Abstract
Ca+ sparklets are subcellular Ca2+ signals produced by the opening of sarcolemmal L-type Ca2+ channels. Ca2+ sparklet activity varies within the sarcolemma of arterial myocytes. In this study, we examined the relationship between Ca2+ sparklet activity and sarcoplasmic reticulum (SR) Ca2+ accumulation and release in cerebral arterial myocytes. Our data indicate that the SR is a vast organelle with multiple regions near the sarcolemma of these cells. Ca2+ sparklet sites were located at or <0.2 μm from SR-sarcolemmal junctions. We found that while Ca2+ sparklets increase the rate of SR Ca2+ refilling in arterial myocytes, their activity did not induce regional variations in SR Ca2+ content or Ca2+ spark activity. In arterial myocytes, L-type Ca2+ channel activity was independent of SR Ca2+ load. This ruled out a potential feedback mechanism whereby SR Ca2+ load regulates the activity of these channels. Together, our data suggest a model in which Ca2+ sparklets contribute Ca2+ influx into a cytosolic Ca2+ pool from which sarco(endo)plasmic reticulum Ca2+-ATPase pumps Ca2+ into the SR, indirectly regulating SR function.
Keywords: L-type Ca2+ channels, total internal reflection fluorescence microscopy, ryanodine receptors
the opening of sarcolemmal L-type Ca2+ channels produces subcellular Ca2+ signals called Ca2+ sparklets. They play a critical role in the regulation of intracellular Ca2+ concentration ([Ca2+]i) in smooth muscle at physiological membrane potentials (i.e., −50 to −30 mV) and extracellular Ca2+ (i.e., 2 mM) (2, 22, 36). By controlling [Ca2+]i, Ca2+ sparklets could modulate multiple physiological and pathological processes in arterial myocytes including excitability, Ca2+ release from intracellular stores, contraction, and gene expression (24, 26).
The release of Ca2+ from the SR via small clusters of Ca2+-activated ryanodine receptors (RyRs) in smooth muscle cells results in the generation of a local [Ca2+]i signal referred to as a “Ca2+ spark” (25). L-type Ca2+ channels are known to modulate Ca2+ spark activity in arterial myocytes (6, 7, 10). However, unlike ventricular myocytes, where L-type Ca2+ channel-mediated Ca2+ influx (i.e., a Ca2+ sparklet) can activate nearby RyRs to produce a Ca2+ spark via a tight, local Ca2+-induced Ca2+-release mechanism (36), in smooth muscle, functional coupling of L-type Ca2+ channels and Ca2+ sparks is deemed to be “loose” or indirect (7, 10). In this model, the probability of Ca2+ spark activation during an L-type Ca2+ channel opening is low. Ca2+ influx via L-type Ca2+ channels modulates Ca2+ spark activity by controlling SR Ca2+ load. Increasing SR Ca2+ load increases the opening probability and Ca2+ flux via RyRs, resulting in higher Ca2+ spark amplitude and frequency. Consistent with this model, increasing the opening probability of L-type Ca2+ channels by the application of Bay K-8644 or membrane depolarization increases SR Ca2+ load and thus Ca2+ spark frequency and amplitude in these cells (10, 16, 17, 25).
Optical recordings of Ca2+ sparks and Ca2+ sparklets have revealed an interesting feature of the channels underlying these events: that their activity varies within arterial myocytes (14, 21–23). These regional variations in Ca2+ entry and the interdependency between L-type Ca2+ channel and SR function raise a series of important questions. What is the relationship between Ca2+ sparks and Ca2+ sparklet sites in smooth muscle? Does SR Ca2+ load vary locally depending on nearby Ca2+ sparklet activity? Do Ca2+ sparklets modulate the rate of refilling of SR with Ca2+? And, finally, does Ca2+ sparklet activity depend on SR Ca2+ load?
To address these specific questions, we developed an experimental approach to image Ca2+ sparklets and their relative impact on SR Ca2+ load and release (e.g., Ca2+ sparks) in cerebral arterial myocytes with high temporal and spatial resolution. We found that the SR occupies a vast area with multiple SR-sarcolemmal junctions. Our data indicate that SR Ca2+ content and Ca2+ spark activity are not modulated locally by nearby Ca2+ sparklets, although their activity was necessary for faster SR Ca2+ refilling. We also found that L-type Ca2+ channel activity was independent of SR Ca2+ load in arterial myocytes. This observation ruled out a potential feedback mechanism whereby SR Ca2+ load regulates L-type Ca2+ channel function. Our data suggest a model whereby L-type Ca2+ channel activity induces Ca2+ influx into a cytosolic Ca2+ pool from which sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps Ca2+ into an SR network, in which Ca2+ is homogeneously distributed (20).
MATERIALS AND METHODS
Isolation of arterial myocytes.
Sprague-Dawley rats were used in this study in strict accordance with protocols approved by the Animal Care and Use Committee of the University of Washington. Rats were euthanized by an intraperitoneal injection of pentobarbital sodium (250 mg/kg ip). Myocytes were dissociated from cerebral (middle, posterior, and basilar) arteries using standard enzymatic techniques described elsewhere (3). After dissociation, cells were maintained in a nominally Ca2+-free Ringer solution until used. Thapsigargin (TG; 1 μM) was included in some of the solutions used to record Ca2+ sparklets and whole cell Ca2+ currents (ICa) to eliminate Ca2+ release from intracellular stores and depletes Ca2+ from the SR during experimentation.
Plasmid construction and preparation of adenovirus expressing ER-RFP.
Tagged red fluorescent protein (RFP) was modified to contain a calreticulin signal sequence in the NH2-terminal and an ER retention signal, KDEL (11), in the COOH-terminal. This construct is referred to as ER-RFP. The adenovirus expressing ER-RFP was generated with the ViraPower Adenoviral Expression System using Gateway Technology following the manufacturer's guidelines (Invitrogen). The adenovirus entry clone was created by subcloning ER-RFP into the entry vector pENTR 1A. The adenovirus ER-RFP expression clone was generated via LR recombination to the destination vector pAD/CMV/DEST. Virus particles were generated by transfecting 293A cells with PacI linearized ER-RFP pAD/CMV/DEST DNA with Jet Pei. Virus particles were harvested from 293A cells exhibiting a cytopathic effect using a freeze/thaw method. The virus was tittered to determine the multiplicity of infection.
