Abstract
There is increasing evidence that inflammation plays a pivotal role in the pathogenesis of some forms of pulmonary hypertension (PH). We recently demonstrated that deficiency of adiponectin (APN) in a mouse model of PH induced by eosinophilic inflammation increases pulmonary arterial remodeling, pulmonary pressures, and the accumulation of eosinophils in the lung. Based on these data, we hypothesized that APN deficiency exacerbates PH indirectly by increasing eosinophil recruitment. Herein, we examined the role of eosinophils in the development of inflammation-induced PH. Elimination of eosinophils in APN-deficient mice by treatment with anti-interleukin-5 antibody attenuated pulmonary arterial muscularization and PH. In addition, we observed that transgenic mice that are devoid of eosinophils also do not develop pulmonary arterial muscularization in eosinophilic inflammation-induced PH. To investigate the mechanism by which APN deficiency increased eosinophil accumulation in response to an allergic inflammatory stimulus, we measured expression levels of the eosinophil-specific chemokines in alveolar macrophages isolated from the lungs of mice with eosinophilic inflammation-induced PH. In these experiments, the levels of CCL11 and CCL24 were higher in macrophages isolated from APN-deficient mice than in macrophages from wild-type mice. Finally, we demonstrate that the extracts of eosinophil granules promoted the proliferation of pulmonary arterial smooth muscle cells in vitro. These data suggest that APN deficiency may exacerbate PH, in part, by increasing eosinophil recruitment into the lung and that eosinophils could play an important role in the pathogenesis of inflammation-induced PH. These results may have implications for the pathogenesis and treatment of PH caused by vascular inflammation.
Keywords: pulmonary hypertension, eosinophils, pulmonary artery smooth muscle cells, adiponectin, mouse model
pulmonary hypertension (PH) can occur in association with multiple disorders, many of which share a common pathological appearance characterized by inflammation and remodeling of the pulmonary vasculature (3, 13, 22). Accumulating evidence suggests that pulmonary vascular inflammation is an important stimulus for the pathological changes seen in various types of PH (3, 8, 13, 19, 59). For example, it is now felt that pulmonary vascular eosinophilic inflammation in response to parasite eggs is a primary factor in the development of PH in schistosomiasis, the most common cause of PH worldwide (4, 6). Similarly, it has been demonstrated that, when pulmonary eosinophilic (allergic) inflammation is induced in mice by sensitization and challenge with high-dose ovalbumin (OVA), mice develop prominent pulmonary vascular remodeling that mimics human disease (8, 45, 61, 62, 65, 66, 71, 75). It is likely that inflammation is a direct stimulus for vascular remodeling possibly via the release of growth factors and other mediators, or via metabolic changes such as focal hypoxia (28). Together, these data suggest that inflammation can be an important pathogenic factor in the development of PH and thus could be an effective therapeutic target for some forms of the disease.
Recent experimental evidence suggests that adipose tissue may contribute to the pathogenesis of inflammatory vascular diseases such as atherosclerosis through the secretion of multiple bioactive mediators (adipokines), which influence energy homeostasis, inflammation, and tissue remodeling (30, 63, 70). One of the most important adipokines is adiponectin (APN), which has a wide range of metabolic, anti-inflammatory, and antiproliferative activities (55). Interestingly, obese individuals have lower amounts of circulating APN compared with lean individuals, suggesting that decreased APN levels may contribute to the increased incidence of vascular diseases associated with obesity. Links between APN and pulmonary vascular disease are not fully defined; however, recent data in mouse models of PH from our group and others suggest that APN deficiency can increase the severity of pulmonary vascular inflammation, pulmonary vascular remodeling, and PH (19, 45, 49, 66). In our prior study (45), prominent vascular remodeling and PH was seen in APN-deficient (APN−/−) mice utilized in a low-dose OVA model of pulmonary allergic inflammation, but not in wild-type (WT) control mice. There was also increased pulmonary eosinophil recruitment in the APN−/− mice compared with WT mice in this model. Low-dose OVA was used for these experiments, since high-dose OVA overwhelmed the anti-inflammatory activity of APN in the WT control mice. Our data also suggested that APN deficiency increased eosinophil recruitment by enhancing the production of the eosinophil-active chemokine CCL11 (45). Based on these data, we hypothesized that eosinophilic inflammation of the pulmonary vasculature can promote pulmonary artery smooth muscle cell (PASMC) proliferation and, thus, APN could indirectly suppress vascular remodeling by inhibiting eosinophil accumulation in the lung. In the current study, we sought to follow up on our previous findings and investigate this hypothesis to better define the mechanisms of APN-mediated suppression of PH. In the data presented here, we demonstrate that inhibition of eosinophil accumulation in the lung prevents the development of pulmonary vascular remodeling induced by inflammation. Additionally, we show that APN deficiency is associated with elevated chemokine production from alveolar macrophages isolated from the lungs of APN−/− mice compared with WT mice. Finally, we report in vitro data showing that eosinophil granule extracts can stimulate PASMC proliferation and signaling. These data support an important role for APN in the pathogenesis of PH by modulating pulmonary vascular inflammation.
MATERIALS AND METHODS
Mice.
APN−/− mice (45) and PHIL mice (32) were all backcrossed more than seven generations onto a C57BL/6 background. WT C57BL/6 control mice were obtained from the National Cancer Institute (Bethesda, MD). Mice were used at 6–8 wk of age and were age and sex matched for all experiments. There were no baseline differences in weight between genotypes (data not shown). All protocols for mice experiments were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Murine models of PH.
