Abstract
We demonstrated previously that cytochrome P-450 (CYP) 2C29 is the epoxyeicosatrienoic acid (EET) synthase responsible for the EET-mediated flow/shear stress-induced dilation of vessels of female nitric oxide (NO)-deficient mice (Sun D, Yang YM, Jiang H, Wu H, Ojami C, Kaley G, Huang A. Am J Physiol Regul Integr Comp Physiol 298: R862–R869, 2010). In the present study, we aimed to identify which specific CYP isoform(s) is the source of the synthesis and release of EETs in response to stimulation by shear stress in vessels of rats. Cannulated mesenteric arteries isolated from both sexes of NG-nitro-l-arginine methyl ester (l-NAME)-treated rats were perfused with 2 and 10 dyn/cm2 shear stress, followed by collection of the perfusate to determine EET concentrations and isoforms. Shear stress stimulated release of EETs in the perfusate of female (but not male) NO-deficient vessels, associated with an EET-mediated vasodilation, in which 11,12- and 14,15-EET contributed predominantly to the responses. Rat CYP cDNA array screened a total of 32 CYP genes of mesenteric arteries, indicating a significant upregulation of CYP2C7 in female l-NAME-treated rats. Endothelial RNA and protein were extracted from intact single vessels. Expression of CYP2C7 mRNA and protein in pooled extractions of endothelial lysate was identified by PCR and Western blot analyses. Transfection of the vessels with CYP2C7 short interfering RNA eliminated the release of EETs, consequently abolishing the EET-mediated flow-induced dilation; these responses, however, were maintained in vessels transfected with nonsilencing short interfering RNA. Knockdown of endothelial CYP2C7 was confirmed by PCR and Western blot analyses. In conclusion, CYP2C7 is an endothelial EET synthase in the female rat vasculature, by which, in NO deficiency, shear stress stimulates the release of EETs to initiate vasodilation.
Keywords: cytochrome P-450 2C7, epoxyeicosatrienoic acids, endothelium, estrogen
cytochrome p-450 (cyp) epoxygenase catalyzes the oxidative metabolism of arachidonic acid to epoxyeicosatrienoic acids (EETs) that regulate various biological processes (3, 8, 29). In a variety of blood vessels, CYP2C, 2J, as well as 2B subfamilies have been identified (10, 35, 39, 40) and are believed to be the major source of endothelium-derived hyperpolarizing factors (EDHF) that mediate vasodilator responses, when endothelial nitric oxide (NO) synthesis is impaired. Indeed, published studies from our laboratory provided strong evidence that EETs are the EDHF that elicit flow/shear stress-induced dilation and hyperpolarization in vessels of NO-deficient mice/rats, via an estrogen-dependent activation of phosphatidylinositol 3/Akt signaling (12, 13, 15, 16, 34, 35, 37).
Despite the fact that much of the work on EDHF has been performed in rats, little is known about the CYP isoform(s) that produces EETs in this species. Compared with the vasculature of humans and mice, in which CYP2C8/9 and 2C29 genes are confirmed to be the specific CYP isoforms responsible for the EET-mediated vasodilator responses (6, 35), no one, up to date, has determined the relative contribution of rat CYP isoforms to the production of EETs, nor is it known whether the expression of CYP isoforms in the endothelium has any role in the regulation of vascular function. Although three isoforms of CYP2C subfamily (CYP2C11, 2C23, and 2C24) were reported to be expressed in renal microvessels of rats (10, 41), the lack of evidence for their localization in the vascular endothelium (9) challenges their roles in the synthesis of vascular EETs and control of vascular tone. Additionally, increasing evidence has revealed the estrogen-dependent potentiation of EDHF-dependent responses in mesenteric arteries of rats (23, 24, 36), but the specific gene(s) and related mediators responsible for the responses were not determined. These unresolved issues, therefore, provide a rationale for the present study, aimed to identify the specific CYP isoform(s) that is targeted/activated by estrogen in vascular endothelium and functions as the EET synthase responsible for the shear stress-stimulated release of EETs that, in turn, dilate vessels of female NO-deficient rats (15, 16, 37). As products of the identified specific CYP gene/enzyme, EETs released from single vessels were quantitatively and qualitatively analyzed, followed by evaluation of their functional significance in mediating flow-induced dilation. The results were confirmed by transfection of the vessels with specific short interfering RNA (siRNA) oligonucleotides.
MATERIALS AND METHODS
Animals
Nine-week-old male and female Wistar rats were purchased from Charles River Laboratories (Wilmington, MA). They were administered NG-nitro-l-arginine methyl ester (l-NAME) in their drinking water (50 mg/100 ml) for 3 wk. All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Rat P-450 cDNA Array
Mechanism.
The array is a plate-based hybridization profiling analysis for monitoring the expression of dozens of genes through reverse transcription of mRNA into cDNA. Targeted genes are specifically captured on a plate precoated with the gene-specific oligonucleotides and detected by a microplate luminometer.
Protocols.
