Abstract
Maternal use of selective serotonin (5-HT) reuptake inhibitors (SSRIs) is associated with an increased risk for persistent pulmonary hypertension of the newborn (PPHN), but little is known about 5-HT signaling in the developing lung. We hypothesize that 5-HT plays a key role in maintaining high pulmonary vascular resistance (PVR) in the fetus and that fetal exposure to SSRIs increases 5-HT activity and causes pulmonary hypertension. We studied the hemodynamic effects of 5-HT, 5-HT receptor antagonists, and SSRIs in chronically prepared fetal sheep. Brief infusions of 5-HT (3–20 μg) increased PVR in a dose-related fashion. Ketanserin, a 5-HT 2A receptor antagonist, caused pulmonary vasodilation and inhibited 5-HT-induced pulmonary vasoconstriction. In contrast, intrapulmonary infusions of GR127945 and SB206553, 5-HT 1B and 5-HT 2B receptor antagonists, respectively, had no effect on basal PVR or 5-HT-induced vasoconstriction. Pretreatment with fasudil, a Rho kinase inhibitor, blunted the effects of 5-HT infusion. Brief infusions of the SSRIs, sertraline and fluoxetine, caused potent and sustained elevations of PVR, which was sustained for over 60 min after the infusion. SSRI-induced pulmonary vasoconstriction was reversed by infusion of ketanserin and did not affect the acute vasodilator effects of acetylcholine. We conclude that 5-HT causes pulmonary vasoconstriction, contributes to maintenance of high PVR in the normal fetus through stimulation of 5-HT 2A receptors and Rho kinase activation, and mediates the hypertensive effects of SSRIs. We speculate that prolonged exposure to SSRIs can induce PPHN through direct effects on the fetal pulmonary circulation.
Keywords: persistent pulmonary hypertension of the newborn, ketanserin, SB206553, GR127935
the fetal pulmonary circulation is characterized by high pulmonary vascular resistance (PVR) and low pulmonary blood flow. Mechanisms that maintain high PVR are poorly understood but include a low pulmonary artery Po2 and an imbalance between vasoconstrictors and vasodilators (1, 9). After birth PVR falls, allowing for an increase in pulmonary blood flow. A failure of this decrease in PVR results in the clinical syndrome known as persistent pulmonary hypertension of the newborn (PPHN). Factors that contribute to the pathogenesis of PPHN are multifactorial, including altered vascular reactivity, vascular remodeling, and abnormal growth (18). Understanding the basic mechanisms that regulate pulmonary vascular resistance is crucial to the development of novel therapies for severe PPHN.
Although the role of serotonin (5-HT) in the pathogenesis of adult pulmonary hypertension (PH) has been extensively studied, little is known about the role of 5-HT in the normal perinatal pulmonary circulation or in models of PPHN. 5-HT is a potent vasoconstrictor and stimulates smooth muscle cell growth and proliferation in the adult pulmonary circulation (11, 13, 38, 51). Activation of the 5-HT 1B, 2A, and 2B receptors and the 5-HT transporter (SERT) mediate 5-HT signaling in the adult lung (34, 38, 39). Pharmacological or genetic strategies that augment 5-HT expression worsen, and inhibition of 5-HT signaling decreases the severity of pulmonary hypertension in adult animal models (10, 12, 23, 27, 29, 35). Recent studies suggest that selective 5-HT reuptake inhibitor (SSRI) therapy reduces PH in adult animal models and in the clinical setting (26, 31, 37, 53).
In contrast to the effects of SSRIs in adults with PH and in experimental PH, epidemiological studies suggest that SSRI treatment of maternal depression after the 20th wk of gestation increases the risk of PPHN in the infant by sixfold (7). This striking developmental difference in response to SSRIs on the pulmonary circulation reflects the lack of our present understanding on the roles of 5-HT in the developing lung. The association between the maternal use of SSRIs and PPHN remains controversial, and only one animal study to date has examined whether SSRIs cause PPHN (16, 55). Treatment of pregnant rats with SSRIs leads to mild PPHN in rat pups, as seen by increased right ventricular hypertrophy and hypoxemia at birth; however, the mechanisms underlying this response remain uncertain. Specifically, whether this effect is due to the direct action of SSRIs on the developing pulmonary circulation or through changes in maternal utero-placental circulation is unknown. The paradoxical findings of the potential differences of SSRIs in the adult and fetal circulation highlight the critical need to study 5-HT signaling and the direct effect of SSRIs in the fetal lung.
Therefore, we hypothesize that 5-HT plays a key role in maintaining high PVR in the normal fetus and that fetal exposure to SSRIs increases 5-HT activity, which increases pulmonary vascular tone, leading to sustained PH at birth. We studied the effect of acute intrapulmonary infusions of 5-HT, the 5-HT receptor antagonists, GR127935, ketanserin, SB206553, and the SSRIs, sertraline and fluoxetine, in the chronically prepared ovine fetus.
MATERIALS AND METHODS
Pregnant, mixed-breed (Colombia-Rambouillet) ewes were used in this study. All procedures and protocols were reviewed and approved by the Animal Care and Use Committee of the University of Colorado Denver and followed the Guide for the Care and Use of Laboratory Animals established by the National Research Council.
