Abstract
Human lung research has made remarkable progress over the last century largely through the use of animal models of disease. The challenge for the future is to translate these findings into human disease and bring about meaningful disease modification or even cure. The ability to generate transformative therapies in the future will require human tissue, currently scarce under the best of circumstances. Unfortunately, patient-derived somatic cells are often poorly characterized and have a limited life span in culture. Moreover, these cells are frequently obtained from patients with end-stage disease exposed to multiple drug therapies, leaving researchers with questions about whether their findings recapitulate disease-initiating processes or are simply the result of pharmacological intervention or subsequent host responses. The goal of studying early disease in multiple cell and tissue types has driven interest in the use of induced pluripotent stem cells (iPSCs) to model lung disease. These cells provide an alternative model for relevant lung research and hold promise in particular for studying the initiation of disease processes in genetic conditions such as heritable pulmonary arterial hypertension as well as other lung diseases. In this Perspective, we focus on potential iPSC use in pulmonary vascular disease research as a model for iPSC use in many types of advanced lung disease.
Keywords: induced pluripotent stem cells, iPSCs, pulmonary arterial hypertension, bone morphogenetic protein receptor type 2, BMPR2
the health needs of an aging world population have brought stem cells to the forefront of regenerative medicine. Current research approaches aim at harnessing and enhancing the regenerative potential of endogenous stem cells (12) or at manipulating stem cells ex vivo for the development of cell replacement therapies. Several stem cell populations have been isolated and studied, but embryonic stem cells (ESCs) (13, 56) appear to hold particular promise for widespread clinical application due to their unparalleled differentiation repertoire (37). ESCs are isolated from the inner cell mass at the blastocyst stage of embryo development, can proliferate indefinitely in culture without loss of differentiation potential and are pluripotent, i.e., they can give rise to cells of all three germ layers. However, the use of human ESCs derived from the blastocyst is fraught with ethical and technical issues, such as the destruction of human embryos, difficulty deriving new cell lines, limited genetic diversity of existing cell lines, and the need for immune suppression if derivatives of these cells are transplanted into allogeneic human recipients. Hence, pluripotent cells generated by alternative methods, such as cell fusion or somatic cell nuclear transfer, have been proposed for application to the study or future treatment of human disease (26, 34). The most promising new tool is induced pluripotent stem cells (iPSCs). In this technology somatic cells, such as fibroblasts, are reprogrammed into pluripotent cells via overexpression of specific transcription factors. iPSCs display the critical features that define pluripotent stem cells, including self-renewal, multilineage differentiation, and functional contribution to tissues of all three primary germ layers.
In their seminal 2006 paper, Takahashi and Yamanaka (55) reported that retrovirus-mediated overexpression of four transcription factors (Oct4, Sox2, c-Myc, and Klf4) in mouse fibroblasts resulted in the subsequent derivation of iPSCs with morphological, molecular and cellular and functional similarity to ESCs. By 2007, a similar approach had been applied to derive human iPSCs by reprogramming human dermal fibroblasts (54, 62).The rapid advance of iPSC technology has required the establishment of strict standards for human iPSC use (10) and has also facilitated its application widely to research in all organs, including the cardiopulmonary system. Although several technical hurdles remain for the safe use of iPSC in cell replacement therapies, disease modeling and drug development using these cells are already underway.
Since iPSCs are pluripotent by definition (can give rise to three germ layers and any differentiated cell type), they can be used to model disease progression and pathophysiology in vitro (8). The general approach is to reprogram somatic cells from diseased and healthy (control) subjects to iPSCs, differentiate the latter to the cell type affected by the disease, and perform genetic, epigenetic, and molecular characterization of the differentiated iPSC progeny. Differentiated cells can be used for many purposes (Table 1), including drug screening to discover new molecules that can reverse or mitigate the disease phenotype, and potentially as cell-based therapies.
