Abstract
Glucagon-like peptide-1 (GLP-1) is produced by and released from the small intestine following ingestion of nutrients. GLP-1 receptor (GLP-1R) agonists applied peripherally or centrally decrease food intake and increase glucose-stimulated insulin secretion. These effects make the GLP-1 system an attractive target for the treatment of type 2 diabetes mellitus and obesity. In addition to these more frequently studied effects of GLP-1R stimulation, previous reports indicate that GLP-1R agonists suppress water intake. The present experiments were designed to provide greater temporal resolution and site specificity for the effect of GLP-1 and the long-acting GLP-1R agonists, exendin-4 and liraglutide, on unstimulated water intake when food was and was not available. All three GLP-1R ligands suppressed water intake after peripheral intraperitoneal administration, both in the presence of and the absence of food; however, the magnitude and time frame of water intake suppression varied by drug. GLP-1 had an immediate, but transient, hypodipsic effect when administered peripherally, whereas the water intake suppression by IP exendin-4 and liraglutide was much more persistent. Additionally, intracerebroventricular administration of GLP-1R agonists suppressed water intake when food was absent, but the suppression of intake showed modest differences depending on whether the drug was administered to the lateral or fourth ventricle. To the best of our knowledge, this is the first demonstration of GLP-1 receptor agonists affecting unstimulated, overnight intake in the absence of food, the first test for antidipsogenic effects of hindbrain application of GLP-1 receptor agonists, and the first test of a central effect (forebrain or hindbrain) of liraglutide on water intake. Overall, these results show that GLP-1R agonists have a hypodipsic effect that is independent of GLP-1R-mediated effects on food intake, and this occurs, in part, through central nervous system GLP-1R activation.
Keywords: exendin-4, liraglutide, hypodipsia, thirst
glucagon-like peptide-1 (GLP-1) is produced primarily by L-cells in the intestine and neurons in the nucleus of the solitary tract (NTS) of the hindbrain in response to nutrient entry in the gastrointestinal tract (22, 27, 28). Administration of GLP-1, or GLP-1 receptor (GLP1-R) agonists, decreases food intake, inhibits gastric emptying, and enhances the production of insulin in response to glucose (11, 23). The role of GLP-1 as an incretin hormone has made the GLP-1 system an attractive treatment strategy for type-2 diabetes mellitus (T2DM); however, the rapid enzymatic metabolism of the endogenous peptide limits its half-life to ∼2 min (14), thus making GLP-1 clinically impractical. Accordingly, GLP-1R agonists with increased resistance to rapid enzymatic degradation have been developed. Two examples currently used clinically for T2DM treatment are exendin-4, with a half-life of ∼2.5 h, and liraglutide, which has a half-life of ∼13 h (29). Interestingly, these same GLP-1R agonists are also potential treatments for obesity due to the potent suppressive effects on food intake and body weight that have been reported in both humans and animal models (2, 25, 26, 36, 43).
A small number of studies also show a role for GLP-1R ligands in drinking behavior in both rats and humans (9, 21, 30, 40, 45). These studies, however, did not sufficiently determine whether the observed effects on drinking were more directly related to the suppressive effects on feeding, especially with respect to unstimulated water intake. This is of critical importance because water and food intakes often occur together. Indeed, most of the daily water intake in rats occurs during bouts of feeding (7). Accordingly, treatments that reduce food intake also are likely to decrease water intake, making it difficult to determine from these earlier studies whether GLP-1R agonists have an effect on water intake, independent of any effects on food intake.
In the present experiments, we tested the effect of three GLP-1R ligands (GLP-1, exendin-4, and liraglutide) on 24-h water intake in the presence or absence of food. Drugs were administered intraperitoneally or centrally (lateral or fourth ventricle) to evaluate the potential for a forebrain and/or hindbrain central site of action. Moreover, automated intake measures provided detailed and novel temporal and ligand-specific characteristics of the effects of the GLP-1R agonists on water intake. To the best of our knowledge, this is the first test of GLP-1 receptor agonists on unstimulated, overnight intake in the absence of food, the first test for antidipsogenic effects of hindbrain application of GLP-1 receptor agonists, and the first test of a central effect (forebrain or hindbrain) of liraglutide on water intake. Collectively, the results are consistent with the hypothesis that GLP-1R agonists produce a potent hypodipsic effect, independent of any effects on food intake suppression after either peripheral or central administration.
METHODS
Animals.
Forty-four adult male Sprague-Dawley rats (325–349 g at time of purchase; Harlan Laboratories, Indianapolis, IN) were used for the experiments. Animals were housed individually in hanging stainless-steel wire mesh cages (Unifab, Kalamazoo, MI) in a temperature- and humidity-controlled room and maintained on a reverse 12:12-h light-dark schedule (lights off at 10:00 AM and on at 10:00 PM). Standard rodent chow and tap water were available ad libitum except where noted. A repeated-measures design was used within each experiment, but animals were not used in multiple experiments. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo and conformed to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Intracerebroventricular cannula implantation and placement verification.