Fetal Surgical Preparation
Surgery was performed at 124–129 days gestation (full term = 147 days) after ewes had fasted for 24 h and thirsted overnight. Animals were given intramuscular penicillin G (600,000 U) and intravenous gentamicin (80 mg) immediately before surgery. Ewes were sedated with intravenous ketamine (1,000 mg) and diazepam (10 mg) and intubated and ventilated with 1–3% isoflurane for the duration of surgery. Under sterile conditions, a midline abdominal incision was made, and the uterus was externalized. The left fetal forelimb was exposed through hysterotomy. Polyvinyl catheters (20-gauge) were placed in the left axillary artery and vein and advanced in the ascending aorta and superior vena cava, respectively. A left thoracostomy and pericardial incision provided access to the heart and great vessels. With the use of a 16-gauge intravenous placement unit (Angiocath, Travenol, Deerfield, IL), a 22-gauge catheter was placed through purse-string sutures in the left pulmonary artery (LPA) to allow for selective drug infusions. A 14-gauge intravenous placement unit (Angiocath) was used to place 20-gauge catheters in the main pulmonary artery (MPA) and left atrium (LA). After gentle, blunt dissection of the bifurcation of the MPA, a flow transducer (Transonic Systems, Ithaca, NY) was placed around the LPA to measure blood flow to the left lung (QLPA). A catheter was placed in the amniotic cavity to serve as a pressure referent. The uterus was sutured, and a dose of ampicillin (500 mg) was given in the amniotic cavity. The catheters and flow transducer cable were externalized to a flank pouch on the ewe after the abdominal wall was closed. Postoperatively, ewes were allowed to eat and drink ad libitum and were generally standing within 1 h. All animals were treated with scheduled buprenorphine (0.6 mg) for 48 h postoperatively and then as indicated (based on veterinary assessment of pain). All catheters were gently flushed daily with 1–2 ml heparinized normal (0.9%) saline to maintain catheter patency.
Western Blot Analysis
Western blot analysis for lung SERT, 5-HT receptor 1B, 2A, and 2B was performed by standard methods. Blots were incubated overnight at 4°C with SERT (catalog no. sc-14514; Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:200), 5-HT 1B receptor (catalog no. sc-1460; Santa Cruz Biotechnology; dilution 1:200), 5-HT 2A receptor (catalog no. sc-32538; Santa Cruz Biotechnology; dilution 1:200), or 5-HT 2B receptor antibodies (catalog no. sc-15080; Santa Cruz Biotechnology; dilution 1:200). Blots were washed and incubated for 1 h at room temperature with donkey anti-goat IgG-horseradish peroxidase (catalog no. sc-2033; Santa Cruz Biotechnology; 1:4,000 dilution). Bands of interest were visualized using the Enhanced Chemiluminescence Plus kit and identified by molecular weight as designated by the manufacturer for protein of interest. Blots were stripped and reprobed with an antibody to β-actin (catalog no. A5316; Sigma, St. Louis, MO). Densitometry was performed using NIH Image J software. Changes in protein expression were analyzed after normalization for β-actin expression.
Drug Preparation
A solution of 5-HT, 5-HT creatinine sulfate monohydrate complex (3 μg/ml; Sigma H7752), was made immediately before each study by dissolving the drug in normal saline. Ketanserin (50 mg/ml DMSO; Sigma S006), GR127935 (10 mg/ml H2O; Sigma G5793), and SB206553 (10 mg/6 ml H20; 1661; Tocris Bioscience, Ellisville, MO) solutions were made immediately before each experiment. Fasudil, HA-1077 (100 μg/ml; H-2330; LC Laboratories, Woburn, MA) was dissolved in normal saline. Sertraline hydrochloride (20 mg/ml DMSO; Sigma S6319), fluoxetine (4 mg/ml H2O; Sigma F132) and acetylcholine chloride (15 μg/ml sterile normal saline; Sigma A6625) were made and stored at −20°C.
General Study Design
Ewes were allowed to recover from surgery for a minimum of 24 h before the initiation of physiological studies. During each study, pulmonary arterial, aortic, and left atrial pressures were measured by connecting externalized catheters to computer-driven pressure transducers (model MP100A; Biopac Systems, Santa Barbara, CA). Pressure measurements were referenced to simultaneously recorded amniotic pressure. The flow transducer was connected to an internally calibrated flowmeter (Transonics Systems) to measure QLPA. Before infusion of study drugs, a 30-min period of stable baseline hemodynamics was established. Hemodynamic variables, including main pulmonary artery pressure (MPAP), left atrial pressure (LAP), aortic pressure (AoP), and QLPA, were measured continuously for the duration of each study protocol. Left lung PVR was calculated as follows: PVR = (MPAP − LAP)/QLPA. Heart rate (HR) was determined from phasic pressure traces. Arterial blood gas measurements included pH, Pco2, and Po2 (model ABL 800; Radiometer, Copenhagen, Denmark) and were obtained before and after each drug infusion.
Experimental Design
Protocol 1: pulmonary hemodynamic effects of 5-HT in the fetal lamb.
The purpose of this study was to investigate the pulmonary effects of selective 5-HT infusions. After a 24-h recovery from surgery, baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements were stable for a 30-min period, 5-HT (3–20 μg) was infused into the LPA over 20 min. Preliminary studies showed dose-related pulmonary vascular effects at these doses with limited systemic effects. Hemodynamic measurements were recorded every 10 min for 40 min after the infusion concluded. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 2: pulmonary hemodynamic effects of the 5-HT receptor blockers, ketanserin, GR127935, and SB206553.
The purpose of these studies was to investigate whether endogenous 5-HT maintains normal fetal vascular tone through actions on the 5-HT 2A, 1B, or 2B receptor. Baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements were stable for a 30-min period, either ketanserin (10 mg), GR127935 (10 mg), or SB206553 (10 mg) were infused, on random days, into the LPA over 20 min. Hemodynamic measurements were recorded every 10 min for 40 min after the infusion concluded. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 3: effects of 5-HT 2A receptor blockade on the hemodynamic response of exogenous 5-HT administration.
The purpose of this protocol was to investigate whether exogenous 5-HT causes pulmonary vasoconstriction through the 5-HT 2A receptor. Baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements 5-HT (20 μg) was either infused alone or after pretreatment with ketanserin (10 mg), a 5-HT 2A receptor blocker. Hemodynamic measurements were recorded every 10 min for 40 min after the infusion concluded. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 4: effects of Rho kinase inhibition on the hemodynamic response of exogenous 5-HT administration.
