Abstract
The epithelial and endothelial cells lining the alveolus form a barrier essential for the preservation of the lung respiratory function, which is, however, vulnerable to excessive oxidative, inflammatory, and apoptotic insults. Whereas profound breaches in this barrier function cause pulmonary edema, more subtle changes may contribute to inflammation. The mechanisms by which cigarette smoke (CS) exposure induce lung inflammation are not fully understood, but an early alteration in the epithelial barrier function has been documented. We sought to investigate the occurrence and mechanisms by which soluble components of mainstream CS disrupt the lung endothelial cell barrier function. Using cultured primary rat microvascular cell monolayers, we report that CS induces endothelial cell barrier disruption in a dose- and time-dependent manner of similar magnitude to that of the epithelial cell barrier. CS exposure triggered a mechanism of neutral sphingomyelinase-mediated ceramide upregulation and p38 MAPK and JNK activation that were oxidative stress dependent and that, along with Rho kinase activation, mediated the endothelial barrier dysfunction. The morphological changes in endothelial cell monolayers induced by CS included actin cytoskeletal rearrangement, junctional protein zonula occludens-1 loss, and intercellular gap formation, which were abolished by the glutathione modulator N-acetylcysteine and ameliorated by neutral sphingomyelinase inhibition. The direct application of ceramide recapitulated the effects of CS, by disrupting both endothelial and epithelial cells barrier, by a mechanism that was redox and apoptosis independent and required Rho kinase activation. Furthermore, ceramide induced dose-dependent alterations of alveolar microcirculatory barrier in vivo, measured by two-photon excitation microscopy in the intact rat. In conclusion, soluble components of CS have direct endothelial barrier-disruptive effects that could be ameliorated by glutathione modulators or by inhibitors of neutral sphingomyelinase, p38 MAPK, JNK, and Rho kinase. Amelioration of endothelial permeability may alleviate lung and systemic vascular dysfunction associated with smoking-related chronic obstructive lung diseases.
Keywords: antioxidants, sphingolipids, cell death, inflammation, cytoskeleton
cigarette smoking (CS) is the most common etiological factor of chronic obstructive pulmonary disease (COPD), a highly prevalent and morbid disease that includes pulmonary emphysema and chronic bronchitis. In susceptible individuals, CS induces inflammation of the airways together with enlargement of peripheral airspaces attributable to destruction of alveolar walls. In contrast to the enlargement of alveolar spaces, which is a late manifestation of emphysema (9), the earliest pathological changes induced by CS in the lung include a breach in the airway epithelial barrier (5, 49), along with indices of oxidative stress and apoptosis (8, 27, 33, 53). However, CS and its highly diffusible oxidative free radicals may also directly injure endothelial cells (26) and alter their barrier function, which could fuel an inflammatory response to contribute to the development of emphysema. We sought to identify whether soluble components of mainstream CS directly affect endothelial barrier function and to determine the mechanism by which this occurs.
Previous reports have documented that CS induces epithelial cell permeability in vitro in A549 (23) and Calu-9 cell lines or primary cultured human bronchial epithelial cells (42, 43), as well as in vivo in guinea pigs (6) and rats (23, 31). Oxidative stress and active cytoskeletal rearrangement were critical for the development of epithelial barrier disruption induced by CS (24, 46). Whereas breaches of the epithelial barrier may induce wound-repair inflammatory responses, disruption of the endothelial barrier may directly increase the access of circulating proteins, plasma, and inflammatory cells to the interstitium and alveolar spaces. Together, these processes may synergize to promote inflammation in lung parenchyma. The mechanisms that disrupt the endothelial barrier typically engage specific signaling pathways that either weaken intercellular junctions or cell-matrix tethering structures, or increase the contractility of the actin-myosin cytoskeleton (13). These signaling pathways involve components of the Rho family of GTPases and mitogen-activated protein kinases (MAPK) that alter the phosphorylation of key cytoskeletal or junctional proteins to eventually increase endothelial cell permeability (11, 19). In addition to protein signaling, bioactive sphingolipids, such as ceramide or sphingosine-1 phosphate, may modulate endothelial cell responses, including barrier function (21, 50). Of note, increased ceramide has been shown to augment endothelial cell permeability, both when applied directly to endothelial cells in cultures (25) and as a mediator of platelet-activating factor-induced pulmonary edema, in a pump-perfused mouse lung preparation (16, 21). We previously reported a significant increase in ceramide in the lungs of patients with emphysema and in response to experimental CS exposure (36, 37). Furthermore, we have shown that ceramide is a key mediator of alveolar cell apoptosis and airspace enlargement in a model of murine emphysema (37) and that the upregulated ceramides trigger oxidative stress-dependent apoptosis in the lung (36, 37). The role of ceramide as a mediator of the acute effect of CS on endothelial cell barrier function is unknown. Stimuli such as CS may elevate ceramide levels by acid or neutral sphingomyelinase (SMase)-catalyzed sphingomyelin hydrolysis, via de novo synthesis, or by recycling it from sphingosine or other metabolites (30, 47).
Using primary murine or human lung endothelial cells cultured on microelectrodes of an electrical cell impedance sensor (ECIS), we investigated here whether the upregulation of ceramide is involved in the acute effects of CS on the lung endothelium. We complemented these studies with intravital assessment of the effect of ceramide on the alveolar microcirculation in vivo in intact rats, using two-photon excitation microscopy. We report the novel finding that soluble components of CS increase the permeability of the endothelial cell barrier via redox-dependent activation of a complex network of signaling molecules that include ceramides, caspases, Rho kinase, and p38 MAPK, which interact to ultimately cause a loss of intercellular tight junctions.
MATERIALS AND METHODS
Reagents.
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise stated. Ceramides with C16:0 fatty acyl chain (conjugated with polyethylene glycol 2000, to allow solubilization; 10–20 μM) or C6:0 fatty acyl chain (20 μM) were purchased from Avanti Polar Lipids (Alabaster, AL).
Cells.
Primary rat lung microvascular cells (RLMEC) were kindly provided by Dr. Troy Stevens (University of South Alabama) and maintained in DMEM-high glucose supplemented with 10% FBS and 1% penicillin-streptomycin. Human primary lung microvascular endothelial cells and their culture media EBM2-MV and bullet kit were from Lonza (Walkersville, MD). Primary rat lung epithelial cells L2 were purchased from ATCC and maintained in Hams-F12 media supplemented with 10% FBS and 1% penicillin-streptomycin. Primary mouse lung endothelial cells (MLEC) were a kind gift from Dr. Patty Lee (Yale University) and maintained in DMEM-high glucose supplemented with 20% FBS and 1% penicillin-streptomycin.
CS extract.
Filtered research-grade cigarettes (1R3F) from the Kentucky Tobacco Research and Development Center (University of Kentucky, Lexington, KY) were used for preparing aqueous CS extract. CS (100%) was prepared by bubbling smoke from two cigarettes into 20 ml of PBS at a rate of 1 cigarette/min to 0.5 cm above the filter (7), followed by pH adjustment to 7.4 and 0.2-μm filtration. A similar procedure was followed for air control extract (AC) preparation, bubbling ambient air instead of smoke, followed by a similar preparation as for CS extract. Treatments were performed with CS extract concentrations ranging from 1–10% (vol:vol), as indicated.
TER measurements.
Electrical resistance across cell monolayers was measured using ECIS (Applied Biophysics, Troy, NY), as previously described (38). Cells were cultured on gold microelectrodes, and the total resistance was measured in real time across monolayers. Experiments were conducted on confluent cells monolayers when transcellular electrical resistance (TER) achieved a steady state. TER values (Ohm) for each time point were normalized to the initial TER value (at the beginning of the recording) and plotted as normalized TER. Only wells with an initial TER >5,000 Ohm were utilized.
Ceramide determination.