Table 1.
| Application | Examples |
|---|---|
| In vitro cell derivation for humanized model systems | Neurons |
| Cardiomyocytes | |
| Drug development studies | Cardiomyocytes: Long QT syndrome (21) |
| Derivation of cells from compartments not readily accessible or amenable to culture | Lung cells (48)
|
| Studies of epigenetic variants, and other modifiers of cell phenotypes | Epigenetic modification of gene expression and cell phenotype (38) |
| In vitro and ex vivo tissue engineering to reconstruct tissues for study and/or therapy | |
| Cell-based therapies | No human studies to date
|
An alternative approach, still in its infancy, is the direct reprogramming of fibroblasts to differentiated cells without the intermediate iPSC step, as has recently been reported with hepatocyte-like cells (17, 49), cardiomyocytes (19), and neurons (59, 61). Currently, the existence of efficient directed differentiation protocols for several cell types favors the use of iPSCs over direct reprogramming. If the repertoire of possible conversions is considerably expanded in the future, the latter may become the method par excellence for the de novo derivation of differentiated cells. Several human disease-specific iPSC lines have been created (20, 40, 48, 51) from skin fibroblasts obtained from individuals with a wide spectrum of disorders. These range from single gene defects, such as α1-antitrypsin deficiency and cystic fibrosis (51), to complex multifactorial diseases such as Type 1 diabetes (32). Cell lineages derived from these iPSCs recapitulate key molecular aspects of the represented diseases to provide promising research opportunities (Fig. 1) (11, 28, 33).
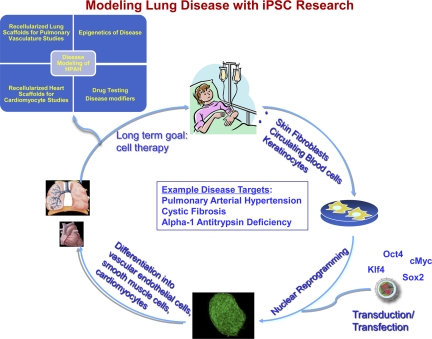
Fig. 1.
Generation and utilization of human induced pluripotent stem cells (iPSCs) to study health and disease states. Somatic cells are obtained from consenting donors and reprogrammed to iPSCs. The somatic cells from which iPSCs are derived may represent subjects with varying degrees of disease complexity and phenotype. iPSCs may be differentiated into specific cell lineages that can be characterized by using a wide variety of assays and used to attempt organ regeneration or other experiments. HPAH, heritable pulmonary arterial hypertension.
Several of these disorders are neurodegenerative and neurodevelopmental. Since protocols for differentiation of human ESCs and iPSCs to motor and dopaminergic neurons are well established (24, 29, 41), in vitro derivation of neurons from diseased iPSCs has already been reported (11, 28, 33). For example, Dimos et al. (11) reported highly efficient derivation of motor neurons (as witnessed by coexpression of TUJ1 and HB9) from amyotrophic lateral sclerosis patient-specific iPSCs. In another study, iPSCs from Rett syndrome patients were coaxed to differentiate to neurons deficient for the MeCP2 protein (33). These neurons exhibited reduced number of synapses and electrophysiological defects, thus recapitulating key molecular aspects of the disease. Finally, iPSCs from patients with familial dysautonomia (FA), a rare neurodegenerative disease due to IKBKAP aberrant splicing, were differentiated to cells of the central and peripheral nervous system that exhibited a high ratio of mutant-to-normal transcript (28). Functional levels of the latter were restored after exposure to the plant hormone kinetin, a promising candidate molecule for treatment of FA.
The feasibility of using human iPSCs for drug development was also demonstrated in the case of the long QT syndrome, an autosomal dominant disease characterized by delayed repolarization of cardiomyocytes that results in potentially lethal tachycardia. This syndrome is due to mutations of genes encoding potassium channel subunits such as KCNQ1 and KCNH2. Two independent groups demonstrated that iPSC-derived cardiomyocytes from long QT syndrome patients show longer action potentials (characteristic signature of the disease) compared with control cardiomyocytes (21, 35). Furthermore, aberrant ion flux in these cells in vitro was responsive to drugs, such as beta blockers that ameliorate the disease phenotype in vivo (21).
