Abstract
Previously we demonstrated that chronic hypoxia (CH) induces an inflammatory condition characterized by immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. It is well established that chronic inflammatory pain induces the expression of acid-sensitive ion channels (ASIC) in primary sensory neurons, where they contribute to hyperalgesia and allodynia. The present study examines the effect of CH on ASIC expression in petrosal ganglion (PG), which contains chemoafferent neurons that innervate oxygen-sensitive type I cells in the carotid body. Five isoforms of ASIC transcript were increased ∼1.5–2.5-fold in PG following exposure of rats to 1, 3, or 7 days of hypobaric hypoxia (380 Torr). ASIC transcript was not increased in the sympathetic superior cervical ganglion (SCG). In the PG, CH also increased the expression of channel-interacting PDZ domain protein, a scaffolding protein known to enhance the surface expression and the low pH-induced current density mediated by ASIC3. Western immunoblot analysis showed that CH elevated ASIC3 protein in PG, but not in SCG or the (sensory) nodose ganglion. ASIC3 transcript was likewise elevated in PG neurons cultured in the presence of inflammatory cytokines. Increased ASIC expression was blocked in CH rats concurrently treated with the nonsteroidal anti-inflammatory drug ibuprofen (4 mg·kg−1·day−1). Electrophysiological recording of carotid sinus nerve (CSN) activity in vitro showed that the specific ASIC antagonist A-317567 (100 μM) did not significantly alter hypoxia-evoked activity in normal preparations but blocked ∼50% of the hypoxic response following CH. Likewise, a high concentration of ibuprofen, which is known to block ASIC1a, reduced hypoxia-evoked CSN activity by ∼50% in CH preparations. Our findings indicate that CH induces inflammation-dependent phenotypic adjustments in chemoafferent neurons. Following CH, ASIC are important participants in chemotransmission between type I cells and chemoafferent nerve terminals, and these proton-gated channels appear to enhance chemoreceptor sensitivity.
Keywords: inflammation, protons, chemotransmission, sensory adaptation, oxygen sensing
multicellular organisms have evolved complex adaptive mechanisms that mitigate the adverse effects of diminished ambient O2. In mammals, exposure to acute hypoxia excites peripheral arterial chemoreceptors in the carotid body that reflexly elicit an immediate increase in lung ventilation. Chronic exposure to a hypoxic environment, on the other hand, initiates additional adaptive adjustments that further enhance the uptake and delivery of O2. Prominent among these is ventilatory acclimatization to hypoxia (VAH), a process in which ventilation gradually increases (31, 32). In rats, VAH is complete after ∼4–7 days exposure to steady-state hypoxia (31) and is correlated with a progressive increase in hypoxic sensitivity in the carotid body (32). Electrophysiological studies of carotid sinus nerve (CSN) impulse traffic show a graded increase in sensitivity between days 1 and 9 of chronic hypoxia (CH; at 380 Torr), resulting in an approximately twofold increase in the chemoreceptor response evoked by a standardized acute hypoxic stimulus (5).
It is widely accepted that O2 sensing in the carotid body occurs in specialized type I (glomus) cells that contain abundant mitochondria, a highly developed endoplasmic reticulum, and numerous clear-core and dense-core vesicles (11). Recent studies suggest that type I cells possess multiple O2-sensing mechanisms and that cell depolarization in response to hypoxia involves diverse subsets of K+ channels, some of which are gated by molecular O2 (15). Hypoxia evokes the release of multiple neurotransmitter agents from type I cells, which act upon the terminals of primary sensory neurons whose cell bodies reside in the petrosal ganglion (PG) of the IXth cranial nerve (28).
Multiple studies have demonstrated that type I cells are highly adaptable to a low-O2 environment. Hypoxia elevates the level of the transcription factor hypoxia inducible factor-1 (HIF-1), as well as selected hypoxia-sensitive genes including endothelin-1, VEGF, and tyrosine hydroxylase (TH), the enzyme that controls the rate of catecholamine synthesis in type I cells (6, 8, 22, 49). In adult rabbit type I cells, CH lowers the expression of a specific set of non-O2-sensitive voltage-gated K+ channels (KV 3.4), consistent with increased cell excitability via O2-sensitive K+ channels (21). Moreover, rat type I cells have been shown to increase Na+ channel (NaV 1.1) expression in CH, an adaptation that may contribute to enhanced cell depolarization and neurotransmitter release (3). Finally, in the normoxic carotid body, it is known that nitric oxide and superoxide anion modulate chemoreceptor excitability; consequently the finding that upregulation of nitric oxide synthase and NADPH oxidase occurs during CH suggests that the degree of hyperexcitability may be closely regulated (17, 19, 33).
Recent studies in our laboratory have documented that CH elicits an inflammatory condition in the rat carotid body, and that concurrent treatment with common anti-inflammatory drugs prevents inflammation as well as chemoreceptor adaptation (25). Chemoreceptor sensitivity is commonly evaluated by electrophysiological recording of afferent impulse activity in the CSN. The increased responsiveness following CH is thought to be related, at least in part, to well-documented functional changes in type I cells. But it has largely remained untested whether CH also induces adaptive changes in chemoafferent nerve terminals that could also contribute to this hyperexcitability. In another sensory system, namely nociception, numerous studies of chronic pain have shown that inflammation induces multiple changes in nociceptor afferents that result in neuronal hypersensitivity (53). For one, chronic inflammatory pain elevates the expression of acid-sensitive ion channels (ASIC) in dorsal root ganglia (DRG), an effect that is blocked by concurrent treatment with anti-inflammatory drugs (26, 50). Multiple isoforms of these proton-gated channels have been described, and they are known to form homomeric and heteromeric Na+-conducting channels in primary sensory neurons (23). Moreover, a recent study in mice has implicated ASIC in the mediation of ischemic pain in muscle (24), and an earlier study showed that ASIC3 gene deletion prevents hyperalgesia following repeated injections of acid in muscle (42).
