Abstract
The respiratory epithelium forms an important barrier against inhaled pollutants and microorganisms, and its barrier function is often compromised during inflammatory airway diseases. Epithelial activation of hypoxia-inducible factor-1 (HIF-1) represents one feature of airway inflammation, but the functional importance of HIF-1 within the respiratory epithelium is largely unknown. Using primary mouse tracheal epithelial (MTE) cells or immortalized human bronchial epithelial cells (16HBE14o−), we evaluated the impact of HIF-1 activation on loss of epithelial barrier function during oxidative stress. Exposure of either 16HBE14o− or MTE cells to H2O2 resulted in significant loss of transepithelial electrical resistance and increased permeability to fluorescein isothiocyanate-dextran (4 kDa), and this was attenuated significantly after prior activation of HIF-1 by preexposure to hypoxia (2% O2; 6 h) or the hypoxia mimics CoCl2 or dimethyloxalylglycine (DMOG). Oxidative barrier loss was associated with reduced levels of the tight junction protein occludin and with hyperoxidation of the antioxidant enzyme peroxiredoxin (Prx-SO2H), events that were also attenuated by prior activation of HIF-1. Involvement of HIF-1 in these protective effects was confirmed using the pharmacological inhibitor YC-1 and by short-hairpin RNA knockdown of HIF-1α. The protective effects of HIF-1 were associated with induction of sestrin-2, a hypoxia-inducible enzyme known to reduce oxidative stress and minimize Prx hyperoxidation. Together, our results suggest that loss of epithelial barrier integrity by oxidative stress is minimized by activation of HIF-1, in part by induction of sestrin-2.
Keywords: hydrogen peroxide, peroxiredoxin, occludin, sestrin-2, lung, permeability
the respiratory epithelium forms a critical barrier that acts as a first line of defense against environmental allergens, particulates, and microorganisms and produces numerous mediators involved in first-line host defense and innate airway immune responses. Being continuously subjected to hazardous and injurious environmental insults, the airway epithelium is endowed with effective defense and repair mechanisms to prevent loss of barrier function and restore epithelial integrity after injury. Disruption of epithelial integrity allows for increased invasion of infectious agents and may promote allergic sensitization and is an important pathological feature of several respiratory diseases, including allergic asthma (17, 33). Oxidative mechanisms, originating from environmental pollutants or from endogenous production of reactive oxygen species (ROS) during airway inflammation, are thought to contribute importantly to the pathology of several pulmonary inflammatory diseases, including asthma, cystic fibrosis, and chronic obstructive pulmonary disease (COPD) (11, 47, 57), and promote epithelial barrier injury and dysfunction by inducing apoptotic cell death or by the modulation of cytoskeletal networks and tight junctions (TJ) (4, 5). Therefore, the epithelium is equipped with abundant antioxidant enzyme systems to maintain cellular redox homeostasis and to counter the deleterious effects of enhanced ROS production, as well as several other proteins involved in remodeling and metabolism that can contribute to epithelial maintenance and repair (17, 28, 46).
A recently recognized feature of chronic inflammatory airway diseases is the activation of the heterodimeric transcription factor hypoxia-inducible factor-1 (HIF-1) within the airway or alveolar epithelium (36, 45, 56). Although HIF-1 is traditionally known as a master regulator of O2 homeostasis, and a key mediator of adaptive responses to tissue hypoxia (53), HIF-1 has more recently emerged as an important mediator in immune and inflammatory responses (18, 40). HIF-1 is largely regulated at the level of its oxygen-labile α-subunit (HIF-1α), which is rapidly hydroxylated and degraded during normoxic conditions and stabilized during conditions of hypoxia, allowing for its nuclear translocation and association with HIF-1β to initiate transcription of a number of hypoxia-responsive genes (53). In addition, HIF-1α is also subject to transcriptional or posttranscriptional regulation by various inflammatory mediators, which can enhance HIF-1 activation even under normoxic conditions (18, 40, 42). Because of the interdependence of innate immune and hypoxic responses to infection and tissue damage, activation of HIF-1 is common in conditions associated with infection or inflammation. HIF-1 activation promotes bactericidal properties of phagocytic cells (16) and supports innate immune functions of dendritic cells, mast cells, and epithelial cells (40) and thus has important consequences for both the pathogen and the host.
The functional consequences of HIF-1 activation within the airway epithelium are still unclear. While several studies have suggested contributing functions of HIF-1 to allergic airway inflammation in models of allergic asthma (23, 26, 32) and in mucus hypersecretion and globlet cell hyperplasia (20, 45), others have also suggested protective functions of epithelial HIF-1 against inflammation associated with hypoxia (50). Moreover, several studies in intestinal epithelia have implicated HIF-1 in transcriptional programs that facilitate barrier protection in models of experimental colitis or in response to hypoxia (22, 30), although it is still unclear whether airway epithelial HIF-1 activation could similarly protect airway epithelial integrity. The well-known protective effect of hypoxic preconditioning against oxidant-induced airway injury and edema (2, 61) would suggest a potential beneficial role of HIF-1 in airway epithelial barrier maintenance, although this has not been formally tested to date. The objective of the present studies was therefore to explore the impact of HIF-1 activation on airway epithelial barrier function in an in vitro model of oxidant-induced epithelial injury. Our results demonstrate that preactivation of HIF-1 indeed protects airway epithelia against oxidant-induced barrier dysfunction, which was associated with induction of the antioxidant protein sestrin-2 (Sesn2) and maintenance of occludin status and attenuation of peroxiredoxin (Prx) hyperoxidation.
MATERIALS AND METHODS
Cell culture.
Experiments were performed with the SV40-transformed human bronchial epithelial cell line 16HBE14o−, kindly provided by Dr. D. Gruenert (15) and cultured as described previously (41). Additional experiments were performed using primary mouse tracheal epithelial (MTE) cells isolated from C57BL/6J mice (The Jackson Laboratories), isolated and cultured as described previously (41). For measurements of epithelial barrier function, cells were plated in Transwell culture inserts (PET membrane pore size 0.4 μm; Corning) or on electric cell substrate impedance sensing (ECIS) cultureware electrode arrays (array model 8W10E+; Applied BioPhysics) and coated with a solution consisting of LHC basal medium (Invitrogen), BSA (Invitrogen), bovine collagen I (BD Laboratories), and human fibronectin (Sigma-Aldrich). MTE cells were seeded at a density of 1.5 × 105 in Transwell culture inserts coated with rat tail collagen I (BD Laboratories). Studies of epithelial barrier function were initiated when transepithelial electrical resistance (TER) was >300 Ω·cm2.
