Abstract
Our recent studies employing HPLC-tandem mass spectrometry to analyze venous perfusate from isolated, perfused kidneys demonstrate that intact kidneys produce and release into the extracellular compartment 2′,3′-cAMP, a positional isomer of the second messenger 3′,5′-cAMP. To our knowledge, this represents the first detection of 2′,3′-cAMP in any cell/tissue/organ/organism. Nuclear magnetic resonance experiments with isolated RNases and experiments in isolated, perfused kidneys suggest that 2′,3′-cAMP likely arises from RNase-mediated transphosphorylation of mRNA. Both in vitro and in vivo kidney experiments demonstrate that extracellular 2′,3′-cAMP is efficiently metabolized to 2′-AMP and 3′-AMP, both of which can be further metabolized to adenosine. This sequence of reactions is called the 2′,3′-cAMP-adenosine pathway (2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine). Experiments in rat and mouse kidneys show that metabolic poisons increase extracellular levels of 2′,3′-cAMP, 2′-AMP, 3′-AMP, and adenosine; however, little is known regarding the pharmacology of 2′,3′-cAMP, 2′-AMP, and 3′-AMP. What is known is that 2′,3′-cAMP facilitates activation of mitochondrial permeability transition pores, a process that can lead to apoptosis and necrosis, and inhibits proliferation of vascular smooth muscle cells and glomerular mesangial cells. In summary, there is mounting evidence that at least some types of cellular injury, by triggering mRNA degradation, engage the 2′,3′-cAMP-adenosine pathway, and therefore this pathway should be added to the list of biochemical pathways that produce adenosine. Although speculative, it is possible that the 2′,3′-cAMP-adenosine pathway may protect against some forms of acute organ injury, for example acute kidney injury, by both removing an intracellular toxin (2′,3′-cAMP) and increasing an extracellular renoprotectant (adenosine).
Keywords: 2′-AMP, 3′-AMP, kidney
Discovery of 2′,3′-cAMP in a Biological System
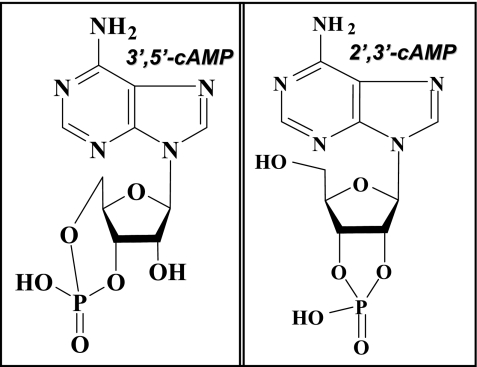
liquid chromatography-tandem mass spectrometry (LC-MS/MS) combines the resolving power of HPLC with the detection sensitivity and specificity of tandem mass spectrometry (MS/MS). With MS/MS, a precursor ion is selected for further fragmentation and a product ion is selectively detected and quantified, an analytic procedure known as selected reaction monitoring (SRM). While investigating, using LC-MS/MS in the SRM mode, the release of 3′,5′-cAMP from isolated, perfused rat kidneys, we noted an unexpected chromatographic peak that was not 3′,5′-cAMP (82). Despite the risks of being distracted by “analytic trash,” this serendipitous observation was too intriguing to ignore. So, we deviated from our original experimental plan and instead focused on determining the identity of this unknown substance. We knew that the mass-to-charge ratio (m/z) of the precursor ion for the unknown was the same as the m/z for the precursor ion of 3′,5′-cAMP and that the m/z for the product ion for the unknown was the same as the m/z of the product ion for 3′,5′-cAMP (hence the SRM signal designed to detect only 3′,5′-cAMP also detected the endogenous unknown). We concluded therefore that the unknown must be structurally quite similar to 3′,5′-cAMP. However, the HPLC retention time (RT) of the unknown molecule on an Agilent Zorbax eclipse XDB-C-18 column (3.5-μm beads; 2.1 × 100 mm; Agilent Technologies, Santa Clara, CA) was unequivocally shorter, with complete baseline separation between the unknown (RT = 2.9 min) and authentic 3′,5′-cAMP (RT = 6.3 min) (82), thus definitively differentiating the unknown from 3′,5′-cAMP. Guided by parsimony, we hypothesized that the mystery substance was 2′,3′-cAMP, structurally the simplest possible positional isomer of 3′,5′-cAMP consistent with the SRM data (Fig. 1). This hypothesis was confirmed by demonstrating that the unknown substance had the same RT and mass spectral properties as authentic 2′,3′-cAMP (82). Because to our knowledge this was the first confirmed detection of 2′,3′-cAMP from any cell/tissue/organ/organism, we decided to pursue further the significance of 2′,3′-cAMP. Meanwhile, using quadrupole time-of-flight mass spectrometry, Pabst and coworkers (80) detected 2′,3′-cAMP in tobacco plants, thus confirming the existence of 2′,3′-cAMP in living systems and suggesting that 2′,3′-cAMP is, on the evolutionary time scale, a very ancient biological molecule.
Fig. 1.
Except for the attachment of one of the phosphodiester bonds, the chemical structure of 2′,3′-cAMP (right) otherwise is like that of 3′,5′-cAMP (left).
A Brief Digression: the 3′,5′-cAMP-Adenosine Pathway
In 1991, we proposed a transmembrane negative feedback mechanism controlling renin release from juxtaglomerular cells (42) that is now known as the first explicit instantiation of the extracellular 3′,5′-cAMP-adenosine pathway (Fig. 2). The extracellular 3′,5′-cAMP-adenosine pathway involves 1) intracellular production of 3′,5′-cAMP by adenylyl cyclases; 2) active extrusion of 3′,5′-cAMP to the extracellular compartment by 3′,5′-cAMP pumps (e.g., MRP4) (11); 3) metabolism of extracellular 3′,5′-cAMP to extracellular 5′-AMP by ecto-3′,5′-cAMP-3′-phosphodiesterases; and 4) metabolism of 5′-AMP to adenosine by ecto-5′-AMPases (e.g., CD73) (43, 50). [Note: in this review, we use the plural form for protein names because it is possible that a given enzymatic/transport process is mediated by multiple distinct proteins or multiple related protein isoforms and that the involved proteins may vary depending on the cellular environment. However, whether single or multiple proteins are involved is unknown.]