The purpose of this protocol was to investigate whether 5-HT causes vasoconstriction through Rho kinase activation. After a 24-h recovery from surgery, baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements were stable for a 30-min period, 5-HT (20 μg) was infused into the LPA over 20 min. Once hemodynamic measurements returned to baseline, fasudil (100 μg) was infused into the LPA. When hemodynamic measurements returned to baseline, 5-HT (20 μg) and fasudil (100 μg) were infused into the LPA over 20 min.
Protocol 5: pulmonary hemodynamic effects of the SSRIs, sertraline and fluoxetine, in the fetal lamb.
Baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements were stable for a 30-min period, sertraline (10 mg) or fluoxetine (10 mg) was infused into the LPA over 40 or 20 min, respectively. The drugs were infused over different time intervals to deliver similar infusion rates. Doses were based on preliminary studies demonstrating pulmonary vascular effects and limited systemic effects. We then studied the pulmonary hemodynamic effects of ketanserin infusion following sertraline administration to determine whether antagonism of the 5-HT 2A receptor would attenuate the hemodynamic effects of sertraline. After baseline measurements were stable for a 30-min period, sertraline (10 mg) was infused into the LPA over 40 min. Immediately after the completion of the sertraline infusion, the 5-HT 2A receptor antagonist ketanserin was infused (10 mg) directly into the LPA over 20 min. Hemodynamic measurements were recorded for 60 min after the end of the infusion. Acetylcholine (15 μg) was infused after sertraline administration to determine whether sertraline increases PVR through inhibition of endothelial nitric oxide synthase (eNOS). After baseline measurements were stable for a 30-min period, acetylcholine was infused over 10 min. Following the return of hemodynamic measurements to baseline, the SSRI sertraline (10 mg) was infused into the LPA over 40 min. Immediately following the end of the sertraline infusion, acetylcholine was again infused into the LPA over 10 min. Hemodynamic measurements were recorded for another 80 min. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 6: Western blot analysis of fetal vs. adult ovine whole lung expression of the SERT and the 5-HT 1B, 2A, and 2B receptors.
Lung whole lung lysates were collected from the ovine fetus (gestational age 136–143 days, term 147 days, N = 4) and the adult ewe (N = 4) to compare the developmental protein expression of the SERT and the 1B, 2A, and 2B 5-HT receptors, as described above.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism version 5.0 for Mac, GraphPad Software (San Diego, CA). Hemodynamic variables over time were compared using repeated-measures ANOVA with Newman-Keul's post hoc testing. Statistical comparisons were performed using unpaired t-tests and paired t-tests. Data are presented as means ± SE. The significance level was set at P < 0.05.
RESULTS
Protocols
Protocol 1: pulmonary hemodynamic effects of brief 5-HT infusion in the fetal lamb.
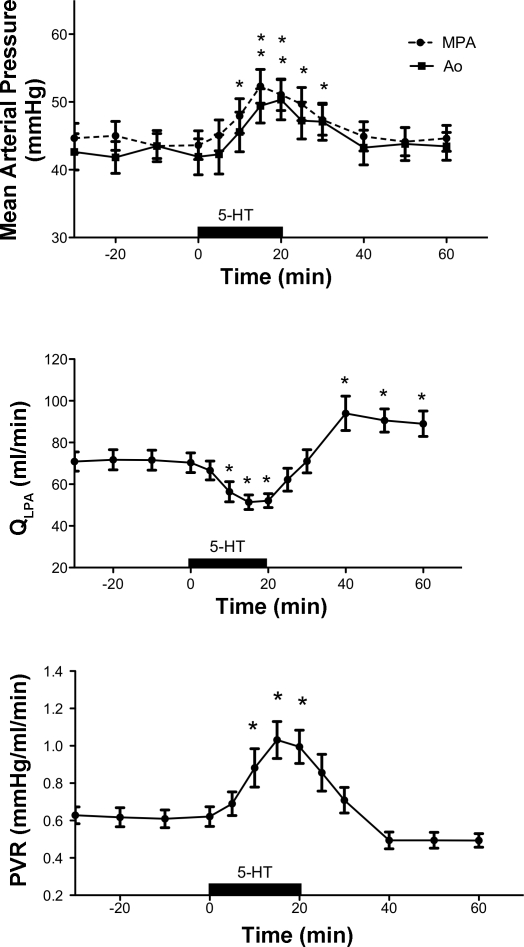
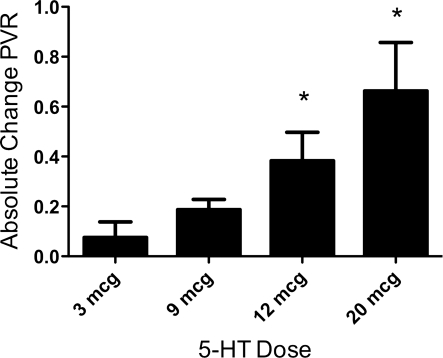
Brief intrapulmonary infusions of 5-HT decreased QLPA by 29% (72 ± 19 vs. 52 ± 13 ml/min, P < 0.0001) and increased PVR by 61% from baseline (0.62 ± 0.19 vs. 1.00 ± 0.36 mmHg/ml per min, P < 0.0001; Fig. 1). These effects were not limited to the pulmonary circulation, as brief intrapulmonary infusions of 5-HT increased both MPAP (from 44 ± 18 vs. 51 ± 19 mmHg, P < 0.001) and AoP (41 ± 9 vs. 49 ± 10, P < 0.001) (Fig. 1). The effects of 5-HT were short-lived, as QLPA and PVR returned to baseline shortly after the end of the infusion. 5-HT induced pulmonary vasoconstriction in a dose-dependent fashion at doses ranging from 3–20 μg (Fig. 2). HR decreased from 176 ± 16 (baseline) to 165 ± 21 beats per min (bpm) at the end of the infusion (P < 0.05), and pH decreased from 7.38 ± 0.03 to 7.37 ± 0.03, P < 0.05. LAP, PaO2, and PaCO2 did not change in response to 5-HT.
