Abstract
Hypothalamic orexin neurons project to the hindbrain, and 4th-ventricle intracerebroventricular (4th-icv) injection of orexin-A treatment increases food intake. We assessed the effects of hindbrain orexin-A and the orexin-1-receptor antagonist SB334867 on meal pattern in rats consuming standard chow. When injected 4th-icv shortly before dark onset, lower doses of orexin-A increased food intake over a 2-h period by increasing the size of the first meal relative to vehicle, whereas the highest dose increased food intake by causing the second meal to be taken sooner. Conversely, hindbrain SB334867 reduced food intake by decreasing the size of the first meal of the dark phase. We also examined the effects of 4th-icv orexin-A and SB334867 on locomotor activity. Only the highest dose of orexin-A increased activity, and SB334867 had no effect. In addition, hindbrain SB334867 induced c-Fos in the nucleus of the solitary tract. These data support the suggestion that endogenous hindbrain orexin-A acts to limit satiation. Both orexin-A and the pancreatic satiation hormone amylin require an intact area postrema to affect food intake, so we asked whether 4th-icv orexin-A impairs the satiating effect of peripheral amylin treatment. Amylin reduced the size of the first meal of the dark cycle when rats were pretreated with 4th-icv saline, yet amylin was ineffective after 4th-icv orexin-A pretreatment. Using double-label immunohistochemistry, we determined that some orexin-A fibers in the area postrema are located in proximity to amylin-responsive neurons. Therefore, hindbrain orexin-A may increase food intake, in part, by reducing the ability of rats to respond to amylin during a meal.
Keywords: orexin, amylin, food intake, satiation, meal pattern
a variety of evidence supports the idea that orexin neurons, which reside exclusively in the lateral and perifornical areas of the hypothalamus, play a role in the control of food intake. Lateral- or 3rd-intracerebroventricular (icv) injection of orexin-A increases food intake, as does site-specific injection of orexin-A into several hypothalamic nuclei (8, 11). The orexin-1 receptor (OX1R) is thought to mediate these effects on feeding, because OX1R-selective antagonists reduce food intake, and activation of the orexin-2 receptor by orexin-B has little effect on feeding (4, 7). Although most of the previous research has focused on orexin action within the hypothalamus, orexin-A can also increase food intake when administered to the caudal brainstem (27). Orexin neurons project to several locations within the medulla, including the dorsal vagal complex (DVC), the area postrema (AP), nucleus of the solitary tract (NTS), and dorsal motor nucleus of the vagus nerve, and OX1R are expressed by neurons within these nuclei (10, 14, 16).
Detailed behavioral analysis of hindbrain orexin-A's effect on food intake can provide useful insights into its mechanism of action. The first question to address is whether orexin-A increases food intake by increasing meal size or by increasing the frequency of meals. Baird et al. (2) recently examined the dose response function for 3rd- and 4th-icv orexin-A treatment in rats licking for sucrose solutions during the light phase of the day. They reported that 1 nmol orexin-A increased the size of rats' meals, whereas 10 nmol orexin-A increased food intake by causing the rats to take more meals during the 90-min test period, with no effects of any dose of orexin-A on palatability-related measures, such as lick rate or licking burst size. Here, we investigated whether hindbrain orexin-A increases meal size, meal frequency, or both during dark-phase spontaneous chow intake, which differs from ingestion of sucrose solution during the light-phase in several respects (e.g., taste and palatability of food, circadian cues, spontaneous vs. conditioned eating). We report a dose response function similar to that obtained by Baird et al., in which lower doses affect meal size exclusively, while our highest dose affects the frequency of meals.
Previous studies have established that 4th-icv injection of orexin-A can increase food intake (2, 27), but it is possible that this is purely a pharmacologic effect. Hindbrain OX1R stimulation by exogenous administration of the agonist can clearly drive an orexigenic response, but that does not speak to the question of whether endogenous stimulation of hindbrain OX1R promotes ingestion under normal physiologic conditions. If endogenous hindbrain OX1R activation does have an orexigenic effect, then blockade of those receptors should reduce food intake. Peripheral administration of the selective OX1R antagonist SB334867 does decrease food intake (7, 8), but that effect could be mediated by OX1R in any number of locations. Here, we target caudal brainstem OX1R by examining the pattern of ingestive behavior in response to 4th-icv administration of SB334867.
Orexin-A plays a role in spontaneous physical activity (11), and it has been reported that 4th-icv injection of 1 nmol orexin-A during the light cycle increased locomotor activity (27). Such changes in motor behavior could contribute to the observed effects on food intake. Therefore, we performed a dose response analysis for locomotion in a separate experiment, using two doses of orexin-A that increased feeding in our meal pattern study. If endogenous stimulation of hindbrain OX1R contributes to the control of locomotor activity, then one might expect that blockade of these receptors would decrease activity. Such an effect could potentially explain reduced food intake; if SB334867 induces lethargy or sleep, treated rats would be less likely to eat for reasons unrelated to satiation. To address these possibilities, we examined the effect of hindbrain injection of the OX1R antagonist SB334867 on locomotor activity, using a dose that effectively reduces meal size.
