Abstract
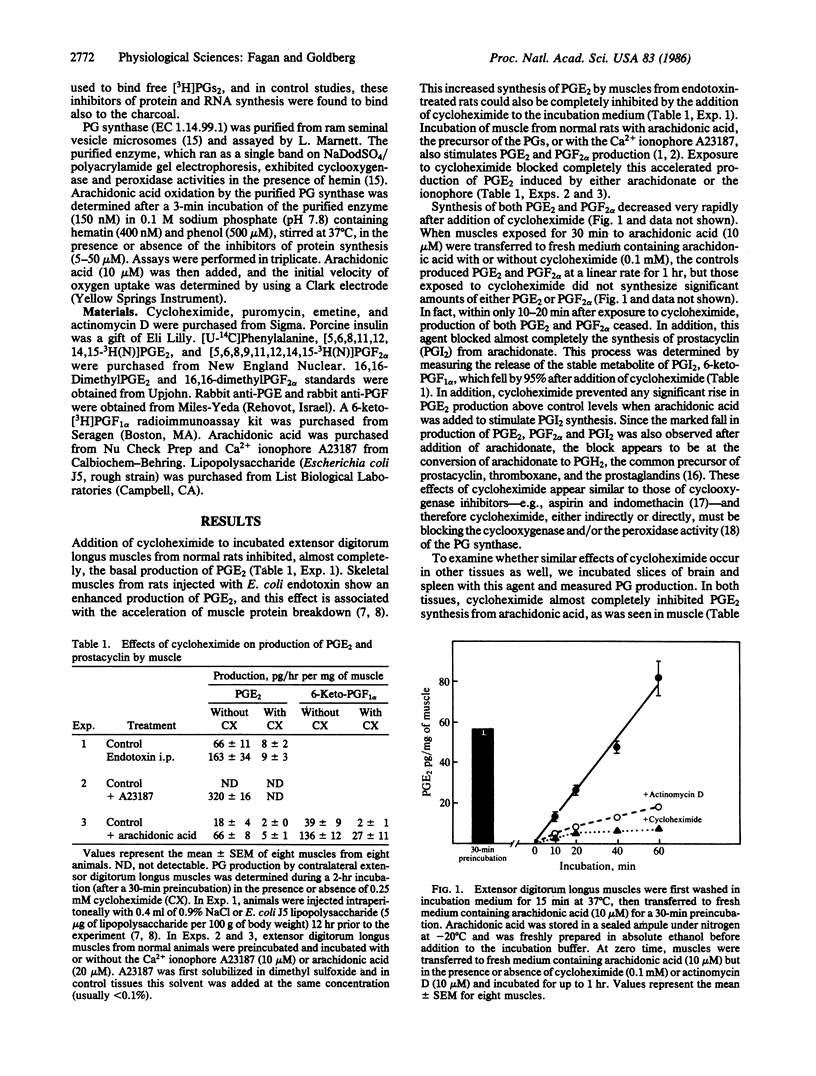
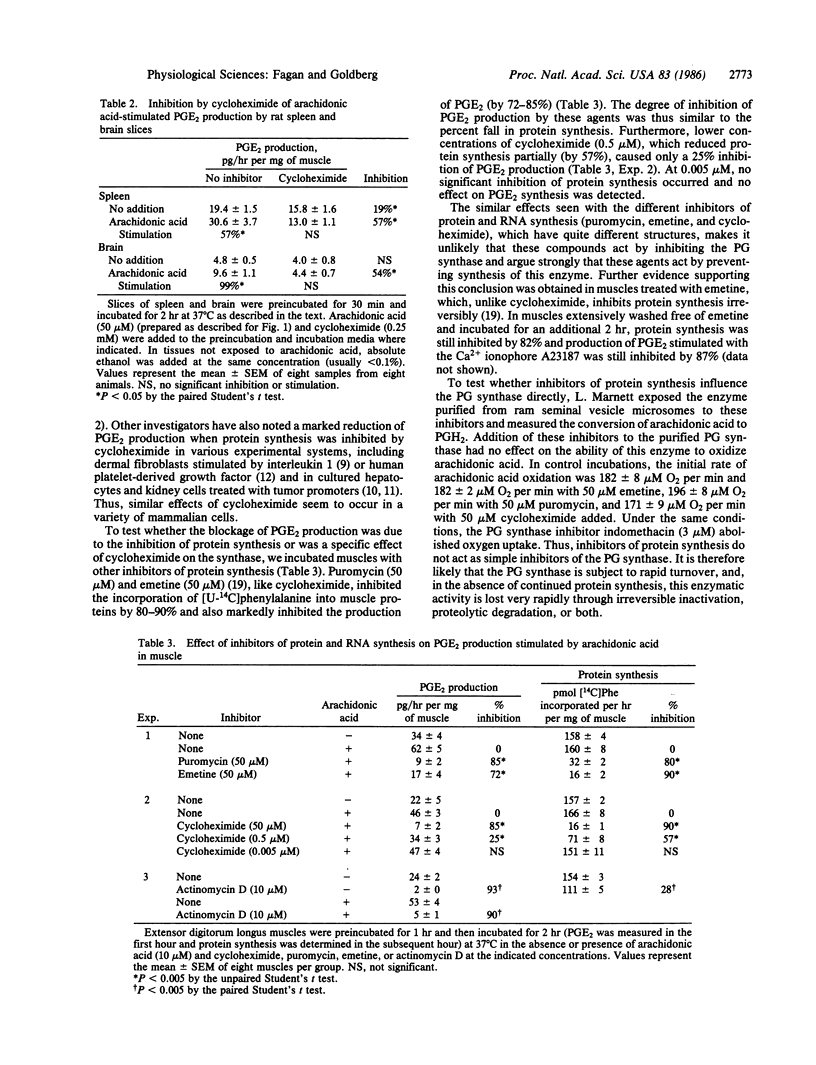
Inhibitors of protein or RNA synthesis prevented prostaglandin (PG) production in isolated skeletal muscles, brain, and spleen. Incubation of rat muscles with cycloheximide prevented the stimulation of PGE2 production induced in vitro by the Ca2+ ionophore A23187 and in vivo by injection of endotoxin. Cycloheximide also inhibited the stimulation by arachidonic acid of PGE2, PGF2 alpha, and prostacyclin. These observations suggest that the block in prostanoid production results from a loss of PG synthase activity (EC 1.14.99.1). These effects were detectable within 10 min after exposure of the muscle to cycloheximide. The degree of inhibition of PG production correlated with the degree of inhibition of protein synthesis. Other inhibitors of protein synthesis, puromycin and emetine, also prevented conversion of arachidonate into PGE2 in these tissues, but they did not inhibit purified PG synthase. Exposure of muscles to actinomycin D for 20 min also reduced PGE2 production from arachidonate by 90%. Thus, both the PG synthase and its mRNA appear to be inactivated rapidly (t1/2 less than 10 min) in muscle and other mammalian tissues. The block in PG production induced by inhibitors of protein and RNA synthesis may account for their antipyrogenic actions and certain of their other physiological effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Beetens J. R., Claeys M., Herman A. G. Antioxidants increase the formation of 6-oxo-PGF1 alpha by ram seminal vesicle microsomes. Biochem Pharmacol. 1981 Oct;30(20):2811–2815. doi: 10.1016/0006-2952(81)90419-6. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Marsh J. M., LeMaire W. J. Mechanism of luteinizing hormone regulation of prostaglandin synthesis in rat granulosa cells. J Biol Chem. 1978 Nov 10;253(21):7757–7761. [PubMed] [Google Scholar]
- Craig N., Kostura M. Inhibition of protein synthesis in CHO cells by actinomycin D: lesion occurs after 40S initiation complex formation. Biochemistry. 1983 Dec 20;22(26):6064–6071. doi: 10.1021/bi00295a004. [DOI] [PubMed] [Google Scholar]