In the present study we have evaluated the hypothesis that CH-induced hyperexcitability involves the expression of ASIC in chemoafferent neurons. We have used quantitative real-time PCR (qPCR) and Western immunoblotting to examine expression of ASIC in PG following CH. We have also evaluated the effect of concurrent treatment with the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen on ASIC expression. Amplified RNA (aRNA) technology has been used to assess the effect of inflammatory cytokines on ASIC3 expression in cultured PG neurons. We have also compared the expression of ASIC in PG vs. sensory neurons in the nodose ganglion (NG) and postganglionic neurons in the superior cervical sympathetic ganglion (SCG).
The potential involvement of ASIC in chemotransmission between type I cells and chemoafferent nerve terminals has been examined by quantifying the effect of the specific ASIC antagonist A-317567 on CSN activity recorded in preparations superfused in vitro. Also, because high concentrations of selected NSAIDs have been shown to directly block ASIC1a-mediated membrane currents (50), separate experiments evaluated the effects of bath-applied ibuprofen vs. naproxen, on chemoreceptor activity.
Finally, previous studies have shown that peripheral nerve injury or inflammation elicits transient cytokine production and macrophage invasion in dorsal root ganglia (DRG) (36, 53). Thus we have also examined whether the inflammation in carotid body induced by CH (25) is likewise associated with cytokine upregulation in the PG, which contains the chemoafferent neuron cell bodies.
METHODS
Animals and exposure to chronic hypoxia and ibuprofen.
Thirty-eight rats exposed in a hypobaric chamber were housed in standard rodent cages with food and water. Pressures were reduced from ambient barometric pressure (BP) at the University of Utah (i.e., BP ∼630 Torr; 1,500 m) until a pressure equivalent to ∼5,500 m (380 Torr) was reached, and maintained for a selected period (up to 10 days). The chamber was opened every one or two days to replenish food and water and change litter. Nineteen control, normal animals were maintained outside the chamber in ambient conditions. Selected animals received ibuprofen (sodium salt; 4 mg·kg−1·day−1 ip; Sigma-Aldrich) dissolved in 0.9% NaCl at a concentration of 4.0 mg/ml. Control animals were injected with a similar volume of 0.9% NaCl. Animal protocols were approved by the University of Utah Institutional Animal Care and Use Committee.
Real-time qPCR.
As previously described (25), PG and SCG were harvested from rats anesthetized with a mixture of ketamine (10 mg/100 g) plus xylazine (0.9 mg/100 g). Tissues were rapidly excised, cleaned of surrounding connective tissue in cold modified Tyrode's solution, and then immediately frozen on Al-foil on dry ice. In accord with the kit instructions (RNAqueous-Micro, Ambion, Austin, TX), total RNA was extracted from homogenized tissue samples pooled from groups of five rats for each experiment. Following removal of contaminating DNA (DNase treatment), first-strand complementary DNA was synthesized from 1 μg total RNA (quantified with a NanoDrop ND-1000 spectrophotometer) with use of RETROscript (Ambion). Aliquots of cDNA corresponding to 2 ng of total RNA were introduced into a SYBR Green reaction mix (25 μl; Qiagen) containing “upstream” and “downstream” primers for a selected ASIC isoform (ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3), or a selected cytokine [monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6)]. All primer pairs were “blasted” against known rat gene sequences. qPCR was conducted in an MJ Research PTC-200 thermocycler. From each pooled group of cDNA, three to five PCR reactions were initiated at 95°C for 15 min followed by 40 cycles consisting of 30 s at 94°C, 30 s at 58°C, and 30 s at 72°C, with the final cycle extended to 5 min at 72°C. Product purity was evaluated by determination of the melting curve, after which samples were stabilized at 4°C. Sample comparisons were based on the relative standard curve method (47) and data are normalized to 18S rRNA expression. Amplifications of samples not treated with RETROscript were performed to exclude possible contamination with genomic DNA. The means of data obtained from normal animals or untreated cells are valued as 1.0 ± SE. Data from experimental groups are expressed as a factor of normal or untreated, ± SE.
Western blotting.
PG, NG, and SCG were rapidly removed from anesthetized rats and homogenized at 4°C in buffer consisting of 25 mM Tris·HCl, pH 7.5, 0.42 M NaCl, 1.5 mM MgCl2, 0.5 mM EDTA, 1 mM DTT, 25% sucrose, 1 mM Na3VO4, 5 μg/ml leupeptin, and 1 mm phenylmethylsulfonyl fluoride. Samples were vortexed and the lysates were centrifuged at 10,000 g for 15 min at 4°C. Protein concentration in supernatants was determined by the method of Bradford (2); 20 μg of protein for each sample was denatured at 95°C for 5 min in SDS buffer and then loaded onto 10% polyacrylamide gels for electrophoresis. Separated proteins were electroblotted to a polyvinylidene difluoride membrane (Millipore). Following treatment in 5% (wt/vol) nonfat milk and 0.1% Tween in TBS buffer for 1 h, the membrane was incubated overnight with ASIC3 primary antibody diluted 1:500 (Alpha Diagnostics, San Antonio, TX). In control experiments primary antibody was presorbed with ASIC3 synthetic peptide (Alpha Diagnostics). Immunolabeling was evaluated by using a WesternBreeze Chemiluminescent immunodetection kit (Invitrogen).
aRNA.
