Abstract
Sodium-hydrogen exchanger (NHE), the principal sarcolemmal acid extruder in ventricular myocytes, is stimulated by a variety of autocrine/paracrine factors and contributes to myocardial injury and arrhythmias during ischemia-reperfusion. Platelet-activating factor (PAF; 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a potent proinflammatory phospholipid that is released in the heart in response to oxidative stress and promotes myocardial ischemia-reperfusion injury. PAF stimulates NHE in neutrophils and platelets, but its effect on cardiac NHE (NHE1) is unresolved. We utilized quiescent guinea pig ventricular myocytes bathed in bicarbonate-free solutions and epifluorescence to measure intracellular pH (pHi). Methylcarbamyl-PAF (C-PAF; 200 nM), a metabolically stable analog of PAF, significantly increased steady-state pHi. The alkalosis was completely blocked by the NHE inhibitor, cariporide, and by sodium-free bathing solutions, indicating it was mediated by NHE activation. C-PAF also significantly increased the rate of acid extrusion induced by intracellular acidosis. The ability of C-PAF to increase steady-state pHi was completely blocked by the PAF receptor inhibitor WEB 2086 (10 μM), indicating the PAF receptor is required. A MEK inhibitor (PD98059; 25 μM) also completely blocked the rise in pHi induced by C-PAF, suggesting participation of the MAP kinase signaling cascade downstream of the PAF receptor. Inhibition of PKC with GF109203X (1 μM) and chelerythrine (2 μM) did not significantly affect the alkalosis induced by C-PAF. In summary, these results provide evidence that PAF stimulates cardiac NHE1, the effect occurs via the PAF receptor, and signal relay requires participation of the MAP kinase cascade.
Keywords: guinea pig cardiomyocytes, intracellular pH, ischemia reperfusion injury
intracellular ph (pHi) is closely regulated in ventricular myocytes by a well-defined system of sarcolemmal acid extruder and loader proteins (56). Sodium-hydrogen exchanger (NHE1) is the most important acid extruder, activated principally by intracellular acidosis. Numerous extracellular stimuli including neurohumoral mediators and growth factors can also stimulate NHE1 activity by binding to G protein-coupled membrane receptors (5, 12). In addition to its important role in mediating acid extrusion, NHE1 activation contributes to injury and arrhythmias during ischemia-reperfusion (I/R) by promoting calcium overload via the sodium-calcium exchanger (21).
Platelet-activating factor (PAF; 1-o-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a proinflammatory phospholipid with a number of physiological and pathophysiological actions (40, 50). PAF and structurally related phospholipids are released by a variety of cell types including neutrophils, mast cells, platelets, endothelial cells, and cardiac myocytes in response to various stimuli including oxidative stress (32, 49, 50). In the heart, synthesis and release of PAF is normally very low but can be increased markedly after I/R (33). This occurs mainly through the remodeling pathway in which hydrolysis of membrane phospholipids by phospholipase A2 is followed by acetylation by an acyltransferase (45) to yield PAF (32, 50). The actions of PAF and PAF-like molecules generated from oxidative fragmentation of membrane phospholipids (27) are mediated through a G protein-coupled receptor, which is linked to a variety of intracellular signaling pathways (18, 19). PAF and PAF-like phospholipids are hydrolyzed by PAF acetylhydrolases (PAF-AHs). The plasma form of PAF-AH is also known as lipoprotein-associated phospholipase A2 and PLA2G7 (49).
The cardiac actions of PAF are diverse and include induction of arrhythmias in canine ventricular myocytes (17) and negative inotropic effects on rat ventricular myocytes (29, 39). At the whole heart level, considerable evidence indicates that PAF promotes myocardial I/R injury (32, 52). However, it has recently been proposed that PAF can have cardioprotective effects at very low concentrations (37).
Given the importance of PAF as a G protein-linked signaling molecule and its multiple actions on the heart, we sought to determine whether signaling through this pathway modulates NHE1 activity since this is a well-characterized, key contributor to myocardial injury and arrhythmia during I/R. PAF has been shown to stimulate NHE in neutrophils and platelets (16, 43). However, to our knowledge no studies have addressed whether PAF-mediated signaling regulates cardiac NHE activity. In this study we present evidence that PAF stimulates NHE1 in ventricular myocytes from adult hearts and provide mechanistic evidence suggesting that the MAP kinase signaling axis participates in this response.
MATERIALS AND METHODS
Myocyte isolation.
The experiments were performed using adult ventricular myocytes isolated from healthy guinea pigs by enzymatic digestion, as described (61). All procedures involving animals were approved by the Animal Care and Use Committee of the University of Utah and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Briefly, guinea pigs were anesthetized with sodium pentobarbital (50 mg/kg ip), and then the excised heart was attached to an aortic cannula and perfused with solutions gassed with 100% O2 and held at 37°C, pH 7.2. Perfusion with a Ca2+-free solution for 5 min was followed by 14 min of perfusion with the same solution containing 0.1 mg/ml collagenase P (Roche Diagnostic, Manheim, Germany), 0.01 mg/ml protease (type XIV; Sigma-Aldrich, St. Louis, MO), and 0.05 mM CaCl2. The heart was then perfused for 5 min with the same solution containing no enzymes. The ventricle was isolated and minced, shaken for 10 min, and then filtered through a nylon mesh. Cells were stored at room temperature in normal HEPES-buffered solution. All cells used in this study were rectangular, had well-defined striations, and did not spontaneously contract. All experiments were conducted within 10 h of isolation.