Nevertheless, most human diseases, even if genetic in origin, manifest their phenotype as a result of a complex interplay of genetic and environmental factors; thus their correct in vitro modeling using iPSCs presents formidable challenges (48). For instance, final manifestation of cystic fibrosis phenotype is not just a function of CFTR mutation but also includes the lung microbiome, anatomy, drug therapy, and other factors (6). Such challenges will be discussed in the context of iPSC modeling of pulmonary arterial hypertension (PAH), a highly morbid cardiopulmonary disease resulting from aberrant remodeling of the pulmonary vasculature. We have previously derived more than 100 iPSC lines from patients with diseases affecting the epithelial, endothelial, or interstitial compartments of the lung (48). Cells from these compartments are not readily accessible or amenable to culture; thus deriving these differentiated cell types de novo in culture from iPSCs may provide important in vitro correlates to their naturally occurring in vivo counterparts. We believe the same approach can be successfully applied to PAH, since it involves several cell types.
Use of iPSC Technology To Model Lung Diseases, Exemplified by PAH
PAH is characterized by elevated pulmonary artery pressures and widespread obstruction and obliteration of small pulmonary arteries (Fig. 2) (3, 47). Arterial changes occur in all the layers of the vascular wall. This includes occlusion, or near occlusion, of the lumen due to neointimal formation characterized by the expansion of endothelial cells, cells with markers of vascular smooth muscle cells, and inflammatory cells, as well as obstructive microthrombi. A fibroproliferative response characterized by an increase in extracellular matrix components may be seen, and vessels may display extensive development of endothelial channels (plexiform lesions) characteristic of PAH. The surrounding adventitia is typically thickened, as well (14, 43). Progressive vascular remodeling results ultimately in right ventricular (RV) failure. Whereas PAH is associated with a wide array of comorbid conditions, it also occurs as a primary pulmonary vascular disease known as either idiopathic (IPAH) (no known family history or genetic abnormality) or heritable (HPAH) PAH (50) (positive family history and/or genetic abnormality).
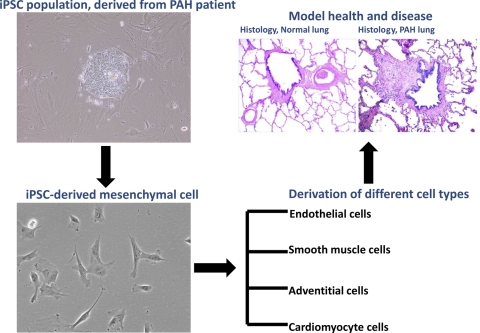
Fig. 2.
Schematic to describe a method by which iPSCs may be used to study pulmonary arterial hypertension (PAH). First, iPSCs would be derived from a PAH patient, with or without a known causative genetic variant (such as BMPR2 gene mutation). Those iPSCs may be used to derive cells of relevance to PAH pathogenesis to proceed with various studies, such as those outlined in the text and in Table 1. Further steps of action may include the ultimate derivation of tissues of interest for disease modeling, cell-based therapeutic approaches, etc.
The majority of HPAH cases are due to mutations in bone morphogenetic protein receptor type 2 (BMPR2) (31). BMPR2 expression is typically reduced in the lungs of not only HPAH patients with BMPR2 gene mutations, but also among those with IPAH (1). Therefore, understanding the exact mechanisms by which dysfunctional bone morphogenetic protein (BMP) signaling results in PAH should be important for the development of effective therapies more broadly than just for those with HPAH. However, ∼20% of HPAH cases and at least 60% of IPAH cases are associated with non-BMPR2 variants, the majority of which are not described (31). iPSC should facilitate the study of PAH regardless of its genetic underpinnings, and human iPSC offer a unique platform for mechanistic studies of and drug development for PAH. For example, direct comparison of specific patient genotype drug responses, in variable cell types with the same genotype, may lead to novel information not only for genotype and cell-specific drug discovery, but also for drug toxicities in PAH. The technology also holds the potential for direct cell transplant to treat PAH in patients. Such cell therapies could be directed toward the pulmonary microvasculature and the failing right heart (57). iPSC could be used to generate mesenchymal stem and endothelial cells, which have been shown to be effective in animal models of pulmonary hypertension directly (2, 22, 63) or with further molecular modification of expression of key genes like eNOS to improve microvasculature structure and function (5, 23, 64). For example, the PHACeT (Pulmonary Hypertension: Assessment of Cell Therapy) Trial (clinicaltrials.gov, identifier NCT00469027) is a phase I trial of autologous progenitor cell-based gene therapy currently underway; in theory, autologous iPSCs derived from skin or circulating cells could be differentiated and manipulated to effectively deliver cell-based therapies in a similar manner.