- Cranston W. I., Hellon R. F., Townsend Y. Suppression of fever in rabbits by a protein synthesis inhibitor, anisomycin. J Physiol. 1980 Aug;305:337–344. doi: 10.1113/jphysiol.1980.sp013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Beaudry G. A., King L., Waite M. Regulation of arachidonic acid metabolism in Madin-Darby canine kidney cells. Comparison of A23187 and 12-O-tetradecanoyl-phorbol-13-acetate. Biochim Biophys Acta. 1984 Jan 17;792(1):33–38. doi: 10.1016/0005-2760(84)90279-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Baptista E. M., Kennicott K. L., VandenHeuvel W. J., Walker R. W., Fagerness P. E., Kuehl F. A., Jr Oxidation reactions by prostaglandin cyclooxygenase-hydroperoxidase. J Biol Chem. 1981 Jul 25;256(14):7352–7361. [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Beveridge G. C., Marnett L. J., Kuehl F. A., Jr Direct and indirect involvement of radical scavengers during prostaglandin biosynthesis. Adv Prostaglandin Thromboxane Res. 1980;6:153–155. [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Kuehl F. A., Jr Reduction of hydroperoxides in the prostaglandin biosynthetic pathway by a microsomal peroxidase. J Biol Chem. 1979 May 10;254(9):3295–3302. [PubMed] [Google Scholar]
- Egan R. W., Paxton J., Kuehl F. A., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Friedman P. A. Antibiotics and nucleic acids. Annu Rev Biochem. 1971;40:775–810. doi: 10.1146/annurev.bi.40.070171.004015. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G. The prevention by inhibitors of brain proptein synthesis of the hyperactivity and hyperpyrexia produced in rats by monoamine oxidase inhibition and the administration of L-tryptophan or 5-methoxy-N,N-dimethyltryptamine. J Neurochem. 1972 Oct;19(10):2409–2422. doi: 10.1111/j.1471-4159.1972.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Grollman A. P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Grulich J., Rothe D., Gronwald R., Loth U., Schettler G., Kommerell B., Ross R. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest. 1985 Apr;75(4):1381–1387. doi: 10.1172/JCI111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham E. A., Egan R. W., Soderman D. D., Gale P. H., Kuehl F. A., Jr Peroxidase-dependent deactivation of prostacyclin synthetase. J Biol Chem. 1979 Apr 10;254(7):2191–2194. [PubMed] [Google Scholar]
- Huslig R. L., Fogwell R. L., Smith W. L. The prostaglandin forming cyclooxygenase of ovine uterus: relationship to luteal function. Biol Reprod. 1979 Oct;21(3):589–600. doi: 10.1095/biolreprod21.3.589. [DOI] [PubMed] [Google Scholar]