Following treatments, culture media was washed off, and cells were collected by scraping, followed by lipid extraction utilizing a modified Bligh and Dyer method (3). Total lipid phosphorus (Pi) content of each lipid extract was measured by Pi labeling with NH4-molybdate (10). Sphingolipid analyses were performed via combined liquid chromatography-tandem mass spectrometry using AB-Sciex API4000 triple quadrupole mass spectrometer (Foster City, CA) interfaced with an Agilent 1100 series liquid chromatograph (Agilent Technologies, Wilmington, DE), as previously described (37). The ceramide analytes were ionized via positive ion electrospray ionization. Elution of the ceramides was detected by multiple reactions monitoring characteristics for 14:0, 16:0, 18:0, 18:1, 20:0, 24:0 and 24:1 ceramides. C17:0-ceramide was employed as internal standard. All ceramide measurements were normalized by lipid Pi.
Ceramide synthesis inhibition studies.
The following inhibitors were utilized by treating cells for the indicated time before the addition of CS extract: ceramide synthase inhibitor fumonisin B1 (FB1; 10 μM, 2 h; Cayman Chemical, Ann Arbor, MI), serine palmitoyl transferase inhibitor myriocin (My; 50 nM; 2 h; Biomol International, Plymouth Meeting, PA), neutral SMase inhibitor GW4869 (15–20 μM), and acid SMase inhibitor imipramine (50 μM; Calbiochem, San Diego, CA).
siRNA experiments.
Neutral SMase knockdown was achieved in RLMEC using targeted siRNA against rat neutral SMase2 (50 nM; Dharmacon, Lafayette, CO). Routine transient transfections using siRNA were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following manufacturer's specifications.
Real-time PCR.
Total RNA was extracted from RLMEC using the RNeasy kit (Qiagen, Valencia, CA) following manufacturer's instructions, and cDNA was transcribed using the SuperScript III First-strand Synthesis kit (Invitrogen). Primers for rat Smpd2 gene (accession number NM_031360) amplification were as follows: sense, 5′-gtacagccggcagaaggata-3′; antisense, 5′-cacaacaggaccacatttgc-3′. Primers for GAPDH were as follows: sense, 5′-gaaatcccctggagctctgt-3′; antisense, 5′-ctggcaccagatgaaatgtg-3′. Real-time PCR was performed using the Power SYBR Green PCR Master Mix kit (Applied Biosystems, Carlsbad, CA) using an Applied Biosystems 7500 Real-Time thermal cycler. Cycling was performed, following an initial denaturation at 95°C for 15 min, at 95°C for 15 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s, and repeated for 40 cycles. Relative expression was assessed by the ΔΔCT method, compared with GAPDH.
Antioxidants, apoptosis inhibitors, and MAPK pathway signaling inhibitors.
Antioxidants, apoptosis inhibitors, and MAPK pathway signaling inhibitors were as follows: catalase (1–1.5 mM; converts hydrogen peroxide to hydrogen and oxygen), N-acetylcysteine (NAC, 0.5–1 mM; free-radical scavenger and precursor to glutathione), superoxide dismutase (SOD) mimetics Mn(III)TMPyP (a manganese-based metalloporphyrin) and tempol (a nitroxide) (25–50 μM and 1–2 mM, respectively; Calbiochem), general caspase inhibitor ZVAD-fmk (250 nM; MBL, Woburn, MA), ERK1/2 inhibitors, PD98059 (50 μM), U0126 (20 μM), Rho kinase inhibitor (Y27632; 1–5 μM), p38 MAPK inhibitor SB203580 (5 μM; Santa Cruz Biotechnology, Santa Cruz, CA), and JNK/SAPK MAPK inhibitor SP600125 (30–50 μM) from Calbiochem.
Immunoblotting.
Cell lysates were collected using standard RIPA buffer with protease and phosphatase inhibitors (Complete and Phostop, respectively; Roche, Indianapolis, IN). Samples with equal protein amount, determined by BCA protein analysis (Pierce, Rockford, IL), were resolved using SDS-PAGE, were transferred onto PVDF using semidry transfer (Bio-Rad, Hercules, CA) following manufacturers specifications, and were probed with the following primary antibodies raised against: phospho-p38 (1:500 dilution; Cell Signaling, Beverly, MA), total p38 (1:500, Cell Signaling), phospho-JNK (1:500, Cell Signaling), total JNK (1:500, Cell Signaling), phospho-ERK1/2 (1:500, Cell Signaling), and total ERK1/2 (1:500, Cell Signaling). Protein expression was detected using enhanced chemiluminescence ECL-plus (Amersham, Piscataway, NJ), quantified by densitometry, and normalized by housekeeping protein, using specific β-actin antibody (1:50,000, Sigma).
Immunocytochemistry.
RLMEC cells were grown on gelatin-coated coverslips overnight and treated as indicated for 4 h. After fixation in paraformaldehyde (4%), cells were stained for actin using Texas red phalloidin (1:200, Molecular Probes/Invitrogen), as previously described (34). Junctional proteins were visualized using immunostaining for zonula occludens (ZO)-1 (FITC-conjugated ZO-1, 5 μg/ml; Invitrogen), and nuclei were visualized with 4,6-diamidino-2-phenyl indole dihydrochloride. Confocal images were collected using an Olympus FV1000-MPE confocal microscope mounted on an Olympus IX81 inverted microscope stand with an Olympus Plan Apo ×60 water immersion objective, NA 1.4.
Animal experiments.
All animal studies were conducted in compliance with the approval of the Institutional Animal Care and Use Committee (IACUC) guidelines of Indiana University. DBA/2J mice, females, 3 mo of age (Jackson Laboratories, Bar Harbor, ME) were exposed to 3R4F cigarettes for 5 h/day, 5 days/wk, for 4 mo. Lungs were harvested 24 h following the last CS exposure, snap-frozen, and later homogenized for protein extraction and routine Western blotting. Adult male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 250–350 g were utilized for intravital microscopy (the detailed procedure is described in Ref. 40). Briefly, rats were ventilated using a mechanical ventilator via orotracheal intubation. The lungs of animals were exposed by intercostal incision, which allowed the interface with a custom-made imaging window, mounted on an imaging tray that was then placed on the objective of a Bio-Rad two-photon excitation microscopy system. Physiological parameters were maintained throughout imaging (for up to 3 h) (40). Ceramide C16:0 was administered via jugular vein injection, at a dose (10 mg/kg) previously shown to induce lung apoptosis and airspace enlargement following 24–48 h of intratracheal instillation (37).
Statistical analysis.
Statistical analysis was performed using SigmaStat 3.5. Comparisons among groups were made using ANOVA. For experiments in which two conditions were being compared, a two-tailed Student's t-test was used. All experiments were performed at least three times. All data are expressed as means ± SE, and statistically significant differences were considered if P < 0.05.
RESULTS
CS disrupts endothelial cell monolayer integrity in a time- and dose-dependent manner.