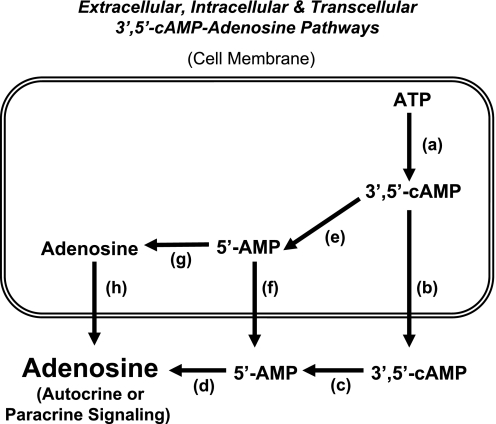
Fig. 2.
Schematic summarizes the biochemical steps in the 3′,5′-cAMP-adenosine pathways. a: Production of 3′,5′-cAMP from ATP catalyzed by adenylyl cyclases (many isoforms are known to exist). b: Active transport of 3′,5′-cAMP into the extracellular compartment by 3′,5′-cAMP pumps (e.g., MRP4 and MRP5). c: Metabolism of extracellular 3′,5′-cAMP to 5′-AMP by ecto-3′,5′-cAMP-3′-phosphodiesterses. d: Metabolism of extracellular 5′-AMP to adenosine by ecto-5′-AMPases (e.g., CD73). e: Metabolism of intracellular 3′,5′-cAMP to 5′-AMP by endo-3′,5′-cAMP-3′-phosphodiesterses (many isoforms are known to exist). f: Active transport of 5′-AMP into the extracellular compartment by 5′-AMP pumps. g: Dephosphorylation of 5′-AMP to adenosine by endo-5′-AMPases (e.g., cytosolic 5′-nucleotidase). h: Efflux of adenosine to the extracellular compartment mediated by equilibrative nucleoside transporters (many isoforms are known to exist). Extracellular 3′,5′-cAMP-adenosine pathway = a → b → c → d; transcellular 3′,5′-cAMP-adenosine pathway = a → e → f → d; intracellular 3′,5′-cAMP-adenosine pathway = a → e → g → h.
Studies confirm the existence of the extracellular 3′,5′-cAMP-adenosine pathway in a number of biological systems including intact kidneys (46, 55, 68–70), preglomerular microvessels (45) and preglomerular vascular smooth muscle cells (PGVSMCs) (47), glomerular mesangial cells (GMCs) (24), proximal tubules and proximal tubular epithelial cells (56), collecting ducts and collecting duct epithelial cells (49), cardiac fibroblasts (22, 23), aortic vascular smooth muscle (21, 25, 27), pial microvessels (40), skeletal muscle (13), ileum (36), adipocytes (73), and a catecholaminergic neuronal cell line (20). The extracellular 3′,5′-cAMP-adenosine pathway may also provide a chemical connection by which the liver can affect the kidney (48), and very recent studies by Kuzhikandathil et al. (61) support the concept that the extracellular 3′,5′-cAMP-adenosine pathway is also involved in regulating renal D1 dopamine receptor expression.
Although most experimental evidence speaks to the existence of the extracellular 3′,5′-cAMP-adenosine pathway, it is also conceivable that a transcellular 3′,5′-cAMP pathway may function in which 3′,5′-cAMP is metabolized intracellularly to 5′-AMP (by a variety of endo-3′,5′-cAMP-3′-phosphodiesterases), followed by release of 5′-AMP to the extracellular compartment with subsequent metabolism to adenosine (Fig. 2). Also conceivable is an intracellular 3′,5′-cAMP-adenosine pathway in which 3′,5′-cAMP is metabolized inside cells to 5′-AMP, and intracellular 5′-AMP is converted to adenosine by endo-5′-AMPases. Intracellular adenosine would then exit to the cell surface escorted by equilibrative nucleoside transporters (Fig. 2). However, work by Eltzschig and colleagues (29, 72) clearly demonstrates that hypoxia/inflammation induces adenosine biosynthesis mostly in the extracellular compartment via metabolism of released adenine nucleotides to 5′-AMP (mediated by CD39) followed by metabolism of 5′-AMP to adenosine (mediated by CD73). This suggests that intracellular production of adenosine followed by transport to the extracellular compartment is unimportant compared with extracellular production of adenosine, at least when hypoxia/inflammation is the stimulus. Nonetheless, it is possible that with some stimuli intracellular production of adenosine is important. Along these lines, the reader should be alerted to the fact that adenosine biosynthesis can occur through several mechanisms that are engaged by different stimuli and produce adenosine in different compartments. For example, S-adenosylhomocysteine hydrolase produces intracellular adenosine by converting S-adenosylhomocysteine to adenosine plus homocysteine (18, 19, 58, 66).
