Abstract
The incidence of obesity is now at epidemic proportions and has resulted in the emergence of nonalcoholic fatty liver disease (NAFLD) as a common metabolic disorder that can lead to liver injury and cirrhosis. Excess sucrose and long-chain saturated fatty acids in the diet may play a role in the development and progression of NAFLD. One factor linking sucrose and saturated fatty acids to liver damage is dysfunction of the endoplasmic reticulum (ER). Although there is currently no proven, effective therapy for NAFLD, the amino sulfonic acid taurine is protective against various metabolic disturbances, including alcohol-induced liver damage. The present study was undertaken to evaluate the therapeutic potential of taurine to serve as a preventative treatment for diet-induced NAFLD. We report that taurine significantly mitigated palmitate-mediated caspase-3 activity, cell death, ER stress, and oxidative stress in H4IIE liver cells and primary hepatocytes. In rats fed a high-sucrose diet, dietary taurine supplementation significantly reduced hepatic lipid accumulation, liver injury, inflammation, plasma triglycerides, and insulin levels. The high-sucrose diet resulted in an induction of multiple components of the unfolded protein response in the liver consistent with ER stress, which was ameliorated by taurine supplementation. Treatment of mice with the ER stress-inducing agent tunicamycin resulted in liver injury, unfolded protein response induction, and hepatic lipid accumulation that was significantly ameliorated by dietary supplementation with taurine. Our results indicate that dietary supplementation with taurine offers significant potential as a preventative treatment for NAFLD.
Keywords: oxidative stress, endoplasmic reticulum stress, inflammation, lipids, sucrose
nonalcoholic fatty liver disease (NAFLD) is a burgeoning metabolic disorder characterized by fatty infiltration of the liver (steatosis) with progression, in some people, to nonalcoholic steatohepatitis (NASH) and liver failure (35, 39). Presently, NAFLD is estimated to affect upwards of 24% of the general population, as well as 3–9% of all children in the United States (48, 78). Of great concern are statistics indicating that, within 10 years of diagnosis, nearly 20% of the patients presenting with NASH progress to cirrhosis (1, 8, 51). Recent data also suggest that NAFLD is independently associated with the development of cardiovascular disease and overall-obesity-related mortality (41, 59–61). Thus, in light of these data, there is a critical need to elucidate the mechanisms that mediate the development and progression of NAFLD and to identify potential therapies for the disease.
Although the pathogenesis of NAFLD remains uncertain, it has been suggested that various factors, including oxidative stress, proinflammatory cytokines, gut-derived bacterial endotoxin, and disruptions in lipid metabolism may contribute to the progression of the disease (20). Accumulation of lipids in nonadipose tissues can lead to cell dysfunction, such as insulin resistance and cell death, a phenomenon known as lipotoxicity (65). Experimental and clinical data indicate that excess fatty acids, in particular, long-chain saturated fatty acids, are an important determinant of liver cell integrity, liver function, and potentially, an independent risk factor for the progression from NAFLD to NASH (24, 64, 67).
An accumulating body of literature suggests that an important factor linking excess fatty acids to liver damage is dysfunction of the endoplasmic reticulum (ER). One of the largest subcellular organelles, the ER plays an important role in the correct assembly of proteins destined for intracellular organelles and the cell surface (47). We and others have demonstrated that saturated fatty acids induce markers of ER stress, an indicator of disrupted ER homeostasis, in liver cells (13, 30). We have also demonstrated that diets that produce hepatic steatosis characterized by increased saturated fatty acids (e.g., high-saturated fat or high-sucrose diets) lead to ER stress and liver injury (67). Furthermore, multiple markers of ER stress have been observed in livers taken from genetic models of obesity (43) and in livers from obese patients with NAFLD (10, 52). There is no proven, effective therapy for NAFLD; thus, lifestyle modifications, similar to those recommended for obesity, remain the primary treatment option (5). However, this option remains fraught with problems of compliance, and there is a pressing need for therapeutic strategies that might prevent NAFLD in the continuing presence of high-fat and/or high-sugar diets.
Taurine, 2-aminoethanesulfonic acid, is a major intracellular amino sulfonic acid with diverse physiological functions. Taurine supplementation may be beneficial in preventing various metabolic disorders, including obesity, insulin resistance, and atherosclerosis (15, 50, 77). Increasing evidence also suggests that taurine may have beneficial effects on NAFLD. For example, plasma taurine levels are decreased in various forms of liver cirrhosis (73, 76). Also, taurine supplementation prevents alcoholic fatty liver disease in experimental animals (7) and the development of hepatic steatosis in hamsters provided a high-fat/cholesterol diet (6). Moreover, mice characterized by hetero- and homozygous knockout of the taurine transporter develop chronic liver disease characterized by fibrosis, inflammation, and hepatocyte apoptosis (69). Interestingly, the beneficial effects of taurine are often accompanied by reductions in ER stress, suggesting a link between the therapeutic properties of taurine and restoration of ER homeostasis (36, 40, 80).
The present study was undertaken to examine the effects of taurine supplementation on cell and animal models of NAFLD with a view to assessing its potential as a preventative treatment. Our findings demonstrate that taurine mitigates palmitate-induced ER stress and cell death in primary hepatocytes and H4IIE liver cells. Taurine also significantly reduced lipid accumulation and liver damage in rats fed a high-sucrose diet and in mice injected with the ER stress-inducing compound tunicamycin. Collectively, our results indicate that part of the protective effects of taurine in diet-induced NAFLD are related to the amelioration of ER stress and that dietary supplementation with this compound offers significant potential as a preventative treatment for this disease.
EXPERIMENTAL PROCEDURES
Experimental agents.
Fatty acids (Sigma, St. Louis, MO) were complexed to bovine serum albumin at a 2:1 molar ratio (72). Taurine and tunicamycin were purchased from Sigma.
Cell culture.
The rat hepatoma liver cell line H4IIE (American Type Culture Collection, Manassas, VA) was cultured in DMEM containing 8 mM glucose and supplemented with 10% fetal bovine serum, penicillin, and streptomycin sulfate. Experiments were performed at 80–100% cell confluence. In preparation for primary cell culture, hepatocytes were isolated from male, Wistar rats (Charles River Laboratories, Wilmington, MA) by collagenase perfusion (2, 68). All procedures involving rats were reviewed and approved by the Colorado State University Institutional Animal Care Committee. Cells were first incubated in Roswell Park Memorial Institute media (RPMI; HyClone, Logan, UT) containing 11 mM glucose, 10−7 M dexamethasone, and 10−7 M insulin on Matrigel-coated plates (for RNA) or on collagen-coated plates containing 5% fetal bovine serum (for protein) for 4 h (attachment period). The medium was then changed to one containing RPMI, 8 mM glucose, 10−7 M dexamethasone, and 10−8 M insulin. The following morning, experimental treatments were performed using RPMI that contained 8 mM glucose and 10−7 M dexamethasone (control low-glucose medium). Each independent experiment was performed in triplicate, and taurine, when present, was 1% wt/vol.