Cerebral arteries were infected with 1 ml of ER-RFP pAD diluted in 1 ml of DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. Expression of ER-RFP pAD in freshly isolated arterial myocytes was evaluated 48 h postinfection (Fig. 1). To label the sarcolemma of these cells, we used Alexa 488-conjugated wheat germ agglutinin (WGA), as previously described (24). Cells were imaged (512 × 512 pixel images) using our confocal system coupled with a Nikon ×60 oil immersion lens [numerical aperture (NA): 1.4] and a zoom of 3.5 (pixel size = 0.1 μm). The point spread function of our confocal system showed that it has lateral and axial resolutions of 0.25 and 0.80 μm, respectively.
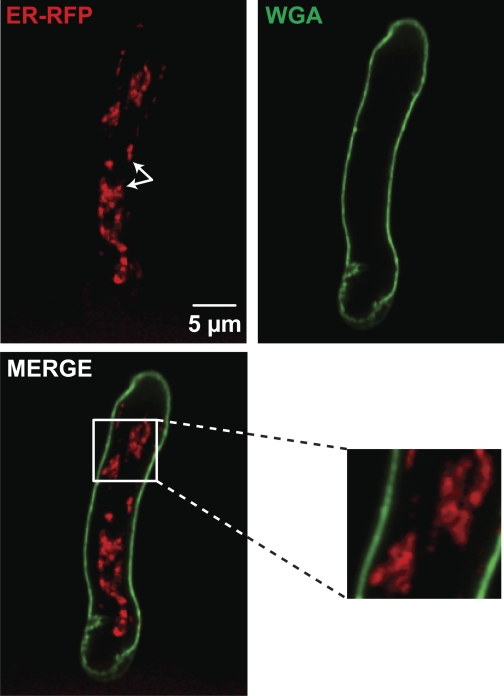
Fig. 1.
The sarcoplasmic reticulum (SR) occupies a vast area with multiple SR-sarcolemmal junctions in cerebral arterial myocytes. Shown are two-dimensional confocal images of ER-red fluoresent protein (RFP)- and wheat germ agglutinin (WGA)-associated fluorescence in representative arterial myocytes. Arrows show the proximity of the SR to the nuclear area. Shown below the ER-RFP image is a two-dimensional image produced by merging the ER-RFP and WGA images. The inset to the right of the merged image shows an enlarged image of the region in the white box. n = 6 cells.
Patch-clamp electrophysiology.
We used the conventional whole cell patch-clamp technique to control membrane voltage and to record ICa with an Axopatch 200B amplifier. During experiments, cells were continuously superfused with a solution containing (in mM) 120 N-methyl-d-glutamate, 5 CsCl, 1 MgCl2, 10 glucose, 10 HEPES, and 20 CaCl2 and adjusted to pH 7.4. Pipettes were filled with a solution composed of (in mM) 87 Cs-aspartate, 20 CsCl, 1 MgCl2, 5 MgATP, 10 HEPES, and 10 EGTA and adjusted to pH 7.2 with CsOH. For Ca2+ sparklet experiments, 0.2 mM fluo-5F (or rhod-2) was added to the pipette solution. ICa was recorded using a step depolarization of 200 ms from a holding potential of −70 to +20 mV. ICa was measured as the difference between the peak and sustained currents at the end of the pulse. Under our experimental conditions, these currents have been shown to be produced by nifedipine-sensitive L-type Ca2+ channels. A voltage error of 10 mV, attributable to the liquid junction potential of these solutions, was corrected offline. ICa was sampled at 20 kHz and low-pass filtered at 2 kHz.
Total internal reflection fluorescence and confocal microscopy and signal mass analysis.
Ca2+ sparklets were recorded in patch-clamped (whole cell configuration) cerebral arterial myocytes as previously described (2). Cells were held at a hyperpolarized potential of −70 mV to increase the driving force for Ca2+ entry and thus allow the recording of quantal Ca2+ sparklets. We used a through-the-lens total internal reflection fluorescence (TIRF) microscope built around an inverted Olympus IX-70 microscope equipped with an Olympus PlanApo (×60, NA: 1.45) oil-immersion lens and an Andor iXON charge-coupled device camera (South Windsor, CT). To monitor [Ca2+]i, cells were loaded with the Ca2+ indicator fluo-5F or rhod-2 (200 μM). As in previous studies (2, 22, 23), we used the relatively slow Ca2+ buffer EGTA (10 mM) (on rate ≈ 100-fold slower than fluo-5F or rhod-2) to restrict the fluorescence of the indicator to near the site of Ca2+ entry (≤1 μm) (40). A similar strategy has been used to record Ca2+-release sites in ventricular myocytes using confocal microscopy (1, 35). For further details about this protocol, readers are referred to Amberg et al. (2).
Images were acquired at 100–300 Hz. As previously reported (22, 23), we determined the activity of Ca2+ sparklets by calculating the nPs of each Ca2+ sparklet site, where n is the number of quantal levels and Ps is the probability that a quantal Ca2+ sparklet event is active. Because Ca2+ sparklet activity has a bimodal distribution, Ca2+ sparklet sites were grouped into three categories: silent (by default having an nPs of 0), low (nPs between 0 and 0.2), and high (nPs higher than 0.2). A detailed description of this analysis can be found in Navedo et al. (23).
Background fluorescence was subtracted from the total fluorescence signal of fluo-5F- or rhod-2-loaded arterial myocytes. The fluo-5F or rhod-2 fluorescence signal was then converted to Ca2+ concentration units using the following “Fmax” equation (19):
as previously described before (2, 23). In brief, F is fluorescence, Fmax is the fluorescence intensity of fluo-5F or rhod-2 in the presence of saturating free Ca2+, Kd is the dissociation constant of the indicator (fluo-5F: 1,280 nM and rhod-2: 600 nM), and Rf is the Fmax-to-Fmin ratio of the indicators (fluo-5F: 286 and rhod-2: 150). Fmin is the fluorescence intensity of the indicator in a solution where the Ca2+ concentration is 0. Kd and Rf values were determined in vitro using standard methods and were similar to those reported by others (38). Fmax was determined at the end of each experiment by exposing cells to the Ca2+ ionophore ionomycin (10 μM) and 20 mM external Ca2+.