The low-dose OVA (45) and high-dose OVA (8) models were performed as previously described. Briefly, for the low-dose model, mice were immunized with three intraperitoneal injections of 50 μg of OVA in 0.1 ml PBS on days 1, 4, and 7. Starting on day 12, mice were challenged by intranasal injection with 20 μg OVA in 30 μl PBS or PBS alone (control mice) weekly for 4 wk. For the high-dose model, mice were immunized with intraperitoneal injections of 50 μg OVA (Sigma-Aldrich, St. Louis, MO) bound to 2 mg Alum (Sigma-Aldrich) in 0.5 ml PBS on day 1 and day 14. The mice were then challenged with aerosolized OVA or PBS for 45 min at a concentration of 10 mg/ml on days 28, 29, 30, 35, 36, 37, 41, and 42 and with OVA at a concentration of 25 mg/ml on day 43 only. Mice were analyzed 24 h after the last challenge in both models.
Administration of antibody directed against interleukin-5.
APN−/− mice in the low-dose OVA model were injected intraperitoneally with 1 mg of anti-interleukin (IL)-5 antibody [obtained from the TRFK-5 cell line (ATCC, Manassas, VA), purified by BioXCell (West Lebanon, NH)] or isotype IgG control antibody (Abcam, Cambridge, MA) 1 h before each intranasal injection of OVA.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was performed as previously described (46). Mice were anesthetized with a lethal injection of ketamine (100 mg/kg). The cells recovered from the BAL were washed in PBS and enumerated in a hemocytometer. The differential cell count on cells isolated from the BAL were determined by enumerating mononuclear cells (macrophages, monocytes, and lymphocytes), neutrophils, and eosinophils on cytocentrifuge preparations of the cells stained with Diff-Quick (Dade Behring, Newark, DE). At least 200 cells were counted on each slide.
Histological analyses.
For histopathological examination, lungs were flushed free of blood, inflated with 10% buffered formalin to 25 cmH2O of pressure, and prepared and evaluated as previously described (45). Briefly, sections of paraffin-embedded lungs were stained with hematoxylin-eosin. For measurement of vessel wall thickness, sections were stained with an antibody directed against α-smooth muscle actin (Abcam) according to the manufacturers' recommended protocol. The quantitative analysis of vessel wall thickness was performed as previously described (75). Briefly, the external diameter of the vessel of interest was measured using NIS Elements AR imaging analysis software (Nikon, Melville, NY). The distance between the endothelial and the adventitia components of the vessel wall at two diametrically opposed locations was measured. The vessel wall thickness was represented as the percentage of the sum of the two endothelia-to-adventitia distances over the external diameter. One hundred to 150 small- and medium-sized preacinar pulmonary arteries per mouse were analyzed. Genotypes of mice were blinded to examiners who performed the measurements.
Hemodynamic studies.
Right ventricular systolic pressure (RVSP) was measured as previously described (45). In brief, mice were anesthetized, and a PE-10 polyethylene catheter was placed in the left carotid artery for monitoring heart rate and systemic arterial pressure. A 1.2-Fr high-fidelity pressure catheter (FTS-1211B-0018; Scisense, London, ON, Canada) was advanced into the right ventricle via the jugular vein to measure RVSP. All signals were recorded and analyzed using a data acquisition system (AD Instruments, Colorado Springs, CO).
Isolation of eosinophil granule extracts.
Eosinophil granules were isolated as previously described (37). Briefly, eosinophils were isolated and purified from blood of IL-5 transgenic mice. Heparinized blood was layered on a Percoll E gradient [60% Percoll E, 1× Hanks' balanced salt solution, 15 mM HEPES (pH 7.4), and 0.003 N HCl] and centrifuged (45 min, 3,000 rpm, 4°C). The buffy coat was recovered and washed in PBS plus 2% FCS. Eosinophils were isolated using a magnetic cell separation system (Miltenyi Biotec, Auburn, CA). The isolated eosinophils were lysed with 0.25 M sucrose, 300 U/ml heparin, and 200 U/ml DNase. Granules were recovered by centrifuging the lysate (20 min, 10,000 g, 4°C). The pellet was then resuspended in 0.01 M HCl, sonicated, and recentrifuged at 300 g. The supernatant was removed, and the acid soluble granule proteins were stored at a concentration of protein equivalent to 1 × 108 cells/ml by adding 0.01 M HCl as needed.
In vitro proliferation assay of PASMCs.
PASMCs were isolated from the main pulmonary arteries of male C57BL/6 mice and cultured as previously described (75). After three to six passages, cells were used for the proliferation assay. PASMCs were seeded in 96-well plates at 8,000 cells/well. After starvation in 0.1% BSA media for 24 h, PASMCs were cultured with an aliquot of eosinophil granule extracts isolated from 106 eosinophils or an equivalent volume of vehicle. After 48 h, the number of cells was determined using the CyQUANT NF Cell Proliferation Assay Kit (Invitrogen, Camarillo, CA).
Protein kinase B and extracellular signal-regulated kinase phosphorylation.