Animals.
Male Wistar Crl(WI)BR rats (Charles River, Wilmington, MA) weighing ∼180 g (7–8 wk of age) on arrival were provided free access to a purified high-starch diet (68% of energy from corn starch, 12% from corn oil, and 20% from casein; Research Diets, New Brunswick, NJ) (46) and water for 1 wk. Rats were housed individually in a temperature- and humidity-controlled environment with a 12:12-h light-dark cycle. All procedures were reviewed and approved by the Colorado State University institutional animal care committee. After the 1-wk acclimation period, rats were fed either the high-starch diet (STD) or a high-sucrose diet (HSD; 68% of energy from sucrose, 12% from corn oil, 20% from casein; Research Diets) (46) for 4 wk. Dietary groups were randomly assigned to either normal drinking water (n = 6 per diet) or to drinking water that contained taurine (2% wt/vol; n = 6 per diet). Water and food intake were measured every other day, and body weight was measured weekly. To evaluate the effects of both diet and taurine on ER stress in hepatocytes, freshly isolated hepatocytes were taken from a separate group of rats (n = 4 per diet per treatment).
Male, C57BL/6J mice (7–11 wks) were housed in colony cages and maintained on a 12:12-h light-dark cycle. Mice were randomly assigned to one of four treatment groups (n = 6 for each group): 1) control, intraperitoneally injected with PBS; 2) taurine, mice were provided taurine (2% wt/vol added to the drinking water) for 1 wk prior to sham injection with PBS; 3) tunicamycin, intraperitoneally injected with tunicamycin; and 4) tunicamycin + taurine, mice were provided taurine (2% wt/vol added to the drinking water) for 1 wk prior to intraperitoneal injection with tunicamycin. Previous work has shown that the dose of tunicamycin used (0.5 mg/kg body wt) results in lassitude, lack of grooming, weight loss, liver injury, and hepatic steatosis that peaks between days 4 and 5 postinjection (79). In the taurine and tunicamycin + taurine groups, taurine treatment was maintained until death at 4 days after vehicle/tunicamycin injection. Four days postinjection, all mice were anaesthetized (isoflourane/oxygen) and killed by decapitation, and blood samples were collected for plasma analysis. Livers were removed and weighed, and samples were then either immersion fixed overnight in 4% paraformaldehyde in PBS (pH 7.3) for subsequent histological analysis or were snap frozen in liquid nitrogen. The animal care and procedures were approved by the Animal Care and Use Committee of the University of Colorado at Denver Health Sciences Center and the Guide for the Care and use of Laboratory Animals prepared by the National Academy of Sciences.
RNA isolation and analysis.
Total RNA was extracted with Trizol reagent using the manufacturer's protocol (Invitrogen, Carlsbad, CA). For analysis of XBP1 splicing, a two-step protocol was used for reverse transcription polymerase chain reaction (PCR) using Superscript II reverse transcriptase and Taq polymerase (68). For real-time PCR, reverse transcription was performed using 0.5 μg of DNase-treated RNA, Superscript II RnaseH−, and random hexamers. PCR reactions were performed in 96-well plates using transcribed cDNA and IQ-SYBR green master mix (Bio-Rad, Hercules, CA). Primer sets can be found in a previous publication (72). PCR efficiency was between 90 and 105% for all primer and probe sets and linear over five orders of magnitude. The specificity of products generated for each set of primers was examined for each fragment using a melting curve and gel electrophoresis. Reactions were run in triplicate, and data was calculated as the change in cycle threshold (ΔCT) for the target gene relative to the ΔCT for β2-microglobulin and cyclophilin (control genes), according to the procedures of Muller et al. (38). Results were similar regardless of the control gene; therefore data in results are reported using β2-microglobulin.
Immunoblot analysis.
Cells and liver tissue were processed as described previously (67). Equivalent amounts of protein (50 μg) were subjected to SDS-PAGE, transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Piscataway, NJ), and the membranes were incubated with antibodies against glucose regulated protein 78 (GRP78; Stressgen, Ann Arbor, MI), phospho-eukaryotic initiation factor 2-α (p-eIF2α; Cell Signaling, Waverly, MA), eIF2α (Cell Signaling), TNF-α (Cell Signaling), phospho-IKKα/β (IKKβ; Cell Signaling), C/EBP homologous protein (CHOP; Santa Cruz Biotechnology, Santa Cruz, CA), activating transcription factor-4 (ATF4; Santa Cruz Biotechnology), protein kinase-like ER kinase (PERK; Santa Cruz Biotechnology), phospho-PERK (Santa Cruz Biotechnology), and/or β-actin (Sigma). Proteins were detected using horseradish peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence reagent (Pierce, Rockford, IL). Density was quantified using a UVP Bioimaging system (Upland, CA).
Determination of caspase activity and cell death.
Activity of the caspase-3 class of cysteine proteases was determined with the Colorimetric Caspase-3 Activation Assay, which uses a caspase-specific peptide that is conjugated to the color reporter molecule p-nitroanaline (R&D Systems, Minneapolis, MN). Caspase activity was normalized to cell lysate protein concentration. Cell death was examined using the Cell Death Detection ELISA kit (Roche Diagnostics, Penzberg, Germany). The assay is based on the quantitative sandwich-enzyme immunoassay-principle using mouse monoclonal antibodies directed against DNA and histones. This allows specific determination of mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates.
2,7-Dichlorofluorescein fluorescence and protein carbonyl formation.
Oxidative stress was estimated using 2,7-dichlorofluorescein di-acetate (DCFH-DA) (14). This assay is based on the ability of DCFH-DA to diffuse across the cell membrane and, following enzymatic hydrolysis to DCFH, oxidized to the fluorescent compound 2′,7′-dichlorodihydrofluorescein (DCF). Following treatments, cells were loaded with 5 μM DCFH-DA (Molecular Probes), using serum-free media, for 45 min at 37°C (17). Fluorescence was monitored with excitation and emission wavelengths of 490 and 535 nm, respectively. Data are reported as the fold increase in median fluorescence over control cells. Protein carbonyls were measured using an ELISA-based kit (Biocell, New Zealand). Briefly, protein samples were reacted with dinitrophenylhydrazine at room temperature for 45 min, after which time samples were rinsed, and a blocking solution was added for 1 h. Antidinitrophenylhydrazine antibody and, subsequently, a secondary antibody, were both added for 1 h. Finally, substrate buffer was added for 10 min, and absorbance was measured at 405 nm.
Histology.
Paraffin-embedded sections were stained with either hematoxylin and eosin or Masson trichrome. Histological examination was performed in a blinded fashion using the procedures described by Brunt et al. (4) as modified by Kleiner et al. (25). Images were captured on an Olympus BX51 microscope equipped with a four-megapixel Macrofire digital camera (Optronics; Goleta, CA) using the PictureFrame Application 2.3 (Optronics).
Immunohistochemistry.