PG were dissected free of surrounding connective tissue and transferred to Ham's F-12 medium (Ca2+- and Mg2+-free) containing 0.2% collagenase and 0.2% trypsin. Each organ was cut into 6 to 12 pieces and incubated for 40 min in a CO2 incubator (5% CO2-95% air) at 36.5°C. Tissue fragments were rinsed (2 × 10 min, room temperature) in F-12 medium (Ca2+ and Mg2+ free), transferred to poly-l-lysine-coated glass coverslips, and triturated in a small volume of medium, plus 10% fetal calf serum and 5 μg/ml insulin. The coverslips containing dissociated sensory neurons were maintained in the CO2 incubator for 2 h, at which time medium was replaced. Selected coverslips were treated with media containing IL-1β (50 ng/ml), TNF-α (25 ng/ml), and IL-6 (50 ng/ml) (Sigma-Aldrich). Following 24 h incubation, small to medium-sized sensory neurons collected via a patch-clamp pipette were immersed in buffer provided in a PicoPure RNA isolation kit (Microgenomics/Arcturus), and total RNA was isolated according to kit directions. The RNA was further purified with RNeasy MinElute Cleanup kit (Qiagen). mRNA was amplified according to directions in the MessageBOOSTER cDNA synthesis kit (Epicentre Biotechnologies). In accord with kit manufacturer specifications, results assume equal amplification factors for polyA ASIC3 mRNA in normal vs. cytokine-treated sensory neurons.
Electrophysiological recording of CSN activity.
As has been described previously (5), the carotid bifurcations were excised from rats under ketamine-xylazine anesthesia and placed in a Lucite chamber containing 100% O2-equilibrated modified Tyrode solution at 0–4°C. Each carotid body along with its attached nerve was carefully dissected from the artery and cleaned of surrounding connective tissue. Preparations were then placed in a conventional flow chamber where the carotid body was continuously superfused (up to 4 h) with modified Tyrode solution maintained at 37°C and equilibrated with a selected gas mixture. The CSN was drawn up into the tip (∼100 μm ID) of a glass suction electrode for monopolar recording of chemoreceptor activity. Basal neural activity was established in superfusates maintained at Po2 = 450 Torr. The Po2 was lowered to 120 Torr in superfusates equilibrated with air to provide a moderately hypoxic stimulus. Neural activity was led to an alternating current-coupled preamplifier, filtered, and transferred to a window discriminator and a frequency-to-voltage converter. Signals were processed by an AD/DA converter for display of frequency histograms on a PC computer monitor. Data were expressed as impulses per second and analyzed by Student's t-test and ANOVA. Ibuprofen and naproxen (sodium salts; Sigma-Aldrich) were dissolved in water to a concentration of 50 mM and stored at −20°C. The thawed stock solutions were diluted in modified Tyrode solution to a final concentration of 500 μM. The specific ASIC antagonist, A-317567 (a gift from Dr. Alan Light, University of Utah Department of Anesthesiology), was dissolved in 5% DMSO-95% water plus a few microliters of 1 M HCl to a concentration of 10 mM, then quick frozen and stored at −20°C. Aliquots were thawed and diluted in Tyrode to a final concentration of 100 μM (24).
RESULTS
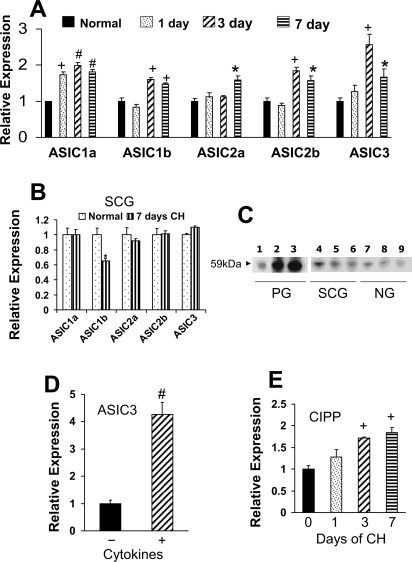
Figure 1A shows data from qPCR assays of ASIC gene expression in PG. Our experiments evaluated the effect of CH on the relative expression of five ASIC isoforms following 0, 1, 3, and 7 days at 380 Torr, equivalent to exposure at an altitude of ∼5,500 m (∼18,000 ft). Data show that following 1 day of CH only ASIC1a is elevated, and expression of this isoform remained increased throughout the 7-day CH exposure. ASICS1b, ASIC2b, and ASIC3 expression were increased following days 3 and 7, whereas ASIC2a was increased only after 7 days. Parallel studies of gene expression in the SCG (Fig. 1B), which contains sympathetic neurons, glial cells, and small intensely fluorescent (SIF) cells, indicate that 7 days of CH failed to increase ASIC; in fact, CH elicited a ∼40% decrease of ASIC1b in this tissue. Western blot comparisons shown in Fig. 1C demonstrate that following 3 and 7 days of CH ASIC3 protein expression is elevated in PG, but not in the SCG, nor in the NG, which contains primary sensory neurons. Data in Fig. 1D, obtained by using aRNA technology, quantify transcript in isolated neurons that were cultured for 24 h in the absence or presence of inflammatory cytokines. Results show that exposure to a cocktail containing IL-1β (50 ng/ml), TNF-α (25 ng/ml), and IL-6 (50 ng/ml) induces a 4.2-fold increase in expression of ASIC3 transcript. Finally, qPCR results in Fig. 1E show that, in PG, CH elevates expression of channel-interacting PDZ domain protein (CIPP), a scaffolding protein known to enhance the surface expression and the low pH-induced current density mediated by ASIC3 (1). Significant elevation of CIPP corresponds to the elevation of ASIC3 in PG on days 3 and 7 of CH.
Fig. 1.