PASMCs were cultured in serum-free medium for 24 h. After exposure to eosinophil granule extracts or vehicle for varying durations of time, cells were washed in PBS and lysed in Nonidet P-40 cell lysis buffer (Invitrogen) containing phosphatase inhibitors (Calbiochem, La Jolla, CA) and protease inhibitor cocktail tablets (Roche, Indianapolis, IN). Lysates were cleared by a centrifugation at 12,000 rpm and 4°C for 15 min, and protein concentrations were determined. Cell proteins (15 μg) were subjected to SDS-PAGE on a 4–20% gradient Tris-glycine gel (Invitrogen) under reducing conditions. Proteins were then transferred onto a nitrocellulose membrane (Invitrogen). Membranes were blocked in Tris-buffered saline and Tween 20 (TBST) with 5% nonfat milk for 1 h at room temperature and incubated overnight at 4°C with primary antibodies in TBST containing 5% BSA. Membranes were washed three times in TBST, each for 5 min, and then incubated 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA). Bound antibodies were detected with enhanced chemiluminescence reagents (GE Healthcare). The dilution of primary antibodies was 1:1,000 and 1:2,000 for the secondary antibodies. Primary antibodies to protein kinase B (Akt) 1 and phospho-Akt1 (Ser473) were obtained from Cell Signaling Technology. Primary antibodies to extracellular signal-regulated kinase (ERK) and phospho-ERK (Tyr204) (detects both ERK1 and ERK2 in mice) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Lung macrophage sorting.
Single-cell suspensions of lungs were freshly obtained from collagenase-digested lung tissues as previously described (47). CD11c+ cells were then isolated via magnetic bead separation according to the manufacturer's introduction (Miltenyi Biotec). Alveolar macrophages were isolated using a BD FACSAria cell sorter (BD Biosciences, Rockville, MD) based on high levels of CD11c (CD11chigh) and high autofluorescence in the FL1 channel (FL1high) as previously described (47, 73).
Quantification of phosphorylated signal transducer and activator of transcription 6.
RAW 264.7 cells were plated into a 96-well plate (2 × 105 cells/well). The cells were incubated in media alone or media with 10 μg/ml of APN for 24 h. The cells were then stimulated with 100 ng/ml IL-4 for 7 min and analyzed for phosphorylated signal transducer and activator of transcription (STAT) 6 using a commercial In-Cell ELISA kit (Thermo Scientific, Rockford, IL) according to the manufacturer's protocol.
Quantification of gene expression.
RNA was purified from the lung or isolated macrophages and analyzed by quantitative RT-PCR as previously described (46). Primer sequences used were selected using the Massachusetts General Hospital PrimerBank (pga.mgh.harvard.edu/primerbank/).
Statistical analysis.
Results are shown as mean ± SE values. Graphpad Prism software was used to analyze the results. Two groups were compared using a Student's t-test. Between-group comparison of means with two independent variables was performed by two-way ANOVA. A value of P < 0.05 was regarded as a significant difference.
RESULTS
Anti-IL-5 antibody treatment attenuates pulmonary vascular remodeling and hypertension.
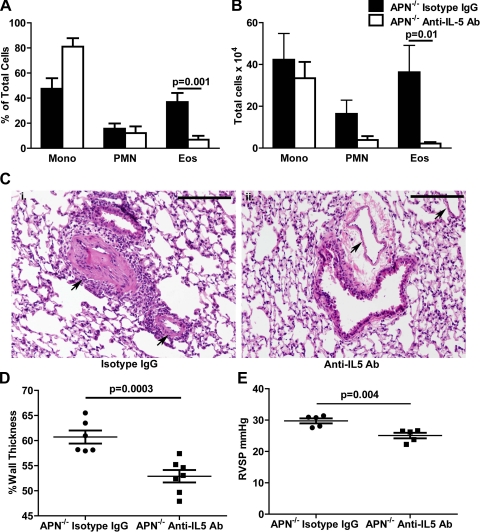
We have previously reported that APN−/− mice develop increased pulmonary arterial muscularization, pulmonary eosinophilia, and PH compared with WT mice in a murine model that utilizes low-dose OVA sensitization and challenge to induce eosinophilic pulmonary vascular inflammation (45). In those previous experiments, we used a low-dose OVA model rather than a previously published high-dose OVA model (8) to limit the inflammatory stimuli from overwhelming the activity of APN. To examine if eosinophils play a role in pulmonary vascular remodeling associated with allergic inflammation, we treated APN−/− mice with antibodies against IL-5 before mice were challenged with OVA. IL-5 is essential for the proliferation of eosinophil lineage-committed cells in the bone marrow and has direct effects facilitating eosinophil maturation and survival (12, 16). Treatment with anti-IL-5 antibodies reduced the number and percentage of eosinophils in BAL fluid from OVA-challenged APN−/− mice compared with APN−/− mice treated with isotype control antibodies (Fig. 1, A and B). PBS challenge did not result in inflammation in both groups of mice (data not shown). Histological assessment of the lung tissue revealed that pulmonary arterial inflammation and muscularization were less in APN−/− mice given anti-IL-5 antibody than in mice treated with isotype control antibody (Fig. 1C, i and ii). The cells within the vasculature stained positive for α-smooth muscle cell (SMC) actin (data not shown) consistent with a mesenchymal origin for the cells, and image analysis indicated a reduction in pulmonary arterial wall thickness in the anti-IL-5-treated mice compared with vessels in control mice (Fig. 1D). We also measured right ventricular pressures in OVA-challenged APN−/− mice treated with anti-IL-5 antibody or isotype control antibody. Consistent with the remodeling data, anti-IL-5-treated mice had reduced right ventricular pressures compared with isotype control-treated mice (Fig. 1E). Other hemodynamic parameters such as heart rate, systolic blood pressure, and right ventricular diastolic pressure were not different between the two groups (data not shown).