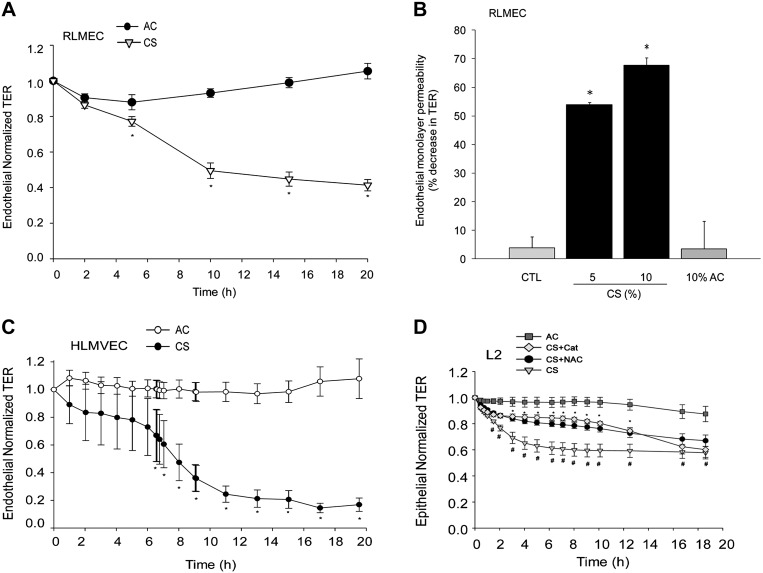
We studied the effect of an aqueous extract of mainstream CS on lung endothelial monolayer integrity. Exposure of primary RLMEC to CS markedly decreased TER (RLMEC barrier function) compared with control ambient AC (Fig. 1A). The decrease in TER reached statistical significance 5 h after CS exposure and was maximal at 20 h (Fig. 1A, and data not shown). The decrease in RLMEC barrier function induced by CS occurred in a dose-dependent manner (Fig. 1B). We noted that CS extract concentrations of 10% induced a 60% decrease in TER, concentrations of less than 3% caused little changes in the barrier function, and concentrations equal to or higher than 20% caused cell death with total loss of barrier function (data not shown). Similar effects of CS were seen in primary human lung endothelial cells (Fig. 1C), which were more sensitive to the barrier disruptive effects of CS because a CS concentration of 3% induced an 80% decrease in TER. In comparison, primary rat lung epithelial cells (Fig. 1D) had an earlier response to CS, exhibiting significant decreases in TER after only 1–2 h, albeit of a similar magnitude as that of endothelial cell responses.
Fig. 1.
Cigarette smoke extract (CS) disrupts endothelial monolayer integrity. A: transendothelial resistance (TER) measured in primary rat lung microvascular endothelial cells (RLMEC) following treatment with CS (10%) compared with ambient air control (AC; 10%); means ± SE; n = 11–13, *P < 0.05; Student's t-test. B: dose-dependent increase in RLMEC permeability in response to CS (5% or 10%; 20 h) compared with AC (10%); means ± SE; n = 3–7; *P < 0.05 vs. AC; ANOVA. C: kinetics of normalized TER across primary human lung microvascular endothelial cells monolayers in response to CS (3%) compared with AC (3%); means ± SE; n = 4–6, *P < 0.05; Student's t-test. D: normalized TER of primary rat lung epithelial cell (L2) monolayers treated with AC (4%) or CS (4%), CS + N-acetylcysteine (NAC) (500 μM), or CS + catalase (Cat) (1 mM); means ± SE; n = 4–15, *P < 0.05 vs. CS; #P < 0.05 vs. AC; ANOVA.
Effect of antioxidants on lung endothelial cell monolayer integrity.
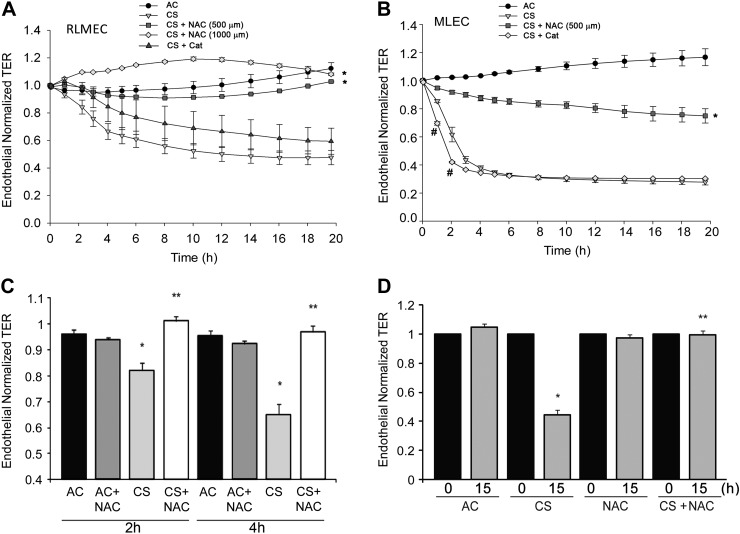
CS is a rich source of reactive oxygen and nitrogen species. CS is known to induce oxidative stress-dependent increase in epithelial cells monolayer permeability (41). Indeed, the antioxidant and glutathione modulator NAC or the antioxidant catalase significantly ameliorated the TER across rat lung epithelial cell monolayers exposed to CS aqueous extract (Fig. 1D). To investigate the role of oxidative stress in the endothelial cell barrier response to CS, we treated primary RLMEC or MLEC with NAC or catalase, followed by CS extract. Treatment with NAC significantly and dramatically protected the endothelial barrier function in cells exposed to CS, in a dose-dependent manner (Fig. 2, A–D). Unlike NAC, the catalase effect on TER was species and cell-type dependent. Whereas epithelial cells treated with catalase demonstrated significant protection against CS-induced permeability (Fig. 1D), endothelial cells of rat or mouse origin had either a marginal early, or no protection, respectively (Fig. 2, A and B). Surprisingly, catalase worsened TER early in the course of MLEC treatment with CS (Fig. 2B). Additionally, catalase treatment did not exhibit a synergistic effect with NAC in RLMEC (data not shown). Treatment with the SOD mimetics Mn(III)TMPyp or tempol also failed to attenuate the CS-induced decrease in TER and did not exhibit any synergy with each other or with catalase (data not shown). Treatment with AC or with NAC in the absence of CS had no significant effect on the baseline endothelial barrier function at early or late time points (Fig. 2, C and D).
Fig. 2.
Effects of antioxidants NAC and catalase on CS-induced decrease in TER. A: TER of primary RLMEC monolayers treated with AC (10%), CS (10%), CS + NAC (500 μM, or 1 mM), or CS + Cat (1.5 mM); means ± SE; n = 3–11, *P < 0.05 vs. CS alone all time points; ANOVA. B: TER measured in mouse lung endothelial cell (MLEC) monolayers treated with AC (4%) or CS (4%), CS + NAC (500 μM), or CS + Cat (1 mM); means ± SE; n = 3–8, *P < 0.05 vs. CS at all time points; #P < 0.05 vs. CS at the indicated time points; ANOVA. C and D: bar graphs with mean TER at the indicated early (C) or late (D) time points (0 h indicating the start of TER measurements) in RLMEC monolayers treated with AC (10%; n = 14), CS (10%; n = 19), AC + NAC (500 μM; n = 14), or CS + NAC (n = 13); means ± SE; *P < 0.001 vs. AC, **P < 0.001 vs. CS; ANOVA.
Role of caspases on CS-induced barrier disruption in endothelial cells.
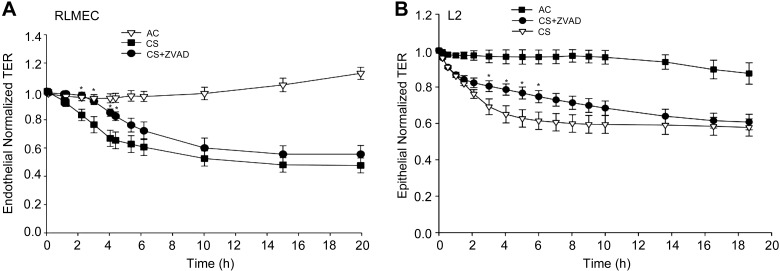
RLMEC treated with CS at concentrations similar to those causing barrier disruption (5–10%) did not exhibit markers of apoptosis, such caspase-3/7 or caspase-9 activation, or terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling-positive staining (data not shown). Treatment of either RLMEC or rat epithelial cells with the general caspase inhibitor ZVAD-fmk failed to attenuate the decrease in TER at 10 h or 20 h following CS exposure, respectively. However, both endothelial and epithelial cells treated with the caspase inhibitor exhibited a significant, albeit transient, protection from CS-induced permeability during the first several hours of CS exposure (Fig. 3, A and B). In the absence of CS, caspase inhibition with ZVAD-fmk had no effect on the baseline barrier function (data not shown). These results suggest that caspases plays a minor and transient role in the CS effect on the endothelial and epithelial barrier function in this model.