To further examine the neural effects of SB334867, we investigated whether 4th-icv injection of the same dose that reduces meal size induces c-Fos within caudal brainstem nuclei typically associated with satiation, the NTS, and AP. Zheng et al. (27) reported that hindbrain orexin-A treatment induces c-Fos expression in AP and NTS. Here, we used a double-labeling technique to assess possible activation of hindbrain catecholamine neurons by SB334867, because these cells are known to respond to satiation signals (e.g., 17, 18).
Based on the findings of Baird et al. (2) and our data showing that the hindbrain OX1R activation increases meal size, we hypothesized that hindbrain orexin-A dampens the response to within-meal satiation signals. Meal size is strongly influenced by signals that are released in response to the presence of nutrients in the gastrointestinal tract. These signals provide negative feedback during ingestion and promote meal termination (i.e., satiation). The effectiveness of these satiation signals can be increased or decreased by other hormones and neurotransmitters that do not have a direct relation to food in the gastrointestinal tract (20). We propose that orexin-A acts in this manner, increasing food intake by reducing the response to satiation signals. Further support for this idea comes from immunohistochemical evidence that orexin-A fibers can be found in close proximity with hindbrain neurons that express c-Fos in response to a gastric nutrient load (27). Here, we asked whether 4th-icv orexin-A treatment can blunt the anorexic response to one particular satiation signal, the pancreatic hormone amylin. We chose to investigate a possible interaction between orexin-A and amylin, as opposed to other satiation signals, because both orexin-A and amylin require an intact AP to affect food intake (2, 13). Together, the data we present here support the hypothesis that hindbrain OX1R activity promotes food intake by limiting satiation.
MATERIALS AND METHODS
Subjects.
Naïve male Wistar rats (Charles River, Wilmington, MA) were individually housed in Plexiglas chambers fitted with the BioDaq (Research Diets, New Brunswick, NJ) continuous food intake monitoring system for experiments 1, 3, and 6. Rats in experiments 2 and 4 were housed in Plexiglas chambers that sat on top of a custom device for the measurement of locomotor activity (described below). Rats in experiments 5 and 7 were individually housed in Plexiglas cages with food hoppers. The room was temperature controlled and maintained on a 12:12-h light-dark cycle. Distilled water and rat chow (5001; Purina, St. Louis, MO) were available ad libitum unless otherwise noted. All animals were handled daily and habituated to intracerebroventricular and intraperitoneal injections of saline before the experiments began. Body weight was measured daily, and food intake was measured continuously. All experimental procedures were approved by the Florida State University institutional animal care and use committee, and conform to the standards of the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Drugs and injection procedure.
Orexin-A and amylin (Bachem, Torrance, CA) were each dissolved in sterile 0.9% saline. For experiment 2, the OX1R antagonist SB334867 (Tocris Bioscience, Ellisville, MO) was dissolved in 66% DMSO (Sigma Aldrich, St. Louis, MO) and 3% hydroxypropyl-β-cyclodextrin (Fisher Scientific, Pittsburgh, PA) in sterile deionized water. The corresponding vehicle was also 66% DMSO and 3% hydroxypropyl-β-cylcodextrin. For experiments 4 and 5, we were able to dissolve the SB334867 in 2% DMSO and 10% hydroxypropyl-β-cyclodextrin in sterile deionized water by heating to 45°C while stirring. Therefore, the vehicle in experiments 4 and 5 was 2% DMSO and 10% hydroxypropyl-β-cyclodextrin.
All 4th-icv injections were made using a 10-μl syringe (Hamilton, Reno, NV) connected to a 33G injector (Plastics One, Roanoke, VA) via Tygon tubing (VWR, Radnor, PA). Drugs or vehicle were delivered at a rate of 1 μl/min.
Surgery.
A 26-gauge guide cannula (Plastics One), was implanted 2.0 mm above the 4th ventricle under 2 to 4% isoflurane in 1 liter oxygen/minute inhaled continuously during surgery. Stereotaxic coordinates for 4th-icv cannula placement were 1) on midline, 2.5 mm anterior to the occipital suture; and 2) 5.2 mm ventral to the skull surface. The cannula was cemented to three jeweler's screws attached to the skull and closed with an obturator. Buprenorphine hydrochloride (0.3 mg/kg im) (Butler Schein Animal Health Supply, Columbus, OH) or carprofen (5 mg/kg sc) (Butler Schein Animal Health Supply) was administered before the start of surgery and again if rats showed signs of distress (i.e., lethargy, lack of grooming, porphyrin staining around eyes or nose) over the next 2 days. Food intake and body weight were monitored while rats recovered for at least 5 days before experimental procedures began. Cannula placements were verified before the start of experiments through the measurement of a sympathetically mediated increase in plasma glucose 60 min after injection of 210 μg 5-thio-d-glucose into the 4th ventricle (19).
Experiment 1: effect of 4th-icv orexin-A on meal pattern.