Paraffin-embedded liver samples were cut as 4-μm-thick serial sections. The unfolded protein response (UPR) was analyzed using an anti-KDEL (a tetrapeptide located at the COOH-terminal sequence of luminal proteins) monoclonal antibody (SPA-827), which recognizes both glucose-regulated protein (GRP)78/BiP and GRP94 (StressGen Biotechnologies Ann Arbor, MI). The anti-CHOP polyclonal Ab (sc-575) was purchased from Santa Cruz Biotechnology. Anti-KDEL was diluted 1:50 and used without antigen retrieval. Rabbit anti-CHOP polyclonal antibody was diluted 1:40 and applied after heat retrieval in Retrieve-all 2 (Signet Laboratories) at 95°C for 30 min and a 10-min incubation in 0.1% Triton-X. Sections were deparaffinized, and the endogenous peroxidase activity was blocked with 0.5% H2O2 in methanol for 10 min. After blocking with 5% normal goat serum, sections were incubated with primary antibody for 1 h at room temperature followed by goat anti-rabbit or rabbit anti-mouse biotinylated secondary antibodies (Vector Laboratories); diluted 1:500 in 0.05 mol/l Tris buffer, pH 7.5, for 30 min, and streptavidin-peroxidase (Zymed Laboratories); and diluted 1:20, for 5 min. Sections were developed in Nova Red peroxidase substrate (Vector Laboratories) and counterstained with hematoxylin. Nonspecific immunostaining was not detected in sections stained with nonimmune IgG as the primary antibody or with the secondary antibody alone.
Hepatic and hepatocyte lipids.
Liver lipid was extracted using the procedure of Bligh and Dyer (3). Triglyceride concentration was determined using a kit (Sigma).
Plasma measures.
Glucose was measured with an automated analyzer (Beckman Instruments, Fullerton, CA). Insulin and C-reactive protein were analyzed by ELISA (Linco Research, St. Charles, MO and Helica, Fullerton, CA, respectively). Plasma TNF-α levels were determined by ELISA (R&D systems). To assess the level of systemic oxidative stress, plasma levels of reactive aldehydes formed by lipid peroxidation were determined using a thiobarbituric acid reactive substances (TBARS) assay. TBARS determinations were carried out with an OXI-TEK TBARS Assay Kit (Alexis Biochemicals, San Diego, CA), according to the manufacturer's standard protocol with modifications as described previously (26). Results were expressed as nanomoles malondialdehyde (MDA) equivalents per milliliter for plasma samples. Plasma triglycerides were determined enzymatically (Sigma) and free fatty acid levels were determined using the WAKO NEFA-C kit (Richmond, VA). Aspartate aminotransferase and alanine aminotransferase were analyzed using kits (ThermoDMA, Arlington, TX).
Data analysis and statistics.
Statistical comparisons were calculated using ANOVA and post hoc comparisons among means using the Scheffé or Tukey tests. Statistical significance was set at P < 0.05. All data are reported as the means ± SD.
RESULTS
Effects of taurine on palmitate-mediated cell death in liver cells.
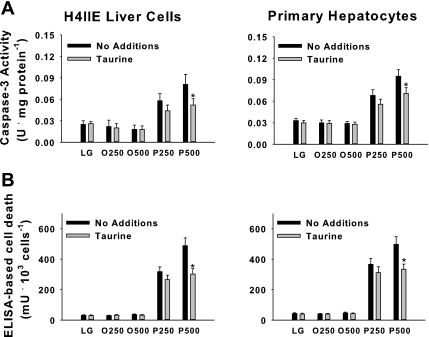
We have previously reported that saturated fatty acids, but not unsaturated fatty acids, cause liver cell death (71, 72). Palmitate-mediated cell death appears to be caspase dependent and is consistently present after 16-h but not 6-h incubations (71, 72). In the present study, we first examined the effects of taurine on palmitate-mediated caspase-3 activity and liver cell death. The unsaturated fatty acid oleate was used as a negative control. Taurine reduced (P < 0.05) but did not normalize triglyceride accumulation in response to both palmitate and oleate (data not shown). Taurine significantly reduced palmitate-mediated induction of caspase-3 activity and cell death in H4IIE liver cells and primary hepatocytes following 16-h incubations at palmitate concentrations of 500 μM, but not at 250 μM (Fig. 1). Taurine did not significantly affect the cellular utilization of glucose or free fatty acids based on the net loss of these nutrients from the medium (data not shown).
Fig. 1.
Fatty acid-mediated cell death in H4IIE liver cells and primary hepatocytes. Caspase-3 activity (A); ELISA-based cell death in H4IIE liver cells (B, left) and primary hepatocytes (B, right). Cells were incubated for 16 h in control media [low-glucose medium (LG)] or LG supplemented with albumin-bound oleate at 250 μM (O250) or 500 μM (O500), palmitate at 250 μM (P250), or 500 μM (P500) in the absence (no additions) or presence of taurine (1% wt/vol). Values are means ± SD for n = 6–8 independent experiments. *Significantly different from same condition without taurine.
Effects of taurine on palmitate-mediated ER stress in liver cells.
