Abstract
The highly reactive nature of dopamine renders dopaminergic neurons vulnerable to oxidative damage. We recently demonstrated that loss-of-function mutations in the Drosophila gene Catecholamines up (Catsup) elevate dopamine pools but, paradoxically, also confer resistance to paraquat, an herbicide that induces oxidative stress-mediated toxicity in dopaminergic neurons. We now report a novel association of the membrane protein, Catsup, with GTP cyclohydrolase rate-limiting enzyme for tetrahydrobiopterin (BH4) biosynthesis and tyrosine hydroxylase, rate-limiting enzyme for dopamine biosynthesis, which requires BH4 as a cofactor. Loss-of-function Catsup mutations cause dominant hyperactivation of both enzymes. Elevated dopamine levels in Catsup mutants coincide with several distinct characteristics, including hypermobility, minimal basal levels of 3,4-Dihydroxy-Phenylacetic Acid, an oxidative metabolite of dopamine, and resistance to the Vesicular Monoamine Transporter inhibitor, reserpine, suggesting that excess dopamine is synaptically active and that Catsup functions in the regulation of synaptic vesicle loading and release of dopamine. We conclude that Catsup regulates and links the dopamine synthesis and transport networks.
Keywords: Dopamine; Drosophila,; Parkinson’s disease; oxidative stress; neuroprotection
Dopamine (DA) regulates movement, cognition, attention and reward (Calabresi et al., 2007; Palmiter, 2007; Sillitoe and Vogel, 2008), while dysfunction of dopaminergic signaling has been implicated in numerous neurological diseases and abnormal behaviors. The highly reactive nature of DA itself places dopaminergic neurons in the substantia nigra at heightened risk of oxidative damage, the primary route to cellular death in Parkinson’s disease (PD) (Goedert, 2001; Lotharius and Brundin, 2002, Maries et al., 2003). Moreover, elevated levels of DA or BH4, a cofactor required for DA synthesis, are neurotoxic in several model systems (Berman and Hastings, 1999; Choi et al., 2003; Gomez-Santos et al., 2006). Recently, it has been reported that DA in Drosophila PD models similarly enhances neurodegeneration (Bayersdorfer et al., 2010).
In an earlier study, we found that tyrosine hydroxylase (TH; EC1.14.16.2), the rate-limiting enzyme in DA biosynthesis, is dominantly activated by a post-translational mechanism in Drosophila strains heterozygous for loss-of-function Catecholamines up (Catsup) mutations (Stathakis et al., 1999). Subsequently, we investigated the effects of Catsup and other genes that regulate DA synthesis and homeostasis on sensitivity to the oxidative toxin, paraquat (PQ), which we found to selectively trigger dopaminergic neuron degeneration and parkinsonian-like movement disorders (Chaudhuri et al., 2007). Surprisingly, loss-of-function Catsup mutations, despite having strongly elevated levels of DA and tetrahydrobiopterin (BH4), were dominantly neuroprotective against PQ exposure (Chaudhuri et al., 2007).
Here, we report the results of our investigation of the mechanisms by which Catsup mutations can enhance BH4 and DA synthesis and yet simultaneously confer neuroprotection against PQ-induced oxidative insult. We find that Catsup acts to negatively regulate BH4 and DA synthesis pathways through physical interactions with the rate-limiting enzymes of both pathways, GTP cyclohydrolase I (GTPCH; EC3.5.4.16) and TH, respectively. Moreover, we provide evidence that Catsup is localized to synaptic termini and acts as a negative regulator of vesicular monoamine transporter (VMAT), which is responsible for synaptic vesicle uptake of DA. We conclude that Catsup has the ability to regulate DA homeostasis by effectively integrating the synthesis of both the BH4 and DA pathways through their respective rate-limiting enzymes, with the transport and synaptic release of DA.
Materials and Methods
Drosophila strains
The strain y w1118, which is wild type for the Catsup and VMAT genes, and the wild type strain, Canton S, were employed as control strains. Catsup loss-of-function mutant strains were y w1118; Catsup26/CyO and y w1118; Catsup12/CyO (Stathakis et al., 1999). Catsup26, a deletion from immediately upstream of the start codon, extending approximately 600 bp into the gene, and Catsup12, a frameshift mutation, produce no detectable protein. As Catsup mutant alleles are homozygous lethal, all experiments in this report were conducted using heterozygous strains. The VMAT loss-of-function mutant strain w; VMATΔ14/CyO, (Romero-Calderón et al., 2008), was provided by David Krantz (UCLA); the transgenic TH-Gal4 strain (Friggi-Grelin et al., 2003) was obtained from Jay Hirsh (University of Virginia). The transgenic Catsup line UAS-FLAG::Catsup was a gift from Dr. Lawrence Reiter (University of Tennessee Health Science Center). All stocks were maintained on standard Drosophila medium at 25° C.
Immunocytochemistry
Immunolocalization in whole mount Drosophila brain was performed as described previously (Chaudhuri et al., 2007). Primary antibodies and their dilutions were: guinea pig anti-Catsup, 1: 1000 (Stathakis et al., 1999); rabbit anti-Drosophila TH (DTH; a gift from Wendi Neckameyer, St. Louis University School of Medicine), 1:8000 (Neckameyer et al., 2000), and rabbit anti-Drosophila VMAT-A (DVMAT), 1:100 (a gift of David Krantz, UCLA) (Chang et al., 2006). Mouse anti-GFP (Jackson Immunoresearch) and mouse anti-FLAG (Sigma) were used at 1:5000 and 1:500 dilutions, respectively. FITC- and Cy3-conjugated IgG secondary antibodies corresponding to the appropriate species (Jackson Immunoresearch) and Alexafluor 488-conjugated secondary antibody (Santa Cruz) were used at a 1:5000 dilution. Images were acquired using a Leica TCS SP2 AOBS confocal microscope (Wetzlar, Germany) and processed in Adobe Photoshop.
Immunoblotting
The following primary antibodies were used for detection of proteins on immunoblots: chicken anti-DGTPCH at 1:40,000 (a gift from Dr. Lawrence Reiter, University of Tennesse Health Science Center), guinea pig anti-Catsup at 1:8,000, rabbit anti-DVMAT at 1:1000, rabbit anti-DTH at 1:15,000, mouse anti-ß tubulin (Sigma) at 1:20,000, mouse anti-late bloomer (Developmental Studies Hybridoma Bank) at 1:1000. Goat anti-rabbit (Jackson Immunoresearch), anti-guinea pig (Jackson Immunoresearch), and anti-mouse IgG (Santa Cruz) conjugated to horseradish peroxidase, were used at 1:5,000 dilution. Goat anti-chicken IgY-horseradish peroxidase was diluted 1:5,000 (Sigma) or 1:10,000 (Jackson Immunoresearch). The proteins were visualized with a SuperSignal West Pico Chemiluminescent detection system (Pierce).