Rats in experiments 3 and 4 were implanted with chronic indwelling intracerebroventricular cannulas aimed at the lateral or fourth cerebral ventricle, respectively. Rats were anesthetized using a combination of ketamine (70 mg/kg im; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (5 mg/kg im; Spectrum Chemical, Gardena, CA) before being secured in a stereotaxic apparatus. A small burr hole was drilled, and guide cannulas (26 gauge; Plastics One, Roanoke, VA) were implanted using the following coordinates: lateral ventricle (LV), 0.9 mm posterior to bregma, 1.4 mm lateral to midline, and 1.8 mm ventral to dura; fourth ventricle (4V), 2.5 mm anterior to the occipital structure, on midline, and 4.8 mm ventral to skull. Cannulas were affixed to the skull with bone screws and dental cement, and rats were given a single postoperative injection of carprofen (5 mg/kg im; Pfizer Animal Health, New York, NY). At least 5 days after surgery, accurate cannula placement was verified by the response to injection of 10 ng ANG II (LV) or 210 μg 5-thio-d-glucose (4V). Rats that drank at least 6 ml of water in 30 min after ANG II or that had at least a twofold increase in blood glucose after 5-thio-d-glucose were included in subsequent testing. All animals were verified again using the same protocol after the last experimental day and data from any rat that did not meet verification requirements were removed from the analysis.
Drug injections and intake measures.
Injections were given ∼30 min before lights out. Peripheral injections were all intraperitoneal, and central injections were made with a 33-gauge injection cannula inserted into polyethylene-50 tubing attached to a 2-μl Hamilton syringe. Injection cannulas were left in place for ∼1 min after each injection. Water bottles were weighed and returned to the cages immediately after each injection. Total water intake during the testing period was calculated by the difference in pre- and post-test water bottle weight, and the distribution of intake was assayed by counting licks in 20-min bins using a contact lickometer (designed and constructed by the Psychology Electronics Shop, University of Pennsylvania, Philadelphia, PA). The lickometer interfaced with a computer using an integrated USB digital I/O device (National Instruments, Austin, TX) and was processed in a MATLAB (MathWorks, Natick, MA) software environment before being ported to Excel for final analysis. Water spouts were behind an electrically isolated metal plate with a 3.175 mm-wide opening through which the rat needed to lick to reach the spout, minimizing the possibility of non-tongue contact with the spout. Food hoppers were measured before and after food intake tests. Spillage was collected on a plastic transparency under each cage and was included in the measures as uneaten food.
Experiment 1: peripheral administration of GLP-1R agonists on water and food intake.
Using a repeated-measures design, rats (n = 12) were injected intraperitoneally with GLP-1 (7–36) (500 μg/kg; American Peptide, Sunnyvale, CA), exendin-4 (3 μg/kg; American Peptide), liraglutide (100 μg/kg; Bachem, Torrance, CA), or vehicle (1 ml/kg 0.9% saline). The doses were selected on the basis of previous studies showing that each was within the effective range for a hypophagic response following intraperitoneal administration (12, 46). Food and water intakes were measured over the subsequent 24 h. Each rat received each treatment condition in a partial Latin squares design with each testing day separated by 3 or 4 days.
Experiment 2: peripheral administration of GLP-1R agonists on water intake in the absence of food.
The procedures employed in experiment 1 were repeated using the same number of rats and the same injection protocol except that food was removed immediately before the injections and remained unavailable for 24 h, during which time water intake was measured. Food was returned after the 24-h drinking test.
Experiment 3: lateral ventricle administration of GLP-1R agonists on water intake in the absence of food.
Experiment 3 was identical to experiment 2, but drugs were injected into the LV. Ten rats received injections of GLP-1 (10 μg), exendin-4 (0.1 μg), liraglutide (2 μg), or vehicle (0.9% saline), doses that reliably suppress food intake (13, 46) (unpublished pilot work). All injections were 1 μl. A partial Latin-squares design was used with 4–6 days between treatments.
Experiment 4: 4V administration of GLP-1R agonists on water intake in the absence of food.
Experiment 4 was identical to experiment 3, except drugs were injected into the 4V.
Statistical analysis.
All data were analyzed using Statistica (StatSoft, Tulsa, OK). For each experiment a two-way repeated-measures ANOVA was used to test for within-subjects effects of drug and time. When there were more than four levels in any factor (e.g., 12 time bins), a Greenhouse-Geisser correction was used to determine statistical significance. Dark-phase (12-h) water intake, 24-h water intake, and 24-h food intake were each analyzed by repeated-measures one-way ANOVA. Significant main or interaction effects (P < 0.05) were further analyzed using Newman-Keuls post hoc tests.
RESULTS
Experiment 1: peripheral administration of GLP-1R agonists on water and food intake.
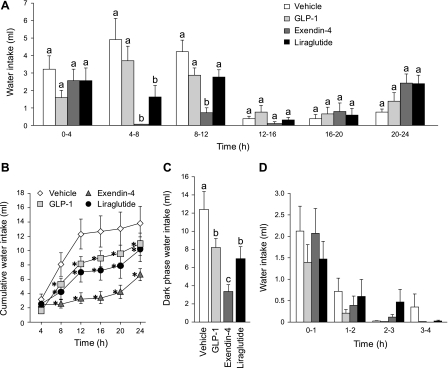
Analysis of the cumulative and noncumulative water intake revealed that the GLP-1R agonists each suppressed water intake, but the magnitude and time course of suppression were drug specific (Fig. 1). GLP-1 and exendin-4 rapidly suppressed water intake, whereas the effect of liraglutide was slower to develop. Additionally, the effect of GLP-1 was transient compared with the longer-lasting effect of exendin-4 or liraglutide. A two-way repeated-measures ANOVA on noncumulative intake in 4-h bins revealed main effects of drug (F1.95, 21.44 = 49.16, P < 0.001), time (F2.37, 26.06 = 111.23, P < 0.001), and a drug × time interaction (F4.74, 52.19 = 34.96, P < 0.001; Fig. 1A). Post hoc tests confirmed a statistically significant suppression of water intake in the first 4 h after exendin-4 administration (P < 0.001 compared with vehicle). This immediate suppression was followed by elevated intake during the final 4-h bin (20 h through 24 h), during which time, water intake was greater than that observed by the vehicle group (P < 0.05). Liraglutide suppressed water intake during the first 4-h bin compared with vehicle treatment (P < 0.001), but this suppression was not as large as that observed after exendin-4 treatment (P < 0.001). The suppressive effect of liraglutide became increasingly robust throughout the first 12 h of testing. No differences in water intake were observed during the majority of the subsequent light phase, which began during hour 12, but water intake was low during this period in all groups. Similar to exendin-4, rats treated with liraglutide also drank more water during the last 4 h of testing. Indeed, water intake by rats treated with liraglutide was greater than all other groups in the last 4-h bin (all P values < 0.01).