- Kent R. S., Diedrich S. L., Whorton A. R. Regulation of vascular prostaglandin synthesis by metabolites of arachidonic acid in perfused rabbit aorta. J Clin Invest. 1983 Aug;72(2):455–465. doi: 10.1172/JCI110993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett L. J., Siedlik P. H., Ochs R. C., Pagels W. R., Das M., Honn K. V., Warnock R. H., Tainer B. E., Eling T. E. Mechanism of the stimulation of prostaglandin H synthase and prostacyclin synthase by the antithrombotic and antimetastatic agent, nafazatrom. Mol Pharmacol. 1984 Sep;26(2):328–335. [PubMed] [Google Scholar]
- Marnett L. J., Wlodawer P., Samuelsson B. Co-oxygenation of organic substrates by the prostaglandin synthetase of sheep vesicular gland. J Biol Chem. 1975 Nov 10;250(21):8510–8517. [PubMed] [Google Scholar]
- Miyamoto T., Ogino N., Yamamoto S., Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976 May 10;251(9):2629–2636. [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979 May 17;300(20):1142–1147. doi: 10.1056/NEJM197905173002006. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Mode of action of aspirin-like drugs. Adv Intern Med. 1979;24:1–22. [PubMed] [Google Scholar]
- Moore P. K., Hoult J. R. Anti-inflammatory steroids reduce tissue PG synthetase activity and enhance PG breakdown. Nature. 1980 Nov 20;288(5788):269–270. doi: 10.1038/288269a0. [DOI] [PubMed] [Google Scholar]
- Moore P. K., Hoult J. R. Pathophysiological states modify levels in rat plasma of factors which inhibit synthesis and enhance breakdown of PG. Nature. 1980 Nov 20;288(5788):271–273. doi: 10.1038/288271a0. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Chandrasekaran K., Hoffman J. C., McFarland V. W. Quantitation of a 55K cellular protein: similar amount and instability in normal and malignant mouse cells. Mol Cell Biol. 1982 Jul;2(7):763–771. doi: 10.1128/mcb.2.7.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. R., Moritz H., Needleman P. Mechanism of enhanced renal prostaglandin biosynthesis in ureter obstruction. Role of de novo protein synthesis. J Biol Chem. 1978 Nov 25;253(22):8210–8212. [PubMed] [Google Scholar]