Cell superfusion chamber.
Lipid-free bathing solutions were held in glass reservoir bottles and delivered by gravity to the cell bath. Bathing solutions containing 1-O-alkyl-2-N-methylcarbamyl-sn-glycero-3-phosphocholine (C-PAF; Enzo Life Science, Plymouth Meeting, PA) or 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POV-PC; Cayman Chemical, Ann Arbor, MI) were held in plastic reservoir bottles and delivered by gravity. The temperature of the solutions in the superfusion chamber was 36 ± 0.3°C. The 1-ml Plexiglas cell bath had a clear glass bottom and was mounted on the stage of an inverted microscope (Diaphot; Nikon, Japan). Bathing solutions flowed continuously through the bath at 3 to 4 ml/min, and solution depth was held at ∼1 mm. Exchange of the bath solution required ∼5 s. The bottom of the bath was coated with laminin (Collaborative Research, Bedford, MA) to improve cell adhesion.
Bathing solution and drugs.
Myocytes were continuously superfused with normal HEPES-buffered solution containing (in mM) 126.0 NaCl, 11.0 dextrose, 4.4 KCl, 1.0 MgCl2, 2.0 CaCl2, and 24.0 HEPES titrated to pH 7.4 with 1 M NaOH. In some experiments, NHE was blocked with either 60 μM cariporide (Sanofi-Aventis, Frankford, Germany) or by replacing external NaCl with an equimolar concentration of N-methyl-d-glucamine and adjusting the pH to 7.4 with 1.04 M HCl. Selective blockade of PAF receptors was accomplished using WEB 2086 (10 μM; Tocris Bioscience, Ellisville, MO). PKC was inhibited using two different agents, GF109203X (1 μM; Tocris Bioscience) and chelerythrine chloride (2 μM; Sigma-Aldrich). To inhibit signaling through the MAP kinase axis, we exposed the cells to 25 μM PD98059 (Calbiochem, San Diego, CA). This compound inhibits MEK, the kinase that phosphorylates MAP kinase (also known as ERK). All these inhibitors were present at least 15 min before measurements of pHi. Cariporide was supplemented as a solid briefly before the experiments were initiated. C-PAF was prepared as a 10 mM stock solution dissolved in ethanol. Similarly, POV-PC, an oxidatively fragmented phospholipid with biological activities comparable with those of PAF (36, 54) and WEB 2086 were dissolved in ethanol to generate 16.8 and 100 mM stock solutions, respectively. Our stocks of GF109203X and PD98059 (25 and 50 mM, respectively) were prepared in dimethyl sulfoxide (DMSO). Chelerythrine chloride was dissolved directly in the bathing solutions. The final concentrations of ethanol and DMSO were less than 0.1%; appropriate vehicle controls served to rule out potential nonspecific effects.
Measurement of pHi and cell shortening.
The experimental conditions were designed to increase the likelihood of detecting changes in pHi induced by NHE. Thus all studies were performed on quiescent cells to minimize changes in pHi resulting from increased metabolic acid production (7). Because intracellular calcium activates NHE (57, 58), the use of resting cells also reduces the likelihood of pacing-induced changes in intracellular calcium affecting NHE activity. The use of HEPES-buffered bathing solutions containing no added CO2 or bicarbonate blocked trans-sarcolemmal transport of acid equivalents via sodium bicarbonate cotransport, NBC (61), and Cl−-HCO3− exchange (60). This is important since PAF may affect the activity of these bicarbonate-dependent transporters and thus make it difficult to attribute changes in pHi to NHE.
pHi was measured in single myocytes with an epifluorescence system coupled to a Nikon inverted microscope. Carboxy-seminaphthorhodafluor-1 (carboxy-SNARF-1), a pH-sensitive fluorophore, was used as the fluorescent pH indicator, as described previously (48, 61). Briefly, myocytes were incubated at 36°C for 5 min in normal HEPES-buffered solution containing 10–13 μM SNARF-AM (Molecular Probes, Eugene, OR), the acetoxymethyl ester of SNARF-1. The cells were then placed in the cell bath, where they were incubated in HEPES-buffered solution for at least 15 min before pHi measurements began.
Excitation at 515 nm was provided by a 150-watt xenon lamp and was directed to the bath via a 40× oil-immersion objective lens (numerical aperture, 1.3). Emitted fluorescence was simultaneously collected by two photomultiplier tubes equipped with band-pass filters centered at 640 ± 20 nm and 580 ± 20 nm. The fluorescence emission ratio (640/580) was digitized at 5–10 kHz (Digidata 1322A; Axon Instruments, Foster City, CA). Myocytes displayed negligible autofluorescence, and the small level of background fluorescence was subtracted from the individual wavelengths. The emission ratio was calibrated as described previously (25, 48) using solutions of varying pH that also contained 10 μM nigericin. We used the best-fit equation resulting from the calibration curves from several myocytes to calculate pHi of the cells used in this study.
To insure that overall myocyte function was preserved during C-PAF exposure, we measured myocyte shortening during field stimulation (cycle length = 2 s). Changes in cell length were measured with an intensified charge-coupled device camera coupled to a video edge-detector device (53). Individual cells without SNARF loading were first paced in control solution until shortening stabilized (∼1 min). The stimulus was shut off, and C-PAF (200 nM) was applied to the quiescent cells for 15 min at the end of which time pacing was resumed. The results were analyzed as shortening before and after C-PAF. The cells were not continuously paced during the entire 15 min of C-PAF exposure to mimic the quiescent conditions of the pH experiments.