Rats were killed by inhalation of 100% CO2. Isolated mesenteric arteries/arterioles were pulverized in liquid nitrogen. Total RNA was extracted using a commercial RNA isolation kit (TRI REAGENT, Sigma). Total RNA samples were the mixtures of equal amounts of RNA prepared from three female or three male rats. Five micrograms of total RNA from each group were then reverse transcripted into cDNA with the presence of biotin-dUTP in the reaction mixture. The labeled cDNA samples were added to 96-well plates (Signosis) that had been precoated with 32 rat CYP gene-specific oligonucleotides, including CYP1A1, 1A2, 1B1, 2A1, 2A2, 2B1, 2B2, 2C6, 2C11, 2C12, 2D1, 2D2, 2D3, 2D4, 2D5, 2E1, 2J4, 2S1, 3A1/3A23, 3A2, 4B1, 2F1, 4A2, 4A3, 2C7, 2C13, 2C23, 2C24, 2D10, 2D18, 2J3, 2J10, and β-actin. These isoforms of CYP genes were specifically captured in individual wells on the plates. The captured cDNA samples were detected with streptavidin-horseradish peroxidase. The luminescence of each well was read by a plate reader (Bio-TEK, Synergy HT) and reported as relative light units. The expression of individual genes was proportional to the intensity of luminescence of the corresponding well. Results were reported as background-corrected (blank well) luminescent intensity for each gene with normalization to the cDNA of β-actin.
Isolation of Endothelial RNA and Protein From Single Vessels
Endothelial expression of CYP isoforms was evaluated via specific extraction of endothelial RNA and protein from intact vessels. A freshly isolated single second-order mesenteric artery (∼320 μm in diameter and ∼15 mm in length with all branches ligated) was cannulated and perfused with PSS-MOPS in a vessel chamber. From inflow tubing, 200 μl of the air were injected through the vessel to expel intraluminal contents, followed by 30 μl of TRIzol reagent passing through the vessel in 30 s. The TRIzol reagent with lysate of the endothelium was collected from the outflow tubing and then underwent regular procedures for RNA isolation, reverse transcription, and PCR. Similarly, in separate vessels, 30 μl of 1× Laemmli sample buffer with 2-mercaptoethanol was continually injected into vessels for 2 min, followed by collecting endothelial protein. Approximately 12 vessels were isolated from each rat, and their endothelial cell (EC) RNA or EC protein was pooled as one sample, and concentrations of RNA or protein in the sample were measured. From each sample, ∼0.1 μg RNA or 10 μg protein were used for RT-PCR and Western blot analyses, respectively. After endothelial RNA or protein was obtained, the remaining parts of vessels were used as samples for smooth muscle cells (SMCs).
Quantitative Real-Time RT-PCR
Isolated endothelial RNA was subjected to real-time quantitative PCR (LightCycler, Roche Diagnostics, Indianapolis, IN). Oligonucleotide primers for CYP2C7, as well as 2C11, were purchased from Qiagen (QT01080590), and its expression was normalized to GAPDH. A relative quantitation method (ΔΔCt) was used to evaluate the expression of the gene in different groups of animals. All primer products were verified on a 1.5% agarose gel.
Western Blot Analysis
Ten micrograms of isolated endothelial protein from each sample were loaded on a 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane. Custom polyclonal antibodies against rat CYP2C7 were produced by Thermo Fisher Scientific Open Biosystems. A peptide of 19 amino acids (DYKDKEMLTFMEKVNENLK, Cyp2c7-188:206) was specifically designed and analyzed by the company using accession no. P05179 to limit cross-reactivity while improving overall antibody specificity. Two rabbits were immunized using a 70-day protocol with a primary immunization and three sequential boosts. Both antiserum and affinity-purified antibodies were obtained and used in the experiments (see Figs. 5A and 7D, respectively). Specific bands were normalized to GAPDH or β-actin.
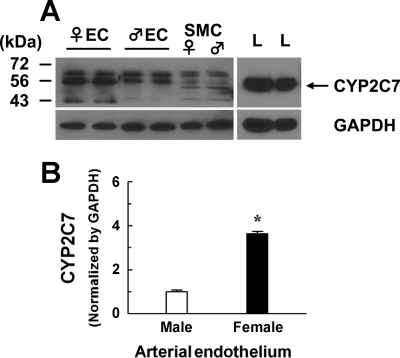
Fig. 5.
Original (A) and summarized (B) mean ± SE data showing the expression of CYP2C7 protein (56 kDa) in the endothelial lysates (EC) and smooth muscle cells (SMC) collected from mesenteric arteries of l-NAME-treated female (♀ EC, n = 3 blots) and male (♂ EC, n = 3 blots) rats. Rat liver (L) was used as positive control. The membrane was probed by CYP2C7 antiserum. GAPDH was used as loading control. *Significant difference from males, P < 0.05.
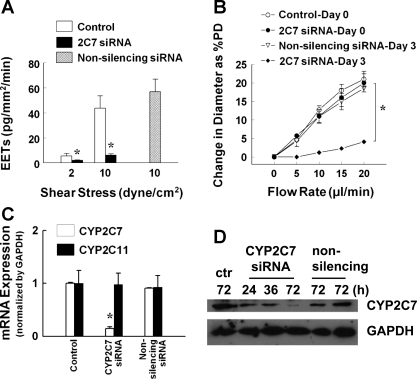
Fig. 7.