Another cAMP-Adenosine Pathway: Discovery of the 2′,3′-cAMP-Adenosine Pathway
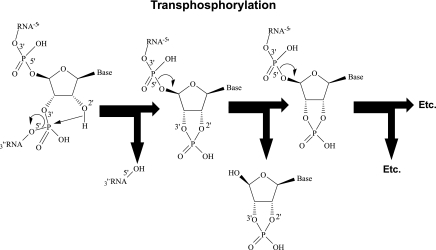
Based on our work on the extracellular 3′,5′-cAMP-adenosine pathway, the thought occurred to us that in addition to the extracellular 3′,5′-cAMP-adenosine pathway, an extracellular 2′,3′-cAMP-adenosine pathway (Fig. 3) might exist (54). This hypothesis was further motivated by the following facts. 1) Stimulation of mRNA turnover involves the actions of multiple RNases that first cleave the phosphodiester bonds in the polyadenine (poly-A) tail of mRNA, followed by 5′-decapping and then degradation of the mRNA (96). 2) mRNA is degraded by the action of ribonucleases (RNases) that catalyze the hydrolysis of the P-O5′ bond of RNA. Importantly, this reaction precedes in two steps, i.e., transphosphorylation of RNA to produce 2′,3′-cyclic phosphodiester intermediates, followed by hydrolysis of the cyclic intermediates to form 3′-phosphomonoesters. Elegant 31P-nuclear magnetic resonance (NMR) spectroscopy experiments by Thompson et al. (89) indicate that only 0.1% of RNA substrates are both transphosphorylated and hydrolyzed before dissociating from RNases. In other words, 99.9% of the RNA substrates are released from the RNase as 2′,3′-cyclic phosphodiester intermediates. Thus 2′,3′-cAMP is most likely formed from adenine nucleotide residues in mRNA by transphorylation reactions catalyzed by RNases (Fig. 4) (89). 3) Because of the large number of adenine nucleotide residues in mRNA (particularly in the poly-A tail) (3), mRNA turnover could produce large quantities of 2′,3′-cAMP per molecule of mRNA degraded. 4) Active transporters, such as MRP4 and MRP5, rapidly transport nucleosides and nucleotides (including cyclic nucleotides) of diverse chemical structures and would likely extrude 2′,3′-cAMP from the cell (8, 15, 60, 91). 5) There exist various enzymes that could possibly serve as ecto-2′,3′-cAMP-3′-phosphodiesterases, ecto-2′,3′-cAMP-2′-phosphodiesterases, ecto-2′-AMPases, and ecto-3′-AMPases to hydrolyze extracellular 2′,3′-cAMP to 2′-AMP, extracellular 2′,3′-cAMP to 3′-AMP, extracellular 2′-AMP to adenosine, and extracellular 3′-AMP to adenosine, respectively. For example 2′,3′-cyclic nucleotide-3′-phosphodiesterase is a membrane-bound enzyme that in vitro can convert 2′,3′-cAMP to 2′-AMP (88, 90, 93), and some RNases, such as ptRNase 1, are secreted by cells into the extracellular compartment and can hydrolyze 2′,3′-cAMP to 3′-AMP (86, 87). Finally, there are ecto-enzymes, for example alkaline phosphatases, that could possibly function to process extracellular 2′-AMP and 3′-AMP to adenosine.
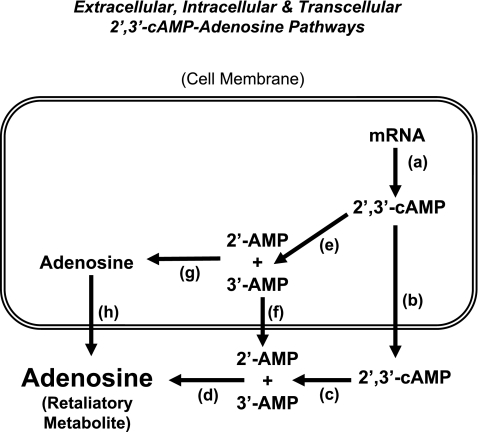
Fig. 3.
Schematic summarizes the biochemical steps in the 2′,3′-cAMP-adenosine pathways. a: Production of 2′,3′-cAMP from mRNA catalyzed by RNases (many isoforms are known to exist). b: Active transport of 2′,3′-cAMP into the extracellular compartment by 2′,3′-cAMP pumps (e.g., MRP4 and MRP5). c: Metabolism of extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP by ecto-3′,5′-cAMP-3′-phosphodiesterses (e.g., extracellular CNPase) and ecto-3′,5′-cAMP-2′-phosphodiesterses (e.g., extracellular RNases), respectively. d: Metabolism of extracellular 2′-AMP and 3′-AMP to adenosine by ecto-2′-AMPases and ecto-3′-AMPases, respectively. e: Metabolism of intracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP by endo-2′,3′-cAMP-3′-phosphodiesterses (e.g., intracellular CNPase) and endo-2′,3′-cAMP-2′-phosphodiesterses (e.g., intracellular RNases), respectively. f: Active transport of 2′-AMP/3′-AMP into the extracellular compartment by 2′-AMP/3′-AMP pumps. g: Dephosphorylation of 2′-AMP and 3′-AMP to adenosine by endo-2′-AMPases and endo-3′-AMPases. h: Efflux of adenosine to the extracellular compartment mediated by equilibrative nucleoside transporters (many isoforms are known to exist). Extracellular 2′,3′-cAMP-adenosine pathway = a → b → c → d; transcellular 2′,3′-cAMP-adenosine pathway = a → e → f → d; intracellular 2′,3′-cAMP-adenosine pathway = a → e → g → h.
Fig. 4.
Proposed biochemical mechanism for the production of 2′,3′-cAMP by RNase-mediated transphosphorylation of mRNA.
We first tested the concept of an extracellular 2′,3′-cAMP-adenosine pathway in isolated, perfused rat kidneys (54). In these experiments, we noted that arterial infusions of 2′,3′-cAMP (30 μmol/l) increased the mean renal venous secretions of 3′-AMP, 2′-AMP, and adenosine by 3,400-, 26,000-, and 53-fold, respectively. Also, arterial infusions of 2′-AMP and 3′-AMP increased renal venous secretion of adenosine similar to that achieved by 5′-AMP. This is noteworthy because 5′-AMP is considered the most important precursor of endogenous adenosine, and these experiments show that 2′-AMP and 3′-AMP can also be processed to adenosine. To determine whether our observations translate to the in vivo situation, we infused 2′,3′-cAMP, 2′-AMP, 3′-AMP, and 5′-AMP into the renal artery of anesthetized rats (54). In these experiments, 2′,3′-cAMP increased urinary excretion of 2′-AMP, 3′-AMP, and adenosine, and infusions of 2′-AMP and 3′-AMP increased urinary excretion of adenosine as efficiently as did 5′-AMP (again demonstrating that 5′-AMP is not the only AMP capable of generating adenosine).