Fig. 3.
Effect of caspase inhibition on CS-induced endothelial and epithelial barrier disruption. A: effect of the general caspase inhibitor Z-VAD-FMK (ZVAD) on RLMEC barrier treated with CS (10%), CS + ZVAD (250 nM), compared with AC (10%); means ± SE; n = 3–11 *P < 0.05 vs. CS; ANOVA. B: normalized TER across primary rat lung epithelial cell (L2) monolayers exposed to AC (4%), CS (4%), or to CS + ZVAD (250 nM); means ± SE; n = 4–6; *P < 0.05 vs. CS; ANOVA.
CS-induced endothelial barrier dysfunction requires oxidative stress-dependent production of ceramide.
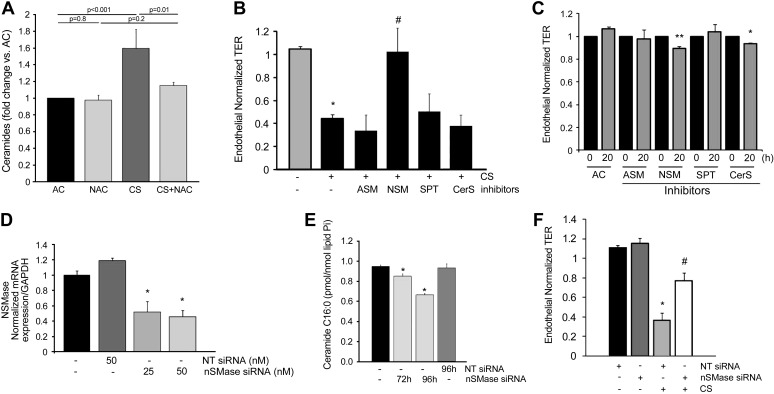
We have previously shown that CS induces ceramides in the whole lung in vivo (36) and in cultured human lung endothelial cells and MLEC (29, 37). Lindner et al. (25) have demonstrated that exogenous ceramides induce endothelial cell barrier dysfunction independently of apoptosis. To determine the role of ceramides in CS-induced barrier dysfunction in rat lung endothelial cells, we first determined robust and significant increases in ceramides in RLMEC exposed to CS (Fig. 4A). CS-induced ceramide production was attenuated by treatment of endothelial cells with NAC (Fig. 4A). To identify whether the CS-induced upregulation of ceramide was responsible for CS-induced barrier dysfunction, we treated endothelial cells with pharmacological inhibitors of enzymes involved in ceramide synthesis. Of these, inhibition of acid SMase (imipramine), serine palmitoyl transferase (My), or ceramide synthases (FB1) did not attenuate CS-induced barrier dysfunction (Fig. 4B). However, inhibition of neutral SMase with GW4869 significantly attenuated CS-induced barrier dysfunction (Fig. 4B), suggesting that the activation of neutral SMase by CS is implicated in its effect on endothelial permeability. The protective effect of neutral SMase inhibitor against the CS-induced barrier dysfunction was remarkable considering that cells treated with the inhibitor GW4869 alone exhibited a mild decrease in endothelial barrier. A similar effect on baseline TER was noted when cells were treated with the ceramide synthase inhibitor FB1, suggesting that a tonic activation of ceramide synthases and neutral SMase is required for the baseline maintenance of the endothelial barrier function (Fig. 4C). To determine the specificity of the neutral SMase2 involvement in the CS effect on endothelial barrier, we knocked down its expression by treatment of RLMEC with targeted siRNA (Fig. 4D). Compared with control untreated or with nontarget siRNA-treated conditions, neutral SMase siRNA treatment significantly decreased ceramide C16:0, one of the most abundant intracellular ceramides in the murine endothelium (29) (Fig. 4E). In contrast to nonspecific fluorescently labeled siRNA (siGLO; data not shown) or to nontargeting siRNA, treatment with targeted neutral SMase2 siRNA significantly attenuated the CS-induced decrease in TER (Fig. 4F).
Fig. 4.
Ceramide synthesis involvement in CS-induced permeability. A: total ceramide levels (normalized by lipid phosphorus, Pi) in RLMEC treated with AC (10%), AC + NAC (500 μM), CS (10%; 4 h), or CS + NAC (500 μM); means + SE; n = 3–6. B: normalized TER (20 h) RLMEC monolayers following treatment with AC (10%; n = 14), CS (10%; n = 19), or CS and each of the following inhibitors (n = 7–8): acid sphingomyelinase (ASM) inhibitor (Imipramine, 50 μM), neutral sphingomyelinase (NSM) inhibitor (GW4869, 15 μM), serine palmitoyl transferase (SPT) inhibitor (myriocin; 50 nM), or ceramide synthase (CerS) inhibitor (fumonisin B1, FB1, 1 μM); means ± SE; *P < 0.05 vs. AC; #P < 0.05 vs. CS; ANOVA. C: mean normalized TER (0 or 20 h) of RLMEC monolayers following treatment with AC alone (n = 14), or AC and each of the following inhibitors (n = 3–15): ASM inhibitor (Imipramine, 50 μM), NSM inhibitor (GW4869, 15 μM), SPT inhibitor (myriocin; 50 nM), or CerS inhibitor (FB1, 1 μM); means ± SE; *P < 0.05 vs. AC; **P < 0.01 vs. AC; ANOVA. D: neutral SMase (nSMase) mRNA expression in RLMEC treated with specific siRNA or nontarget (NT) siRNA for 72 h and measured by real-time PCR; means + SE; n = 3, *P < 0.01 vs. NT siRNA. E: ceramide C16:0 levels (normalized by lipid, Pi) in RLMEC treated with AC (10%) alone or with specific nSMase siRNA or NT siRNA (100 nM) for the indicated time; means + SE; n = 3, *P < 0.05 vs. AC; ANOVA. F: TER in RLMEC following exposure to AC (10%; 20 h) or CS (10%; 20 h) in the presence of NT siRNA or nSMase siRNA (50 nM; 96 h); means ± SE; n = 4–9, *P < 0.05 vs. AC; #P < 0.05 vs. CS 10%; ANOVA.
CS induces oxidative stress-dependent cytoskeletal changes in lung endothelial cells.
We next investigated whether treatment with CS at concentrations shown to decrease endothelial TER induces cytoskeletal changes in RLMEC. Texas Red phalloidin staining of actin and FITC-conjugated ZO-1 antibody immunostaining of intercellular junctions revealed that RLMEC exhibited profound changes in the actin cytoskeletal organization within 4 h of CS exposure, compared with controls (Fig. 5, A and C). We noted increased stress fiber formation causing thick bundles of actin across the cytoplasm. At high, toxic concentrations, CS caused dissolution of actin cytoskeleton (data not shown). Furthermore, CS caused a dramatic reduction and fragmentation of the expected linear ZO-1 staining at the cell plasma membrane and increased intercellular gaps (Fig. 5C). Both the actin rearrangement and the fragmentation of the intercellular ZO-1 staining were attenuated when cells were preincubated with NAC (500 μM, Fig. 5D), suggesting that reactive oxygen species mediated the effect of CS on the cytoskeleton. Treatment with the neutral SMase inhibitor GW4869 also attenuated stress fiber formation, and it protected against the loss of ZO-1 immunostaining induced by CS (Fig. 5E). In the absence of CS, there were no discernable effects of NAC (Fig. 5C, Fig. 7I) or GW4869 on the endothelial cytoskeleton (data not shown).
