Abstract
Peroxisome proliferator-activated receptor (PPAR) γ activation attenuates hypoxia-induced pulmonary hypertension (PH) in mice. The current study examined the hypothesis that PPARγ attenuates hypoxia-induced endothelin-1 (ET-1) signaling to mediate these therapeutic effects. To test this hypothesis, human pulmonary artery endothelial cells (HPAECs) were exposed to normoxia or hypoxia (1% O2) for 72 h and treated with or without the PPARγ ligand rosiglitazone (RSG, 10 μM) during the final 24 h of exposure. HPAEC proliferation was measured with MTT assays or cell counting, and mRNA and protein levels of ET-1 signaling components were determined. To explore the role of hypoxia-activated transcription factors, selected HPAECs were treated with inhibitors of hypoxia-inducible factor (HIF)-1α (chetomin) or nuclear factor (NF)-κB (caffeic acid phenethyl ester, CAPE). In parallel studies, male C57BL/6 mice were exposed to normoxia (21% O2) or hypoxia (10% O2) for 3 wk with or without gavage with RSG (10 mg·kg−1·day−1) for the final 10 days of exposure. Hypoxia increased ET-1, endothelin-converting enzyme-1, and endothelin receptor A and B levels in mouse lung and in HPAECs and increased HPAEC proliferation. Treatment with RSG attenuated hypoxia-induced activation of HIF-1α, NF-κB activation, and ET-1 signaling pathway components. Similarly, treatment with chetomin or CAPE prevented hypoxia-induced increases in HPAEC ET-1 mRNA and protein levels. These findings indicate that PPARγ activation attenuates a program of hypoxia-induced ET-1 signaling by inhibiting activation of hypoxia-responsive transcription factors. Targeting PPARγ represents a novel therapeutic strategy to inhibit enhanced ET-1 signaling in PH pathogenesis.
Keywords: pulmonary hypertension, hypoxia, peroxisome proliferator-activated receptor γ, endothelin, endothelial cells
pulmonary hypertension (PH), defined as an elevation of the mean pulmonary artery pressure >25 mmHg at rest or 30 mmHg with exercise causes significant morbidity and mortality (13, 31). The pathogenesis of PH involves endothelial dysfunction with increased production of vasoconstrictors, e.g., endothelin-1 (ET-1), and reduced production of vasodilators, e.g., prostacyclin and nitric oxide (NO) (5, 14). Despite the availability of existing PH therapies that attenuate these derangements, PH morbidity and mortality remains unacceptably high (20, 44), indicating an urgent need for novel therapeutic strategies. Recent studies indicate that the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ), may play an important role in the pathophysiology of PH. Treatment with thiazolidinedione (TZD) PPARγ ligands attenuates PH in several experimental models (6, 19, 27, 36, 39). PPARγ ligands not only attenuate the development of, but also reverse established, chronic hypoxia-induced PH, right ventricular hypertrophy, and pulmonary vascular muscularization in mice (39). Reduced PPARγ expression is associated with PH (1, 16, 18), and smooth muscle- and endothelium-targeted depletion of PPARγ caused PH in mice (16, 18). Collectively, these reports suggest that PPARγ may regulate pathways contributing to PH pathogenesis, but the mechanisms for these effects remain to be established.
ET-1, an endothelium-derived 21-amino acid peptide, is the product of a gene on chromosome 6 that encodes the inactive 212-amino acid preprohormone, prepro-ET-1. The latter is cleaved by endopeptidases to the 39-amino acid big ET-1. A final cleavage step, performed by endothelin-converting enzyme (ECE), converts big ET-1 to the 21-amino acid product ET-1 (23, 56). ET-1, one of the strongest endogenous vasoconstrictors and mitogens (56), is expressed in and released by endothelial cells (ECs), epithelial cells, and mesenchymal cells in the normal lung (7, 9). Once released, ET-1 binds to two G protein-coupled endothelin receptors A and B (ETAR and ETBR) (2, 4). ETAR, located mainly on vascular smooth muscle cells, is responsible for mediating vasoconstriction, migration, and proliferation (37, 56). In contrast, ETBR is present predominantly on ECs and mediates vasorelaxation and release of NO and prostacyclin, leading to vasodilation and clearance of ET-1 from the pulmonary circulation (5, 56).
ET-1 contributes to the pathogenesis of pulmonary arterial hypertension (PAH) (14, 37). Plasma ET-1 concentrations are elevated in patients with PAH (46, 57), and ET-1 mRNA and protein expression is increased in PAH ECs and correlated with increased pulmonary vascular resistance (46). The importance of ET-1 signaling in PH pathogenesis is further emphasized by the development and implementation of nonselective (7) and ETAR-selective (9) receptor antagonists in the treatment of PH (25, 41). Despite successful implementation of ET receptor antagonism in the clinical management of PH, the clinical efficacy of these agents has been less than ideal (41). The current study therefore explored alternative intervention strategies to enhance attenuation of ET-1 signaling and PH by focusing on the PPARγ receptor.
PPARγ is a ligand-activated transcription factor that belongs to the nuclear hormone receptor superfamily (24, 28). The PPARγ receptor is activated by a diverse spectrum of ligands that include naturally occurring fatty acids and their metabolites as well as synthetic TZD medications such as rosiglitazone and pioglitazone, which are currently used in the United States to treat patients with type 2 diabetes (10). Although candidate mechanisms for the therapeutic effects of PPARγ ligands in PH have been reviewed (47), the precise mechanisms by which PPARγ activation regulates PH have not been defined. Once activated, PPARγ forms a heterodimer with the retinoid X receptor, RXR, and binds to PPAR response elements in the promoter region of responsive genes to increase their expression. PPARγ activation can also reduce expression of selected genes though transrepression mechanisms (43). For example, activation of PPARγ inhibited hypoxia-inducible factor-1α (HIF-1α) (29) and nuclear factor-κ light-chain-enhancer of activated B cells (NF-κB) (29, 34) through PPARγ-induced transrepression. In this study, we postulate that these transrepression effects of PPARγ are critical for modulation of ET-1 signaling in PH.