For short-term food restriction during the light cycle, the gates that allowed access to food hoppers were closed 5 h before the start of the dark cycle. One hour before the start of the dark cycle, rats (n = 7) received 4th-icv injections of either saline, 0.05, 0.1, or 1 nmol of orexin-A in 2 μl. Food access was returned immediately before the onset of the dark cycle. Food intake was continuously measured thereafter. This experiment used a counterbalanced within-subjects design in which all rats received all conditions separated by at least 48 h.
Experiment 2: effect of 4th-icv OX1R antagonist on meal pattern.
The procedure for this experiment was identical to that for experiment 1, except the rats (n = 9) received either vehicle or 20 nmol of the OX1R antagonist in 5 μl. All the rats received the vehicle and SB334867 conditions in counterbalanced order separated by at least 72 h. Two rats were removed from this study due to poor response to the DMSO/β-cyclodextrin vehicle (these rats lost ∼30 g body wt during the 48 h after vehicle injection and displayed lethargy and poor grooming), leaving seven subjects that tolerated this vehicle well (no significant weight loss or signs of distress). Only the results from those seven rats are presented here.
Experiment 3: effect of 4th-icv orexin-A on locomotor activity.
Rats were housed in customized individual cages designed to measure locomotor activity, as described previously (25). Briefly, the chambers are positioned on a plastic platform with stiff strain-gauge load-beam transducers (model L2330; Futek, Irvine, CA) that allow localization of the animal's position in two dimensions over time. Locomotor activity is defined as movements that exceed 1-cm distance traveled without direction reversal, and the cumulated distance of locomotor activity is sampled every 30 s. Food and water are positioned on opposite sides of the cage, maximizing the likelihood of movement by the rat.
The rats were habituated to these chambers for 1 wk before the experiment began. On experimental treatment days, rats (n = 8) received 4th-icv injections of either saline, 0.1, or 1 nmol of orexin-A in 2 μl, administered within 60 min of dark cycle onset. The computer program for measurement of activity was started immediately before dark onset. Short-term food intake was not assessed in this study, but 24-h intake and body weight were measured daily to monitor the rats' general health. This experiment used a counterbalanced within-subjects design in which all rats received all conditions separated by at least 72 h.
Experiment 4: effect of 4th-icv OX1R antagonist on locomotor activity.
The methods for this experiment were identical to those of experiment 3 except for the drug treatments, and the same subjects (n = 8) were used beginning 6 days after the completion of the orexin-A dose response. In this study, rats received 4th-icv injections of either vehicle or 20 nmol of the OX1R antagonist in 5 μl. Each rat was treated with vehicle and SB334867 in counterbalanced order separated by 72 h. Because we dissolved SB334867 differently in this experiment compared with experiment 2, we verified the anorexic effect of this preparation. The rats were returned to standard cages and given 1 wk to acclimate before the food intake test was conducted. Rats were then tested as described in experiment 2, with vehicle and drug conditions counterbalanced and separated by 72 h. Food measurements were taken manually at 30 and 60 min after the onset of the dark cycle.
Experiment 5: effect of 4th-icv OX1R antagonist on hindbrain c-fos.
Food was removed from rats (n = 12) 3 h prior to 4th-icv injections, when six rats received 20 nmol SB334867 and six rats received vehicle in 5 μl. At 90 min postinjections, rats were deeply anesthetized (180 mg/kg ketamine and 30 mg/kg xylazine ip) and transcardially perfused with cold PBS followed by 4% paraformaldehyde. The brains were removed and sunk in 30% sucrose in 10 mM PBS and were then frozen in isopentane at −37°C. Coronal cryostat sections (16 μm) through the caudal brainstem were slide mounted and stored at −80°C.
For each rat, we selected four anatomically matched sections through the hindbrain at the level of the AP and double-labeled for c-Fos and dopamine-β-hydroxylase (DβH), a marker of catecholamine neurons. The primary antibodies were rabbit anti-c-Fos Ab-5 (Calbiochem, San Diego, CA) at 1:5,000 concentration and mouse anti-DβH (Millipore, Billerica, MA) at 1:1,000, both diluted in 0.1% BSA in 10 mM PBS. The secondary antibodies were donkey anti-mouse Alexa Fluor-488 (Invitrogen, Carlsbad, CA) at 1:200, and donkey anti-rabbit Cy3 (Jackson Laboratories, West Grove, PA) at 1:600, both diluted in 0.1% BSA in 10 mM PBS. Sections were rinsed with PBS and then incubated in blocking solution of 5% normal donkey serum in PBS for 2 h at room temperature. Sections were next incubated with the primary antibodies overnight at 4°C. The sections were then rinsed with PBS and incubated with the secondary antibodies simultaneously for 2.5 h at room temperature. DAPI (Sigma-Aldrich) diluted at 0.1 mg/ml in PBS was applied for 15 min, and then sections were rinsed with PBS prior to coverslipping. Negative control sections (primary antibodies left out) showed no staining.