Cell treatments.
Cells cultured in Transwells or ECIS electrode arrays were exposed to hypoxia (2% O2) for 6 h and returned to normoxia for 18 h, or treated with the hypoxia mimetics cobalt chloride (CoCl2; 200 μM) or dimethyloxalylglycine (DMOG; 1 mM) for 24 h. Following these preincubations, CoCl2 or DMOG were removed, and epithelial barrier function was challenged by addition of H2O2 (100–500 μM) or poly-l-lysine (5 μg/ml; GIBCO). In some cases, cells were pretreated with the HIF-1 inhibitor YC-1 (10 μM) or the cGMP analog 8-bromoguanosine 3′,5′-cyclic monophosphate (8-BrcGMP) (10 μM) for 30 min before treatment with hypoxia. For treatment of cells in Transwell inserts, all reagents were added to both apical and basolateral compartments. Unless indicated otherwise, all reagents used were obtained from Sigma-Aldrich.
Determination of TER.
Epithelial barrier function in Transwell culture plates was evaluated by measuring TER using an EVOM Epithelial Voltohmmeter (World Precision Instruments). TER values were corrected for background resistance of coated culture inserts and medium without cells. Alternatively, epithelial resistance was measured using ECIS (60). For these measurements, 16HBE14o− cells were seeded at a density of 75 × 103 cells/well in coated electrode arrays (array model 8W10E+; Applied BioPhysics) in 400 μl of medium. Epithelial resistance was measured using an ECIS Zθ instrument (Applied BioPhysics) set to the frequency scan mode. Resistance measurements were corrected for baseline resistance values established by measuring the resistance of coated arrays and culture medium (400 μl/well) in the absence of cells. Data are presented from resistance values measured at a frequency of 4,000 Hz.
Analysis of epithelial permeability.
The permeability of 16HBE14o− or MTE monolayers was determined by measuring the transepithelial passage of fluorescein isothiocyanate-dextran (FITC-dextran; relative molecular mass 4 kDa) from the apical to the basolateral compartment of cells cultured on Transwells. Apical medium was replaced with 300 μl of phenol red-free medium containing 25 mg/ml FITC-dextran, before cell exposure to H2O2. Aliquots (50 μl) of the apical and basolateral medium were collected at various time points, and fluorescence (excitation 492 nm; emission 530 nm) was analyzed using a Biotek Synergy HT plate reader. Epithelial permeability was expressed as percent leakage of FITC-dextran from apical to basolateral compartments.
Stable silencing of HIF-1α by short-hairpin RNA.
Expression of HIF-1α was silenced in 16HBE14o− cells by transduction with lentiviral particles encoding short-hairpin RNAs (shRNAs) directed against HIF-1α (MISSION TRC shRNA, clones TRCN0000003810 and TRCN0000010819; Sigma-Aldrich). 16HBE14o− cells transduced with MISSION Nontarget shRNA encoding particles (product no. SHC002V; Sigma-Aldrich) were used as controls. Cells were transduced according to the manufacturer's instructions with a multiplicity of infection of one, and transduced cells were selected by prolonged exposure to puromycin (4 μg/ml; Sigma-Aldrich). Effects on HIF-1α expression were verified by RT-PCR and Western blot analysis. Transduced cell lines are further referred to as HIF-1α shRNA targets 1 and 2 (T1 and T2, respectively) and nontarget (NT).
Western blot analysis.
Cellular monolayers were extracted in reducing Laemmli buffer on ice for 2 min and collected by scraping. Whole cell lysates were sonicated on ice for 15 s, boiled for 5 min at 100°C, and cleared by centrifugation (5 min; 14,500 rpm). Samples were separated by 8 or 10% SDS-PAGE and transferred to nitrocellulose membranes. For Western blot analysis, membranes were blocked for ≥1 h in 5% milk or 5% BSA and incubated with primary antibodies against HIF-1α (1:20,000; NB100–479, Novus Biologicals, Littleton, CO), occludin (1:1,000; Zymed, Carlsbad, CA), claudin-1 (1:500; Zymed), thioredoxin (Trx) I (1:1,000; Cell Signaling, Danvers, MA), PrxI (1:5,000; Abcam, Cambridge, MA), Prx-SO2H (1:1,000; Abfrontier, Seoul, Korea), or β-actin (1:5,000). Primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) and visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
Semiquantitative and quantitative RT-PCR.
RNA was isolated from cells using an RNeasy mini Kit (Qiagen, Valencia, CA). cDNA was prepared and amplified as described (41) and visualized on 1% agarose gels stained with ethidium bromide. For quantitative real-time PCR analysis, 5 ng of cDNA was used as template for amplification by SYBR Green methods (Applied Biosystems, Foster City, CA) with GAPDH (FAM channel) assay on demand (Applied Biosystems) as a housekeeping gene (Table 1). The amplification reaction was run for 35 cycles, and relative quantity was calculated using ΔΔCt analysis.
Table 1.
Primer sequences for genes analyzed by RT-PCR
| Primer Sequence | |
|---|---|
| h-Occludin | Left: 5′-tttgtgggacaaggaacaca-3′ |
| Right: 5′-gcaggtgctctttttgaagg-3′ | |
| h-Claudin-1 | Left: 5′-gccccagtggaggatttact-3′ |
| Right: 5′-agccagacctgcaagaagaa-3′ | |
| Sesn2 | Left: 5′-gcattacctgctgctgcata-3′ |
| Right: 5′-aaggcctggatatgctcctt-3′ | |
| h-Catalase | Left: 5′-acatggtctgggacttctgg-3′ |
| Right 5′-accttggtgagatcgaatgg-3′ | |
| h-Trx1 | Left: 5′-ccgctcgtcagactccagc-3′ |
| Right: 5′-ctggttatattttcagaaaacatg-3′ | |
| h-PrxI | Left: 5′-atgtcttcaggaaatgctaaaat-3′ |
| Right: 5′-tcacttctgcttggagaaatattc-3′ | |
| h-PrxII | Left: 5′-ccgagatcatcgcgttcag-3′ |
| Right: 5′-ctggtcacgtcagcaagca-3′ | |
| h-β-Actin | Left: 5′-tgacggggtcacccacactgtgcccatcta-3′ |
| Right: 5′-ctagaagcatttgcggtggacgatggaggg-3′ |
h, Human; Sesn2, sestrin-2; Trx, thioredoxin; ; Prx, peroxiredoxin.