The current study examines the hypothesis that PPARγ regulates hypoxia-induced PH by modulating ET-1 signaling. To explore this hypothesis, mice or human pulmonary artery endothelial cells (HPAECs) were exposed to hypoxia and then treated with or without the PPARγ ligand rosiglitazone. The results demonstrate that hypoxia activates transcriptional mechanisms that upregulate multiple components in the ET-1 signaling pathway. Rosiglitazone attenuates hypoxia-induced PH (39) and concomitantly prevents enhanced ET-1 signaling. Collectively, these findings suggest that targeting PPARγ represents a novel strategy to successfully inhibit multiple steps of ET-1 signaling that contribute to PH pathogenesis.
MATERIALS AND METHODS
Reagents.
Human ET-1 and mouse ET-1 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R & D Systems (Minneapolis, MN). HPAECs were obtained from Invitrogen (Carlsbad, CA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from ATCC (Manassas, VA). ET-1, ECE-1, ETAR, ETBR, HIF-1α, NF-κB, and CDK4 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The following reagents were supplied by Sigma-Aldrich (St. Louis, MO): Trypan blue, FBS, dimethyl sulfoxide (DMSO), methyl cellulose, HIF-1α inhibitor chetomin, and NF-κB inhibitor caffeic acid phenethyl ester (CAPE). The PPARγ ligand rosiglitazone was obtained from Cayman Chemical (Ann Arbor, MI).
HPAECs.
Pulmonary artery ECs were isolated from the lungs of control or idiopathic pulmonary arterial hypertension (IPAH) patients as described (35), and lysates of these cells were generously provided by Dr. Suzy Comhair (Cleveland Clinic, Cleveland, OH).
Exposure of C57BL/6 mice to hypoxia.
Male C57BL/6 mice aged 8–12 wk old were purchased from the Jackson Laboratory (Bar Harbor, ME) and exposed to 10% O2 (hypoxia) or room air (control) for 3 wk as reported (39). All animals were given unrestricted access to water and standard mouse chow. Mice were housed socially in groups of five. During the last 10 days of the experiment, each animal was gavaged daily with rosiglitazone (10 mg·kg−1·day−1 in 100 μl 0.5% methyl cellulose) or with an equivalent volume of vehicle alone as reported (39). All animal studies were approved by the Institutional Animal Care and Use Committee of the Atlanta Veterans Affairs Medical Center.
Hypoxia-exposed HPAEC and cell proliferation assay.
HPAECs (passages 2–7) were exposed to hypoxia in a Biospherix (Lacona, NY) exposure chamber. HPAECs were placed in a standard incubator or hypoxia chamber that maintains temperature (37°C), O2 concentration (1%), and CO2 levels (5%) for 72 h. To examine the effect of rosiglitazone on hypoxia-induced cell proliferation, HPAECs were treated during the last 24 h of normoxia or hypoxia exposure by adding rosiglitazone (0–20 μM) or an equivalent volume of vehicle directly to the culture media, and cell proliferation was measured using MTT assays. In selected studies, HPAECs were pretreated with DMSO or the HIF-1α inhibitor chetomin (25 nM) or the NF-kB inhibitor CAPE (20 μM) for 3 h and then for the entire 72-h exposure to normoxia or hypoxia. All manipulations of cells exposed to hypoxia were performed in a glove box that maintains the hypoxic environment to avoid effects of reoxygenation during sample processing. The impact of hypoxia with or without rosiglitazone on HPAEC function was determined by assessing HPAEC proliferation using cell counting and MTT assays. HPAECs were counted on a hemocytometer. Cell viability was determined by Trypan blue exclusion assay. HPAECs were stained with 0.08% Trypan blue at room temperature for 5 min. The number of live cells was calculated as: total number of cells − number of stained cells. The data were then normalized to the cells in the control preparation. In selected experiments, proliferation was determined using a colorimetric method based on metabolic reduction of the soluble yellow tetrazolium dye MTT to its insoluble purple formazan as recently reported (17, 33).
ET-1 ELISA.
C57BL/6 mouse lung tissue was homogenized in tissue protein extraction reagent buffer (Thermo Scientific) and analyzed with a quantitative sandwich enzyme immunoassay for ET-1 (R&D Systems). Media from HPAECs exposed to normoxia or hypoxia with or without rosiglitazone were collected, and human ET-1 levels in HPAEC supernatants were measured using a high-sensitivity chemiluminescence ELISA kit (R&D Systems). ET-1 levels were normalized to total protein content of samples measured with a bicinchoninic acid assay (Pierce, Rockford, IL).
Immunohistochemistry.
Lungs from normoxia- or hypoxia-exposed C57BL/6 mice following treatment with vehicle or rosiglitazone were subjected to histological examination as described previously (39). Briefly, the lungs were isolated, pressure perfused with calcium-free EDTA-containing buffer followed by optimum-cutting temperature compound (Fisher), frozen, sectioned, fixed, and stained with primary antibodies to ET-1. Lung sections (8 μm) were incubated with biotinylated donkey antirabbit secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) followed by horseradish peroxidase-streptavidin (Vectastain kit; Vector Laboratories, Burlingame, CA). Color was developed with 3,3′-diaminobenzidine tetrahydrochloride substrate (Vector Laboratories). Nonimmune rabbit IgG isotype control primary antibody was used to control for nonspecific antibody binding. Multiple high-power photomicrographs were obtained using a Leica DM4000B microscope (Wetzlar, Germany).
Quantitative real-time PCR analysis.