Experiments in intact tissues suggest that energy depletion in tissues stimulates the degradation of mRNA (1, 2, 5), and as noted above, studies with isolated RNases suggest that 2′,3′-cAMP is a product of mRNA degradation (89). Therefore, we examined the effects of energy depletion on the renal venous secretion of 2′,3′-cAMP and its downstream metabolites. In this regard, we treated isolated, perfused rat kidneys with two metabolic poisons, i.e., iodoacetate plus 2,4-dinitrophenol for 30 min. We observed that renal injury with metabolic inhibitors increased the mean renal venous secretion of 2′,3′-cAMP, 3′-AMP, 2′-AMP, and adenosine by 29-, 16-, and 4.2-fold, respectively. To further test the hypothesis that 2′,3′-cAMP in intact kidneys arises from mRNA degradation, we treated isolated, perfused rat kidneys with rapamycin, a drug that activates mRNA turnover via the mammalian target of rapamycin pathway (4, 7, 39). In this experimental series, rapamycin caused a time-related and large (∼1,000% of basal) increase in renal venous 2′,3′-cAMP secretion while inhibiting the renal secretion of 3′,5′-cAMP (∼50% of basal) (82).
We also tested whether isolated, perfused mouse kidneys express an extracellular 2′,3′-cAMP-adenosine pathway (51). In this regard, we administered into the renal artery 2′,3′-cAMP and 3′,5′-cAMP to isolated, perfused mouse kidneys and measured renal venous secretion rates of 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenosine, and inosine. Arterial infusions of 2′,3′-cAMP increased the mean venous secretion of 2′-AMP (390-fold), 3′-AMP (497-fold), adenosine (18-fold), and inosine (adenosine metabolite; 7-fold), but did not alter 5′-AMP secretion. In contrast, infusions of 3′,5′-cAMP did not affect venous secretion of 2′-AMP or 3′-AMP, but increased secretion of 5′-AMP (5-fold), adenosine (17-fold), and inosine (6-fold). Dividing the concentrations of cAMPs in the renal venous perfusate by the concentrations in the renal arterial perfusate revealed that ∼95% of arterial 2′,3′-cAMP was removed from the vascular compartment during a single pass through the mouse kidney, whereas only ∼88% of 3′,5′-cAMP was extracted. This suggests that the kidney more efficiently metabolizes extracellular 2′,3′-cAMP than extracellular 3′,5′-cAMP. As with the rat kidney, in the isolated, perfused mouse kidney, energy depletion with metabolic inhibitors increased the secretion of 2′,3′-cAMP (8-fold), 2′-AMP (4-fold), 3′-AMP (4-fold), 5′-AMP (3-fold), adenosine (2-fold), and inosine (7-fold), but did not increase 3′,5′-cAMP secretion.
Taken together, the results of the aforementioned studies support the existence of an extracellular 2′,3′-cAMP-adenosine pathway in kidneys. However, this pathway may exist in a broad array of cells/tissues/organs. For example, our most recent studies demonstrate efficient metabolism of 2′,3′-cAMP, 2′-AMP, and 3′-AMP to downstream purines in preglomerular vascular smooth muscle cells (53), glomerular mesangial cells (53), aortic vascular smooth muscle cells (52), coronary artery vascular smooth muscle cells (52), microglia (92), and astrocytes (92). Thus the extracellular 2′,3′-cAMP-adenosine pathway may exist not only in kidneys but in most cells/tissues/organs. Although the data to date support primarily the extracellular 2′,3′-cAMP-adenosine pathway, other configurations of the 2′,3′-cAMP-adenosine pathway are possible, for example, the transcellular and intracellular 2′,3′-cAMP-adenosine pathways (Fig. 3).
What enzymes mediate the 2′,3′-cAMP-adenosine pathway? Although the answer to this important question is currently unknown, one candidate is 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase). Importantly, CNPase is the third most abundant protein in myelin sheaths in the brain. Although neurologists have long viewed this protein as merely a structural protein and convenient marker for oligodendrocytes, the fact is that CNPase, at least in vitro, converts 2′,3′-cAMP to 2′-AMP and is the only known enzyme that promotes this reaction (85, 90, 93). Although there is only a single gene for CNPase, there are two CNPase mRNAs that are generated by alternative splicing (85, 88, 90, 93). One of these gives rise to the CNPase I isoform of the enzyme, whereas the other has two potential translation initiation sites and can give rise to both isoforms of the enzyme. CNPase II differs from CNPase I only in having an additional 20 amino acids on the N terminus. The function of CNPase and its isoforms remains enigmatic. Both isoforms are modified by isoprenylation at their C terminus and have multiple closely spaced hydrophobic domains, and these features result in the attachment of CNPase to membranes. The recent availability of CNPase knockout mice will permit an assessment of the role of CNPase in the 2′,3′-cAMP-adenosine pathway in the kidney as well as other organ systems, a line of research being actively pursued in our laboratory.
Although CNPase can metabolize 2′,3′-cAMP to 2′-AMP, it does not metabolizes 2′,3′-cAMP to 3′-AMP. Importantly, a large array of 2′,3′-cyclic nucleotide-2′-phosphodiesterases have been identified in microorganisms (for a listing, see http://www.brenda-enzymes.org/php/result_flat.php4?ecno=3.1.4.16); however, these enzymes were identified using in vitro assay systems with synthetic substrates. Recent experiments by Rao et al. (81) identify six phosphohydrolases from microorganisms that metabolize 2′,3′-cAMP mostly to 3′-AMP. This near-exclusive production of 2′,3′-cAMP to 3′-AMP is likely related to the lower activation energy of the P-O2′ bond of 2′,3′-cAMP. The main point is that probably most organisms express a wide array of 2′,3′-cyclic nucleotide-2′-phosphodiesterases that can serve as ecto- and/or endo-2′,3′-cAMP-2′-phosphodiesterases that metabolize extracellular and intracellular 2′,3′-cAMP to 3′-AMP.