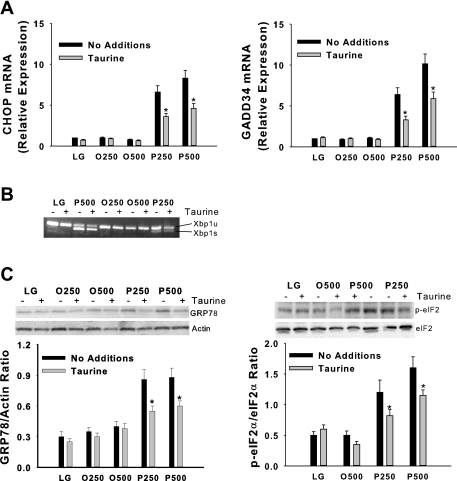
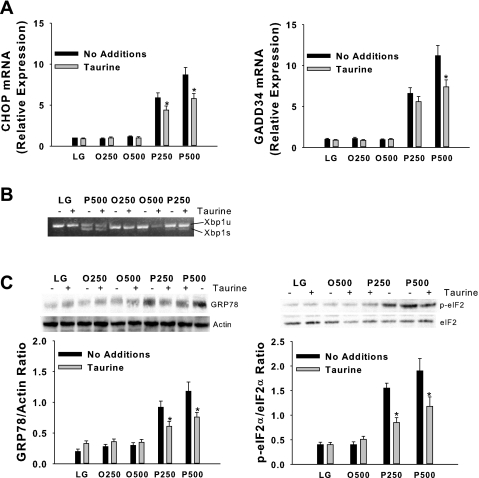
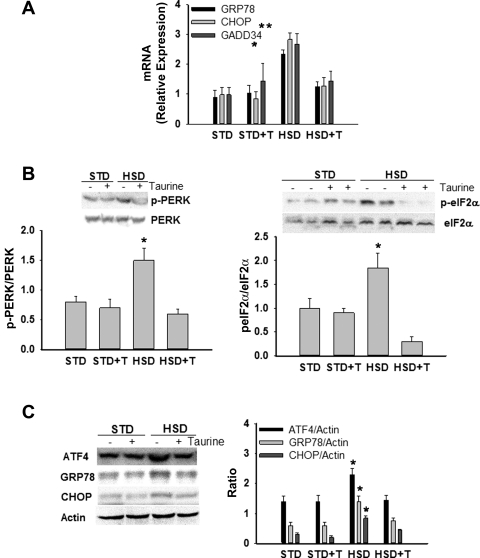
Disruption of ER homeostasis, collectively termed ER stress, activates the UPR (23). The UPR is initiated by three ER transmembrane proteins, inositol requiring ER-to-nucleus signaling protein-1α (IRE1α), RNA-dependent protein kinase-like ER eukaryotic initiation factor-2α kinase (PERK), and activating transcription factor-6 (ATF6) (53). Activation of IRE1α promotes the splicing of X-box-binding protein-1 (Xbp1) mRNA and subsequent transcription of molecular chaperones (e.g., GRP78) and genes involved in ER-associated degradation (57). PERK activation leads to phosphorylation of the α-subunit of the translation initiation factor eIF2 and subsequent attenuation of translation initiation, as well as increased expression of GRP78, CHOP, a proapoptotic gene, and growth arrest and DNA damage-inducible protein 34 (GADD34) (22, 57). GADD34 mediates dephosphorylation of eIF2α and therefore reversal of translational attenuation (53). Activation of ATF6 can also lead to increased expression of both molecular chaperones and CHOP. Taurine reduced, but did not prevent, the palmitate-mediated increase in CHOP and GADD34 mRNA, GRP78 protein, and phosphorylation of eIF2α in H4IIE liver cells (Fig. 2) and primary hepatocytes (Fig. 3). Taurine had little or no effect on palmitate-mediated Xbp1 splicing in either cell type (Figs. 2 and 3).
Fig. 2.
Fatty acid-mediated endoplasmic reticulum (ER) stress in H4IIE liver cells. C/EBP homologous protein (CHOP) and growth arrest and DNA damage-inducible protein 34 (GADD34) mRNA (A); unspliced (u) and spliced (s) X-box-binding protein-1 (Xbp1) mRNA (B); and GRP78, phosphorylated eIF2α (p-eIF2α), total eIF2α, and actin protein (C). Incubations were exactly as described in Fig. 1 with the exception that the duration of the incubations was 6 h. In graphs, values are means ± SD for n = 6–8 independent experiments, and gels are representative of n = 6–8. Note the different order of treatments between blot and graph for eIF2α. *Significantly different from same condition without taurine (1% wt/vol).
Fig. 3.
Fatty acid-mediated ER stress in primary hepatocytes. CHOP and GADD34 mRNA (A); unspliced (u) and spliced (s) Xbp1 mRNA (B); and GRP78, p-eIF2α, total eIF2α, and actin protein (C). Incubations were exactly as described in Fig. 1 with the exception that the duration of the incubations was 6 h. Values in graphs are means ± SD for n = 6 independent experiments, and gels are representative of n = 6. Note the different order of treatments between blot and graph for GRP78 and eIF2α. *Significantly different from same condition without taurine (1% wt/vol).
Effects of taurine on palmitate-mediated oxidative stress.
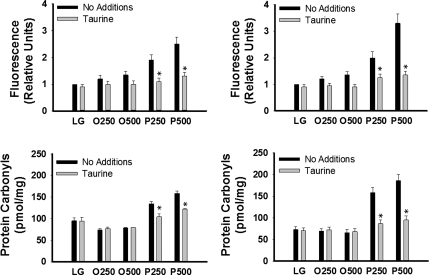
Reduced oxidative stress has been identified as a potential mechanism by which taurine exerts its protective effects. The antioxidant properties of taurine include the ability to scavenge reactive oxygen species and reduce lipid peroxidation (11, 49). Thus, we next examined the effects of taurine on palmitate-mediated oxidative stress. Palmitate, but not oleate, increased (P < 0.05) DCF fluorescence in both H4IIE liver cells and primary hepatocytes, and these effects were prevented by coincubation with taurine (Fig. 4A). Time course studies revealed that the palmitate-mediated increase in DCF fluorescence was observed after 2 h and was maximal after 6 h (data not shown). Dose-response studies demonstrated that 0.25 and 0.5% taurine did not effectively reduce DCF fluorescence (data not shown). Additionally, palmitate, but not oleate, increased protein carbonyls, and the presence of taurine significantly reduced this effect in both cell types (Fig. 4B).
Fig. 4.
Fatty acid-mediated oxidative stress in H4IIE liver cells and primary hepatocytes. 2,7-Dichlorofluorescein (DCF) fluorescence in H4IIE liver cells (Top, left) and primary hepatocytes (Top, right); protein COOH formation in H4IIE liver cells (Bottom, left) and primary hepatocytes (Bottom, right). Incubations were exactly as described in Fig. 1 with the exception that the duration of the incubations was 6 h. Values are means ± SD for n = 3–6 independent experiments. *Significantly different from same condition without taurine (1% wt/vol).
Effects of taurine on high-sucrose diet-induced hepatic steatosis, ER stress, inflammation, and liver injury.
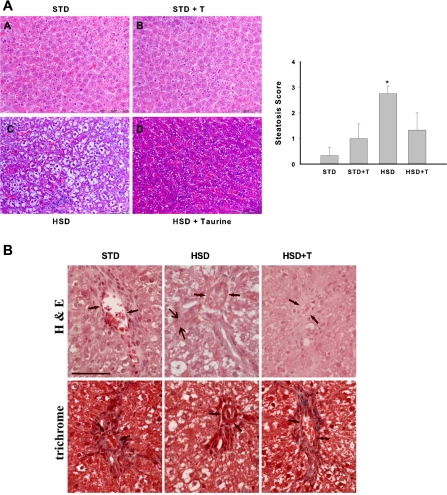
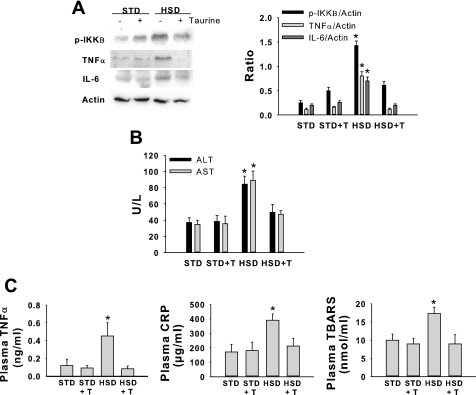
To date, the role of dietary fat and blood lipids in obesity and NAFLD/NASH have probably attracted the majority of attention. However, previous work in our laboratory has shown that a high-sucrose diet induces hepatic steatosis characterized by increased saturated fatty acids, ER stress, liver injury, and insulin resistance in rats (45, 63, 67). To further investigate the therapeutic potential of taurine, we examined the effects of taurine supplementation in a rat model of high-sucrose, diet-induced NAFLD. The high-sucrose diet significantly increased plasma insulin, plasma triglycerides, and liver triglycerides (Table 1, Fig. 5A). Histological analysis also demonstrated that lipid was present in a larger percentage of hepatocytes in sucrose-fed rats compared with all other groups (Fig. 5A). Taurine supplementation effectively reduced sucrose-mediated increases in insulin, triglycerides, and hepatic steatosis (Table 1 and Fig. 5A). Hyalinization of the portal vein wall was also present in sucrose-fed rats but not in the other groups (Fig. 5B). None of the dietary groups were characterized by the presence of ballooned cells, acidophil cells, pigmented macrophages, or glycogenated nuclei. Sucrose-fed rats were also characterized by hepatic ER stress (Fig. 6), increases in or activation of inflammatory pathway proteins (Fig. 7A), liver injury (Fig. 7B), increases in markers of systemic inflammation (Fig. 7C), and systemic oxidative stress (Fig. 7C), all of which were reduced by taurine supplementation. To examine whether hepatocytes were also characterized by changes in ER stress markers we examined freshly isolated hepatocytes. Hepatocytes isolated from sucrose-fed rats were characterized by an increase in ER stress markers, all of which were reduced by taurine supplementation (Table 2).