Slides were examined with an Olympus BX41 fluorescence microscope, and monochromatic digital images were acquired with a Retiga EXI Aqua camera and Q-Capture software (Hunt Optics, Pittsburgh, PA). We used Photoshop CS4 (Adobe, San Jose, CA) to adjust contrast, add color, and merge images of DβH and c-Fos immunoreactivity. c-Fos-positive nuclei, DβH-positive cells, and double-labeled cells in the AP and NTS were counted by eye on four sections per rat.
Experiment 6: effect of 4th-icv orexin-A on amylin-induced satiation.
As in experiments 1 and 2, food was restricted starting at 5 h before the start of the dark cycle. One hour before the start of the dark cycle, the rats (n = 12) received 4th-icv injections of either saline or 0.05 nmol orexin-A. Ten minutes before dark onset, the rats received intraperitoneal injections of either saline or amylin (5 μg/kg). After the intraperitoneal injections were completed, food access was returned, and food intake was continuously measured thereafter. All rats received all four conditions (saline/saline; saline/amylin; orexin-A/saline; orexin-A/amylin) in counterbalanced order separated by 48 h.
Experiment 7: proximity of orexin fibers to amylin-responsive neurons.
The procedure for this study was identical to experiment 5, except in this case rats (n = 8) received intraperitoneal injections of either 5 μg/kg amylin (n = 5) or saline (n = 3).
Anatomically matched hindbrain sections from these rats were double-labeled for c-Fos and orexin-A. The primary antibodies were: rabbit anti-c-Fos Ab-5 (Calbiochem, San Diego, CA) diluted 1:5,000 in 0.1% BSA in 10 mM PBS; and goat anti-orexin-A (C-19; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:5,000 in SignalStain Diluent (Cell Signaling Technology, Beverly, MA). The secondary antibodies were donkey anti-rabbit Alexa Fluor-488 (Invitrogen, Carlsbad, CA) diluted 1:200 in 0.1% BSA in 10 mM PBS, and donkey anti-goat Cy3 (Jackson Laboratories, West Grove, PA) diluted 1:600 in 0.1% BSA in 10 mM PBS. The staining procedure was similar to that described above with the following differences. Prior to blocking, the sections were incubated with 0.5% sodium borohydride for 20 min at room temperature. Blocking solution of 5% normal donkey serum in 0.05% Triton-X-100 in PBS was applied for 2.5 h at room temperature. Sections were incubated with the anti-c-Fos primary antibody overnight at 4°C, and then the anti-orexin-A primary antibody for 96 h at 4°C. Negative control sections (primary antibodies left out) showed no staining.
Slides were examined as described above. c-Fos-positive nuclei in the AP NTS were counted by eye on three to four sections per rat. Orexin-A-positive fibers were not quantified.
Meal pattern analysis.
Analysis of meal pattern was performed with BioDaq Viewer Software (Research Diets, New Brunswick, NJ). A meal was defined as the consumption of at least 0.25 g of chow separated by at least 15 min (900 s) from subsequent intake. These criteria were chosen based on previous studies (6, 15, 24). We varied the analytical criteria for minimum meal size from 0.1 to 0.5 g, and intermeal interval from 5 to 20 min, and saw no substantial difference in the results (not shown). Intermeal interval was defined as the time between two meals, and latency to begin the first meal was defined as the number of seconds between the time when food access resumed and the start of the first meal.
Statistical analysis.
Data from our dose response for 4th-icv orexin-A were analyzed by within-subjects one-way ANOVA. The effects of the 4th-icv OX1R antagonist were examined with paired one-way Student's t-tests. The effects of orexin-A and SB334867 on locomotor activity were analyzed by two-way ANOVA with drug and time as factors. The effect of orexin-A pretreatment on amylin-induced satiation was analyzed by within-subjects two-way ANOVA, with orexin-A and amylin as factors. Planned comparisons were conducted with Bonferroni-adjusted t-tests. Between-subjects one-way Student's t-tests were used to assess the effects of amylin on c-Fos in the AP. For all analyses, P values < 0.05 were taken to be statistically significant.
RESULTS
Experiment 1: effect of 4th-icv orexin-A on meal pattern.
All three doses of hindbrain orexin-A significantly increased food intake relative to saline during the first 2 h of the dark phase [F(3, 18) = 6.15, P < 0.05], and intakes did not differ significantly across the three doses (each relative to vehicle, P < 0.05) (see Fig. 1A). However, the pattern of intake observed was dose dependent with significant effects on meal size [F(3, 18) = 4.21, P < 0.05] and intermeal interval [F(3, 18) = 3.54, P < 0.05]. The size of the first meal of the dark phase was significantly elevated after 0.05 and 0.1 nmol orexin-A (each relative to vehicle, P < 0.05), whereas the tendency toward an increase in meal size after 1 nmol orexin-A was not significant (see Fig. 1B). The sizes of the second and subsequent meals were not affected by any dose (data not shown). The 1-nmol dose of orexin-A increased total 2-h intake by decreasing the intermeal interval between the first and second meals. The 0.05- and 0.1-nmol doses of orexin-A tended to increase the interval between the first and second meals, but these increases were not significant, whereas the 1-nmol dose significantly reduced the intermeal interval by 44% (P < 0.05) (see Fig. 1C).