Determination of sarcolemmal acid efflux [JH].
Intracellular acid loading was achieved using the ammonium prepulse (15 mM NH4+) technique (25, 61). As noted above, the use of HEPES-buffered cell bathing solutions ensured inactivation of NBC (25, 61). The rate of acid efflux (JH) was evaluated as the indicator of NHE activity and plotted as a function of pHi. JH was calculated as −βint·dpHi/dt during recovery from an acid load, where βint is intrinsic intracellular buffering power. We determined previously the relationship between βint and pHi over the pHi range 6.70–7.30 in adult guinea pig ventricular myocytes (63).
Statistics.
Summarized results are expressed as means ± SE. A paired Student's t-test was used to test significance between results obtained with each cell serving as its own control. An unpaired t-test was used to test significance between results obtained on different cells. P < 0.05 was considered significant.
RESULTS
Effect of C-PAF and POV-PC on steady-state pHi.
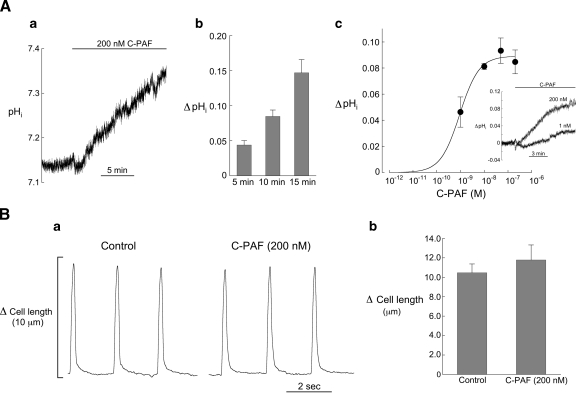
In the first series of experiments we examined the effect of C-PAF and POV-PC (PAF analog) on steady-state pHi in quiescent myocytes bathed in HEPES-buffered solution containing no added CO2 or bicarbonate. As noted above, these experimental conditions increase the likelihood of detecting changes in pHi attributable to NHE activity. C-PAF induced a dose-dependent rise in steady-state pHi, an example of which is shown in Fig. 1Aa. By 15 min the mean increase in pHi in 200 nM C-PAF was 0.15 ± 0.02 units (Fig. 1Ab). Reapplication of the control solution for 5 min following 15 min of C-PAF typically attenuated or blocked the rise in pHi but did not restore it to its control value (not shown), perhaps reflecting the low level of metabolic acid production and absence of Cl−-HCO3− exchange. Recovery did occur in response to short periods (∼5 min) of C-PAF exposure (not shown). Smaller increases in pHi occurred when the concentration of C-PAF was reduced (Fig. 1Ac). Because 200 nM C-PAF gave a maximum pH response, this concentration was used in all subsequent experiments.
Fig. 1.
Response of intracellular pH (pHi) and cell shortening to methylcarbamyl-PAF (C-PAF). Aa: example record showing the rise in pHi induced by 200 nM C-PAF. Ab: summarized results (5 min, n = 23; 10 min, n = 16; 15 min, n = 5) showing the time course of increase in pHi induced by 200 nM C-PAF, expressed as ΔpHi (C-PAF minus control). Ac: relationship between ΔpHi at 10 min and C-PAF concentration (200 nM, n = 16; 50 nM, n = 6; 10 nM, n = 5; 1 nM, n = 7). The apparent EC50 is 0.9 nM. Inset shows response of 2 myocytes to 200 nM and 1 nM C-PAF. Ba: example record showing that 15 min superfusion with C-PAF (200 nM) had little effect on cell shortening (pacing cycle length = 2 s). Bb: summarized results for myocyte shortening showing no significant changes in cell shortening following 15 min of 200 nM C-PAF (n = 5, paired).
Ten minutes of superfusion with a PAF analog (200 nM), which is recognized by the PAF receptor in human macrophages (36), also elicited a significant increase in steady-state pHi of 0.08 ± 0.01 units (n = 4, paired, P < 0.01), demonstrating that bioactive phospholipids other than C-PAF can induce this effect.
There was no evidence that superfusion with either C-PAF or POV-PC degraded cell viability during the course of the experiments. The myocytes remained quiescent and rod-shaped in appearance with well-defined striations and without spontaneous contractions or the appearance of blebs. Further evidence that C-PAF did not affect cell function is the absence of any significant changes in myocyte shortening following 15 min of superfusion with 200 nM C-PAF (Fig. 1B).
Effect of cariporide and external sodium on C-PAF-induced changes in steady-state pHi.
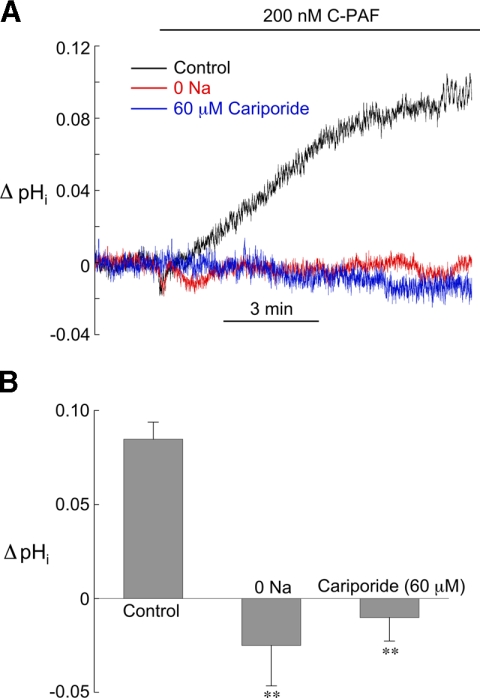
To identify the mechanism responsible for the intracellular alkalosis produced by C-PAF, we performed experiments with myocytes bathed in solutions containing either 60 μM cariporide, a highly selective NHE1 inhibitor (28), or no external sodium, to inhibit sodium-hydrogen exchange. NHE1 blockade was initiated at least 5 min before exposure to 200 nM C-PAF and continued throughout the experiment. Both interventions completely inhibited the rise in pHi, strongly suggesting it is mediated by acid extrusion via NHE1 (Fig. 2, A and B).