Co-immunoprecipitation
Drosophila head extracts was used for co-immunoprecipitation studies as described previously (Zhang et al., 2007) with some modifications. Briefly, extracts containing 200 μg of total protein were immunoprecipitated overnight at 4°C with 20 μg of anti-Catsup, anti-dTH, or anti-dGTPCH antibodies. The immunoprecipitates were adsorbed onto Protein A-/G-Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C. The precipitated proteins were then subjected to Western blot analysis by standard methods.
Yeast Two-Hybrid Assay
Cloning of full-length and truncated GTPCH and TH cDNA into the activation domain vector pGAD-C1 and the DNA-binding domain vector pGBD-C1 and the assay methods were described previously (Bowling et al., 2008). The sequence encoding the large cytoplasmic loop of Catsup as well as N-terminal and C-terminal halves of this loop were similarly subcloned, using the following primers: (Catsup full-length cytoplasmic loop) AGTGAATTCGTACGCATTCTAAAGGGC and ATAGGATCCCCTGGAACAGCCAGACTT; (N-terminal Catsup cytoplasmic loop) ATAGAATTCCCAGAAGCGGAGCCA and ATAGGATCCCCTGGAACAGCCAGACTT; (C-terminal Catsup cytoplasmic loop) AGTGAATTCGTACGCATTCTAAAGGGC and CTCGGATCCCGCTTCTGGTTTCTT.
Reserpine, L-DOPA, paraquat and 3-iodotyrosine feeding
Separated male and female flies, 48-72 hrs post-eclosion, were fed on filter paper saturated with 5% (wt/vol) sucrose, or 5% sucrose containing 1, 30 or 120 mM reserpine (Sigma-Aldrich), 30 mM 3-iodotyrosine or 1 mM L-DOPA, and 5mM paraquat (Sigma-Aldrich). Unless otherwise noted, drug feeding periods were 12 hrs.
Neurochemical and enzyme assays
Neurochemical separations were performed as described in Chaudhuri et al. (2007). Monoamines were detected with ESA electrochemical analytical cell, Model 5011, (channel 1 at −50mV, channel 2 at 300 mV), and pteridines were detected with a Linear Model LC305 fluorescence detector at excitation wavelength 360 nm and emission wavelength 465 nm. Analysis was performed using ESA CoulArray software.
TH activity assays were performed on fly head tissue extracts using the method of Vie et al. (1999) modified as previously described (Bowling et al., 2008). TH catalytic activity was determined by HPLC analysis as previously described and expressed as nmoles L-DOPA generated per min. per mg protein (Bowling et al., 2008).
GTPCH activity assays were conducted as previously described with some modifications (Funderburk et al., 2006). Briefly, head extracts were mixed with GTP (Sigma-Aldrich) to a final substrate concentration of 200 μM and subsequently incubated for 1 hr at 37°C. The product of the reaction, 7,8-dihydroneopterin triphosphate, was oxidized by incubation for 1 hr in 1% (wt/vol) iodine (Sigma-Aldrich); 2% (wt/vol) potassium iodide (Sigma-Aldrich) in 1M HCL (Fisher). The oxidation reaction was terminated and the samples decolorized with the addition of 3% ascorbic acid (Sigma-Aldrich). The resulting mixture was then incubated at 37°C for 30 min with calf intestine alkaline phosphatase (Roche Biochemicals) to produce neopterin. The filtered supernatants were then separated by HPLC and the neopterin pools were quantified via fluorescence detection as described above.
Behavioral assays
Time-in-motion locomotion assays were performed as described previously (Carbone et al., 2006), using 15-20 males and females of each genotype, 3-5 days post-eclosion. Relative locomotion activity was measured as the number of seconds each fly spent in motion during a 45 sec period immediately following gentle mechanical disturbance. Grooming assays (Chang et al., 2006) were performed using 20 flies, 3-5 days post-eclosion, of each genotype. Flies were observed individually for a 2 min period in each of 3 separate trial periods. A single grooming event was recorded when a fly rubbed its leg over any part of its body.
Statistical analysis
Analyses of all data were conducted using GraphPad Prism (San Diego, CA) using two-tailed Student’s T-test assuming equal variances or one-way ANOVA followed by Bonferroni’s post test as required. Details of analyses are described in figure legends.
Results
Catsup mutants have elevated TH and GTPCH enzyme activity
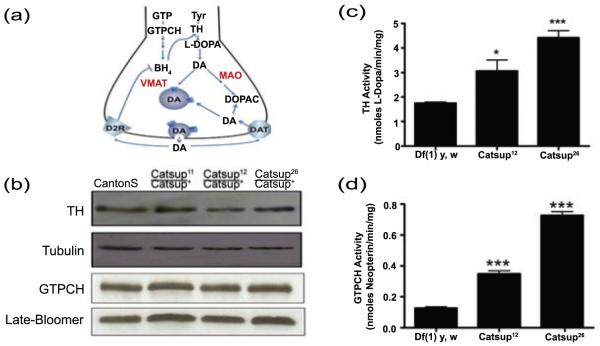
Catsup (Genbank AAF53744.1) is a 449 amino acid polypeptide containing eight highly hydrophobic regions, seven of which were predicted to be membrane-spanning helices (TMHMM program; probability >95%) (Krogh et al., 2001). The eighth region lies within a predicted 123 amino acid cytoplasmic loop, spanning residues 242 to 316 (Suppl. Fig. 1). Unexpectedly, sequence alignments place Catsup in a family of zinc transporters, closely related to mammalian protein, KE4 (Taylor et al., 2004), and no structural features could be linked to known mechanisms of DA regulation. Therefore, we conducted a behavioral and biochemical characterization of Catsup function that might provide an explanation for the distinctive phenotypes of Catsup mutants. Figure 1a depicts the components of DA synthesis and transport that may be targets of Catsup regulation. Since DA and BH4 are elevated in the heads of heterozygous loss-of-function Catsup mutants (Chaudhuri et al., 2007), we expected that their respective rate-limiting biosynthesis enzymes, TH and GTPCH, to be over-expressed or hyperactive. Immunoblot experiments revealed that the levels of both proteins in Catsup mutant heads are indistinguishable from levels in a wild type control (Fig. 1b). However, the activity of TH was 2-fold and 3-fold higher, respectively, in the Catsup12 and Catsup26 mutants, relative to the wild type control (Fig. 1c), while GTPCH was elevated 3-fold in the Catsup12 strain and nearly 7-fold in the Catsup26 strain (Fig. 1d). These results demonstrate that Catsup mutations do not affect expression of the TH or GTPCH-encoding genes (pale and Punch, respectively), but rather activate both enzymes post-translationally.
Figure 1.