Although dysfunction of the BMP signaling pathway in HPAH results in severe vascular remodeling and occlusion of small peripheral pulmonary microvasculature, the precise mechanisms responsible for disease pathogenesis are elusive (36). With the advent of iPSCs, new venues of research are now available to elucidate the underlying mechanisms in several ways. For example, since multiple cell types are likely contribute to HPAH pathogenesis, the ability to differentiate iPSCs into multiple lineages will allow the analysis of multiple cell types derived from a single patient, including vascular endothelial cells (ECs), smooth muscle cells and cardiomyocytes (25). In addition, multiple developmental pathways of lineage differentiation may be evaluated. For example, one may evaluate the commitment of iPSCs into mesenchymal cells (4) or iPSCs into ECs via hemangioblast (7) stages of commitment.
Once purified differentiated cells are available, in vitro and ex vivo tissue engineering approaches may be used to reconstruct arteries and study the development of the disease in a physiologically relevant system (48). For example, we and others previously described a “decellularization-recellularization” approach to create bioartificial lungs for autologous lung transplantation (39, 42). This platform may also be relevant for ex vivo studies of HPAH using iPSCs. ECs, smooth muscle cells, and lung epithelial cells could conceivably be derived in vitro and used to seed decellularized lungs. Liquid or air ventilation in combination with continuous media perfusion in the endothelial compartment would create an experimental setup that approximates the function of the native lung. This model may also be used to evaluate potential disease modifiers, such as suspected environmental insults previously associated with PAH [e.g., fenfluramine derivatives (18) and serotonergic agents (30)].
In addition to pulmonary vascular remodeling, PAH is associated with RV dysfunction (15). On average, patients with HPAH die at a younger age than those with IPAH (53). Because RV function is the primary determinant of mortality in PAH, RV dysfunction exacerbated by BMPR2 mutation or reduced BMPR2 expression may underlie this finding. Consistent with this possibility, BMP signaling appears critical to proper cardiac development (58), and mutations in BMPR2 have been linked to congenital heart disease-associated PAH (45, 46).
Given the critical role of BMP signaling in cardiac differentiation (58), it is conceivable that iPSCs derived from HPAH patients will manifest differences in cardiogenic differentiation compared with iPSCs from healthy wild-type subjects, and this may yield new mechanistic insights. In vitro studies show that the differentiation of iPSCs into cardiovascular progenitors, and ultimately to mature ECs and cardiomyocytes, is enhanced by proper exposure to BMP morphogens at specific time points of the differentiation process (25, 60). When directing the differentiation of normal ESCs or iPSCs, antagonists of BMP signaling, such as Noggin and PRDC, or chemical compounds such as dorsomorphin, influence both the yield and physiological properties of stem cell-derived cardiomyocytes (16). Thus iPSCs derived from HPAH patients carrying BMPR2 mutations may manifest differences in cardiomyocyte differentiation compared with iPSCs from healthy individuals without BMPR2 mutations; this would provide a useful model for comparative studies. In addition to disease modeling of HPAH, employing iPSC lines with known mutations in receptors that activate BMP signaling will help to advance our understanding of one of the key signaling pathways of cardiovascular development, embryonic patterning, and musculoskeletal formation. On this basis, the novel tool of iPSCs with known BMPR2 mutations may present a unique opportunity to address the cellular and molecular basis of cardiovascular diseases more broadly.