Fig. 1.
Hemodynamic response to serotonin (5-HT) (12–20 μg). mPAP, mean pulmonary artery pressure; AoP, systemic pressure; QLPA, left pulmonary artery flow; PVR, pulmonary vascular resistance. *P < 0.001 vs. baseline (N = 16).
Fig. 2.
Dose-dependant fetal pulmonary vasoconstrictor response, expressed as absolute change in baseline PVR, for 5-HT doses 3–20 μg. *P < 0.05, 3 μg vs. both 12- and 20-μg doses.
Protocol 2: pulmonary hemodynamic effects of the 5-HT receptor blockers, ketanserin, GR127935, and SB206553.
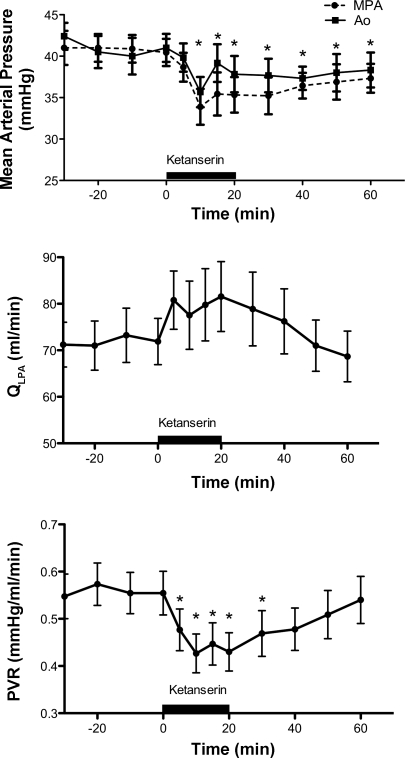
Brief intrapulmonary infusions of ketanserin (10 mg), a 5-HT 2A receptor antagonist, decreased PVR by 24% from 0.56 ± 0.13 at baseline to 0.43 ± 0.12 mmHg/ml per min (P < 0.005 Fig. 3). Ketanserin decreased MPAP from 40 ± 15 vs. 35 ± 6 mmHg, P < 0.05 (Fig. 3). AoP briefly decreased during the infusion (from 41 ± 4 vs. 36 ± 14 mmHg, P < 0.05) but returned to baseline values for the remainder of the study. Ketanserin did not change HR or arterial blood gas tensions. Intrapulmonary infusions of the 5-HT 1B antagonist (GR127935) or the 5-HT 2B antagonist (SB206553) had no effect on hemodynamics or blood gas tensions (data not shown).
Fig. 3.
Hemodynamic response to ketanserin, 5-HT 2A receptor antagonist. *P < 0.05 vs. baseline (N = 12).
Protocol 3: effects of 5-HT 2A receptor blockade on the hemodynamic response of exogenous 5-HT administration.
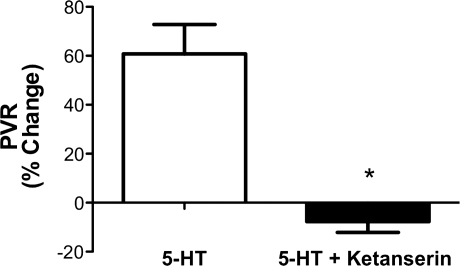
The hemodynamic effects of infusions of 5-HT (20 μg) alone or after pretreatment with ketanserin (10 mg) were studied to determine whether the effects of 5-HT are mediated through 5-HT 2A receptor activation. The infusion of 5-HT alone increased PVR by 60%, which was abolished after pretreatment with ketanserin (Fig. 4). The administration of 5-HT increased PVR from 0.61 ± 0.12 to 0.9 ± 0.23 mmHg/ml per min (P < 0.05). Pretreatment with ketanserin blocked 5-HT-mediated vasoconstriction, baseline PVR 0.64 ± 0.1 vs. 0.53 ± 0.06 mmHg/ml per min. 5-HT administration alone decreased QLPA from 68 ± 17 to 51 ± 13 ml/min, P < 0.05. In comparison, 5-HT administered after pretreatment with ketanserin resulted in no change in QLPA, 65 ± 9 vs. 69 ± 14 ml/min. There were no changes in blood gas tensions or HR.
Fig. 4.
Pulmonary vascular response to infusion of 5-HT, expressed as change from baseline PVR. Pretreatment with ketanserin (5-HT + Ketanserin) completely abolished the 60% rise in PVR induced by 5-HT. *P < 0.05 vs. 5-HT.
Protocol 4: effects of Rho kinase inhibition on the hemodynamic response of exogenous 5-HT administration.
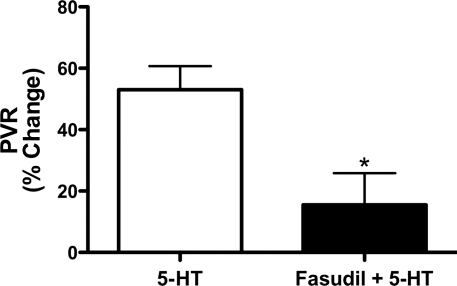
The hemodynamic response of 5-HT (20 μg) infusion alone was compared with the combination of fasudil (100 μg) and 5-HT (20 μg) administration. The infusion of 5-HT alone increased PVR by 53%, which was attenuated with the coadministration of fasudil with 5-HT (P < 0.05, Fig. 5). 5-HT alone increased PVR from 0.50 ± 0.19 to 0.69 ± 0.21 mmHg/ml per min, P < 0.05. MPAP increased from 38 ± 8.1 to 43 ± 7.3, P < 0.05. The coadministration of fasudil with 5-HT blocked 5-HT-mediated vasoconstriction (0.50 ± 0.16 vs. 0.57 ± 0.13 mmHg/ml per min) and increase in MPAP (37 ± 6.9 to 44 ± 6.7, ns).