Catsup mutations dominantly elevate TH and GTPCH activity through a post-translational mechanism. (a) Key components of DA synthesis, transport and metabolism. GTP is converted via three enzymatic reactions to the cofactor BH4. GTPCH catalyzes the rate-limiting step. TH converts tyrosine (tyr) to L-DOPA, in a BH4-dependent reaction. Aromatic amino acid decarboxylase subsequently converts L-DOPA to DA. DA is then transported into synaptic vesicles via VMAT or is converted by a two-step pathway into DOPAC by Monoamine Oxidase (MAO) and aldehyde dehydrogenase. After synaptic release, residual extracellular DA may be transported by into the pre-synaptic terminus by Dopamine Transporter (DAT) or may interact with the D2 autoreceptor (D2R) to trigger a G-protein coupled signaling pathway that leads to dephosphorylation of TH, which down-regulates its activity. (b) Immunoblot analysis of 3-5 day old flies was performed using antibodies against dTH, dGTPCH and loading controls, ß-tubulin and late-bloomer. Levels of TH and GTPCH protein are indistinguishable in the wild type (Canton S) and heterozygous Catsup mutant head extracts. (c, d) Extracts of adult male and female heads (in equal numbers), collected 24-48 hrs post-eclosion, were assayed for TH and GTPCH activity. Activities are expressed as nmoles of product per min. per mg protein. TH and GTPCH activities are elevated in head extracts of Catsup26/ Catsup+ and Catsup12/Catsup+ mutants relative to the wild type control (*, p< 0.05, **, p<0.01, *** p<0.001, n=5).
Catsup co-localizes with TH and VMAT in dopaminergic neurons
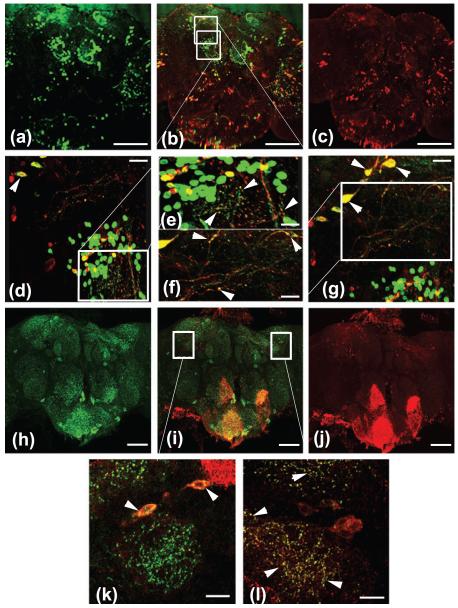
We next tested whether Catsup is expressed in dopaminergic neurons or is acting through expression in other cell types. Catsup, detected with anti-Catsup antibody, was found in dopaminergic neurons identified by expression of a TH>GFP reporter and by anti-dTH antibody (Fig. 2a-f; Suppl. Fig. 2a-c). Catsup was observed in a punctate pattern within dopaminergic neuron cell bodies and in axonal processes and termini, exhibiting strong co-localization with TH. We also found co-localization of Catsup with synaptobrevin::GFP expressed in dopaminergic termini (Supplementary Fig. 2d-f, arrows). Finally, FLAG::Catsup expressed in dopaminergic neurons (Fig. 2d-g) and endogenous Catsup (Suppl. Fig. 3) co-localized with the synaptic vesicle protein, VMAT, in cell bodies and synaptic termini (Fig. 2k,l), in a pattern consistent with the previously reported localization of dVMAT (Greer et al, 2005). While these data demonstrate that Catsup is expressed in dopaminergic cell bodies and synaptic termini, we also noted that the protein is expressed in other neurons and termini that apparently are not dopaminergic. Studies are in progress to explore possible non-dopaminergic functions of Catsup.
Figure 2.
Catsup co-localizes in cell bodies and synaptic regions of dopaminergic neurons with TH and VMAT. (a-g) Co-localization (yellow, b) of TH (green, a) and Catsup (red, c) in adult CNS, detected with anti-dTH and anti-Catsup antibodies. (a-c) TH and Catsup co-expressed in DA neuron clusters in the central brain. Scale bar = 100 μm. (d,g) enlargements of indicated regions of (c) showing co-expression in cell bodies and synaptic termini. Scale bars = 50 μm. (e and f) Enlargements of (d) and (g), respectively, showing further details of synaptic co-localization of TH and Catsup. Scale bars = 10 μm. (h-l) Co-localization (yellow, i) of VMAT (green, h) and FLAG::Catsup (red, j) in CNS of w; TH-Gal4; UAS-FLAG::Catsup adults, detected with anti-VMAT and anti-FLAG antibodies. (h-j) VMAT and FLAG::Catsup expression in the central brain, Catsup is densely expressed in the lower brain as well as in specific regions in the central brain. Scale bars = 50 μm (k, l) Enlargements of indicated regions of (i), showing Catsup and VMAT co-localization within intracellular compartments (k, arrow heads) and in synaptic bouton-like puncta (l, arrowheads). Scale bars 20 μm.
Catsup physically associates with TH and GTPCH
We previously found that Drosophila TH and GTPCH directly interact when both are phosphorylated and that this interaction mutually regulates both enzymes, increasing BH4 and DA production beyond that achieved by phosphorylation in the absence of interaction (Krishnakumar et al., 2000; Bowling et al., 2008). This regulated interaction, in combination with our observation that the partial reduction in Catsup protein in heterozygous mutants is sufficient to elevate the activities of these enzymes and their pathway end-products, suggests a possible mechanism of Catsup action. We hypothesized that Catsup might engage in stoichiometric interactions with TH and GTPCH, preventing access of their regulatory domains to activating kinases and their association with each other. Limiting Catsup expression in this model would reduce activity-suppressing interactions. We tested whether Catsup could associate with either enzyme by performing yeast two-hybrid interaction assays, focusing on the highly conserved 123 amino acid predicted cytoplasmic domain of Catsup (Suppl. Fig. 1). Both enzymes interacted with this domain, requiring the C-terminal half of the Catsup cytoplasmic loop, but not the N-terminal half (Table 1). These interactions are mediated through sites in the N-terminal half of TH and N-terminal regulatory domains of two GTPCH isoforms, RA (NP_726038; GTPCHc in our original nomenclature (McLean et al., 1993)) and RC (NP_523801, GTPCHb in our original nomenclature). No interactions were observed between Catsup and the C-terminal catalytic domains of either enzyme. The yeast two-hybrid results were confirmed by co-immunoprecipitation of TH and GTPCH with Catsup from adult head extracts (Fig. 3).
Table 1.
Cytoplasmic loop of Catsup interacts physically with TH and GTPCH in a yeast two hybrid assay1
| Catsup Loop2 | TH Fragments3 | SD(-T,-W,-H) | LacZ |
|---|---|---|---|
| Full-length | Full-length | + | + |
| C-terminal domain | Full-length | + | + |
| Full-length | N-terminal half | + | + |
| C-terminal domain | N-terminal half | + | + |
| Full-length | C-terminal half | − | − |
| C-terminal domain | C-terminal half | − | − |
| N-terminal domain | Full-length | − | − |
| Catsup Loop 1 | GTPCH Fragments 4 | SD(-T,-W,-H) | LacZ |
| C-terminal domain | Full-length GTPCHa/Pu-RA | + | + |
| C-terminal domain | N-terminal GTPCHa/Pu-RA | + | + |
| N-terminal domain | Full-length GTPCHa/Pu-RA | − | − |
| C-terminal domain | Full-length GTPCHc/Pu-RC | + | + |
| C-terminal domain | N-terminal GTPCHc/Pu-RC | + | + |
| N-terminal domain | Full-length GTPCHc/Pu-RC | − | − |
| C-terminal domain | GTPCH catalytic domain | − | − |
The full length Catsup cytoplasmic loop and its C-terminal and N-terminal halves in the pGAD-C1 vector were used as bait; prey constructs were the full length, N-terminal and C-terminal fragments of TH, and the full length protein and N-terminal regulatory and catalytic domain fragments of GTPCH isoforms RA and RC in the pGBD-C1 vector. (+): yeast cells grew on SD/-Trp-Ade-His plates and developed blue coloration after incubation with X-gal. (−): no growth on the selection medium.