To label RyRs or SERCA in the SR of arterial myocytes, freshly dispersed cells were incubated with either fluorescent BODIPY FL ryanodine (10 μM) or fluorescent BODIPY FL TG (10 μM), respectively, for 20–30 min. Myocytes were then patch clamped and dialyzed with a pipette solution containing rhod-2 to monitor Ca2+ sparklets with our TIRF microscope. TIRF microscopy was used for these experiments as it allows the recording of Ca2+ sparklets in relation to subsarcolemmal RyRs and SERCA with high resolution. During analysis, RyR or SERCA images were overlaid onto the Ca2+ sparklet stack, and the distance between the center of the sparklet site and the RyR or Ca2+-ATPase pump islets was measured. To exclude nonspecific labeling of FL ryanodine (10 μM) or FL TG (10 μM), freshly isolated arterial myocytes were incubated with 10-fold higher concentrations of nonlabeled ryanodine or nonlabeled TG for 10 min before the addition of the fluorescent compounds and imaging of these cells as described above. Under these conditions, labeling of RyRs and SERCA with FL ryanodine and FL TG was significantly decreased to 4 ± 1% (n = 5 cells) and 10 ± 4% (n = 6 cells), respectively (P < 0.05; Fig. 2C).
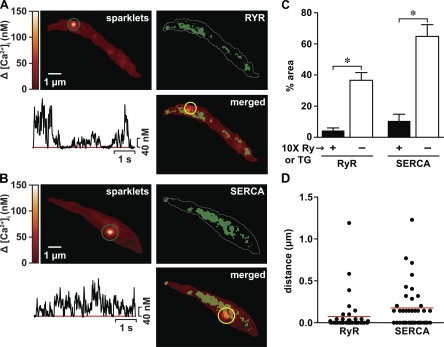
Fig. 2.
Ca2+ sparklet sites coincide with junctional SR expressing sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and ryanodine (Ry) receptors (RyRs). Shown are total internal reflection fluorescence (TIRF) images of representative arterial myocytes exposed to FL Ry (top right corner in A) or FL thapsigargin (TG; top right corner in B) to label RyRs and SERCA, respectively, in the SR. The white dotted lines represent the sarcolemmal footprint of the cells. Ca2+ sparklets were imaged in these cells after they were loaded with rhod-2 via a patch pipette with TIRF microscopy (top left corners in A and B). The traces below the sparklet images are time courses of intracellular Ca2+ concentration ([Ca2+]i) in the sites indicated by the green circles. The images in the bottom right corners in A and B were produced by superimposing the FL Ry or FL TG images with the respective Ca2+ sparklet images. C: bar graph of the percent area (in μm2) occupied by FL Ry (n = 12 cells) or FL TG (n = 8 cells) labeling in the presence (+) or absence (−) of 10-fold higher concentrations of nonfluorescence Ry or TG in TIRF images of arterial myocytes. D: scatterplot of the distance between FL Ry or FL TG labeling and Ca2+ sparklet sites in arterial myocytes. The red lines indicate the median values in each group. *P < 0.05.
To assess the intraluminal SR Ca2+ concentration ([Ca2+]SR) and Ca2+ sparklets simultaneously using TIRF, we loaded cells with the low-affinity Ca2+ indicator mag-fluo-4 (Kd= 22 μm) and the high-affinity dye rhod-2 to differentially load the SR and cytosol, respectively. Because of the thin excitation field created by TIRF (i.e., z-axis resolution of ∼100 nm), we cannot image SR Ca2+ content in central SR regions. To overcome this limitation, in some experiments, intraluminal [Ca2+]SR was assessed using a Nikon swept-field confocal microscope. Cells were incubated with 10 μM mag-fluo-4 at 37°C for 45 min, washed with indicator-free solution, and stored at 4°C to unload the cytosolically located mag-fluo-4. Cells were then voltage clamped using the whole cell patch-clamp technique with an internal solution containing rhod-2 to monitor cytosolic Ca2+. Cells were illuminated with 488 nm to excite mag-fluo-4 and 568 nm to excite rhod-2. A dichroic mirror and a long-pass interference filter separated the fluorescence from both indicators. Ca2+ stores were identified on the basis of the response obtained from SR-loaded cells with mag-fluo-4 to a solution containing 0 mM Ca2+ and 10 mM caffeine to release Ca2+ from the SR. Under our experimental conditions, using 20 mM Ca2+ in the extracellular solution could lead to a larger [Ca2+]SR than in the presence of 2 mM Ca2+. Note, however, that while the absolute levels of [Ca2+]SR and rate of loading are likely to be higher in 20 mM external Ca2+ than in 2 mM external Ca2+, the experimental conditions of our experiments are not likely to alter the spatial distribution of Ca2+ in the SR lumen and its relationship to nearby Ca2+ sparklet sites.
Simultaneous imaging of Ca2+ sparks and sparklets was performed on isolated arterial myocytes loaded with the fluorescent Ca2+ indicator fluo-4 AM (50 μM) (30) using a Nikon Swept Field confocal system coupled to a Nikon TE300 inverted microscope equipped with a Nikon ×60 water-immersion lens (NA: 1.4). Images were analyzed with custom software written in IDL language (Research Systems, Boulder, CO). Ca2+ sparks were identified with a computer algorithm similar to the one described by Cheng et al. (5). Images were normalized by dividing the fluorescence intensity of each pixel (F) by the average resting fluorescence intensity (F0) of a confocal image to generate an F/F0 image. For analysis, we determined the amplitude and frequency of Ca2+ signals before and after the application of the RyR blocker tetracaine, the L-type Ca2+ channel inhibitor nifedipine, or both. Tetracaine was used for these experiments instead of ryanodine to identify Ca2+ sparks because it is membrane permeable and reversibly blocks RyRs in cardiac and arterial myocytes (6, 8, 28, 34).
We used the signal mass approach developed by Zou et al. (41) to determine the amount of Ca2+ flux associated with a Ca2+ spark or a Ca2+ sparklet before and after pharmacological manipulation. For this analysis, the total fluorescence intensity (Ftotal) associated with a Ca2+ signal is determined from raw images by summing the fluo-4 fluorescence from all the pixels within the same area of the image larger than the entire fluorescence signal produced before and after the indicated pharmacological manipulation. The change in Ftotal (ΔFtotal) was then determined by subtracting the total fluorescence before a Ca2+ signal from the total fluorescence at each point during that Ca2+ signal progression. Signal mass was calculated by determining the peak of the integral of ΔFtotal trace over time for each Ca2+ signal before and after pharmacological manipulation. For a more detailed description of this analysis, see Amberg et al. (2).
