Abstract
Combined heat acclimation (AC) and exercise training (EX) enhance exercise performance in the heat while meeting thermoregulatory demands. We tested the hypothesis that different stress-specific adaptations evoked by each stressor individually trigger similar cardiac alterations, but when combined, overriding/trade-off interactions take place. We used echocardiography, isolated cardiomyocyte imaging and cDNA microarray techniques to assay in situ cardiac performance, excitation-contraction (EC) coupling features, and transcriptional programs associated with cardiac contractility. Rat groups studied were controls (sedentary 24°C); AC (sedentary, 34°C, 1 mo); normothermic EX (treadmill at 24°C, 1 mo); and heat-acclimated, exercise-trained (EXAC; treadmill at 34°C, 1 mo). Prolonged heat exposure decreased heart rate and contractile velocity and increased end ventricular diastolic diameter. Compared with controls, AC/EXAC cardiomyocytes demonstrated lower l-type Ca2+ current (ICaL) amplitude, higher Ca2+ transient (Ca2+T), and a greater Ca2+T-to-ICaL ratio; EX alone enhanced ICaL and Ca2+T, whereas aerobic training in general induced cardiac hypertrophy and action potential elongation in EX/EXAC animals. At the genomic level, the transcriptome profile indicated that the interaction between AC and EX yields an EXAC-specific molecular program. Genes affected by chronic heat were linked with the EC coupling cascade, whereas aerobic training upregulated genes involved with Ca2+ turnover via an adrenergic/metabolic-driven positive inotropic response. In the EXAC cardiac phenotype, the impact of chronic heat overrides that of EX on EC coupling components and heart rate, whereas EX regulates cardiac morphometry. We suggest that concerted adjustments induced by AC and EX lead to enhanced metabolic and mechanical performance of the EXAC heart.
Keywords: echocardiography, isolated cardiomyocytes, Ca2+ signaling, genomic responses
heat acclimation (AC) and exercise training (EX) are powerful stressors, causing structural cardiac remodeling and improving mechanical performance. Each stressor has its own adaptive requirements, e.g., increased peripheral blood flow to enhance heat dissipation, required for AC, or increased muscle blood flow to augment the oxygen supply and muscle power output needed during bouts of exercise. Evidence derived from isolated rat heart preparations prompted the hypothesis that different stress-specific adaptive signaling mechanisms trigger the similar cardiac alterations evoked by each stressor alone. Upon AC, an increase in left cardiac ventricular compliance and systolic pressure with a concomitant decrease in oxygen consumption suggests enhanced cardiac efficiency (25–26). At the cellular level, greater Ca2+ transient amplitude compensates for decreased myofilament Ca2+ responsiveness and plays a major role in greater force development (4). Myosin isoform redistribution from the predominantly fast myosin isoform (V1) to slow myosin isoform (V3) predominance and the altered expression of Ca2+ regulatory proteins decrease the contraction and relaxation velocities of the AC heart (20, 28).
In contrast to AC, endurance EX increases cardiac muscle mass (hypertrophy) and oxygen consumption (6, 36). Fast myosin with high ATPase activity (V1) predominance and increased contractility is induced by changes in the regional contractile properties of the heart (7). Increased myofilament sensitivity to Ca2+ in intact (40) and skinned cardiomyocytes (8, 30) has been demonstrated, although the intracellular Ca2+ transient remains unchanged (24). Taken together, the available data suggest that both AC and EX alter the mechanisms associated with the EC coupling cascade, Ca2+ regulation, and contractile response, as well as metabolic responses, but each mechanism is affected differently by each stressor.
As performance in a hot environment occurs frequently, the scientific consensus is that a combined AC and EX training protocol is required to enhance exercise performance and to meet thermoregulatory demands. In humans undergoing combined AC and EX (EXAC), stroke volume increases and heart rate decreases (33, 35). Surprisingly, our knowledge of the cellular mechanisms affecting cardiac hemodynamics and metabolic performance is sparse. In isolated hearts subjected to combined AC and swimming training, the mechanical and metabolic performance improved and exceeded that induced by each stressor alone (22, 29). These studies did not provide a cellular or molecular explanation or examine whether the improvements in the EXAC group were due to a superior/enhanced response of the same pathways reacting to each individual stressor or were caused by interactions between possible stressor-specific recruited adaptive responses.
When stressors are combined, either additive, overriding, or exclusion processes could occur, whereby the mechanisms provoking the long-term adaptive responses and functional remodeling depend on transcriptional programs (9, 16). By characterizing the adapted phenotype in tandem with alterations in gene profiles in response to stressors, the interactions between competitive stressors may become clearer.
The goal of this investigation was twofold: 1) to test the hypothesis that combined AC and EX has a different effect on cardiac contractile properties than either stressor alone and 2) to investigate the mode of interaction (e.g., enhancement/interference/no interaction) between the two stressors in the EXAC cardiac phenotype. The specific effects of AC and EX on cardiac contractile responses of the EXAC phenotype were following exposure to each treatment, individually and combined, in vivo using echocardiography and ex vivo using an adult rat cardiomyocyte preparation. The transcriptional program evoked by each stressor alone and in combination, using a cDNA array was studied to discover whether reprogramming of gene expression contributes to the evolvement of the EXAC phenotype. Our findings indicate that in the EXAC cardiac phenotype: 1) heat and EX have differential effects on the EC coupling cascade in an overriding or trade-off manner, and 2) the interaction between the two stressors involves an EXAC-specific molecular program.
MATERIALS AND METHODS
All animal procedures were approved by the Ethics Committee for Animal Experimentation of The Hebrew University and complied with the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 85–23, 1996).
Animals and Treatments
Three- to four-week-old male rats (Rattus norvegicus, Sabra strain albino variation), initially weighing 80–100 g, were kept in a 12:12-h light-dark cycle, with food (Ambar Lab chow) and water ad libitum. Rats were randomly divided into four groups: control, AC, normothermic EX, and EXAC. Rats were assigned either to echocardiography and cellular-physiological experimentation (5–6 animals) or to genomic studies (6 animals). Control rats were maintained at 24°C with no further treatment. Heat-acclimated rats (AC and EXAC) were subjected to continuous exposure for 30 days to environmental heat at 34 ± 1°C with 30–40% relative humidity, which induces heat-acclimatory homeostasis as monitored by growth rate and rectal temperatures (e.g., 14). The EX and the EXAC groups were subjected for 1 mo to a progressively increasing aerobic exercise protocol on a treadmill, starting with 13.6 m/min, incline 0%, 35′ in the first week up to 20 m/min, incline 6%, 60′ in the last week (23). Body weight and colonic temperature (Tc) were measured weekly before and after an exercise session by using a digital balance (Precisa; PAG, Zurich, Switzerland) and YSI thermistor probes no. 402 (model ITS-90; Eutech Instruments, Singapore) inserted 6 cm beyond the anal sphincter, respectively, as previously detailed (23). Exercise sessions were conducted between 9:00 AM and 1:00 PM to avoid the effects of circadian rhythm at normothermic temperature. Experimental procedures on the EX and EXAC heart phenotypes were initiated 1 or 2 days after termination of the 1-mo exposure periods.
In Vivo Morphological and Physiological Measurements
Measurements were performed at the onset and at the end of EXAC training periods. As no differences were found in the preacclimation/preexercise phase among the groups, only data obtained at the end of acclimation/exercise regimens are presented. Measurements were conducted on lightly anesthetized (ketamine in 0.5% xylazine 8.5 mg/100 g body wt, injected ip) spontaneously breathing rats, with anterior chest hair shaved, and positioned in left lateral mode. A two-dimensional short-axis view of the left ventricle at the level of the papillary muscles was obtained using an echocardiograph (model Vivid 7; GE, Horten, Norway) equipped with a 13-MHz transducer for small animals (model i13L; GE, Tirat Hakarmel, Israel). M-mode tracings were recorded through the anterior and posterior left ventricular (LV) walls at a sweep speed of 100 mm/s using a commercially available off-line analysis system (EchoPAC; GE, Tirat Hakarmel, Israel) (Fig. 1). We measured heart rate, LV end-diastolic (LVDd) and LV end-systolic (LVDs) dimensions, thickness of the interventricular septum (IVST), and LV posterior wall thickness (LVPWT) at end-diastole. Also, LV fractional shortening (LVFS): [(LVDd − LVSd)/LVEDd·100], velocity of circumferential shortening (VCF): [(LVIDd − LVIDs)/LVIDd·ejection time], and LV mass (LVMASS): 0.8·{1.04·[(LVDd+LVPWT+IVST)3 − (LVDd)3]} + 0.6 g) were calculated. Doppler flow imaging was recorded from a parasternal long axis to obtain heart rate.