Little is known regarding the identity of either extracellular or intracellular 2′-AMPases or 3′-AMPases that metabolize extracellular and intracellular 2′-AMP and 3′-AMP to adenosine. What is known is that ecto-2′-AMPases and ecto-3′-AMPases are distinct from CD73 (also called ecto-5-nucleotidase) that functions as an ecto-5′-AMPase (i.e., converts extracellular 5′-AMP to adenosine). This conclusion is based on the observations that pharmacological inhibition of CD73 does not alter the metabolism of extracellular 2′-AMP and 3′-AMP to adenosine in PGVSMCs (53), GMCs (53), aortic vascular smooth muscle cells (52), coronary artery vascular smooth muscle cells (52), microglia (92) or astrocytes (92), and the metabolism of extracellular 2′,3′-cAMP to adenosine is similar in kidneys from CD73 knockout vs. wild-type mice (51).
Pharmacology and Biochemistry of 2′,3′-cAMP, 2′-AMP, and 3′-AMP: A Brief Literature Overview
Very little is known about the pharmacology and biochemistry of 2′-AMP, 3′-AMP, and 2′,3′-cAMP, and most of what is known comes from work performed decades ago. In 1963, Denatale et al. (17) reported that in cats intravenous boluses of 2′-AMP, 3′-AMP, and 2′,3′-cAMP caused a delayed reduction in arterial blood pressure (reaching a maximum in ∼5 min) followed by recovery over 20–30 min, and the hypotensive response was accompanied by tachycardia (presumably reflex induced). In the guinea pig perfused hindlimb preparation, intra-arterial delivery of 2′-AMP, 3′-AMP, and 2′,3′-cAMP caused vasodilation, yet there was no effect of these purines on sympathetic or parasympathetic responses in the cat nictitating membrane preparation. Wahn et al. (94) in 1975 reported that 2′,3′-cAMP did not affect the induction of neural differentiation in cultures of amphibian epidermis, and in 1975 Garrison et al. (35) observed that 2′,3′-cAMP had no effect on glucose production or CO2 fixation in liver cells from fasted rats. In 1976, Fleming et al. (31) noted that only extremely high concentrations (>10 mM) of 2′,3′-cAMP inhibited “to varying degrees” the formation of granulocyte-macrophage colonies in cultures of normal mouse bone marrow, and Hartzell (38) in 1979 found that unlike adenosine, 2′,3′-AMP did not hyperpolarize pacemaker cells in the frog heart.
A few additional reports appeared in the 1980s. In this regard, in 1980 Furh et al. (32) showed that 2′,3′-cAMP inhibited leucine incorporation in a mouse lymphoma cell line, but provided no explanation of the mechanism or physiological significance of this finding. Ichikawa et al. (41) in 1980 reported that adenosine and 5′-AMP, but not 3′-AMP, inhibited growth of mastocytoma P-815 cells. Willemot et al. (95) in 1981 observed that 2′,3′-cAMP inhibited neurotransmission in the rat vas deferens, and because the effect was not abolished by adenosine deaminase, the authors concluded that 2′,3′-cAMP acted directly on a receptor independent of metabolism to adenosine. In 1981, Lee et al. (65) showed that 2′-AMP decreased evoked potentials in the rat hippocampal slice preparation; however, the effects were only partially antagonisted by adenosine deaminase, and the authors concluded that 2′-AMP per se can modulate synaptic transmission. Fiszman et al. (30) in 1984 reported that in the rat vas deferens 2′,3′-cAMP inhibited the motor response to nerve stimulation and the effect was increased by dipyridamole and impaired by theophylline, and in 1986, Oshima et al. (79) reported that 2′,3′-cAMP had effects on damselfish motil iridophores that were blocked by theophylline.
The 1990s did little to clarify the role of 2′,3′-cAMP, 2′-AMP, or 3′-AMP. In 1992, Nakane et al. (74) reported that 2′,3′-cAMP did not vasodilate the isolated, perfused canine coronary artery. However, Bushfield et al. (9) in 1990 demonstrated that 3′-AMP is found in tissues and inhibits adenylyl cyclase via the P-site. Importantly, the presence of 3′-AMP in tissues has been confirmed by Fujimori and colleagues (33, 34, 71).
Physiological Role of the 2′,3′-cAMP-Adenosine Pathway: Possible Mechanism for Protecting Against Tissue Injury
Even taken together, the world's literature on 2′,3′-cAMP, 2′-AMP, or 3′-AMP is scant, incomplete, and inconsistent. Given the lack of information regarding the pharmacology and biochemistry of 2′,3′-cAMP, 2′-AMP, or 3′-AMP, what can be said about the possible physiological roles of the 2′,3′-cAMP-adenosine pathway? To answer this question, it is enlightening to consider that tissue injury is well known to activate mRNA degradation (1, 2, 5, 10, 12), and mRNA breakdown is one of the earliest events during apoptosis (16). Therefore, tissue injury would be expected to activate the 2′,3′-cAMP-adenosine pathway. A recent benchmark study by Azarashvili et al. (6) shows beyond a reasonable doubt that 2′,3′-cAMP opens mitochondrial permeability transition pores (MPTPs), and it is well known that the opening of MPTPs leads to apoptosis and necrosis (59). It is also well known that extracellular adenosine protects cells/tissues/organs from injury/insults. This is especially true for the kidney. For example, landmark studies by Okusa and coworkers (14, 75–78) demonstrate that adenosine, via A2A receptors, protects the kidney from ischemia-reperfusion injury (and other forms of injury) primarily by anti-inflammatory actions mediated by A2A receptors. In addition, recent studies show that both the adenosine A2B receptor (37) and A1 receptor (57, 62–64) protect the kidney from ischemia-reperfusion injury. Therefore, intracellular metabolism of 2′,3′-cAMP to 2′-AMP and 3′-AMP followed by conversion of 2′-AMP and 3′-AMP to adenosine followed by export of intracellular adenosine would be protective because these reactions would 1) reduce the intracellular levels of an intracellular toxin (2′,3′-cAMP) and 2) increase extracellular levels of a retaliatory/protective metabolite (adenosine). Similarly, active transport of extracellular 2′,3′-cAMP to the cell surface followed by extracellular metabolism of 2′,3′-cAMP to corresponding AMPs and to adenosine would also help maintain low intracellular levels of 2′,3′-cAMP and high extracellular levels of adenosine. Thus the 2′,3′-cAMP-adenosine pathway may protect the kidney, as well as many other organ systems, from injury.