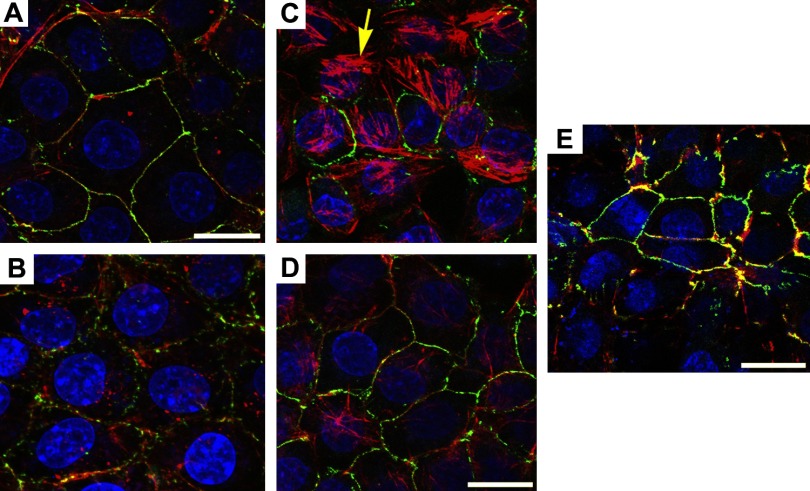
Fig. 5.
Cytoskeletal changes induced by CS in lung endothelial cells. Representative 3D-reconstructed images comprised of 4–17 confocal slices captured with the fluorescence microscope of RLMEC treated with AC (A; 10%; 4 h), AC + NAC (B; 500 μM), CS (C; 10%; 4 h), CS + NAC (D), or CS + GW4869 (E; 15 μM), and stained for actin (red), zonula occludens (ZO)-1 (green; arrowhead), and nuclei (blue). Note the actin reorganization in stress fibers (arrow), intercellular gap formation, and disruption of ZO-1 expression in CS-treated cells and their amelioration with antioxidant or nSMase inhibitor treatment. Scale bar = 17 μm.
Fig. 7.
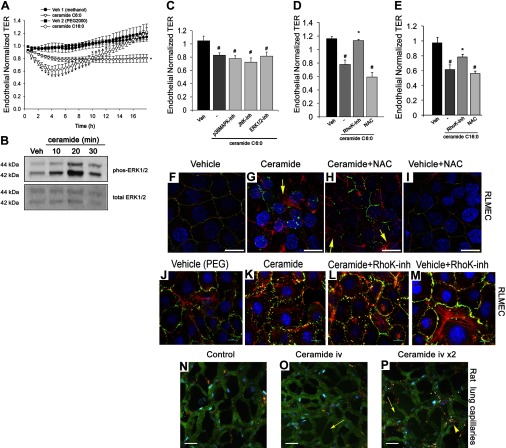
Effect of ceramides on endothelial barrier function in vitro and in vivo. A: normalized TER in RLMEC treated with ceramide C6:0 (20 μM), ceramide C16:0 (20 μM), or their respective vehicles methanol (%) or polyethylene glycol (PEG)2000 (10 μl); means ± SE; *P < 0.05 vs. vehicle, n = 3–15. B: Western blot of phosphorylated and total ERK1/2 in RLMEC treated with ceramide 6:0 (20 μM) for the indicated time. C: normalized TER measurements of RLMEC exposed to vehicle (methanol) or ceramide 6:0 (20 μM; 19 h) alone or with the p38 MAPK inhibitor SB203580 (5 μM), JNK1/3 inhibitor SP600125 (50 μM), or ERK1/2 inhibitor U0126 (20 μM); means + SE; #P < 0.05 vs. vehicle. D: mean normalized TER in RLMEC treated with vehicle (methanol), ceramide 6:0 (20 μM; 19 h), ceramide plus Rho Kinase inhibitor Y27632 (4 μM), or ceramide plus NAC (500 μM); n = 7–15; #P < 0.001 vs. vehicle; *P < 0.005 vs. ceramide. E: normalized TER in RLMEC treated with vehicle (PEG2000), ceramide-16:0 (20 μM; 5 h), ceramide plus Rho Kinase inhibitor Y27632 (4 μM), or ceramide plus NAC (500 μM); means + SE; n = 7–12; #P < 0.001 vs. vehicle; *P < 0.05 vs. ceramide. F–I: representative confocal fluorescence micrographs of RLMEC treated with vehicle (PEG2000; 10 μM; 2 h), ceramide C16:0-PEG (10 μM; 2 h), ceramide + NAC (500 μM; 2 h), or vehicle + NAC (500 μM; 2 h) and stained for actin (red), ZO-1 (green; arrowhead), and nuclei with 4,6-diamidino-2-phenyl indole dihydrochloride (DAPI) (blue). Note patchy actin reorganization in stress fibers (arrow) and the marked disruption of ZO-1 expression in ceramide-treated cells and lack of amelioration with NAC treatment. Scale bar = 17 μm. J–M: representative immunofluorescence micrographs of RLMEC treated with vehicle (PEG2000; 10 μM; 2 h), ceramide C16:0-PEG (10 μM; 2 h), ceramide + RhoK inhibitor (0.5 μM; 2 h), or vehicle + RhoK inhibitor and stained for actin (red), ZO-1 (green; arrowhead), and nuclei with DAPI (blue). Note that the marked disruption of ZO-1 expression in ceramide-treated cells was markedly inhibited in cells treated with RhoK inhibitor. Scale bar = 10 μm. N–P: effect of ceramide on the lung microcirculation captured in real time in the pulmonary microvasculature of a living rat (Supplemental Movies). Three-dimensional reconstruction of FITC-labeled vessels (green) surrounding alveoli (dark regions) and Rho-G6-labeled neutrophils (red) imaged via intravital 2-photon microscopy before (N) and after (O and P) intravenous administration of ceramide C16:0 (10 mg/kg). Nuclei were stained with intravenous Hoechst (blue). Note increasing neutrophil trafficking and plasma extravasation into airspaces captured 6 min postceramide administration (O), and then 2 min following a second dose of ceramide (P). Scale bars = 25 μm.
CS-induced endothelial barrier dysfunction requires activation of MAPK pathways.