Chemicals and statistics.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Normally distributed data are presented as means ± SE. Two-sample comparisons were made using a Student's t-test. Nonparametric statistical analyses (Mann-Whitney test) were used for nonnormally distributed data. P values of <0.05 were considered statistically significant. In the figures, asterisks indicate a significant difference between groups.
RESULTS
Arterial smooth muscle cells have an extensive SR network.
We determined the spatial distribution of the SR in freshly isolated myocytes from cerebral arteries. To do this, we imaged arterial myocytes infected with a modified tagged RFP fused with a calreticulin entrance sequence in its NH2-terminal and a four amino acid sequence ER/SR retention signal (KDEL) in its COOH-terminal. We refer to this protein as ER-RFP. Figure 1 shows a two-dimensional confocal image of a representative arterial myocyte expressing ER-RFP. The SR appears in the images as a vast, dense, cell-wide structure that seems to extend from areas adjacent to the nucleus (arrows) to subsarcolemmal regions (n = 6 cells). WGA tagged to Alexa-488 binds sialic acid residues in glycoproteins and, thus, was used to more clearly determine the relationship between the SR and sarcolemma of arterial myocytes in cells infected with ER-RFP (Fig. 1). As previously reported for gastrointestinal and portal vein myocytes (14, 15), ER-RFP fluorescence was observed throughout the cell and in regions of the cell near the WGA fluorescence (Fig. 1, inset).
Ca2+ sparklet sites are located near the junctional SR expressing SERCA and RyRs.
The data described above suggest that there are multiple sites within a cell where functional interactions via Ca2+ signals between and among Ca2+ sensitive proteins in the SR and sarcolemma could take place. Thus, we examined the relationship between Ca2+ sparklet sites and regions of SR likely involved in Ca2+ uptake (i.e., expressing SERCA) and release (i.e., expressing RyRs) using a TIRF microscope. We recorded Ca2+ sparklets in cells that had been exposed to fluorescent FL ryanodine (10 μM) or FL TG (10 μM) to label RyRs and SERCA, respectively (Fig. 2, A and B). Consistent with other studies (12, 15, 27, 32, 33) and the confocal data above, we found in TIRF images that RyRs and SERCA were expressed broadly in regions near the sarcolemma of arterial myocytes. Indeed, quantitative analysis of FL ryanodine and FL TG images suggested that 37 ± 0.5% (n = 12 cells) and 65 ± 0.7% (n = 8 cells) of the sarcolemmal footprint was within close proximity to SR regions expressing RyRs and SERCA, respectively (Fig. 2C). In addition, we found that the majority of Ca2+ sparklet sites overlapped with regions of the SR expressing RyR or SERCA. The median distance between Ca2+ sparklet sites and RyR- or SERCA-associated fluorescence was <0.2 μm (Fig. 2D). These findings suggest that most Ca2+ sparklet sites are located near or at regions of the SR involved in Ca2+ uptake and release.
Localization of Ca2+ sparklets and Ca2+ sparks in arterial myocytes.
We investigated whether Ca2+ sparks occur near or at Ca2+ sparklet sites in cerebral arterial myocytes. Figure 3A shows a local, transient Ca2+ signal that was detected in close proximity to the sarcolemma of an arterial myocyte. To determine the specific contribution of Ca2+ sparks and Ca2+ sparklets to these Ca2+ signals, we imaged [Ca2+]i before and after application of the RyR blocker tetracaine (50 μM) (8) or the L-type Ca2+ channel inhibitor nifedipine (10 μM) (2, 22, 23). Note that 50 μM tetracaine had no effect on whole cell L-type Ca2+ current density (control: −1.5 ± 0.2 pA/pF vs. tetracaine: −1.2 ± 0.2 pA/pF, P > 0.05, n = 10 cells; Fig. 3A) (8).
Fig. 3.
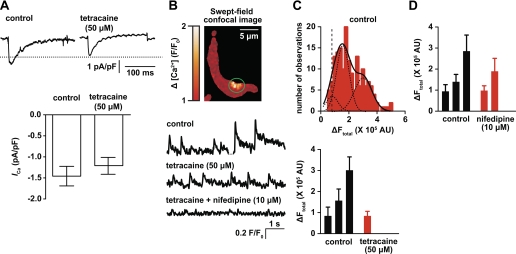
Ca2+ sparklets occur near Ca2+ spark sites in arterial myocytes. A, top: representative Ca2+ current (ICa) recordings under control conditions and after the application of 50 μM tetracaine. Bottom, plot of means ± SE of the peak ICa density before (−1.5 ± 0.2 pA/pF) and after treatment with tetracaine (−1.2 ± 0.2 pA/pF, P > 0.05, n = 10 cells). ICa was elicited by a step depolarization of 200 ms to +20 mV. B: representative swept-field confocal image of an arterial myocyte showing a transient [Ca2+]i signal. The traces below the image show the time course of [Ca2+]i in the area highlighted by the green circle during control conditions and after the application of 50 μM tetracaine and tetracaine + 10 μM nifedipine. F/F0, fluorescence intensity/resting fluorescence intensity. C: amplitude histogram of the signal mass amplitude [change in total fluorescent intensity (ΔFtotal), in arbitrary units (AU)] during control conditions (top; n = 10 cells). Bottom, graph plotting means ± SE of the signal mass amplitude of Ca2+ signals in control conditions and after the application of 50 μM tetracaine. D: means ± SE of the signal mass amplitude of Ca2+ signals before and after the application of 10 μM nifedipine (n = 11 cells).
Transient Ca2+ signals under control conditions had mean amplitude of 0.7 ± 0.07 ΔF/F0 and a mean frequency of 0.8 ± 0.08 Hz (Fig. 3B, bottom). In the presence of tetracaine, these Ca2+ signals were significantly smaller in amplitude (0.2 ± 0.01 ΔF/F0) than under control conditions (P < 0.05; Fig. 3B, bottom). Tetracaine-insensitive events were subsequently eliminated by the simultaneous application of 10 μM nifedipine + tetracaine in the solution (Fig. 3B, bottom). We constructed an amplitude histogram of the signal mass (ΔFtotal) of these Ca2+ signals. Under control conditions, the histogram could be fit with the sum of three Gaussian functions with centers (i.e., mean) at 0.8 × 105, 1.6 × 105, and 3.0 × 105 ΔFtotal (χ2 = 2.4, n = 10 cells; Fig. 3C, top). In the presence of tetracaine, only events with a mean signal mass amplitude of 0.8 × 105 ΔFtotal were observed (χ2 = 0.2; Fig. 3C, bottom). These events were eliminated with the simultaneous application of tetracaine + nifedipine. These results suggest that tetracaine-insensitive events are produced by Ca2+ sparklets, whereas events with a significantly larger signal mass, which were tetracaine sensitive, are produced by Ca2+ sparks in the same microdomain (Fig. 3B).