Fig. 5.
Pulmonary vascular response to infusion of 5-HT alone or in combination with the Rho kinase inhibitor fasudil, expressed as percent change from baseline PVR. The 53% increase in PVR induced by 5-HT alone was attenuated with the combination of 5-HT and fasudil to a 15% increase in PVR from baseline. *P < 0.05 vs. 5-HT.
Protocol 5: pulmonary hemodynamic effects of the SSRIs, sertraline and fluoxetine, in the fetal lamb.
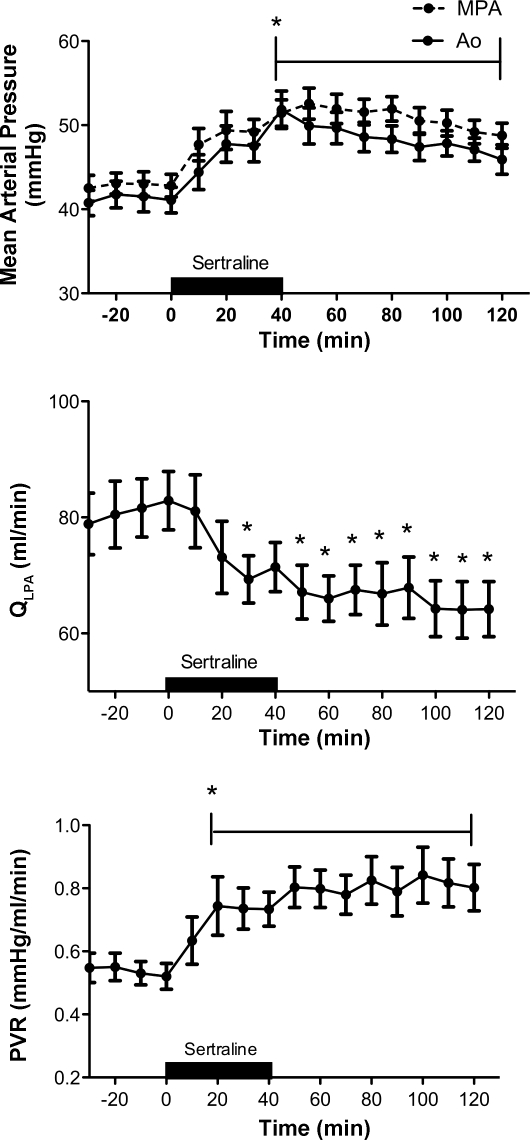
Infusions of the SSRI sertraline decreased QLPA by 18% (81 ± 21 vs. 67 ± 19 ml/min, P < 0.05) (Fig. 6) and increased PVR by 48% (Fig. 6) from 0.54 ± 0.16 (baseline) to 0.80 ± 0.26 mmHg/ml per min (50 min), P < 0.05. The pulmonary vasoconstrictor response to sertraline was sustained for at least 80 min after the completion of the infusion. Systemic (41 ± 5 to 50 ± 8 mmHg; P < 0.05) and pulmonary artery pressures (43 ± 5.4 to 51 ± 6.3 mmHg; P < 0.05) were significantly increased following the infusion of sertraline (Fig. 6). Acute infusions of sertraline did not significantly alter HR (171 ± 14 baseline vs. 184 ± 23 bpm) or LAP (2.5 ± 0.6 baseline vs. 3.0 ± 1.2 mmHg). Direct intrapulmonary infusions of sertraline decreased pH (7.37 ± 0.02 vs. 7.32 ± 0.05; P < 0.05) and Po2 (22 ± 4 vs. 20 ± 4 Torr; P < 0.05) and increased Pco2 (45 ± 3 vs. 49 ± 4 Torr; P < 0.05).
Fig. 6.
Hemodynamic response to the selective 5-HT reuptake inhibitor (SSRI), sertraline. *P < 0.05 vs. baseline (N = 16).
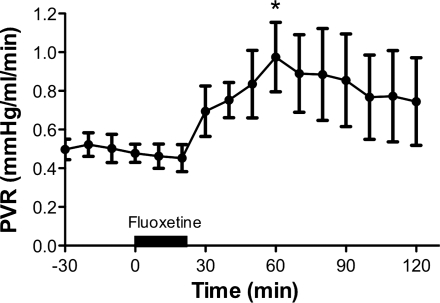
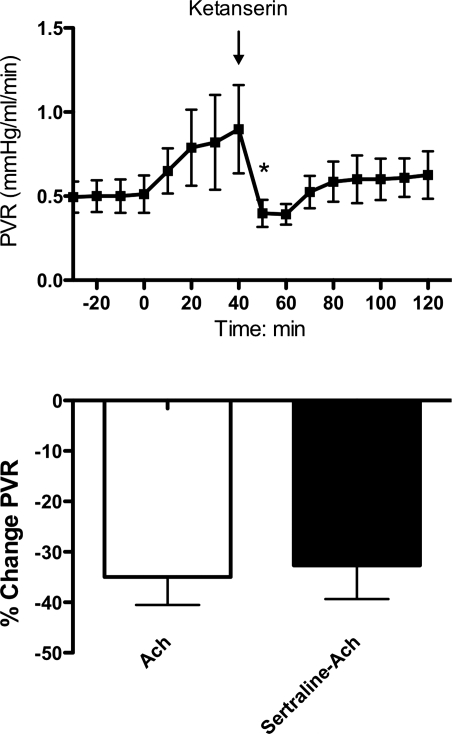
As observed with sertraline, infusions of a second SSRI, fluoxetine, also caused potent and sustained pulmonary vasoconstriction (Fig. 7). Fluoxetine increased PVR by 38%, from 0.55 ± 0.14 (baseline) to 0.76 ± 0.16 (P < 0.05), and QLPA decreased 14% from 80 ± 15 (baseline) to 69 ± 22 ml/min. Sertraline administration resulted in a potent increase in PVR. However, ketanserin, a 5-HT 2A receptor antagonist, decreased PVR to baseline values (Fig. 8, top). Acetylcholine (15 μg) decreased PVR by 33% when infused before and after the infusion of sertraline, suggesting that sertraline did not acutely impair eNOS activity or signaling (Fig. 8, bottom).