Table 1.
General and metabolic characteristics
| Variable | STD | STD+T | HSD | HSD+T |
|---|---|---|---|---|
| Daily food intake, kcal | 93.5 ± 4.4 | 99.9 ± 10.4 | 100.0 ± 10.0 | 105.1 ± 5.7 |
| Daily taurine intake, g1 | 0.8 ± 0.1 | 0.9 ± 0.2 | ||
| Weight gain, g | 166.8 ± 15.3 | 191.7 ± 29.1 | 185.2 ± 22.5 | 188.8 ± 16.9 |
| Epidydymal fat, g | 7.2 ± 1.4 | 8.5 ± 1.9 | 9.9 ± 2.2‡ | 7.4 ± 0.9 |
| Retroperitoneal fat, g | 6.1 ± 3.0 | 8.2 ± 3.7 | 8.0 ± 2.4 | 6.2 ± 0.9 |
| Plasma glucose, mg/dl | 139.0 ± 6.0 | 145.0 ± 4.0 | 153.0 ± 6.0 | 149.0 ± 6.0 |
| Plasma insulin, ng/ml | 0.9 ± 0.2 | 1.0 ± 0.2 | 2.0 ± 0.3* | 1.5 ± 0.1 |
| Plasma FFA, mM | 0.30 ± 0.04 | 0.32 ± 0.03 | 0.33 ± 0.04 | 0.37 ± 0.03 |
| Plasma TG, mM | 0.39 ± 0.09 | 0.48 ± 0.09 | 1.10 ± 0.08* | 0.55 ± 0.10 |
| Liver TG, μmol/g | 6.4 ± 0.7 | 7.1 ± 0.6 | 12.1 ± 0.6† | 10.2 ± 0.5†§ |
Values are means ± SD. STD, high-starch diet; STD+T, STD+taurine; HSD, high-sucrose diet; HSD+T, HSD+taurine; FFA, free fatty acids; TG, triglycerides. 1Taurine intake estimated based on daily water consumption.
Significantly different from all other groups;
significantly different from STD and STD+T;
significantly different from STD;
significantly different from HSD.
Fig. 5.
Effects of taurine on hepatic steatosis and liver histology in rats provided a high-starch (STD) or high-sucrose diet (HSD). Hematoxylin and eosin (H&E) staining and analysis of steatosis score (A) and H&E and trichrome staining (B). Liver sections from animals in each of the dietary groups was stained with either H&E or Masson trichrome and examined histologically. STD, for 4 wks; STD+T, STD and drinking water with 2% (wt/vol) taurine for 4 wks; HSD, for 4 wk; HSD+T, HSD for 4 wk and drinking water with 2% taurine (wt/vol). In the graph, dietary groups are compared using a point score: 0 = < 10% of hepatocytes exhibiting steatosis; 1 = 10–33% exhibiting steatosis; 2 = 34–66% exhibiting steatosis; 3 = > 66% exhibiting steatosis. B: representative, equal-sized periportal areas are shown (small arrows). Periportal region from sucrose-fed rat appeared thickened and more uniformly red, indicative of hyalinization. Large arrows in the H&E stain from sucrose-fed rat show the hepatic artery, in which no hyalinization was present. Scale bar = 100 microns. Values in graph are means ± SD for n = 6 per group. *Significantly different from other dietary groups.
Fig. 6.
Effects of taurine on ER stress markers in rats provided STD or HSD. GRP78, CHOP, and GADD34 mRNA (A); phosphorylation of protein kinase-like ER kinase (p-PERK) relative to total PERK and p-eIF2α relative to total eIF2α (B); ATF4, GRP78, CHOP, and actin protein (C). Groups are exactly as described in Fig. 5. Values are means ± SD for n = 4–6 per group and blots shown are representative. *Significantly different from other dietary groups.
Fig. 7.
Effects of taurine on hepatic and systemic markers of inflammation, liver injury, and oxidative stress in rats provided an HSD or STD. Phosphorylation of IKKβ (p-IKKβ), TNF-α, IL-6, and actin protein (A); plasma alanine aminotransferase, (ALT) and aspartate aminotransferase (AST) (B); plasma TNF-α, C-reactive protein (CRP), and thiobarbituric acid reactive substances (TBARS) (C). Groups are described in Fig. 5. Values are means ± SD for n = 4–6 per group and Western blots are representative. *Significantly different from other dietary groups.
Table 2.
Endoplasmic reticulum stress markers in hepatocytes isolated from rats fed an STD or HSD
| Variable | STD | STD+T | HSD | HSD+T |
|---|---|---|---|---|
| p-PERK/Actin | 0.77 ± 0.19 | 0.84 ± 0.17 | 1.84 ± 0.32* | 0.92 ± 0.21 |
| p-eIF2α/eIF2α | 1.1 ± 0.24 | 1.0 ± 0.21 | 2.05 ± 0.36* | 1.23 ± 0.20 |
| ATF4/Actin | 1.41 ± 0.34 | 1.29 ± 0.30 | 2.45 ± 0.37* | 1.37 ± 0.29 |
| GRP78/Actin | 0.79 ± 0.16 | 0.71 ± 0.17 | 1.35 ± 0.27* | 0.89 ± 0.19 |
| CHOP/Actin | 0.23 ± 0.05 | 0.20 ± 0.06 | 0.97 ± 0.11* | 0.31 ± 0.07 |
Values are arbitrary optical density units and are reported as means ± SD. PERK, protein kinase-like ERK; p-eIF2α, phospho-eukaryotic initiation factor-2α; eIF2α, eukaryotic initiation factor-2α; ATF4, activating transcription factor-4; GRP78, glucose regulated protein-78; CHOP, C/EBP homologous protein.