Analysis of cellular glutathione status and cell viability.
Cellular glutathione (GSH) status after H2O2 treatments was determined by HPLC after derivatization of cell lysates with 2 mM monobromobimane, as described previously (13). Cellular toxicity was determined using an LDH Cytotoxicity Detection Kit (Takara BIO) according to the manufacturer's instructions.
Data presentation and statistical analysis.
Quantitative data are presented as means ± SE from two to three experiments performed in duplicate or triplicate. Statistical significance was evaluated using GraphPad Prism (GraphPad Software, La Jolla, CA), and results were considered significant when P < 0.05 using one-way ANOVA followed by Tukey's posttest for multiple comparisons.
RESULTS
Preexposure to hypoxia protects against airway epithelial cell oxidative barrier dysfunction.
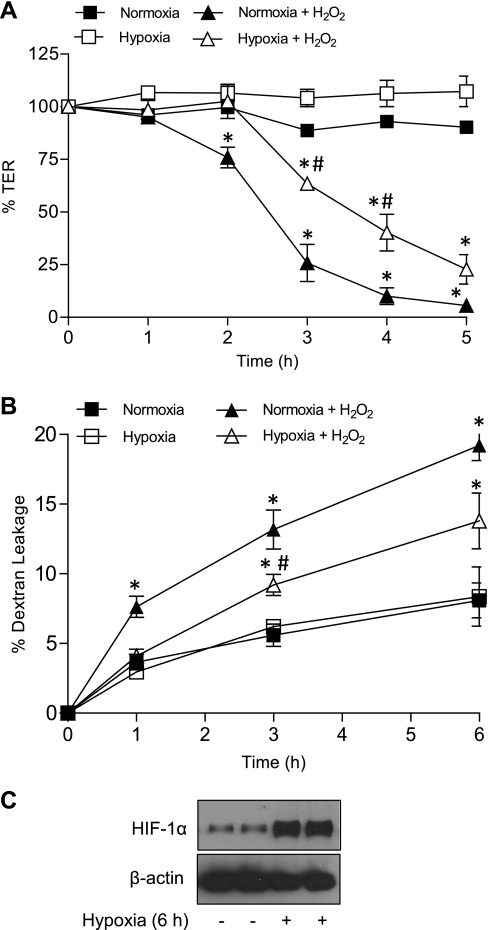
To model epithelial injury by environmental or endogenous oxidative mechanisms, epithelial monolayers of MTE or 16HBE14o− cells were exposed to H2O2, which was previously shown to disrupt epithelial barrier integrity in other epithelial cell lines (4, 5). Although H2O2 did not significantly affect barrier properties of MTE cell monolayers at concentrations up to 250 μM over 24 h (data not shown), exposure to 500 μM H2O2 resulted in loss of barrier integrity over several hours, as assessed by loss of TER (Fig. 1A) and increased permeability to FITC-dextran (4 kDa) (Fig. 1B). These effects of H2O2 were not associated with persistent changes in cellular GSH levels or acute toxicity, as measured by lactate dehydrogenase release (data not shown), suggesting that these effects on barrier function result from effects on cytoskeletal networks or junctions rather than nonspecific changes due to global redox changes or loss of viability.
Fig. 1.
Preexposure to hypoxia attenuates H2O2-induced epithelial barrier dysfunction. Primary mouse tracheal epithelial (MTE) cells were seeded on Transwell inserts and exposed to hypoxia (2% O2) for 6 h, followed by 18 h normoxia before challenge with H2O2 (500 μM). Epithelial barrier function was determined at the indicated time points after H2O2 by monitoring transepithelial electrical resistance (TER) (A) and permeability to fluorescein isothiocyanate (FITC)-dextran (relative mol mass 4 kDa) (B). P < 0.05 compared with corresponding control (*) and with H2O2 + normoxia (#).
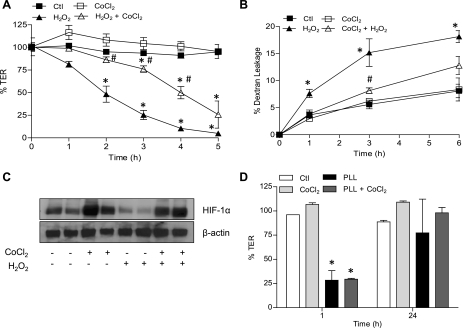
Following suggested protective effects of hypoxia on intestinal epithelial barrier integrity (22, 30), we next determined whether preexposure to hypoxia was similarly protective in airway epithelia. As shown in Fig. 1A, preexposure of MTE cells to hypoxia (2% O2) for 6 h followed by normoxia significantly delayed decreases in TER by H2O2 administration (initiated 24 h after onset of hypoxia). Similarly, preexposure to hypoxia also attenuated H2O2-induced permeability to FITC-dextran (Fig. 1B). No such protective effects of hypoxia were seen when cells were exposed to H2O2 directly following the 6-h hypoxia period, suggesting that its protective effects are mediated by a hypoxia-mediated transcriptional program. To extend these findings, MTE cells or 16HBE14o− cells were pretreated for 24 h with the hypoxia mimetic CoCl2 (200 μM) before treatment with H2O2. As shown in Fig. 2, preincubation with CoCl2 significantly delayed H2O2-mediated TER loss and epithelial permeability to FITC-dextran in 16HBE14o− cells. Nearly identical findings were also observed using MTE cells (data not shown). No protection against TER loss was observed when CoCl2 was added simultaneously with H2O2, again indicating that preincubation was required. We next evaluated whether preincubation with CoCl2 could also prevent epithelial barrier disruption by other mechanisms, such as by polycationic protein release from activated inflammatory cells (65). This was modeled by exposure of MTE cells to the synthetic polycationic protein poly-l-lysine (5 μg/ml), which led to a rapid and transient decline in TER (65), but this was not affected by pretreatment with CoCl2 (Fig. 2D). These collective findings indicate that preexposure to hypoxia primarily appears to protect against epithelial barrier dysfunction induced by oxidative mechanisms.
Fig. 2.