Fig. 1.
Effects of 4th-ventricle intracerebroventricular (4th-icv) injection of orexin-A on total food intake during the first 2 h of the dark cycle (A), first meal size (B), and interval between the first and second meals of the dark cycle (C). IMI, intermeal interval. All data are means ± SE. *P < 0.05 vs. vehicle.
Experiment 2: effect of 4th-icv OX1R antagonist on meal pattern.
Hindbrain ventricular injection of SB334867 significantly reduced the size of the first meal of the dark cycle by 33% relative to the vehicle condition (P < 0.05) (See Fig. 2A). Rats' latency to begin the first meal was not affected by the OX1R antagonist (see Fig. 2B). The sizes of the second and subsequent meals were also similar across conditions (data not shown). However, the interval between the first and second meals was significantly decreased by 4th-icv SB334867 (P < 0.05) (see Fig. 2C). Due to this decreased intermeal interval, rats were able to compensate for the smaller first meal after OX1R antagonist so that total intakes across conditions were similar within 4 h into the dark phase (data not shown).
Fig. 2.
Effects of 4th-icv SB334867 on first meal size (A), latency to begin the first meal (B), and IMI between the first and second meals (C). All data are means ± SE. *P < 0.05 vs. vehicle.
Experiment 3: effect of 4th-icv orexin-A on locomotor activity.
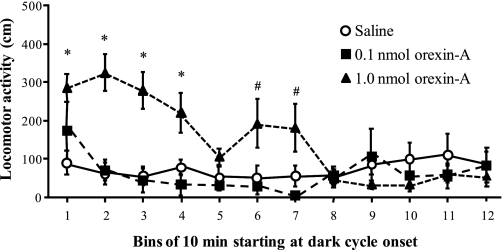
Hindbrain administration of orexin-A increased locomotor activity, but this effect was observed exclusively at the 1-nmol dose (see Fig. 3). There were significant main effects of orexin-A [F(2,14) = 11.73, P < 0.01] and of time [F(11, 77) = 3.72, P < 0.001], and a significant interaction between orexin-A and time [F(22, 154) = 3.17, P < 0.0001]. The 1-nmol dose of orexin-A increased activity relative to saline and 0.1 nmol orexin-A treatment starting at the onset of dark and continuing for 40 min (P < 0.05), with another near-significant elevation in activity between 50 and 70 min after dark onset (P = 0.05). After the 70-min point, there were no further effects of 4th-icv orexin-A. Activity after the 0.1-nmol dose was not significantly different from that observed after saline at any time.
Fig. 3.
Effect of 4th-icv orexin-A on locomotor activity (cm traveled) in 10-min bins across the first 2 h of the dark phase. Data are means ± SE. *1.0 nmol orexin-A significantly differs from saline and 0.1 nmol orexin-A, P < 0.05. #1.0 nmol orexin-A compared with saline and 0.1 nmol, P = 0.05.
Experiment 4: effect of 4th-icv OX1R antagonist on locomotor activity.
Fourth-icv injection of 20 nmol SB334867 had no significant effect on locomotor activity at any point during the first 2 h of the dark phase (see Fig. 4). As a positive control for drug effectiveness in these subjects, we assessed the anorexic effect of SB334867 in a subsequent test. SB334867 significantly reduced 30-min dark-cycle food intake (vehicle mean 3.9 ± 0.2 g reduced to SB334867 mean 2.6 ± 0.6 g, P < 0.05).
Fig. 4.
Effect of 4th-icv SB334867 on locomotor activity (cm traveled) in 10-min bins across the first 2 h of the dark phase. Data are means ± SE.
Experiment 5: effect of 4th-icv OX1R antagonist on hindbrain c-fos.
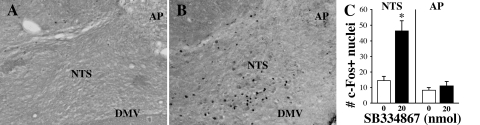
SB334867 significantly increased the mean number of c-Fos-positive nuclei per section within the NTS (P < 0.01), but had no effect on c-Fos immunoreactivity in the AP (see Fig. 5). Within the NTS, we identified a mean of 51.5 ± 0.5 DβH-positive cell bodies per section in those from vehicle-injected rats, and 51.8 ± 1.6 DβH-positive cells in those treated with SB334867. Few NTS DβH-positive cells were also c-Fos-positive regardless of drug condition (vehicle mean 1 ± 0.4; SB334867 mean 4.5 ± 1.5, NS). In the AP, we also observed no effect on mean number of DβH-positive cells (vehicle mean 64.6 ± 2; SB334867 mean 64 ± 2.6) or double-labeled cells (vehicle mean 0.3 ± 0.1; SB334867 mean 0.8 ± 0.4).
Fig. 5.