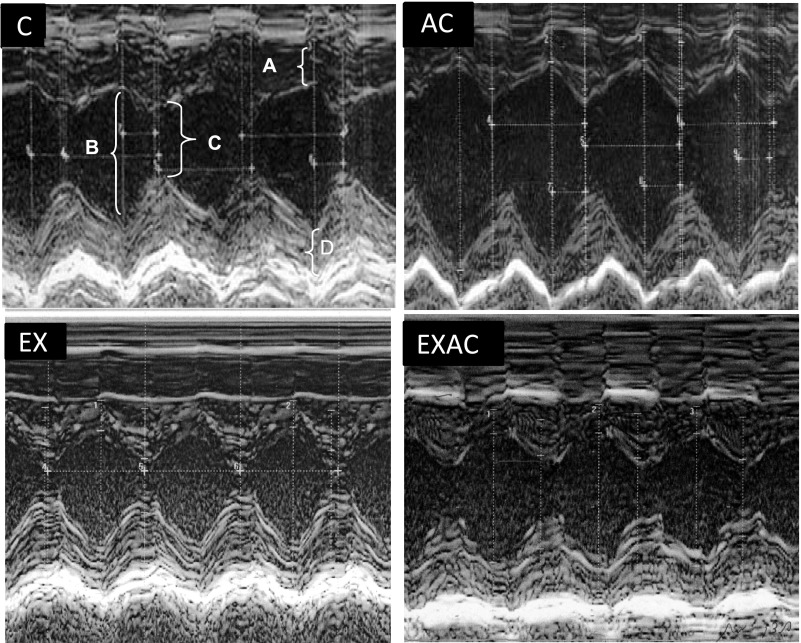
Fig. 1.
Representative echographic images of the instantaneous dimensional changes by M-mode in control (C), heat acclimated (AC), exercise training under normothermic conditions (EX), and combined AC and EX (EXAC) groups. A, IVSd-interventricular septum thickness at diastole; B, left ventricular end-diastolic diameter; C, left ventricular end-systolic diameter; D, left ventricle posterior wall thickness at diastole.
The growth rates of AC, EX, and EXAC rats are markedly slower than those of normothermic rats (14, 23), resulting in significant body weight differences between the groups. Hence, all relevant parameters were normalized to body mass.
Cellular Features of Isolated Cardiomyocytes
Cardiomyocyte isolation.
The animals were euthanized, and the hearts were rapidly removed and placed in a 4°C physiological solution. Briefly, for cardiomyocyte isolation (4, 10) the hearts were retrogradely perfused through the aorta in sequence with modified tyrode solution containing (in mM): 1 Ca2+, 120 NaCl, 15 NaHCO3, 5.4 KCl, 5 HEPES Na+ salt, 0.25 NaH2PO4, 0.5 MgCl2, pH 7.4 ( 2–3 min), Ca2+-free modified tyrode (5 min), 100 ml of modified tyrode solution containing 0.25 mM CaCl2, 17 mg collagenase type II (Worthington), and 0.8 mg protease type XIV (Sigma) (10 min). The hearts were then removed from the cannula, and the atria were cut away. The ventricles were soaked in 3 ml KB (Kraft-Brühes) solution (in mM: 70 KOH, 50 glutamic acid, 40 KCl, 20 taurine, 20 KH2PO4, 10 glucose, 10 HEPES, 0.5 EGTA, 3 MgCl2, pH 7.4 with KOH), cut into small pieces, and triturated in KB with a wide-bore plastic pipette. The resulting soup-like solution was filtered through silk, and the cells were stored in KB solution at 4–8°C. The cell preparations, containing 50 to 70% rod-shaped cells, were used within 15 h.
Cardiomyocyte contractility and Ca2+ transients.
Cell shortening [amplitude systolic motion (ASM)] and Ca2+T were measured at 37°C. The intracellular Ca2+ concentration [Ca2+]i was measured using fura-2 (4). Myocytes, perfused with KHB solution, were examined in a quartz-based chamber placed on an inverted epifluorescence microscope (Nikon Diaphot 200). The myocytes were field-stimulated (0.5 Hz, square waves) and contractions (ASM) were measured using a video motion edge detector (Crescent Electronics, Sandy, UT). For fura-2 measurements, the cells were alternately excited at 340 and 380 nm with 510-nm emission by using a fluorimetric system (Photon Technology International, Birmingham, NJ). Ca2+ levels were recorded as the ratio of 340/380 fluorescence (11). To calibrate basal information on cardiomyocyte performance, we calculated fractional shortening (% of resting cell length), Ca2+T amplitude and Ca2+ fluxes (±dCa2+T/dt and ±dCa2+T·dt−1·PA−1) when PA = Ca2+Tmax.
Electrophysiological Recordings
Action potentials (APs) and ionic currents of rat ventricular cardiomyocytes were measured during continuous superfusion, using only rod-shaped myocytes with no spontaneous contractions. All electrophysiological measurements were performed at room temperature (≈25°C) using a whole-cell configuration in current or discontinuous voltage-clamp modes (Axoclamp 2B; Axon Instruments, Foster City, CA). Pipettes were pulled with a pp-830 puller (Narishige, Japan) with a two-step procedure. Pipette resistance was 2–4 MΩ.
AP.
AP was measured using a standard whole-cell, single-electrode, current-clamp technique in a bridge mode. The voltage change over the series resistance was corrected manually by the bridge circuit. The APs were elicited by current injection (duration 2 ms, amplitude 3–5 nA, 0.2 Hz) from a holding potential of −90 mV. The superfusion solution contained (in mM): 150 NaCl, 5.4 KCl, 10 HEPES, 2 MgCl, 2 CaCl2, 20 glucose, pH 7.4. The pipette solution contained (in mM): 40 KCl, 8 NaCl, 100 d,l-K-aspartate, 5 Mg-ATP, 5 EGTA, 2 CaCl2, 10 M HEPES, 0.1 Tris-GTP, pH 7.4. Free Ca2+ concentration, calculated according to Christ et al. (3), was ∼50 nM (EQCAL computer program, Biosoft, Cambridge, UK).
Measurement of Ca2+ currents.
The Ca2+ currents in the isolated cardiomyocytes were measured in the whole-cell discontinuous voltage-clamp mode. The sampling rate of the discontinuous voltage-clamp was 7–10 kHz for all current measurements. l-type Ca2+ current (ICaL) was evoked by 50-ms voltage steps from a holding potential of −40 mV to 0 mV. The cardiomyocytes were perfused in Na+-free external solution for isolation of ICaL from Na+ currents. K+ currents were blocked by tetraethyl ammonium chloride (TEA-Cl) and by replacing K+ with Cs+. The Na+-free superfusion solution contained (in mM): 120 TEA-Cl, 10 CsCl, 10 HEPES, 2 CaCl2, 1 MgCl2 and 20 glucose, pH 7.4 (adjusted with CsOH). The pipette solution (pH 7.2) included (in mM): 90 cesium methane sulfonate, 20 CsCl, 10 HEPES, 4 Mg-ATP, 0.4 Tris-GTP, 10 EGTA, and 3 CaCl2. The calculated free Ca2+ concentration according to Christ et al. (3) was ∼60 nM (EQCAL computer program, Biosoft). The peak transient current amplitude was determined as the difference between peak inward current and the steady-state current at the end of the depolarizing step.
Data Storage and Analysis
Data were analyzed digitally by using a LabVIEW software program and sampled at 20 kHz. Capacitance was calculated by integrating the capacitive current evoked by a 10-mV voltage step at a low clamping gain and dividing it by the amplitude of the voltage step.
Global genomic responses.
Animals were anesthetized, and hearts were rapidly placed in liquid nitrogen and stored at −80°C until analysis. For gene expression, a Clontech cDNA Atlas array was used containing 1,187 genes representing a variety of homeostatic functional groups of the rat genome, spotted on a nylon membrane (Rat 1.2, no. 1467; Clontech Laboratory, Palo Alto, CA). The left ventricles from two animals were pooled, and three pools were prepared for each experimental group. TRI reagent (Molecular Research Center) was used to extract total RNA. The quantity and quality of the RNA were determined from its absorbance at 260 and 280 nm (Nano Drop, NP-1000) and by denaturing 1% agarose gel electrophoresis (16, 34). The probes were labeled for 1 h by reverse transcription of total RNA (3 μg) at 42°C in a primer mix (Clontech) containing [32P]dATP (Amersham Biosciences, Buckinghamshire, UK). The reaction was terminated by adding 0.1 M EDTA and 1 mg/ml glycogen (Sigma). The unincorporated 32P-labeled nucleotides were removed using Nucleo-Spin extraction columns (Clontech).
cDNA array hybridization.