A: quantitation of EETs in the perfusate collected from shear stress-stimulated (2 and 10 dyn/cm2) mesenteric arteries of l-NAME-treated female rats in control conditions (N/n = 6/12) and after transfected with CYP2C7 short interfering RNA (siRNA; N/n = 5/15) or nonsilencing siRNA (N/n = 5/12) for 72 h, respectively. B: flow-induced dilation in control conditions (N/n = 5/10) (day 0) and after incubation with CYP2C7 siRNA (N/n = 5/8) or nonsilencing siRNA (N/n = 5/5) for 72 h (day 3). %PD, percent passive diameter. C and D: endothelial CYP2C7 and 2C11 mRNA (C) and CYP2C7 protein (D) expressions in mesenteric arteries in control conditions and transfected with CYP2C7 siRNA or nonsilencing siRNA for 24–72 h. Values are means ± SE. *Significant difference from controls and nonsilencing siRNA, P < 0.05.
Immunohistochemistry
Isolated single mesenteric arteries were fixed with 4% paraformaldehyde, followed by embedding in OCT compound. Frozen sections (10 μm thick) of the vessels were incubated with the purified CYP2C7 antibody (1:200) overnight at 4°C. Control staining was performed by prior incubation of the vessel section with CYP2C7 peptide for 1 h, followed by the CYP2C7 antibody. After that, vessel sections were probed with a second antibody of CY3-conjugated goat anti-rabbit IgG (1:500). The fluorescent image was captured with an Olympus BX60 microscopy (×100 oil objective) and a digital camera (CoolSNAP, RS Photometrics).
Perfused Arteries and Sample Collection
See Ref. 35. Second-order mesenteric arteries were isolated and cannulated in a vessel chamber filled with physiological salt solution (37°C) at 80 mmHg of intraluminal pressure. After equilibration, a known level of shear stress (2 and 10 dyn/cm2) was continually applied to the vessel for 5 min, during which the perfusate was collected in the outflow tubing.
Quantitation and Qualification of EETs in Perfusate
Perfusate EETs were quantified by gas chromatography mass spectroscopy (GC-MS) and qualitatively analyzed by liquid chromatography mass spectroscopy (LC-MS) (35), respectively. Two nanograms of 11,12-EET-d8 were added to the perfusate as an internal standard. After extraction, samples for GC-MS analysis were separated by HPLC to obtain total EET fractions, followed by derivatization and GC-MS measurement. Endogenous EETs were determined by comparison of GC retention time with internal standard (mass-to-charge ratio = 327.2), quantified by calculating the ratio of abundance and further normalized by endothelial area of the vessels (mm2). Extracted samples for LC-MS analysis were directly reconstituted in acetonitrile and injected into a LC-MS. EET isoforms were characterized based on their retention time with mass-to-charge ratio = 319.2 and expressed as the percentage of total EETs.
Flow/Shear Stress-Induced Dilation
After 1-h equilibration, isolated and cannulated mesenteric arteries developed spontaneous tone at 80-mmHg pressure. Perfusate flow was increased from 0 to 20 μl/min, in steps of 5 μl/min, and flow-diameter relationships were recorded in control vessels and vessels transfected with specific siRNA. At the conclusion of experiments, vessels were incubated in calcium-free physiological salt solution for 10 min; passive diameter (PD) of arteries at 80 mmHg was then obtained. Changes/increases in diameter of vessels, as a function of increases in flow, were normalized to their PD and expressed as “percentage (%) of PD”.
Experimental Protocols
Control study.
Perfusate samples from 2 and 10 dyn/cm2 shear stress-stimulated vessels were collected from control vessels; and the vessels were subjected to 6-(2-proparglyoxyphenyl) hexanoic acid (PPOH, 5 × 10−5 M) for 45 min to inhibit CYP/epoxygenase, for the measurement of EETs. In separate experiments, flow-induced dilation was also assessed. At the end of experiments, perfused vessels were injected intraluminally with TRI Reagent or Laemmli buffer, followed by collecting endothelial lysate, in which the expression of CYP2C7 mRNA or protein was determined.
RNA interference study.
The efficiency and specificity for the transfection of siRNA into the endothelium of isolated vessels have been demonstrated in our previous study (35). Vessel culture perfusion system and transfection procedures were also described in detail previously (34, 35). Briefly, in each experiment, six arteries were cannulated in perfusion chambers and superfused with Dulbecco's modified Eagle's medium (DMEM) with 1% antibiotic antimycotic solution without serum. After equilibration, three vessels were transfected with CYP2C7 siRNA. Three microliters of siRNA were mixed initially with 6 μl HiPerFect transfection reagent (Qiagen) per 100 μl DMEM at room temperature for 10 min. The mixture was further diluted 1:9 with DMEM to a final concentration of 25 nmol/l. The siRNA mixture was then injected intraluminally into the cannulated vessels at 37°C for 4–6 h without flow. The other vessels were either untransfected as time course controls, or transfected with nonsilencing siRNA. The vessels were then incubated at 40 mmHg of intravascular pressure with 17β-estradiol (10−9 M) and 2 dyn/cm2 shear stress for 24–72 h. After that, shear stress-induced release of EETs and flow-induced dilation were assessed by repeating the control experiments. The efficiency/specificity of CYP2C7 siRNA transfection was evaluated by perfusing incubated vessels with TRI Reagent or Laemmli buffer for extraction of endothelial RNA and protein, in which expressions of CYP2C7, as well as 2C11, mRNA and enzyme were evaluated by real-time RT-PCR and Western blot analyses.