Fig. 1.
Eosinophils are necessary for pulmonary vascular remodeling in adiponectin-deficient (APN−/−) mice. A and B: percentage and number of mononuclear cells (Mono), neutrophils (PMN), and eosinophils (Eos) in the bronchoalveolar lavage (BAL) from APN−/− mice following ovalbumin (OVA) immunization and challenge and pretreatment with anti-interleukin (IL)-5 or isotype control antibodies (n = 6–7 mice/group). C: representative hematoxylin- and eosin-stained lung sections of APN−/− mice following OVA immunization and challenge and treatment with isotype control (i) or anti-IL-5 (ii) antibodies (Ab) (×100 magnification). Pulmonary arteries are indicated with black arrows. Black bars are 100 μm (representative images from n = 6–7 mice/group). D: vessel wall thickness (% of total) in small and medium preacinar blood vessels in lung sections from APN−/− mice following OVA immunization and challenge and treatment with isotype or anti-IL-5 control antibodies (n = 6–7 mice/ group). E: right ventricular systolic pressure (RVSP) measured in APN−/− mice following OVA immunization and challenge and treatment with isotype or anti-IL-5 control antibodies (n = 5 mice/group).
PHIL mice do not develop pulmonary vascular remodeling.
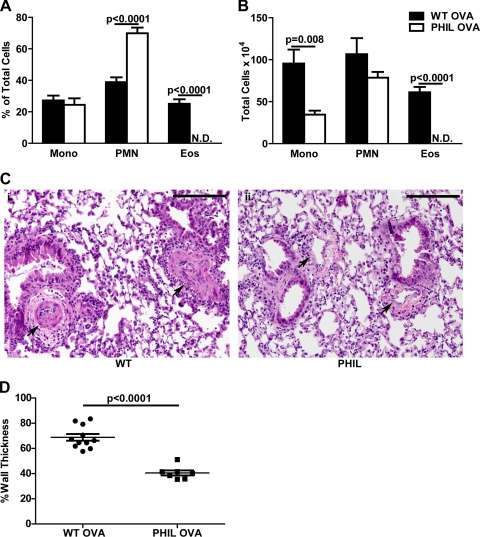
To confirm the importance of eosinophils for pulmonary vascular remodeling induced with allergic inflammation, we utilized a mouse strain that is specifically devoid of eosinophils without disturbing the production of other hematopoietic lineages (PHIL mice) (32). We used these mice in a recently described model of pulmonary vascular remodeling that utilizes high-dose OVA to induce muscularization of pulmonary arteries in WT mice (8). High-dose OVA was used instead of low-dose OVA because WT mice do not develop significant pulmonary vascular remodeling in the low-dose OVA model (only APN−/− mice). Following OVA challenge, WT mice developed prominent BAL eosinophilia, but PHIL mice did not have any eosinophils in the BAL (Fig. 2, A and B). PBS challenge did not induce lung inflammation in either group of mice (data not shown). Consistent with our studies in anti-IL-5-treated mice, PHIL mice developed less pulmonary vascular remodeling in response to OVA challenge than did similarly treated WT mice (Fig. 2C, i and ii) and had less pulmonary arterial wall thickening (Fig. 2D). The high-dose OVA model used in these experiments does not induce PH under normoxic conditions so we did not measure right ventricular pressures in these mice (8). Together these data suggest that recruitment of eosinophils to the lung is necessary for the development of pulmonary vascular remodeling in models of PH induced by eosinophilic lung inflammation. Thus, if APN suppresses eosinophil accumulation in the lung, it could indirectly suppress vascular remodeling.
Fig. 2.
PHIL mice have attenuated pulmonary vascular remodeling. A and B: percentage and number of mononuclear cells, neutrophils, and eosinophils in the BAL of wild-type (WT) and PHIL mice following OVA immunization and challenge (n = 8–11 mice/group). C: representative hematoxylin- and eosin-stained lung sections of WT (i) and PHIL (ii) mice (×100 magnification). Pulmonary arteries are indicated with black arrows. Black bars are 100 μm (representative images from n = 8–11 mice/group). D: vessel wall thickness (% of total) in preacinar blood vessels in lung sections from WT and PHIL mice after OVA immunization and challenge (n = 8–11 mice/group). N.D., not detected.
Macrophages from APN−/− mice secrete more CCL11 and CCL24.