The hypoxia mimetic CoCl2 protects against airway epithelial barrier loss by H2O2 but not by poly-l-lysine. Monolayers of 16HBE14o− cells seeded on Transwell inserts were treated with CoCl2 (200 μM) for 24 h and subsequently challenged with 500 μM H2O2 (A and B), and epithelial barrier function was monitored by measuring changes in TER or permeability to FITC-dextran. C: effects of CoCl2 and/or H2O2 on hypoxia-inducible factor (HIF)-1α accumulation in 16HBE140- cells as determined by Western blot. D: MTE cell monolayers were pretreated with CoCl2 (200 μM) for 24 h and exposed to 5 μg/ml poly-l-lysine for 1 or 24 h for measurement of TER. P < 0.05 compared with control (*) and with H2O2 (#).
Protective actions of hypoxia against H2O2-induced airway epithelial barrier dysfunction are mediated by HIF-1.
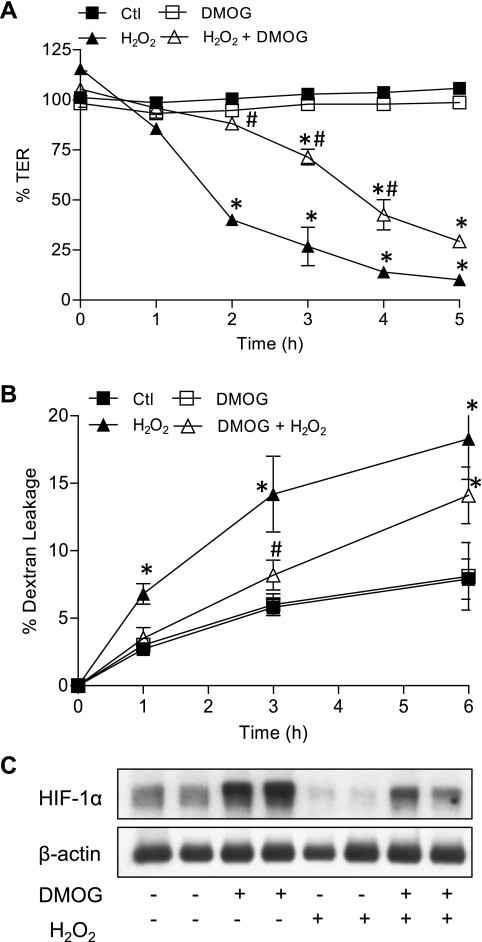
The transcription factor HIF-1 is one of the major mediators of cellular responses to hypoxia, and we therefore suspected a central role of HIF-1 in the protective effect of hypoxia against H2O2-induced epithelial barrier loss. Both hypoxia (Fig. 1C) and the hypoxia mimetic CoCl2 (Fig. 2C) caused significant accumulation of HIF-1α, the oxygen-sensitive component of HIF-1. Cell exposure to H2O2 led to decreased HIF-1α protein levels, which was prevented by CoCl2 pretreatment (Fig. 2D). Using the prolyl hydroxylase inhibitor DMOG as an alternative approach to stabilize HIF-1α and activate HIF-1, we observed that preincubation with DMOG similarly enhanced cellular HIF-1α levels and protected against loss of epithelial barrier function by H2O2 (Fig. 3).
Fig. 3.
HIF-1 activation by the prolyl hydroxylase inhibitor dimethyloxalylglycine (DMOG) protects against airway epithelial barrier loss by H2O2. Monolayers of 16HBE14o− cells seeded on Transwell inserts were treated with DMOG (1 mM) for 24 h and subsequently challenged with 500 μM H2O2, and epithelial barrier function was monitored by measuring changes in TER (A) or permeability to FITC-dextran (B). Effects of DMOG and/or H2O2 on HIF-1α accumulation were determined by Western blot (C). P < 0.05 compared with control (*) and with H2O2 (#).
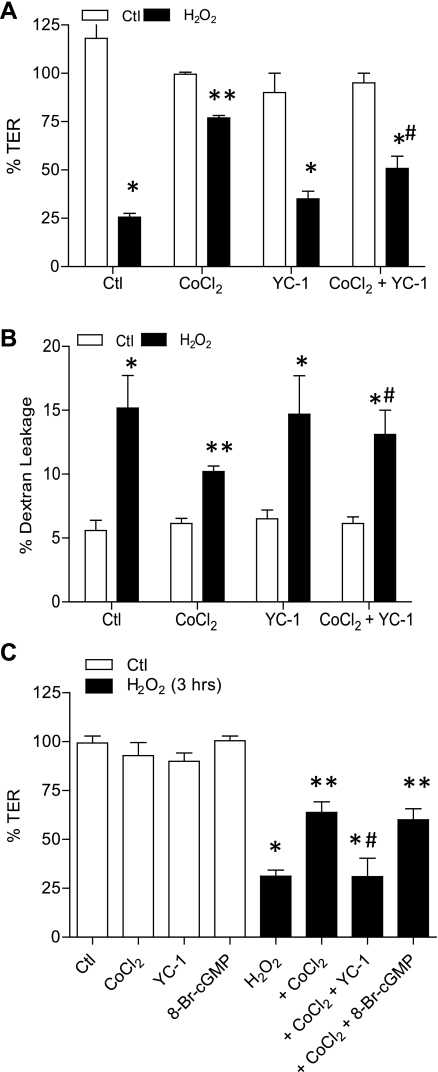
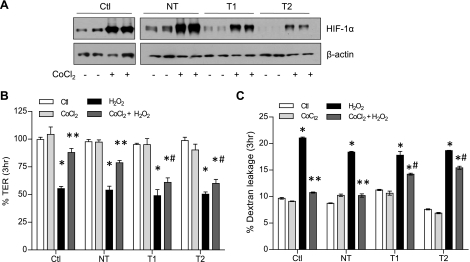
To more directly determine the involvement of HIF-1, we first performed similar experiments in the presence of the pharmacological HIF-1 inhibitor YC-1. Addition of YC-1 was found to significantly attenuate the ability of CoCl2 to prevent H2O2-induced TER loss and dextran permeability (Fig. 4B). Because YC-1 is also known to activate soluble guanylyl cyclase, we used the nonhydrolyzable cGMP analog 8-BrcGMP to verify that these effects of YC-1 were not due to cGMP-mediated signaling (Fig. 4C). As a more definitive approach to establish the importance of HIF-1 signaling in the protective effects of hypoxia, we silenced HIF-1α in 16HBE14o− cells by stable transduction of HIF-1α shRNA using lentiviral particles. As shown in Fig. 5A, basal and CoCl2-induced levels of HIF-1α were suppressed significantly in T1 and T2 shHIF-1α cells, whereas cells transduced with NT shRNA expressed HIF-1α at levels comparable to untransfected cells. Moreover, while CoCl2 pretreatment significantly attenuated H2O2-induced TER loss and epithelial permeability in untransfected or NT shRNA transfected cells, these effects were attenuated markedly in both T1 and T2 shHIF-1 cell lines (Fig. 5, B and C). Collectively, these experiments conclusively demonstrate the importance of HIF-1 activation in protective effects of hypoxia or hypoxia mimics against oxidant-induced barrier dysfunction.