Effect of chronic hypoxia (CH) and cytokines on acid-sensitive ion channel (ASIC) expression in rat petrosal (PG), nodose (NG), and superior cervical (SCG) ganglia. A: time course of expression for 5 ASIC isoforms. mRNA transcript levels are expressed relative to normal following 1, 3, and 7 days of CH at 380 Torr. B: ASIC expression in SCG is not affected by 7 days of CH, with the exception that ASIC1b is decreased by ∼40%. C: Western blot showing effect of CH on expression of ASIC3 in PG (blot exposed for 2 min to Kodak BioMax XAR film), SCG, and NG (blot exposed for 3 min). Lanes 1, 4, and 7: normal; lanes 2, 5, and 8: 3 days CH; lanes 3, 6, and 9: 7 days CH. D: effect of 24-h exposure to cytokine cocktail [TNF-α (25 ng/ml), IL-1β (50 ng/ml), and IL-6 (50 ng/ml)] on expression of ASIC3 transcript in cultured PG neurons. Fifteen small to medium neurons in each group were collected and processed for amplification of mRNA, followed by quantitative PCR. E: CH induces upregulation of channel-interacting PDZ domain protein (CIPP), a scaffolding protein for ASICs. *, +, and #P < 0.05, 0.01, and 0.001, respectively, vs. normal. Data are means ± SE.
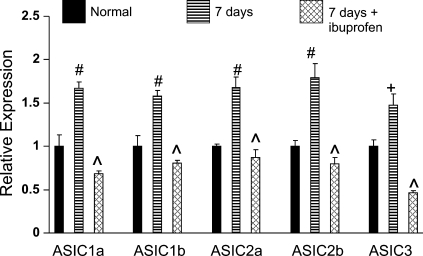
Figure 2 shows the effect of a common anti-inflammatory drug, ibuprofen, on the expression of ASIC isoforms in PG. In these experiments, comparisons were made between rats exposed to CH for 7 days, with or without concurrent daily injection of ibuprofen (4 mg·kg−1·day−1 ip). The data indicate that ibuprofen inhibits upregulation of all isoforms of ASIC in PG following 7 days of CH. In all cases the levels of ASIC were significantly less than expression measured in untreated CH animals (P < 0.01–0.001).
Fig. 2.
Effect of nonsteroidal anti-inflammatory drug ibuprofen on CH-induced ASIC expression in PG. mRNA transcript levels for 5 ASIC isoforms are expressed relative to normal (means ± SE). Rats were exposed to CH (380 Torr) for 7 days with or without ibuprofen (4 mg/kg ip). Normal (control) rats and 7-day CH rats were injected with saline. + and #P < 0.01 and 0.001 vs. normal; ^P < 0.001 vs. 7 days CH.
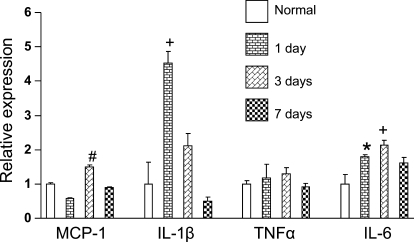
Data in Fig. 3 quantify inflammatory cytokine transcript expression in PG following 0, 1, 3, and 7 days at 380 Torr. One day of CH elicits a 4.5-fold and a 1.8-fold increase in IL-1β and IL-6, respectively. Levels of IL-1β are partially recovered at 3 days and fully recovered following 7 days of CH. IL-6 remains elevated on day 3 and is partially recovered following 7 days of hypoxia. TNF-α levels remained constant during CH, and MCP-1 was elevated on day 3 (∼1.5-fold) and then recovered after 7 days of CH.
Fig. 3.
Effect of CH on expression of inflammatory cytokines in rat PG. Tissues were harvested from rats following 0 (i.e., normal), 1, 3, or 7 days at 380 Torr. mRNA transcript levels determined in quantitative PCR assays are expressed relative to normal (means ± SE) for monocyte chemoattractant protein-1 (MCP-1), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), respectively. *, +, and #P < 0.05, 0.01, and 0.001, respectively, vs. normal.
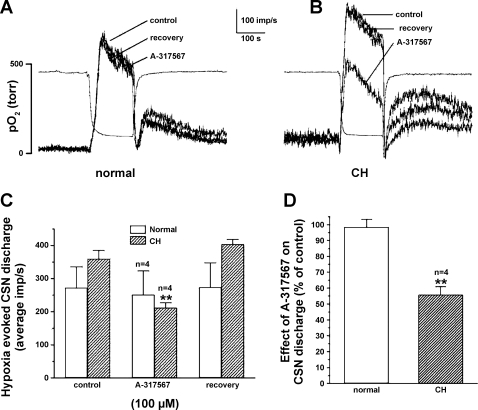
Figure 4 demonstrates the effects of the specific ASIC antagonist A-317567 on CSN activity recorded in preparations superfused in vitro. This drug blocks both the transient and sustained ASIC currents, unlike amiloride, which reduces only the transient current (10). Each preparation was exposed to three standardized hypoxic challenges consisting of an initial control response, followed by hypoxia in the presence of 100 μM A-317567, and finally, a hypoxic challenge after a 20-min washout of the drug. The drug was introduced 2.5 min prior to the second hypoxic challenge. Figure 4A shows results (superimposed traces of integrated CSN activity) from a typical normal preparation in which A-317567 did not alter either the basal or the hypoxia-evoked nerve activity. Basal activity was likewise not altered by the antagonist following 10 days of CH, but the much larger evoked activity shown in Fig. 4B was substantially blocked by the drug. Summary data (Fig. 4, C and D) indicate that the ASIC antagonist was minimally effective in normal preparations (n = 4) but reduced the hypoxia-evoked discharge by 47% in preparations from CH animals (P < 0.01; n = 4).