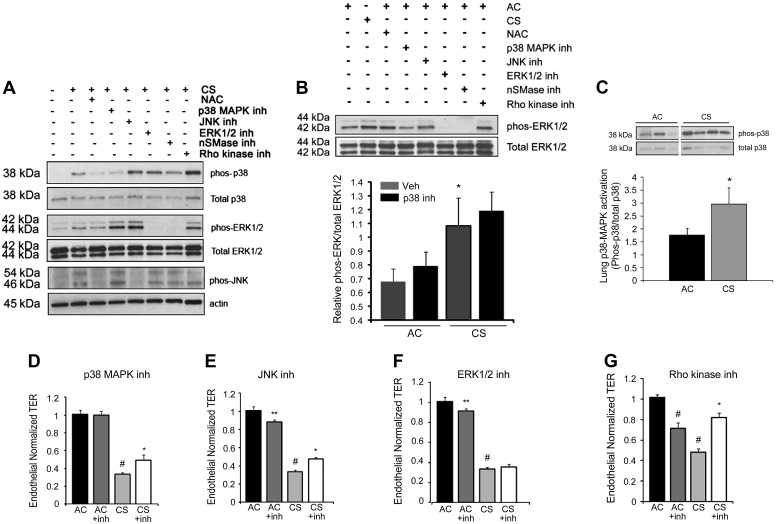
We investigated whether the endothelial barrier dysfunction induced by CS is mediated by MAPK signaling pathways. CS induced robust phosphorylation and therefore activation of the MAPKs p38, JNK, and ERK1/2 in RLMEC at 1 h, as detected by immunoblotting with specific antibodies (Fig. 6, A and B). Although the CS activation of ERK1/2 was somewhat enhanced by the specific inhibitors of p38 and/or JNK, SB203580 and SP600125, respectively (Fig. 6A), this effect was not always reproducible, and, on average, it did not reach statistical significance (Fig. 6B). To ensure the specificity of the MAPK inhibitors for their respective substrate, we measured their effects on the phosphorylation of ERK1/2 in the absence of CS exposure (Fig. 6B). As expected, only treatment with the specific ERK inhibitor PD98059, but not the specific inhibitors of p38 MAPK or JNK, blocked the ERK1/2 activation (Fig. 6B). Interestingly, in both CS-exposed and AC-exposed cells, ERK1/2 phosphorylation was abolished following treatment with the neutral SMase inhibitor GW4869. These data suggest that CS exposure of endothelial cells is associated with a net activation of all three MAPK pathways, ERK1/2, p38, and JNK. The effect of neutral SMase on ERK activity both at baseline and with CS exposure is intriguing, but its significance remains to be investigated. The antioxidant NAC (500 μM) attenuated the CS-induced MAPK-phosphorylation, indicating a mechanism of activation that is oxidative stress dependent (Fig. 6A). To ensure that CS-induced p38 MAPK phosphorylation was not limited to culture conditions, we confirmed that p38 is activated by CS in vivo, in lung tissue protein lysates collected from DBA/2J mice, which are susceptible to develop emphysema after CS exposure for 4 mo (45) (Fig. 6C). We next investigated whether MAPK signaling contributes to the decrease in TER in response to CS. RLMEC were preincubated with SB203580 (p38 inhibitor), SP600125 (JNK1–3 inhibitor), or PD98059 or U0126 (ERK1/2 inhibitors), in the presence of CS or AC controls (Fig. 6, D–F). Only inhibition of p38 MAPK and JNK significantly attenuated CS-induced permeability within 3 h and 5 h, respectively; this protective effect was evident throughout the duration of the experiment, for up to 24 h; results at 15 h are shown in Fig. 6, D–F. An early trend for protection from CS-induced permeability was observed following ERK inhibition, but this effect was transient (for only the first 2 and 3 h of exposure) and failed to reach statistical significance (data not shown and Fig. 6F). Because Rho family proteins and Rho kinase play a central role in actin cytoskeletal rearrangement and endothelial cell barrier function, we analyzed the effect of Rho kinase inhibition on TER of RLMEC challenged with CS. The Rho kinase inhibitor Y27632 (3 μM) significantly and persistently ameliorated the CS-induced decrease in TER (Fig. 6G). Higher concentrations of the inhibitor (e.g., 5 μM) failed to protect the barrier from CS and were associated with cell toxicity (data not shown). Of note, Rho kinase inhibition did not attenuate MAPK activation (Fig. 6A), suggesting that Rho kinase activation is not required for MAPK pathways activation in response to CS.
Fig. 6.
Role of MAPK activation in CS-induced barrier dysfunction in endothelial cells. A: Western blot of p38, ERK1/2, and JNK phosphorylation in RLMEC exposed to AC (10%; 1 h), CS (10%; 1 h), or CS + NAC (500 μM), p38 inhibitor SB203580 (5 μM), JNK inhibitor SP600125 (50 μM), ERK1/2 inhibitor PD98059 (50 μM), nSMase inhibitor GW4869 (15 μM), or Rho kinase inhibitor Y27632 (3 μM). Representative of n = 5 independent experiments. B: top: Western blot of ERK1/2 phosphorylation in RLMEC exposed to AC (10%; 1 h), CS (10%; 1 h), or AC + NAC (500 μM), p38 inhibitor SB203580 (5 μM), JNK inhibitor SP600125 (50 μM), ERK1/2 inhibitor PD98059 (50 μM), nSMase inhibitor GW4869 (15 μM), or Rho kinase inhibitor Y27632 (3 μM). Representative of n = 3 independent experiments. Bottom: densitometry ERK1/2 phosphorylation in response to AC (10%, 1 h), AC + p38 MAPK inhibitor SB203580 (5 μM), CS (10%, 1 h), and CS + p38 MAPK inhibitor; means + SE, n = 12, *P < 0.05 vs. AC. C: Western blot of p38 phosphorylation in total lung homogenates isolated from DBA/2J mice exposed to CS for 4 mo compared with AC control with densitometric analysis of Western blot data; means + SE; n = 6, *P < 0.05, Student's t-test. D–G: normalized TER measurements of RLMEC exposed to AC (10%) or CS (10%; 15 h), without or with a p38 MAPK inhibitor SB203580 (D; 5 μM; n = 3–8; #P < 0.001 vs. AC; *P < 0.01 vs. CS), JNK1/3 inhibitor SP600125 (E; 50 μM; n = 3–8; #P < 0.001 vs. AC; *P < 0.01 vs. CS; **P < 0.05 vs. AC), ERK1/2 inhibitor PD98059 (F; 50 μM; n = 3–8; #P < 0.001 vs. AC; **P < 0.05 vs. AC), or Rho kinase inhibitor Y27632 (G; 3 μM; n = 6–17; #P < 0.001 vs. AC; *P < 0.05 vs. CS).
Treatment with exogenous ceramides leads to loss of lung endothelial barrier function.
As expected (25), short-chain ceramide C6:0 treatment (10–20 μM) caused a significant TER decrease in RLMEC (Fig. 7, A and C), which was caspase activation independent (data not shown). Although C6:0 is present in lung tissue, it is of relatively low abundance (39). In contrast, ceramide C16:0 is one of the most abundant ceramides in the lung. The study of longer-chain ceramides is hampered by their poor solubility. We utilized ceramide C16:0 conjugated with polyethylene glycol (PEG)2000, testing it against a vehicle control with a similar amount of PEG2000. Compared with ceramide C6:0 treatment, the effect of ceramide C16:0 (10 μM) on TER was more rapid, with an onset after 1 h and a maximum magnitude at 5 h, but also more transient because TER recovered to baseline after 12 h (Fig. 7A). Treatment with ceramide induced marked activation of ERK1/2 within 10 min, which peaked at 20 min, as detected by Western blotting (Fig. 7B). However, the decrease in TER induced by ceramide treatment was not attenuated by pretreatment with p38, JNK, or ERK1/2 inhibitors (Fig. 7C). However, the Rho kinase inhibitor Y27632 significantly restored the endothelial barrier function following treatment with either ceramide tested (Fig. 7, D and E). Somewhat unexpected, because ceramides are potent inducers of oxidative stress, NAC treatment did not rescue the TER decreases, nor did it attenuate intercellular gap formation and junctional protein ZO-1 disruption induced by ceramide treatment (Fig. 7, F–I). In contrast, Rho kinase inhibition did protect the intercellular gaps from disruptive effects of ceramides and had a modest inhibitory effect on stress fiber formation (Fig. 7, J–M). These data are supportive of the notion that ceramide produced during CS exposure may be sufficient to induce barrier dysfunction through a mechanism involving Rho kinase activation and that oxidative stress is acting upstream of ceramide, stimulating its production. Because longer chain ceramides have not been studied in vivo and the response of the microcirculatory barrier to ceramides has not been investigated in the intact animal, we next asked whether augmentation of ceramide by intravenous administration of PEG-conjugated (solubilized) C16:0 is sufficient to cause alveolar capillary leakage in vivo. Using two-photon excitation microscopy of the lung in a living rat with intact circulation (Fig. 7N, corresponding to Supplemental Video S1; supplemental material for this article is available online at the American Journal of Physiology Lung Cellular and Molecular Physiology website), we noted a dose-dependent increase in the extravasation of FITC-labeled rat serum albumin from the alveolar microcirculation into the alveolar airspaces in response to ceramide C16:0 (depicted in Supplemental Video S2, corresponding to Fig. 7O, and Supplemental Video S3, corresponding to Fig. 7P).