Fig. 2.
Evidence that C-PAF stimulates sodium-hydrogen exchanger (NHE1). A: example pHi signals from 3 different myocytes showing the response of pHi to 200 nM C-PAF under control conditions and in the presence of zero [Na+]o and 60 μM cariporide, both of which block the C-PAF-induced rise in pHi. B: summarized results of ΔpHi induced by 10 min superfusion with 200 nM C-PAF under control conditions (n = 16), zero [Na+]o (n = 4), and 60 μM cariporide (n = 4). **P < 0.01, unpaired control vs. NHE1 blockade.
Effect of C-PAF on pHi recovery from intracellular acidosis.
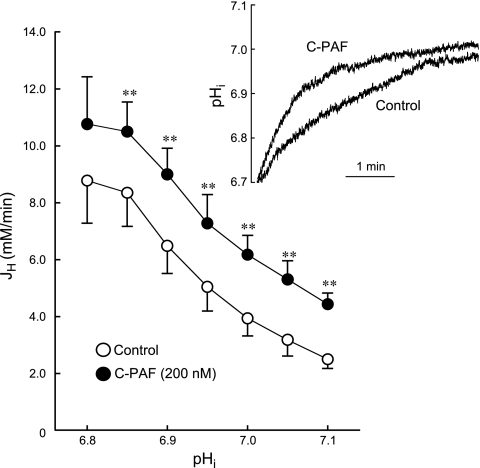
Intracellular acidosis is the major activator of NHE1, and pHi recovery from ammonia prepulses is mediated entirely by NHE1 in guinea pig ventricular myocytes bathed in HEPES-buffered solutions (25, 61). Figure 3 shows that C-PAF (200 nM) significantly increased the rate of pHi recovery from acid loading producing an upward shift in the JH versus pHi curve at all values of pHi. Thus C-PAF stimulates NHE1-mediated acid extrusion over a wide range of pHi values.
Fig. 3.
Effect of C-PAF on the relationship between net acid efflux via NHE1 (JH) and pHi. JH was significantly increased by C-PAF (200 nM) at all values of pHi (**P < 0.01, paired). N values for each point range from 7 to 10 myocytes.
Effect of WEB 2086 on C-PAF-induced rise in steady-state pHi.
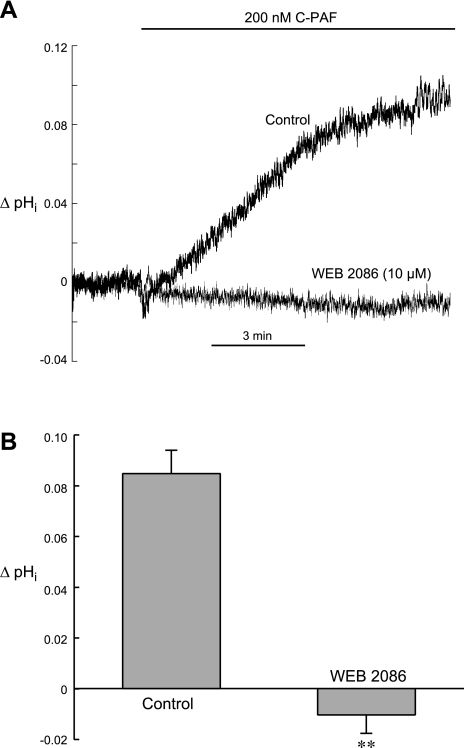
In many cells and organs, PAF transduces signals through a G protein-coupled receptor, the PAF receptor (18, 19). To determine whether the effect of C-PAF on steady-state pHi required engagement of the PAF receptor, we preincubated ventricular myocytes with WEB 2086 (5 min, 10 μM), a highly specific PAF receptor antagonist (8). The cells were then exposed to C-PAF (200 nM) in the continued presence of receptor antagonist. We found that WEB 2086 completely inhibited the ability of C-PAF to raise pHi (Fig. 4), indicating that C-PAF-mediated stimulation of NHE1 requires functional PAF receptors.
Fig. 4.
Effect of PAF receptor inhibition on C-PAF-induced stimulation of NHE1. A: example pHi signals from 2 myocytes illustrating the ability of PAF receptor blockade with WEB 2086 (10 μM) to inhibit the stimulatory effect of C-PAF (200 nM) on NHE1. B: summarized results of ΔpHi induced by 10 min superfusion with 200 nM C-PAF under control conditions (n = 16) and in the presence of 10 μM WEB 2086 (n = 5). **P < 0.01, unpaired control vs. WEB 2086.
Role of MAP kinase and PKC signaling in C-PAF-induced alkalosis.