A testable prediction of this hypothesis is that treatment of arterial myocytes with nifedipine should preserve tetracaine-sensitive events. Consistent with this, the application of 10 μM nifedipine eliminated events with a mean signal mass amplitude of 0.9 × 105ΔFtotal and slightly decreased the mean amplitude of remaining events from 1.4 × 105 and 2.9 × 105 ΔFtotal to 1.0 × 105 and 1.9 × 105 ΔFtotal, respectively (n = 11 cells; Fig. 3D). The decrease in the mean signal mass amplitude of nifedipine-insensitive events (i.e., Ca2+ sparks), which were abolished by tetracaine, is likely due to a decrease in SR Ca2+ load (6, 7, 10). Taken together, these data indicate that although Ca2+ sparklets occur near Ca2+ spark sites, they are not directly required for Ca2+ spark ignition in cerebral arterial myocytes.
SR Ca2+ load does not vary regionally with nearby Ca2+ sparklet activity.
A mechanism by which Ca2+ sparklets could potentially modulate Ca2+ spark activity is by controlling SR Ca2+ load, especially in areas near sparklet sites. To investigate this possibility, we simultaneously monitored SR Ca2+ and Ca2+ sparklet activity using TIRF microscopy. TIRF allowed us to detect Ca2+ sparklets and measure potential relative changes in near-membrane SR Ca2+ content with a high signal-to-noise ratio. The SR of arterial myocytes was selectively loaded with the low-affinity Ca2+ indicator mag-fluo-4 (see materials and methods). Cells were then patch clamped in the whole cell configuration to allow for the dialysis of mag-fluo-4, limiting the fluorescence of this fluorophore to the SR lumen.
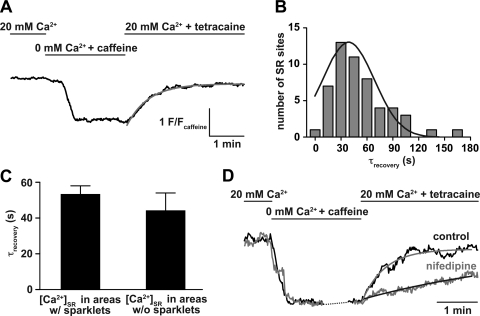
To ensure SR loading, cells were patch clamped with a pipette solution containing 100 nM free [Ca2+]i. Rhod-2 was also included in this solution to image cytosolic Ca2+ signals. To prevent Ca2+ sparks (i.e., Ca2+-release events via RyRs located in the SR), cells were continually perfused with the RyR blocker tetracaine (50 μM). As shown in Fig. 4A, cells subjected to this protocol had well-defined zones of mag-fluo-4 fluorescence. The application of a solution containing 10 mM caffeine and no tetracaine resulted in a decrease in mag-fluo-4 fluorescence, thus confirming that these signals arose from Ca2+-bound mag-fluo-4 in the lumen of the SR (n = 14 cells; Fig. 4A, bottom).
Fig. 4.
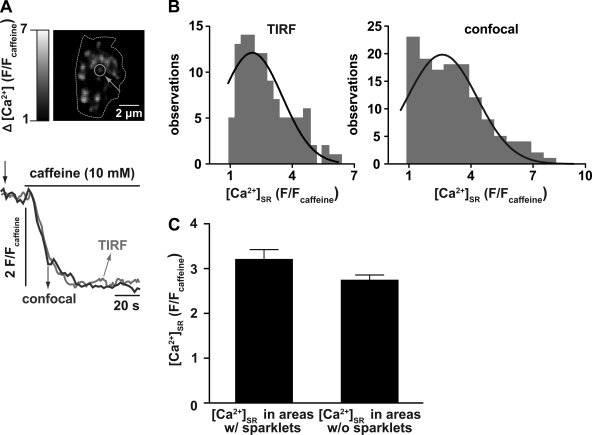
Ca2+ sparklet activity does not contribute to regional variations in SR Ca2+ concentration ([Ca2+]SR). A, top: representative TIRF image of an arterial myocyte in which the SR was differentially loaded with the slow fluorescence indicator mag-fluo-4 to monitor [Ca2+]SR. Bottom, time course of [Ca2+]SR in the region indicated by the green circle in the TIRF image above and the time course of [Ca2+]SR obtained from a different arterial myocyte using a confocal microscope. The TIRF [Ca2+]SR image was obtained at the time point indicated by the arrow. The dotted line indicates the sarcolemmal footprint obtained with TIRF. B: amplitude histogram of [Ca2+]SR [in F/fluorescent intensity of caffeine (Fcaff) units] in multiple SR regions obtained from cells imaged with TIRF (left; n = 14 cells) or confocal (right; n = 7 cells) microscopy. Each histogram could be fit with a single Gaussian function (solid line) with a center at 2.2 ± 0.09 F/Fcaff (TIRF) and 2.6 ± 0.2 F/Fcaff (confocal). C: bar graph of [Ca2+]SR in areas with and without nearby (≤1 μm) Ca2+ sparklet activity (P > 0.05).
The amplitude distribution of Ca2+ pools within the SR was assessed by fitting an amplitude histogram of [Ca2+]SR [in F/caffeine fluorescent intensity (Fcaff) units] in multiple SR regions, as shown in Fig. 4B. The histogram could be fit (χ2 = 2.1) with a Gaussian function with a center at a [Ca2+]SR of 2.2 ± 0.09 F/Fcaff. This analysis suggests that the [Ca2+]SR distribution within arterial myocytes has a single mode, indicating that [Ca2+]SR is similar throughout the SR in these cells. Similar results were obtained in mag-fluo-4-loaded cells imaged with a confocal microscope (2.6 ± 0.17 F/Fcaff, χ2 = 2.8, n = 7 cells; Fig. 4B, right). Consistent with the data described above, we found that [Ca2+]SR was similar in SR regions independently of their association or not with Ca2+ sparklet sites (P > 0.05; Fig. 4C).