RNA was extracted from lung tissue or HPAEC using an RNeasy kit (Quiagen, Valencia, CA) according to the manufacturer's protocol. The concentration of mRNA was quantified using an ND-1000 Spectrophotometer (NanoDrop Technology, Wilmington, DE). One microgram of RNA was reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR (qRT-PCR) was performed on samples using a Bio-Rad iCycler and analyzed by its software. The qRT-PCR specific primers were designed using Probe-Finder web-based software (Roche, Indianapolis, IN). ET-1, ECE-1, ETAR, and ETBR mRNA levels were normalized to 9S rRNA reference gene (Table 1). The comparative threshold cycles (Ct) values were normalized to the 9S rRNA reference gene using the 2−ΔΔCt method (32).
Table 1.
Primer information for real-time quantitative PCR
| Gene Symbol | mRNA Product Length, bp | Annealing Temperature, °C | Primer Sequences (5′-3′) | GenBank Accession No. |
|---|---|---|---|---|
| Human | ||||
| ET-1 | 359–418 | 60 | F: TCTCTGCTGTTTGTGGCTTG | NM_001955 |
| (60 bp) | R: GAGCTCAGCGCCTAAGACTG | |||
| ECE-1 | 552–624 | 60 | F: CATCAAGCACCTCCTCGAA | NM_001397 |
| (73 bp) | R: ACGGTAGTATACTTGCGCCTTT | |||
| ETAR | 1,222–1,287 | 60 | F: GCGCTCTTAGTGTTGACAGGT | NM_001957 |
| (66 bp) | R: GAATCCCAATTCCCTGAACA | |||
| ETBR | 158–240 | 60 | F: CTTGGAGTCTGGACATCTGAAA | NM_000115 |
| (83 bp) | R: CTGCATGCTGCTACCTGCT | |||
| 9S rRNA | 237–338 | 60 | F: CTGACGCTTGATGAGAAGGAC | NM_001013 |
| (102 bp) | R: CAGCTTCATCTTGCCCTCAT | |||
| Mouse | ||||
| ET-1 | 447–531 | 60 | F: CTGCTGTTCGTGACTTTCCA | NM_010104 |
| (85 bp) | R: TCTGCACTCCATTCTCAGCTC | |||
| ECE-1 | 773–846 | 60 | F: CCCTGATGGAGCTGATTGA | NM_199307 |
| (74 bp) | R: GTCCTGGAAGTTGTCCTTGG | |||
| ETAR | 919–979 | 60 | F: GGGCATCACCGTCTTGAA | NM_010332 |
| (61 bp) | R: GGAAGCCACTGCTCTGTACC | |||
| ETBR | 1,135–1,226 | 60 | F: CGGTATGCAGATTGCTTTGA | NM_007904 |
| (92 bp) | R: AACAGAGAGCAAACACGAGGA | |||
| 9S rRNA | 437–552 | 60 | F: ATCCGCCAACGTCACATT | NM_029767 |
| (116 bp) | R: CCGCCGCCATAAGGAGAAC |
ET-1, endothelin-1; ECE-1, endothelin-converting enzyme-1; ETAR, endothelin receptor A; ETBR, endothelin receptor B; F, forward; R, reverse.
Western blot analysis.
After treatment with normoxia or hypoxia with or without rosiglitazone, mouse lung or HPAEC protein lysates were subjected to Western blot analysis as reported (39). Primary antibodies included ET-1, ECE-1, ETAR, ETBR, and CDK4 antibodies. Proteins were visualized using a peroxidase-coupled anti-goat, anti-rabbit, or anti-mouse IgG in the presence of LumiGlo reagent (Thermo Scientific). Bands were identified by chemiluminescence, quantified by laser densitometry (Bio-Rad), and normalized to CDK4 levels within the same lane.
Analysis of transcription factor activation.
Electrophoretic mobility shift assay (EMSA) or p65 nuclear translocation were employed to examine hypoxia and rosiglitazone-induced alterations in the nuclear binding of NF-κB or HIF-1α. Nuclear extracts from HPAECs were prepared using a commercial kit (ActiveMotif, Carlsbad, CA). Double-stranded consensus oligonucleotides or mutated probes for HIF-1α (50, 51) and NF-κB (30) were radiolabeled with [32P-γ]ATP using T4 polynucleotide kinase enzyme. Nuclear protein (5 μg) was incubated with radiolabeled probes, and then DNA-protein complexes were separated on 6% native polyacrylamide. Gels were fixed and exposed to X-ray film. DNA-protein bands were quantified by densitometric scanning using a GS-800 laser densitometer (Bio-Rad). To analyze NF-κB p65 activation and nuclear translocation, HPAEC were seeded in eight-well chamber slides (Lab-Tek, Vernon Hills, IL) and grown to confluency. After treatment with control or hypoxic conditions with or without rosiglitazone, HPAEC were washed, fixed with 4% (vol/vol) paraformaldehyde, and then permeabilized with 0.1% (vol/vol) Triton X-100. After being blocked with 0.5% BSA/PBS, primary anti-p65 rabbit polyclonal antibody (1:100 dilution; Santa Cruz Biotechnology) was added. HPAEC were then incubated overnight at 4°C followed by incubation with DyLight 488 conjugated secondary goat anti-rabbit antibody (1:500 dilution; Thermo Scientific, Waltham, MA). The chamber slides were then washed and mounted with Vectashield mounting medium with DAPI reagent (Vector Laboratories). Confocal images were captured on an Olympus laser scanning microscope equipped with the appropriate filter sets.
Statistical analysis.
In all experiments, data were analyzed by one-way ANOVA followed by post hoc analysis with Student-Newman-Keuls test to examine differences between specific groups using the software GraphPad Prism, version 4.0 (La Jolla, CA). The level of statistical significance was taken as P < 0.05.
RESULTS
Rosiglitazone attenuates hypoxia-induced ET-1 levels in C57BL/6 mouse lung in vivo and in HPAEC in vitro.