Chemicals
CYP2C7 interference RNA (Rn_CYP2C7–5/1 FlexiTubesiRNA, SI03056634/02874214) and nonsilencing siRNA (ALLStars Negative Controls), as well as the primers, were purchased from Qiagen. CYP2C7 antiserum, followed by purified antibody for Western blot analyses, were specially synthesized by Open Biosystems. Oligonucleotide primers for endothelial NO synthase (eNOS) and α-smooth muscle actin (α-SMA) (QT00140119) were purchased from Qiagen. Primary antibodies for eNOS and α-SMA (ab5694) were purchased from Abcam. All other chemicals were obtained from Sigma (St. Louis, MO).
Statistics
Data are expressed as means ± SE. N/n refers the number of rats/vessels. Statistical analysis was performed using repeated-measures ANOVA, followed by the Tukey-Kramer post hoc test and Student's t-test. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Shear Stress-Stimulated Release of EETs in Female NO-Deficient Vessels
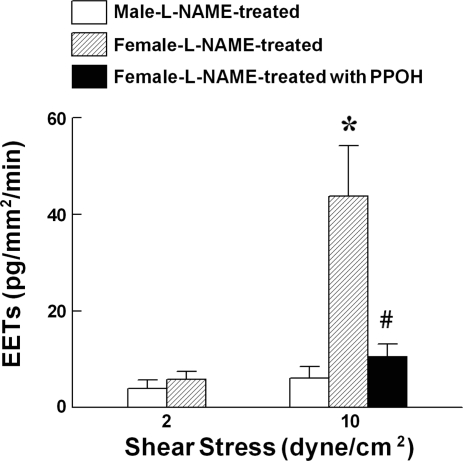
Based on our laboratory's previous findings that flow/shear stress-induced dilation in NO-deficient vessels of females was solely mediated by CYP/epoxygenase metabolites of arachidonic acid, as indicated by the elimination of the responses by epoxygenase inhibitors (15, 16, 37), we perfused isolated vessels of l-NAME-treated rats with different levels of shear stress, followed by measurement of EET concentrations and determination of EET regiosomers in the perfusate. Average diameter and length of perfused vessels were 332.6 ± 9.3 μm and 18.8 ± 1.2 mm in male, and 317.9 ± 10.1 μm and 15.7 ± 0.5 mm in female rats, respectively. In response to the stimulation with 2 and 10 dyn/cm2 shear stress, female vessels exhibited a dose-dependent release of EETs, a response that was prevented by PPOH. The release of EETs in male vessels reached only the lowest detectable margin (Fig. 1), suggesting a negligible contribution of EETs to the mediation of flow/shear stress-induced dilation of male arteries, which was mediated by prostaglandins (15, 37). Thus the following studies were conducted on female NO-deficient arteries that release EETs in response to shear stress.
Fig. 1.
Quantitation of epoxyeicosatrienoic acids (EETs) in the perfusate collected from mesenteric arteries stimulated with shear stress (2 and 10 dyn/cm2) for 5 min in control conditions and in the presence of 6-(2-proparglyoxyphenyl) hexanoic acid (PPOH; 5 × 10−5 M) of NG-nitro-l-arginine methyl ester (l-NAME)-treated male (N/n = 5/5) and female (N/n = 7/18) rats. Values are means ± SE; N/n, no. of rats/vessels. *Significant difference from males. #Significant difference from female controls: P < 0.05.
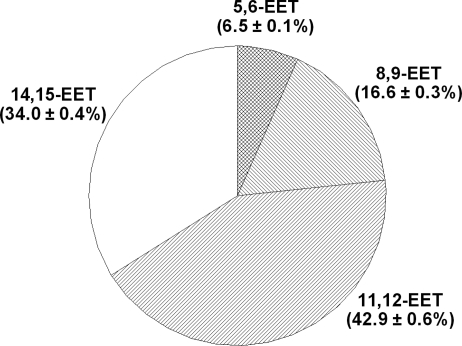
To identify the regioisomeric EET(s) that contribute to the mediation of flow/shear stress-induced dilations, perfusate EETs were analyzed by LC-MS. Figure 2 shows that 11,12- and 14,15-EET are the major mediators that account for 42.9 ± 0.6 and 34 ± 0.4% of total EETs released from shear stress-stimulated vessels, respectively. 8,9- and 5,6-EET provide lesser contributions (by 16.6 ± 0.3 and 6.9 ± 0.1%, respectively) to the responses.
Fig. 2.
Percentage of EETs released in response to 10 dyn/cm2 shear stress in mesenteric arteries of l-NAME-treated female rats (N/n = 5/10).
Predominant Expression of CYP2C7 in Arterial Endothelium of Female NO-deficient Rats
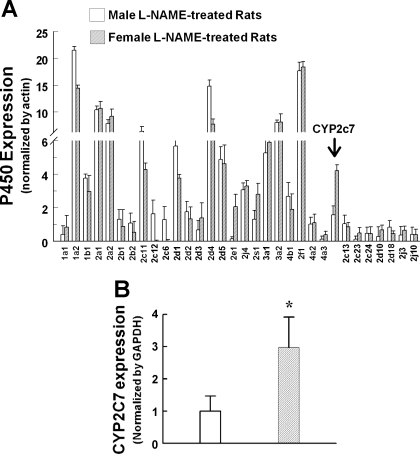
Rat CYP cDNA array was used to screen CYP genes in female NO-deficient vessels. We found that, among total captured genes, there was an upregulation of CYP2C7 (2.8-fold) in vessels of l-NAME-treated female compared with male rats (Fig. 3A), a result that was validated by real-time PCR assay (Fig. 3B). The significance of sex-specific-related changes in other CYP genes, such as CYP2C11 or CYP2E1, is currently being assessed in our laboratory.