Fig. 7.
Pulmonary vasoconstrictor response to the SSRI, fluoxetine. *P < 0.05 vs. baseline.
Fig. 8.
Top: pulmonary vasoconstrictor response to sertraline is abolished with the infusion of ketanserin. *P < 0.05 end of sertraline infusion vs. ketanserin infusion. Bottom: pulmonary vascular response to acetylcholine (ACh) alone or acetylcholine infusion following sertraline infusion, expressed as percent change from baseline.
Protocol 6: Western blot analysis of fetal vs. adult ovine whole lung expression of the SERT and the 5-HT 1B, 2A, and 2B receptors.
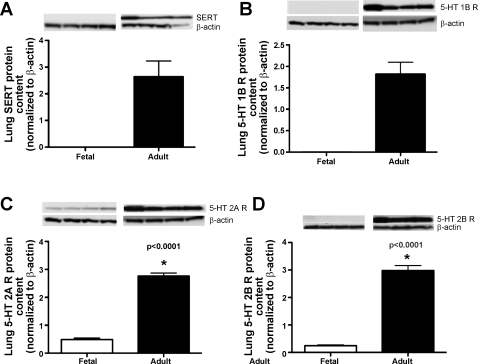
Western blot analysis demonstrated absent expression of the SERT and the 5-HT 1B receptor in the lung of the late-gestation ovine fetus compared with the adult ewe. The ovine fetus does express the 5-HT 2A and 2B receptors, but in much lower levels than the adult (Fig. 9).
Fig. 9.
Compared with adult values, fetal lung protein content for 5-HT transporter (SERT) (A), 5-HT 1B receptor (B), 5-HT 2A receptor (C), and 5-HT 2B receptor (D) are markedly decreased. Values are presented as means ± SE (*P < 0.0001).
DISCUSSION
We found that exogenous 5-HT is a potent pulmonary vasoconstrictor in the late-gestation ovine fetus and that the pulmonary hemodynamic effects of 5-HT are mediated through the 5-HT 2A receptor and at least partially through Rho kinase activation. In addition, ketanserin causes fetal pulmonary vasodilation, suggesting that endogenous 5-HT partially contributes to the maintenance of normal pulmonary vascular tone in the ovine fetus thorough the 5-HT 2A receptor. These effects were not observed after treatment with 5-HT 1B or 2B receptor antagonists. We also found that acute intrapulmonary infusions of the SSRIs, sertraline and fluoxetine, cause potent and sustained increases in pulmonary vascular resistance and decreases in pulmonary blood flow and that these effects were mediated through the action of the 5-HT 2A receptor. These agents also caused a sustained increase in systemic pressure. Finally, acetylcholine-induced vasodilation persists after sertraline treatment, suggesting that sertraline did not directly impair NOS activity.
These results are interesting because this is the first report describing the direct pulmonary effects of 5-HT in the developing pulmonary circulation in vivo and the mechanisms through which 5-HT induces fetal pulmonary vasoconstriction. This is the first study to show that acute selective pulmonary infusions of two commonly prescribed SSRIs, sertraline and fluoxetine, directly causes sustained and potent fetal pulmonary vasoconstriction in vivo. SSRI-induced pulmonary vasoconstriction was abolished with the administration of ketanserin, the 5-HT 2A receptor antagonist, but acetylcholine-induced pulmonary vasodilation was not altered by sertraline treatment, supporting our hypothesis that SSRIs cause pulmonary vasoconstriction through an increase in free local or circulating 5-HT and not through inhibition of eNOS.
The first potential link between 5-HT and the development of PPHN was recognized several years ago when Chambers et al. (7) reported that the maternal use of SSRIs after the 20th wk of gestation was associated with a sixfold increased risk of the development of PPHN (7). SSRIs are the most common agents used to treat depression (2, 49). Maternal depression is extremely prevalent with between 10–15% of women of reproductive age diagnosed with depression (33). When untreated, maternal depression is associated with numerous adverse consequences including low birth weight, prematurity, and maternal substance abuse (5, 8, 54). SSRIs readily cross the placenta, as reflected by fetal cord blood levels, corresponding to 30–70% of maternal levels (22, 50). Some infants exposed to SSRIs during the last trimester of pregnancy are reported to develop self-limited signs of withdrawal from these drugs, manifested as sleep disturbances, gastrointestinal and neurological symptoms, and respiratory distress, suggesting significant fetal drug delivery during late gestation (6, 14, 46). Past studies in pregnant ewes have shown that maternal infusion of SSRIs decreases uterine artery blood flow and causes fetal hypoxemia (41). Recent studies have demonstrated that fluoxetine-exposed neonatal rats develop pulmonary hypertension, as evidenced by pulmonary vascular remodeling, right ventricular hypertrophy, decreased oxygenation, and higher mortality at birth (16). However, the pulmonary vascular effects of SSRIs were not directly measured in vivo.