The membranes were prehybridized for 1 h at 68°C in a hybridization solution (Clontech) containing 0.1 mg/ml sheared salmon testes DNA (Sigma) to block nonspecific binding. A radiolabeled cDNA probe (5 to 15 × 106 cpm) was applied to each membrane and hybridized overnight at 68°C. Cot-1 DNA (Clontech) (1 g/ml) was added to block nonspecific binding. The membranes were exposed for 4, 12, and 24 h to a phosphor screen and detected using a Bioimaging Analyzer BAS2000 (Fuji Photo Film). Atlas Image 2.01 software (Clontech) was used to record spot pixel density and for background subtraction before further analysis (16, 34).
Quantitative RT-PCR.
To confirm the results obtained from the array: 1) heat shock protein 70 (HSP70), the consensus stress gene, responding to both AC (16) and EX (42); 2) the inward rectifying potassium channel (Kir6.2), a K+-ATP sensitive channel that is influenced by the metabolic state of the cell; 3) a gene encoding the L-type Ca2+-channel (CACNA1D); and 4) vascular endothelial growth factor receptor 1 were detected by quantitative real-time PCR (ABI Prism 7000 Sequence Detection System; Applied Biosystems) and normalized to the level of the endogenous control β-actin (27). The primers were designed using Primer Express software (Applied Biosystems; Table 1). The results were analyzed using the change in cycle threshold method, which reflects the difference in threshold for the target gene relative to that of β-actin in each sample.
Table 1.
Real-time PCR primer sequences
| Clontech ID/Gene Bank Accession No. | Gene | Primers |
|---|---|---|
| Z27118 | HSP70 | Sense: 5′-caagaatgcgctcgagtccta-3′ |
| Antisense: 5′-ctctttctcagccagcgtgtta-3′ | ||
| M57682 | Ca2+-Channel CACNA1D | Sense: 5′-GCAAACTATGCAAGAGGCACC-3′ |
| Antisense: 5′-TGGCCTGTCTAGCAGCATCA-3′ | ||
| X83581 | Potassium channel, inward rectifier | Sense: 5′-ATCCGCTTCAGCTATTTCGC-3′ |
| Antisense: 5′-CGGTCGGAAGTCACCTATGC-3′ | ||
| D28498 | VEGFR1 | Sense: 5′-CCCTCAGCCTACCATCAAGTG-3′ |
| Antisense: 5′-CTCCCAAAGCAGAAGTCATTCC-3′ | ||
| β-Actin | Sense: 5′-tgtggcatccatgaaactac-3′ | |
| Antisense: 5′-atttgcggtgcacgatggag-3′ |
Calibration and normalization.
A linear regression performed at the individual time points (4, 12, and 24 h) was averaged with the intensity of the middle time point. Each value was rescaled using the average expression of all probes in that hybridization. Relative expression of the visible genes was presented as treatment/control Base-2 log ratio. As we have found a 1.5-fold change reliable for interpreting expression changes caused by EXAC (16, 34), this number was chosen as the value for significance.
Correlations and comparisons.
The gene expression visibility threshold was 1,200 pixels. Significant change was considered as a +0.7 or −0.7 log2 ratio to control. Biological function analyses were performed on genes with significantly altered expression (34). The genes were sorted according to the Gene Ontology (GO) database categories for biological processes. For annotation, we used Intelligent System and Bioinformatics Laboratories (http://vortex.cs.wayne.edu), DAVID (Database for Annotation Visualization and Integrated Discovery, http://david.abcc.ncifcrf.gov), and Affymetrix (http://www.NetAffx.com). Using the Spotfire DecisioSite 9.1.1 Data Analysis Package with the Functional Genomics Companion, we detected genes that were either common to all treatments or responded specifically to a certain stressor. Uniqueness of gene activation in response to a specific treatment was defined as no change in expression in the other groups [i.e., levels fell between −0.7 and 0.7 (log2)]. The data discussed in this publication have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession no. GSE8329.
Statistical analyses
For statistical analysis of the physiological experiments, we used the commercial GraphPad Prism 5 software. Treatments were taken as the independent categorical variables, and the individual rats were considered as a random sample from the population. One-way and two-way ANOVA were used to test the effects of treatment on the dependent variables. Dunnett's test was used for post hoc tests to compare each treatment group with controls. For multiple comparisons between groups, the Tukey's test was conducted. Data are expressed as means ± SE. For genomic analysis, the pooled cardiac samples prepared for each treatment group were considered as random samples from the population. We performed multiple test correction assuming hypergeometric distribution of gene expression to sort the statistically significant expressed genes (biologically meaningful). The expected proportion of false rejections among all rejections, and the Fisher exact score correction were used. A P < 0.05 was considered statistically significant.
RESULTS
Body and Heart Weights and Heart Rate
The data presented in Table 2 shows that AC rats weighed less than controls (P < 0.05). The body weights of EX rats were similar to AC rats; EXAC rats were smaller than all other groups, however, their weight was significantly smaller than control rats only (P < 0.05). These results are consistent with our earlier studies on AC rats (e.g., Ref. 14) and EX and EXAC swimmers (19, 26). The left ventricle-to-body weight ratio, an indicator of cardiac hypertrophy, increased significantly in both exercising groups (P < 0.05). Additionally, chronic heat exposure (AC and EXAC groups) caused a significant drop in basal heart rate compared with controls (AC: −8%, EXAC: −13% P < 0.05). No significant differences in basal Tc were found among the groups (average Tc: 37.3 ± 0.09), but in the EXAC group, the Tc elevation at the end of an exercise bout was 0.7°C less than matched EX rats (39.6 ± 0.16, 38.6 ± 0.1, P < 0.01) (see Ref. 26).
Table 2.
Body weights, colonic temperature, heart rate, and LV mass in the experimental groups
| Number | Control | AC | EX | EXAC | |
|---|---|---|---|---|---|
| Body weight,g | 12 | 291 ± 9.5 | 254 ± 12.4* | 255 ± 9.7* | 225 ± 7.4* |
| Heart rate, beats/min | 5 | 332 ± 8.3 | 307 ± 6.3* | 334 ± 30.5 | 292 ± 7.7* |
| LV mass, g | 5 | 1.207 ± 0.04 | 1.1 ± 0.03* | 1.028 ± 0.05* | 0.961 ± 0.06* |
| LV mass/body wt ×10−1 | 5 | 4.07 ± 0.1 | 4.1 ± 0.2 | 4.4 ± 0.2* | 4.9 ± 0.3*† |
Values are mean ± SE. AC, heat acclimated; EX, exercise training; EXAC, combined AC and EX; LV, left ventricular.
Significance vs. control,
EX vs. EXAC (P = /< 0.05).
EXAC Affect Morphometric and Physiological Parameters (Echocardiography)
EX and chronic heat had differential effects on the EXAC phenotype (Fig. 1, Table 3). Training induced cardiac hypertrophy, displayed by greater left ventricle posterior wall thickness in trained (EX, EXAC) than in control and AC hearts (P < 0.05). The EXAC rats also had increased septal thickness (EXAC vs. control and AC P < 0.05). A profound impact of chronic heat, found only in the sedentary rats, was a significant increase in internal ventricle diameter (LVID) during both diastole and systole. In contrast, systolic LVID in both exercise groups decreased and diastolic LVID remained unchanged, implying greater force generation.
Table 3.
Ventricular dimension in rats, following 1-mo exposure to exercise and heat factors
| Control | AC | EX | EXAC | |
|---|---|---|---|---|
| Anatomical parameters, mm/body wt | ||||
| IVST-diastolic | 0.005 ± 0.0003 | 0.0046 ± 0.0002 | 0.005 ± 0.00029 | 0.007 ± 0.00071*†‡ |
| IVST-systolic | 0.007 ± 0.0004 | 0.008 ± 0.0003 | 0.0085 ± 0.0004* | 0.01 ± 0.000857*† |
| LVPW-diastolic | 0.006 ± 0.0010 | 0.007 ± 0.0008 | 0.008 ± 0.001012* | 0.01 ± 0.001847* |
| LVPW-systolic | 0.008 ± 0.00075 | 0.009 ± 0.0007 | 0.011 ± 0.0003* | 0.012 ± 0.001*† |
| LVID-diastolic | 0.024 ± 0.00199 | 0.028 ± 0.0011* | 0.023 ± 0.001996 | 0.025 ± 0.001933 |
| LVISd-systolic | 0.0153 ± 0.00051 | 0.0181 ± 0.001* | 0.0137 ± 0.0008‡ | 0.0164 ± 0.001079 |
| Contractile features | ||||
| VCF, ms−1 | 5.804 ± 0.2778 | 4.622 ± 0.3127* | 6.108 ± 0.7253 | 4.648 ± 0.36§ |
| FS, % | 37.16 ± 0.994 | 35.08 ± 1.433 | 39.38 ± 4.63 | 33.77 ± 1.67§ |
Values are mean ± SE. IVST, intraventricular septal thickness; LBPW, left ventricular posterior wall thickness; LVIDd, left ventricular internal diastolic diameter; LVISd, left ventricular internal systolic diameter; VCF, velocity circumferential shortening; FS, fractional shortening.