Recent studies showed that ET-1 mRNA levels were increased in pulmonary microvascular ECs isolated from patients with IPAH compared with control groups (8). To our knowledge, the present study is the first to report that pulmonary artery ECs isolated from patients with IPAH demonstrate increased ET-1 mRNA levels compared with control groups (Fig. 1A). Because rosiglitazone administered by gavage attenuated hypoxia-induced PH, right ventricular hypertrophy, and pulmonary vascular remodeling in mice (39), we used this model in the current studies, as shown in Fig. 1, to examine ET-1 signaling in the hypoxic mouse model. Hypoxia increased ET-1 levels in C57BL/6 mouse lung nearly sevenfold, and treatment with the PPARγ ligand rosiglitazone reduced hypoxia-mediated increases in lung ET-1 (Fig. 1B). Similarly hypoxia (1% O2 × 72 h)-induced ET-1 release from HPAECs was attenuated by rosiglitazone treatment (Fig. 1C). As shown in Fig. 1D, lungs from hypoxia-exposed C57BL/6 mice demonstrated increased intensity of staining with the ET-1 antibody, and staining intensity was attenuated in lungs from hypoxia-exposed mice treated with rosiglitazone. Immunostaining (brown coloration) was observed in small arterioles and alveolar structures in each treatment group. These findings provide additional evidence that hypoxia significantly increases ET-1 expression in the pulmonary vasculature. There was no staining with nonimmune IgG isotype control primary antibody (data not shown). To examine the effect of rosiglitazone on hypoxia-induced cell proliferation, HPAECs were treated with rosiglitazone (0–20 μM) and examined using MTT assays. Figure 1E shows that a low concentration of rosiglitazone (1 and 5 μM) had no effect on hypoxia-induced HPAEC proliferation, whereas 10 μM rosiglitazone treatment significantly decreased hypoxia-induced HPAEC proliferation compared with control conditions. Therefore, we used 10 μM concentrations of rosiglitazone in all subsequent experiments.
Fig. 1.
Treatment with rosiglitazone (RSG) attenuates hypoxia-induced endothelin (ET)-1 protein levels in vivo and in vitro. A: pulmonary artery endothelial cells were isolated from the lungs of control or idiopathic pulmonary arterial hypertension (IPAH) patients and characterized as reported (35). Quantitative real-time PCR was performed for ET-1. Each bar presents the mean ± SE ET-1 mRNA levels relative to 9S. *P < 0.05 vs. control, n = 3 experiments. B: whole lung homogenates were collected from mice exposed to normoxia (NOR, 21% O2) or hypoxia (HYP, 10% O2) for 3 wk. During the last 10 days of this exposure, selected animals were also treated ± RSG (10 mg·kg−1·day−1 by gavage) as reported (39). Lung ET-1 levels were measured with enzyme-linked immunosorbent assay (ELISA). P < 0.05 vs. NOR (*) and vs. HYP (+); n = 5–6. C: human pulmonary artery endothelial cells (HPAECs) were exposed to NOR (21% O2) or HYP (1% O2) for 72 h. Selected cells were treated during the final 24 h of exposure with RSG (10 μM). At the conclusion of the study, HPAEC media were collected, subjected to ET-1 ELISA assay, and normalized to total cell protein. Each bar represents the average ET-1 level ± SE from 6–9 samples in each treatment group expressed relative to control values. P < 0.05 vs. NOR (*) and vs. HYP (+). D: immunohistochemical detection of ET-1 in lungs from C57BL/6 mice following exposure to NOR or HYP and treatment with vehicle or RSG. Representative 8-μm sections are presented. Immunostaining (brown coloration) was noted in small arterioles and alveolar structures in each treatment group. Scale bar = 50 μm. There was no staining with nonimmune IgG isotype control primary antibody (data not shown). E: HPAEC were exposed to NOR or HYP ± RSG (as described in C), and cell proliferation was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. DMSO, dimethyl sulfoxide. Each bar represents the average HPAEC proliferation level ± SE from 6–9 samples in each treatment group expressed relative to control values. P < 0.05 vs. NOR (*) and vs. HYP (+).
Rosiglitazone attenuates hypoxia-induced increases in ET-1, ECE-1, and ET-1 receptors in vivo and in vitro.
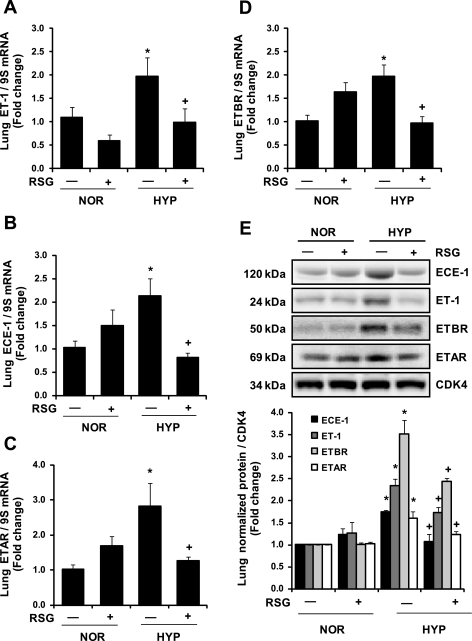
Biological effects of ET-1 can be regulated by the expression of ECE-1, an enzyme involved in ET-1 processing, as well as by the expression of endothelin receptors, ETAR and ETBR. With the use of qRT-PCR, expression of ECE-1, ETAR, and ETBR was examined in lung tissue from mice exposed to hypoxia with or without rosiglitazone as described in Fig. 1B. Hypoxia increased ET-1, ECE-1, ETAR, and ETBR mRNA and protein levels compared with control animals, and treatment with rosiglitazone attenuated those increases (Fig. 2). Similarly, in HPAEC in vitro (as described in Fig. 1C), hypoxia-induced increases in ET-1, ECE-1, and ETBR mRNA and protein levels were attenuated by rosiglitazone (Fig. 3). Although rosiglitazone attenuated hypoxia-induced increases in HPAEC ETAR mRNA and protein levels, at baseline, ETAR mRNA levels were only 1/30th the level of ETBR, indicating that ETBR is the major ET receptor expressed in HPAECs (data not shown).
Fig. 2.