Effect of 4th-icv SB334867 on c-Fos in dorsomedial hindbrain. Representative images of the nucleus of the solitary tract (NTS) of a vehicle-treated rat (A) and an SB334867-treated rat (B) at approximately −14 mm to bregma. AP, area postrema; DMV, dorsal motor nucleus of the vagus nerve. C: means ± SE c-Fos-positive cells per section through NTS and AP. *P < 0.05 vs. vehicle.
Experiment 6: effect of 4th-icv orexin-A on amylin-induced satiation.
Peripheral amylin treatment significantly reduced the size of the first meal relative to intraperitoneal saline when rats were pretreated with 4th-icv saline (P < 0.001) (see Fig. 6). When intraperitoneal amylin was delivered after 4th-icv orexin-A, however, it failed to reduce meal size relative to either intraperitoneal saline condition. Although intakes after 0.05 nmol orexin-A prior to intraperitoneal saline tended to increase, first meal size for that condition was not significantly different from that seen after 4th-icv saline and intraperitoneal saline. ANOVA showed a significant interaction between orexin-A and amylin [F(1, 11) = 6.97, P < 0.05]. Latency to begin the first meal was not affected by either treatment alone or in combination (means ± SE across all conditions: 21.5 ± 2.9 s). There was a trend for shorter intervals between the first and second meals when rats received vehicle/amylin treatment (means ± SE: 70.7 ± 9.0 min) compared with vehicle/vehicle (means ± SE: 101.8 ± 12.8 min), but this effect failed to reach significance (P = 0.08). Intermeal intervals after orexin-A/vehicle (means ± SE: 112 ± 11.9 min) and orexin-A/amylin (means ± SE: 106.0 ± 15.7 min) did not differ from one another or vehicle/vehicle. The size of the second meal was not affected by any drug treatment (means ± SE across all conditions: 3.3 ± 0.4 g).
Fig. 6.
Effect of amylin (5 μg/kg ip) injection on the size of the first meal of the dark cycle when rats were pretreated with either 4th-icv saline or 0.05 nmol orexin-A. Data are means ± SE. *P < 0.05 vs. all other conditions.
Experiment 7: proximity of orexin-A fibers to amylin-responsive neurons.
Compared with vehicle, amylin treatment significantly increased c-Fos in the AP (saline: means ± SE, 17.7 ± 7.9; amylin means ± SE, 128.8 ± 7.9; P < 0.0001). Amylin also induced c-Fos in the NTS, but to a lesser degree and with greater variation across rats (saline: means ± SE, 8.0 ± 1.4; amylin: means ± SE, 71.6 ± 12.8; P < 0.001). The density of orexin-A-positive fibers varied somewhat across rats, as has been previously reported (24), but the pattern of distribution was consistent and did not appear to be affected by amylin treatment (see Fig. 7). Orexin-A fibers were most dense near the borders of the AP, although fibers were observed throughout the AP and NTS. In amylin-treated rats, c-Fos-positive nuclei were distributed throughout the AP. Orexin-A fibers were sometimes, though not always, observed in close proximity to c-Fos-positive nuclei (see Fig. 7, B, C, E, and F).
Fig. 7.
Images of sections through the AP (approximately −14 mm to bregma) that were double-labeled for orexin-A fibers (green) and intraperitoneal amylin-induced c-Fos (magenta). A and D: sections from 2 different subjects that were treated with saline. B and E: sections from 2 different subjects that were treated with amylin. C and F: higher magnification images of areas in E, containing orexin-A fibers in close proximity to c-Fos-positive nuclei.
DISCUSSION
The results presented here support the hypothesis that hindbrain OX1R activation increases intake by increasing meal size, an effect that may be mediated at least, in part, through an interaction with the satiation hormone amylin. We confirmed that 4th-icv orexin-A increases chow intake and showed that this effect can occur even during the hours immediately after dark onset, when rats spontaneously take their largest meals. Hindbrain orexin-A treatment increased meal size at the lower doses, but affected intermeal interval at the higher dose, similar to the dose response described by Baird et al. (2) for orexin-A's effect on licking for sucrose solutions. Based on the results of experiment 2, however, we suggest that the meal frequency effect at the higher dose is pharmacologic in nature. Hindbrain administration of SB334867 reduced meal size and meal frequency. If endogenous OX1R stimulation promoted intake by causing rats to take meals more frequently, then one would expect to see the antagonist increase the interval between meals. We found the opposite, and speculate that the decrease in intermeal interval reflects a compensatory response that allowed the rats to quickly catch up to typical intakes for vehicle conditions. The effect of the lower doses of 4th-icv orexin-A on first meal size, along with the observation that the OX1R antagonist reduces first meal size, supports the hypothesis that endogenous stimulation of hindbrain OX1R plays a physiologic role in meal size control.