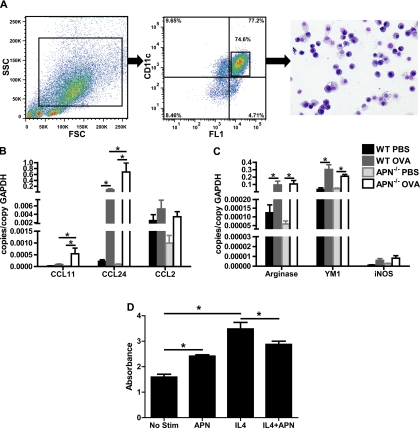
Our previous work has suggested that APN may suppress eosinophil recruitment into the lung via inhibition of eosinophil-active chemokine secretion in the lung (45). We hypothesized that APN could decrease chemokine secretion largely through its effects on lung macrophages. To better define the effects of APN on lung macrophages in vivo, we isolated lung macrophages using cell sorting from single cell suspensions of the lungs from WT and APN−/− mice immunized with OVA and challenged with OVA or PBS. As seen in previous studies (47, 73), examination of the sorted cells demonstrated that they were >95% pure based on their morphology on cytospins (Fig. 3A). We then measured RNA expression levels of a panel of chemokines, cytokines, and growth factors. In addition, we quantified the levels of arginase-I and YM1, markers of alternatively activated (M2-type) macrophages, and inducible nitric oxide synthase (iNOS), a marker of M1-type macrophages. Consistent with our prior results, the levels of the eosinophil-active chemokines CCL11 and CCL24 were upregulated with OVA exposure in WT and APN−/− mice, but the mRNA levels were greater in macrophages from APN−/− mice (Fig. 3B). The mRNA levels of CCL2, IL-4, IL-6, IL-10, tumor necrosis factor, interferon (IFN)-γ, vascular endothelial growth factor, platelet-derived growth factor (PDGF), and epidermal growth factor in these cells were also induced by OVA challenge, but there were no differences in the expression levels of these genes in macrophages from WT and APN−/− mice (Fig. 3B and data not shown). There was also increased expression of arginase-1, YM1, and iNOS with OVA challenge in WT and APN−/− mice, but again there was no difference in the levels between the genotypes (Fig. 3C). Interestingly, work from others has suggested that APN could prime vascular macrophages into an M2-type phenotype; however, we did not observe a similar effect in the lung in this model (40, 53). To explore this further, we stimulated a murine macrophage cell line (RAW 264.7) with IL-4 after pretreatment with APN and analyzed STAT6 phosphorylation (activated STAT6). Interestingly, APN treatment of these cells alone increased STAT6 phosphorylation relative to baseline (Fig. 3D). However, when the cells were stimulated with IL-4, APN limited the increase in phosphorylated STAT6 compared with cells that were stimulated with IL-4 without APN pretreatment (Fig. 3D). Taken together, these data suggest that APN modulates eosinophil recruitment into the lung in response to an allergic inflammatory stimulus by suppressing expression of macrophage-derived chemokines. This suppression may occur through effects on nuclear factor (NF)-κB activation and STAT6 activation.
Fig. 3.
Increased levels of CCL11 and CCL24 RNA in macrophages from OVA-immunized and -challenged APN−/− and WT mice. A: representative flow sorting method for isolation of pulmonary macrophages. Last panel shows a cytospin of the isolated cells. B: levels of RNA encoding CCL11, CCL24, and CCL2 in lung macrophages isolated from the lungs of WT and APN−/− mice following OVA immunization and OVA or PBS challenge (n = 7–8 mice/group, P < 0.05 by 2-way ANOVA for genotype and OVA vs. PBS, *P < 0.05 by t-test). C: levels of RNA encoding arginase-1, YM1, and inducible nitric oxide synthase (iNOS) in lung macrophages isolated from the lungs of WT and APN−/− mice following OVA immunization and OVA or PBS challenge (n = 7–8 mice/group, P < 0.05 by 2-way ANOVA for OVA vs. PBS but not for genotype, *P < 0.05 by t-test). D: levels of phosphorylated signal transducer and activator of transcription (STAT) 6 in RAW 264.7 macrophages following pretreatment with APN (10 μg/ml) and IL-4 (100 ng/ml) (each condition was done in triplicate, *P < 0.05 by t-test). No Stim, no stimulation.
Eosinophil granule extracts are mitogenic for PASMCs.
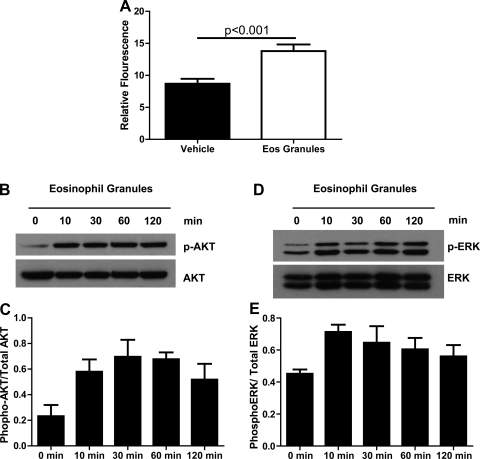
To explore the mechanism linking eosinophils to pulmonary vascular remodeling, we cultured PASMCs in the absence and presence of eosinophil granule extracts. PASMCs were cultured as previously described (75) and serum starved. Cells were then incubated with media containing the granule contents from 106 eosinophils/culture dish well or vehicle (37). PASMC proliferation was twofold greater in PASMC incubated with eosinophil granules than in cells incubated with vehicle (Fig. 4A). To further explore the mechanism of the mitogenic effect of eosinophil granule proteins, we examined the effects of the granule extracts on phosphorylation of Akt1 and ERK, downstream mediators known to be important in PASMC proliferation (1, 38, 39, 60). Consistent with the proliferation data, treatment with eosinophil granules increased the levels of phosphorylated Akt and ERK in PASMCs (Fig. 4, B–E). These data suggest that eosinophils could stimulate pulmonary vascular remodeling by releasing their granule contents and directly stimulating PASMC proliferation in the vasculature.
Fig. 4.