Fig. 4.
Effect of ASIC antagonist A-317567 (100 μM) on basal and hypoxia-evoked carotid sinus nerve (CSN) activity. A: integrated CSN activity recorded in vitro in a superfused normal rat carotid body/CSN preparation. Three superimposed traces show a control response to a standardized hypoxic stimulus, followed by a response in the presence of A-317567, and finally a response following a 20-min wash. The drug was introduced 2.5 min prior to the 2nd hypoxic challenge. Separate trace shows bath Po2. B: in a preparation from a 9-day CH rat hypoxia-evoked CSN activity is enhanced; A-317567 causes a large reduction in the hypoxic response but has no effect on basal activity. C: data (means ± SE) from 4 normal and 4 CH preparations show that A-317567 caused an insignificant change in normal preparations, but following 9 or 10 days of CH in 4 animals the drug reduced the hypoxia evoked activity by ∼44%. **P < 0.01 vs. control response in CH preparations. D: summary data expressed as a percent of control responses for normal vs. CH preparations show that effect of A-317567 is significantly larger following CH; **P < 0.01 vs. normal.
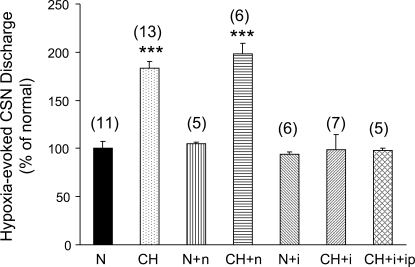
Separate experiments examined the effect of high concentrations of ibuprofen vs. naproxen on hypoxia-evoked CSN activity recorded in vitro. We also evaluated the effects of bath-applied ibuprofen in CH carotid bodies harvested from rats concurrently treated with ibuprofen at a dose (4 mg·kg−1·day−1 ip) that blocks chemoreceptor adaptation (25) and CH-induction of ASIC in PG. Superfused carotid body/CSN preparations were exposed to hypoxia and drugs in accord with the procedures described above for testing the effects of A-317567. Data in Fig. 5, expressed as percent of hypoxia-evoked impulses per second (minus basal nerve activity) in control trials, show that naproxen (500 μM) did not alter the hypoxic response in normal or CH preparations. Ibuprofen (500 μM) was likewise ineffective in normal preparations, but this drug inhibited ∼50% of the hypoxic CSN activity following CH. In preparations from CH animals concurrently treated with ibuprofen, bath application of ibuprofen had no effect on activity evoked by the acute hypoxic challenge. Neither ibuprofen nor naproxen significantly altered the basal discharge rate in CSN.
Fig. 5.
Effect of high concentrations of bath-applied nonsteroidal anti-inflammatory drugs on hypoxia-evoked CSN activity. Data are expressed as percent of evoked activity (168.4 ± 12.1 impulses/s; means ± SE) in normal (N) preparations. CH, 380 Torr for 8–10 days; n, naproxen (500 μM); i, ibuprofen (500 μM); ip: ibuprofen injected intraperitoneally (4 mg·kg−1·day−1 during CH). Numbers in parentheses = number of preparations. ***P < 0.001 vs. normal.
DISCUSSION
The present study shows that CH upregulates mRNA for multiple ASIC isoforms in PG, a cranial nerve sensory ganglion, which like the DRG contains primary sensory neurons subserving multiple sensory modalities. Moreover, the data indicate that exposure to inflammatory cytokines in vitro, or hypoxia in vivo, elevates levels of ASIC3 transcript and/or protein, respectively, in PG neurons. CH also increased transcript expression of CIPP, a scaffolding protein known to enhance ASIC3 localization in cell membranes, and increase currents evoked by low pH (1). Importantly, a putative link between increased ASIC expression and inflammation is indicated by the observation that concurrent treatment with a low dose of ibuprofen blocked CH-induced ASIC expression. The finding that bath application of the specific ASIC channel antagonists A-317576 or ibuprofen reduces the excess chemoreceptor response to hypoxia is consistent with the hypothesis that ASIC participate in chemotransmission between type I cells and chemoafferent neurons following CH.
In the carotid body, CH elicits immune cell invasion and a robust elevation of inflammatory cytokine expression including a two- to threefold increase in transcripts for IL-1β, IL-6, and TNF-α within the first 1–3 days of exposure (25). Importantly, signs of inflammation in CH were long lasting, as evidenced by a 5.5-fold increase in IL-6 expression following 28 days of CH (25). In view of the fact that chemoafferent PG neurons terminate within this site of robust inflammation, the observed elevation of ASIC in PG is consistent with the study of Voilley et al. (50), who showed that hind paw injection of complete Freund's adjuvant elicited similarly increased ASIC expression in L4 and L5 DRG neurons. These authors also demonstrated that with the exception of ASIC2a, all isoforms of ASIC are upregulated in DRG 2 days following injection of Freund's adjuvant into rat hind paw. Our time course data indicate that ASIC2a transcript is likewise not elevated following 1 or 3 days of CH but that this isoform is expressed at ∼1.5-fold above normal after 7 days of hypoxia. Similar to the findings of Voilley et al., our data show that CH induces early increases (following 1 or 3 days of CH) of the other four ASIC isoforms. Moreover, the robust 2.5-fold increase in ASIC3 following 3 days of hypoxia is consistent with the fact that this is the most abundant isoform in primary sensory DRG neurons (51). Voilley et al. likewise showed that a dose of ibuprofen, equal to the dose used in our study, blocked ASIC expression induced by Freund's adjuvant (50). It was also reported that ibuprofen, as well as other NSAIDs, did not alter ASIC expression in normal sensory neurons. Ibuprofen is a well established inhibitor of cyclooxygenases (COX1 and COX2), resulting in lowered synthesis of inflammatory prostanoids (34). In addition, ibuprofen inhibits the nuclear translocation of the transcription factor, NF-κB, which mediates production of inflammatory cytokines (37). In the CH carotid body, ibuprofen treatment significantly lowered but did not completely prevent increased expression of inflammatory cytokines (25). Thus the observation that ibuprofen completely blocks CH-induced expression of ASIC may indicate a COX/prostanoid-mediated mechanism of ASIC gene expression. Finally, Voilley et al. reported that neurons expressing increased ASIC transcript were relatively small and they also expressed substance P and/or binding sites for isolectin B4, markers of C-fiber sensory neurons (20, 41). Although our study did not identify the sensory modality of PG neurons expressing ASIC, the data are nonetheless consistent with the notion that peripheral inflammation induces phenotypic adjustments in chemoafferent neurons. Moreover, the observation that A-317567 and ibuprofen partially block the chemoreceptor discharge in CH, but not in normal preparations, further implicates ASIC in chemoafferent neuron adaptation.