Recent studies by Eltzschig and colleagues (28, 29, 83) demonstrate that hypoxia and inflammation trigger increases in extracellular adenosine, which reduces inflammation through A2B receptors, due to direct interaction of adenosine with A2B receptors and perhaps indirectly through netrin-1, which also activates A2B receptors. Importantly, as reviewed by Sakar and Fisher (84), inflammatory cytokines induce a number of RNA degradation enzymes that by degrading viral RNA are involved in innate immunity. For example, polynucleotide phosphorylase is a type I interferon-inducible 3′,5′ exoribonuclease. It is conceivable, therefore, that hypoxia/inflammation would activate the extracellular 2′,3′-cAMP-adenosine pathway, and this hypothesis merits exploration.
Interestingly, the increase in serum creatinine induced by renal ischemia-reperfusion injury is attenuated, rather than enhanced, in mice null for either CD39 or CD73 (67), and renal histopathology induced by ischemia-reperfusion injury is not changed in CD39 knockout mice and is actually improved in CD73 knockout mice (67). CD39 (converts ATP/ADP to 5′-AMP) and CD73 (converts 5′-AMP to adenosine) are thought to be the primary mediators of adenosine production. However, the findings that renal adenosine is protective yet knockdown of CD39 or CD73 does not worsen outcome suggest that alternative metabolic pathways are more important in forming adenosine during renal ischemia-reperfusion, and the renal 2′,3′-cAMP-adenosine pathway is a possible candidate.
In addition to possibly protecting against acute kidney injury, it is also conceivable that the renal 2′,3′-cAMP-adenosine may guard against glomerulosclerosis induced by diseases such as diabetes and hypertension and protect against chronic kidney disease induced by previous acute kidney injuries. Long term, many kidney injuries result in abnormal proliferation of PGVSMCs and GMCs, which contributes to glomerulosclerosis and compromises renal function (26). Importantly, recent studies show that 2′,3′-cAMP potently and efficaciously inhibits proliferation of both PGVSMCs and GMCs (44). Also, 2′,3′-cAMP inhibits aortic vascular smooth muscle cell proliferation and proliferation of coronary artery vascular smooth muscle cells (52). These effects of 2′,3′-cAMP are partially mediated by metabolism to 2′-AMP and 3′-AMP with subsequent metabolism of the AMPs to adenosine, which then acts on A2B receptors to inhibit proliferation of vascular smooth muscle cells and GMCs. However, 2′,3′-cAMP per se appears to exert antiproliferative effects on these cell types independently of 2′-AMP, 3′-AMP, adenosine, or adenosine receptors.
The End of the Beginning: And a Lot More To Do!
With regard to understanding the 2′,3′-cAMP-adenosine pathway, this brief review is not the end, or even the beginning of the end, of the story; rather, this review marks the end of the beginning of this research direction. We have much more to do and to learn. Our current working model is that cell/tissue/organ injury stimulates mRNA breakdown and thus engages the 2′,3′-cAMP-adenosine pathway, which may protect against further injury by reducing levels of intracellular 2′,3′-cAMP and increasing levels of extracellular adenosine. However, presently these concepts should be viewed with healthy skepticism because at this writing the notion that the 2′,3′-cAMP-adenosine pathway is tissue protective is speculative. Hopefully, future research will corroborate some of these ideas and will correct the rest.
GRANTS
This work was supported by National Institutes of Health Grants HL069846, DK068575, DK079307, DK091190, and NS070003.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Akahane M, Ono H, Ohgushi H, Takakura Y. Viability of ischemia/reperfused bone determined at the gene expression level. J Reconstr Microsurg 17: 203–209, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Akahane M, Ono H, Ohgushi H, Tamai S. Viability of ischemia/reperfused muscles in rat: a new evaluation method by RNA degradation. J Orthop Res 19: 559–564, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. The CELL Nucleus. In: Molecular Biology of The Cell (2nd ed.). New York: Garlan, 1989, p. 481–550 [Google Scholar]
- 4. Albig AR, Decker CJ. The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Mol Biol Cell 12: 3428–3438, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almeida A, Paul Thiery J, Magdelenat H, Radvanyi F. Gene expression analysis by real-time reverse transcription polymerase chain reaction: influence of tissue handling. Anal Biochem 328: 101–108, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol 296: C1428–C1439, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Banholzer R, Nair AP, Hirsch HH, Ming XF, Moroni C. Rapamycin destabilizes interleukin-3 mRNA in autocrine tumor cells by a mechanism requiring an intact 3′ untranslated region. Mol Cell Biol 17: 3254–3260, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflügers Arch 453: 661–673, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Bushfield M, Shoshani I, Johnson RA. Tissue levels, source, and regulation of 3′-AMP: an intracellular inhibitor of adenylyl cyclases. Mol Pharmacol 38: 848–853, 1990 [PubMed] [Google Scholar]
- 10. Catts VS, Catts SV, Fernandez HR, Taylor JM, Coulson EJ, Lutze-Mann LH. A microarray study of post-mortem mRNA degradation in mouse brain tissue. Brain Res 138: 164–177, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Cheng D, Ren J, Jackson EK. Multidrug resistance protein 4 mediates cAMP efflux from rat preglomerular vascular smooth muscle cells. Clin Exp Pharmacol Physiol 37: 205–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chevyreva I, Faull RLM, Green CR, Nicholson LFB. Assessing RNA quality in postmortem human brain tissue. Exp Mol Pathol 84: 71–77, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Chiavegatti T, Costa VL, Jr, Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol 153: 1331–1340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Deeley RG, Westlake C, Cole SPC. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86: 849–899, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Del Prete MJ, Robles MS, Guao A, Martinez- AC, Izquierdo M, Garcia-Sanz JA. Degradation of cellular mRNA is a general early apoptosis-induced event. FASEB J 16: 2003–2005, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Denatale G, Causa P, Coscia L. Su alcune attività farmacologiche del 2′ AMP, del 3′ AMP e del 2′-3′ AMP in confront con il 5′ AMP. Arch Ital Sci Farmacol 13: 169–172, 1963 [PubMed] [Google Scholar]