Full length Catsup loop: residues 242-363; N-terminal fragment: residues 242- 295; C-terminal fragment: residues 293-363.
Full length TH: residues 1-508; N-terminal half: residues 1-269; C-terminal half: residues 269-508.
Full length GTPCHa/RA: residues 1-324; N-terminal GTPCHa/RA: residues 1-117. Full length GTPCHc/RC: residues 1-308; N-terminal GTPCHc/RC: residues 1-101. Catalytic domain (shared by GTPCH-RA and –RC): residues 118 in RA and 102 in RC to C- terminus.
Figure 3.
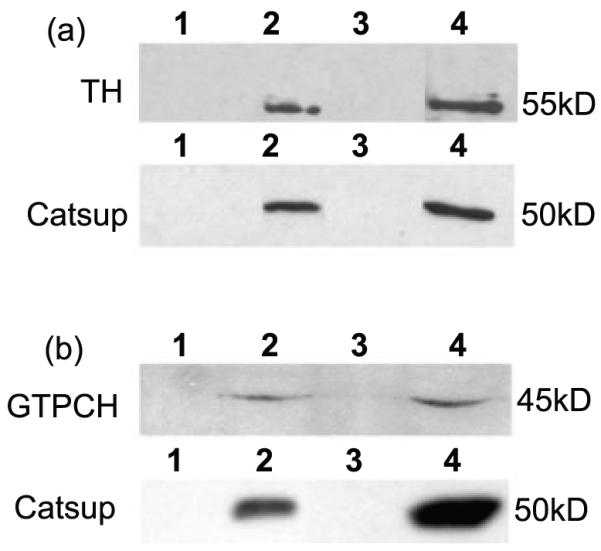
TH and GTPCH co-immunoprecipitate with Catsup. (a) TH interacts with Catsup. (b) GTPCH interacts with Catsup. The upper panels in (a) and (b) show the results of anti-Catsup immunoprecipitation from head extracts; the blots were probed with anti-dTH (a) and anti-GTPCH (b). The lower panels shows the results of anti-dTH (a) and anti-GTPCH (b) immunoprecipation from head extracts; the blots were probed with anti-Catsup antibody. The lanes in each panel are in the following order: Lane 1- Beads with no antibody + extract- a control for non-specific retention of proteins. Lane 2- Co-immunoprecipitated protein Lane 3: blank. 4: Extract only-detecting endogenous proteins of the same molecular mass as the co-precipitated proteins in lanes 2.
In summary, these results demonstrate that Catsup associates with TH and GTPCH, the rate-limiting enzymes for DA synthesis. The regions of these enzymes that mediate interactions with Catsup are identical to or overlap the domains of TH and GTPCH interactions with each other, results that support a model of competitive inhibitory (Catsup-TH and Catsup-GTPCH) and activating (TH-GTPCH) protein complexes.
Catsup mutants display elevated synaptic activity
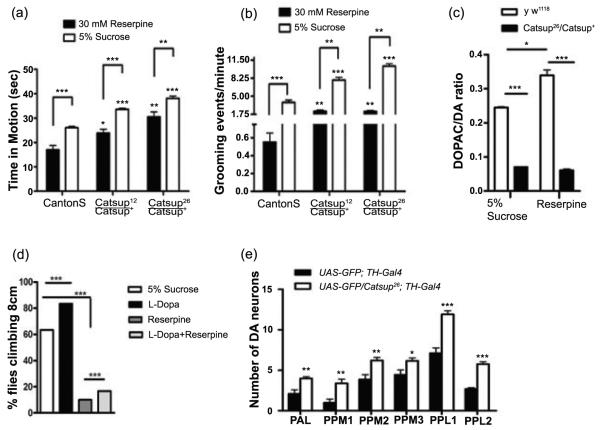
Since Catsup is expressed in synaptic termini, we next tested whether the excess DA in Catsup mutants is synaptically active. DA is required for movement in flies, as in mammals; simple mobility assays, therefore, allow a sensitive assessment of its synaptic function. Both Catsup26 and Catsup12 mutants are hyperactive, as determined by time-in-motion assays (Fig. 4a). Similarly, Catsup mutants also exhibit elevated grooming frequency (Fig. 4b), another behavior modulated by the synaptic activity of amines (Chang et al., 2006). We conclude that the consequence of loss of Catsup function is elevated DA release coordinated with elevated biosynthetic enzyme activity.
Figure 4.
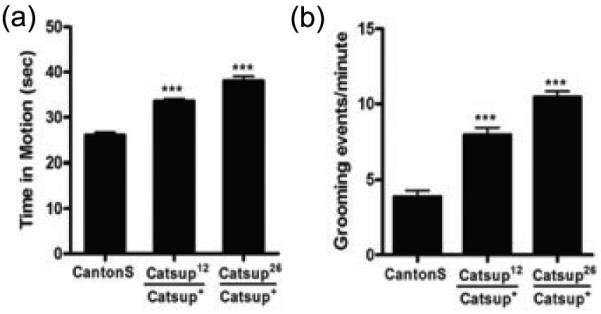
Amine-linked mobility is elevated in heterozygous Catsup mutants. (a) Overall activity measured as the portion of 45 sec intervals that each of 20 adult males of each genotype (3-5 days post-eclosion) was in motion. Both Catsup12/Catsup+ and Catsup26/Catsup+, were hyperactive relative to wild type (Canton S) flies. (b) Grooming, quantified as the number of grooming events in 60 sec intervals, was determined using 20 adult male flies (3-5 days post-eclosion). Catsup mutants had higher grooming frequencies than the wild type flies. Statistical analysis was performed using one-way ANOVA. Error bars represent mean SEM. (*** p< 0.001).
VMAT activity is enhanced in Catsup loss-of-function mutants
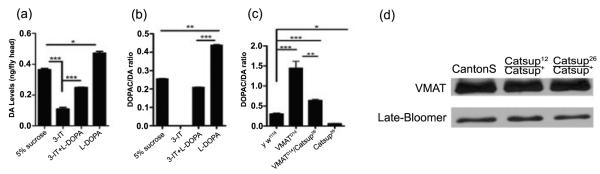
Alterations in dVMAT expression affect mobility (Chang et al., 2006; Simon et al., 2009). The hypermobility of Catsup mutant flies suggested that vesicular transport of cytosolic DA by VMAT also is affected by Catsup mutations. Before testing this hypothesis, we first modulated DA pools pharmacologically in wild type adults and compared the neurochemical profiles with those of Catsup mutants in which DA pools are altered genetically. Similar profiles would suggest that Catsup function is restricted to regulation of DA biosynthesis. Catsup+ flies that ingested 30 mM 3-iodotyrosine (3-IT), a competitive inhibitor of TH, had depleted DA pools in heads, while ingestion of 1 mM L-DOPA, the immediate product of TH catalysis and the precursor of DA (Fig. 1a), resulted in elevation of DA pools (Fig. 5a). Simultaneous ingestion of 3-IT and L-DOPA, restored DA levels.