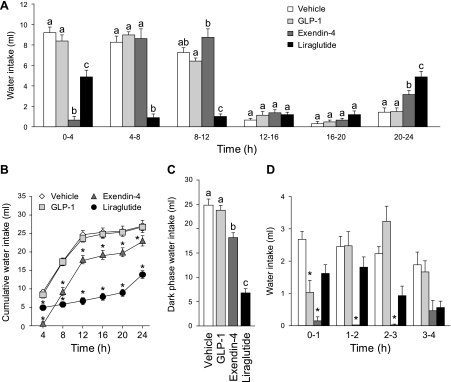
Fig. 1.
Unstimulated water intake with food available after intraperitoneal injection of vehicle (0.9% saline), GLP-1 (500 μg/kg), exendin-4 (3 μg/kg), or liraglutide (100 μg/kg). A: noncumulative 24-h water intake collapsed into 4-h bins. Both exendin-4 and liraglutide had a robust suppressive effect on water intake during the first 12 h of testing (dark phase). a,bBars with different letters, and within the same bin, are significantly different (P < 0.05). B: cumulative 24-h water intake. Cumulative analysis illustrates that the initial hypodipsia after exendin-4 or liraglutide administration was not compensated for during the testing period. *Significant differences from vehicle (P < 0.05). C: dark-phase water intake. Exendin-4 or liraglutide suppressed water intake over the dark phase, with liraglutide administration causing the greatest suppression. a,b,cBars with different letters are significantly different (P < 0.05). D: noncumulative water intake in 1-h bins. Examination of shorter bins revealed an immediate, but transient, effect of GLP-1 on water intake. *Significant differences from controls within a given bin (P < 0.05; data from the whole 24-h test were analyzed, but for clarity, only the first 4 bins are shown in the figure). Data are shown as means ± SE.
Analysis of cumulative intake largely mirrored the noncumulative data, but provides an additional, perhaps clearer, illustration that the decrease of water intake after liraglutide and exendin-4 was maintained throughout the 24-h period (Fig. 1B). A two-way repeated-measures ANOVA on the cumulative 4-h data confirmed main effects of drug (F1.87, 20.52 = 73.78, P < 0.001), time (F1.66, 18.22 = 410.44, P < 0.001), and a drug × time interaction (F3.97, 43.64 = 49.26, P < 0.001), and post hoc tests showed that the suppressive effect of exendin-4 and liraglutide was statistically significant by the end of the first 4 h. Water intake after liraglutide or exendin-4 injection remained lower at every time during the testing period (P < 0.001 and P < 0.001, respectively) compared with intakes after vehicle injection. This indicates that at the end of the 24-h test, the animals that received liraglutide and exendin-4 did not fully compensate for the initial suppression of water intake.
As expected, the vast majority of water intake occurred during the 12-h dark phase. Analysis of dark-phase intake (0–12 h) confirmed the findings described above. Specifically, a repeated-measures ANOVA detected a main effect of drug (F3,33 = 83.37, P < 0.001), and post hoc tests confirmed that both exendin-4 and liraglutide suppressed total water intake during the dark phase (P < 0.001 and P < 0.001 compared with vehicle; Fig. 1C).
Although the effect of GLP-1 was not apparent in the above analyses, a transient effect of GLP-1 was detected when data were analyzed in shorter time bins (Fig. 1D). When 24-h intake was broken into 1-h bins, ANOVA confirmed main effects of drug (F1.95, 21.44 = 49.16, P < 0.001), time (F5.38, 59.21 = 19.55, P < 0.001), and a drug × time interaction (F8.51, 93.56 = 6.93, P < 0.001). Post hoc tests confirmed a transient effect of GLP-1 that was limited to the first hour after treatment and is illustrated in Fig. 2. The initial effect of GLP-1 disappeared quickly, and no further effects of GLP-1 on water intake were observed.
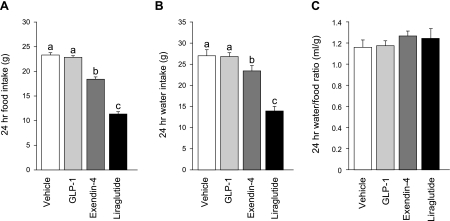
Fig. 2.
Total 24-h intake after intraperitoneal injection of vehicle (0.9% saline), GLP-1 (500 μg/kg), exendin-4 (3 μg/kg), or liraglutide (100 μg/kg). Analysis of 24-h food intake (A) showed a hypophagic response to exendin-4 or liraglutide that was markedly similar in direction and magnitude to the suppression of 24 h water intake (B). C: effect was normalized when the data were analyzed as the ratio of water intake to food intake. a,b,cBars with different letters within a given panel differed from each other significantly (P < 0.05). Data are shown as means ± SE.