RSG attenuates HYP-induced increases in ET-1 signaling pathway mRNA levels in mouse lung. C57BL/6 mice were exposed to NOR (21% O2) or HYP (10% O2) ± treatment with RSG as described in Fig. 1. Each bar represents the mean ± SE ET-1 (A), endothelin-converting enzyme (ECE)-1 (B), endothelin receptor A (ETAR, C), and endothelin receptor B (ETBR, D) mRNA relative to ribosomal 9S and expressed relative to control. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 5. Mouse lungs were collected for Western blotting using antibodies directed against ECE-1, ET-1, ETBR, ETAR, and CDK4 (E). Representative blots from 3 separate experiments are presented above average densitometric values ± SE for each protein. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 3.
Fig. 3.
RSG attenuates HYP-induced increases in HPAEC ET-1 signaling components and proliferation. HPAEC were exposed to NOR (21% O2) or HYP (1% O2) for 72 h. Selected cells were treated during the final 24 h of exposure with RSG (10 μM). Each bar represents the mean ± SE ET-1 (A), ECE-1 (B), and ETBR (C) mRNA levels relative to ribosomal 9S normalized to the control value from 6–9 samples in each treatment group. P < 0.05 vs. NOR (*) and vs. HYP (+). HPAECs were collected for Western blotting using antibodies directed against ECE-1, ET-1, ETBR, and CDK4 (D). Representative blots from 3 separate experiments are presented above average densitometric values ± SE for each protein. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 3. The impact of HYP ± RSG on HPAEC proliferation was determined using cell counting and MTT assays (E). Each bar represents the mean ± SE relative to control. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 6.
Rosiglitazone inhibits hypoxia-induced HPAEC cell proliferation.
To investigate whether PPARγ activation could inhibit hypoxia-induced proliferation of HPAECs, HPAECs were placed in normoxic or hypoxic conditions for 72 h. During the last 24 h, dishes were exposed to vehicle (DMSO) or rosiglitazone (10 μM). As shown in Fig. 3E, hypoxia-induced increases in endothelin signaling pathway components were associated with increased HPAEC cell proliferation. In contrast, these alterations were attenuated by the PPARγ ligand rosiglitazone.
Rosiglitazone decreases nuclear binding of transcription factors involved in ET-1 expression.
The comparable regulation of multiple components within the ET-1 signaling pathway by PPARγ following hypoxic activation suggested that PPARγ targets common proximal upstream regulators of ET-1 pathway components and led us to consider PPARγ-mediated transcriptional regulation. The ET-1, ECE, ETAR, and ETBR promoters have binding sites for transcription factors known to be activated by hypoxia such as HIF-1α and NF-κB (26, 38, 42, 48, 52). Because previous reports have demonstrated that chronic hypoxia upregulates HIF-1α through transcriptional mechanisms (48) (in addition to the originally described hypoxia-mediated prolyl hydroxylase inhibition and HIF-1α protein stabilization) (3), EMSAs were performed to evaluate the effect of hypoxia and rosiglitazone treatment on HIF-1α and NF-κB nuclear binding in HPAECs. As shown in Fig. 4, hypoxia increased nuclear binding of HIF-1α (Fig. 4, A and B) and NF-κB (Fig. 4, C and D) compared with control conditions, and rosiglitazone decreased HIF-1α and NF-κB nuclear binding. Consistent with these findings, hypoxia-induced nuclear translocation of NF-κB p65 was attenuated by rosiglitazone (Fig. 4E).
Fig. 4.
RSG attenuates HYP-induced HYP-inducible factor (HIF)-1α and nuclear factor (NF)-κB nuclear binding in HPAEC. HPAEC were exposed to NOR (21% O2) or HYP (1% O2) for 72 h. During the final 24 h of exposure, selected cells were treated with RSG (RSG, 10 μM). Nuclear proteins were extracted from HPAECs and then incubated with radiolabeled HIF-1α (A) and NF-κB (C) oligonucleotides and subjected to electrophoretic mobility shift assay analysis. DNA-protein complexes were separated on a native polyacrylamide gel, and densitometric analysis of bands was performed (B and D). Arrows, probe shift due to HIF-1α and NF-κB protein binding and unbound free probe; P, probe alone; C, control 50× unlabeled probe; Cm, control mutated 50× probe. Each bar represents the mean ± SE. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 3. In E, the intracellular localization of the NF-κB subunit p65 was investigated by immunofluorescence by using specific p65 antibody. Images are representative of 3 experiments. Scale bar = 10 μm. There was no staining with nonimmune IgG isotype control primary antibody (data not shown).
Rosiglitazone attenuates HIF-1α and NF-κb mRNA expression.
We also investigated whether exposure to hypoxia would increase HIF-1α and NF-κB mRNA levels. As shown in Fig. 5, rosiglitazone attenuated hypoxia-induced increases in HIF-1α (Fig. 5A) and NF-κB (Fig. 5B) mRNA levels in mouse lung. Similarly, hypoxia-induced increases in HPAEC HIF-1α (Fig. 5C) and NF-κB (Fig. 5D) mRNA levels were also attenuated by rosiglitazone.
Fig. 5.
RSG attenuates HYP-induced increases in HIF-1α and NF-κB mRNA levels in mouse lung and in HPAEC. C57BL/6 mice (A and B) or HPAEC (C and D) were exposed to NOR or HYP ± RSG as described in Fig. 1. Each bar represents the mean ± SE HIF-1α or NF-κB mRNA level relative to ribosomal 9S and expressed relative to control. P < 0.05 vs. NOR (*) and 9s. HYP (+); n = 5–9.