Figure 5.
Pharmacological and genetic manipulation of cytosolic dopamine pools. (a, b) Pharmacological modulation of DA levels in y w1118 flies. Adults, 48 h post-eclosion, were fed 5% sucrose, 30 mM 3-iodo-tyrosine (3-IT), 30 mM 3-IT + 1mM L-DOPA, or 1mM L-DOPA for 12 hrs. (a) DA levels were significantly depleted in the presence of 3-IT, and significantly elevated when fed L-DOPA. Co-feeding 3-IT and L-DOPA resulted in near normal DA pools. (b) DOPAC:DA ratios respond to pharmacological alteration of DA pools. The TH inhibitor, 3-IT, reduced the DOPAC:DA ratio to a negligible level, while flies treated with L-DOPA had significantly higher DOPAC:DA ratios relative to controls. These results indicate that removal of DA from the cytosol is not solely dependent on cytosolic DA levels. (c) Double mutant analysis of DOPAC:DA ratios in VMATD14/Catsup26 reveals a functional interaction. VMATD1 /VMAT+ and Catsup26/Catsup+ mutants display strongly elevated and reduced DOPAC:DA ratios, respectively, in comparison to the y w1118 flies. The transheterozygous combination of Catsup26 and VMATD14 mutations partially rescues the DOPAC:DA ratio relative to VMATD1 /VMAT+ flies. Statistical analysis was performed using one-way ANOVA. Error bars represent mean SEM. (*, p<0.05, **, p<0.01, ***, p<0.001, n=5). (d) Mutations in Catsup do not alter VMAT protein levels. Immunoblot analysis was performed using antibodies against dVMAT and loading control, Late-bloomer.
We then measured the metabolic conversion of cytosolic DA into dihydroxyphenyl acetate (DOPAC). The ratio of DOPAC:DA is a well-established index for the turnover of cytosolic DA in mammalian neurons (Di Monte et al., 1996; Drolet et al., 2004). Elevated DOPAC:DA ratios indicate diminished vesicular packaging and increased DA turnover. In contrast, low DOPAC:DA levels indicate reduced cytosolic DA pools and, where DA synthesis per se is not diminished, more efficient removal of DA from the cytosol by VMAT. We previously found elevated DOPAC levels after exposure of wild type flies to the oxidative stressor paraquat (Chaudhuri et al., 2007) and employed this neurochemical assay here, in the absence of paraquat, to monitor cytosolic clearance of DA.
DOPAC levels in control animals were very low, yielding a DOPAC:DA ratio of approximately 0.05 (Fig. 5; Suppl. Fig. 4). DOPAC pools were undetectable when DA was depleted with 30 mM 3-IT. Administration of 1 mM L-DOPA led to significantly increased DA pools and DOPAC:DA ratios, demonstrating that much of the excess DA remains in the cytosol. The positive correlation between pharmacologically manipulated DA levels and DOPAC:DA ratios suggests that vesicular transport is not modulated solely by DA levels in the adult brain and that the level of DOPAC present in dopaminergic neurons is dependent partly upon the level or activity of VMAT in Drosophila, as in mammalian systems. We tested this hypothesis using a DVMAT deletion mutant, VMATD14. VMATD14/VMAT+ heterozygotes displayed a diminished DA pool and elevated DOPAC pool and DOPAC:DA ratio (Fig. 5c; Suppl. Fig. 4b,c), demonstrating that DOPAC levels inversely reflect VMAT function.
The results of the above experiments led to the prediction that DOPAC pools and DOPAC:DA ratios would be elevated in Catsup mutant heads, if loss-of-function Catsup mutations affect only DA synthesis. On the other hand, if vesicular transport also is elevated, diminished or unaltered DOPAC:DA ratios should be observed. Despite the nearly three-fold elevation in TH activity in Catsup26/Catsup+ adult heads (Fig. 1c), and the concomitantly elevated DA pools (Chaudhuri et al., 2007), the DOPAC:DA ratio in extracts of heterozygous Catsup mutant heads was approximately three-fold less than the ratio in wild type controls (Fig. 5c; Suppl. Fig. 4c). Cumulatively, these results suggest that the reduction of Catsup protein expression enhanced VMAT function, leading to diminished DOPAC levels and elevated synaptic packaging and release.
Immunoblot comparisons of VMAT in Catsup mutant and wild type head extracts revealed no significant differences, suggesting that Catsup does not modulate VMAT expression or turn-over (Fig. 5d). Significantly, the elevated DOPAC:DA ratio of the VMAT mutant can be rescued by the presence of a single Catsup mutant allele, in VMAT D14/Catsup26 transheterozygotes (Fig. 5c; Suppl. Fig. 4c). These results demonstrate that Catsup function is partially epistatic to VMAT in that loss of Catsup function enhances remaining VMAT function in VMAT mutants.
Catsup mutations affect VMAT susceptibility to reserpine
The status of VMAT function was assessed further by administration of reserpine, which inhibits both mammalian and Drosophila VMAT (Greer et al., 2005). Ingestion of 30 mM reserpine induced robust effects on behavior in wild type flies, with reduction of mobility and grooming frequency (Fig. 6a,b). Mobility and grooming frequency also were diminished in Catsup26/Catsup+ and Catsup12/Catsup+ mutants after reserpine treatment, an expected result since both lines retain one normal allele of Catsup. However, reserpine-treated mutants displayed higher levels of mobility and grooming relative to reserpine-fed wild type animals (p<0.05).
Figure 6.
Administration of VMAT inhibitor, reserpine, reveals a functional interaction between VMAT and Catsup. Mutations in Catsup diminish the effects of reserpine on overall mobility (a) and grooming (b) relative to wild type controls. Reserpine reduced mobility in all cases, but was unable to restore mobility to a wild type level in Catsup mutants. (c) Reserpine ingestion causes an increase in the DOPAC:DA ratio of wild type, but not Catsup26/Catsup+ mutant, heads. (a-c) Adult males of each genotype were fed 30 mM reserpine in 5% sucrose. N = 20 for each test and genotype. (d) Elevation of DA pools alone (in absence of Catsup mutation) does not rescue mobility from the effects of reserpine. Adult males were fed 1 mM L-DOPA and/or 10 mM reserpine for 12 hrs, after a 12 hr pre-feeding of 1 mM L-DOPA. Mobility was assayed as the percent of the flies assayed in three replicas that were able to climb 8 cm in 4 sec. Each replica consisted of 5 adult males per vial, 10 vials. (e) Acute exposure to reserpine (120 mM, 48 hrs) causes dopaminergic neurodegeneration in Catsup+, but not Catsup26/Catsup+ flies. Both Catsup wild type and mutants were combined with UAS-GFP/+; TH-Gal4/+ to visualize dopaminergic neurons. Individual dopaminergic clusters were scored in 10-12 brains per genotype. PAL (protocerebral anterolateral), PPM1, PPM2, PPM3 (protocerebral posterior medial), PPL1 and PPL2 (protocerebral posterolateral) neurons were counted individually. Wild type brains displayed significantly greater loss of DA neurons in all clusters, relative to Catsup mutant brains. The significance of differences between Catsup mutant and wild type neurons in each cluster *** p<0.0001; ** p <0.001 and * p<0.05.