Eosinophil granules are mitogenic for pulmonary artery smooth muscle cells (PASMCs). A: number of PASMCs after 48 h of stimulation with eosinophil granule extract or vehicle control as measured by a fluorescent assay (n = 3 for both groups). PASMCs were prepared from 5 WT mice and pooled for analysis. B: representative Western blot of PASMC protein extracts following incubation with eosinophil granule extract for the indicated times and reacted with antibody to phosphorylated protein kinase B (Akt) and total Akt. The experiment was repeated 3 times. C: relative quantification of the ratio of phosphorylated Akt (p-AKT) and total Akt levels in PASMC protein extracts after stimulation with eosinophil granule extracts for the indicated times (data pooled from 3 separate experiments). D: representative Western blot of PASMC protein extracts following incubation with eosinophil granule extract for the indicated times and reacted with antibody to phosphorylated ERK and total ERK. The experiment was repeated 3 times. E: relative quantification of the ratio of phosphorylated ERK and total ERK levels in PASMC protein extracts after stimulation with eosinophil granule extracts for the indicated times (data pooled from 3 separate experiments).
DISCUSSION
In a previous study, we demonstrated that, in response to allergic inflammation, APN−/− mice have increased eosinophil recruitment into the lung associated with increased pulmonary artery remodeling and elevated pulmonary pressures (45). Based on these data, we hypothesized that the increased remodeling seen in APN−/− mice could result from two different mechanisms: 1) APN deficiency leads to loss of APN-mediated direct suppression of PASMC proliferation; and/or 2) increased eosinophil accumulation around the pulmonary vasculature in APN−/− mice indirectly leads to increased stimulation of the remodeling response. We have recently demonstrated that APN can suppress pulmonary vascular remodeling via direct antiproliferative effects on PASMCs (75). In the studies presented here, we test the other hypothesis, namely that APN may indirectly modulate pulmonary vascular remodeling through effects on eosinophil recruitment. We provide evidence that eosinophils are necessary for the development of pulmonary vascular remodeling in murine models of PH induced by allergic pulmonary vascular inflammation. We also demonstrate that APN may control eosinophil recruitment into the lung via effects on macrophage-mediated chemokine secretion. Finally, we show that eosinophil granule contents are mitogenic for PASMCs and induce proproliferative signaling in these cells. Overall, these studies add more support to the growing body of literature linking metabolism, inflammation, and pulmonary vascular disease (8, 18–20, 22, 59) and suggest a potential therapeutic role for manipulation of inflammatory mediators and adipokine activity in the treatment of PH.
Although most of the evidence linking metabolism to vascular inflammation and remodeling is derived from studies of systemic vascular processes (29), there is an increasing appreciation of effects of metabolism on the development of pulmonary vascular disease. Studies in human samples and from animal models have supported a mechanistic role for insulin resistance, apoE deficiency, and peroxisome proliferator-activated receptor-γ (PPARγ) activity in the pathogenesis of PH (2, 15, 18–20). Furthermore, treatment with PPARγ agonists has been shown to mitigate PH and pulmonary vascular remodeling in animal models, similar to effects of these agents on systemic vascular remodeling (7, 27, 52). There also appears to be an increased incidence of PH and pulmonary vascular remodeling in obesity (21, 44, 69). In light of the obesity-associated downregulation of APN expression, these data suggest that APN could play a mechanistic role connecting obesity and metabolism with increased pulmonary vascular inflammation and remodeling.
Although our recently published results suggest that APN can directly suppress vascular remodeling via effects on PASMCs, we were also interested in examining the effects of APN on pulmonary vascular inflammation. A role for inflammation in the pathogenesis of PH is suggested by studies demonstrating the presence of increased levels of cytokines in patients with PH (14, 25) and the presence of leukocytes in and around the remodeled vasculature of the lung (10, 51, 72). Clinical studies of idiopathic forms of PH have demonstrated that proinflammatory cytokines and chemokines are biomarkers for PH (14, 25, 64) and have better prognostic value than hemodynamic measurements (64). Furthermore, in animal models, pulmonary vascular inflammation induces vascular remodeling and PH (8, 45, 61, 62, 65, 66, 71). This may be most relevant for forms of PH with prominent vascular inflammation such as PH related to shistosomiasis infection (the most common form of PH worldwide) (6) and autoimmunity, but may also be important in other forms of PH (17). It has been suggested that inflammatory cells release mediators that stimulate remodeling of the vessel wall in part by directly promoting PASMC proliferation (22, 48, 59). Eosinophils, in particular, are known to be especially potent sources of mitogenic mediators (42). Alternatively, inflammation could induce metabolic changes such as focal hypoxia that could induce remodeling (28).