To further establish the importance of hypoxic activation of HIF-1α and NF-κB in enhanced ET-1 signaling, the ability of rosiglitazone to inhibit hypoxic induction of ET-1 was compared with the HIF-1α inhibitor chetomin and with the NF-κB inhibitor CAPE (Fig. 6). Consistent with the findings in Fig. 1C, rosiglitazone attenuated hypoxic increases in HPAEC ET-1 mRNA and protein levels. Treatment with chetomin (25 nM) or CAPE (20 μM) similarly attenuated hypoxia-induced ET-1 mRNA (Fig. 6A) and protein (Fig. 6B) levels. These results confirm that modulation of hypoxia-activated transcription factors regulates ET-1 signaling in human ECs and that targeting these transcription factors with PPARγ ligands may represent a novel and selective approach to attenuate ET-1-induced alterations in the pulmonary circulation.
Fig. 6.
Modulation of HYP-induced ET-1 mRNA and protein levels by inhibitors of HIF-1α or NF-κB in HPAECs. HPAECs were pretreated with DMSO, with the HIF-1α inhibitor chetomin (CTM, 25 nM), or with the NF-κB inhibitor caffeic acid phenethyl ester (CAPE, 20 μM) for 3 h. HPAECs were then exposed to NOR (21% O2) or HYP (1% O2) for 72 h in the presence of absence of CTM or CAPE. During the final 24 h of exposure, selected HPAEC were treated with RSG (10 μM). In A, each bar represents the mean ± SE ET-1 mRNA level relative to 9S and expressed relative to control. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 3. In B, representative Western blots for ET-1 and CDK4 are presented. In C, average ET-1 densitometric values ± SE are presented. P < 0.05 vs. NOR (*) and vs. HYP (+); n = 3.
DISCUSSION
ET-1 plays a critical role in endothelial dysfunction, an early event in the pathogenesis of PH. Pharmacological blockade of ET receptors constitutes one of the currently available therapies for PH; however, therapeutic trials with ET receptor antagonists in patients with PH have met with mixed results (11). Furthermore, treatment with ET receptor antagonists is not recommended in subsets of PH patients such as those with PH related to chronic obstructive pulmonary disease (11, 41). While a recent meta-analysis demonstrates that ET receptor blockers provide benefits in some PH patients (11, 13, 44), PH morbidity and mortality remain high. Collectively, these findings support an important pathophysiological role for ET-1 signaling in PH pathogenesis and suggest that new strategies beyond ET receptor antagonism that regulate ET-1 signaling may contribute to novel approaches to PH therapy.
Recent studies indicate that stimulating PPARγ with TZD ligands, such as rosiglitazone or pioglitazone, attenuates PH in several experimental models (6, 19, 27, 36, 39). Because previous evidence suggests that PPARγ can regulate ET-1 signaling in the systemic circulation (21, 22, 40), the current study examines PPARγ and its ability to regulate ET-1 signaling in mouse lung and HPAECs. Our studies provide novel evidence that hypoxia activates transcriptional mechanisms involved in upregulating multiple components of the ET-1 signaling pathway and that treatment with rosiglitazone mediates coordinated reductions in hypoxia-induced ET-1 signaling pathway components. These findings suggest that targeting PPARγ may represent a novel strategy to successfully inhibit multiple steps in ET-1 signaling that contribute to PH pathogenesis.
Hypoxia stimulates PH pathogenesis in human (15) and animal subjects (6, 27, 39). Chronic hypoxia-induced PH is a frequent clinical problem that contributes to pulmonary vasoconstriction, pulmonary vascular remodeling, endothelial dysfunction, right ventricular failure, and death. We recently reported that C57BL/6 mice exposed to chronic hypoxia developed increases in right ventricular systolic pressure, right ventricular hypertrophy, and pulmonary vascular remodeling that were attenuated by treatment with rosiglitazone (39). In the current study using this same model, hypoxia (10% O2 for 3 wk) increased ET-1 levels in C57BL/6 mouse lung, and treatment with rosiglitazone attenuated hypoxia-induced lung ET-1 levels. These observations are consistent with recent reports demonstrating that treatment with rosiglitazone reduced ET-1 levels in Sprague-Dawley rats exposed to 12% O2 for 4 wk (27). To our knowledge, the current studies provide the first demonstration that PPARγ ligands also attenuate hypoxic increases in other components of the ET-1 signaling pathway, including ECE, ETAR, and ETBR. Our in vitro studies further indicate that hypoxia-induced ET-1 release from HPAECs was attenuated by rosiglitazone treatment. These findings provide novel evidence that PPARγ can coordinately regulate the expression of a program of ET-1 signaling components. Because pharmacological ligands for the PPARγ receptor are currently employed in the treatment of type 2 diabetes in the United States, these findings suggest that PPARγ ligands could be examined in clinical trials of PH therapy. The relevance of these observations is supported by the current finding that ET-1 mRNA levels are increased in HPAECs derived from patients with PH and by previous reports of HIF activation (12) and impaired NO production(53, 54) in IPAH ECs.
PPARγ not only regulated hypoxia-induced ET-1 levels, but also other ET-1 signaling pathway components, such as ECE-1, ETAR, and ETBR mRNA and protein in vitro and in vivo. Furthermore, hypoxia-induced increases in ET-1 signaling components were associated with increased HPAEC proliferation and were attenuated by treatment with rosiglitazone. Significant effects of rosiglitazone treatment on components of the ET-1 signaling pathway were observed only in hypoxia-exposed mice or cells and were not observed following exposure to control conditions. Furthermore, these in vitro and in vivo effects on the ET-1 signaling pathway were accomplished by administering rosiglitazone only during the latter third of the hypoxia exposure period. These findings indicate that PPARγ activation with rosiglitazone can attenuate and reverse a diverse spectrum of hypoxia-induced increases in ET-1 signaling pathway components even when introduced late in the course of hypoxia exposure.