Significantly different from all other groups.
Effects of taurine on high-sucrose diet-induced hepatic gene expression.
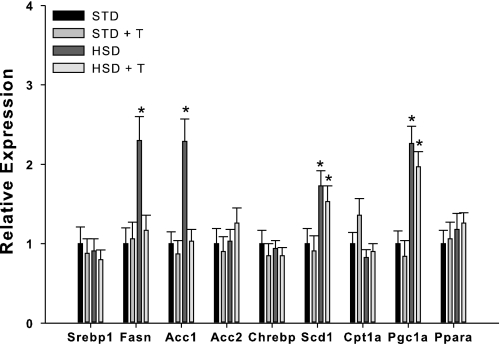
To examine potential molecular mechanisms for taurine-mediated changes in hepatic steatosis we examined gene expression profiles for several lipogenic and oxidative genes. The high-sucrose diet increased fatty acid synthase (Fasn), acetyl-CoA carboxylase 1 (Acc1), stearoyl-CoA desaturase (Scd1) and PPAR-γ coactivator 1-α (Pgc1a) mRNA levels (Fig. 8). Taurine supplementation reduced sucrose-mediated induction of Fasn and Acc1 gene expression (Fig. 8).
Fig. 8.
Effects of taurine on hepatic gene expression in rats provided STD or HSD. Srebp1, sterol regulatory element binding protein-1; Fasn, fatty acid synthase; Acc1 and -2, acetyl CoA carboxylase 1 and 2; Chrebp, carbohydrate response element binding protein; Scd1, steroyl-CoA desaturase-1; Cpt1a, carnitine palmitoyl transferase 1a; Pgc1a, peroxisome proliferator-activated receptor-γ coactivator 1; Ppara, peroxisome proliferator-activated receptor-α mRNA. Groups are described in Fig. 5. Values are means ± SD for n = 6 per group. *Significantly different from other dietary groups.
Effects of taurine on tunicamycin-induced hepatic steatosis, liver injury, and UPR induction.
The experiments described above indicate that taurine may be exerting its protective effects in diet-induced NAFLD by ameliorating oxidative stress, inflammation, and ER stress. A major complication to our understanding of the protective mechanisms of taurine supplementation is the fact that these pathogenic features are interrelated phenomena with each capable of inducing the other (32, 33). In an effort to dissect the protective mechanisms of taurine in NAFLD, we used an animal model of tunicamycin-induced steatosis and liver injury as described in materials and methods. Tunicamycin causes ER stress and induction of the UPR by inhibiting UDP-N-acetylglucosamine:dolichol phosphate N-acetylglucosamine-1-P transferase, and thereby blocking protein N-glycosylation. In this model, ER stress is the primary initiating factor, and any resultant oxidative stress and/or inflammation will be downstream consequences of the treatment.
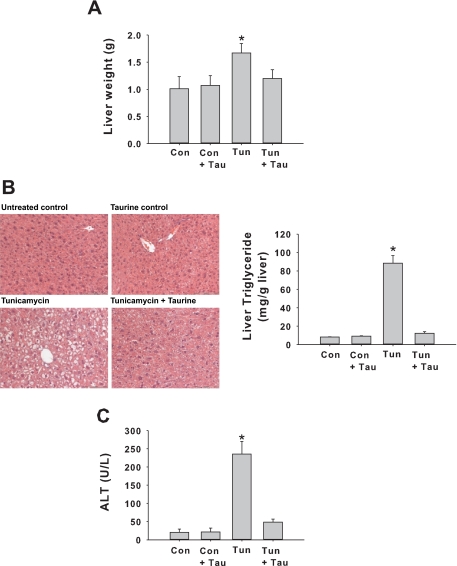
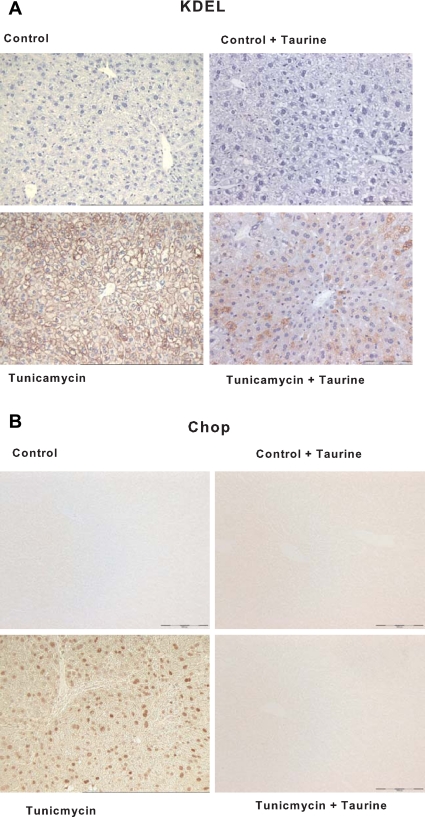
Tunicamycin injection resulted in hepatic steatosis and concomitant increases in liver weight, plasma alanine aminotransferase, and liver lipid content (Fig. 9). Hepatic lipid accumulation and liver injury were significantly attenuated by taurine supplementation (Fig. 9). The effect of taurine upon tunicamycin mediated induction of the UPR was analyzed by immunohistochemistry. This analysis revealed that taurine supplementation reduced the induction of proteins containing the KDEL signal sequence and completely ablated tunicamycin induced expression of CHOP (Fig. 10). These results are consistent with the possibility that taurine exerts hepatoprotective effect in NAFLD at least in part by reducing ER stress.
Fig. 9.
Effects of taurine in mice injected with tunicamycin. Liver weight (A); H&E stain and liver triglycerides (B); ALT (C). Con, control and carrier injected; Con + T, carrier injected and provided taurine in drinking water; Tun, tunicamycin injected; Tun + T, tunicamycin injected and provided taurine in drinking water. Values are means ± SD for n = 6/group. *Significantly different from all other groups.
Fig. 10.
Effects of taurine on ER stress in mice injected with tunicamycin. Immunostaining for KDEL (a tetrapeptide located at the COOH-terminal sequence of luminal proteins) (A) and C/EBP homologous protein (CHOP) (B) in liver tissue. Groups are described in Fig. 9. Scale bar = 200 μm.