Two factors may contribute to the resistance of Catsup mutants to reserpine. First, since reserpine is a competitive inhibitor of VMAT, the elevation of DA synthesis in Catsup mutants could enhance the ability of DA to compete with reserpine for access to VMAT. Second, loss of Catsup function may result in a higher threshold for inhibition of mobility due to an increase in the transport activity of VMAT. These alternatives were tested by determining the DOPAC: DA ratios in Catsup26/Catsup+ heterozygotes after exposure to 30 mM reserpine. Reserpine ingestion had no effect on the DOPAC:DA ratios of Catsup26/Catsup+ mutant flies, in striking contrast to the strong elevation of DA turnover in wild type flies (Fig. 6c; Suppl. Fig. 4d,e). This result suggests that the reduction of Catsup function allows more efficient clearing of cytosolic DA, indicating that VMAT in Catsup mutants is partially refractory to inhibition by reserpine. Accordingly, we found that pharmacological elevation of DA pools by L-DOPA feeding has a modest effect only on reserpine suppression of mobility in wild type flies (Fig 6d).
Catsup mutations protect dopaminergic neurons against reserpine-induced neurodegeneration
During reserpine inhibition, cytosolic DA metabolism forms reactive oxygen species that lead to cell death (LaVoie and Hastings, 1999; Asanuma et al., 2003). We tested whether reserpine administration caused dopaminergic neuron degeneration in Drosophila CNS and whether Catsup26 mutants exhibited neuroprotection against reserpine-induced neurotoxicity. The reserpine exposure time was prolonged from 12 to 48 hrs and the reserpine concentration was increased to 120mM in order to induce an acute neuronal response. Dopaminergic neurons in the adult Drosophila CNS were visualized by GFP expression under TH-Gal4 control and by anti-DTH immunostaining (data not shown), with comparable results. After reserpine treatment, all six dopaminergic neuron clusters scored in UAS-GFP/+; TH-Gal4/+ brains had significantly fewer DA neurons than UAS-GFP/ Catsup26; TH-Gal4/+ brains (Fig. 6e). The remaining dopaminergic neurons of the Catsup+ brains exhibited rounding of cell bodies and retraction of processes (Suppl. Fig. 5h,k,l). Catsup26 mutant brains retained more dopaminergic neurons after reserpine exposure, and most surviving neurons were morphologically normal (Suppl. Fig. 5g,i,j). Having previously determined that mutations in Catsup, in the absence of neurotoxic treatments, have no effect on dopamineric neuron numbers or structure (Chaudhuri et al., 2007), we conclude that the enhancement of VMAT function in heterozygous Catsup mutant brains protects against the toxicity of cytosolic DA.
Discussion
Close coordination of DA synthesis and transport is a necessity since dopaminergic neurons are at risk from DA itself due to its propensity for forming free radicals and DA quinones, which subsequently can attack proteins and lipids (Berman and Hastings, 1999; LaVoie and Hastings, 1999; Sulzer et al., 2000; Asanuma et al., 2003). Increasingly, it is becoming appreciated that regulated protein interactions play crucial roles in integrating synthesis and transport, minimizing accumulation of cytosolic or extracellular DA. Numerous reports have suggested mechanisms for facilitating the packaging of newly synthesized DA, beginning with early observations that TH exists in both cytosolic and membrane-associated forms (Kuczenski et al., 1972; Kuhn et al., 1990). More recently, Chen et al. (2003) reported that transport of newly synthesized DA from membrane-associated biosynthesis enzymes in synaptosomal preparations was more efficient than transport of DA added exogenously, while Wang et al. (2009) reported the unexpected association of TH with mitochondrial membranes suggesting that further novel mechanisms remain to be discovered. Cartier et al. (2010) demonstrated functional and physical interactions between cytosolic domains of VMAT2 and both TH and aromatic amino acid decarboxylase, which converts L-DOPA to DA.
We previously reported that synthesis of DA in Drosophila is tightly integrated with the synthesis of the TH cofactor, BH4, which in turn, is dependent upon the activity of GTPCH (Krishnakumar et al., 2000). Subsequently, we discovered that TH and GTPCH physically associate in a phosphorylation-dependent interaction, which has functional consequences for both enzymes (Bowling et al., 2008). In this report, we demonstrate another component of this critical integration process, the membrane protein Catsup, which adds a previously unknown negative regulatory link in the complex coordination mechanism of DA synthesis and transport. We have reported that loss-of-function Catsup mutations cause elevated levels of BH4 and DA in adult heads (Chaudhuri et al., 2007), and here we demonstrate that these elevated pools result from the negative regulation of both TH and GTPCH activities by Catsup. We show that both enzymes associate with a cytoplasmic domain of Catsup and do so, through their respective N-terminal regulatory domains, which would have the effect of blocking access of kinases to the regulatory serine residues found within the interaction zones (Funderburk et al., 2006).
Consistent with mounting evidence for tightly coordinated synthesis and transport of DA, we also identified a functional interaction between Catsup and VMAT. The hyperactivity of Catsup mutants first suggested this interaction. Other studies of polymorphisms in Catsup also show an association with locomotion (Carbone et al., 2006), as well as modulation of day sleep (inactivity) patterns (Harbison et al., 2009), indicating that the mobility phenotypes of the mutants tested here reflect a normal function of this gene. Comparison of the effects of reserpine on wild type control, L-DOPA-supplemented and Catsup mutant flies further implicate VMAT modulation. Loss of Catsup function limits the effect of reserpine on mobility and neuron loss and also results in low DOPAC pools and DOPAC:DA ratios, indicating enhanced vesicular transport. Pharmacological manipulation of DA levels does not produce similar outcomes, ruling out the hypothesis that the Catsup mutant phenotypes are a consequence of elevated DA pools alone. This evidence therefore defines a central role for Catsup in the integration of DA synthesis and transport into vesicles for storage and synaptic release, adding a novel component to the active synthesis-transport complex demonstrated by Cartier et al. (2010). It is important to note that in vitro confirmation of TH interactions with VMAT in the Cartier et al. study relied on a his6-tagged Drosophila TH cDNA constructed in our laboratory. Thus, their study also confirms the conservation of this functional interaction across species. Our preliminary results suggest that a Catsup ortholog in dopaminergic neurons of the human SN physically associates with human TH, indicating the possibility of a conserved regulatory mechanism.