Ca2+ sparklets increase the rate of SR Ca2+ refilling in arterial myocytes.
Having established that Ca2+ sparklets are not associated with significant variations in local SR Ca2+ load in areas close to these Ca2+ signals, we investigated whether they influence the rate of loading of the SR with Ca2+. In these experiments, we imaged [Ca2+]SR before, during, and after the application of 10 mM caffeine using TIRF (Fig. 5A). [Ca2+]SR decreased rapidly after the application of 10 mM caffeine but recovered back to control levels after the removal of caffeine from the extracellular solution (Fig. 5). The recovery phase of [Ca2+]SR records could be fit with a single exponential function with a time constant of recovery of 38 ± 2 s (n = 11 cells; Fig. 5B). The rate of recovery of [Ca2+]SR after caffeine at multiple SR sites was similar whether or not they were within 1 μm from an active Ca2+ sparklet site (P > 0.05; Fig. 5C). In cells where L-type Ca2+ channel activity was inhibited with nifedipine, and consequently low activity and persistent Ca2+ sparklet events were blocked (2), the rate of recovery of [Ca2+]SR was nearly 12-fold slower (638 ± 170 s, n = 5 cells; Fig. 5D) than under control conditions. This is consistent with the view that Ca2+ sparklets contribute to faster SR Ca2+ refilling. Together, these findings suggest that while Ca2+ sparklets do not contribute to the creation of a localized, heterogeneous SR Ca2+ gradient in areas near these events, they are required for faster Ca2+ refilling of the SR after it has been depleted.
Fig. 5.
Ca2+ sparklets increase the rate of SR Ca2+ load in arterial myocytes. A: representative time course of [Ca2+]SR before, during, and after the application of a solution containing 0 mM external Ca2+ + 10 mM caffeine to deplete the Ca2+ from the SR. The red line indicates the best fit of the recovery phase of [Ca2+]SR with a single exponential function. B: histogram of the rate of [Ca2+]SR recovery after the application of caffeine. This histogram was fitted with a single exponential function with a time constant of recovery (τrecovery) of 38 ± 2 s (n = 11 cells). C: bar graph of the mean ± SE rate of [Ca2+]SR recovery in areas with and without Ca2+ sparklet activity (P > 0.05). D: nifedipine decreased the rate of [Ca2+]SR recovery after the application of caffeine (n = 5 cells).
Ca2+ sparklet activity is independent of SR Ca2+ content.
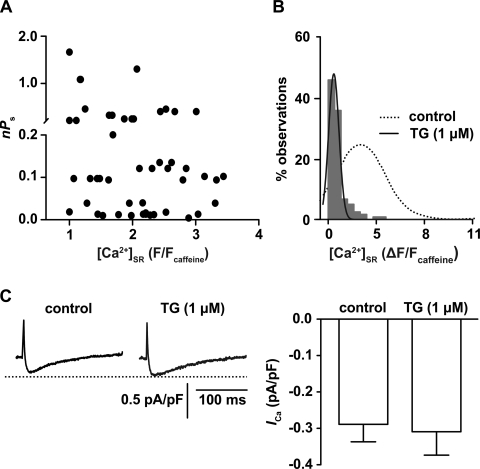
Recent studies (29, 37) have reported significant inhibition of L-type Ca2+ channel activity after SR Ca2+ depletion. Thus, using the approach described above, we determined whether Ca2+ sparklet activity depends on [Ca2+]SR in freshly isolated cerebral arterial myocytes. A testable prediction of the aforementioned studies is that depletion of SR Ca2+ load should inhibit Ca2+ sparklet activity in arterial myocytes. To test this hypothesis, we recorded Ca2+ sparklet activity before, during, and after depletion of SR Ca2+ load (Fig. 6). We found that Ca2+ sparklet activity did not change (P > 0.05) with varied [Ca2+]SR (n = 9 cells; Fig. 6A). Indeed, our data suggest that there is poor correlation (r2 = 0.08) between Ca2+ sparklet activity (nPs) and [Ca2+]SR in arterial myocytes.
Fig. 6.
Ca2+ sparklet activity is independent of SR Ca2+ load in arterial myocytes. A: scatterplot of Ca2+ sparklet activity at different [Ca2+]SR (n = 9 cells). Note that Ca2+ sparklet activity was poorly correlated with SR Ca2+ content (r2 = 0.08). n, Number of quantal levels; Ps, probability that a quantal Ca2+ sparklet is active. B: amplitude histogram of [Ca2+]SR (in ΔF/Fcaff units) in multiple SR regions obtained from cells treated with TG to deplete the SR of Ca2+ (n = 7 cells). The histogram could be fit with a single Gaussian function (solid line) with a center at 0.4 ± 0.2 ΔF/Fcaff (χ2 = 3.1). The dotted line represents the [Ca2+]SR distribution obtained from cells that were not exposed to TG, as in Fig. 4B, right. C, left: representative ICa recordings under control conditions and after SR depletion with 1 μM TG. Right, plot of the means ± SE of the peak ICa density (in pA/pF, at +20 mV) before and after SR depletion with 1 μM TG (n = 4 cells, P > 0.05).
To determine the implications of these results on cell-wide Ca2+ influx, we recorded whole cell ICa from freshly isolated arterial myocytes before and after depletion of SR Ca2+ with 1 μM TG. For analysis, we only used arterial myocytes that maintained their characteristic spindle-shaped morphology after SR depletion. The amplitude histogram of [Ca2+]SR (in ΔF/Fcaff units) shown in Fig. 6B demonstrates that [Ca2+]SR was significantly decreased in cells treated with TG compared with untreated cells (Fig. 4A), thus confirming that this treatment depletes SR Ca2+ stores (n = 7 cells). Treatment of cells with TG did not induce a change in peak ICa density (−0.31 ± 0.06 pA/pF) compared with control peak ICa (−0.29 ± 0.05 pA/pF, P > 0.05, n = 4 cells; Fig. 6C). Collectively, these findings strongly suggest that L-type Ca2+ channel activity does not depend on [Ca2+]SR in arterial myocytes.