Fig. 3.
Rat cytochrome P-450 (CYP) isoform gene expressions by rat P-450 cDNA plate array (A) and endothelial CYP2C7 mRNA expression by real-time PCR (B) in mesenteric arteries of l-NAME-treated male and female rats (N/n = 3/9). Values are means ± SE. *Significant difference from males, P < 0.05.
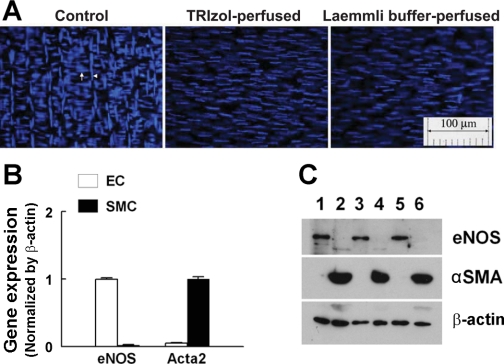
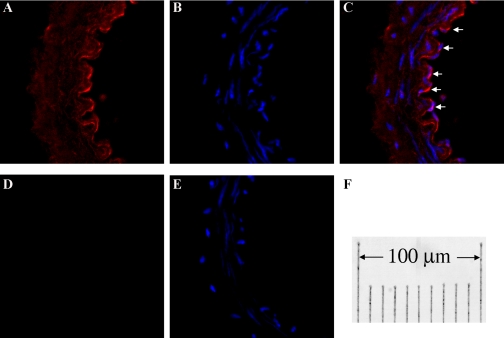
Given that shear stress-stimulated release of EETs to initiate flow-induced dilator responses has been shown to be an endothelium-dependent response (11–13, 15, 37), and that upregulation of CYP2C7 in arteries of female NO-deficient rats was expected to be responsible for the response, the specific localization of CYP2C7 in arterial endothelium needed to be further proven by extraction of endothelial RNA and protein from single intact vessels. The specificity of the isolated endothelial RNA and protein was confirmed by data shown in Fig. 4. Vessels, after being perfused with TRIzol (Fig. 4A, middle) or Laemmli buffer (Fig. 4A, right), and unperfused controls (Fig. 4A, left) were cut longitudinally, placed on a slide with the lumen facing up, and stained with DAPI, with the nuclei (blue) of ECs aligned vertically and those of SMCs aligned horizontally. Figure 4B shows RT-PCR analysis for eNOS and α-SMA (acta2), indicating that the endothelial RNA contains eNOS gene exclusively with a minimum of acta2 that is expressed predominantly in SMCs. Figure 4C depicts protein expressions of eNOS and α-SMA, showing that eNOS exists only in the endothelial lysate (EC, lanes 1, 3, and 5), and α-SMA exists only in the remaining part of the vessels (SMC, lanes 2, 4, and 6). By using this method, expression of CYP2C7 enzyme in the endothelium of vessels was proven in Fig. 5A, showing that CYP2C7 protein is predominantly expressed in ECs (lanes 1–4) and minimally expressed in SMC (lanes 5 and 6). Rat liver was used as a positive control (lane 7). Consistent with CYP2C7 mRNA expression (Fig. 3B), endothelial expression of CYP2C7 protein was significantly greater in vessels of l-NAME-treated female than male rats (Fig. 5B). Localization of CYP2C7 to arterial endothelium was confirmed further by the immunohistochemistry (Fig. 6), indicating that strong staining for CYP2C7 was observed in ECs of the vessel (Fig. 6, A–C), which was abolished by coincubation of the vessels with CYP2C7 peptide (Fig. 6, D and E).
Fig. 4.
A: DAPI-stained vessels in control conditions (left), and after perfused with TRIzol (middle) or Laemmli buffer (right). Endothelial (EC) and smooth muscle cells (SMC) are aligned vertically (arrowhead) and horizontally (arrow), respectively. B and C: expression of endothelial nitric oxide synthase (eNOS) and α-smooth muscle actin (αSMA) mRNA by RT-PCR (n = 5) and protein by Western blotting (n = 3/3) in the endothelial lysates (EC) collected from TRIzol- or Laemmli buffer-perfused vessels, and SMCs (the remaining portions of vessels after collection of endothelial lysates). C: lanes 1, 3, and 5 were loaded with endothelial protein, and lanes 2, 4, and 6 were loaded with protein of SMC.
Fig. 6.
Sections of mesenteric arteries (endothelial layer faces to the right) of female l-NAME-treated rats were stained for CYP2C7 and nuclei with Cy3 (red) and DAPI (blue), respectively. A and B: section stained for CYP2C7 and nuclei, both of which are merged shown in C, indicating a prominent staining for CYP2C7 in ECs (arrows). D: section for negative control staining, which was additionally stained with DAPI shown in E. F: the final magnification.