P < 0.05 vs. control,
P < 0.05 vs. AC,
P < 0.05 vs. EX,
P < 0.05 vs. control t-test.
The VCF and the changes in left ventricular diameter between contraction and relaxation [referred to as the FS (%)], are indicators of the cardiac contractile response, a measure of LV performance. VCF was slower in both heat-treated groups (AC and EXAC) (P < 0.05), but the %FS decreased only in the EXAC group (P < 0.05). The VCF of the EX group was insignificantly faster than controls.
Cardiomyocyte Contractility
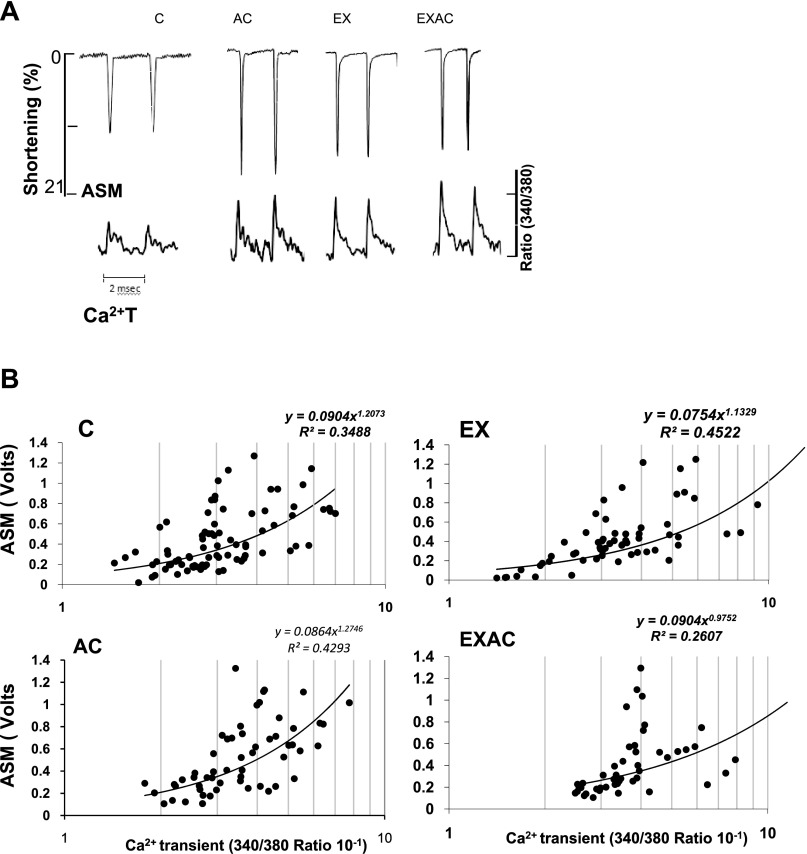
In view of the echocardiograph implications of chronic heat/exercise on cardiac contractility, we studied several contractile features in isolated cardiomyocytes. Initially, cardiomyocyte shortening to Ca2 signal (ASM-Ca2+T) relations were analyzed under basal conditions (Fig. 2, A and B). Both AC and EX cardiomyocytes demonstrated significantly greater ASM than controls (Table 4), with the AC group having the greatest percentage of shortening (P < 0.05). All experimental groups had greater Ca2+T than controls (P < 0.05). The Ca2+T derivatives (+dCa2+T·dt−1·PA−1) of the heat-treated groups were significantly smaller than in the normothermic groups (P < 0.05), implying slower calcium flux velocities (Table 4). Ca2+ responsiveness curves (Fig. 2B) show that the AC and EXAC groups had decreased Ca2+ responsiveness, i.e., more Ca2+ transients were required to induce cell shortening. In these heat-treated groups, fewer cells contracted when the 340/380 fluorescence ratio, namely Ca2+ level analog, was < 2.3 (Z test, P < 0.013).
Fig. 2.
Contractile response of cardiomyocytes from controls, AC, EX, and EXAC rats. A: individual recordings of traces of twitch peak force development [amplitude systolic motion (ASM)] and calcium transients (Ca2+T) using fura-2 in cardiomyocytes at a stimulation rate of 0.5 Hz. B: ASM-Ca2+T relations in the cardiomyocytes of the treated groups. The 2 heat-exposed groups show rightward shift of the curves.
Table 4.
Contractile kinetics of the myocytes (cardiomyocyte shortening; Ca2+ transients and positive/negative time derivatives, and ICaL duration)
| Control | AC | EX | EXAC | |
|---|---|---|---|---|
| ASM, % | 11.5 ± 1.26 | 20.6 ± 2.12* | 14.5 ± 1.34* | 12.8 ± 0.92 |
| Ca2+T, 340/380 | 0.30 ± 0.02 | 0.35 ± 0.03* | 0.41 ± 0.03* | 0.37 ± 0.01* |
| +dCa2+T·dt−1·PA−1 | 0.40 ± 0.025 | 0.272 ± 0.010* | 0.327 ± 0.037 | 0.27 ± 0.014* |
| −dCa2+T·dt−1·PA−1 | 0.24 ± 0.015 | 0.19 ± 0.009 | 0.20 ± 0.024 | 0.17 ± 0.009* |
| ICaL integral, AUC | 2.010 ± 0.170 | 1.433 ± 0.55* | 2.146 ± 0.127 | 1.541 ± 0.127* |
Values are mean ± SE. n = 5-6 rats (5-6 cells/rat). ASM, amplitude systolic motion; Ca2+T, Ca2+ transients; PA, peak Ca amplitude; ICaL, L-type calcium current; AUC, area under the curve.
Significant difference from controls.
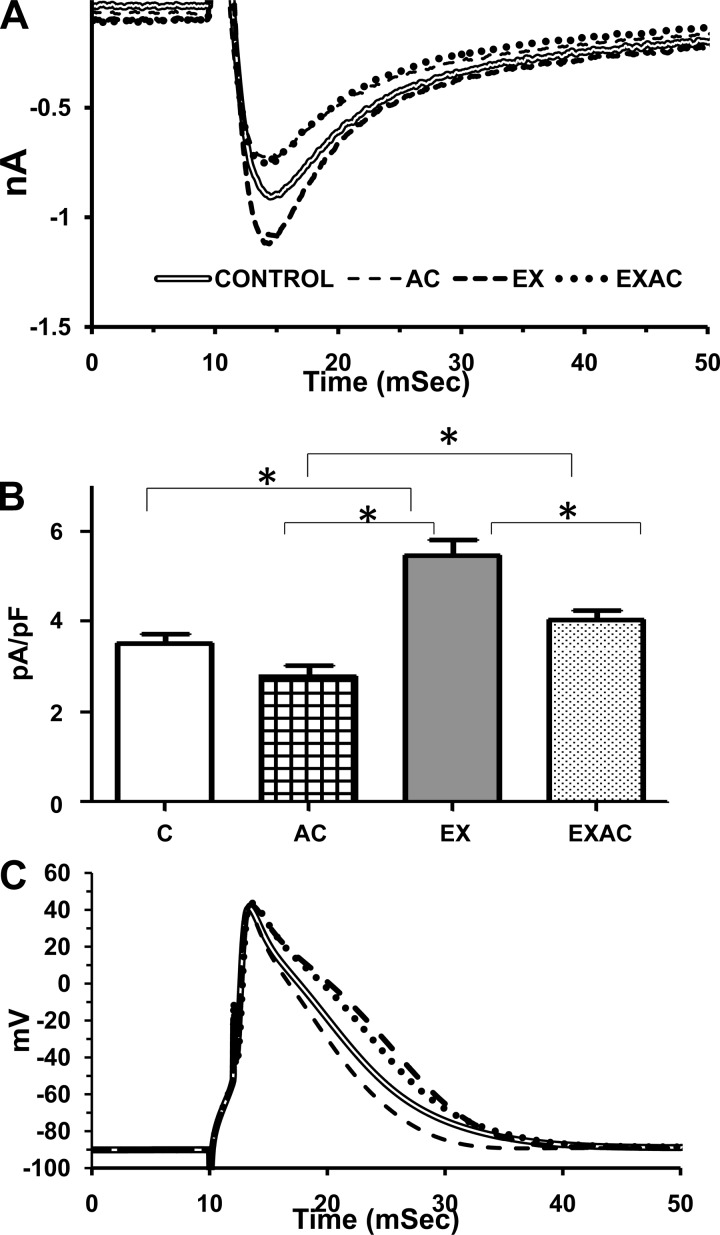
On the basis of these findings, indicating that Ca2+ fluxes are an adaptive target of AC, two additional parameters in the EC coupling cascade, L-type Ca2+ current and AP duration (APD) were studied. Fig. 3, A and B, demonstrates that both AC and training affect ICaL amplitude and density. Chronic heat decreased peak ICaL amplitude vs. the normothermic groups (by 17%, P < 0.05). In contrast, aerobic EX increased peak ICaL amplitude by 25% (P < 0.05) compared with the sedentary groups. Peak ICaL density (Fig. 3B) was also significantly lower in the AC group vs. the EX or the EXAC (P < 0.05) and significantly higher in the EX rats compared with controls or EXAC (P < 0.05). The current integral (0 mV) was significantly smaller in AC and EXAC vs. the normothermic groups (P < 0.05, Table 4). As seen in Fig. 3B, AC alone shortened APD, EX induced a reciprocal response, and EXAC did not differ significantly from control and EX. Collectively, the changes in Ca2+ currents and APD imply that Ca2+ current is affected by chronic exposure to heat and exercise, reciprocally.