DISCUSSION
The breach in endothelial integrity, along with that of the epithelial layer, may constitute some of the earliest elements of lung injury in response to CS exposure. Both insults are dependent on oxidative stress and are dramatically reversed by the antioxidant NAC. Neither of the two SOD mimetics used to scavenge superoxides and/or peroxynitrites (2) protected against CS-induced permeability changes. Similarly, scavenging hydrogen peroxide by catalase treatment alone, or in combination with SOD mimetics, was not sufficient to attenuate the effects of CS on the endothelial barrier function, despite their protective effects on the epithelium. These results were somewhat surprising, given the role of superoxide and hydrogen peroxide in mediating effects of CS on endothelial cells (12). Because NAC has complex, even opposing, effects on the net intracellular hydrogen peroxide content (18), it is possible that the broader detoxifying effects of the glutathione system are critical for protecting the endothelium from CS-induced barrier dysfunction. There is considerable evidence for increased oxidative stress in the lungs of patients with COPD, which may be contributed directly by CS components, or may be a consequence of the stress response (18). Ceramide itself may trigger oxidative stress, but, although the ceramide production in response to CS was redox sensitive, the effect of ceramide on permeability was not abolished by antioxidants. This finding is in contrast to a more chronic in vivo model, where ceramide-induced apoptosis and airspace enlargement (measured at 48 h) were attenuated by antioxidants such as Cu/Zn SOD overexpression (36). This apparent discrepancy may be due to the complexity of the in vivo models, the timing of the ceramide effect, or the specificity of the mutual amplification of ceramide and oxidative stress to cause apoptosis and airspace destruction, rather than vascular barrier dysfunction. Our findings place reactive oxygen species upstream of neutral SMase-mediated ceramide production induced by CS. A redox-dependent neutral SMase activation by CS was first reported by Levy et al. (22), demonstrating that the neutral SMase activation in the context of CS-induced epithelial cells apoptosis was glutathione dependent. Apoptosis was not a prominent feature of CS-induced endothelial or epithelial barrier dysfunction in our models. The minor role for caspase activation in CS-induced barrier dysfunction in both endothelial and epithelial cells was exerted in the early stages of permeability increase and is not unexpected because both TNF-α and ceramide were reported to induce barrier dysfunction in vitro in an apoptosis-independent manner (25, 38). In contrast, the monolayer permeability measurements indicated an important role of neutral SMase in mediating the increased permeability induced by CS. Interestingly, both neutral SMase as well as ceramide synthases appear to be required for the maintenance of the endothelial barrier in baseline conditions.
Our results revealed that CS exposure leads to oxidative stress-dependent, but ceramide-independent p38 MAPK phosphorylation in lung endothelial cells. The involvement of p38 MAPK and Rho kinase in the CS-induced endothelial cell permeability was expected because sidestream CS exposure, particulate matter, TNF-α, thrombin, hydrogen peroxide, and pertussis toxin, among others, also required p38 MAPK activation for their endothelial barrier-disruptive effects (4, 14, 26, 35, 48). In models of endothelial cell barrier dysfunction induced by TNF-α, the p38 MAPK phosphorylation and activation occurred in a Rho kinase-dependent manner (32), whereas, in the context of the edemogenic effect of pertussis toxin, the p38 MAPK phosphorylation occurred independent of Rho kinase activation (14). In our model of CS-induced permeability, the Rho kinase activation was not required for p38 MAPK phosphorylation, but both were important in mediating the effect of CS on the endothelial cell cytoskeleton and barrier function (schematic in Fig. 8). An explanation for these results may be that both kinases could independently modulate endothelial cell shape via distinct substrate phosphorylation. Whereas Rho kinase may modulate actin-myosin interaction via myosin light chain phosphatase phosphorylation and inactivation, p38 MAPK may act via phosphorylation of caldesmon (4) or ezrin, radixin, and moesin proteins (20). It remains to be determined whether these or other specific substrates targeted by p38 MAPK and Rho kinase during CS exposure are responsible for the dramatic cytoskeletal changes and loss of cell junction protein ZO-1 in lung endothelial cells. The relevance of the signaling pathways activated by CS in vitro was tested in DBA/2J mice, a strain that is susceptible to CS-induced emphysema (1, 45). The lungs of mice exhibited increased p38 MAPK phosphorylation at 24 h after a 5-h exposure period to CS, in a chronic CS exposure model, in which mice were exposed 5 h/day, 5 days/wk for 4 mo. These data corroborate previous reports of p38 MAPK activation several hours (6–24 h) in an acute CS exposure model in strains of rat and mouse that, similar to our model, exhibit inflammation and oxidative stress following several days of CS exposure (28, 52). Because these studies were performed in the whole lung, it remains unknown whether the p38 MAPK is specifically activated in endothelial cells or in other cells in the lung.
Fig. 8.
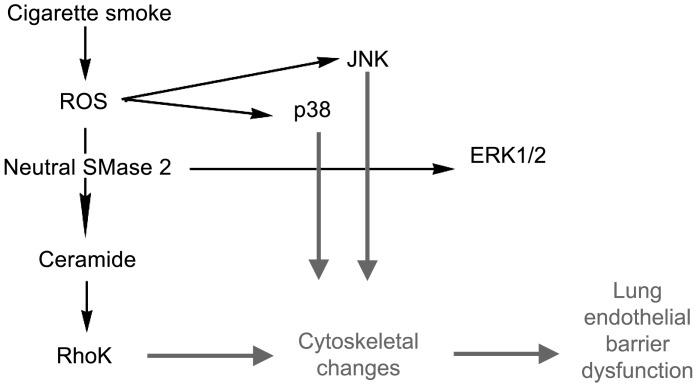
Schematic with the proposed mechanisms by which soluble components of CS induce barrier dysfunction in lung endothelial cells. ROS, reactive oxygen species.
Our study has relevance to the development of CS-induced emphysema because both ceramides and oxidative stress are thought to be involved in its pathogenesis, on the basis of data gathered in experimental disease models. Our data presented here reveal that ceramide is both required by CS to induce endothelial cell barrier dysfunction and by itself is sufficient to increase both epithelial and endothelial permeability. Ryan et al. (44) have previously demonstrated that direct administration of ceramide to the trachea increases the permeability of the airway, and, consistent with this notion, Lindner et al. (25) demonstrated that ceramide treatment increases endothelial monolayer permeability and is edemogenic in vivo when administered intravascularly in an isolated perfused lung model (16). Our data extend these observations in vivo, in a physiological rat preparation, where the intravenous administration of long-chain ceramide expressed abundantly in the lung increased the extravasation of fluorescently labeled rat albumin within minutes. Because only very high doses of ceramide (repeated injection of 10 mg/kg iv) induced marked alveolar flooding, it is unlikely that ceramides released by CS in vivo will ever achieve such high levels within a short period of time. However, whereas routine CS exposure by itself does not cause clinical pulmonary edema in humans, it may be an important risk factor for the development of pulmonary edema in the adult respiratory distress syndrome (ARDS) (17). The mechanisms responsible for this association are not known. The observation that experimental exposures to CS increased alveolar permeability in rabbits without causing structural changes in the alveolo-capillary membrane (51) may indicate a possible neurogenic mechanism (15). However, our data suggest that CS induces subtle changes in the alveolo-capillary barrier directly, via oxidative stress-dependent and ceramide-mediated cytoskeletal changes. This mechanism may render endothelial cells more susceptible to edemogenic effects of subsequent injuries associated with ARDS and may independently contribute, over time, to chronic inflammatory changes associated with COPD. It may therefore be of interest to determine whether amelioration of the endothelial barrier function by detoxifying agents, ceramide synthesis inhibitors, or Rho kinase inhibitors could be a useful therapeutic adjunct in COPD.