In striking contrast to the effect of SSRIs on the fetal pulmonary circulation, SSRI treatment has been shown to attenuate disease severity in both animal models of adult PH and in adult patients with established PH. SERT inhibition, with either SSRIs or SERT knockout mice, attenuates hypoxia-induced PH in rodents (20, 37). Clinical studies suggest that SSRI use in patients with PH is associated with a reduction in the risk of death (26, 53). Why SSRIs exert such differential effects in the fetal and adult pulmonary circulation remains unanswered but may be due to developmental differences in the pulmonary expression of the SERT and 5-HT receptors. We also report that, compared with adult expression, fetal lung content of SERT and the 5-HT 1B, 2A, and 2B receptor proteins were markedly decreased. In fact, SERT protein was not detected in the fetal lung. Whether these differences in SERT expression contribute to developmental changes in SSRI effects and potential mechanistic implications is not understood. We speculate that SSRIs may act in part through effects on platelets, enhancing the release of 5-HT in the fetal circulation and increasing pulmonary vasoconstriction. Alternatively, SSRIs may potentially cause vasoconstriction through nonspecific effects, such as inhibition of voltage-gated potassium channels (56). Additional studies are needed to clarify these mechanisms. Although this study was conducted to address the direct effects of SSRI infusions on the lung, studies of chronic maternal administration of SSRIs are needed to assess the effect of SSRIs on the uterine placental circulation and whether chronic administration has similar effects to acute administration in the fetal circulation.
We found that SSRIs induce both pulmonary vasoconstriction and systemic hypertension, which may be attributable to an acute increase in circulating levels of 5-HT, by SSRI blockade of 5-HT uptake into platelets, endothelial cells, and other cell types that express the SERT. Infusions of fluoxetine in sheep results in an acute increase in plasma 5-HT levels, whereas chronic fetal exposure to SSRIs reduces mean cord blood 5-HT concentrations and platelet 5-HT concentrations in newborns (3, 28, 42). We found that the 5-HT 2A receptor antagonist, ketanserin, reverses SSRI-induced pulmonary vasoconstriction, supporting the hypothesis that SSRIs induce pulmonary vasoconstriction through increased 5-HT levels. Another proposed mechanism for SSRI-induced vasoconstriction is through direct inhibition of NOS activity. Previous studies have shown that paroxetine, another commonly used SSRI, inhibits NOS activity and expression (4, 15). However, we found that acetylcholine-induced vasodilation remained following sertraline administration, showing that eNOS was not inhibited by sertraline.
In adult rodents, 5-HT-induced vasoconstriction is primarily mediated through the 5-HT 1B receptor (34, 38). In contrast, inhibition of fetal 5-HT 1B receptors had no significant effect on fetal pulmonary blood flow, whereas 5-HT 2A receptor inhibition decreased PVR. Prior studies in isolated rabbit pulmonary arteries have shown that 5-HT-induced fetal pulmonary vasoconstriction is mediated through the 5-HT 2A receptor (40).
One of the effectors of 5-HT signaling in adult pulmonary artery smooth muscle cells is Rho kinase (21, 25, 32, 36). Activation of RhoA/ROCK pathway contributes to vasoconstriction and pulmonary vascular remodeling (25, 44, 47). In the fetus, Rho kinase activation has been shown to maintain PVR and is increased in experimental PPHN (19, 48). In this study, we found that 5-HT-mediated vasoconstriction was attenuated with coadministration of fasudil, the Rho kinase inhibitor.
We conclude that 5-HT causes potent vasoconstriction in the ovine fetal pulmonary circulation through activation of the 5-HT 2A receptor and in part through Rho kinase signaling. The SSRIs, sertraline and fluoxetine, cause potent and sustained pulmonary vasoconstriction in the normal ovine fetus, which is mediated through stimulation of the 5-HT 2A receptor. We speculate that endogenous 5-HT likely contributes to the maintenance of pulmonary vascular resistance during fetal life. We further suggest that prolonged exposure to SSRIs may lead to abnormal 5-HT metabolism, which may alter the expression of 5-HT receptors, specifically the 2A receptor, leading to increased Rho kinase activity contributing to sustained abnormalities of pulmonary vascular tone, reactivity, or structure, which may lead to impaired transition of the pulmonary circulation at birth (PPHN).
GRANTS
C. Delaney is supported by NIH-NICHD grant T32 HD007186-31 and Gilead Young Investigator Award. J. Gien is supported by NIH grant 1K08HL102261 and Actelion Pharmaceuticals (Entelligence Young Investigator Award). S. Abman is supported by NIH grants HL007670, HL085703, and HL068702.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors are grateful for statistical advice from Dr. Paul Rozance.
REFERENCES
- 1. Abman SH. Abnormal vasoreactivity in the pathophysiology of persistent pulmonary hypertension of the newborn. Pediatr Rev 20: e103–e109, 1999 [PubMed] [Google Scholar]
- 2. American College of Obstetrics and Gynecology Treatment with selective serotonin reuptake inhibitors during pregnancy. Obstet Gynecol 108: 1601–1603, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Anderson GM, Czarkowski K, Ravski N, Epperson CN. Platelet serotonin in newborns and infants: ontogeny, heritability, and effect of in utero exposure to selective serotonin reuptake inhibitors. Pediatr Res 56: 418–422, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Angulo J, Peiro C, Sanchez-Ferrer CF, Gabancho S, Cuevas P, Gupta S, Saenz de Tejada I. Differential effects of serotonin reuptake inhibitors on erectile responses, NO-production, and neuronal NO synthase expression in rat corpus cavernosum tissue. Br J Pharmacol 134: 1190–1194, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 49: 726–735, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Boucher N, Bairam A, Beaulac-Baillargeon L. A new look at the neonate's clinical presentation after in utero exposure to antidepressants in late pregnancy. J Clin Psychopharmacol 28: 334–339, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Chambers C, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354: 579–587, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med 63: 830–834, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Dawes GS, Mott JC. The vascular tone of the foetal lung. J Physiol 164: 465–477, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dempsie Y, Morecroft I, Welsh DJ, MacRitchie NA, Herold N, Loughlin L, Nilsen M, Peacock AJ, Harmar A, Bader M, MacLean MR. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation 117: 2928–2937, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Dunn JA, Lorch V, Sinha SN. Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: norepinephrine, epinephrine, serotonin, and potassium chloride. Pediatr Res 25: 360–363, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 108: 1141–1150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, Adnot S. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol Heart Circ Physiol 272: H1173–H1181, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Ferreira E, Carceller AM, Agogue C, Martin BZ, St-Andre M, Francoeur D, Berard A. Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatrics 119: 52–59, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Finkel MS, Laghrissi-Thode F, Pollock BG, Rong J. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull 32: 653–658, 1996 [PubMed] [Google Scholar]