Fig. 4.
Protective effects of CoCl2 are prevented by the HIF-1 inhibitor YC-1. 16HBE14o− cells were preincubated with CoCl2 as in Fig. 2 and exposed to H2O2 for 3 h in the absence or presence of the HIF-1 inhibitor YC-1 (10 μM, added 30 min before incubation with CoCl2), and changes in TER (A) and FITC-dextran permeability (B) were measured. C: comparison of effect of YC-1 with the cGMP homolog 8-bromoguanosine 3′,5′-cyclic monophosphate (8-BrcGMP, 10 μM). P < 0.05 compared with control (*), with H2O2 (**), and with H2O2 + CoCl2 (#).
Fig. 5.
HIF-1α silencing prevents protective effects of CoCl2 against H2O2-induced barrier loss. HIF-1α was silenced in 16HBE14o− cells by lentiviral transfection of short-hairpin RNA (shRNA) against HIF-1α or nontarget (NT) shRNA as a control. Efficiency of HIF-1α silencing in transfected cells was evaluated by Western blot analysis of lysates of untreated or CoCl2-treated cells (A). Effect of HIF-1α shRNA on protective effects of CoCl2 preincubation against 3 h treatment with H2O2 was determined by analysis of TER using electric cell-substrate impedance sensing (ECIS), measured at a frequency of 4,000 Hz (B), or permeability to FITC-dextran of cells seeded in Transwell inserts. Ctl, nontransduced control; NT, nontarget shRNA; T1, HIF-1α shRNA target 1; T2, HIF-1α shRNA target 2. P < 0.05 compared with corresponding untreated control (*), with corresponding H2O2 group (**), and with NT H2O2 + CoCl2 (#).
HIF-1 activation prevents oxidative loss of the TJ protein occludin.
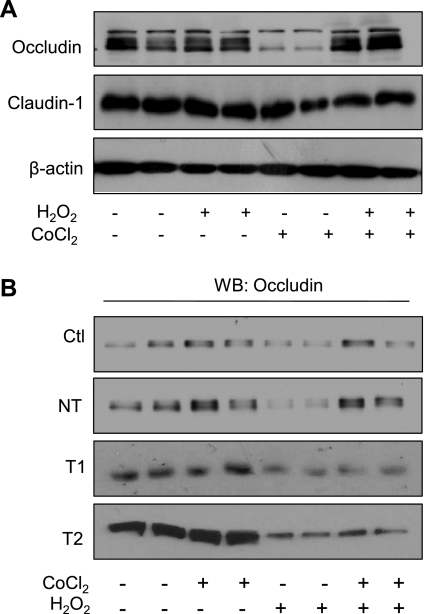
Airway epithelial barrier function is largely maintained by TJs, comprised of a complex series of interacting proteins localized to the apical aspect of columnar cells (33), that prevent the diffusion of soluble mediators or proteins between apical and basolateral cell surfaces. We therefore investigated whether oxidant-induced epithelial barrier dysfunction was associated with altered levels of TJ proteins. While no changes were observed in overall levels of claudin-1 in response to H2O2 (500 μM; 5 h), levels of occludin were reduced markedly (Fig. 6A). Because this was not accompanied by a loss of occludin mRNA expression (data not shown), this was most likely due to oxidative degradation (4). Oxidant-induced loss of occludin was prevented by pretreatment with CoCl2 before administration of H2O2 (Fig. 6A). CoCl2 treatment alone did not enhance expression of occludin protein (Fig. 6A) nor enhance mRNA transcript levels (data not shown) over this time period. The protective effects of CoCl2 pretreatment with respect to occludin loss were again mediated by HIF-1, as they were not observed in 16HBE14o− cells transfected with T1 or T2 shHIF-1 in contrast to cells transfected with NT shRNA (Fig. 6B). Similarly, effects of CoCl2 on H2O2-mediated occludin degradation were also prevented in the presence of YC-1 (data not shown).
Fig. 6.
Activation of HIF-1 prevents oxidative loss of the tight junction protein occludin. 16HBE14o− cells were treated with CoCl2 and H2O2 as described in Fig. 3, and whole cell lysates were analyzed by Western blot for the expression of the tight junction proteins occludin and claudin-1 compared with β-actin (A). H2O2-dependent loss of occludin was not observed in 16HBE14o− cells transfected with HIF-1α shRNA (T1 or T2) compared with control (NT) transfected cells (B).
Preactivation of HIF-1 prevents hyperoxidation of Prx.
The protective effects of HIF-1 activation against H2O2-dependent epithelial barrier dysfunction suggest that HIF-1 preactivation may alter cellular defenses against H2O2 or cellular ability to regenerate oxidized proteins. Indeed, previous studies have suggested that hypoxia and HIF-1 can augment expression of the H2O2-metabolizing peroxidase PrxI and the redox repair protein TrxI (19, 31, 58). However, no significant changes were observed in mRNA transcripts for PrxI, TrxI, or catalase in 16HBE14o− cells in response to CoCl2 (data not shown). Moreover, Western blot analysis did not reveal significant changes in either PrxI or TrxI in response to either hypoxia (2% O2) or CoCl2 (200 μM) (data not shown).