As expected, exendin-4 and liraglutide reliably decreased 24-h food intake, but we did not find a 24-h hypophagic response after GLP-1 administration (Fig. 2A). Analysis of the 24-h food intake revealed a main effect of drug (repeated-measures ANOVA, F3,33 = 121.65, P < 0.001). Post hoc tests revealed that rats given exendin-4 or liraglutide ate less than rats in the vehicle (P < 0.001; P < 0.001 respectively) or GLP-1 groups (P < 0.001; P < 0.001 respectively). Moreover, the magnitude of suppression by liraglutide was greater than by exendin-4 treatment (P < 0.001).
The availability of food during the water intake measures made it impossible for us to estimate whether the observed effect on water intake was independent of the effect on food intake. Indeed, the percent suppression in 24-h water intake was remarkably similar to percent suppression in 24-h food intake within each drug condition (Fig. 2, A and B). Moreover, when the data were expressed as a ratio of water to food, intake differences were neutralized (F3, 33 = 0.94, P = 0.43; Fig. 2C), making it necessary to test whether the effect on water intake was secondary to an effect on food intake.
Experiment 2: peripheral administration of GLP-1R agonists on water intake in the absence of food.
To test the possibility that the observed hypodipsia was secondary to the suppression of food intake, we repeated the experiment but removed food immediately before drug administration. The absence of food did not, however, prevent the antidipsogenic effects of GLP-1R agonists, and the direction, timing, and magnitude of suppression were remarkably similar to what we observed in the previous experiment (Fig. 3). Analysis of noncumulative, 4-h binned water intake confirmed main effects of drug (F2.08, 22.90 = 5.19, P < 0.05), time (F1.46, 16.10 = 25.36, P < 0.001) and a drug × time interaction (F6.03, 66.37 = 14.91, P < 0.001; Fig. 3A). Similar to the previous results, exendin-4 administration quickly and robustly suppressed water intake (P < 0.001 vs. vehicle). After the initial 4 h, intake was similar to controls for all of the remaining bins except the last, during which there was again a moderate, but statistically significant, increase in water intake (P < 0.01 compared with vehicle). The effect of liraglutide on water intake was also similar to that observed in the previous experiment. Suppression of water intake did not occur immediately after drug administration, but was observed in the second and third 4-h bins (P < 0.001 compared with vehicle in the 4–8-h bin; P < 0.001 compared with vehicle in the 8–12-h bin). Rats given liraglutide showed a moderate, but statistically significant, increase in water intake during the final 4-h bin (P < 0.001 compared with vehicle).
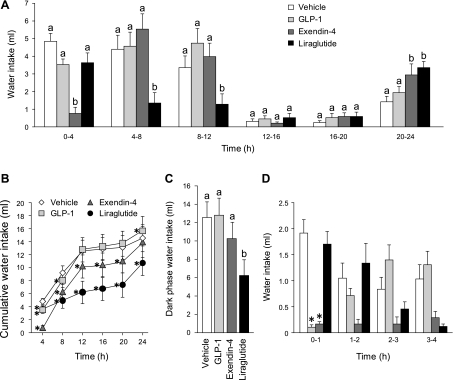
Fig. 3.
Unstimulated water intake without food available after intraperitoneal injection of vehicle (0.9% saline), GLP-1 (500 μg/kg), exendin-4 (3 μg/kg), or liraglutide (100 μg/kg). A: noncumulative 24-h water intake collapsed into 4-h bins confirms that the hypodipsia produced by these drugs could not be explained by a primary suppression of food intake. a,bBars with different letters, and within the same bin, are significantly different (P < 0.05). B: cumulative 24-h water intake illustrates that hypodipsia after GLP-1 or exendin-4 was compensated for by the end of the testing period. *Significant differences from vehicle (P < 0.05). C: dark-phase water intake. Of the three GLP-1R agonists, only liraglutide suppressed water intake over the entire dark phase. a,bBars with different letters are significantly different (P < 0.05). D: noncumulative water intake in 1-h bins. Examination of shorter bins revealed an immediate, but transient, effect of GLP-1 on water intake, as was observed in experiment 1. *Significant differences from controls within a given bin (P < 0.05; data from the whole 24-h test were analyzed, but for clarity, only the first 4 bins are shown in the figure). Data are shown as means ± SE.
Analysis of cumulative water intake confirmed the robust hypodipsia induced by peripheral exendin-4 and liraglutide (Fig. 3B). A two-way repeated-measures ANOVA found main effects of drug (F1.91, 21.04 = 10.17, P < 0.001), time (F1.06, 11.63 = 36.65, P < 0.001), and a drug × time interaction (F2.96, 32.51 = 12.79, P < 0.001). By examining the data in this way, it is evident that animals that received liraglutide maintained a decrease in overall water intake throughout the entire 24 h (P < 0.05 compared with vehicle at 4 h; P < 0.001 compared with vehicle at all other time points). Rats that received exendin-4, however, increased intake near the end of the test (hours 20–24), thereby eliminating cumulative 24-h differences from controls.
Dark-phase water intake again was examined and a repeated-measures ANOVA confirmed a main effect of drug (F3,33 = 13.62, P < 0.001; Fig. 3C). Post hoc tests revealed a suppressive effect of liraglutide, but GLP-1 and exendin-4 were not significantly different from vehicle. Although this analysis did not detect an effect from exendin-4, the analysis of 4-h binned and cumulative intake clearly indicated that GLP-1R agonists suppressed water intake in the absence of food.