In our prior work, we demonstrated that the increased number of eosinophils in the lung of APN−/− mice in a model of PH induced by allergic inflammation was associated with elevated levels of the eosinophil-active chemokine CCL11 in the lung (45). These data are consistent with human data which revealed an inverse correlation between serum APN levels and CCL11 levels in patients with obesity (24). In the studies presented here, we now show that levels of the eosinophil-active chemokine CCL24 are also modulated by APN and that APN may control chemokine secretion via effects on lung macrophages. CCL11 and CCL24 have been shown to be key mediators of allergen-induced pulmonary eosinophila in mice (58) and are produced by multiple cell types in the lung during allergic inflammation. However, it appears that macrophages are the primary source in the lung (31, 34, 35, 56–58, 78). Previous data indicate that APN can influence immune responses through actions on NF-κB signaling in macrophages (76, 77, 79), a cellular pathway that has been linked to the development of allergic inflammation and CCL11 production (9, 36, 43). Furthermore, in vitro and in vivo studies on macrophages have demonstrated that APN inhibits the expression of NF-κB-responsive genes such as IFN-γ and CXCL10/IP-10 (54, 67, 68). Studying ex vivo macrophages, we demonstrate that CCL11 and CCL24 expression was greater in macrophages isolated from the lungs of OVA-challenged APN−/− mice than in those from WT mice. We did not find differences in the expression of growth factors or other cytokines from these cells. Studies have demonstrated that APN can promote macrophage polarization toward the M2 phenotype in vitro (40, 53), and, consistent with this, we demonstrate enhanced STAT6 activity in a macrophage cell line exposed to APN alone. However, APN limited STAT6 activation when these cells were stimulated with IL-4, suggesting that APN may also reduce STAT6 activity in macrophages in response to inflammatory stimuli. Interestingly, we did not see differences in the expression of a panel of markers for the M2 phenotype on ex vivo macrophages isolated from the lungs of WT and APN−/− mice used in the models of PH, suggesting that the effects of APN on macrophage phenotype may be less important in vivo during allergic inflammation. Overall, these data suggest that APN directly suppresses chemokine expression in macrophages, possibly via effects on NF-κB and STAT6 signaling.
Eosinophils are known to be fibrogenic and potent sources of growth factors, cytokines, and other mediators that are mitogenic for SMCs (42, 50, 74). In the OVA-induced chronic model of allergic airway inflammation, eosinophils have been shown to be necessary for airway remodeling (5, 26). Similarly, in our model of allergic inflammation-induced PH, eosinophils are necessary for the development of pulmonary vascular remodeling. Given that APN−/− mice have increased vascular inflammation with enhanced eosinophil recruitment into the lung, it follows that some of the increased remodeling seen in these mice may result from the increased inflammation. Together, these data suggest that inflammation can be an important pathogenic factor in the development of PH and could be an effective therapeutic target for forms of the disease induced by inflammation. Our results may be most relevant to PH associated with schistosomiasis infection, where eosinophilic pulmonary vascular inflammation is very prominent and is thought to stimulate pulmonary vascular remodeling.
It is clear that, in response to growth factors, such as PDGF, and other mediators, such as serotonin, multiple pathways that mediate mitogenic activity in PASMCs are activated in parallel (23, 33, 39). For example, the 3-phosphoinositide-dependent kinase/Akt1 pathway is an important regulator of SMC survival and proliferation (11). Akt is activated via phosphorylation by a number of receptor tyrosine kinases following the binding of growth factors or hormones. Most notably, inhibition of Akt activation reduces proliferation of PASMCs in vitro (38, 41). ERK1 and ERK2 are mitogen-activated protein kinases that have also been shown to be principal mediators of PASMC proliferation in response to PDGF and serotonin stimulation (33, 39, 60). Our data demonstrate that incubation of PASMCs with secretory granule proteins isolated from eosinophils leads to phosphorylation of Akt and ERK and that this activity is associated with increased PASMC proliferation. These data suggest that eosinophilic inflammation could stimulate pulmonary vascular remodeling via direct activation of proproliferative signaling pathways in PASMCs by mediators contained in eosinophil granules. Given that eosinophilic granules are a rich source of growth factors, cytokines, and other mediators that can stimulate proliferation, it is likely that multiple pathways in PASMCs are activated in response to eosinophilic inflammation and exposure to granule contents (50, 74). Future studies are necessary to identify the exact components in eosinophil granules that mediate the stimulatory effects on the growth of PASMCs.
In summary, we have established a critical role of eosinophils in the pathogenesis of vascular remodeling in models of PH induced by allergic inflammation. Our data suggest that eosinophils may be directly mitogenic for PASMCs via degranulation. Furthermore, APN may indirectly modulate pulmonary vascular remodeling by inhibiting the recruitment of eosinophils into the lung by decreasing the secretion of CCL11 and CCL24 by macrophages.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-088297 to B. D. Medoff, T32 HL-07874 to B. D. Medoff in support of M. Weng, and HL-074352 to K. D. Bloch and by a Foundation Leducq award to K. D. Bloch.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Barry Sandall for technical support with this research.