Our data, in concert with those of Cartier et al. (2010), suggest a model of dynamic interactions that integrate the modulation of synthesis and transport (Fig. 7). We propose that Catsup and VMAT are engaged in a dynamic exchange of the biosynthetic complex. When TH and GTPCH associate with Catsup, they are sequestered from activating kinases and from VMAT association, suppressing DA synthesis. Upon potentiation of the neuron, the biosynthesis complex is released from Catsup, allowing its interaction with VMAT, linking synthesis with transport. After synaptic release, Catsup reacquires the complex, once again sequestering the potentially dangerous enzymes, resulting in basal vesicular transport of free DA. In the basal state, VMAT can be accessed by the inhibitor reserpine (Rudnick et al., 1990; Zheng et al., 2006), which blocks DA transport into the vesicle. In this state, even basal levels of DA would enhance the danger of oxidative reactions. In contrast, occupation of VMAT by the biosynthesis complex in the activated state would limit reserpine access.
Figure 7.
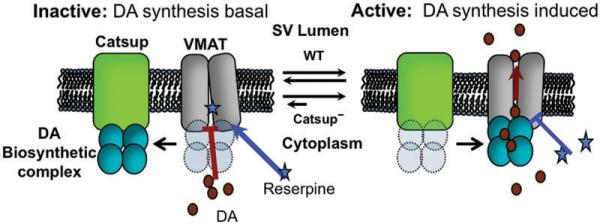
Model for functional interaction between Catsup and VMAT. Catsup is located either in the synaptic vesicle membrane or in another membrane in close proximity. The BH4 and DA biosynthetic complexes are proposed to interact with Catsup in the absence of neuron stimulation. In this state, N-terminal regulatory domains of TH and GTPCH are blocked from phosphorylation by ser/thr kinases, minimizing biosynthetic activity. Upon neuron stimulation, Catsup releases the complex, which then may undergo phosphorylation and interaction with VMAT, allowing efficient vesicular transport of DA. In the inactive state, reserpine has ready access to its binding site on VMAT, preventing DA transport. In the active state, reserpine access is partially blocked. In Catsup mutants, diminished levels of Catsup result in increased interactions between the biosynthesis complex and VMAT, prolonging activation and blockade of reserpine binding.
Catsup loss-of-function mutations are dominant, suggesting a balanced stoichiometric relationship between Catsup and VMAT, as a 50% reduction in Catsup protein is sufficient to elevate DA pools (Stathakis et al., 1999), while simultaneously minimizing the generation of DOPAC. We propose that in the absence of Catsup protein, VMAT is more likely to retain the biosynthesis complex and in consequence, may be less accessible to reserpine.
As presented in Fig. 7, Catsup is in the vesicle membrane with VMAT. However, co-localization in the same membrane compartment is not an essential feature of this model. The relevant membranes simply need to be in close proximity, as demonstrated by Egana et al. (2009) who discovered that the plasma membrane protein Dopamine Transporter (DAT) physically associates with the synaptic vesicle protein synaptogyrin 3. We have shown that Catsup is located in dopaminergic termini; ongoing membrane fractionation analysis and protein interaction studies will clarify the interaction mechanism.
Pharmacological inhibition of VMAT or its genetic knockdown increases neuronal sensitivity to oxidative damage (Gainetdinov et al., 1998; Choi et al., 2005; Caudle et al., 2007; Vergo et al., 2007), whereas increased VMAT expression is neuroprotective (Liu et al., 1992; Vergo et al., 2007). Importantly, dVMAT2 has comparable effects on Drosophila monoaminergic neurons (Greer et al., 2005; Lawal et al., 2010). Part of our analysis of VMAT function relies upon the metabolic conversion of cytosolic DA to DOPAC. In vertebrates, the enzyme monoamine oxidase (MAO) catalyzes the first step in this process (Severson et al., 1981a; Di Monte et al., 1996). The ratio of DOPAC to DA pools then serves as an indicator of the efficiency of vesicle loading (Severson et al., 1981a; 1981b). Drosophila does not possess a highly conserved MAO, but a functional MAO activity has been reported (Dewhurst et al., 1972). Others have reported that application of the vertebrate MAO inhibitor hydrazaline enhances monoamine-linked behaviors, presumably by preventing the breakdown of monoamine neurotransmitters (Yellman et al., 1997). We previously found that ingestion of the oxidative stressor paraquat by adult flies leads to dramatic elevation of DOPAC and DOPAC:DA ratios (Chaudhuri et al., 2007). Here, we have demonstrated that in wild type animals, uncoupling of transport from DA synthesis by reserpine inhibition or VMAT mutation, also leads to elevated DOPAC levels. Upon L-DOPA administration, we discovered a direct correlation between increased DA levels and its turnover, suggesting that VMAT transport activity can be saturated under conditions of high cytosolic DA pools. It is, therefore, noteworthy that Catsup mutants, despite having 3-7 fold elevated DA pools, exhibit barely detectable DOPAC levels.
In summary, we have demonstrated a role for Catsup in coupling synthesis to transport, based on the evidence that Catsup physically interacts with TH and GTPCH, is present in synaptic termini, and functionally interacts with VMAT. Loss of Catsup allows elevated DA synthesis, transport and synaptic release. Catsup may aid in tethering these proteins in a complex that facilitates and coordinates their efficient access to VMAT. Such enzymatic compartmentalization, in addition to being bioenergetically favorable for the cell, has implications for cellular protection from oxidative stress that results from the metabolic by-products of DA. Our finding that Catsup regulates key steps in DA neurotransmission in Drosophila has implications for mammalian systems and neurological diseases such as PD since the results reported here demonstrate a correspondence with reserpine-induced Parkinsonian symptoms in mammalian models (Schwartz et al., 1996; Nash et al., 1999). This report adds another facet to studies that have established Drosophila as an important model for the study of PD and other neurodegenerative diseases (Feany and Bender, 2000; Bonini and Fortini, 2003). Given the unique regulatory role played by Catsup in DA homeostasis, further investigations may yield potential therapeutic treatments for DA-related disorders.
Supplementary Material
Acknowledgements
This work was supported by a grant from the National Institutes of Health (GM62879) to J.M.O. and by funds from the University of Alabama.