- 16. Fornaro E, Li D, Pan J, Belik J. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am J Respir Crit Care Med 176: 1035–1040, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106: 1071–1083, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol 11: 525–549, 1984 [PubMed] [Google Scholar]
- 19. Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Chronic intrauterine pulmonary hypertension increase endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol 295: L680–L687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G, Pacaud P. RhoA and rho kinase activation in human pulmonary hypertension Role of 5-HT signaling. Am J Respir Crit Care Med 179: 1151–1158, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Hendrick V, Smith LM, Suri R, Hwang S, Haynes D, Altshuler L. Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol 188: 812–815, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Hironaka E, Hongo M, Sakai A, Mawatari E, Terasawa R, Okumura N, Yamazaki A, Ushiyama Y, Yazaki Y, Kinoshita O. Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res 60: 692–699, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Homma N, Nagaoka T, Morio Y, Ota H, Gebb A, Karoor V, McMurtry I, Oka M. Endothelin-1 and serotonin are involved in activation of rhoA/rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Kawut SM, Horn EM, Berekashvili KK, Lederer DJ, Widlitz AC, Rosenzweig EB, Barst RJ. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther 19: 370–374, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Keegan A, Morecroft I, Smillie D, Hicks M, MacLean M. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT 1B-receptor knockout mice and the 5-HT 1B/1D receptor antagonist GR127935. Circ Res 89: 1231–1239, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns, and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry 60: 720–726, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Launay J, Herve P, Peoch K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8: 1129–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. Persistent pulmonary hypertension of the newborn infant. J Pediatr 89: 626–630, 1976 [DOI] [PubMed] [Google Scholar]
- 31. Li XQ, Hong Y, Wang Y, Zhang XH, Wang HL. Sertraline protects against monocrotaline-induced pulmonary hypertension in rats. Clin Exp Pharmacol Physiol 33: 1047–1051, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Suzuki Y, Day R, Fanburg B. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Llewellyn AM, Stowe ZN, Nemeroff CB. Depression during pregnancy and the puerperium. J Clin Psychiatry 58: 26–32, 1997 [PubMed] [Google Scholar]
- 34. Maclean MR, Sweeney G, Baird M, McCulloch KM, Houslay M, Morecroft I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br J Pharmacol 119: 917–930, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacLean MR, Deuchar G, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation 109: 2150–2155, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Mair KM, MacLean MR, Morecroft I, Dempsie Y, Palmer TM. Novel interactions between the 5-HT transporter, 5-HT 1B receptors and rho kinase in vivo and in pulmonary fibroblasts. Br J Pharmacol 155: 606–616, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, Eddahibi S. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med 168: 487–493, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Morecroft I, Heeley R, Prentice H, Kirk A, MacLean MR. 5-hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol 128: 730–734, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, Harmar A, MacLean MR. Functional interactions between 5-Hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther 313: 539–548, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Morecroft I, MacLean MR. 5-hydroxytryptamine receptors mediating vasoconstriction and vasodilation in perinatal and adult rabbit small pulmonary arteries. Br J Pharmacol 125: 69–78, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morrison JL, Chien C, Riggs KW, Gruber N, Rurak D. Effect of maternal fluoxetine administration on uterine blood flow, fetal blood gas status, and growth. Pediatr Res 51: 433–442, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Morrison JL, Riggs KW, Chien C, Gruber N, McMillen IC, Rurak DW. Chronic maternal fluoxetine infusion in pregnant sheep: effects on the maternal and fetal hypothalamic-pituitary-adrenal axes. Pediatr Res 56: 40–46, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Morrison JL, Riggs KW, Rurak DW. Fluoxetine during pregnancy: impact on fetal development. Reprod Fertil Dev 17: 641–650, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol 100: 996–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Ni Wei Watts SW. 5-Hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter. Clin Exp Pharmacol Physiol 33: 575–583, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Nordeng H, Lindemann R, Perminov KV, Reikvam A. Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr 90: 288–291, 2001 [PubMed] [Google Scholar]
- 47. Oka M, Homma N, Tarasevicience-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Pirraglia PA, Stafford RS, Singer DE. Trends in prescribing of selective serotonin reuptake inhibitors and other newer antidepressant agents in adult primary care. Prim Care Companion J Clin Psychiatry 5: 153–157, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rampano J, Proud S, Hackett LP, Kristensen JH, Ilett KF. A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int J Neuropsychopharmcol 7: 329–334, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Rondelet B, Van Beneden R, Kerbaul F, Motte S, Fesler P, McEntee K, Brimioulle S, Ketelslegers JM, Naeije R. Expression of the serotonin 1B receptor in experimental pulmonary hypertension. Eur Respir J 22: 408–412, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Rudolph AM, Paul MH. Pulmonary and systemic vascular response to continuous infusion of 5-hydroxytryptamine (serotonin) in the dog. Am J Physiol 189: 263–268, 1957 [DOI] [PubMed] [Google Scholar]
- 53. Shah SJ, Gomberg-Maitland M, Thenappan T, Rick S. Selective serotonin reuptake inhibitors and the incidence and outcome of pulmonary hypertension. Chest 136: 694–700, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Steer RA, School TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol 45: 1093–1099, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Wilson KL, Zelig CM, Harvey JP, Cummingham BS, Dolinsky BM, Napolitano PG. Persistent pulmonary hypertension is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol 28: 19–24, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Yeung SY, Millar JA, Mathie A. Inhibition of neuronal Kv potassium currents by the antidepressant drug, fluoxetine. Br J Pharmacol 128: 1609–1615, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]