci (Lond) 96: 349–355, 1999 [PubMed] [Google Scholar]
- 25. Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14: 1315–1323, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Garratt KN, Holmes DR, Jr, Molina-Viamonte V, Reeder GS, Hodge DO, Bailey KR, Lobl JK, Laudon DA, Gibbons RJ. Intravenous adenosine and lidocaine in patients with acute mycocardial infarction. Am Heart J 136: 196–204, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Gebremedhin D, Weinberger B, Lourim D, Harder DR. Adenosine can mediate its actions through generation of reactive oxygen species. J Cereb Blood Flow Metab 30: 1777–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glavind-Kristensen M, Matchkov V, Hansen VB, Forman A, Nilsson H, Aalkjaer C. KATP-channel-induced vasodilation is modulated by the Na,K-pump activity in rabbit coronary small arteries. Br J Pharmacol 143: 872–880, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R546–R552, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Headrick JP, Lasley RD. Adenosine receptors and reperfusion injury of the heart. Handb Exp Pharmacol: 189–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heaps CL, Bowles DK. Gender-specific K+-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol 92: 550–558, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Hein TW, Wang W, Zoghi B, Muthuchamy M, Kuo L. Functional and molecular characterization of receptor subtypes mediating coronary microvascular dilation to adenosine. J Mol Cell Cardiol 33: 271–282, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Hein TW, Yuan Z, Rosa RH, Jr, Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci 46: 2113–2119, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol 105: 1–5, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Hove-Madsen L, Prat-Vidal C, Llach A, Ciruela F, Casado V, Lluis C, Bayes-Genis A, Cinca J, Franco R. Adenosine A2A receptors are expressed in human atrial myocytes and modulate spontaneous sarcoplasmic reticulum calcium release. Cardiovasc Res 72: 292–302, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Iwamoto T, Umemura S, Toya Y, Uchibori T, Kogi K, Takagi N, Ishii M. Identification of adenosine A2 receptor-cAMP system in human aortic endothelial cells. Biochem Biophys Res Commun 199: 905–910, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5: 247–264, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson TD, Marrelli SP, Steenberg ML, Childres WF, Bryan RM., Jr Inward rectifier potassium channels in the rat middle cerebral artery. Am J Physiol Regul Integr Comp Physiol 274: R541–R547, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Kemp BK, Cocks TM. Adenosine mediates relaxation of human small resistance-like coronary arteries via A2B receptors. Br J Pharmacol 126: 1796–1800, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kerbaul F, Benard F, Giorgi R, Youlet B, Carrega L, Zouher I, Mercier L, Gerolami V, Benas V, Blayac D, Gariboldi V, Collart F, Guieu R. Adenosine A2A receptor hyperexpression in patients with severe SIRS after cardiopulmonary bypass. J Investig Med 56: 864–871, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Kolachala VL, Wang L, Obertone TS, Prasad M, Yan Y, Dalmasso G, Gewirtz AT, Merlin D, Sitaraman SV. Adenosine 2B receptor expression is post-transcriptionally regulated by microRNA. J Biol Chem 285: 18184–18190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 92: 518–525, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Liclican EL, McGiff JC, Falck JR, Carroll MA. Failure to upregulate the adenosine2A receptor-epoxyeicosatrienoic acid pathway contributes to the development of hypertension in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 295: F1696–F1704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marala RB, Mustafa SJ. Immunological characterization of adenosine A2A receptors in human and porcine cardiovascular tissues. J Pharmacol Exp Ther 286: 1051–1057, 1998 [PubMed] [Google Scholar]
- 46. Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 101: 2154–2159, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 23: 1224–1230, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 282: H437–H444, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol: 161–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2AAR-mediated relaxation using A2AAR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295: H2068–H2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nayeem MA, Ponnoth DS, Boegehold MA, Zeldin DC, Falck JR, Mustafa SJ. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am J Physiol Regul Integr Comp Physiol 296: R567–R574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olanrewaju HA, Gafurov BS, Lieberman EM. Involvement of K+ channels in adenosine A2A and A2B receptor-mediated hyperpolarization of porcine coronary artery endothelial cells. J Cardiovasc Pharmacol 40: 43–49, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A2A and A2B receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 279: H650–H656, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Park WS, Han J, Kim N, Ko JH, Kim SJ, Earm YE. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 289: H2461–H2467, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Pich EM, Epping-Jordan MP. Transgenic mice in drug dependence research. Ann Med 30: 390–396, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Pohl U, De Wit C, Gloe T. Large arterioles in the control of blood flow: role of endothelium-dependent dilation. Acta Physiol Scand 168: 505–510, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. Absence of adenosine-mediated aortic relaxation in A2A adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol 297: H1655–H1660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am J Physiol Cell Physiol 269: C1112–C1118, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem 268: 16887–16890, 1993 [PubMed] [Google Scholar]
- 60. Rivers RJ, Hein TW, Zhang C, Kuo L. Activation of barium-sensitive inward rectifier potassium channels mediates remote dilation of coronary arterioles. Circulation 104: 1749–1753, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 45: 1775–1780, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Sabouni MH, Ramagopal MV, Mustafa SJ. Relaxation by adenosine and its analogs of potassium-contracted human coronary arteries. Naunyn Schmiedebergs Arch Pharmacol 341: 388–390, 1990 [DOI] [PubMed] [Google Scholar]
- 63. Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol 288: H1633–H1640, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Sousa T, Pinho D, Morato M, Marques-Lopes J, Fernandes E, Afonso J, Oliveira S, Carvalho F, Albino-Teixeira A. Role of superoxide and hydrogen peroxide in hypertension induced by an antagonist of adenosine receptors. Eur J Pharmacol 588: 267–276, 2008 [DOI] [PubMed] [Google Scholar]
- 65. Sun Park W, Kyoung Son Y, Kim N, Boum Youm J, Joo H, Warda M, Ko JH, Earm YE, Han J. The protein kinase A inhibitor, H-89, directly inhibits KATP and Kir channels in rabbit coronary arterial smooth muscle cells. Biochem Biophys Res Commun 340: 1104–1110, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Talukder MA, Morrison RR, Jacobson MA, Jacobson KA, Ledent C, Mustafa SJ. Targeted deletion of adenosine A3 receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol 282: H2183–H2189, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Talukder MA, Morrison RR, Ledent C, Mustafa SJ. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol 41: 562–570, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Tawfik HE, Teng B, Morrison RR, Schnermann J, Mustafa SJ. Role of A1 adenosine receptor in the regulation of coronary flow. Am J Physiol Heart Circ Physiol 291: H467–H472, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teng B, Muir WW., 3rd Effects of xylazine on canine coronary artery vascular rings. Am J Vet Res 65: 431–435, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Teng B, Qin W, Ansari HR, Mustafa SJ. Involvement of p38-mitogen-activated protein kinase in adenosine receptor-mediated relaxation of coronary artery. Am J Physiol Heart Circ Physiol 288: H2574–H2580, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Ueeda M, Thompson RD, Padgett WL, Secunda S, Daly JW, Olsson RA. Cardiovascular actions of adenosines, but not adenosine receptors, differ in rat and guinea pig. Life Sci 49: 1351–1358, 1991 [DOI] [PubMed] [Google Scholar]
- 73. van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, Siebes M. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol; doi:10.1016/j.yjmcc.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 74. Wang H, Long C, Duan Z, Shi C, Jia G, Zhang Y. A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovasc Res 73: 497–503, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Woodcock EA, Du XJ, Reichelt ME, Graham RM. Cardiac α1-adrenergic drive in pathological remodelling. Cardiovasc Res 77: 452–462, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, Liu Y, Hu W, Qu X. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol 88: 165–171, 2010 [DOI] [PubMed] [Google Scholar]
- 77. Zatta AJ, Headrick JP. Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol 144: 576–587, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhao JL, Yang YJ, Pei WD, Sun YH, Chen JL, Go RL. Intravenous adenosine reduces myocardial no-reflow by decreasing endothelin-1 via activation of the ATP-sensitive K+ channel. Acta Cardiol 63: 355–359, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Zingman LV, Zhu Z, Sierra A, Stepniak E, Burnett CM, Maksymov G, Anderson ME, Coetzee WA, Hodgson-Zingman DM. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J Mol Cell Cardiol 51: 72–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]