It has long been known that only ∼20% of BMPR2 mutation carriers develop PAH. Differences in epigenetic modification(s) among carriers have been proposed as one hypothesis to explain this observation. If epigenetic modifications are important for the manifestation of BMPR2 mutation defects in vitro, the “epigenetic memory” of the original somatic cells from which iPSCs are derived may be important; this is a potential deficiency of fibroblast-derived iPSCs. Epigenetic memory refers to the presence of epigenetic marks from the cell type of origin in the resulting iPSCs that continues to influence gene expression. However, this epigenetic memory appears to be transient and is lost at later passages following the onset of reprogramming (44). In the context of PAH, if environmental insults mediate reversible epigenetic modifications in patients that exhibit the HPAH phenotype, then early passage iPSCs should retain epigenetic modifications peculiar to their cell of origin (e.g., lung vascular cells) whereas late passage iPSCs will not. Because using the somatic cell from which the iPSC is derived may improve this memory, novel iPSC derivation techniques, such as those that derive iPSC directly from the particular diseased cell type (for example, a pulmonary microvascular endothelial cell) rather than skin fibroblasts, may provide a more effective technology from which to derive iPSC depending upon the goals of the study. In fact, derivation of a given cell type may be more efficient if the iPSC is derived from that somatic cell type both because of and also of irrespective of epigenetic memory (38). This may be a challenge in PAH research given the paucity of lung tissue for study.
Although HPAH has the luxury of a known mutation in the majority of cases, we and others are identifying candidate genes that underlie susceptibility to development of a number of lung diseases including those involving the airways, such as chronic obstructive pulmonary disease, asthma, idiopathic pulmonary fibrosis, and familial interstitial pneumonia (9, 27). The same iPSC techniques discussed above could be used to study how these identified genes affect epithelial lineage differentiation, molecular signatures, cell phenotype, and function.
Thus iPSC technology offers the potential to investigate the full complement of disease spectrum, from early to advanced, and to dissect genetic and environmental influences on phenotype. Moreover, in the years ahead iPSCs should have enhanced applicability to human lung disease by 1) providing broad access to a patient-derived expandable pluripotent human cell population, 2) recapitulating disease pathophysiology in a cell-specific manner, and 3) giving rise in vitro to multiple cell lineage derivatives with the same genetic makeup. The opportunity for cell-based therapies exists, although a number of impediments exist, including the choice of what cell type to reprogram (may be different for different diseases and/or therapeutic goals), need for high-throughput reprogramming techniques with lower costs, as well as safety issues including but not limited to genetic stability of the derived cells (52). Nonetheless, iPSCs should ultimately help us translate science from “bench to bedside” in the most meaningful form: healing human lung disease.
GRANTS
This work was supported by the following grants: L. Ikonomou and D. N. Kotton: NIH PO1 HL047049 and USAMRC 07138002; A. R. Hemnes: NIH K08 HL093363; G. Bilousova: DEBRA AWD-100972; R. Hamid: NIH R01 HL102020; J. E. Loyd: NIH P01 HL 72058; A. K. Hatzopoulos: HL083958 and HL100398; S. M. Majka: AHA-GIA and NIH R01 HL091105; E. D. Austin: NIH K23 HL098743 and VUMC Turner-Hazinski Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
A special thanks to Dr. James West for critical input in the review of this manuscript, as well as to Dr. Joyce Johnson for contribution of the normal pulmonary artery photo shown in Fig. 2.