We observed a significant increase in locomotor activity only after 1 nmol orexin-A, but both 0.1 nmol and 1 nmol orexin-A increased food intake significantly over the first 2 h of the dark phase. Therefore, we can conclude that an increase in physical activity is not required for hindbrain orexin-A to increase food intake. However, the locomotor activity effect at the 1-nmol dose of orexin-A may play a role in the pattern of meal-taking behavior observed here. At that dose, we saw no significant increase in first meal size, but the rats took their second meal 50% sooner than under vehicle conditions, such that their total intake over 2 h was significantly elevated. It is possible that rats treated with this dose of 4th-icv orexin-A terminate their first meal before they are truly satiated, due to increased motivation to engage in physical activity, and thus return to take a second meal earlier than they might have otherwise. Alternatively, this dose may produce exclusively pharmacologic effects on both feeding behavior and locomotor activity. Our observation that locomotor activity is not affected by 4th-icv administration of the OX1R antagonist at a dose that significantly reduces meal size suggests that endogenous stimulation of hindbrain receptors does not strongly influence physical activity. This finding also eliminates the possibility that rats treated with 4th-icv SB334867 decreased food intake simply because they are lethargic or sleeping, strengthening the conclusion that hindbrain OX1R are involved in the control of meal size.
OX1R are expressed by neurons in caudal brainstem nuclei that play an important role in the behavioral response to satiation signals, and orexin neurons project to these locations. Zheng et al. (27) showed that gastric nutrient infusion activates hindbrain neurons in close proximity with orexin-A fibers. Here, we present the first behavioral evidence of an interaction between hindbrain orexin-A and a satiation signal. We examined the ability of 4th-icv orexin-A to reduce the anorexic response to amylin, specifically, because both treatments require the AP to affect feeding (2, 13). Our finding that an effective dose of amylin failed to suppress intake when it followed 4th-icv orexin-A supports the suggestion that orexin-A may increase food intake, in part, by reducing the satiating effect of amylin. It is important to note that in this experiment, we used a dose of orexin-A that had no effect when delivered in conjunction with intraperitoneal saline. Thus, the lack of amylin-induced anorexia when rats were pretreated with orexin-A is not simply the expression of an orexigenic effect of orexin-A. On the contrary, the fact that a subthreshold dose of orexin-A blocked amylin's effect on food intake supports the hypothesis that hindbrain OX1R are capable of modulating the response to amylin. A more detailed exploration of the potential interaction between these two systems would require multiple dose combinations of agonists and antagonists for each. Without such analysis, it is not possible to draw firm conclusions about the nature of the interaction that we observed here. Additional research will also be necessary to determine whether orexin-A alters the effectiveness of other satiation signals in addition to amylin. It has been reported that 3rd-icv orexin-A reduces the effectiveness of CCK in the mouse (1), but those data are difficult to interpret because the study did not include a comparison of food intake after orexin-A alone with intake after orexin-A plus CCK under the same food deprivation conditions.
Previous studies of the orexigenic effects of 4th-icv orexin-A have examined food intake during the light cycle, a time when rats are unlikely to spontaneously consume a great deal of food (9, 23). In our experiments, the test compounds were delivered shortly before the onset of dark. We obtained potent drug effects during the first several hours of the dark cycle, a time when rats spontaneously take their largest meals. This appears to be a point of contrast between the effects of forebrain and caudal brainstem OX1R stimulation. Others have shown that lateral-icv orexin-A treatment, at doses that significantly increase intake during the light phase, fails to affect intake when administered immediately prior to the dark phase (22). It is also notable that we observed an increase in locomotor activity with the 1-nmol dose of orexin-A early in the dark phase, when rats are already aroused and active in the absence of any additional stimulation.
The 4th-icv route of administration of orexin-A and OX1R antagonist in our experiments supports the hypothesis that the hindbrain OX1R plays a role in the control of feeding behavior, but the specific nuclei that mediate these effects are not known. OX1R are expressed in neurons of DVC, an area known to play a significant role in the control of feeding (14). Zheng et al. (27) showed that direct injection of 0.4 nmol orexin-A into the DVC during the midlight phase failed to affect standard chow intake, but did increase intake of high-fat diet within 30 min of treatment. In the same study, they reported that a higher dose instead suppressed food intake. These data do not strongly support the suggestion that the DVC is a major mediator of 4th-icv orexin-A's effects, but there are few other hindbrain structures that have a significant density of OX1R expression and are also known to play a role in the control of ingestive behavior. Further research will be necessary to clarify the nuclei that are most relevant for hindbrain orexin-A's intake-stimulatory effect.