REFERENCES
- 1. Agbani EO, Coats P, Mills A, Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulm Pharmacol Ther 24: 100–109, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 121: 2045–2066, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation 118: 1758–1766, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest 113: 551–560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crosby A, Jones FM, Southwood M, Stewart S, Schermuly R, Butrous G, Dunne DW, Morrell NW. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am J Respir Crit Care Med 181: 279–288, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 205: 361–372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desmet C, Gosset P, Pajak B, Cataldo D, Bentires-Alj M, Lekeux P, Bureau F. Selective blockade of NF-kappa B activity in airway immune cells inhibits the effector phase of experimental asthma. J Immunol 173: 5766–5775, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Dorfmuller P, Humbert M, Perros F, Sanchez O, Simonneau G, Muller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 38: 893–902, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dumas de la Roque E, Savineau JP, Bonnet S. Dehydroepiandrosterone: a new treatment for vascular remodeling diseases including pulmonary arterial hypertension. Pharmacol Ther 126: 186–199, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Elsas PX, Elsas MI. Eosinophilopoiesis at the cross-roads of research on development, immunity and drug discovery. Curr Med Chem 14: 1925–1939, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Fartoukh M, Emilie D, Le Gall C, Monti G, Simonneau G, Humbert M. Chemokine macrophage inflammatory protein-1alpha mRNA expression in lung biopsy specimens of primary pulmonary hypertension. Chest 114: 50S–51S, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice causes PDGF receptor-β-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 297: L1082–L1090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamelmann E, Oshiba A, Loader J, Larsen GL, Gleich G, Lee J, Gelfand EW. Antiinterleukin-5 antibody prevents airway hyperresponsiveness in a murine model of airway sensitization. Am J Respir Crit Care Med 155: 819–825, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Hamid R, Newman JH. Evidence for inflammatory signaling in idiopathic pulmonary artery hypertension: TRPC6 and nuclear factor-kappaB. Circulation 119: 2297–2298, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansmann G, Rabinovitch M. The protective role of adiponectin in pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol 298: L1–L2, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 115: 1275–1284, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Haque AK, Gadre S, Taylor J, Haque SA, Freeman D, Duarte A. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med 132: 1397–1404, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1378: F79–F113, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Herder C, Hauner H, Haastert B, Rohrig K, Koenig W, Kolb H, Muller-Scholze S, Thorand B, Holle R, Rathmann W. Hypoadiponectinemia and proinflammatory state: two sides of the same coin?: results from the Cooperative Health Research in the Region of Augsburg Survey 4 (KORA S4). Diabetes Care 29: 1626–1631, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 151: 1628–1631, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science 305: 1776–1779, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 15: 659–668, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol 184: 4062–4068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krenning G, Moonen JR, Harmsen MC. Pleiotropism of adiponectin: inflammation, neovascularization, and fibrosis. Circ Res 104: 1029–1031, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 18: 313–325, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Lamkhioued B, Renzi PM, Abi-Younes S, Garcia-Zepada EA, Allakhverdi Z, Ghaffar O, Rothenberg MD, Luster AD, Hamid Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol 159: 4593–4601, 1997 [PubMed] [Google Scholar]
- 32. Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305: 1773–1776, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol Lung Cell Mol Physiol 277: L282–L291, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol 162: 2477–2487, 1999 [PubMed] [Google Scholar]
- 35. Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med 163: 1669–1675, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda EA, Rothenberg ME, Drazen JM, Luster AD. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest 99: 1767–1773, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun 77: 4976–4982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol 299: H656–H663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo C, Yi B, Bai L, Xia Y, Wang G, Qian G, Feng H. Suppression of Akt1 phosphorylation by adenoviral transfer of the PTEN gene inhibits hypoxia-induced proliferation of rat pulmonary arterial smooth muscle cells. Biochem Biophys Res Commun 397: 486–492, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Masu K, Ohno I, Suzuki K, Okada S, Hattori T, Shirato K. Proliferative effects of eosinophil lysates on cultured human airway smooth muscle cells. Clin Exp Allergy 32: 595–601, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol 163: 6876–6883, 1999 [PubMed] [Google Scholar]
- 44. McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 104: 2797–2802, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol 41: 397–406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. CARMA1 is critical for the development of allergic airway inflammation in a murine model of asthma. J Immunol 176: 7272–7277, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, Kuperman DA, Erle DJ, Luster AD. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J Immunol 182: 623–635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakagawa Y, Kishida K, Kihara S, Funahashi T, Shimomura I. Adiponectin ameliorates hypoxia-induced pulmonary arterial remodeling. Biochem Biophys Res Commun 382: 183–188, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Neves JS, Weller PF. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol 21: 694–699, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 26: 1110–1118, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285: 6153–6160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 102: 218–225, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 110: 267–278, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest 97: 604–612, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem 280: 13952–13961, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 175: 5341–5350, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118: 2372–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ren W, Watts SW, Fanburg BL. Serotonin transporter interacts with the PDGFβ receptor in PDGF-BB-induced signaling and mitogenesis in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L486–L497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rydell-Tormanen K, Johnson JR, Fattouh R, Jordana M, Erjefalt JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol 39: 61–67, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Rydell-Tormanen K, Uller L, Erjefalt JS. Remodeling of extra-bronchial lung vasculature following allergic airway inflammation (Abstract). Respir Res 9: 18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol 115: 925–927, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122: 920–927, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Summer R, Fiack CA, Ikeda Y, Sato K, Dwyer D, Ouchi N, Fine A, Farber HW, Walsh K. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol 297: L432–L438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol 294: L1035–L1042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117: 375–386, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Taraseviciute A, Voelkel NF. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res 11: 198–202, 2006 [PubMed] [Google Scholar]
- 70. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Tormanen KR, Uller L, Persson CG, Erjefalt JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am J Respir Crit Care Med 171: 19–25, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994 [PMC free article] [PubMed] [Google Scholar]
- 73. Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 61: 170–177, 2004 [DOI] [PubMed] [Google Scholar]
- 74. Weller PF. Human eosinophils. J Allergy Clin Immunol 100: 283–287, 1997 [DOI] [PubMed] [Google Scholar]
- 75. Weng M, Raher MJ, Leyton P, Combs TP, Scherer PE, Bloch KD, Medoff BD. Adiponectin decreases pulmonary arterial remodeling in mouse models of pulmonary hypertension. Am J Respir Cell Mol Biol, 2010. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316: 924–929, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett 579: 6821–6826, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol 163: 6321–6329, 1999 [PubMed] [Google Scholar]
- 79. Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96: 1723–1732, 2000 [PubMed] [Google Scholar]