- 18. Deussen A, Lloyd HG, Schrader J. Contribution of S-adenosylhomocysteine to cardiac adenosine formation. J Mol Cell Cardiol 21: 773, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Deussen A, Pexa A, Loncar R, Stehr SN. Effects of homocysteine on vascular and tissue adenosine: a stake in homocysteine pathogenicity? Clin Chem Lab Med 43: 1007–1010, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Do T, Sun Q, Beuve A, Kuzhikandathil EV. Extracellular cAMP inhibits D1 dopamine receptor expression in CAD catecholaminergic cells via A2a adenosine receptors. J Neurochem 101: 619–631, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Dubey RK, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway induces nitric oxide synthesis in aortic smooth muscle cells. Hypertension 31: 296–302, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension 36: 337–342, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension 37: 1095–1100, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Extracellular 3′,5′-cyclic AMP-adenosine pathway inhibits glomerular mesangial cell growth. J Pharmacol Exp Ther 333: 808–815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubey RK, Gillespie DG, Shue H, Jackson EK. A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension 35: 267–272, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Dubey RK, Jackson EK, Rupprecht HD, Sterzel RB. Factors controlling growth and matrix production in vascular smooth muscle and glomerular mesangial cells. Curr Opin Nephrol Hypertens 6: 88–105, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Dubey RK, Mi Z, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension 28: 765–771, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118: 3301–3315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med 202: 1493–1505, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiszman ML, Stefano FJ. Amphetamine-clonidine interaction on neurotransmission in the vas deferens of the rat. Naunyn Schmiedebergs Arch Pharmacol 328: 148–153, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Fleming WA, McNeill TA. Cellular responsiveness to stimulation in vitro: increased responsiveness to colony stimulating factor of bone marrow colony-forming cells treated with surface-active agents and cyclic 3′5′ AMP. J Cell Physiol 88: 323–329, 1976 [DOI] [PubMed] [Google Scholar]
- 32. Fuhr JE, Stidham JD. Inhibitory effect of cyclic adenosine 2′,3′-monophosphate on leucine incorporation by L5178Y cells. J Cell Physiol 103: 71–75, 1980 [DOI] [PubMed] [Google Scholar]
- 33. Fujimori H, Pan-Hou H. Formation of adenosine 3′-monophosphate in rat liver mitochondria. Biol Pharm Bull 21: 624–627, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Fujimori H, Sato R, Yasuda M, Pan-Hou H. A specific and rapid method for determination of adenosine 3′-monophosphate (3′-AMP) content and 3′-AMP forming enzyme activity in rat liver mitochondria, using reversed-phase HPLC with fluorescence detection. Biol Pharm Bull 21: 1348–1351, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Garrison JC, Haynes RC., Jr The hormonal control of gluconeogenesis by regulation of mitochondrial pyruvate carboxylation in isolated rat liver cells. J Biol Chem 250: 2769–2777, 1975 [PubMed] [Google Scholar]
- 36. Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134: 1116–1126, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS 5: e137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartzell HC. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol 293: 23–49, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem 273: 14424–14429, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Hong KW, Shin HK, Kim HH, Choi JM, Rhim BY, Lee WS. Metabolism of cAMP to adenosine: role in vasodilation of rat pial artery in response to hypotension. Am J Physiol Heart Circ Physiol 276: H376–H382, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Ichikawa A, Esumi K, Takagi M, Yatsunami K, Negishi M, Yokoyama K, Tomita K. Effect of adenosine and adenosine 5′-monophosphate on cell division of cultured mastocytoma P-815 cells. J Pharmacobiodyn 3: 123–135, 1980 [DOI] [PubMed] [Google Scholar]
- 42. Jackson EK. Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol 31: 1–35, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol 281: F597–F612, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Jackson EK, Gillespie DG, Dubey RK. 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. J Pharmacol Exp Ther 337: 444–450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther 295: 23–28, 2000 [PubMed] [Google Scholar]
- 46. Jackson EK, Mi Z. Regulation of renal ectophosphodiesterase by protein kinase C and sodium diet. J Pharmacol Exp Ther 325: 210–216, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. J Pharmacol Exp Ther 283: 177–182, 1997 [PubMed] [Google Scholar]
- 48. Jackson EK, Mi Z, Zacharia LC, Tofovic SP, Dubey RK. The pancreatohepatorenal cAMP-adenosine mechanism. J Pharmacol Exp Ther 321: 799–809, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther 307: 888–896, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol 66: 571–599, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol 301: F565–F573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol 301: H391–H401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension 56: 151–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jackson EK, Ren J, Zacharia LC, Mi Z. Characterization of renal ecto-phosphodiesterase. J Pharmacol Exp Ther 321: 810–815, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther 317: 1219–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Kim M, Chen SWC, Park SW, Kim M, D′Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kloor D, Osswald H. S-Adenosylhomocysteine hydrolase as a target for intracellular adenosine action. Trends Pharmacol Sci 25: 294–297, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Kruh GD, Zeng H, Rea PA, Liu G, Chen ZS, Lee K, Belinsky MG. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr 33: 493–501, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Kuzhikandathil EV, Clark L, Li Y. The extracellular cAMP-adenosine pathway regulates expression of renal D1 dopamine receptors in diabetic rats. J Biol Chem 286: 32454–32463, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee HT, Emala CW. Adenosine attenuates oxidant injury in human proximal tubular cells via A1 and A2a adenosine receptors. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Lee KS, Schubert P, Emmert H, Kreutzberg GW. Effect of adenosine versus adenine nucleotides on evoked potentials in a rat hippocampal slice preparation. Neurosci Lett 23: 309–314, 1981 [DOI] [PubMed] [Google Scholar]