DISCUSSION
In this study, we used confocal and TIRF microscopy to investigate with high temporal and spatial resolution the relationship between Ca2+ sparklets and SR function in arterial myocytes. We report four fundamental observations. First, Ca2+ sparklets are located close to SR regions expressing RyRs and SERCA. Second, consistent with these data, Ca2+ sparklets occur near Ca2+ spark sites in arterial myocytes. Third, local SR Ca2+ load and recovery rate do not vary regionally due to the activation of nearby Ca2+ sparklets. Yet, sparklets are necessary for faster overall SR Ca2+ replenishment. To our knowledge, this is the first time that [Ca2+]SR dynamics and Ca2+ sparklet activity have been directly and simultaneously visualized in freshly isolated cerebral arterial myocytes. Finally, our data suggest that L-type Ca2+ channel activity is independent of [Ca2+]SR content. This observation is important because it rules out any potential feedback mechanism whereby SR Ca2+ load regulates the activity of these channels in arterial myocytes.
Our data suggest that the SR of living cerebral artery smooth muscle is a vast, convoluted, nonuniform organelle that extends throughout the cell and approaches the sarcolemma in multiple regions of the cell. Similar findings have been reported in cultured and freshly isolated arterial myocytes from the gastrointestinal tract, portal vein, and aorta using electron (9) and confocal (12, 13, 15, 32, 39) microscopy. Together with these studies, our data suggest that this SR organization is a conserved feature in smooth muscle from different tissues that creates areas of specialized communication between and among Ca2+-sensitive proteins (i.e., L-type Ca2+ channels, RyRs, and SERCA) located in the sarcolemma and SR.
The issue of whether L-type Ca2+ channels can activate RyRs in the SR that comes into close (10–30 nm) apposition to the surface membrane (i.e., junctional SR) of arterial myocytes has been extensively examined (7, 10, 18). Using a combination of patch-clamp electrophysiology and line-scan confocal microscopy, Kotlikoff's laboratory (7) found that membrane depolarization evoked L-type Ca2+ currents and increased the probability of Ca2+ spark occurrence. Interestingly, they observed that in most cases, the activation of Ca2+ sparks occurred long (>10 ms) after the onset of depolarization and resultant Ca2+ currents. On the basis of these data, a model was proposed in which RyRs and L-type Ca2+ channels were “loosely” coupled in arterial myocytes. This model is supported by recent data from Moosmang's laboratory (10) demonstrating that Ca2+ spark frequency and amplitude are significantly reduced, but not eliminated, in arterial myocytes from a mouse with the Cav1.2 gene inactivated. These findings suggest that local, tight coupling between L-type Ca2+ channels and RyRs is not necessary for the ignition of Ca2+ sparks. However, these results did not address the issue of whether Ca2+ sparks and Ca2+ sparklets can coexist in the same microenvironment in arterial myocytes.
The data in this study provide compelling evidence in support of the hypothesis that Ca2+ sparklets could occur near Ca2+ spark sites in cerebral arterial myocytes. First, Ca2+ sparklets can occur in regions expressing RyRs in the SR of arterial mycoytes (Fig. 2). Second, the signal mass of Ca2+ signals revealed three prominent peaks, two of which were completely eliminated after the application of the RyR blocker tetracaine. The remaining peak seems to correspond to the activity of Ca2+ sparklets, since it was eliminated by application of the L-type Ca2+ channel blocker nifedipine in the presence of tetracaine. Third, consistent with the “loose coupling” model above, the signal masses of Ca2+ signals that seem to correspond to Ca2+ sparks were not abolished by nifedipine. Indeed, nifedipine abolished events with a smaller signal mass amplitude but only slightly decreased the amplitude of the remaining larger events. Together, these results suggest that Ca2+ sparks and Ca2+ sparklets can coexist in the same microenvironment, where Ca2+ sparklets can indirectly modulate RyRs activity via the regulation of [Ca2+]SR by cytosolic [Ca2+]i (7, 10).
Our [Ca2+]SR data are consistent with recent studies from the Bers (31) and McCarron (20) laboratories in cardiac and portal vein myocytes that suggest that the SR is an interconnected organelle in which Ca2+ can freely diffuse. We found that the [Ca2+]SR distribution was unimodal (Fig. 4, B and C) with a relatively low coefficient of variation (defined as SD/mean) of 0.68 for TIRF data and 0.65 for confocal data. Interestingly, [Ca2+]SR was similar in regions of the SR near high-activity Ca2+ sparklet sites and regions of the cells without visible Ca2+ sparklet activity. Based on these data, we propose a model for the contribution of Ca2+ sparklets to [Ca2+]SR in cerebral arterial myocytes. In this model, Ca2+ sparklets contribute to a general cytosolic pool of Ca2+ from which the SR can draw to accelerate store refilling. [Ca2+]SR could be transiently higher in areas associated with storage (e.g., calsequestrin), uptake (e.g., SERCA pumps), or release (e.g., RyRs or inositol 1,4,5-trisphosphate receptors), but normally will exist in a free diffusional equilibrium throughout the SR of cerebral arterial myocytes, which is not influenced by the activity of nearby Ca2+ sparklets. Future studies should examine this model.
An important observation in the present study is that the activity of Ca2+ sparklets is not dependent on [Ca2+]SR. Recent data suggest that stromal interaction molecule 1 (STIM1), which is the main activator of stored-operated channels, directly binds and modulates L-type Ca2+ channel activity in rat cortical neurons and cultured A7r5 smooth muscle cells, respectively (29, 37). These two studies have reported significant inhibition of L-type Ca2+ channel activity after SR Ca2+ depletion, in stark contrast with our results. While the reasons for these opposing findings are unclear, it is possible that variation in the expression of STIM1 in cultured versus freshly isolated cells (used in this study) may contribute to differences in the modulation of L-type Ca2+ channels after SR Ca2+ depletion. Indeed, a recent report (4) has suggested that STIM1 is abundantly expressed in cultured cells, but very low levels were detected in freshly isolated arterial myocytes. Alternatively, it is possible that L-type Ca2+ channels are not regulated by STIM1 after SR Ca2+ depletion in cerebral arterial myocytes or that differences in experimental conditions (e.g., different cell types, recording conditions, or depletion methods) may account for the apparent disparities. Regardless, our results rule out a potential feedback mechanism whereby SR Ca2+ load regulates sparklet activity in cerebral arterial myocytes. Thus, the physiological relevance of STIM1 regulation of L-type Ca2+ channels during SR Ca2+ depletion in cerebral arterial myocytes is unclear.
Limitations of the study.