Fig. 3.
A: averaged lines of L-type Ca2+ currents. B: current density amplitude. C: averaged lines of action potential of cardiomyocytes. *Significance vs. control P < 0.05, 1-way ANOVA. Action potential duration in milliseconds at 70 and 30% of the amplitude height are: 70%: control, 5.6 ± 0.5; AC, 4.6 ± 0.2;* EX, 7.4 ± 0.3;* EXAC, 6.0 ± 0.5. 30%: control, 12.5 ± 0.85; AC, 10.4 ± 0.6;* EX, 15.1 ± 0.4;* EXAC, 13.0 ± 0.7. *Significance vs. control P < 0.05, 1-way ANOVA.
Global Genomic Response
Long-term environmental adaptive responses and functional remodeling depend largely on transcriptional programs. Gene profiling revealed that collectively, the exercise groups showed the largest number of genes with altered expression (EX-478, EXAC-448), whereas the AC group showed the least (384). Most changes in expression were in upregulation. Among the visible genes, 232 genes were significantly upregulated/downregulated (by at least 1.5-fold) in all treatment groups.
Analysis I: Functional Analysis
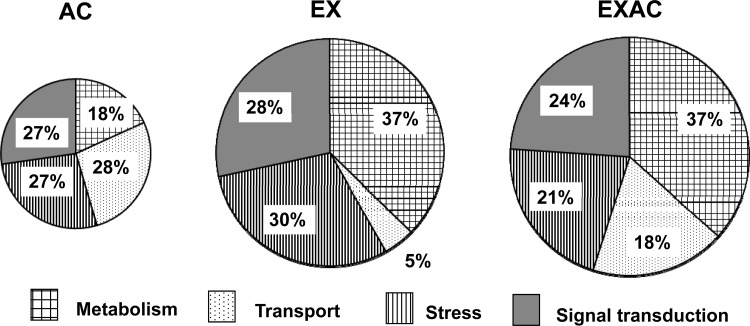
Only genes with significant upregulation/downregulation were analyzed. We focused on four GO groups for biological processes: metabolism, transport, signal transduction, and stress and demonstrated that the respective number of stressor-specific genes were 68, 62, and 25 for the EX, EXAC, and AC groups. In the EX and EXAC groups, the genes categorized under the metabolism GO functional group constitute the largest proportion (37% vs. 18% in the AC group) (Fig. 4). In contrast, in the two heat-treated groups (AC and EXAC), the number of genes associated with the transport GO category group were significantly elevated (AC 25%, EXAC 21%) compared with the EX group (4%).
Fig. 4.
Distribution (%) of genes with significant changes in their expression levels (≤/≥ 1.5-fold) by their association with specific treatment, according to functional categories. Pie chart area corresponds to the number of the significantly upregulated/downregulated genes in each group. The majority of the genes were upregulated. Enriched functional categories were sorted using Onto-Tools Suite Software provided by Intelligent Systems and Bioinformatics Laboratory.
Analysis II: Enriched Specific Pathways
Genes involved in EC coupling and Ca regulatory proteins.
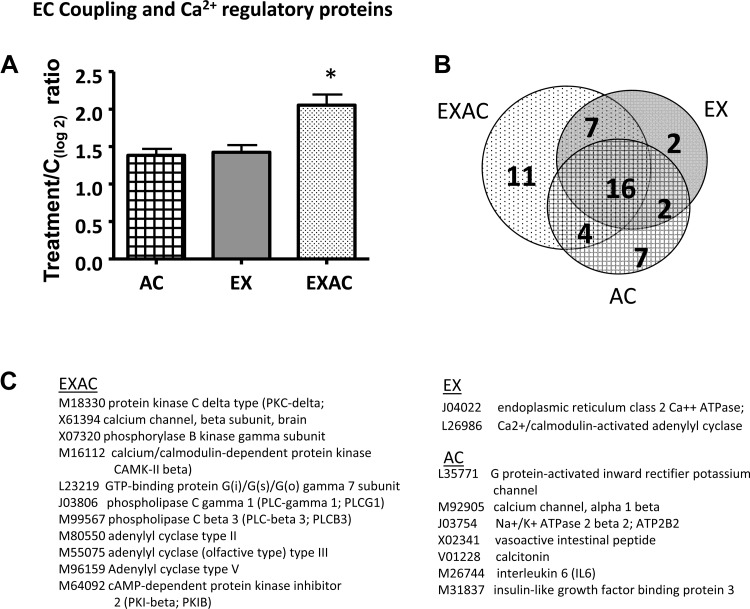
The 61 genes spotted on the array encoded excitation/contraction coupling and calcium regulatory proteins. Among those 61 genes, EXAC activated the largest number: 37 compared with 27 in the EX and 28 in the AC groups. The transcriptome profile highlights the following: 1) 16 genes, among which voltage-gated dihydropyridine-sensitive L-type Ca2+ channel, calcium-transporting sarcoplasmic reticulum-type ATPase, and inositol 1,4,5-trisphosphate, directly involved in the EC coupling cascade, were upregulated by more than twofold in all treatments groups (see Supplemental Table S1 available with the online version of this article); 2) differences in the magnitude of expression of several genes that were altered by all treatments, suggesting that for these genes, interference or additive interactions are involved in forming the EXAC phenotype (Supplemental Table S1); 3) uniqueness of gene activation in response to a specific treatment. We found that the EXAC group has 11 stressor-specific genes of which adenylyl cyclase, phospholipase control, GTP-binding protein, calcium/calmodulin-dependent protein kinase, Ca2+ channel β-subunit, and protein kinase C δ-type are associated with calcium signaling, EC coupling, and calcium regulation. AC hearts had seven stressor-specific responding genes (2 in the EC coupling cascade), whereas the EX group had only two uniquely responding genes, both associated with Ca2+ binding/regulation. The average mRNA expression was significantly different, with EXAC rats having the highest mRNA levels (P < 0.01, Fig. 5).
Fig. 5.
Stress-specific responding genes associated with EC coupling and Ca2+ regulatory protein pathways. A: average expression of the genes. Data are presented as log2 of treatment/control ratio. Each bar represents the average data of three arrays (pooled samples, n = 6). Values > 0.7 were considered significant upregulation/downregulation compared with controls. Values are means ± SE, *P < 0.001 (EXAC vs. AC or EX). B: Venn diagram marking those genes that were impacted uniquely by the stress type. C: stress-specific genes. Uniqueness of gene activation in response to a specific treatment was defined as significant change vs. no change in expression in the other groups [i.e., levels fell between −0.7 and 0.7 (log2)].
Genes involved in energy metabolism.