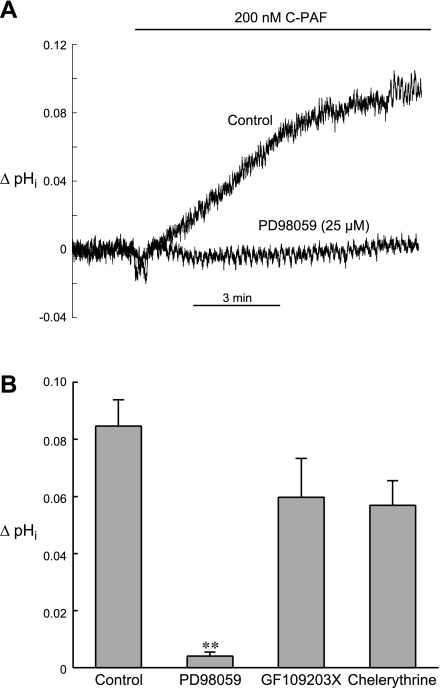
Previous work has shown that phosphorylation of cardiac NHE1 by various agonists involves several protein kinases, including members of the MAP kinase signaling axis such as MEK, MAP kinase/ERK, and p90RSK, as well as PKC (5, 12, 34). To assess possible involvement of the MAP kinase pathway in the pHi response to C-PAF, we performed experiments in the presence of PD98059 (25 μM), a MEK inhibitor (11). As shown in the example record in Fig. 5A and summarized in Fig. 5B, PD98059 completely blocked the rise in pHi elicited by 200 nM C-PAF, suggesting that signals downstream from MEK, perhaps MAP kinase (ERK) and RSK, are involved.
Fig. 5.
Effects of PD98059 (MEK inhibitor), GF109203X (PKC inhibitor), and chelerythrine (PKC inhibitor) on C-PAF-induced stimulation of NHE1. A: example pHi signals from 2 myocytes illustrating the ability of PD98059 (25 μM) to completely block the stimulatory effect of 200 nM C-PAF on NHE1. B: summarized results of ΔpHi induced by 10 min superfusion with C-PAF (200 nM) under control conditions (n = 16) and in the presence of either 25 μM PD98059 (n = 6), 1 μM GF109203X (n = 4), 2 μM chelerythrine (n = 7). In contrast with PKC inhibition, MEK blockade significantly reduced the stimulatory effect of C-PAF on NHE1. **P < 0.01, unpaired control vs. PD98059.
Although alkalosis induced by 200 nM C-PAF was somewhat reduced by PKC blockade with GF109203X (1 μM) and chelerythrine (2 μM), the effect was not statistically significant (Fig. 5B), suggesting that PKC activation does not play a major role in the stimulation of NHE1 by C-PAF.
DISCUSSION
The present work demonstrates that C-PAF stimulates NHE1 in ventricular myocytes at both normal resting pHi and during intracellular acidosis. A classical PAF receptor mediates these effects. The downstream signaling appears to involve primarily the MAP kinase pathway with little or no contribution by PKC activation. Earlier work reported stimulation of NHE in neutrophils and platelets by PAF (16, 43), but to our knowledge this is the first report of PAF-induced modulation of NHE in myocytes from adult mammalian heart.
Modulation of NHE1 activity by endogenous ligands.
Under normal conditions [pHi ∼7.2, pHo ∼7.4] the rate of acid extrusion via cardiac NHE1 is low. However, it is markedly increased by a fall in pHi (56). This stimulation is attributed to allosteric control of carrier activity by proton occupancy of the cytosolic proton sensor on the transporter domain of NHE1 (59). Transport activity is also significantly increased in ventricular myocytes by a variety of paracrine and autocrine factors, including angiotensin (14, 30), endothelin (24), thrombin (62), and phenylephrine (47). These agents act through G protein-coupled receptors to phosphorylate the cytoplasmic COOH-terminal regulatory domain of the transporter and increase the affinity of the proton sensor (5, 12, 59). Phosphorylation of NHE1 by these agents involves several protein kinases, including key members of the MAP kinase signaling axis (i.e., MAP kinase/ERK and p90RSK), as well as PKC (5, 12, 34). MAP kinase/ERK and p90RSK have been shown to directly phosphorylate myocardial NHE1 (4, 9, 34). In contrast, PKC apparently does not have this effect, even though it has a significant influence on transporter activity (13). Our data are consistent with participation of the MAP kinase signaling pathway on PAF-induced activation of NHE and suggest little, if any, role for PKC in this process.
Occupancy of PAF receptors in noncardiac cells activates numerous signal transduction pathways that vary among cell types and include members of the MAP kinase axis, PKCs, protein tyrosine kinases, and phosphatidylinositol 3-kinases (19, 50). PAF-induced signaling in cardiac cells includes PKC activation in cultured neonatal rat ventricular myocytes (29) and adult rat heart (38), p38 MAP kinase activation in H9c2 cultured cardiomyocytes (64), and phosphatidylinositol 3-kinase activation leading to nitric oxide (NO) production in adult mice atrium (1). PAF-induced NO production has also been reported in adult guinea pig papillary muscle (2). Interestingly, we have found that NO appears to inhibit NHE1 in adult rat ventricular myocytes (20).