REFERENCES
- 1. Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105: 1672–1678, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 292: H1120–H1128, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54: S55–S66, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bilousova G, Hyun Jun D, King KB, DeLanghe S, Chick WS, Torchia EC, Chow KS, Klemm DJ, Roop DR, Majka SM. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vitro. Stem Cells 29: 206–216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell AI, Kuliszewski MA, Stewart DJ. Cell-based gene transfer to the pulmonary vasculature: Endothelial nitric oxide synthase overexpression inhibits monocrotaline-induced pulmonary hypertension. Am J Respir Cell Mol Biol 21: 567–575, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Castellani C, Cuppens H, Macek M, Jr, Cassiman JJ, Kerem E, Durie P, Tullis E, Assael BM, Bombieri C, Brown A, Casals T, Claustres M, Cutting GR, Dequeker E, Dodge J, Doull I, Farrell P, Ferec C, Girodon E, Johannesson M, Kerem B, Knowles M, Munck A, Pignatti PF, Radojkovic D, Rizzotti P, Schwarz M, Stuhrmann M, Tzetis M, Zielenski J, Elborn JS. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 7: 179–196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi KKM, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development 125: 725–732, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Colman A, Dreesen O. Pluripotent stem cells and disease modeling. Cell Stem Cell 5: 244–247, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cookson WO, Moffatt MF. Genetics of asthma and allergic disease. Hum Mol Genet 9: 2359–2364, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Daley GQ, Lensch MW, Jaenisch R, Meissner A, Plath K, Yamanaka S. Broader implications of defining standards for the pluripotency of iPSCs. Cell Stem Cell 4: 200–201, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321: 1218–1221, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 324: 1673–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154–156, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Ghofrani HA, Barst RJ, Benza RL, Champion HC, Fagan KA, Grimminger F, Humbert M, Simonneau G, Stewart DJ, Ventura C, Rubin LJ. Future perspectives for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol 54: S108–S117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One 3: e2904, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang PY, He ZY, Ji SY, Sun HW, Xiang D, Liu CC, Hu YP, Wang X, Hui LJ. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475: 386–389, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, Cuervo N, Moore KJ, Hodge SE, Knowles JA, Morse JH. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J 20: 518–523, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther 89: 655–661, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471: 225–229, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Jungebluth P, Luedde M, Ferrer E, Luedde T, Vucur M, Peinado VI, Go T, Schreiber C, Richthofen MV, Bader A, Haag J, Darsow KH, Bartel SJ, Lange HA, Furlani D, Steinhoff G, Macchiarini P. Mesenchymal stem cells restore lung function by recruiting resident and non-resident proteins. Cell Transplant 2011 [DOI] [PubMed] [Google Scholar]
- 23. Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 114: I181–I185, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells 27: 806–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kattman SJ, Huber TL, Keller GM. Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11: 723–732, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature 444: 481–485, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Lawson WE, Loyd JE. The genetic approach in pulmonary fibrosis: can it provide clues to this complex disease? Proc Am Thorac Soc 3: 345–349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461: 402–406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 23: 215–221, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98: 818–827, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA, 3rd, Soubrier F, Trembath RC, Chung WK. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 54: S32–S42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA 106: 15768–15773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu YL, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143: 527–539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meissner A, Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature 439: 212–215, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363: 1397–1409, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132: 661–680, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol 13: 541–549, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16: 927–931, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell 134: 877–886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 101: 12543–12548, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science 329: 538–541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43: 25S–32S, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li YS, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 28: 848–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roberts KE, McElroy JJ, Wong WP, Yen E, Widlitz A, Barst RJ, Knowles JA, Morse JH. BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J 24: 371–374, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Rosenzweig EB, Morse JH, Knowles JA, Chada KK, Khan AM, Roberts KE, McElroy JJ, Juskiw NK, Mallory NC, Rich S, Diamond B, Barst RJ. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant 27: 668–674, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 361: 1533–1544, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 5: 584–595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475: 390–393, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, Lafyatis RA, Demierre MF, Weiss DJ, French DL, Gadue P, Murphy GJ, Mostoslavsky G, Kotton DN. Generation of transgene-free lung disease-specific human ips cells using a single excisable lentiviral stem cell cassette. Stem Cells 28: 1728–1740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: considerations before clinical applications. Cell Cycle 9: 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sztrymf BCF, Girerd B, Yaici A, Jais X, Sitbon O, Montani D, Souza R, Simonneau G, Soubrier F, Humbert M. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med 177: 1377–1383, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Toshner M, Morrell NW. Endothelial progenitor cells in pulmonary hypertension — dawn of cell-based therapy? Int J Clin Pract Suppl 165: 7–12, 2011 [DOI] [PubMed] [Google Scholar]
- 58. van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res 74: 244–255, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463: 1035–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453: 524–528, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li YL, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476: 228–231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Zhao Q, Liu Z, Wang Z, Yang C, Liu J, Lu J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg 84: 544–552, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96: 442–450, 2005 [DOI] [PubMed] [Google Scholar]