The specific nuclei that mediate the observed interaction between 4th-icv orexin-A and intraperitoneal amylin also remain to be determined. Lesions of the AP prevent the satiating effect of amylin and also block the orexigenic effect of orexin-A (2, 13). Based on these findings, the AP is an obvious candidate for convergence of these two treatments. Our finding that orexin-A fibers are located in close proximity to some AP neurons that are activated by amylin supports the suggestion that under normal physiologic conditions, an interaction could occur at this site. However, it is possible that amylin and orexin-A act on distinct populations of AP neurons and that an interaction occurs in other nearby neurons or at sites downstream of the AP. Moreover, the AP may not be the primary site through which hindbrain orexin-A affects food intake. Our immunohistochemical results confirm previous findings that orexin-A fibers run through the AP (16, 27), and it has been reported that orexin-A can cause depolarization of isolated AP neurons (26), but this does not rule out the possibility that the involvement of the AP in orexin-A-induced hyperphagia is secondary to orexin action in a different brain nucleus. Orexin-A fibers are present in NTS (16, 27) and both orexin-A and amylin induce c-Fos in NTS neurons (12, 27). We showed here that 4th-icv OX1R antagonist treatment induced c-Fos exclusively in noncatecholaminergic cells of the NTS. Rats in this c-Fos study had no access to food, so this pattern of neuronal activation may differ from that observed when satiation signals are elevated by recent ingestion. However, this effect of SB334867 suggests that endogenous orexin-A exerts tonic inhibition on this subset of NTS cells. Although this does not rule out a role for the AP or other nuclei, the effect of hindbrain OX1R highlights a role for NTS in mediating the effects of endogenous OX1R stimulation and supports the hypothesis that interactions between OX1R and gut-derived satiation signals could occur within this nucleus.
Our understanding of interactions between other feeding-relevant signals has been facilitated by examinations of the neuronal c-Fos response to combined treatment. For example, the adiposity hormone leptin enhances the anorexic response to CCK and also significantly increases CCK-induced c-Fos expression in the NTS (5, 15). If dorsal hindbrain neuronal activation is linked to amylin's effect on feeding, then one might hypothesize that 4th-icv orexin-A reduces amylin-induced c-Fos expression. However, any test of this hypothesis is limited by the fact that both amylin and 4th-icv orexin-A treatments induce c-Fos in some of the same caudal brainstem nuclei (12, 27). It would be impossible to clearly assess which neurons were activated by which stimulus after combined treatment. It will be important to identify markers of neuronal activation that are specific to one or the other compound to determine the neural pathways through which orexin-A and amylin may interact.
Perspectives and Significance
Several recent studies have identified a role for orexin neurons in reward-motivated behavior (21). For example, Choi et al. (3) reported that 3rd-icv orexin-A treatment increased progressive ratio responding for food and that orexin neurons were activated by an environmental cue that predicts the availability of a chocolate treat. This study did not identify projection sites of cue-responsive orexin neurons, but it is possible that a subset of these neurons project to the caudal brainstem. Our data suggest that activation of such a projection would impair satiation. We therefore speculate that hindbrain-projecting orexin neurons could link the detection of a food-related cue with reduced satiation and therefore play a role in animals' ability to consume significant amounts of food in response to the cue.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-078779 (to D. L. Williams).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.M.P., N.L., K.K., A.M.D., and R.S. performed experiments; E.M.P., A.M.D., R.S., J.M.O., and D.L.W. analyzed data; E.M.P., R.S., J.M.O., and D.L.W. interpreted results of experiments; E.M.P. and D.L.W. prepared figures; E.M.P. and D.L.W. drafted manuscript; E.M.P. and D.L.W. edited and revised manuscript; E.M.P., N.L., K.K., A.M.D., R.S., J.M.O., and D.L.W. approved final version of manuscript; J.M.O. and D.L.W. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Benjamin Calisch, Alexis Pizzi, and Kathryn Samuel for their technical assistance.
REFERENCES
- 1. Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Orexin reverses cholecystokinin-induced reduction in feeding. Diabetes Obes Metab 4: 399–401, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, Grigg LA. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology 150: 1202–1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167: 11–20, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res 842: 473–477, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol Regul Integr Comp Physiol 276: R1545–R1549, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept 104: 153–159, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96: 45–51, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JR. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20: 1099–1105, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103: 777–797, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept 104: 27–32, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Lutz TA. Amylinergic control of food intake. Physiol Behav 89: 465–471, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 19: 309–317, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Potes CS, Turek VF, Cole RL, Vu C, Roland BL, Roth JD, Riediger T, Lutz TA. Noradrenergic neurons of the area postrema mediate amylin's hypophagic action. Am J Physiol Regul Integr Comp Physiol 299: R623–R631, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci 23: 10084–10092, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213: 451–452, 1981 [DOI] [PubMed] [Google Scholar]
- 20. Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev 20: 41–46, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Thompson JL, Borgland SL. A role for hypocretin/orexin in motivation. Behav Brain Res 217: 446–53, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Thorpe AJ, Mullett MA, Wang C, Kotz CM. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am J Physiol Regul Integr Comp Physiol 284: R1409–R1417, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Thorpe AJ, Teske JA, Kotz CM. Orexin A-induced feeding is augmented by caloric challenge. Am J Physiol Regul Integr Comp Physiol 289: R367–R372, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams TD, Chambers JB, May OL, Henderson RP, Rashotte ME, Overton JM. Concurrent reductions in blood pressure and metabolic rate during fasting in the unrestrained SHR. Am J Physiol Regul Integr Comp Physiol 278: R255–R262, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Yang B, Ferguson AV. Orexin-A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J Neurosci 22: 6303–6308, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol 485: 127–142, 2005 [DOI] [PubMed] [Google Scholar]