Our results do not provide detailed information concerning the intracellular signaling pathways responsible for stimulation of NHE1 by C-PAF. However, the ability of PD98059, a MEK inhibitor (11), to completely block the effect (Fig. 5) suggests that signals downstream from MEK, perhaps MAP kinase/ERK and p90RSK, are involved. PD98059 also inhibits the stimulation of NHE1 in rat ventricular myocytes by hydrogen peroxide (46), phenylephrine (47), and angiotensin II via the AT1 receptor (14). Our results further suggest that PKC may not be involved in the stimulation of NHE1 since two different PKC inhibitors, GF109203X (55) and chelerythrine (15), did not significantly reduce the alkalosis elicited by 200 nM C-PAF (Fig. 5). It is important to note, however, that although PD98059, GF109203X and chelerythrine, are widely used signaling pathway blockers, there are issues concerning the selectivity of each. For example, PD98059 has been shown to inhibit both MEK and cyclooxygenase 2 (6). In addition, GF109203X at concentrations higher than ∼3 μM has been shown to also inhibit p90RSK (42). However, our use of 1 μM GF109203X decreases the likelihood of this being an issue in the present study.
Possible clinical implications of PAF-induced stimulation of NHE1.
Under normal conditions the synthesis and release of PAF in the heart is very low but is markedly increased following I/R (22, 23, 33). Sources of myocardial PAF production are thought to include endothelial cells, neutrophils, platelets, and myocytes (52). Although the role of PAF in myocardial I/R pathophysiology is not completely understood, numerous studies have documented its negative inotropic and arrhythmogenic actions (32, 52). PAF has also been proposed to have cardioprotective properties, but these effects are thought to occur under conditions of very slow rates of release, as occurs following brief ischemic periods (37).
Our results suggest that NHE1 can be stimulated significantly by conditions that promote the synthesis and release of myocardial PAF and PAF analogs. NHE1 is an established contributor to calcium overload and consequent myocardial damage and arrhythmogenesis during ischemia, and especially reperfusion due to its ability to raise intracellular sodium during acid extrusion and thus reduce Ca2+ efflux and or increase influx via sodium-calcium exchanger (21, 31). Thus it seems likely that release of PAF and PAF analogs in the ischemic and surrounding myocardium may have the undesirable effect of further stimulating NHE1 and exacerbating I/R injury. Thus our results may help explain some of the known beneficial effects of PAF receptor antagonists and PAF-AH in mitigating the effects of I/R on infarct size and arrhythmias (3, 22, 26, 33, 35, 41, 44, 51).
In addition to PAF, oxidative stress also promotes production of several other oxidized phospholipids such as POV-PC (10). POV-PC is reported to bind to PAF receptors (36) and, as with C-PAF, it increased steady-state pHi at a concentration of 200 nM, perhaps by stimulating NHE1. Taken together, our present findings combined with earlier work help clarify the role of PAF-like oxidized phospholipids in myocardial I/R injury and further define the cardioprotective mechanism of NHE blockade.
In summary, our results demonstrate for the first time that PAF promotes acid extrusion from guinea pig ventricular myocytes at both normal steady-state values of pHi and in response to intracellular acid loading. This effect is mediated by stimulation of NHE1 and requires occupancy of the PAF receptor. Inhibition of C-PAF-induced acid extrusion by a MEK blocker suggests that signaling via the MAP kinase axis is involved. The action of PAF and PAF-analogs to stimulate NHE may help explain some of the beneficial effects of PAF receptor blockade in reducing injury to the ventricular myocardium in the setting of I/R.
GRANTS
This work was supported by the National Institutes of Health MERIT award R37HL042873 and the Nora Eccles Treadwell Foundation (both to K. W. Spitzer). Y. Ajiro was supported in part by a Medtronic Japan Fellowship. W. R. Giles holds a Medical Scientist Award from the Alberta Heritage Foundation for Medical Research and is funded by the Canadian Institutes for Health Research and the Heart and Stroke Foundation of Alberta, Canada. D. M. Stafforini was supported by National Heart, Lung, and Blood Institute Grant R01-HL-035828.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.A., N.S., and K.W.S. performed experiments; Y.A., N.S., and K.W.S. analyzed data; Y.A., N.S., and K.W.S. prepared figures; Y.A., N.S., W.R.G., D.M.S., and K.W.S. edited and revised manuscript; Y.A., N.S., W.R.G., D.M.S., and K.W.S. approved final version of manuscript; N.S., W.R.G., D.M.S., and K.W.S. interpreted results of experiments; W.R.G., D.M.S., and K.W.S. contributed to the conception and design of research; K.W.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Juergen Puenter of Sanofi-Aventis, Frankford, Germany, for generously providing cariporide for these experiments.