DISCUSSION
Taurine is a small amino sulfonic acid that can be obtained through the diet or synthesized endogenously from methionine and cysteine (12). Taurine aids in numerous physiological processes, including bile salt formation, osmoregulation, central nervous system function, and calcium homeostasis (19). Epidemiological data demonstrate that dietary taurine intake is inversely related to the prevalence of metabolic diseases, and clinical and experimental studies suggest that taurine supplementation protects against metabolic disturbances, including diabetes and its complications (12, 56). Furthermore, taurine supplementation is protective against various hepatic insults, such as those imposed by cyclosporine-A, acetaminophen, a high-methionine diet, and alcohol (7, 11, 16, 70, 75). In the present study, we extend these findings by demonstrating that taurine reduces nutrient (palmitate, high-sucrose diet)- and chemical (tunicamycin)-induced hepatic steatosis, ER stress, inflammation, and injury. Importantly, the beneficial effects of taurine were consistent across four models: palmitate incubation of isolated primary hepatocytes and H4IIE liver cells, long-term (4-wk) high-sucrose feeding in rats, and acute tunicamycin injection in mice, indicating that dietary taurine supplementation has considerable potential as a preventative strategy in diet-induced NAFLD.
The mechanisms by which taurine exerts its beneficial effects are unknown, although several possibilities exist. For example, substantial evidence indicates that taurine protects a wide variety of cells from oxidative damage by increasing antioxidant defense systems (40, 66), decreasing the formation of reactive oxygen species (28, 56), and interfering with reactive oxygen species activity (18, 37). In the present study, taurine reduced 1) palmitate-mediated protein carbonyl formation in H4IIE liver cells, which is elevated in patients suffering from NAFLD or type 2 diabetes (9, 34, 62); 2) palmitate-mediated DCF fluorescence in H4IIE liver cells and primary hepatocytes; and 3) reduced plasma TBARS in high-sucrose diet-fed rats. Collectively these results suggest that taurine, directly or indirectly, can mitigate oxidative stress in the liver.
The ER provides a unique oxidizing environment for protein folding and disulfide bond formation. Each disulfide bond formed during oxidative protein folding produces a single reactive oxygen species. It has been estimated that secretory cells produce 3–6 million disulfide bonds per minute; thus it has been postulated that protein folding in the ER is intimately linked to oxidative stress (33, 55, 58). Indeed, oxidants not only provoke ER stress but also are signals generated by misfolded proteins in the ER that then activate the UPR and can lead to cell death (33, 55, 58).
In the present study, taurine reduced or prevented palmitate-, sucrose-, and tunicamycin-mediated ER stress and UPR activation. These results are in line with recent reports indicating that taurine mitigates ER stress in lung tissue and vascular cells following various insults (36, 40, 80) and suggest that attenuation of ER stress may be an important mechanism by which taurine leads to reduced inflammation, liver injury, and cell death. These data raise the possibility that, in environments conducive to the generation of either stress (e.g., obesity), a self-perpetuating cycle of ER stress and oxidative stress may hasten the development and progression of NAFLD and culminate, over time, in steatohepatitis and/or end-stage liver disease. Results from the current experiments suggest that taurine may break this cycle and prevent long-term liver damage.
In the present study, taurine reduced or prevented hepatic steatosis in high-sucrose diet-fed rats and in mice injected with tunicamycin. High-sucrose diets produce hepatic steatosis primarily through lipogenesis, whereas tunicamycin-mediated steatosis appears to result from impairments in the expression of genes involved in fatty acid oxidation and is augmented by reduced lipoprotein secretion. Recent studies have linked the UPR to the control of lipid biosynthesis, fatty acid oxidation, and lipoprotein secretion (27, 42, 54); therefore we speculate that the improvement in steatosis mediated by taurine is the result of reduced ER stress and UPR activation.
Chronic ER stress and activation of the UPR has been linked to impairments in insulin action, activation of inflammatory cascades and the innate immune response, and apoptosis (21, 31, 44). Thus, it is attractive to suggest that the ability of taurine to reduce palmitate-mediated cell death, inflammation, and liver injury results from a centralized effect on ER homeostasis. However, a few observations from the present study suggest that the effects of taurine may be more complex. First, in liver cells, taurine reduced ER stress markers at both concentrations of palmitate, but significant reductions in caspase-3 activity and cell death were only observed at high concentrations of palmitate. Thus, chronic ER stress and UPR activation are clearly not the only mediators of palmitate-mediated cell death. Second, taurine did not reduce palmitate-mediated Xbp1 splicing. Although this may be due to the inability to detect small changes in Xbp1 splicing, it suggests that the IRE1 branch of the UPR may remain active in the presence of taurine. The ability to maintain activation of this branch of the UPR has been linked to cell survival (29); thus the maintenance of Xbp1 splicing in cells may be linked to reduced cell death.
Obesity and NAFLD are largely diseases of volition, and despite worldwide public-health campaigns recommending lifestyle and dietary changes, many people are not following these recommendations. It is conceivable that taurine could be used in food/drink fortification strategies in a manner analogous to that of folic acid supplementation of flour. This approach, by acting as a passive therapy has lead to dramatic decreases (up to 78% in some populations) in the incidence of neural tube defects (74). In this context, taurine has a number of advantages as a potential preventative treatment for NAFLD in that it is inexpensive, nontoxic, and can be administered orally. However, there are some limitations of the present study that should be noted. In regards to DCF fluorescence as a marker of oxidative damage, we cannot rule out the possibility that reactive species that do not react with DCFH may remain operative in the presence of taurine, and therefore we have underestimated the contribution of oxidative stress to palmitate-mediated cell damage and death. Also, the use of this probe to estimate oxidative stress during apoptosis should be approached with caution, as cytochrome c is a powerful catalyst of DCFH oxidation (17). Finally, although the results of the current experiments suggest that the beneficial effects of taurine are accompanied by reductions in oxidative stress and ER stress, the methods employed cannot determine causality, nor can they delineate the respective contribution of each stress. In summary, by utilizing four models of liver damage, our results indicate that taurine is protective against nutrient- and ER stress-induced hepatic steatosis and liver injury. These data suggest that taurine has significant potential as a preventative and therapeutic treatment in NAFLD.
GRANTS
Financial support for this study was from National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-072017 and DK-47416, and the Lillian Fountain Smith Endowment (to M. J. Pagliassotti) and National Institute of Diabetes and Digestive and Kidney Diseases DK-074487 (to D. R. Petersen). C. L. Gentile was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1F32-DK-082166 and 1K01-DK-087777.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.L.G., A.M.N., H.J., D.O., D.R.P., M.J.P., and K.N.M. conception and design of research; C.L.G., A.M.N., J.C.G., K.T.P., D.W., Y.W., H.J., D.O., D.R.P., M.J.P., and K.N.M. performed experiments; C.L.G., A.M.N., H.J., D.O., D.R.P., and K.N.M. analyzed data; C.L.G., A.M.N., J.C.G., K.T.P., D.W., Y.W., H.J., D.O., D.R.P., M.J.P., and K.N.M. interpreted results of experiments; C.L.G., A.M.N., J.C.G., K.T.P., H.J., D.O., D.R.P., M.J.P., and K.N.M. prepared figures; C.L.G., A.M.N., H.J., D.O., D.R.P., M.J.P., and K.N.M. drafted manuscript; C.L.G., A.M.N., H.J., D.O., D.R.P., M.J.P., and K.N.M. edited and revised manuscript; C.L.G., A.M.N., J.C.G., K.T.P., D.W., Y.W., H.J., D.O., D.R.P., M.J.P., and K.N.M. approved final version of manuscript.