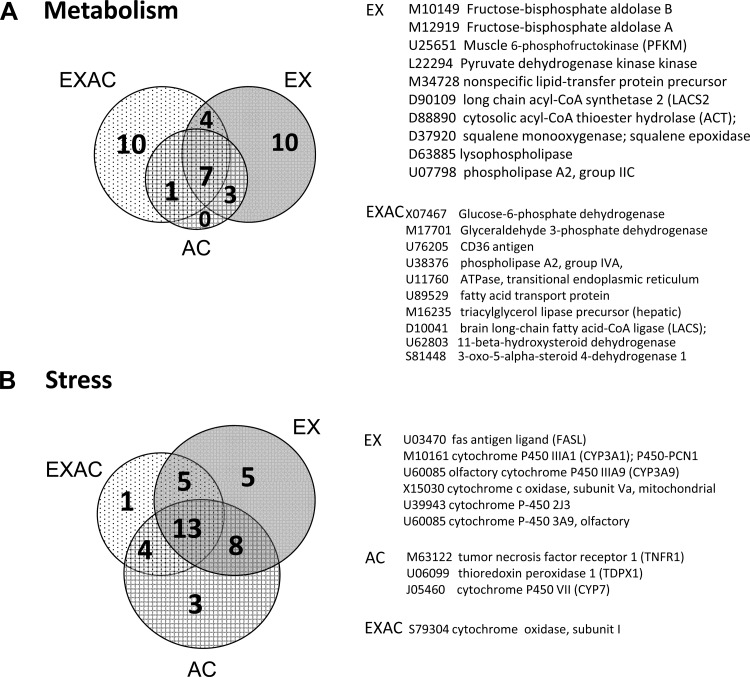
The 50 genes spotted on the array play a role in energy metabolism pathways. Both exercising groups had more upregulated genes than the sedentary AC rats (EX-23, EXAC-22 vs. AC-12); no significant differences in expression levels were found. More genes associated with lipid than carbohydrate metabolism were changed (Supplemental Table S2). The EX and EXAC groups each activated 10 unique genes (Fig. 6A). In the EX group, fructose-bisphosphate aldolase and muscle 6-phosphofructokinase (PFKM) are the rate-limiting enzymes in glycolysis, and pyruvate dehydrogenase kinase controls the glycolytic pyruvate dehydrogenase (PDH) enzyme and other acyl-CoA associated genes, such as long-chain acyl-CoA synthetase 2 (LACS2) and acyl-CoA hydrolase. In the EXAC hearts, notable changes were detected in the expression of GAPDH and glucose-6-phosphate dehydrogenase, enzymes associated with glucose metabolism and NADPH/NADP balance, respectively. Genes associated with lipid metabolism included acyl-CoA synthetase (LACS), lipid transport (fatty acid transport protein), and lipid degradation (phospholipase) protein coding genes.
Fig. 6.
Venn diagram of stress-specific responding genes associated with energy metabolism (A) and stress/cytoprotection (B). Genes that were impacted uniquely by 1 component of the stress are marked (EXAC, EX, or AC). For sorting of stress specific genes see legend to Fig. 5.
Genes related to the stress response.
Fifty-one genes were associated with the stress response, including subunits of cytochrome P450, apoptotic/antiapoptotic, oxidative/antioxidative, DNA damage/repair, and heat shock proteins. EX and AC demonstrated similar numbers of significantly upregulated stress associated genes, whereas the EXAC phenotype activated the least. Thirteen genes were common to all treatment groups. The number of treatment-specific induced genes for AC, EX, or EXAC was small: three, five, and one, respectively (Fig. 6B), and no significant difference in average gene expression was detected among the groups.
Confirmatory Analysis
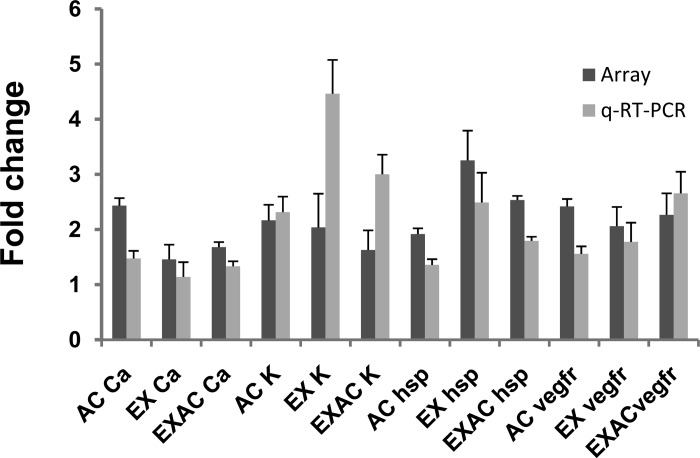
We compared the expression of four genes representing separate functional categories: cytoptotection (HSP70), Ca2+ channel (CACNA1D), potassium channel inward rectifier (KIR) and vascular endothelial growth factor receptor 1. Congruence was found between the results obtained for each individual gene using quantitative real-time PCR and their expression on the array membranes (Fig. 7).
Fig. 7.
Changes in expression level of confirmatory representative genes (in fold change): heat shock protein (HSP70), the consensus stress gene, potassium channel inward rectifier (KIR), a gene encoding the Ca2+ channel (CACNA1D), and vascular endothelial growth factor receptor 1 (VEGFR1). Each bar represents the average of three separate PCR products from three samples.
DISCUSSION
EXAC enhances exercise performance in the heat, but until now, the underlying mechanisms of this phenomenon have remained an enigma. Using an integrated analysis of echocardiography, isolated cardiomyocyte physiology, and transcriptome profiling, we have now deciphered the specific signatures of chronic heat and aerobic EX, when combined, on the contractile performance of the EXAC cardiac phenotype. We argue that the physiological outcome of the interaction between EX and AC is not simply additive but rather results from 1) an overriding and trade-off manner of interaction, and 2) can be considered a stress in its own right, leading to a specific transcriptional program.
What Can We Learn from Echocardiography?
Noninvasive echocardiography in sedated rats provided data on the interaction between chronic heat and EX from the in situ records of AC, EX, and EXAC rats under basal conditions. Although the effects of sedation on cardiovascular hemodynamics and contractility depend on the anesthetic agent used, our between-groups comparisons are valid because all of our experimental animals were sedated similarly, using the same anesthetic agent. Likewise, the morphometric and physiological parameters reported here do not differ significantly among the drugs usually used in rat echocardiography, hence data are provided with values close to those obtained from conscious rats (31, 37).
Chronic heat affects heart rate.
The finding that the heart rate of AC and EXAC rats but not of EX rats was slower than that of controls implies that exercise does not override the impact of chronic heat in the EXAC phenotype. Studies in AC rats (17) and sand rats (Psammomys obesus) (18) have shown that changes in the intrinsic properties of the pacing cells, as well as in commands from the autonomic nervous system, induce such a chronotropic effect.
Echocardiographic assessment of cardiac morphometry and physiology reveals the specific impact of chronic heat.
The adaptive left ventricular hypertrophy developed in both exercise-trained groups (EX, EXAC) indicates that the effects of training are already seen after 1 mo. Adaptive hypertrophy enhances the contractile force via increased muscle mass to match peripheral demands. Thus, our observation of exercise-induced hypertrophy is in harmony with our earlier finding that the isolated EXAC left ventricle generates greater pressure than that of EX hearts (31). In contrast to the quantitative effect of training on the morphometric features of both trained groups, the EX and EXAC cardiac phenotypes showed differential contractile features. In EX hearts, the profound decrease in internal end-systolic ventricular diameter, the inferred increased FS (%), and the VCF implies a greater force generation. In contrast, in EXAC rats, the significant decrease in VCF denotes slower contractile kinetics (see below). Notably, the significant decrease in VCF, also observed in AC hearts, suggests an overriding chronic heat effect, slowing contractile kinetics. The increased internal diastolic diameter in the AC group vs. EX facilitates increased stroke volume and cardiac output without a profound elevation in the beating rate in this phenotype, if challenged. These findings are consistent with our data from isolated AC hearts demonstrating increased ventricular compliance, reduced stiffness, and increased metabolic and mechanical efficiency (15, 26). Rowell et al. (33) demonstrated such a beneficial heat acclimatory effect in human subjects for whom greater cardiac output was achieved via increased stroke volume. Notably, lower VCF in the AC group resulted from a slow myosin isoform (V3) predominance, mediated by the sustained low plasma thyroxin levels occurring during AC (20, 28). Studies on hearts of altitude-adapted rats demonstrated a similar velocity-myosin V3 isoform relationship (2).
Physiological Features: a Lesson from Isolated Cardiomyocytes
Isolated cardiomyocytes provide a good model for studying the mechanisms associated with cardiac contractility and EC coupling. The EC coupling events associated with the Ca2+ dynamics of the EXAC group resembled those of AC myocytes and differed from the EX group: Ca2+T was significantly greater in the EXAC and AC groups than in the controls, even though the EXAC and the AC groups demonstrated markedly lower ICaL amplitude and density (ICaL/capacitance), as well as significantly lower rates of Ca2+ from/to the sarcoplasma Ca2+ pool [±Ca2+T derivatives (Table 4)]. The data from this experiment correspond with the decreased VCF measured in these groups (Table 3), implying a remodeling of the ICaL and Ca2+T generation/relaxation dynamics (see below). These findings are in sharp contrast to those from the EX group in which the increase of Ca2+T coincidentally with ICaL and Ca2+ current density amplitudes, with no changes in Ca2+T derivatives suggests that training alone enhances Ca2+T recruitment without altering its dynamics, namely, training under normothermic conditions has quantitative rather than qualitative affects, which were seen in the heat-exposed rats.