Role of CYP2C7 in the Mediation of Shear Stress-induced Release of EETs and Vasodilation
On the basis of findings showing the release of EETs (Fig. 1) and upregulation of endothelial CYP2C7 (Figs. 3, 5, and 6) in female NO-deficient vessels, Fig. 7 provides a link between CYP2C7 and release of EETs, as well as EET-mediated vasodilation, by transfection of vessels with CYP2C7 siRNA. After treatment of the vessels with CYP2C7 siRNA, shear stress-stimulated release of EETs in the perfusate was significantly reduced by ∼85% compared with control vessels, but was maintained in the vessels that were transfected with nonsilencing siRNA (Fig. 7A), verifying a CYP2C7-dependent response. Consistently, EET-mediated flow-induced dilations were also eliminated after transfection of the vessels with CYP2C7 siRNA, whereas vessels transfected with nonsilencing siRNA maintained dilator responses that were comparable to those recorded before transfection (day 0) (Fig. 7B). The specific knockdown of CYP2C7 mRNA and protein expression was also confirmed by ∼85% downregulation of endothelial expression of CYP2C7 mRNA (Fig. 7C) and protein (Fig. 7D) in siRNA-transfected vessels. In nonsilencing siRNA transfected controls, CYP2C7 expression was maintained. The specificity of CYP2C7 siRNA was additionally confirmed by showing a comparable expression of CYP2C11 mRNA in vessels of CYP2C7 siRNA-treated and control vessels (Fig. 7C). Thus results obtained from biochemical and molecular analyses, in combination with functional studies, strongly suggest that CYP2C7 is the EET synthase responsible for the shear stress-induced release of EETs and vasodilation in vessels of NO-deficient female rats.
DISCUSSION
Our study reveals a crucial role of CYP2C7 in the regulation of a shear stress-dependent mechanism, the most physiologically relevant event in the control of vascular resistance, via the synthesis of EETs to elicit flow-induced dilation in vessels that are deficient in NO synthesis. CYP2C7 had been demonstrated for decades to be one of the major CYP enzymes responsible for the hepatic metabolism of xenobiotics, including drugs, chemicals, and carcinogens (20, 26); its role, however, in the regulation of vascular function was not reported previously. In the present study, we demonstrated for the first time that CYP2C7 is predominantly expressed in rat arterial endothelium, and that it functions as a vascular EET synthase. Moreover, we quantified EET concentrations in the perfusate passing through single rat arteries, in which four regioisomeric EETs were characterized. An additional salient technique was also developed, by which we successfully extracted endothelial RNA and protein from single vessels and applied real-time RT-PCR and Western blot analyses. This technique provides a tool that clarifies whether the observed vascular signaling is specific for the endothelium and allows collecting mostly endothelial RNA and protein from freshly isolated arteries (not cultured ECs), in which the in situ changes, if any, can be revealed.
Published studies from our laboratory demonstrated that, in physiological conditions, co-release of endothelial NO and prostaglandins contributes to flow/shear stress-induced dilator responses in vessels of both sexes of mice/rats (14, 21). However, there is a sex difference in the mediation of shear stress-induced dilation, as a function of NO deficiency, which is characterized as a prostaglandin-mediated dilator response in vessels of males, but EET mediation of the response in females (11, 12, 15, 16, 32, 33, 37), in which EETs are transferable mediators to directly hyperpolarize vascular SMCs to dilate vessels (12, 13). This response is estrogen dependent and occurs via an estrogen receptor-mediated activation of phosphatidylinositol 3-kinase/Akt signaling (15, 16). We also demonstrated that CYP2C29 is the specific CYP gene responsible for the estrogen-dependent, EET-mediated vasodilation/hyperpolarization in vessels of eNOS-knockout mice (35), a response that was also observed in vessels of NO-deficient rats (15, 16, 35, 37). Indeed, in the present study, shear stress-stimulated release of EETs (Fig. 1) in rat vessels was similar to that observed in mice (35), suggesting that the same mechanism is involved, by which shear stress activates endothelial membrane phospholipase to release preformed EETs and de novo synthesized EETs (since PPOH, an inhibitor of epoxygenase reduced EET levels by ∼80%). On the other hand, unlike in mouse vessels, in which 14,15-EET was the major isoform among total 4-regioisomeric EETs (35), in rat vessels, 11,12-EET contributed predominantly to the responses (Fig. 2). This implies that a different CYP isoform is involved, since, as defined by the CYP nomenclature, the last number followed after CYP/2/C identifies individual genes that are specified by their product profile (5). Given that the CYP2C family is the major source for the synthesis of 4-regioisomeric EETs (29), and that an individual CYP gene is characterized by its products, it is reasonable to identify the specific CYP2C isoform that serves as an EET synthase in rat vasculature, accounting for the profile of the four released regioisomeric EETs shown in Fig. 2.
CYP2C7 Responsible for Endothelial Synthesis/Release of EETs
By using rat P-450 cDNA array and real-time PCR, we screened 32 isoforms of CYP genes in vessels isolated from both sexes of NO-deficient rats and found that there was an upregulation of CYP2C7 in vascular endothelium of l-NAME-treated female compared with male rats (Fig. 3). CYP2C7 is persistently expressed in rat liver from puberty through adulthood and is believed to contribute significantly to hepatic drug-metabolizing enzyme activity (27, 28). To date, there was no evidence indicating that CYP2C7 is expressed in vasculature, nor studies revealing its role in the control of vascular tone. Since, in the vascular wall, ECs are the sole source of EETs (3), and since we demonstrated the synthesis/release of EETs in the vessels (Figs. 1 and 2) that overexpressed CYP2C7 (Figs. 3), the question arose as to whether CYP2C7 is the endothelial EET synthase of rats. The answer could be discovered by the study of a cDNA clone that encoded a mouse CYP2C29 with the complete coding region of 490 amino acid residues. This demonstrated that the deduced amino acid sequence of CPY2C29 exhibited 83% identity with rat CYP2C7 (25). Thus the homology of the molecular sequence in both genes provides a structural basis that, in connection with the specific releasing profile of EETs (Fig. 2) and the endothelial location of CYP2C7 (Figs. 5 and 6), supports our hypothesis that CYP2C7 corresponds, indeed, to the mouse CYP2C29 and is an endothelial EET synthase of rat vasculature. This hypothesis was further confirmed by the RNA interference study, indicating that shear stress-stimulated release of EETs and the corresponding vasodilation were eliminated when endothelial CYP2C7 was knocked down (Fig. 7).