It is well established that acute hypoxia elicits a global inflammatory response in the nervous system (4), which might be expected to upregulate ASIC in numerous subpopulations of neurons. However, in rats exposed to hypoxia for 6 days, widespread inflammation recovers (56), a finding consistent with our observation that increased ASIC expression is not present in SCG, nor in NG following 7 days of CH. To our knowledge, ASIC have not been reported in postganglionic sympathetic neurons in the SCG, and the low levels of ASIC we observed in this structure may be expressed by SIF cells in the ganglion. SIF cells are functionally and morphologically similar to carotid body type I cells, which do express ASIC (7, 43, 46). ASIC3 expression has been previously reported in normal NG, as well as PG (13). Thus our observation that CH upregulates ASIC only in PG is consistent with the hypothesis that carotid body inflammation mediates adaptive changes in chemoafferent neurons.
Attempts to localize ASIC in rat carotid body using immunocytochemical techniques have shown that normal type I cells express ASIC1, ASIC2, and ASIC3. It was also reported that “intercluster spaces” were devoid of staining, suggesting that ASIC are not present in nerve fibers and terminals (46). However, these latter results cannot be considered conclusive because the exquisitely slender nerve fibers and terminals that form synapses are not distinct from type I cells in immunofluorescence preparations. Indeed, fluorescent immunocytochemical studies routinely demonstrate the presence of TH in type I cells, whereas TH-positive nerve fibers are not detected (e.g., see Ref. 25). Yet TH is present in chemoafferent nerve terminals, a phenomenon that has been demonstrated by employing immunoelectron microscopy (12). Moreover, ASIC protein levels may be minimal within nerve fibers and terminals in normal carotid body, a notion consistent with our finding that bath-applied A-317567 and ibuprofen inhibit hypoxia-evoked CSN activity in CH animals, but not in normal preparations.
Our data demonstrate for the first time that CH elicits an inflammatory condition in the PG of the IXth cranial nerve. The time course of cytokine transcript upregulation in the PG overlaps with inflammation induced in the carotid body by CH (25). A recent study has demonstrated that inflammation of peripheral nerve terminals induces a transient upregulation of IL-1β and IL-6 proteins in DRG (36). Moreover, experimentally induced inflammation in trigeminal nerve terminals likewise results in increased expression of IL-1β, as well as activation of satellite glial cells in trigeminal ganglion (45).
Our finding that culturing PG neurons in a cytokine cocktail increases ASIC3 transcript is in agreement with Mamet et al. (26), who demonstrated that exposure to IL-1 increases ASIC expression and elevates the depolarizing current evoked by low pH in cultured DRG sensory neurons. The mixture of cytokines used in our study mimics the pattern of upregulation in the carotid body, where on days 1 and 3 of CH, IL-1β, IL-6, and TNF-α are elevated (25). Increased ASIC3 expression in cytokine-treated PG neurons is consistent with the expression of specific cytokine receptors on sensory neurons (55). In addition to IL-1β, which has been shown to upregulate 4 ASIC isoforms in DRG neurons, candidate signaling molecules capable of inducing ASIC include serotonin (5-HT) (26), which is present in type I cells, along with 5-HT type 3 receptors on chemoafferent neurons (29, 52). Moreover, multiple studies indicate that retrograde signaling from inflamed terminal fields alters gene expression and phenotype of primary sensory neurons (9, 27, 30).
A role for ASIC in chemotransmission between type I cells and chemoafferent PG neurons is strongly suggested by the finding that a specific ASIC antagonist blocks ∼50% of CSN activity evoked by an acute hypoxic challenge exclusively in CH preparations. The A-317567 concentration (100 μM) used in our experiments is 3.4- to 50-fold higher than the known IC50 values for blocking low pH-evoked ASIC1-like (IC50 = 2.0 μM), ASIC2-like (IC50 = 29.1 μM), and ASIC3-like (IC50 = 9.5 μM) currents in rat DRG neurons (10), suggesting that completely blocking ASIC eliminates ∼50% of chemotransmission following CH. In addition, ∼50% of hypoxia-evoked activity was blocked by high concentrations (500 μM) of ibuprofen applied in vitro to carotid bodies following CH, whereas an equivalent concentration of naproxen was ineffective. Moreover, treatment of rats with a low dose (4 mg·kg−1·day−1) of ibuprofen during CH prevented hypersensitivity and rendered the high concentration ineffective against CSN activity evoked by acute hypoxia in vitro. These latter findings are consistent with a previous study that showed that elevated levels of bath-applied ibuprofen, but not naproxen, can directly block ASIC1a channels (50). However, functioning ASIC may comprise heteromultimeric complexes (54); thus our findings with ibuprofen do not eliminate the possible participation of other isoforms in chemoafferent plasticity. The effect of ASIC antagonists applied to carotid body could be partially mediated by ASIC channels on type I cells, where they are known to conduct a depolarizing current evoked by low pH (46). The effect of CH on expression of ASIC on type I cells is unknown. However, the specific ASIC antagonists, ibuprofen and A-317567, became effective inhibitors of hypoxia-evoked chemoreceptor activity following increased ASIC expression in PG.