Similar to the results of experiment 1, when we probed the water intake data by examining 1-h binned intake, the main effects of drug (F2.08, 22.90 = 5.19, P < 0.05), time (F4.44, 48.89 = 9.68, P < 0.001), and a drug × time interaction (F6.59, 72.53 = 3.98, P < 0.01) persisted (Fig. 3D). Both the cumulative and noncumulative data show a transient, but robust suppression of water intake by GLP-1 (P < 0.05 compared with vehicle at 4 and 8 h in the cumulative data; P < 0.001 compared with vehicle the first bin in noncumulative data). It is likely that the prolonged effect apparent in the cumulative analysis was due to the large magnitude of intake suppression during the first hour after drug administration. Accordingly, the findings from experiment 2 demonstrate that the antidipsogenic effect of these GLP-1R agonists did not depend on a more proximal effect on food intake.
Experiment 3: lateral ventricle administration of GLP-1R agonists on water intake in the absence of food.
To evaluate the role of central GLP-1R in the observed effects on water intake, we made injections of GLP-1R agonists into the LV. Analysis of noncumulative or cumulative data confirmed that LV injections of liraglutide and exendin-4, but not GLP-1, affected water intake in the absence of food (Fig. 4). Overall, the effects on water intake were similar to those observed after peripheral drug administration, except that we did not find an effect of GLP-1 on water intake. Repeated-measures ANOVA on noncumulative, 4-h binned intake confirmed a main effect of drug (F2.18, 19.60 = 6.81, P < 0.01), time (F1.56, 14.00 = 9.22, P < 0.01), and a drug × time interaction (F2.05, 18.49 = 6.59, P < 0.01; Fig. 5A). Rats given exendin-4 drank moderately more than controls during the first 4-h bin (P < 0.01 compared with vehicle); however, water intake after LV vehicle delivery was considerably lower during this bin than that observed in experiments 1, 2, and 4, suggesting that this increase by exendin-4 is likely an artifact of an abnormally low intake by vehicle-treated rats during this bin rather than being due to drug-induced hyperdipsia. The suppressive effect of exendin-4 and liraglutide was, however, reliable and clear during the next two 4-h bins (P < 0.001 exendin-4 or liraglutide compared with vehicle 4–8-h bin; P < 0.001 exendin-4 compared with vehicle 8–12-h bin; P < 0.01 liraglutide compared with vehicle 2–12-h bin). Unlike our analysis of the intake after peripheral injections, there was no compensatory drinking in the 20–24-h period after drug administration. Evaluation of 1-h intake bins did not reveal any transient differences in intake after GLP-1 administration (Fig. 4D).
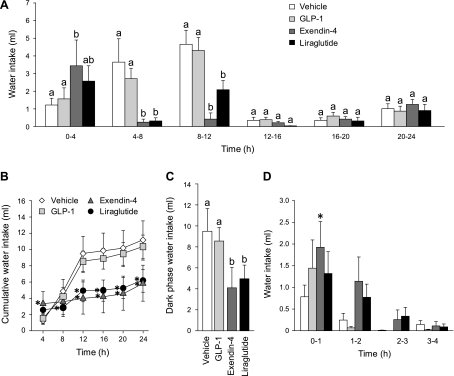
Fig. 4.
Unstimulated intake without food available after lateral ventricular (LV) injection of vehicle (0.9% saline), GLP-1 (10 μg), exendin-4 (0.1 μg), or liraglutide (2 μg). A: noncumulative 24-h water intake collapsed into 4-h bins. Administration of these drugs centrally led to a similar pattern of hypodipsia, as was seen after peripheral injections. a,bBars with different letters, and within the same bin, are significantly different at P < 0.05. B: cumulative 24-h water intake. Cumulative analysis illustrates that the initial hypodipsia after exendin-4 or liraglutide administration was not compensated for during the testing period. Asterisks indicate significant differences from vehicle (P < 0.05). C: dark phase water intake. Exendin-4 or liraglutide administration suppressed water intake over the dark phase to a similar extent. a,bBars with different letters are significantly different (P < 0.05). Data are shown as means ± SE. D: noncumulative water intake in 1-h bins. Examination of shorter bins revealed an effect of exendin-4, but not of any other GLP-1R agonists. *Significant differences from controls within a given bin (P < 0.05; data from the whole 24-h test were analyzed, but for clarity, only the first 4 bins are shown in the figure). Data are shown as means ± SE.
Fig. 5.
Unstimulated intake without food available after 4V injection of vehicle (0.9% saline), GLP-1 (10 μg), exendin-4 (0.1 μg), or liraglutide (2 μg). A: noncumulative 24-h water intake collapsed into 4-h bins. Hindbrain administration of exendin-4 or liraglutide also led to suppression of water intake, suggesting at least a partial contribution of the hindbrain in the observed hypodipsia. a,bBars with different letters, and within the same bin, are significantly different at P < 0.05. B: cumulative 24-h water intake. Cumulative 24-h water intake suggests that GLP-1 also had a suppressive effect on water intake and that the initial hypodipsia after GLP-1, exendin-4, or liraglutide administration was not compensated for during the testing period. *Significant differences from vehicle (P < 0.05). C: dark-phase water intake. GLP-1, exendin-4, or liraglutide administration suppressed water intake over the dark phase, with exendin-4 causing the greatest suppression. a,b,cBars with different letters are significantly different (P < 0.05). Data are shown as means ± SE. D: noncumulative water intake in 1-h bins. Examination of shorter bins did not reveal any significant effects of GLP-1R agonists. *Significant differences from controls within a given bin (P < 0.05; data from the whole 24-h test were analyzed, but for clarity only the first 4 bins are shown in the figure). Data are shown as means ± SE.