Regulation of Vascular CYP2C7 by Estrogen, Shear Stress, and NO
Rat hepatic CYP2C7 has been identified to be a predominant CYP isoform of female (male/female = 1:3) (27), which is in line with our findings (Fig. 3). However, the mechanism responsible for the sexual dimorphisms of CYP2C7 expression in blood vessels seems not to be the same as that found in the liver. Sex difference in the expression of rat hepatic CYP2C7 is regulated by the sex-dependent profiles of circulating growth hormone (GH), shown by the fact that continuous GH secretion in female rats allows a higher expression of CYP2C7 than pulsatile secretion in males (28, 30). In our studies, however, upregulation of CYP2C7 in female vessels is not related to the GH secretion profile, but rather it is dependent on the presence of estrogen per se, since we found previously that in vitro incubation of male vessels with estrogen switched the male phenotype (prostaglandin-mediated) to the female phenotype (EET-mediated) of flow-induced dilation (15, 37), a response that, in the present study, has been demonstrated to be mediated by CYP2C7. Additionally, hepatic CYP2C11 and 2C12 were also reported to be the male- and female-specific isoforms, controlled by the sex-dependent profiles of circulating GH; this sex-specific difference, however, was not observed in rat vessels (Fig. 3). P-450 cDNA array also indicated an upregulation of CYP2E1 in female compared with male vessels. Its contribution to the mediation of shear stress-dependent release of EETs and vasodilation, however, was not supported by the finding that CYP2E1 potentiates renal vasodilation via the mechanism involving the production of 18-HETE and 19-HETE to interact with 20-HETE-induced constriction (1, 4, 40).
Since this is the first study indicating the presence of vascular CYP2C7, little is known about its regulation in the vasculature. We noted in our preliminary studies that, compared with freshly isolated vessels that served as in vivo controls, there was a time-dependent downregulation of endothelial CYP2C7 mRNA in cultured vessels, but when these vessels were exposed to the estrogen plus shear stress, their CYP2C7 mRNA level was comparable to that detected from control vessels. The vessels were cultured with either stimulus (estrogen or shear stress); CYP2C7 mRNA level was ∼40% of control vessels and decreased further in vessels cultured without the stimuli. These findings are consistent with the result observed in cultured ECs, in which CYP2C mRNA and protein levels rapidly decreased after cell isolation, and, moreover, cultured arteries exhibited a CYP-dependent EDHF response only when subjecting them to shear stress (7). The unstable characteristic of vascular CYP highlights the roles of transcriptional processes and the importance of physiological stimuli in the control of its expression. Thus we drew the conclusion that vascular CYP2C7 is induced in response to estrogen and shear stress. Specific mechanism(s) responsible for the regulation of vascular CYP2C7 by estrogen and shear stress remains to be further elucidated.
NO deficiency is believed to be a prerequisite for the backup of EET/EDHF-dependent mechanism in the control of vascular tone (2). We found that the EET-compensatory mechanism did not function in male NO-deficient vessels, nor in those of control female rats in which NO is a primary mediator (12, 37), as evidenced by the lowest detectable level of EETs in the perfusate (Fig. 1) and significantly lesser expression of endothelial CYP2C7 protein in l-NAME-treated male than female rats (Figs. 3 and 5). This supports further our conclusions that there is a sex difference in the compensation for NO deficiency via the release of different mediators to elicit shear stress-dependent responses, characterized as a cyclooxygenase-dependent mechanism in male, but CYP2C7-dependent in female, rats (15, 16, 37). Our findings involving the estrogen-dependent upregulation of EETs/EDHF in the mediation of flow/shear stress-induced dilations provided an explanation for the fact that, in resistance arteries of eNOS/cyclooxygenase-1 double knockout female mice, endothelium-dependent relaxations are preserved and associated with a normal mean arterial blood pressure, whereas the males with these deletions are hypertensive, as a consequence of impaired endothelium-dependent vasodilation (31).