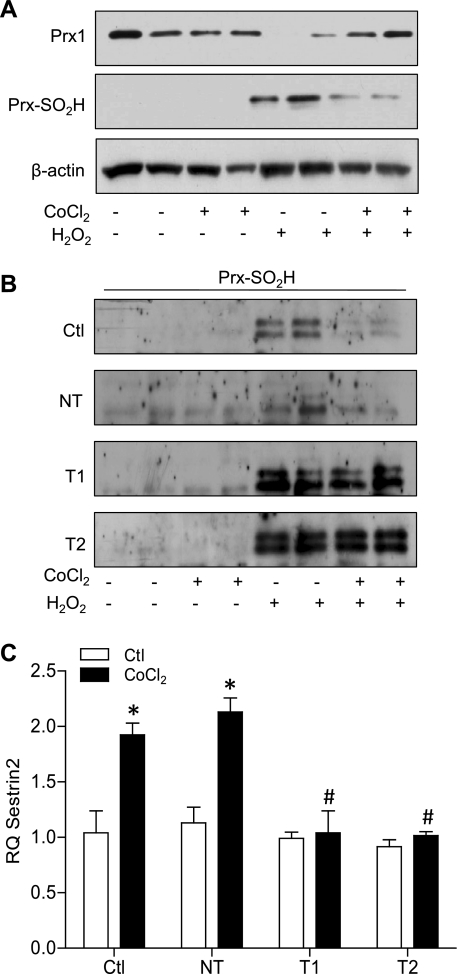
Exposure of 16HBE14o− cells to H2O2 caused significant decreases in PrxI levels, which were prevented by preincubation with CoCl2 (Fig. 7A). Because the peroxidatic cysteine of PrxI/II is susceptible to hyperoxidation to sulfinic acid (Cys-SO2H), resulting in inactivation of its peroxidase activity (48) as well as Prx oligomerization with proposed enhanced chaperone function under conditions of cell stress (44), we assessed Prx-SO2H formation using a specific Prx-SO2H antibody. Indeed, we observed significant accumulation of Prx-SO2H in H2O2-exposed cells (Fig. 7A), which was attenuated markedly upon preincubation with CoCl2, consistent with its ability to preserve PrxI after H2O2 exposure. Moreover, this protective effect of CoCl2 was again mediated by HIF-1, since CoCl2 preincubation did not prevent H2O2-dependent Prx-SO2H formation in cells transfected with HIF-1α shRNA (T1 and T2) in contrast to NT shRNA (Fig. 7B). Because it was recently suggested that Sesn2, a hypoxia-inducible enzyme, can catalyze the reduction of hyperoxidized Prx and reduce cellular oxidative stress (8, 29), quantitative RT-PCR analysis of Sesn2 was performed. Indeed, 16HBE14o− cell treatment with CoCl2 for 24 h resulted in an approximately twofold induction of Sesn2 (Fig. 7C), which was prevented in cells transfected with HIF-1α shRNA, suggesting that HIF-1 activation may reduce epithelial susceptibility to oxidative stress and Prx hyperoxidation at least in part by inducing Sesn2.
Fig. 7.
Preactivation of HIF-1 prevents H2O2-mediated hyperoxidation of peroxiredoxin (Prx), in association with induction of sestrin-2. Monolayers of 16HBE14o− cells were pretreated with CoCl2 and subsequently challenged with H2O2 for 5 h, after which cell lysates were analyzed for the expression of Prx1 and hyperoxidized-Prx (Prx-SO2H) (A). Effects of H2O2 and/or CoCl2 on Prx hyperoxidation (Prx-SO2H) were also evaluated in control 16HBE14o− cells compared with cells transfected with HIF-1α shRNA or nontarget shRNA (B). Control 16HBE14o− cells and HIF-1α shRNA or nontarget (NT) shRNA-transfected cells were treated overnight with CoCl2 and analyzed for expression levels of the transcripts for sestrin-2 (C). P < 0.05 compared with corresponding control (*) and with CoCl2-treated NT control (#).
DISCUSSION
Several recent studies have indicated the importance of HIF-1 signaling in conferring barrier protection in the intestinal epithelium in response to stress induced by hypoxia (22, 55) or under conditions of inflammation (30, 49). These HIF-mediated responses appear to be multifaceted and function through the regulation of genes involved in a broad spectrum of capacities, including mucosal restitution (22), mucin production (37), xenobiotic clearance (12), and nucleotide metabolism and signaling (34, 39, 55). Based on previously reported protective effects of hypoxia against acute oxidative lung injury (2, 61), we anticipated that HIF-1 activation within the respiratory epithelium may similarly promote airway epithelial barrier function during conditions of oxidant stress, and our present findings indeed demonstrate that preactivation of HIF-1 prevents or delays oxidant-induced loss of airway epithelial barrier integrity by preventing oxidative degradation of occludin and hyperoxidation of Prx.
Activation of HIF-1 is increasingly being recognized during inflammatory airway diseases, due to localized hypoxia as a result of active inflammation or increased production of mediators that activate HIF-1 by nonhypoxic mechanisms (42). Indeed, increased levels of HIF-1- and HIF-responsive genes have recently been observed in bronchial biopsy specimens from asthmatic subjects (36) and in epithelia from subjects with COPD (45). However, the role(s) of HIF-1 within airway disease processes remains unclear, and studies with pharmacological inhibitors of HIF or with heterozygous HIF-1α knockout mice or conditional HIF-1β knockout mice have indicated a contribution of HIF-1 to allergic airway inflammation in mouse models of asthma (23, 26, 32). Because HIF-1 is prominently activated in inflammatory cell types during ongoing inflammation (40), these various global approaches to suppress HIF do not address the specific role of HIF within the airway epithelium. Whereas HIF-1 activation in other epithelia has been linked with barrier-protective properties (22, 30), HIF-1 activation has also been implicated in epithelial-to-mesenchymal transition associated with hypoxia and fibrosis (25, 66). Our present results indicate a protective mechanism of HIF-1 activation in maintenance of airway epithelial barrier integrity under conditions of oxidative stress during inflammation. In addition to inducing protection against oxidant-induced barrier dysfunction, HIF-1 activation might also impact on epithelial redox signaling mechanisms such as those activated by the T helper 2 (TH2) cytokine IL-4 (54), and thereby affect TH2 polarized responses. In this regard, it is intriguing to note that targeted deletion of HIF-1α within the airway epithelium was recently found to alter immune responses with TH2-biased inflammation in a model of heavy metal toxicity (51), suggesting protective effects of epithelial HIF-1 against TH2-driven airway inflammation.
The mechanisms by which HIF-1 protects against oxidative epithelial injury are undoubtedly complex. An important aspect of epithelial barrier loss during, e.g., allergic inflammation is the disruption of TJs, apical complexes of integral and peripheral membrane proteins that maintain epithelial polarity and barrier integrity and regulate intercellular transport and allow communication between adjacent cells (59). Limited reports exist to date with respect to a role of HIF-1 in the regulation of TJ proteins, although several recent studies have implicated HIF-1 activation in loss of the TJ proteins zonula occludens-1 and occludin in blood-brain barrier disruption (24, 64). Our studies show that HIF-1 activation prevented oxidative loss of the TJ protein occludin, although HIF activation did not appear to transcriptionally regulate occludin expression. Occludin is subject to proteolytic processing as well as serine/threonine and tyrosine phosphorylation, which promotes its disruption from the TJ complex. Because both of these events can be promoted by oxidant-induced mechanisms (4, 21), the protective effects of HIF-1 are most likely related to altered oxidant metabolism, thereby minimizing these oxidative mechanisms.