- Palmer R. M., Reeds P. J., Atkinson T., Smith R. H. The influence of changes in tension on protein synthesis and prostaglandin release in isolated rabbit muscles. Biochem J. 1983 Sep 15;214(3):1011–1014. doi: 10.1042/bj2141011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Poyser N. L. Effect of actinomycin D on uterine prostaglandin production and oestrous cycle length in guinea-pigs. J Reprod Fertil. 1979 Jul;56(2):559–565. doi: 10.1530/jrf.0.0560559. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Evan G. I., Bishop J. M. The protein encoded by the human proto-oncogene c-myc. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7742–7746. doi: 10.1073/pnas.81.24.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M. The possible involvement of prostaglandin F2 alpha in the stimulation of muscle protein synthesis by insulin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1084–1090. doi: 10.1016/s0006-291x(83)80253-8. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Ruwe W. D., Myers R. D. Fever produced by intrahypothalamic pyrogen: effect of protein synthesis inhibition by anisomycin. Brain Res Bull. 1979 Nov-Dec;4(6):741–745. doi: 10.1016/0361-9230(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Siegert R., Philipp-Dormston W. K., Radsak K., Menzel H. Inhibition of Newcastle disease virus-induced fever in rabbits by cycloheximide. Arch Virol. 1975;48(4):367–373. doi: 10.1007/BF01317435. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Palmer R. M., Reeds P. J. Protein synthesis in isolated rabbit forelimb muscles. The possible role of metabolites of arachidonic acid in the response to intermittent stretching. Biochem J. 1983 Jul 15;214(1):153–161. doi: 10.1042/bj2140153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972 Aug 15;11(17):3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Stimulation and blockade of prostaglandin biosynthesis. J Biol Chem. 1971 Nov;246(21):6700–6702. [PubMed] [Google Scholar]
- Snoek G. T., Levine L. Requirements for protein synthesis and calcium for stimulation of prostaglandin synthesis in cultured rat liver cells by tumor promoters. Cancer Res. 1983 Oct;43(10):4743–4746. [PubMed] [Google Scholar]
- Spindler K. R., Berk A. J. Rapid intracellular turnover of adenovirus 5 early region 1A proteins. J Virol. 1984 Nov;52(2):706–710. doi: 10.1128/jvi.52.2.706-710.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. W., Dowling M. D., Jr Antipyretic effect of cycloheximide, and inhibitor of protein synthesis, in patients with Hodgkin's disease or other malignant neoplasms. Cancer Res. 1975 May;35(5):1218–1224. [PubMed] [Google Scholar]