Abbreviations
- DA
dopamine
- DOPAC
3,4-Dihydroxy-Phenylacetic Acid
- PQ
paraquat
- BH4
tetrahydrobiopterin
References
- Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res. 2003;5:165–76. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- Bayersdorfer F, Voigt A, Schneuwly S, Botella JA. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.02.012. In Press. [DOI] [PubMed] [Google Scholar]
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and Induces permeability transition in brain mitochondria: Implications for Parkinson’s disease. J Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Fortini ME. Human neurodegenerative disease modeling using Drosophila. Annu. Rev. Neurosci. 2003;26:627–656. doi: 10.1146/annurev.neuro.26.041002.131425. [DOI] [PubMed] [Google Scholar]
- Bowling KM, Huang Z, Xu D, Funderburk CD, Karnik N, Ferdousy F, Neckameyer W, O’Donnell JM. Direct binding of GTP cyclohydrolase and tyrosine hydroxylase: Regulatory interactions between key enzymes in dopamine biosynthesis. J Biol Chem. 2008;283:31449–31459. doi: 10.1074/jbc.M802552200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodski C, Vogt Weisenhorn DM, Signore M, Sillaber I, Oesterheld M, Broccoli V, Acam pora D, Simeone A, Wurst W. Location and size of dopaminergic and serotoner gic cell populations are controlled by the position of the midbrain-hindbrain organizer. J Neurosci. 2003;23:4199–4207. doi: 10.1523/JNEUROSCI.23-10-04199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Carbone MA, Jordan KW, Lyman RF, Harbison ST, Leips J, Morgan TJ, DeLuca M, Awadalla P, Mackay TF. Phenotypic variation and natural selection at Catsup, a pleiotropic quantitative trait gene in Drosophila. Curr Biol. 2006;16:912–919. doi: 10.1016/j.cub.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, Egaña L, Torres GE. A biochemical and functional protein complex involving dopamine synthesis and transport in synaptic vesicles. J Biol Chem. 2010;285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Wei J, Fowler SC, Wu JY. Demonstration of functional coupling between dopamine synthesis and its packaging into synaptic vesicles. J Biomed Sci. 2003;10:774–781. doi: 10.1159/000073965. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kim SW, Lee SY, Moon YW, Hwang O. Involvement of apoptosis and calcium mobilization in tetrahydrobiopterin-induced dopaminergic cell death. Exp Neurol. 2003;181:281–290. doi: 10.1016/s0014-4886(03)00054-2. [DOI] [PubMed] [Google Scholar]
- Dewhurst SA, Croker SG, Ikeda K, McCaman RE. Metabolism of biogenic amines in Drosophila nervous tissue. Comp Biochem Physiol B. 1972;43:975–981. doi: 10.1016/0305-0491(72)90241-6. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, DeLanney LE, Irwin I, Royland JE, Chan P, Jakowec MW, Langston JW. Monoamine oxidase-dependent metabolism of dopamine in the striatum and substantia nigra of L-DOPA-treated monkeys. Brain Res. 1996;738:53–59. doi: 10.1016/0006-8993(96)00761-5. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Egaña LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, Peña K, Quiroz M, Hong WC, Dorostkar MM, Janz R, Sitte HH, Torres GE. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Funderburk CD, Bowling KM, Xu D, Huang Z, O’Donnell JM. Atypical N-terminal extensions confer novel regulatory properties on GTP cyclohydrolase isoforms in Drosophila melanogaster. J Biol Chem. 2006;281:33302–33312. doi: 10.1074/jbc.M602196200. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem. 1998;70:1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Gimenez-Xavier P, Ferrer I, Ambrosio S. Intranigral dopamine toxicity and alpha-synuclein response in rats. Neurochem Res. 2006;31:861–868. doi: 10.1007/s11064-006-9090-2. [DOI] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang HY, Houshyar R, Bainton RJ, Diantonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol. 2005;64:239–58. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Harbison ST, Carbone MA, Ayroles JF, Stone EA, Lyman RF, Mackay TRF. Co-regulated transcriptional networks contribute to natural genetic variation in Drosophila sleep. Nat. Genet. 2009;41:371–375. doi: 10.1038/ng.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar S, Burton D, Rasco J, Chen X, O’Donnell J. Functional interactions between GTP cyclohydrolase I and tyrosine hydroxylase in Drosophila. J Neurogenet. 2000;14:1–23. doi: 10.3109/01677060009083474. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kuczenski RT, Mandell AJ. Regulatory properties of soluble and particulate rat brain tyrosine hydroxylase. J Biol Chem. 1972;247:3114–3122. [PubMed] [Google Scholar]
- Kuhn DM, Arthur R, Jr, Yoon H, Sankaran K. Tyrosine hydroxylase in secretory granules from bovine adrenal medull. Evidence for an integral membrane form. J Biol Chem. 1990;265:5780–5786. [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinine formation and protein modification associated with the striatal neurotoxicity of methamphetamine: Evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- McLean JR, Krishnakumar S, O’Donnell JM. Multiple mRNAs from the Punch locus of Drosophila melanogaster encode isoforms of GTP cyclohydrolase I with distinct N-terminal domains. J. Biol. Chem. 1993;268:27191–27197. [PubMed] [Google Scholar]
- Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiol Aging. 2000;21:145–152. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;3:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Romero-Calderón R, Uhlenbrock G, Borycz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Acerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, Krantz DE. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4:e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Steiner-Mordoch SS, Fishkes H, Stern-Bach Y, Schuldiner S. Energetics of reserpine binding and occlusion by the chromaffin granule biogenic amine transporter. Biochemistry. 1990;29:603–608. doi: 10.1021/bi00455a002. [DOI] [PubMed] [Google Scholar]
- Severson JA, Osterburg HH, Finch CE. Aging and haloperidol-induced dopamine turnover in the nigro-striatal pathway of C57BL/6J mice. Neurobiol Aging. 1981a;2:193–197. doi: 10.1016/0197-4580(81)90020-8. [DOI] [PubMed] [Google Scholar]
- Severson JA, Randall PK, Finch CE. Genotypic influences on striatal dopaminergic regulation in mice. Brain Res. 1981b;210:201–215. doi: 10.1016/0006-8993(81)90894-5. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Vogel MW. Desire, disease, and the origins of the dopaminergic system. Schizophr Bull. 2008;34:212–219. doi: 10.1093/schbul/sbm170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Daniels R, Romero-Calderón R, Grygoruk A, Chang HY, Najibi R, Shamouelian D, Salazar E, Solomon M, Ackerson LC, Maidment NT, Diantonio A, Krantz DE. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 2009;108:525–541. doi: 10.1534/genetics.108.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathakis DG, Burton Y, McIvor WE, Krishnakumar S, Wright TRF, O’Donnell JM. The Catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res. 2000;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem J. 2004;377:131–9. doi: 10.1042/BJ20031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dp[a,omergoc meirpms tp dostirbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Vie A, Cigna M, Toci R, Birman S. Differential regulation of Drosophila tyrosine hydroxylase isoforms by dopamine binding and cAMP-dependent phosphorylation. J. Biol. Chem. 1999;274:16788–16795. doi: 10.1074/jbc.274.24.16788. [DOI] [PubMed] [Google Scholar]
- Waldvogel HJ, Curtis MA, Baer K, Rees MI, Faull RL. Immunohistochemical staining of post-mortem adult human brain sections. Nat Protoc. 2006;1:2719–2732. doi: 10.1038/nprot.2006.354. [DOI] [PubMed] [Google Scholar]
- Wang J, Lou H, Pedersen CJ, Smith AD, Perez RG. 14-3-3zeta contributes to tyro sine hydroxylase activity in MN9D cells: localization of dopamine regulatory proteins to mitochondria. J Biol Chem. 2009;284:14011–14019. doi: 10.1074/jbc.M901310200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral re sponses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A. Protein kinase Cδ negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci. 2007;27:5349–5362. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: Role as a novel target for drug development. AAPS J. 2006;8:E682–E692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.