GRANTS
This work was supported by RO1HL077328 (I. Petrache), R21 DA029249-01 (I. Petrache), P50 HL084945 (I. Petrache), T32 HL091816 (M. B. Brown), and NIH 1 S10 RR16798 (W. Hubbard; for funding the API4000 LC-MS/MS system).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Bartalesi B, Cavarra E, Fineschi S, Lucattelli M, Lunghi B, Martorana PA, Lungarella G. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur Respir J 25: 15–22, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med 46: 192–201, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Med Sci 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 4. Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 287: L911–L918, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Boucher RC, Johnson J, Inoue S, Hulbert W, Hogg JC. The effect of cigarette smoke on the permeability of guinea pig airways. Lab Invest 43: 94–100, 1980. [PubMed] [Google Scholar]
- 6. Burns AR, Hosford SP, Dunn LA, Walker DC, Hogg JC. Respiratory epithelial permeability after cigarette smoke exposure in guinea pigs. J Appl Physiol 66: 2109–2116, 1989. [DOI] [PubMed] [Google Scholar]
- 7. Carp H, Janoff A. Inactivation of bronchial mucous proteinase inhibitor by cigarette smoke and phagocyte-derived oxidants. Exp Lung Res 1: 225–237, 1980. [DOI] [PubMed] [Google Scholar]
- 8. Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 31: 1334–1356, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol 5: 257–263, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Dobrowsky RT, Kolesnick RN. Analysis of sphingomyelin and ceramide levels and the enzymes regulating their metabolism in response to cell stress. Methods Cell Biol 66: 135–165, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Edirisinghe I, Arunachalam G, Wong C, Yao H, Rahman A, Phipps RP, Jin ZG, Rahman I. Cigarette-smoke-induced oxidative/nitrosative stress impairs VEGF- and fluid-shear-stress-mediated signaling in endothelial cells. Antioxid Redox Signal 12: 1355–1369, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Garcia JG. Concepts in microvascular endothelial barrier regulation in health and disease. Microvasc Res 77: 1–3, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Garcia JG, Wang P, Schaphorst KL, Becker PM, Borbiev T, Liu F, Birukova A, Jacobs K, Bogatcheva N, Verin AD. Critical involvement of p38 MAP kinase in pertussis toxin-induced cytoskeletal reorganization and lung permeability. FASEB J 16: 1064–1076, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Germonpre PR, Joos GF, Pauwels RA. Characterization of the neurogenic plasma extravasation in the airways. Arch Int Pharmacodyn Ther 329: 185–203, 1995. [PubMed] [Google Scholar]
- 16. Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 10: 155–160, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Iribarren C, Jacobs DR, Jr, Sidney S, Gross MD, Eisner MD. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest 117: 163–168, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax 60: 693–700, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE 2007: re8, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol 176: 1218–1227, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Kuebler WM, Yang Y, Samapati R, Uhlig S. Vascular barrier regulation by PAF, ceramide, caveolae, and NO - an intricate signaling network with discrepant effects in the pulmonary and systemic vasculature. Cell Physiol Biochem 26: 29–40, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Levy M, Khan E, Careaga M, Goldkorn T. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol 297: L125–L133, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li XY, Donaldson K, Rahman I, MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med 149: 1518–1525, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Li XY, Rahman I, Donaldson K, MacNee W. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax 51: 465–471, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindner K, Uhlig U, Uhlig S. Ceramide alters endothelial cell permeability by a nonapoptotic mechanism. Br J Pharmacol 145: 132–140, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Low B, Liang M, Fu J. p38 mitogen-activated protein kinase mediates sidestream cigarette smoke-induced endothelial permeability. J Pharm Sci 104: 225–231, 2007. [DOI] [PubMed] [Google Scholar]
- 27. MacNee W. Oxidants and COPD. Curr Drug Targets Inflamm Allergy 4: 627–641, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 31: 633–642, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Medler TR, Petrusca DN, Lee PJ, Hubbard WC, Berdyshev EV, Skirball J, Kamocki K, Schuchman E, Tuder RM, Petrache I. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol 38: 639–646, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merrill AH, Jr, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E. Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol 142: 208–225, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Mordelet-Dambrine M, Leguern-Stanislas G, Chinet TC, Barritault L, Chretien J, Huchon GJ. Effects of tobacco smoke on respiratory epithelial clearance of DTPA and on lung histology in rats. Eur Respir J 4: 839–844, 1991. [PubMed] [Google Scholar]
- 32. Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol 95: 1889–1895, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD 4: 347–353, 2007. [DOI] [PubMed] [Google Scholar]
- 34. Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R, Romer LH, Garcia JG. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-α-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 17: 407–416, 2003. [DOI] [PubMed] [Google Scholar]
- 35. Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 28: 574–581, 2003. [DOI] [PubMed] [Google Scholar]
- 36. Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, Berdyshev EV, Lungarella G, Tuder RM. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 295: L44–L53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11: 491–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 280: L1168–L1178, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Petrusca DN, Gu Y, Adamowicz JJ, Rush NI, Hubbard WC, Smith PA, Berdyshev EV, Birukov KG, Lee CH, Tuder RM, Twigg HL, 3rd, Vandivier RW, Petrache I. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem 285: 40322–40332, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Presson RG, Jr, Brown MB, Fisher AJ, Sandoval RM, Dunn KW, Lorenz KS, Delp EJ, Salama P, Molitoris BA, Petrache I. Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact. Am J Pathol 179: 75–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys 43: 167–188, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Rusznak C, Mills PR, Devalia JL, Sapsford RJ, Davies RJ, Lozewicz S. Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 23: 530–536, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Rusznak C, Sapsford RJ, Devalia JL, Justin John R, Hewitt EL, Lamont AG, Wood AJ, Shah SS, Davies RJ, Lozewicz S. Cigarette smoke potentiates house dust mite allergen-induced increase in the permeability of human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol 20: 1238–1250, 1999. [DOI] [PubMed] [Google Scholar]
- 44. Ryan AJ, McCoy DM, McGowan SE, Salome RG, Mallampalli RK. Alveolar sphingolipids generated in response to TNF-α modifies surfactant biophysical activity. J Appl Physiol 94: 253–258, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Schweitzer K, Johnstone BH, Garrison J, Rush N, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M, Fisher AJ, Kamocki K, Brown MB, Presson RG, Jr, Broxmeyer HE, March KL, Petrache I. Adipose Stem Cell Treatment in Mice Attenuates Lung and Systemic Injury Induced by Cigarette Smoking. Am J Respir Crit Care Med 183: 215–225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serikov VB, Leutenegger C, Krutilina R, Kropotov A, Pleskach N, Suh JH, Tomilin NV. Cigarette smoke extract inhibits expression of peroxiredoxin V and increases airway epithelial permeability. Inhal Toxicol 18: 79–92, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol 8: 159–167, 1996. [DOI] [PubMed] [Google Scholar]
- 48. Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal 5: 723–730, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Van Driessche W, Kreindler JL, Malik AB, Margulies S, Lewis SA, Kim KJ. Interrelations/cross talk between transcellular transport function and paracellular tight junctional properties in lung epithelial and endothelial barriers. Am J Physiol Lung Cell Mol Physiol 293: L520–L524, 2007. [DOI] [PubMed] [Google Scholar]
- 50. Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 77: 39–45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Witten ML, Lemen RJ, Quan SF, Sobonya RE, Roseberry H, Stevenson JL, Clayton J. Acute cigarette smoke exposure increases alveolar permeability in rabbits. Am Rev Respir Dis 132: 321–325, 1985. [DOI] [PubMed] [Google Scholar]
- 52. Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008. [DOI] [PubMed] [Google Scholar]
- 53. Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, Gandjeva A, Zhen L, Chukwueke U, Mao T, Richter A, Brown E, Ashush H, Notkin N, Gelfand A, Thimmulappa RK, Rangasamy T, Sussan T, Cosgrove G, Mouded M, Shapiro SD, Petrache I, Biswal S, Feinstein E, Tuder RM. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med 16: 767–773, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.