Some of the experimental conditions used in this study could induce changes in [Ca2+]SR that are different to those in arterial myocytes in vivo. For example, as Jaggar's laboratory (6) has shown, incubation of cerebral arteries with tetracaine increases [Ca2+]SR. In addition, the relatively high extracellular [Ca2+] (>2 mM) used in some of the experiments in this study may also contribute to elevated [Ca2+]SR. Thus, we cannot rule out the possibility that the absolute levels of [Ca2+]SR and rates of loading could be different in vivo than under our experimental conditions. Despite these limitations, we contend that novel insights into the bases of SR Ca2+ accumulation and release and its relationship to Ca2+ influx via L-type Ca2+ channels have been obtained in the present study. These insights should form the bases of new hypotheses that can be best tested in appropriate animal models.
Conclusions.
In summary, we have provided the first examination of the relationship between Ca2+ sparklets and [Ca2+]SR dynamics in freshly dissociated cerebral arterial myocytes. Our findings indicate that the SR in cerebral arterial myocytes extends throughout the cell and lines the internal side of the sarcolemma in specialized areas where it can promote functional interactions between Ca2+-sensitive proteins in these two organelles. Ca2+ sparklet activity was not associated with the creation of a localized, heterogeneous SR Ca2+ gradient, but their activity was necessary for faster SR Ca2+ refilling, perhaps due to their influence on steady-state Ca2+ entry and global [Ca2+]i (2). Our data provide important evidence indicating that L-type Ca2+ channel activity is not modulated by SR Ca2+ load in arterial myocytes. Finally, the methods and approaches used here may help determine the mechanisms of regulation of SR function by L-type Ca2+ channels during pathological conditions.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-098200 (to M. F. Navedo) and HL-085686 and HL-085870 (to L. F. Santana) and by American Heart Association Grant 0735251N (to M. F. Navedo). M. A. Nystoriak was supported by the National Institutes of Health-University of Washington Cardiovascular Research Training Program (NHLBI Grant T32-HL-07828). L. F. Santana is an Established Investigator of the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.T., M.A.N., M.N.-C., and M.F.N. performed experiments; Y.T., M.A.N., and M.F.N. analyzed data; Y.T., M.A.N., M.N.-C., L.F.S., and M.F.N. edited and revised manuscript; Y.T., M.A.N., M.N.-C., L.F.S., and M.F.N. approved final version of manuscript; M.A.N., M.N.-C., L.F.S., and M.F.N. interpreted results of experiments; M.A.N. and M.F.N. prepared figures; L.F.S. and M.F.N. conception and design of research; M.F.N. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Rose Dixon for critically reading the manuscript.
Present address of M. A. Nystoriak, M. Nieves-Cintrón, and M. F. Navedo: Dept. of Pharmacology, Univ. of California Davis, Davis, CA.
REFERENCES
- 1. Altamirano J, Bers DM. Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ Res 101: 590–597, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol 579: 187–201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amberg GC, Santana LF. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ Res 93: 965–971, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295: C779–C790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J 76: 606–617, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J Physiol 544: 71–84, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol 115: 653–662, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curtis TM, Tumelty J, Stewart MT, Arora AR, Lai FA, McGahon MK, Scholfield CN, McGeown JG. Modification of smooth muscle Ca2+-sparks by tetracaine: evidence for sequential RyR activation. Cell Calcium 43: 142–154, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Devine CE, Somlyo AV, Somlyo AP. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol 52: 690–718, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between Cav1.2 channels and RyR to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol 584: 205–219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem 264: 21522–21528, 1989 [PubMed] [Google Scholar]
- 12. Gollasch M, Wellman GC, Knot HJ, Jaggar JH, Damon DH, Bonev AD, Nelson MT. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res 83: 1104–1114, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science 275: 1643–1648, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Gordienko DV, Bolton TB, Cannell MB. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle cells. J Physiol 507: 707–720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordienko DV, Greenwood IA, Bolton TB. Direct visualization of sarcoplasmic reticulum regions discharging Ca2+ sparks in vascular myocytes. Cell Calcium 29: 13–28, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol Cell Physiol 274: C1755–C1761, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Kotlikoff MI. Calcium-induced calcium release in smooth muscle: the case for loose coupling. Prog Biophys Mol Biol 83: 171–191, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J 78: 2655–2667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCarron JG, Olson ML. A single luminally continuous sarcoplasmic reticulum with apparently separate Ca2+ stores in smooth muscle. J Biol Chem 283: 7206–7218, 2008 [DOI] [PubMed] [Google Scholar]
- 21. McCarron JG, Olson ML, Currie S, Wright AJ, Anderson KI, Girkin JM. Elevations of intracellular calcium reflect normal voltage-dependent behavior, and not constitutive activity, of voltage-dependent calcium channels in gastrointestinal and vascular smooth muscle. J Gen Physiol 133: 439–457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navedo MF, Amberg G, Votaw SV, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA 102: 11112–11117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol 127: 611–622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 102: e1–e11, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci USA 105: 15623–15628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol 534: 313–326, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Overend CL, O'Neill SC, Eisner DA. The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes. J Physiol 507: 759–769, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 330: 101–105, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 113: 229–238, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Picht E, Zima AV, Shannon TR, Duncan AM, Blatter LA, Bers DM. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ Res 108: 847–856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pucovsky V, Bolton TB. Localisation, function and composition of primary Ca2+ spark discharge region in isolated smooth muscle cells from guinea-pig mesenteric arteries. Cell Calcium 39: 113–129, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Rueda A, Song M, Toro L, Stefani E, Valdivia HH. Sorcin modulation of Ca2+ sparks in rat vascular smooth muscle cells. J Physiol 576: 887–901, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarkozi S, Szentesi P, Cseri J, Kovacs L, Csernoch L. Concentration-dependent effects of tetracaine on excitation-contraction coupling in frog skeletal muscle fibres. J Muscle Res Cell Motil 17: 647–656, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking “Ca2+ spikes” in rat cardiac myocytes. J Physiol 512: 677–691, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410: 592–596, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330: 105–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol 542: 843–854, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium 38: 397–407, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci 23: 2538–2548, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zou H, Lifshitz LM, Tuft RA, Fogarty KE, Singer JJ. Using total fluorescence increase (signal mass) to determine the Ca2+ current underlying localized Ca2+ events. J Gen Physiol 124: 259–272, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]