Other reports on the effect of training on ICaL are controversial. Whereas Mokelke et al. (29) studying female Sprague-Dawley rats trained for 20 wk, found no changes in ICaL, Wang et al. (39) reported unchanged ICaL but decreased ICaL density (ICaL/capacitance), apparently due to altered cardiomyocytes morphometry in rats that had undergone swimming training for 8 wk. Both reports differ from ours.
The increased Ca2+T-to-ICaL ratio and decreased ASM-to-Ca2+T ratio in AC and EXAC hearts suggest that chronic heat enhances the sensitivity of the sarcoplasmic calcium channel, the ryanodine receptor to ICaL (namely less current is required to open the SR Ca2+ pore) and decreases contractile myofilament responsiveness to Ca2+. An enhanced sensitivity of ryanodine receptors to Ca2+ and a decreased SR Ca2+ reuptake compensate for a smaller ICaL, thereby yielding a greater Ca2+T that allows coping with decreased myofibrils sensitivity to adjust to an adequate contractile response. The report that 1) ryanodine receptor blockade abolishes the differences between control and AC hearts, and 2) AC decreases myofilament responsiveness to Ca2+ (4), supports our conclusions. The correlation between decreased ICaL and shorter AP duration in the AC group and increased ICaL with AP elongation in the EX cardiomyocytes also suggests that adaptive modulation of ICaL is a central component in cardiomyocyte adaptation. The resemblance between the EXAC AP and that of EX suggests that EX overrides chronic heat in this respect.
In Vivo-Ex Vivo Integration
Qualitative changes in the EC coupling cascade, leading to decreased contractile velocity in both AC and EXAC, suggests that chronic heat overrides exercise effects on contractile kinetics. Both AC and EXAC increase metabolic efficiency (15, 26), accordingly allowing a velocity/efficiency trade-off. In contrast, the EX signature is manifested by induced hypertrophy and an elongation of APD; hence, both override chronic heat. Given that EX enhances in parallel important intersections along the EC coupling cascade, we can conclude that EX alone has a quantitative effect on the contractile phenotype.
Genomic Responses
Characterizing gene profiles in tandem with physiological alterations of the adapted phenotypes can clarify the interactions between competitive stressors. We used this tool to further decipher the differences between heat and training effects.
EC Coupling and Ca2+ Regulatory Genes
Significant changes in the expression of genes encoding membrane and cytosolic components associated with membrane potential, Ca2+ trafficking and turnover were dominant targets. The EXAC group, with the greatest pressure enhancement (19, 26), had the highest expression level of genes associated with EC coupling and Ca2+ regulatory proteins and the highest number of upregulated stressor-specific transcripts in this functional category (Fig. 5). We speculate that the unique upregulated expression of the L-type channel β-subunit isoform [an auxiliary unit controlling the pore-forming α-subunit of the channel (1)] is associated with the lower amplitude of the ICaL detected in this group. The expression of additional EXAC-specific genes that 1) are associated with enhanced Ca2+T via adrenergic receptor signaling and kinases [causing a positive inotropic response e.g., CamK II and PKC (41)] and 2) affect the delayed rectifier K+ current (e.g., phospholipase C isoforms, for gene details see Fig. 5), agrees with the physiological data obtained from the isolated cardiomyocytes experimental series.
By comparing the transcript profiles of the AC and EX groups with that of EXAC, we can characterize the impact of each individual stressor on specific pathways. First, the profile of the AC group includes both membrane and cytosolic transcripts involved in Ca2+ and K+ (inward rectifier) membrane currents, each affecting the AP and cytosolic Ca2+ turnover. Four of these transcripts are Ca2+ channel α1-isoforms (pore-forming units, see Supplemental Table S1), three of which also changed in the EXAC group but in a different manner. Additionally, the L-type Ca2+ channel α2β-subunit in AC and the β-subunits in EXAC are stressor-specific genes (Fig. 5). Collectively, these findings show, unequivocally, that chronic heat causes a sustained effect on the Ca2+ channel. This view fits with the report of Cohen et al. (4), who demonstrated increased expression of the L-type Ca2+ channel protein dihydropyridine in AC hearts coinciding (as shown here) with a decrease in ICaL amplitude, suggesting that the increased dihydropyridine transcript levels in the heat-treated groups are associated with compensatory responses that maintain the adequate ICaL required for EC coupling. Differential changes in the expression of L-type channel subunit transcripts may affect ICaL amplitude, either by spatial organization or by affecting the responsiveness to kinases (for review see Ref. 1). One limitation of the present study is that the specific role of each channel subunit cannot be studied in our model; nevertheless, our physiological data (Figs. 2 and 3) lend support to the conclusion that interactions between L-type Ca2+ channel α/β-subunits, in both EXAC and AC, decrease ICaL amplitude and that AC affects the EC coupling cascade.
In contrast, EX and EXAC had a greater impact on clusters of genes involved in Ca2+ regulation via their effect on positive inotropic pathways. Only seven genes were common to the exercising groups (EX and EXAC), all of which are related to the adrenergic pathway, to high-energy phosphorylated proteins (GTP binding proteins), and to various kinases, thereby affecting contractility via adrenergic inotropic signaling and induced hypertrophy (e.g., in Ref. 12). Thus, our data imply that EX enhances the regulation of Ca2+ turnover via pathways associated with high-energy compounds. Furthermore, many of the genes upregulated solely in the EXAC (Fig. 6) group are phospholipases that hydrolyze phospholipids into fatty acids, an important energy source for cardiac muscle.
Metabolic Pathways Genes
The AC group, in accordance with its lower metabolic status (17), expressed the fewest upregulated genes involved in lipid and glucose metabolism, with none being stressor specific. In contrast, EX and EXAC, the two groups in which genes categorized under the metabolism GO functional group constituted the largest proportion (Fig. 4), displayed, although different, the same number and magnitude of expression of stressor-specific genes, of both carbohydrate and lipid metabolic pathways (for details see Supplemental Table S2). In agreement with the faster contractile kinetics of EX hearts, PFKM was uniquely upregulated in this group. The observation that lipid metabolism genes had significantly higher expression levels than those involved in glucose metabolism (∼2.5-fold vs. ∼1.8-fold, respectively) agrees with the notion that under normal conditions, the healthy myocardium uses fatty acids as its major energy source, with little contribution from glucose (21), which favors the metabolic impact of prolonged training on both EX and EXAC hearts.
Stress-Associated Genes
As indicators of the stress level of each of the studied groups, we analyzed here the balance between the stress-associated genes. The molecular data agree with the consensus that exposure to both heat and exercise is required to perform in the heat because the EX and AC groups demonstrated similar numbers of significantly upregulated stress-associated genes, whereas the EXAC phenotype activated the least. The number of treatment-specific induced genes for AC, EX, or EXAC was small. The finding of no significant difference in average gene expression among the groups indicates a stressor-independent, universal stress response. The differences among the groups were due only to changes in the balance between constructive/destructive elements.
A large number of stress-associated genes important to the present study include those involved with redox balance. Changes in the intracellular redox environment affect many cellular processes, including the gating properties of ion channels (e.g., Ca2+) and the activity of ion transporters (13, 43). Long-term training can convert harmful redox signals into survival signals, in both heart (5, 22) and skeletal muscles (32). These findings support the hypothesis that enhanced redox buffer systems are involved in the adaptive responses of AC, EX, and EXAC hearts. This topic is beyond the scope of the current investigation, however, and interested readers should refer to Supplemental Table S3.
Collectively, the transcriptome profile highlighting the specific genes responding to each stressor supports this conclusion. The finding that most of the EXAC-specific genes are associated with Ca2+ regulation and EC coupling implies that this cascade is an adaptive target for both chronic heat and exercise training. The physiological outcome of the interaction between EX and AC is not simply additive but rather, their interaction can be considered a stress in its own right, leading to a specific transcriptional program.
Perspectives and Significance
In our young-adult rat experimental model, EC coupling kinetics in the EXAC cardiac muscle phenotype indicate that adaptation to this stressor is determined via an overriding and trade-off mode of interactions, leading to a specific transcriptional program. Molecular memory consolidation of this phenotype occurs during the course of 1 mo of exposure to the acclimating conditions and involves stress-mediated chromatin remodeling (38). We are aware that young adults might be more susceptible than other age groups to adaptive responses. Acclimation is an evolutionary conserved feature, however, and occurs in both genders and at all ages. Because age/gender variations may produce different adaptive impacts, expanding the scope of this investigation to age-dependent or gender-dependent models will provide important information regarding the required threshold for adaptive responses and will clarify whether the transcriptional program is a global phenomenon. Hence, besides pure scientific interest, further characterization of the EXAC stressor will have applicable value.