Perspectives and Significance
This is the first study demonstrating that it is CYP2C7 that functions as a vascular EET synthase in female rats in the absence of NO synthesis. Evidence is provided by the expression of CYP2C7 mRNA/protein in the endothelium of vessels that produce 4-regioisomeric EETs to mediate flow-induced dilation, a response that is prevented by knockdown of vascular CYP2C7. The present study broadens our previous findings, via the identification of the specific gene that is the target of estrogens in the initiation of the preserved shear stress-dependent vasodilation in rat vessels deficient in NO synthesis, suggesting the presence of a CYP/EDHF-mediated mechanism, on which sex differences in susceptibility to cardiovascular disease may be based. The knowledge generated by our findings may lead to better strategies for clinical treatment of cardiovascular diseases, such as hypertension and atherosclerosis, characterized by a reduction in the synthesis or bioactivity of NO. Indeed, given the cardiovascular actions of EETs, the concept can be advanced that EETs and the modulation of their metabolic pathway could be therapeutically targeted for the treatment of hypertension and other cardiovascular diseases. For instance, EET agonists, as well as upregulation of CYP epoxygenase, have been administered chronically in experimental animal models of hypertension and metabolic syndrome and have been demonstrated to decrease blood pressure, attenuate hypertension-induced renal injury (18, 22), and improve insulin signaling and vascular function (17, 19, 38).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL 070653 and HL PO1 43023.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.S. and A.H. conception and design of research; D.S., H.J., H.W., Y.Y., and A.H. performed experiments; D.S. and A.H. analyzed data; D.S. and A.H. interpreted results of experiments; D.S. and A.H. prepared figures; D.S. and A.H. drafted manuscript; D.S., G.K., and A.H. edited and revised manuscript; D.S., G.K., and A.H. approved final version of manuscript.
REFERENCES
- 1. Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol 277: F790–F796, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflügers Arch 459: 881–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll MA, Balazy M, Margiotta P, Huang DD, Falck JR, McGiff JC. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol Regul Integr Comp Physiol 271: R863–R869, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Coon MJ, Ding XX, Pernecky SJ, Vaz AD. Cytochrome P450: progress and predictions. FASEB J 6: 669–673, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Fischer D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Muller M, Busse R, Fleming I, Drexler H. Cytochrome P450 2C9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and in patients with chronic heart failure. Eur J Heart Fail 9: 770–775, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol Res 49: 525–533, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med 18: 20–25, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Heil SG, De Vriese AS, Kluijtmans LA, Dijkman H, van Strien D, Akkers R, Blom HJ. Cytochrome P450–2C11 mRNA is not expressed in endothelial cells dissected from rat renal arterioles. Nephron Physiol 99: 43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest 104: 751–760, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res 96: 376–383, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang A, Sun D, Shesely EG, Levee EM, Koller A, Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am J Physiol Heart Circ Physiol 282: H429–H436, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Huang A, Sun D, Wu Z, Yan C, Carroll MA, Jiang H, Falck JR, Kaley G. Estrogen elicits cytochrome P450–mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ Res 94: 245–252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang A, Wu Y, Sun D, Koller A, Kaley G. Effect of estrogen on flow-induced dilation in NO deficiency: role of prostaglandins and EDHF. J Appl Physiol 91: 2561–2566, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol 56: 329–335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR, Sangras B, Anjaiah S, Manthati VL, Sudarshan RD, Falck JR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Front Physiol 1: 157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang H, Quilley J, Doumad AB, Zhu AG, Falck JR, Hammock BD, Stier CT, Jr, Carroll MA. Increases in plasma trans-EETs and blood pressure reduction in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H1990–H1996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobliakov V, Popova N, Rossi L. Regulation of the expression of the sex-specific isoforms of cytochrome P-450 in rat liver. Eur J Biochem 195: 585–591, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. Am J Physiol Heart Circ Physiol 267: H326–H332, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24: 3770–3781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br J Pharmacol 132: 1035–1046, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu MY, Hattori Y, Sato A, Ichikawa R, Zhang XH, Sakuma I. Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in connexin-43 expression. J Cardiovasc Pharmacol 40: 938–948, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Matsunaga T, Watanabe K, Yamamoto I, Negishi M, Gonzalez FJ, Yoshimura H. cDNA cloning and sequence of CYP2C29 encoding P-450 MUT-2, a microsomal aldehyde oxygenase. Biochim Biophys Acta 1184: 299–301, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6: 1–42, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Oinonen T, Ronis M, Wigell T, Tohmo K, Badger T, Lindros KO. Growth hormone-regulated periportal expression of CYP2C7 in rat liver. Biochem Pharmacol 59: 583–589, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Pampori NA, Shapiro BH. Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology 140: 1245–1254, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Sasamura H, Nagata K, Yamazoe Y, Shimada M, Saruta T, Kato R. Effect of growth hormone on rat hepatic cytochrome P-450f mRNA: a new mode of regulation. Mol Cell Endocrinol 68: 53–60, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 291: H1429–H1435, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ERalpha knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol 293: R1239–R1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun D, Yang YM, Jiang H, Wu H, Ojaimi C, Kaley G, Huang A. Roles of CYP2C29 and RXR gamma in vascular EET synthesis of female mice. Am J Physiol Regul Integr Comp Physiol 298: R862–R869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther 292: 375–380, 2000 [PubMed] [Google Scholar]
- 37. Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol 280: H2456–H2461, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes 59: 997–1005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu Z, Huse LM, Adler P, Graham L, Ma J, Zeldin DC, Kroetz DL. Increased CYP2J expression and epoxyeicosatrienoic acid formation in spontaneously hypertensive rat kidney. Mol Pharmacol 57: 1011–1020, 2000 [PubMed] [Google Scholar]
- 40. Zhang F, Deng H, Kemp R, Singh H, Gopal VR, Falck JR, Laniado-Schwartzman M, Nasjletti A. Decreased levels of cytochrome P450 2E1-derived eicosanoids sensitize renal arteries to constrictor agonists in spontaneously hypertensive rats. Hypertension 45: 103–108, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41: 709–714, 2003 [DOI] [PubMed] [Google Scholar]