Analysis of the cumulative intake highlighted that exendin-4 and liraglutide reduced overall water intake throughout the 24-h testing period (Fig. 4B). Repeated-measures ANOVA revealed main effects of drug (F1.99, 17.91 = 5.31, P < 0.05), time (F1.18, 10.58 = 35.71, P < 0.001), and a drug × time interaction (F2.25, 20.27 = 12.50, P < 0.001). Post hoc testing found significant differences between the liraglutide and control group beginning 8 h after drug administration that persisted through the remainder of the testing (P < 0.05 compared with vehicle at 8 h; P < 0.001 compared with vehicle at all subsequent time points). Analysis of cumulative water intake found that the decreased intake after exendin-4 was not significant until hour 12 (P < 0.001 compared with vehicle at 12–24 h). Both the cumulative and noncumulative data show a clear antidipsogenic effect after LV injection of exendin-4 or liraglutide that did not depend on a concurrent hypophagia.
Dark-phase intake is shown in Fig. 4C and a repeated-measures ANOVA confirmed a main effect of drug (F3,27 = 70.26, P < 0.001). Similar to experiments 1 and 2, exendin-4 and liraglutide both suppressed total 12-h water intake to a similar extent, but there was no effect of GLP-1 (P < 0.01; P < 0.01; P = 0.45, respectively, compared with vehicle).
Experiment 4: 4V administration of GLP-1R agonists on water intake in the absence of food.
GLP-1 receptor agonists also were injected into the 4V to evaluate mediation of water intake suppression by hindbrain substrates. Analysis of noncumulative water intake revealed a main effect of drug (F1.75, 15.78 = 4.13, P < 0.05), time (F2.94, 26.48 = 9.55, P < 0.001), and a drug × time interaction (F4.70, 42.33 = 6.54, P < 0.001; Fig. 5A). Again, after exendin-4 administration, there was robust suppression of water intake during the second 4-h bin (P < 0.001 vs. vehicle) and third 4-h bin (P < 0.001 vs. vehicle). There was also suppression of water intake after liraglutide administration during the second 4-h bin (P < 0.001 vs. vehicle); however, this suppression appeared to be neither as robust, nor as long lasting, as that observed after LV administration. Similar to the results from LV drug administration, there was no effect of GLP-1 on water intake in any bin, nor was there a statistically significant increase in water intake during the last bin of the testing period. Analysis of data in 1-h bins failed to reveal any additional effects (Fig. 5D).
Analysis of cumulative water intake confirmed the effect of liraglutide and exendin-4, and it revealed significant suppression of intake by GLP-1 (Fig. 5B). A two-way repeated-measures ANOVA found a main effect of drug (F1.69, 15.17 = 6.33, P < 0.05), time (F1.52, 13.69 = 44.93, P < 0.001), and drug × time interaction (F2.61, 23.45 = 9.08, P < 0.001). Like the noncumulative analysis, both liraglutide and exendin-4 decreased water intake at 8 h; however, here, it is also evident that cumulative intake remained lower than vehicle intake throughout the rest of the testing period (P < 0.001 for either liraglutide or exendin-4 compared with vehicle at all time points). Cumulative representation of the data also revealed that GLP-1 did have an effect on water intake. Post hoc testing found significant differences between the GLP-1 and control group, beginning 8 h after drug administration that persisted through the remainder of the 24 h (P < 0.001 compared to vehicle at 8–24-h time points).
Analysis of dark-phase intake also detected a suppressive effect of all three GLP-1R agonists after 4V administration (Fig. 5C). A repeated-measures ANOVA confirmed a main effect of drug (F3,27 = 137.91, P < 0.001), and post hoc tests showed similar hypodipsia in GLP-1- or liraglutide-treated rats (P < 0.05 and P < 0.01, respectively, compared with vehicle) and greater suppression of intake after exendin-4 administration (P < 0.001 compared with vehicle, P < 0.05 compared with GLP-1, P < 0.05 compared with liraglutide). Overall, the data from the central injections suggest that both forebrain and hindbrain sites are involved in GLP-1R agonist-induced hypodipsia.
DISCUSSION
The current findings provide a comprehensive evaluation of the time-course of the hypodipsic effects of GLP-1R agonists and provide evidence that suppression of water intake occurred independent of any effects on food intake. Either peripheral or central administration of GLP-1R agonists, at doses that produce hypophagia (12, 13, 46), suppressed water intake, even in the absence of food. Moreover, to the best of our knowledge, this is the first test of water intake after hindbrain injection of these GLP-1R ligands and the first time that this effect has been reported after either forebrain or hindbrain administration of liraglutide. The consistent doses used in the LV and 4V injections provide the first comparison of the efficacy of forebrain and hindbrain routes of administration and highlight the potential for a hindbrain site of action in the antidipsogenic response to GLP-1R agonists.
Peripheral administration of GLP-1, exendin-4, or liraglutide suppressed unstimulated overnight water intake in rats both in the presence and absence of food. The pattern of water intake suppression after peripheral injection was consistent with the known half-lives of the drugs tested in these studies (29). Specifically, GLP-1, which is very rapidly degraded, only suppressed water intake during the first hour of testing, whereas the effect of exendin-4 persisted throughout the first 4 h of testing. Furthermore, liraglutide, which has the longest half-life of the drugs tested, had the longest-lasting effect on water intake, with hypodipsia maintaining for 12 h after intraperitoneal injection. Therefore, it is reasonable to conclude that differences in the time frame of the hypodipsic response to peripheral injections were largely related to the stability of the ligands.