GRANTS
This investigation was supported by the Israel Science Foundation founded by the Israel Academy of Sciences and Humanities Grant 511/01-1, by the USA-Israel Binational Fund BSF Grant 2003-298 and (in part) by the Intramural Research Program of the National Institute on Aging.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K., N.N., and A.S. performed experiments; E.K., N.N., A.S., and M.H. analyzed data; E.K., N.N., A.S., B.H., R.B., D.G., and M.H. interpreted results of experiments; E.K., N.N., A.S., and M.H. prepared figures; E.K., N.N., and M.H. drafted manuscript; E.K., N.N., B.H., R.B., D.G., M.D.S., G.G., and M.H. edited and revised manuscript; E.K., N.N., A.S., B.H., R.B., D.G., M.D.S., G.G., and M.H. approved final version of manuscript; M.D.S., G.G., and M.H. conception and design of research.
Supplementary Material
REFERENCES
- 1. Benitah JP, Alvarez JL, Gomez AM. L-type Ca2+ current in ventricular cardiomyocytes. J Mol Cell Cardiol 48: 26–36, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Cazorla O, AitMou Y, Goret L, Vassort G, Dauzat M, Lacampagne A, Tanguy S, Obert P. Effects of high-altitude exercise training on contractile function of rat skinned cardiomyocyte. Cardiovasc Res 71: 652–660, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Christ T, Wettwer E, Dobrev D, Adolph E, Knaut M, Wallukat G, Ravens U. Autoantibodies against the β1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol 33: 1515–1525, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Cohen O, Kanana H, Zoizner R, Gross C, Meiri U, Stern MD, Gerstenblith G, Horowitz M. Altered Ca2+ handling and myofilaments desensitization underlie cardiomyocyte performance in normothermic and hyperthermic heat acclimated rat hearts. J Appl Physiol 103: (1) 266–275, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Das DK, Maulik N. Conversion of death signal into survival signal by redox signaling. Biochemistry (Mosc) 69: 10–17, 2004. [DOI] [PubMed] [Google Scholar]
- 6. DeMaria AN, Neumann A, Lee G, Fowler W, Mason DT. Alterations in ventricular mass and performance induced by exercise training in man evaluated by echocardiography. Circulation 57: 237–244, 1978. [DOI] [PubMed] [Google Scholar]
- 7. Diffee GM, Nagle DF. Regional differences in effects of exercise training on contractile and biochemical properties of rat cardiac myocytes. J Appl Physiol 95: 35–42, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Diffee GM, Seversen EA, Titus MM. Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. J Appl Physiol 91: 309–315, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Gracey AY, Cossins AR. Application of microarray technology in environmental and comparative physiology. Annu Rev Physiol 65: 231–259, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Griffiths EJ, Stern MD, Silverman HS. Measurement of mitochondrial calcium in single living cardiomyocytes by selective removal of cytosolic indo 1. Am J Physiol Cell Physiol 273: C37–C44, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 12. Hata JA, Williams ML, Koch WJ. Genetic manipulation of myocardial β-adrenergic receptor activation and desensitization. J Mol Cell Cardiol 37: 11–21, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Hool LC. The L-type Ca2+ channel as a potential mediator of pathology during alterations in cellular redox state. Heart Lung Circ 18: 3–10, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Horowitz M. Acclimatization of rats to moderate heat: body water distribution and adaptability of the submaxillary salivary gland. Pflügers Arch 366: 173–176, 1976. [DOI] [PubMed] [Google Scholar]
- 15. Horowitz M. Matching the heart to heat-induced circulatory load: heat-acclimatory responses. News Physiol Sci 18: 215–221, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Horowitz M, Eli-Berchoer L, Wapinski I, Friedman N, Kodesh E. Stress related genomic responses during the course of heat acclimation and its association with ischemic/reperfusion cross-tolerance. J Appl Physiol 97:(4) 1496–1507, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Horowitz M, Meiri U. Central and peripheral contributions to control of heart rate during heat acclimation. Pflügers Arch 422: 386–392, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Horowitz M, Meiri U. Mechanism of heat acclimation induced bradycardia in the sand rat. J Basic Clin Physiol Pharmacol 4: 37–46, 1993. [DOI] [PubMed] [Google Scholar]
- 19. Horowitz M, Parnes S, Hasin Y. Mechanical and metabolic performance of the rat heart: effects of combined stress of heat acclimation and swimming training. J Basic Clin Physiol Pharmacol 4: 139–156, 1993. [DOI] [PubMed] [Google Scholar]
- 20. Horowitz M, Peyser YM, Muhlrad A. Alterations in cardiac myosin isoenzymes distribution as an adaptation to chronic environmental heat stress in the rat. J Mol Cell Cardiol 18: 511–515, 1986. [DOI] [PubMed] [Google Scholar]
- 21. Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res 95: 568–578, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol 297: H144–H152, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kodesh E, Horowitz M. Soleus adaptation to combined exercise and heat acclimation: physiogenomic aspects. Med Sci Sports Exerc 42: 943–952, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Laughlin MH, Schaefer ME, Sturek M. Effect of exercise training on intracellular free Ca2+ transients in ventricular myocytes of rats. J Appl Physiol 73: 1441–1448, 1992. [DOI] [PubMed] [Google Scholar]
- 25. Levi E, Vivi A, Hasin Y, Tassini M, Navon G, Horowitz M. Heat acclimation improves cardiac mechanics and metabolic performance during ischemia and reperfusion. J Appl Physiol 75: 833–839, 1993. [DOI] [PubMed] [Google Scholar]
- 26. Levy E, Hasin Y, Navon G, Horowitz M. Chronic heat improves mechanical and metabolic response of trained rat heart on ischemia and reperfusion. Am J Physiol Heart Circ Physiol 272: H2085–H2094, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1α-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 23: 79–88, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Mirit E, Palmon A, Hasin Y, Horowitz M. Heat acclimation induces changes in cardiac mechanical performance: the role of thyroid hormone. Am J Physiol Regul Integr Comp Physiol 276: R550–R558, 1999. [DOI] [PubMed] [Google Scholar]
- 29. Mokelke EA, Palmer BM, Cheung JY, Moore RL. Endurance training does not affect intrinsic calcium current characteristics in rat myocardium. Am J Physiol Heart Circ Physiol 273: H1193–H1197, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Moore RL, Musch TI, Yelamarty RV, Scaduto RC, Jr, Semanchick AM, Elensky M, Cheung JY. Chronic exercise alters contractility and morphology of isolated rat cardiac myocytes. Am J Physiol Cell Physiol 264: C1180–C1189, 1993. [DOI] [PubMed] [Google Scholar]
- 31. Plante E, Lachance D, Roussel E, Drolet MC, Arsenault M, Couet J. Impact of anesthesia on echocardiographic evaluation of systolic and diastolic function in rats. J Am Soc Echocardiogr 19: 1520–1525, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589: 2129–2138, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rowell LB, Kraning KK, 2nd, Kennedy JW, Evans TO. Central circulatory responses to work in dry heat before and after acclimatization. J Appl Physiol 22: 509–518, 1967. [DOI] [PubMed] [Google Scholar]
- 34. Schwimmer H, Eli-Berchoer L, Horowitz M. Acclimatory-phase specificity of gene expression during the course of heat acclimation and superimposed hypohydration in the rat hypothalamus. J Appl Physiol 100: 1992–2003, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Senay LC, Kok R. Body fluid responses of heat-tolerant and intolerant men to work in a hot wet environment. J Appl Physiol 40: 55–59, 1976. [DOI] [PubMed] [Google Scholar]
- 36. Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 62: 621–634, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Stein AB, Tiwari S, Thomas P, Hunt G, Levent C, Stoddard MF, Tang XL, Bolli R, Dawn B. Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res Cardiol 102: 28–41, 2007. [DOI] [PubMed] [Google Scholar]
- 38. Tetievsky A, Horowitz M. Posttranslational modifications in histones underlie heat acclimation-mediated cytoprotective memory. J Appl Physiol 109: 1552–1561, 2010. [DOI] [PubMed] [Google Scholar]
- 39. Wang S, Ma JZ, Zhu SS, Xu DJ, Zou JG, Cao KJ. Swimming training can affect intrinsic calcium current characteristics in rat myocardium. Eur J Appl Physiol 104: 549–555, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol Heart Circ Physiol 276: H2168–H2178, 1999. [DOI] [PubMed] [Google Scholar]
- 42. Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol 103: 1196–1204, 2007. [DOI] [PubMed] [Google Scholar]
- 43. Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.