REFERENCES
- 1. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri B. A nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 107: 1103–1109, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 43: 506–520, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 4. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467–2474, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Calamita G, Portincasa P. Present and future therapeutic strategies in non-alcoholic fatty liver disease. Expert Opin Ther Targets 11: 1231–1249, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chang YY, Chou CH, Chiu CH, Yang KT, Lin YL, Weng WL, Chen YC. Preventive effects of taurine on development of hepatic steatosis induced by a high-fat/cholesterol dietary habit. J Agric Food Chem 59: 450–457, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology 49: 1554–1562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98: 960–967, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10: 389–406, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das SK, Chu WS, Mondal AK, Sharma NK, Kern PA, Rasouli N, Elbein SC. Effect of pioglitazone treatment on endoplasmic reticulum stress response in human adipose and in palmitate-induced stress in human liver and adipose cell lines. Am J Physiol Endocrinol Metab 295: E393–E400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erman F, Balkan J, Cevikbas U, Kocak-Toker N, Uysal M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids 27: 199–205, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Franconi F, Loizzo A, Ghirlanda G, Seghieri G. Taurine supplementation and diabetes mellitus. Curr Opin Clin Nutr Metab Care 9: 32–36, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem 19: 567–576, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guthrie HC, Hatrick A, Gerrard DJ. Traumatic variation of popliteal artery entrapment syndrome. J R Army Med Corps 152: 161–162, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, Giacca A, Fantus IG. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab 285: E744–E753, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hagar HH. The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett 151: 335–343, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamaguchi T, Azuma J, Schaffer S. Interaction of taurine with methionine: inhibition of myocardial phospholipid methyltransferase. J Cardiovasc Pharmacol 18: 224–230, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17: 330–346, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol 97: 2714–2724, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest 110: 1389–1398, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13: 1211–1233, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr 82: 1178–1184, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med 31: 331–335, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 46: 51–61, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science 318: 944–949, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12093–12101, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin-Gallan P, Carrascosa A, Gussinye M, Dominguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 34: 1563–1574, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116: 1413–1419, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Men X, Han S, Gao J, Cao G, Zhang L, Yu H, Lu H, Pu J. Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis. Acta Orthop 81: 265–269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merezak S, Hardikar AA, Yajnik CS, Remacle C, Reusens B. Intrauterine low protein diet increases fetal β-cell sensitivity to NO and IL-1β: the protective role of taurine. J Endocrinol 171: 299–308, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372–1379, 2002 [PubMed] [Google Scholar]
- 39. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 37: 1202–1219, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Nonaka H, Tsujino T, Watari Y, Emoto N, Yokoyama M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation 104: 1165–1170, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 49: 608–612, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118: 316–332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pagliassotti MJ, Kang J, Thresher JS, Sung CK, Bizeau ME. Elevated basal PI 3-kinase activity and reduced insulin signaling in sucrose-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab 282: E170–E176, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Pagliassotti MJ, Shahrokhi KA, Moscarello M. Involvement of liver and skeletal muscle in sucrose-induced insulin resistance: dose-response studies. Am J Physiol Regul Integr Comp Physiol 266: R1637–R1644, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev 79: 683–701, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr 26: 409–415, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Parildar-Karpuzoglu H, Mehmetcik G, Ozdemirler-Erata G, Dogru-Abbasoglu S, Kocak-Toker N, Uysal M. Effect of taurine treatment on pro-oxidant-antioxidant balance in livers and brains of old rats. Pharmacol Rep 60: 673–678, 2008 [PubMed] [Google Scholar]
- 50. Petty MA, Kintz J, DiFrancesco GF. The effects of taurine on atherosclerosis development in cholesterol-fed rabbits. Eur J Pharmacol 180: 119–127, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 11: 74–80, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15: 829–840, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87: 91–99, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 569: 29–63, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal 11: 2317–2331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 51: 595–602, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54: 3541–3546, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 30: 2119–2121, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Telci A, Cakatay U, Kayali R, Erdogan C, Orhan Y, Sivas A, Akcay T. Oxidative protein damage in plasma of type 2 diabetic patients. Horm Metab Res 32: 40–43, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol 279: R1334–R1340, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 23: 46–52, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta 1585: 202–212, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Vohra BP, Hui X. Taurine protects against carbon tetrachloride toxicity in the cultured neurons and in vivo. Arch Physiol Biochem 109: 90–94, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147: 943–951, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Wang D, Wei Y, Schmoll D, Maclean KN, Pagliassotti MJ. Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology 147: 350–358, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Warskulat U, Borsch E, Reinehr R, Heller-Stilb B, Monnighoff I, Buchczyk D, Donner M, Flogel U, Kappert G, Soboll S, Beer S, Pfeffer K, Marschall HU, Gabrielsen M, Amiry-Moghaddam M, Ottersen OP, Dienes HP, Haussinger D. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J 20: 574–576, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Waters E, Wang JH, Redmond HP, Wu QD, Kay E, Bouchier-Hayes D. Role of taurine in preventing acetaminophen-induced hepatic injury in the rat. Am J Physiol Gastrointest Liver Physiol 280: G1274–G1279, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem 303: 105–113, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 291: E275–E281, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Weisdorf SA, Freese DK, Fath JJ, Tsai MY, Cerra FB. Amino acid abnormalities in infants with extrahepatic biliary atresia and cirrhosis. J Pediatr Gastroenterol Nutr 6: 860–864, 1987 [DOI] [PubMed] [Google Scholar]
- 74. Wolff T, Witkop CT, Miller T, Syed SB. Folic acid supplementation for the prevention of neural tube defects: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 150: 632–639, 2009 [DOI] [PubMed] [Google Scholar]
- 75. Yalcinkaya S, Unlucerci Y, Giris M, Olgac V, Dogru-Abbasoglu S, Uysal M. Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition 25: 436–444, 2009 [DOI] [PubMed] [Google Scholar]
- 76. Yamamoto S. Plasma taurine in liver cirrhosis with painful muscle cramps. Adv Exp Med Biol 403: 597–600, 1996 [DOI] [PubMed] [Google Scholar]
- 77. Yamori Y, Murakami S, Ikeda K, Nara Y. Fish and lifestyle-related disease prevention: experimental and epidemiological evidence for anti-atherogenic potential of taurine. Clin Exp Pharmacol Physiol 31, Suppl 2: S20–S23, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Yu AS, Keeffe EB. Nonalcoholic fatty liver disease. Rev Gastroenterol Disord 2: 11–19, 2002 [PubMed] [Google Scholar]
- 79. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zulli A, Lau E, Wijaya BP, Jin X, Sutarga K, Schwartz GD, Learmont J, Wookey PJ, Zinellu A, Carru C, Hare DL. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension 53: 1017–1022, 2009 [DOI] [PubMed] [Google Scholar]