The molecular mechanisms whereby PPARγ inhibits gene expression in response to hypoxia are not fully defined. PPARγ activation can inhibit gene expression through transrepression mechanisms (43) that involve inhibitory cross talk between PPARγ and other proinflammatory transcription factors, such as HIF-1α (29) or NF-κB (29, 34). Our studies provide several lines of evidence supporting involvement of PPARγ-mediated transrepression in the effects of rosiglitazone on hypoxic stimulation of ET-1 signaling. First, previous reports demonstrated that hypoxia stimulates HIF-1α binding to the ET-1 promoter (29, 55). Our findings not only confirm that hypoxia stimulates HIF-1α and NF-κB activation but demonstrate that rosiglitazone inhibits hypoxia-induced activation of these transcription factors. These results suggest that PPARγ represses HIF-1α or NF-κB transcriptional activity. Second, in silico analysis of the promoter elements of the ET-1 signaling components examined demonstrated that, while the ETBR promoter contains putative PPAR response elements, the ET-1 and ETAR promoters did not. Absence of direct regulation of ET-1, ECE, ETAR, or ETBR expression by rosiglitazone alone further suggests that the observed effects of rosiglitazone are unlikely mediated through activation and binding of PPARγ to ET-1 signaling component promoters. In addition, the ET-1, ECE, ETAR, and ETBR promoters contain binding sites for transcription factors known to be activated by hypoxia (26, 38, 42, 48, 52) such as HIF-1α (45, 55) or NF-κB (reviewed in Ref. 46) (49), indicating that indirect, PPARγ-mediated regulation of HIF-1α and NF-κB transcriptional activity provides plausible mechanisms for the observed regulatory effects of rosiglitazone. Third, applying pharmacological interventions to inhibit hypoxic-mediated activation of HIF-1α or NF-κB produced comparable reductions in hypoxia-induced increases in ET-1 mRNA and protein levels. Collectively, these findings indicate that rosiglitazone attenuated hypoxic upregulation of ET-1 signaling pathway components through PPARγ-mediated transrepression of hypoxic transcriptional activation involving HIF-1α and NF-κB. These hypothetical relationships are depicted in schematic form in Fig. 7.
Fig. 7.
Putative peroxisome proliferator-activated receptor (PPAR) γ-regulated pathways of endothelin signaling in HYP-induced pulmonary hypertension (PH). HYP activates transcription factors, such as HIF-1α or NF-kB, that increase levels of ET-1 and related signaling components (ECE-1, ETAR, and ETBR). RSG attenuates hypoxic stimulation of HIF-1α and NF-kB nuclear binding, thereby reducing hypoxic upregulation of ET-1 and its signaling components that contribute to pathophysiological derangements such as pulmonary vasoconstriction and vascular remodeling. Attenuation of hypoxic activation of ET-1 signaling thereby contributes to the ability of PPARγ ligands to attenuate PH.
The current study has several important limitations that should be recognized. First, hypoxia-induced PH in rodents does not reproduce the characteristic obliterative plexogenic arteriopathy seen in patients with severe forms of PAH. Thus extrapolation of the current findings that targeting PPARγ effectively reduces upregulation of ET-1 signaling pathway components will require confirmation in models that more accurately recapitulate the pathology of PAH. Second, our results provide indirect evidence that hypoxia-induced increases in ET-1 signaling pathway components are mediated by HIF-1α or NF-κB. Ongoing studies in our laboratories will utilize chromatin immunoprecipitation assays to confirm that: 1) hypoxia stimulates binding of either HIF-1α or NF-κB to the promoter elements of ET-1, ECE, ETAR, and ETBR and 2) that PPARγ ligands inhibit HIF-1α or NF-κB binding and promoter activation. Finally, the ultimate application of strategies to target PPARγ to inhibit the ET-1 signaling program in PH would ideally be based on an analysis comparing the effectiveness of that strategy with current endothelin receptor antagonists. Nonetheless, it seems reasonable to postulate that PPARγ ligands, by inhibiting multiple steps in the ET-1 signaling pathway, might provide more effective endothelin signaling inhibition and simultaneously reduce other pathogenic pathways (39) to more effectively attenuate PH pathogenesis.
In summary, the current study extends previous reports to demonstrate that hypoxia increases the expression of ET-1, ECE, ETAR, and ETBR in mouse lung and in HPAECs and provides novel evidence that pharmacological PPARγ ligands can effectively attenuate the upregulation of this program of ET-1 signaling. Furthermore, the current findings provide additional evidence that rosiglitazone mediates its effects through transrepression of the activity of other hypoxia-sensitive transcription factors. These results and related studies provide additional rationale for the continued exploration of nuclear hormone receptors as therapeutic targets in lung disease and vascular biology. The current availability of pharmacological ligands for the PPARγ receptor and their widespread application in the treatment of type 2 diabetes suggest that confirmation of therapeutic benefits in preclinical models could be readily translated to clinical trials. Thus the current report provides additional insight into mechanisms by which targeting the PPARγ receptor modulates PH pathogenesis.
GRANTS
This study was supported by funding from the Research Service of the Atlanta Veterans Affairs Medical Center (C. M. Hart) and from NIH Grants DK-074518 and HL-102167.
DISCLOSURES
None
ACKNOWLEDGMENTS
We thank Kathy Park and Dr. Sinae Kim (Emory University) for technical assistance; Drs. Cherry Wongtrakool and Roy L. Sutliff (Emory University) for thoughtful discussion; and Drs. A. A. Comhair and S. C. Erzurum (Cleveland Clinic) for human pulmonary artery endothelial cells derived from control or idiopathic pulmonary arterial hypertension patients with support of NIH HL-60917 and HL-081064.