REFERENCES
- 1. Alloatti G, Levi R, Malan D, Del Sorbo L, Bosco O, Barberis L, Marcantoni A, Bedendi I, Penna C, Azzolino O, Altruda F, Wymann M, Hirsch E, Montrucchio G. Phosphoinositide 3-kinase gamma-deficient hearts are protected from the PAF-dependent depression of cardiac contractility. Cardiovasc Res 60: 242–249, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Alloatti G, Penna C, De Martino A, Montrucchio G, Camussi G. Role of nitric oxide and platelet-activating factor in cardiac alterations induced by tumor necrosis factor-alpha in the guinea-pig papillary muscle. Cardiovasc Res 41: 611–619, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Auchampach JA, Pieper GM, Cavero I, Gross GJ. Effect of the platelet-activating factor antagonist RP 59227 (Tulopafant) on myocardial ischemia/reperfusion injury and neutrophil function. Basic Res Cardiol 93: 361–371, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Avkiran M, Cook AR, Cuello F. Targeting Na+/H+ exchanger regulation for cardiac protection: a RSKy approach? Curr Opin Pharmacol 8: 133–140, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Avkiran M, Haworth RS. Regulatory effects of G protein-coupled receptors on cardiac sarcolemmal Na+/H+ exchanger activity: signalling and significance. Cardiovasc Res 57: 942–952, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Borsch-Haubold AG, Pasquet S, Watson SP. Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase. J Biol Chem 273: 28766–28772, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Bountra C, Kaila K, Vaughan-Jones RD. Mechanism of rate-dependent pH changes in the sheep cardiac Purkinje fibre. J Physiol 406: 483–501, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casals-Stenzel J, Muacevic G, Weber KH. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther 241: 974–981, 1987 [PubMed] [Google Scholar]
- 9. Cuello F, Snabaitis AK, Cohen MS, Taunton J, Avkiran M. Evidence for direct regulation of myocardial Na+/H+ exchanger isoform 1 phosphorylation and activity by 90-kDa ribosomal S6 kinase (RSK): effects of the novel and specific RSK inhibitor fmk on responses to alpha1-adrenergic stimulation. Mol Pharmacol 71: 799–806, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Deigner HP, Hermetter A. Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr Opin Lipidol 19: 289–294, 2008 [DOI] [PubMed] [Google Scholar]
- 11. English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci 23: 40–45, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fliegel L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Expert Opin Ther Targets 13: 55–68, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Fliegel L, Walsh MP, Singh D, Wong C, Barr A. Phosphorylation of the C-terminal domain of the Na+/H+ exchanger by Ca2+/calmodulin-dependent protein kinase II. Biochem J 282: 139–145, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunasegaram S, Haworth RS, Hearse DJ, Avkiran M. Regulation of sarcolemmal Na+/H+ exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT1 versus AT2 receptors. Circ Res 85: 919–930, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 172: 993–999, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Hidalgo MA, Ojeda F, Eyre P, LaBranche TP, Smith C, Hancke JL, Burgos RA. Platelet-activating factor increases pHi in bovine neutrophils through the PI3K-ERK1/2 pathway. Br J Pharmacol 141: 311–321, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffman BF, Guo SD, Feinmark SJ. Arrhythmias caused by platelet activating factor. J Cardiovasc Electrophysiol 7: 120–133, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem 131: 773–779, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res 39: 41–82, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation 95: 2303–2311, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K. The myocardial Na+-H+ exchange: structure, regulation, and its role in heart disease. Circ Res 85: 777–786, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Katoh S, Toyama J, Kodama I, Koike A, Abe T. Role of platelet activating factor in ischaemia-reperfusion injury of isolated rabbit hearts: protective effect of a specific platelet activating factor antagonist, TCV-309. Cardiovasc Res 27: 1430–1434, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Ko W, Hawes AS, Lazenby WD, Calvano SE, Shin YT, Zelano JA, Antonacci AC, Isom OW, Krieger KH. Myocardial reperfusion injury. Platelet-activating factor stimulates polymorphonuclear leukocyte hydrogen peroxide production during myocardial reperfusion. J Thorac Cardiovasc Surg 102: 297–308, 1991 [PubMed] [Google Scholar]
- 24. Kramer BK, Smith TW, Kelly RA. Endothelin and increased contractility in adult rat ventricular myocytes. Role of intracellular alkalosis induced by activation of the protein kinase C-dependent Na+-H+ exchanger. Circ Res 68: 269–279, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 517: 159–180, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loucks EB, Qayumi AK, Godin DV, English JC, Lim SP, Al Mahmeed T, Gul S. Therapeutic potential of platelet-activating factor antagonism in the management of myocardial infarction. Can J Cardiol 16: 497–504, 2000 [PubMed] [Google Scholar]
- 27. Marathe GK, Harrison KA, Murphy RC, Prescott SM, Zimmerman GA, McIntyre TM. Bioactive phospholipid oxidation products. Free Radic Biol Med 28: 1762–1770, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem 38: 547–554, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Massey CV, Kohout TA, Gaa ST, Lederer WJ, Rogers TB. Molecular and cellular actions of platelet-activating factor in rat heart cells. J Clin Invest 88: 2106–2116, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsui H, Barry WH, Livsey C, Spitzer KW. Angiotensin II stimulates sodium-hydrogen exchange in adult rabbit ventricular myocytes. Cardiovasc Res 29: 215–221, 1995 [PubMed] [Google Scholar]