The ability of ibuprofen to directly block ASIC1a and chemoreceptor activity suggests the possible involvement of a non-anti-inflammatory mechanism in blocking CH-induced ASIC expression and chemoreceptor adaptation. However, microdialysis sampling in humans receiving moderate to high doses of ibuprofen show that drug concentrations in the extracellular spaces of subcutaneous fat and muscle are some two orders of magnitude lower than concentrations in blood plasma (∼100 μM) (48). Similarly, in the rat air pouch model of inflammation, ibuprofen concentration in exudate did not exceed 5 μM following a bolus intravenous dose of 20 mg/kg (44). Rats in our studies of ASIC expression received relatively low doses of ibuprofen (4 mg/kg ip), which would likely result in even lower extracellular drug concentrations. On the other hand, the concentration of ibuprofen required to demonstrate direct effects on ASIC was 500 μM (50).
Evidence obtained within the last decade indicates that normal chemotransmission between rat type I cells and chemoafferent nerve terminals involves both cholinergic and purinergic receptors (28). In a recent study we showed that a common cholinergic antagonist, mecamylamine, blocks ∼80% of CSN activity evoked by hypoxia in normal carotid body, but even higher concentrations of this drug are ineffective following CH (18). In contrast, purinergic P2 receptor antagonists block more than 50% of the hypoxia evoked response in both normal and CH preparations (16). Indications for cotransmission involving protons and ATP following CH resemble findings from recent studies of muscle metaboreceptor and nociceptor afferent neurons, where maximal responses are obtained by combining high concentrations of protons, ATP, and lactate (24). Importantly, the use of selective antagonists suggested involvement of ASIC, TRPV1 channels, and P2 receptors in responses to physiological concentrations of these stimulants. Given that the present data indicate a role for protons in carotid body chemotransmission following CH, it is noteworthy that the contents of dense-cored catecholaminergic synaptic vesicles in PC12 cells are known to maintain an internal pH of 5.0–5.5 (38). These vesicles, which are abundant in type I cells, are the source of dopamine, which is released from type I cells during hypoxia (14). Finally, although we are unaware of any studies of lactate production in carotid body, it is known that in other oxygen-sensitive tissues hypoxia upregulates the expression of anaerobic glycolytic enzymes that are controlled by the transcription factor HIF-1 (40), a known regulator of type I cell plasticity in hypoxia (35, 39).
In summary, CH upregulates ASIC expression in PG, a phenomenon that is blocked by concurrent treatment with a dose of ibuprofen known to prevent CH-induced inflammation and chemoreceptor adaptation in carotid body. Moreover, in vitro application of a specific ASIC channel antagonist or a high concentration of ibuprofen reduces hypoxia-evoked CSN activity to normal levels following CH. In contrast, application of these drugs to normal preparations does not affect chemoreceptor activity. Our findings are consistent with a model of hypoxic chemotransmission following CH that involves multiple neurotransmitter agents, including protons acting on ASIC expressed on chemoafferent nerve terminals and type I cells.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-086508.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Anzai N, Deval E, Schaefer L, Friend V, Lazdunski M, Lingueglia E. The multivalent PDZ domain-containing protein CIPP is a partner of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 277: 16655–16661, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 3. Caceres AI, Obeso A, Gonzalez C, Rocher A. Molecular identification and functional role of voltage-gated sodium channels in rat carotid body chemoreceptor cells. Regulation of expression by chronic hypoxia in vivo. J Neurochem 102: 231–245, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Chao J, Wood JG, Blanco VG, Gonzalez NC. The systemic inflammation of alveolar hypoxia is initiated by alveolar macrophage-borne mediator(s). Am J Respir Cell Mol Biol 41: 573–582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in physiological adaptation of the carotid body during chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Czyzyk-Krzeska M, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem 58: 1538–1546, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Dalmaz Y, Borghini N, Pequignot JM, Peyrin L. Involvement of dopaminergic SIF cells of rat superior cervical ganglion in response to chemoreceptor stimuli. In: Arterial Chemoreception, edited by Eyzaguirre C, Fidone SJ, Fitzgerald RS, Lahiri S, McDonald D, New York: Springer-Verlag, 1990, p. 404–418. [Google Scholar]
- 8. Dinger B, He L, Chen J, Stensaas L, Fidone S. Mechanisms of morphological and functional plasticity in the chronically hypoxic carotid body. In: Oxygen Sensing: Responses and Adaptation to Hypoxia, edited by Lahiri S, Semenza G, Prabhakar N. New York: Dekker, 2003, p. 439–465. [Google Scholar]
- 9. Djouhri L, Dawbarn D, Robertson A, Newton R, Lawson SN. Time course and nerve growth factor dependence of inflammation-induced alterations in electrophysiological membrane properties in nociceptive primary afferent neurons. J Neurosci 21: 8722–8733, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117: 88–96, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fidone SJ, Gonzalez C. Initiation and control of chemoreceptor activity in the carotid body. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda: Am. Physiol. Soc., 1986, sect. 3, Vol. II, pt. 1, chapt. 9, p. 247–312 [Google Scholar]
- 12. Finley JCW, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: Dopaminergic and peptidergic phenotypes. Neuroscience 51: 973–987, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Fukuda T, Ichikawa H, Terayama R, Yamaai T, Kuboki T, Sugimoto T. ASIC3-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain Res 1081: 150–155, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez C, Vaquero LM, Lopez-Lopez JR, Perez-Garcia MT. Oxygen-sensitive potassium channels in chemoreceptor cell physiology: making a virtue of necessity. Ann NY Acad Sci 1177: 82–88, 2009 [DOI] [PubMed] [Google Scholar]