With respect to altered oxidant metabolism, activation of HIF-1 in response to, e.g., hypoxia has been associated with enhanced expression of PrxI as well as TrxI but with reduced expression of superoxide dismutase (SOD) 1 and SOD2 (19, 31, 58). In our studies, we did not detect significant changes in expression of H2O2-metabolizing enzymes such as catalase or PrxI in response to HIF-1 activation but noted significant H2O2-dependent hyperoxidation (and presumably inactivation) of Prx, which was attenuated after prior activation of HIF-1. Prxs have emerged as critical enzymes involved in oxidant sensing and use a redox-active peroxidatic cysteine to metabolize peroxides, resulting in the formation of a cysteine sulfenic acid (Cys-SOH) that is in turn reduced by Trx to regenerate Prx (48). The peroxidatic cysteine is also susceptible to hyperoxidation to sulfinic acid (Cys-SO2H), which can no longer be reduced by Trx and results in loss of peroxidase activity (48), and such Prx hyperoxidation was originally proposed to allow H2O2 to target other proteins and promote redox-mediated signal transduction in, e.g., mitogenic signaling (63). More recent studies, however, have linked formation of Prx-SO2H to stress response signaling mechanisms alerting cells to alterations in oxidant metabolism, demonstrated by organization of accumulated Prx-SO2H in filamentous structures in the cytoplasm in association with actin stress fiber formation (44), thus representing a potential direct mechanism linking oxidative stress to altered epithelial barrier function. In addition, hyperoxidization of PrxII was also reported to promote efficient chaperone function and enhance cellular resistance to H2O2 (38). The antibody used to detect hyperoxidized Prx in the present studies is not specific for any of the Prx isoforms, but, since multiple Prx-SO2H-positive bands were detected (Fig. 7B), several Prx isoforms were likely subjected to hyperoxidation under these conditions, potentially including the mitochondrial PrxIII isoform, which would suggest the involvement of mitochondrial oxidative stress that may be associated with proapoptotic pathways (14).
The prevention of Prx-SO2H accumulation in HIF-activated epithelial cells suggests enhanced minimized formation of Prx-SO2H due to enhanced H2O2 metabolism or accelerated turnover of Prx-SO2H. In this regard, the hypoxia-responsive gene Sesn2, which was originally identified as hypoxia-induced gene 95, was recently reported to be capable of reducing hyperoxized Prxs and regenerate the Prx catalytic cycle (8, 19). The sestrin genes are known targets of p53 (8), and Sesn2 is also regulated by HIF-1 (19), which may be related to previously established associations between HIF-1α accumulation and p53 activation (6, 10). Indeed, induction of Sesn2 by CoCl2 in 16HBE14o− cells was clearly dependent on HIF-1α (Fig. 7C). Several studies have associated Sesn2 with oxidant metabolism and altered oxidative stress signaling, as an important protective response mechanism to prolonged hypoxia or by oxidative stress (9). In fact, overexpression of Sesn2 was found to reduce levels of intracellular ROS in response to cell exposure to exogenous H2O2, whereas inhibition of Sesn2 by short-interfering RNA led to an increase in ROS (8). These findings would suggest that Sesn2 may not directly regenerate Prx from its hyperoxidized state (62) but rather regulate endogenous oxidative metabolism and signaling by alternative mechanisms, such as activation of AMP-activated protein kinase (AMPK) and inhibition of mammalian target of rapamycin (mTOR), which have been implicated in the inhibitory effects of sestrins against genotoxic stress and against age-related diseases (7, 35). The direct contribution of Sesn2 to the protective effects of HIF-1 against oxidative epithelial injury, and the potential effects on AMPK and mTOR signaling, will remain to be further explored in future studies. Alternatively, other HIF-activated target genes, such as netrin-1 (50), trefoil factors (22), and adenosine receptors (34), may contribute to the protective actions of hypoxia on airway epithelia.
Exposure of airway epithelial cells to hypoxia or prolyl hydroxylase inhibitors does not only activate HIF-1 but also HIF-2 (3), which may regulate unique target genes that could also contribute to barrier protection during oxidative stress (1). The fact that HIF-1 shRNA only partially reversed these protective effects (Fig. 5) further suggests the possible contribution of other HIF isoforms. Although HIF-2α was shown to be important for normal lung development (43), little is known regarding its role in airway epithelial function. Recently, activation of airway epithelial protein kinase B (Akt)-mTOR signaling by localized overexpression of Akt was found to result in respiratory distress syndrome (RDS) and was associated with suppression of HIF-2α and vascular endothelial growth factor expression (27), suggesting a potential protective effect of HIF-2 against RDS. Also, deletion of HIF-2α was reported to result in multiple organ pathologies and enhanced oxidative stress, due to reduced expression of primary antioxidant genes (52). These findings suggest that HIF-2 activation may have contributed to the protective effects of hypoxia or prolyl hydroxylase inhibition in our studies as well.
In summary, our present findings indicate the importance of HIF-1 in protective effects against oxidant epithelial injury as a potential mechanism of hypoxic preconditioning, which is associated with reduced oxidative loss of TJ proteins and Prx hyperoxidation and induction of Sesn2. Whether activation of epithelial HIF-1 and induction of Sesn2 are also protective in chronic airway diseases associated with oxidative stress and epithelial injury remains to be demonstrated.
GRANTS
This work was supported by National Institutes of Health Grants HL-074295, HL-068865, and HL-085646 to A. van der Vliet and a T32 training fellowship to N. Olson (ES-007122).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Edwin G. Bovill and Douglas J. Taatjes for assistance with the use of the ECIS system.