- 31. Mentzer RM, Jr, Lasley RD, Jessel A, Karmazyn M. Intracellular sodium hydrogen exchange inhibition and clinical myocardial protection. Ann Thorac Surg 75: S700–S708, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev 80: 1669–1699, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Montrucchio G, Alloatti G, Tetta C, De Luca R, Saunders RN, Emanuelli G, Camussi G. Release of platelet-activating factor from ischemic-reperfused rabbit heart. Am J Physiol Heart Circ Physiol 256: H1236–H1246, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Moor AN, Fliegel L. Protein kinase-mediated regulation of the Na+/H+ exchanger in the rat myocardium by mitogen-activated protein kinase-dependent pathways. J Biol Chem 274: 22985–22992, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Morgan EN, Boyle EM, Jr, Yun W, Kovacich JC, Canty TG, Jr, Chi E, Pohlman TH, Verrier ED. Platelet-activating factor acetylhydrolase prevents myocardial ischemia-reperfusion injury. Circulation 100: II365–II368, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Pegorier S, Stengel D, Durand H, Croset M, Ninio E. Oxidized phospholipid: POVPC binds to platelet-activating-factor receptor on human macrophages. Implications in atherosclerosis. Atherosclerosis 188: 433–443, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Penna C, Bassino E, Alloatti G. Platelet activating factor: the good and the bad in the ischemic/reperfused heart. Exp Biol Med (Maywood) 236: 390–401, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Penna C, Mognetti B, Tullio F, Gattullo D, Mancardi D, Moro F, Pagliaro P, Alloatti G. Post-ischaemic activation of kinases in the pre-conditioning-like cardioprotective effect of the platelet-activating factor. Acta Physiol (Oxf) 197: 175–185, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Pietsch P, Hunger T, Braun M, Roediger A, Baumann G, Felix SB. Effects of platelet-activating factor on intracellular Ca2+ concentration and contractility in isolated cardiomyocytes. J Cardiovasc Pharmacol 31: 758–763, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 69: 419–445, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Qayumi AK, English JC, Godin DV, Ansley DM, Loucks EB, Lee JU, Kim CW. The role of platelet-activating factor in regional myocardial ischemia-reperfusion injury. Ann Thorac Surg 65: 1690–1697, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Roberts NA, Haworth RS, Avkiran M. Effects of bisindolylmaleimide PKC inhibitors on p90RSK activity in vitro and in adult ventricular myocytes. Br J Pharmacol 145: 477–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosskopf D. Sodium-hydrogen exchange and platelet function. J Thromb Thrombolysis 8: 15–24, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Sawa Y, Schaper J, Roth M, Nagasawa K, Ballagi G, Bleese N, Schaper W. Platelet-activating factor plays an important role in reperfusion injury in myocardium. Efficacy of platelet-activating factor receptor antagonist (CV-3988) compared with leukocyte-depleted reperfusion. J Thorac Cardiovasc Surg 108: 953–959, 1994 [PubMed] [Google Scholar]
- 45. Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem 282: 6532–6539, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Snabaitis AK, Hearse DJ, Avkiran M. Regulation of sarcolemmal Na+/H+ exchange by hydrogen peroxide in adult rat ventricular myocytes. Cardiovasc Res 53: 470–480, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Snabaitis AK, Yokoyama H, Avkiran M. Roles of mitogen-activated protein kinases and protein kinase C in alpha1A-adrenoceptor-mediated stimulation of the sarcolemmal Na+-H+ exchanger. Circ Res 86: 214–220, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Spitzer KW, Bridge JH. Relationship between intracellular pH and tension development in resting ventricular muscle and myocytes. Am J Physiol Cell Physiol 262: C316–C327, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovasc Drugs Ther 23: 73–83, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci 40: 643–672, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Stahl GL, Terashita Z, Lefer AM. Role of platelet activating factor in propagation of cardiac damage during myocardial ischemia. J Pharmacol Exp Ther 244: 898–904, 1988 [PubMed] [Google Scholar]
- 52. Stangl V, Baumann G, Stangl K, Felix SB. Negative inotropic mediators released from the heart after myocardial ischaemia-reperfusion. Cardiovasc Res 53: 12–30, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Steadman BW, Moore KB, Spitzer KW, Bridge JH. A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng 35: 264–272, 1988 [DOI] [PubMed] [Google Scholar]
- 54. Subbanagounder G, Leitinger N, Shih PT, Faull KF, Berliner JA. Evidence that phospholipid oxidation products and/or platelet-activating factor play an important role in early atherogenesis: in vitro and in vivo inhibition by WEB 2086. Circ Res 85: 311–318, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991 [PubMed] [Google Scholar]
- 56. Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol 46: 318–331, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Vila-Petroff M, Mundina-Weilenmann C, Lezcano N, Snabaitis AK, Huergo MA, Valverde CA, Avkiran M, Mattiazzi A. Ca2+/calmodulin-dependent protein kinase II contributes to intracellular pH recovery from acidosis via Na+/H+ exchanger activation. J Mol Cell Cardiol 49: 106–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wakabayashi S, Bertrand B, Ikeda T, Pouyssegur J, Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H(+)-sensitive and Ca2+ regulation-defective. J Biol Chem 269: 13710–13715, 1994 [PubMed] [Google Scholar]
- 59. Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls “H+-sensing”. Proc Natl Acad Sci USA 89: 2424–2428, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu P, Spitzer KW. Na-independent Cl−-HCO3− exchange mediates recovery of pHi from alkalosis in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 267: H85–H91, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto T, Swietach P, Rossini A, Loh SH, Vaughan-Jones RD, Spitzer KW. Functional diversity of electrogenic Na+-HCO3− cotransport in ventricular myocytes from rat, rabbit and guinea pig. J Physiol 562: 455–475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yasutake M, Haworth RS, King A, Avkiran M. Thrombin activates the sarcolemmal Na+-H+ exchanger. Evidence for a receptor-mediated mechanism involving protein kinase C. Circ Res 79: 705–715, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Zaniboni M, Swietach P, Rossini A, Yamamoto T, Spitzer KW, Vaughan-Jones RD. Intracellular proton mobility and buffering power in cardiac ventricular myocytes from rat, rabbit, and guinea pig. Am J Physiol Heart Circ Physiol 285: H1236–H1246, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Zhao D, Chu WF, Wu L, Li J, Liu QM, Lu YJ, Qiao GF, Wang ZG, Zhang ZR, Yang BF. PAF exerts a direct apoptotic effect on the rat H9c2 cardiomyocytes in Ca2+-dependent manner. Int J Cardiol 143: 86–93, 2010 [DOI] [PubMed] [Google Scholar]