Hypodipsia was also evident after central administration of GLP-1R agonists, although the pattern of intake was slightly different compared with that observed following peripheral delivery. These differences may provide important information about the site(s) and mechanism(s) of action for the different agonists. Following peripheral injection, liraglutide was more potent at suppressing water intake than was exendin-4. The relative potency of exendin-4 and liraglutide differed, however, when the drugs were injected centrally. Exendin-4 and liraglutide produced similar suppression of intake after LV injections, but exendin-4 was more effective than liraglutide after 4V injection. The different doses used could be partly responsible for the differences between the responses to peripheral and central injections, but it is notable that the direction of the difference was not the same after LV or 4V injection, making it unlikely that it was simply a dose-related artifact. The differences after IP, LV, and 4V injections make it tempting to speculate that an interaction between the mechanism of action of the drugs and the route of administration was responsible. Nevertheless, the hypodipsic effect of GLP-1 agonists after central administration was clear, suggesting that at least part of the effect was mediated by central substrates, similar to studies done on food intake (16).
The comparison of LV and 4V administration revealed differences in the efficacy of GLP-1 that depended on the site of injection. Analysis of cumulative, as well as dark-phase intake, revealed an effect of GLP-1 after 4V, but not after LV, administration. The apparent differences in intake after LV or 4V administration of vehicle may, however, contribute to this effect. We did not find any differences in the response to LV or 4V exendin-4; injection into either ventricle potently suppressed water intake. Concluding that there was no difference based on site of administration is difficult, however, because intake was almost completely eliminated between the 4th and 12th h of testing, creating the possibility of a floor effect. Lastly, liraglutide suppressed water intake, but the magnitude and pattern of the suppression differed based on injection site. The effect of liraglutide was somewhat more robust when administered into the LV than when it was injected into the 4V, raising the possibility of additional forebrain sites at which GLP-1R agonists can act to suppress water intake. Regardless of these nuances in the response, the data clearly demonstrate suppression of water intake by exendin-4 or liraglutide and strongly implicate the hindbrain as a site of action, with the potential for additional receptive sites in the forebrain.
The precise mechanisms underlying peripheral or central GLP-1R-mediated hypodipsia remains to be determined. Meal size suppression following intraperitoneal administration of GLP-1 has been shown to depend upon vagal afferent signaling (34). Vagal afferents synapse on NTS neurons, which, in turn, connect to a network of structures, including the amygdala, paraventricular nucleus (PVN), lateral hypothalamus, and parabrachial nucleus (22, 31, 32). Although these connections have been implicated in the control of food intake, their specific relevance to an effect on water intake remains to be determined, especially with relevance to GLP-1. Moreover, it remains unclear whether peripheral administration of GLP-1R ligands mimics release of GLP-1 from the gut or, instead, activates GLP-1R normally involved in the responses to GLP-1 of central origin. Cells in the caudal NTS synthesize GLP-1, and GLP-1 receptors are found in the NTS, as well as in various structures that receive projections from the NTS (22, 27). Several of these structures, including the lateral septum, amygdala, nucleus accumbens, preoptic area, PVN, supraoptic nucleus, subfornical organ, and area postrema, have a clear relevance to fluid intake and should be included in any preliminary list of structures that may be involved in the observed responses. Further studies are needed, however, to confirm or dismiss a specific role for any of these structures in GLP-1 receptor-mediated suppression of water intake.
The complicated regulation of water intake, and more generally, maintenance of body fluid homeostasis, requires consideration of several alternate explanations for the responses reported here. Specifically, it is important to consider the potential contribution of GLP-1R agonists, injected peripherally or centrally, on diuresis (21, 40), insulin secretion (18, 19, 35), blood pressure (1, 3, 8, 10, 15, 47), and visceral illness (17, 42, 44) before concluding that there is a more direct effect on water intake. Previous reports have shown that GLP-1 administration in rats causes diuresis, as well as increased sodium excretion. It is unlikely, however, that these effects would contribute to the suppression of water intake observed following GLP-1R stimulation, as diuresis and increased sodium excretion would more likely generate compensatory increases in fluid and solute consumption. Whether or not GLP-1R agonists affect sodium intake remains a topic for further investigation. Although insulin affects water intake, the direction of insulin's effect makes it unlikely that this explains GLP-1R-mediated hypodipsia because administration of insulin increases, rather than decreases, water intake in the absence of food (39). Increased blood pressure inhibits water intake (33), but the time course of the pressor response to GLP-1 and the suppression of intake do not support changes in blood pressure as a more direct cause of the observed effects on water intake. Gardiner et al. (8) demonstrates that blood pressure returns to normal ∼120 min after peripheral injection of exendin-4 (using a dose similar to that used in the present studies), but the suppression of water intake in the present study persisted for longer than 120 min. While we cannot completely discount a role for GLP-1R-mediated changes in blood pressure that could influence water intake, it is important to note that these blood pressure changes are more transient than the longer-lasting hypodipsic effects following GLP-1R activation, making it unlikely that the intake suppression was exclusively related to changes in blood pressure. It is also possible that the treatments used in the present study suppressed fluid intake by causing an illness-like response; however, we suggest caution before concluding that this was the primary reason for the decreased fluid intake. First, there is evidence that GLP-1-induced suppression of food intake and illness are separable. Microinjections of GLP-1R ligands into the PVN suppress food intake but do not produce a conditioned taste avoidance (CTA) and microinjections into the central nucleus of the amygdala produce a CTA without any suppression of food intake (17, 24). Second, Kinzig et al. (17) found that an injection of GLP-1 into the 4V was not sufficient to develop CTA, but this route of injection suppressed water intake in the present studies. Third, and perhaps most important, illness-inducing agents may not have the same effect on food intake as they do on fluid intake. Previous studies of water intake in response to LiCl poisoning found that water intake was either unaffected or increased by LiCl (5, 20, 38). These reports are of critical importance here because there is evidence that LiCl-induced illness may involve the GLP-1 system. Specifically, central administration of GLP-1R antagonists block LiCl-induced c-Fos, hypophagia, and pica (37, 41). We are not, however, arguing that illness-inducing effects of the drugs were absent in our studies. There is, indeed, evidence that GLP-1R agonists cause a sensation of visceral illness in humans (4) and a similar effect can be inferred from CTA studies in rats (17, 42, 44). However, on the basis of the evidence presented above, we find this explanation insufficient to account uniformly for the suppressed water intake in the present studies. Thus, it seems reasonable to conclude that any of these alternate explanations cannot entirely account for the hypodipsic effects following GLP-1R activation.