REFERENCES
- 1. Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Arai H, Nakao K, Takaya K, Hosoda K, Ogawa Y, Nakanishi S, Imura H. The human endothelin-B receptor gene. Structural organization and chromosomal assignment. J Biol Chem 268: 3463–3470, 1993 [PubMed] [Google Scholar]
- 3. Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourgeois C, Robert B, Rebourcet R, Mondon F, Mignot TM, Duc-Goiran P, Ferre F. Endothelin-1 and ETA receptor expression in vascular smooth muscle cells from human placenta: a new ETA receptor messenger ribonucleic acid is generated by alternative splicing of exon 3. J Clin Endocrinol Metab 82: 3116–3123, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Cacoub P, Dorent R, Nataf P, Carayon A. Endothelin-1 in pulmonary hypertension. N Engl J Med 329: 1967–1968, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cui B, Cheng YS, Dai DZ, Li N, Zhang TT, Dai Y. CPU0213, a non-selective ETA/ETB receptor antagonist, improves pulmonary arteriolar remodeling of monocrotaline-induced pulmonary hypertension in rats. Clin Exp Pharmacol Physiol 36: 169–175, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med 174: 1025–1033, 2006 [DOI] [PubMed] [Google Scholar]
- 9. DiCarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol Lung Cell Mol Physiol 269: L690–L697, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ Res 102: 283–294, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Dupuis J, Hoeper MM. Endothelin receptor antagonists in pulmonary arterial hypertension. Eur Respir J 31: 407–415, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176: 1130–1138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galie N, Manes A, Negro L, Palazzini M, Bacchi-Reggiani ML, Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J 30: 394–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Goerre S, Wenk M, Bartsch P, Luscher TF, Niroomand F, Hohenhaus E, Oelz O, Reinhart WH. Endothelin-1 in pulmonary hypertension associated with high-altitude exposure. Circulation 91: 359–364, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice causes PDGF receptor-β-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 297: L1082–L1090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther 5: 430–437, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 115: 1275–1284, 2007 [DOI] [PubMed] [Google Scholar]
- 20. He B, Zhang F, Li X, Tang C, Lin G, Du J, Jin H. Meta-analysis of randomized controlled trials on treatment of pulmonary arterial hypertension. Circ J 74: 1458–1464, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN, Schiffrin EL. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol 23: 45–51, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Iglarz M, Touyz RM, Viel EC, Paradis P, Amiri F, Diep QN, Schiffrin EL. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension 42: 737–743, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J Biol Chem 264: 14954–14959, 1989 [PubMed] [Google Scholar]
- 24. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Jasmin JF, Lucas M, Cernacek P, Dupuis J. Effectiveness of a nonselective ET(A/B) and a selective ET(A) antagonist in rats with monocrotaline-induced pulmonary hypertension. Circulation 103: 314–318, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, Yamaguchi I. Novel molecular mechanism of increased myocardial endothelin-1 expression in the failing heart involving the transcriptional factor hypoxia-inducible factor-1alpha induced for impaired myocardial energy metabolism. Circulation 103: 2387–2394, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 15: 659–668, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J 11: 1003–1013, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee KS, Kim SR, Park SJ, Park HS, Min KH, Jin SM, Lee MK, Kim UH, Lee YC. Peroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of mice. J Allergy Clin Immunol 118: 120–127, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Li YD, Ye BQ, Zheng SX, Wang JT, Wang JG, Chen M, Liu JG, Pei XH, Wang LJ, Lin ZX, Gupta K, Mackman N, Slungaard A, Key NS, Geng JG. NF-kappaB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J Biol Chem 284: 4473–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lilienfeld DE, Rubin LJ. Mortality from primary pulmonary hypertension in the United States, 1979–1996. Chest 117: 796–800, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Lu X, Murphy TC, Nanes MS, Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makino N, Sugano M, Satoh S, Oyama J, Maeda T. Peroxisome proliferator-activated receptor-gamma ligands attenuate brain natriuretic peptide production and affect remodeling in cardiac fibroblasts in reoxygenation after hypoxia. Cell Biochem Biophys 44: 65–71, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Matsuda Y, Hoshikawa Y, Ameshima S, Suzuki S, Okada Y, Tabata T, Sugawara T, Matsumura Y, Kondo T. [Effects of peroxisome proliferator-activated receptor gamma ligands on monocrotaline-induced pulmonary hypertension in rats]. Nihon Kokyuki Gakkai Zasshi 43: 283–288, 2005 [PubMed] [Google Scholar]
- 37. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 114: 1417–1431, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Minchenko A, Caro J. Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: role of hypoxia responsive element. Mol Cell Biochem 208: 53–62, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes 55: 3594–3603, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Price LC, Howard LS. Endothelin receptor antagonists for pulmonary arterial hypertension: rationale and place in therapy. Am J Cardiovasc Drugs 8: 171–185, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP. Endothelin 1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells. Diabetes 49: 1561–1570, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta 1771: 926–935, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ryerson CJ, Nayar S, Swiston JR, Sin DD. Pharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysis (Abstract). Respir Res 11: 12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shimada K, Takahashi M, Tanzawa K. Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J Biol Chem 269: 18275–18278, 1994 [PubMed] [Google Scholar]
- 46. Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 114: 464–469, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Sutliff RL, Kang BY, Hart CM. PPARgamma as a potential therapeutic target in pulmonary hypertension. Ther Adv Respir Dis 4: 143–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takanashi M, Miyauchi T, Kakinuma Y, Goto K, Yamaguchi I. Establishment of hypoxia inducible factor-1alpha overexpressing cells that produce endothelin-1. J Cardiovasc Pharmacol 44, Suppl 1: S268–S273, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Taylor CT, Cummins EP. The role of NF-kappaB in hypoxia-induced gene expression. Ann NY Acad Sci 1177: 178–184, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem 268: 21513–21518, 1993 [PubMed] [Google Scholar]
- 51. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270: 1230–1237, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Wort SJ, Ito M, Chou PC, Mc Master SK, Badiger R, Jazrawi E, de Souza P, Evans TW, Mitchell JA, Pinhu L, Ito K, Adcock IM. Synergistic induction of endothelin-1 by tumor necrosis factor alpha and interferon gamma is due to enhanced NF-kappaB binding and histone acetylation at specific kappaB sites. J Biol Chem 284: 24297–24305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 104: 1342–1347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem 276: 12645–12653, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]
- 57. Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, Temma S, Shirakami G, Imura H, Mikawa H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Evidence for increased production of endothelin in pulmonary circulation. Circulation 84: 2280–2285, 1991 [DOI] [PubMed] [Google Scholar]