- 66. Lloyd HG, Deussen A, Wuppermann H, Schrader J. The transmethylation pathway as a source for adenosine in the isolated guinea-pig heart. Biochem J 252: 489–494, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lu B, Rajakumar SV, Robson SC, Lee EKF, Crikis S, d'Apice AJF, Cowan PJ, Dwyer KM. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation 86: 1707–1712, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Mi Z, Herzer WA, Zhang Y, Jackson EK. 3-Isobutyl-1-methylxanthine decreases renal cortical interstitial levels of adenosine and inosine. Life Sci 54: 277–282, 1994 [DOI] [PubMed] [Google Scholar]
- 69. Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. J Pharmacol Exp Ther 287: 926–930, 1998 [PubMed] [Google Scholar]
- 70. Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther 273: 728–733, 1995 [PubMed] [Google Scholar]
- 71. Miyamoto A, Takeshita M, Pan-Hou H, Fujimori H. Hepatic changes in adenine nucleotide levels and adenosine 3′-monophosphate forming enzyme in streptozotocin-induced diabetic mice. J Toxicol Sci 33: 209–217, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Morote-Garcia JC, Rosenberger P, Nivillac NMI, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136: 607–618, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Müller G, Wied S, Over S, Frick W. Inhibition of lipolysis by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes depends on cAMP degradation by lipid droplets. Biochemistry 47: 1259–1273, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Nakane T, Chiba S. Pharmacological analysis of vasodilation induced by extracellular adenosine 3′,5′-cyclic monophosphate in the isolated and perfused canine coronary artery. J Pharmacol Exp Ther 264: 1253–1261, 1993 [PubMed] [Google Scholar]
- 75. Okusa MD. A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282: F10–F18, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol 279: F809–F818, 2000 [DOI] [PubMed] [Google Scholar]
- 77. Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. Enhanced protection from renal ischemia-reperfusion injury with A2A-adenosine receptor activation and PDE 4 inhibition. Kidney Int 59: 2114–2125, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol Renal Physiol 277: F404–F412, 1999 [DOI] [PubMed] [Google Scholar]
- 79. Oshima N, Furuuchi T, Fujii R. Cyclic nucleotide action is mediated through adenosine receptors in damselfish motile iridophores. Comp Biochem Physiol C 85: 89–93, 1986 [PubMed] [Google Scholar]
- 80. Pabst M, Grass J, Fischl R, Léonard R, Jin C, Hinterkörner G, Borth N, Altmann F. Nucleotide and nucleotide sugar analysis by liquid chromatography-electrospray ionization-mass spectrometry on surface-conditioned porous graphitic carbon. Anal Chem 82: 9782–9788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: Implications for PDE screening. Biochem Biophys Res Commun 398: 500–505, 2010 [DOI] [PubMed] [Google Scholar]
- 82. Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328: 855–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nature Immunol 10: 195–202, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Sarkar D, Fisher PB. Human polynucleotide phosphorylase (hPNPase old-35): an RNA degradation enzyme with pleiotrophic biological effects. Cell Cycle 5: 1080–1084, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Schmidt S. Candidate autoantigens in multiple sclerosis. Mult Scler 5: 147–160, 1999 [DOI] [PubMed] [Google Scholar]
- 86. Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci 54: 785–794, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sorrentino S, Libonati M. Structure-function relationships in human ribonucleases: main distinctive features of the major RNase types. FEBS Lett 404: 1–5, 1997 [DOI] [PubMed] [Google Scholar]
- 88. Sprinkle TJ. 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol 4: 235–301, 1989 [PubMed] [Google Scholar]
- 89. Thompson JE, Venegas FD, Raines RT. Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic phosphodiester intermediate. Biochemistry 33: 7408–7414, 1994 [DOI] [PubMed] [Google Scholar]
- 90. Thompson RJ. 2′,3′-cyclic nucleotide-3′-phosphohydrolase and signal transduction in central nervous system myelin. Biochem Soc Trans 20: 621–626, 1992 [DOI] [PubMed] [Google Scholar]
- 91. van Aubel R, Smeets PHE, Peters JGP, Bindels RJM, Russel FGM. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13: 595, 2002 [DOI] [PubMed] [Google Scholar]
- 92. Verrier JD, Exo JL, Jackson TC, Ren J, Gillespie DG, Dubey RK, Kochanek PM, Jackson EK. Expression of the 2′,3′-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem. [Epub before print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vogel US, Thompson RJ. Molecular structure, localization, and possible functions of the myelin-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase. J Neurochem 50: 1667–1677, 1988 [DOI] [PubMed] [Google Scholar]
- 94. Wahn HL, Lightbody LE, Tchen TT, Taylor JD. Induction of neural differentiation in cultures of amphibian undetermined presumptive epidermis by cyclic AMP derivatives. Science 188: 366–369, 1975 [DOI] [PubMed] [Google Scholar]
- 95. Willemot J, Paton DM. Metabolism and presynaptic inhibitory activity of 2′,3′ and 5′-adenine nucleotides in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol 317: 110–114, 1981 [DOI] [PubMed] [Google Scholar]
- 96. Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol 2: 237–246, 2001 [DOI] [PubMed] [Google Scholar]