Perspectives and Significance
The present results provide an important extension of previous findings and help build support for a role for GLP-1 receptors in the regulation of water intake. Moreover, these results are likely generalizable because Gutzwiller et al. (9) found hypodipsia in humans after intravenous injections of GLP-1. Thus, the basic finding that GLP-1 receptor agonists decrease fluid intake could be especially important in clinical settings, especially with respect to geriatric patient populations that are already at risk for dehydration (6). Nevertheless, elucidating the precise role of GLP-1 receptors in fluid intake, determining the underlying neural substrates, and evaluating the possibility that any feeding responses are secondary to fluid intake effects requires further research. Collectively, the data show that either peripheral or central administration of GLP-1 agonists suppresses unstimulated water intake when food is unavailable.
GRANTS
This study was supported by National Institutes of Health Grants DK089752 (to S. E. Kanoski), DK085435 (to M. R. Hayes), and HL091911 (to D. Daniels).
DISCLOSURES
M. R. Hayes receives grant support from Novo Nordisk. No funds or supplies from that grant were used for these studies.
ACKNOWLEDGMENTS
The authors are grateful for the technical assistance provided by Anikó Marshall and Elizabeth Mietlicki.
REFERENCES
- 1. Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol Endocrinol Metab 266: E459–E466, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8: 436–447, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bojanowska E, Stempniak B. Effects of centrally or systemically injected glucagon-like peptide-1 (7–36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul Pept 91: 75–81, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Chia CW, Egan JM. Incretin-based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab 93: 3703–3716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christensen S. Effects of lithium on water intake and renal concentrating ability in rats with vasopressin-deficient diabetes insipidus (Brattleboro strain). Pflügers Arch 396: 106–109, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Ferry M. Strategies for ensuring good hydration in the elderly. Nutr Rev 63: S22–S29, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Fitzsimons TJ, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol 67: 273–283, 1969 [DOI] [PubMed] [Google Scholar]
- 8. Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther 316: 852–859, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 73: 142–150, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Halbirk M, Norrelund H, Moller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Botker HE, Wiggers H. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol 298: H1096–H1102, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100: 503–510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 19: 1342–1349, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48: 612–615, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept 118: 33–38, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115: 3554–3563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes 38: 902–905, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Kristal MB, Steuer MA, Nishita JK, Peters LC. Neophobia and water intake after repeated pairings of novel flavors with toxicosis. Physiol Behav 24: 979–982, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50: 2530–2539, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Leech CA, Chepurny OG, Holz GG. Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm 84: 279–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274: R23–R29, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140: 244–250, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Meier JJ, Gallwitz B, Schmidt WE, Nauck MA. Glucagon-like peptide 1 as a regulator of food intake and body weight: therapeutic perspectives. Eur J Pharmacol 440: 269–279, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Mojsov S, Kopczynski MG, Habener JF. Both amidated and nonamidated forms of glucagon-like peptide I are synthesized in the rat intestine and the pancreas. J Biol Chem 265: 8001–8008, 1990 [PubMed] [Google Scholar]
- 29. Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 124: S3–S18, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, Eng J, Blazquez E. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 67: 1982–1991, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol 150: 217–237, 1973 [DOI] [PubMed] [Google Scholar]
- 32. Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robinson MM, Evered MD. Pressor action of intravenous angiotensin II reduces drinking response in rats. Am J Physiol Regul Integr Comp Physiol 252: R754–R759, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293: R983–R987, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith DF, Amdisen A. Central effects of lithium in rats: lithium levels, body weight and water intake. Acta Pharmacol Toxicol (Copenh) 52: 81–85, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Spitz R. Induction of drinking by insulin in the rat. Eur J Pharmacol 31: 110–114, 1975 [DOI] [PubMed] [Google Scholar]
- 40. Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol Regul Integr Comp Physiol 271: R848–R856, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Thiele TE, Seeley RJ, D'Alessio D, Eng J, Bernstein IL, Woods SC, van Dijk G. Central infusion of glucagon-like peptide-1-(7–36) amide (GLP-1) receptor antagonist attenuates lithium chloride-induced c-Fos induction in rat brainstem. Brain Res 801: 164–170, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996 [DOI] [PubMed] [Google Scholar]
- 44. van Dijk G, Thiele TE, Seeley RJ, Woods SC, Bernstein IL. Glucagon-like peptide-1 and satiety. Nature 385: 214, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Wang T, Edwards GL, Baile CA. Glucagon-like peptide-1 (7–36) amide administered into the third cerebroventricle inhibits water intake in rats. Proc Soc Exp Biol Med 219: 85–91, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110: 43–52, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]