- 16. He L, Chen J, Dinger B, Stensaas L, Fidone S. Effect of chronic hypoxia on purinergic synaptic transmission in rat carotid body. J Appl Physiol 100: 157–162, 2006 [DOI] [PubMed] [Google Scholar]
- 17. He L, Chen J, Liu X, Dinger B, Fidone S. Enhanced nitric oxide-mediated chemoreceptor inhibition and altered cyclic GMP signaling in rat carotid body following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 293: L1463–L1468, 2007 [DOI] [PubMed] [Google Scholar]
- 18. He L, Dinger B, Fidone S. Effect of chronic hypoxia on cholinergic chemotransmission in rat carotid body. J Appl Physiol 98: 614–619, 2005 [DOI] [PubMed] [Google Scholar]
- 19. He L, Liu X, Chen J, Dinger B, Stensaas L, Fidone S. Modulation of chronic hypoxia-induced chemoreceptor hypersensitivity by NADPH oxidase subunits in rat carotid body. J Appl Physiol 108: 1304–1310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jessell TM. Substance P in nociceptive sensory neurons. Ciba Found Symp 225–248, 1982 [DOI] [PubMed] [Google Scholar]
- 21. Kaab S, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. Down regulation of Kv34 channels by chronic hypoxia increases acute oxygen sensitivity in rabbit carotid body. J Physiol 566: 395–408, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 99: 821–826, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, He L, Stensaas L, Dinger B, Fidone S. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am J Physiol Lung Cell Mol Physiol 296: L158–L166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mousa SA, Cheppudira BP, Shaqura M, Fischer O, Hofmann J, Hellweg R, Schafer M. Nerve growth factor governs the enhanced ability of opioids to suppress inflammatory pain. Brain 130: 502–513, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol 95: 657–667, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Phenotypic inflammation switch in rats shown by calcitonin gene-related peptide immunoreactive dorsal root ganglion neurons innervating the lumbar facet joints. Spine (Phila Pa 1976) 26: 1009–1013, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Olson EB, Jr, Dempsey JA. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J Appl Physiol 44: 763–769, 1978 [DOI] [PubMed] [Google Scholar]
- 32. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Prabhakar N, Rao S, Premkumar D, Pieramici SF, Kumar GK, Kalari RK. Regulation of neuronal nitric oxide synthase gene expression by hypoxia: role of nitric oxide in respiratory adaptation to low pO2. Adv Exp Med Biol 410: 345–348, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Rainsford KD. Discovery, mechanisms of action and safety of ibuprofen. Int J Clin Pract Suppl 135: 3–8, 2003 [PubMed] [Google Scholar]
- 35. Roy A, Baby SM, Wilson DF, Lahiri S. Rat carotid body chemosensory discharge and glomus cell HIF-1α expression in vitro: regulation by a common oxygen sensor. Am J Physiol Regul Integr Comp Physiol 293: R829–R836, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Saab CY, Shamaa F, El Sabban ME, Safieh-Garabedian B, Jabbur SJ, Saade NE. Transient increase in cytokines and nerve growth factor in the rat dorsal root ganglia after nerve lesion and peripheral inflammation. J Neuroimmunol 208: 94–103, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Scheuren N, Bang H, Munster T, Brune K, Pahl A. Modulation of transcription factor NF-kappaB by enantiomers of the nonsteroidal drug ibuprofen. Br J Pharmacol 123: 645–652, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schoonderwoert VT, Martens GJ. Proton pumping in the secretory pathway. J Membr Biol 182: 159–169, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol 96: 1173–1177, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Silverman JD, Kruger L. Lectin and neuropeptide labeling of separate populations of dorsal root ganglion neurons and associated “nociceptor” thin axons in rat testis and cornea whole-mount preparations. Somatosens Res 5: 259–267, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Stea A, Alexander SA, Nurse CA. Effects of pHi and pHe on membrane currents recorded with the perforated-patch method from cultured chemoreceptors of the rat carotid body. Brain Res 567: 83–90, 1991 [DOI] [PubMed] [Google Scholar]
- 44. Stevens AJ, Martin SW, Brennan BS, Rowland M, Houston JB. Experimental determination of a drug targeting index for S(+)ibuprofen using the rat air pouch model of inflammation. J Drug Target 2: 333–339, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev 33: 784–792, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res 101: 1009–1019, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 45: 397–407, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Tegeder I, Muth-Selbach U, Lotsch J, Rusing G, Oelkers R, Brune K, Meller S, Kelm GR, Sorgel F, Geisslinger G. Application of microdialysis for the determination of muscle and subcutaneous tissue concentrations after oral and topical ibuprofen administration. Clin Pharmacol Ther 65: 357–368, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Tipoe GL, Fung ML. Expression of HIF-1alpha, VEGF and VEGF receptors in the carotid body of chronically hypoxic rat. Respir Physiol Neurobiol 138: 143–154, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann NY Acad Sci 868: 67–76, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Wang ZY, Bisgard GE. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech 59: 168–177, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 82: 981–1011, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 55. White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4: 834–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wood JG, Mattioli LF, Gonzalez NC. Hypoxia causes leukocyte adherence to mesenteric venules in nonacclimatized, but not in acclimatized, rats. J Appl Physiol 87: 873–881, 1999 [DOI] [PubMed] [Google Scholar]