REFERENCES
- 1. Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci USA 106: 10684–10689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad S, Ahmad A, Gerasimovskaya E, Stenmark KR, Allen CB, White CW. Hypoxia protects human lung microvascular endothelial and epithelial-like cells against oxygen toxicity: role of phosphatidylinositol 3-kinase. Am J Respir Cell Mol Biol 28: 179–187, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Gunzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA 102: 10212–10217, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blasig IE, Bellmann C, Cording J, Vecchio GD, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal 15: 1195–1219, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Boardman KC, Aryal AM, Miller WM, Waters CM. Actin re-distribution in response to hydrogen peroxide in airway epithelial cells. J Cell Physiol 199: 57–66, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor-1 alpha. J Biol Chem 283: 17919–17928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134: 451–460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304: 596–600, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, Skaliter R, Gudkov AV, Chumakov PM, Feinstein E. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 21: 6017–6031, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem 278: 13595–13598, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122: 456–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62: 3387–3394, 2002 [PubMed] [Google Scholar]
- 13. Cotgreave IA, Moldeus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble and protein thiol components of biological systems. J Biochem Biophys Methods 13: 231–249, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J 425: 313–325, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 10: 38–47, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc 6: 678–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dehne N, Brune B. HIF-1 in the inflammatory microenvironment. Exp Cell Res 315: 1791–1797, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Essler S, Dehne N, Brune B. Role of sestrin2 in peroxide signaling in macrophages. FEBS Lett 583: 3531–3535, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 15: 4–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev 57: 883–917, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193: 1027–1034, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo J, Lu W, Shimoda LA, Semenza GL, Georas SN. Enhanced interferon-gamma gene expression in T Cells and reduced ovalbumin-dependent lung eosinophilia in hypoxia-inducible factor-1-alpha-deficient mice. Int Arch Allergy Immunol 149: 98–102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, Ding JY, Dornbos D, Li X, Guthikonda M, Rossi NF, Ding Y. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg 114: 92–101, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huerta-Yepez S, Baay-Guzman GJ, Bebenek IG, Hernandez-Pando R, Vega MI, Chi L, Riedl M, Diaz-Sanchez D, Kleerup E, Tashkin DP, Gonzalez FJ, Bonavida B, Zeidler M, Hankinson O. Hypoxia inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy 66: 909–918, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikeda H, Shiojima I, Oka T, Yoshida M, Maemura K, Walsh K, Igarashi T, Komuro I. Increased Akt-mTOR signaling in lung epithelium is associated with respiratory distress syndrome in mice. Mol Cell Biol 31: 1054–1065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, van der Vliet A. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc 6: 249–255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jonsson TJ, Lowther WT. The peroxiredoxin repair proteins. Subcell Biochem 44: 115–141, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HJ, Chae HZ, Kim YJ, Kim YH, Hwangs TS, Park EM, Park YM. Preferential elevation of Prx I and Trx expression in lung cancer cells following hypoxia and in human lung cancer tissues. Cell Biol Toxicol 19: 285–298, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Kim SR, Lee KS, Park HS, Park SJ, Min KH, Moon H, Puri KD, Lee YC. HIF-1alpha inhibition ameliorates an allergic airway disease via VEGF suppression in bronchial epithelium. Eur J Immunol 40: 2858–2869, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 8: 432–446, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J 20: 2242–2250, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327: 1223–1228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SY, Kwon S, Kim KH, Moon HS, Song JS, Park SH, Kim YK. Expression of vascular endothelial growth factor and hypoxia-inducible factor in the airway of asthmatic patients. Ann Allergy Asthma Immunol 97: 794–799, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616–1627, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Moon JC, Hah YS, Kim WY, Jung BG, Jang HH, Lee JR, Kim SY, Lee YM, Jeon MG, Kim CW, Cho MJ, Lee SY. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J Biol Chem 280: 28775–28784, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136: 607–618, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9: 609–617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olson N, Greul AK, Hristova M, Bove PF, Kasahara DI, van der Vliet A. Nitric oxide and airway epithelial barrier function: regulation of tight junction proteins and epithelial permeability. Arch Biochem Biophys 484: 205–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olson N, van der Vliet A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 25: 125–137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ 15: 628–634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Phalen TJ, Weirather K, Deming PB, Anathy V, Howe AK, van der Vliet A, Jonsson TJ, Poole LB, Heintz NH. Oxidation state governs structural transitions in peroxiredoxin II that correlate with cell cycle arrest and recovery. J Cell Biol 175: 779–789, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Polosukhin VV, Cates JM, Lawson WE, Milstone AP, Matafonov AG, Massion PP, Lee JW, Randell SH, Blackwell TS. Hypoxia-inducible factor-1 signalling promotes goblet cell hyperplasia in airway epithelium. J Pathol 224: 203–211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 726–733, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 533: 222–239, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17: 183–189, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134: 145–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 10: 195–202, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Saini Y, Kim KY, Lewandowski R, Bramble LA, Harkema JR, Lapres JJ. Role of hypoxia-inducible factor 1α in modulating cobalt-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 298: L139–L147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet 35: 331–340, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Sharma P, Chakraborty R, Wang L, Min B, Tremblay ML, Kawahara T, Lambeth JD, Haque SJ. Redox regulation of interleukin-4 signaling. Immunity 29: 551–564, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D, Aidinis V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am J Respir Crit Care Med 176: 1108–1119, 2007 [DOI] [PubMed] [Google Scholar]
- 57. van der Vliet A, Cross CE. Oxidants, nitrosants, and the lung. Am J Med 109: 398–421, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Vengellur A, Woods BG, Ryan HE, Johnson RS, LaPres JJ. Gene expression profiling of the hypoxia signaling pathway in hypoxia-inducible factor 1alpha null mouse embryonic fibroblasts. Gene Expr 11: 181–197, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy 30: 685–698, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res 259: 158–166, 2000 [DOI] [PubMed] [Google Scholar]
- 61. White CW, Jackson JH, McMurtry IF, Repine JE. Hypoxia increases glutathione redox cycle and protects rat lungs against oxidants. J Appl Physiol 65: 2607–2616, 1988 [DOI] [PubMed] [Google Scholar]
- 62. Woo HA, Bae SH, Park S, Rhee SG. Sestrin 2 is not a reductase for cysteine sulfinic acid of peroxiredoxins. Antioxid Redox Signal 11: 739–745, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol 72: 440–449, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Yu XY, Schofield BH, Croxton T, Takahashi N, Gabrielson EW, Spannhake EW. Physiologic modulation of bronchial epithelial cell barrier function by polycationic exposure. Am J Respir Cell Mol Biol 11: 188–198, 1994 [DOI] [PubMed] [Google Scholar]
- 66. Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol 297: L1120–L1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]