Abstract
There is an emerging concept in clinical nephrology that acute kidney injury (AKI) can initiate chronic kidney disease (CKD). However, potential mechanisms by which this may occur remain elusive. Hence, this study tested the hypotheses that 1) AKI triggers progressive activation of selected proinflammatory genes, 2) there is a relative failure of compensatory anti-inflammatory gene expression, 3) proinflammatory lipid accumulation occurs, 4) these changes correspond with “gene-activating” histone acetylation, and 5) in concert, progressive renal disease results. CD-1 mice were subjected to 30 min of unilateral renal ischemia. Assessments were made 1 day, 1 wk, or 3 wk later. Results were contrasted to those observed in uninjured contralateral kidneys or in kidneys from normal mice. Progressive renal injury occurred throughout the 3-wk postischemic period, as denoted by stepwise increases in neutrophil gelatinase-associated lipocalin gene induction and ongoing histologic damage. By 3 wk postischemia, progressive renal disease was observed (massive tubular dropout; 2/3rds reduction in renal weight). These changes corresponded with progressive increases in proinflammatory cytokine/chemokine gene expression (MCP-1, TNF-α, TGF-β1), a relative failure of anti-inflammatory enzyme/cytokine (heme oxygenase-1; IL-10) upregulation, and progressive renal lipid (cholesterol/triglyceride) loading. Stepwise increases in collagen III mRNA and collagen deposition (Sirius red staining) indicated a progressive profibrotic response. Postischemic dexamethasone treatment significantly preserved renal mass, indicating functional significance of the observed proinflammatory state. Progressive gene-activating H3 acetylation was observed by ELISA, rising from 5% at baseline to 75% at 3 wk. This was confirmed by chromatin immunoprecipitation assay of target genes. In sum, these results provide experimental support for the clinical concept that AKI can trigger CKD, this is partially mediated by progressive postischemic inflammation, ongoing lipid accumulation results (potentially evoking “lipotoxicity”), and increasing histone acetylation at proinflammatory/profibrotic genes may contribute to this self-sustaining injury-promoting state.
Keywords: acute kidney injury, progressive renal disease
recent epidemiologic data suggest that the incidence of acute kidney injury (AKI) is increasing, affecting as many as 5% of hospitalized patients (19, 42, 45). It has also been suggested that clinical AKI can initiate the onset of progressive renal disease (11, 22). If both of these assumptions are correct, then AKI could, paradoxically, be a leading cause of chronic kidney failure (11, 22).
Despite these clinical considerations, a direct mechanistic link between AKI and the development of chronic kidney disease (CKD) remains a subject of debate. Indeed, the more traditionally held view is that clinical ischemic and nephrotoxic AKI are largely self limited, reversible diseases (8, 15, 16). The experimental literature strongly supports this traditional view. For example, multiple laboratories demonstrated that following bilateral ischemic injury in rodents, essentially complete renal functional recovery results. For example, Pechman et al. (31) and Basile et al. (37) induced 40–45 min of bilateral renal artery occlusion in the rat, producing severe azotemia at 24 h (e.g., serum creatinine of 3.5 mg/dl). However, by 4 wk postischemia, despite the presence of “healing defects” (e.g., tubulointerstitial capillary dropout; salt-sensitive hypertension), the serum creatinine had returned to baseline values (31, 37). Similar results have been obtained with studies of experimental nephrotoxic acute renal failure (5, 29, 47). For example, Nath et al. (29) found that glycerol-induced acute renal failure (ARF) can only cause CKD when repetitive (weekly ×6 mo) bouts of glycerol injection are imposed.
An experimental limitation could potentially explain why nearly complete post-AKI renal recovery occurs in rodents, but possibly not in humans. When studying the recovery phase of experimental AKI, the severity of the initial insult must be severe enough to cause substantial azotemia, and yet mild enough to permit prompt renal functional recovery to avoid death from uremia. In contrast, with clinical ARF, renal replacement therapy permits sufficient time for the natural course of the disease to be expressed. One way to obviate the above experimental limitation is to study unilateral ischemic renal damage. By avoiding death from uremia, the natural history of postischemic renal damage can be assessed. Using this approach, Koletsky (17) and Finn (7, 9, 10) studied the evolution of postischemic ARF, induced by 60–120 min of unilateral renal artery occlusion. Based on their studies, two conclusions were reached: first, the degree of renal functional recovery is predominantly determined by the length of the initial ischemic insult (e.g., worse with 120 vs. 60 min of ischemia); and second, that the presence of the normal kidney intensifies early postischemic renal vasoconstriction, thereby increasing renal damage. However, whether nonhemodynamic factors, particularly beyond the initial ischemic-reperfusion period, might also impact kidney outcomes was not addressed.
The present study was undertaken to more fully address this latter issue. In particular, we focused on the following questions: 1) is the fate of the kidney solely dictated by the severity of the initial ischemic insult/early postischemic vasoconstriction, or does active tissue injury continue to develop? 2) If delayed tissue injury does, in fact, occur, what role does postischemic tissue inflammation (3, 28, 30, 34, 36, 40) play in this ongoing damage? 3) Because chronic experimental renal failure (e.g., as induced by 5/6ths nephrectomy) causes massive renal tubular lipid accumulation and associated “lipotoxicity” (14, 18, 21, 33, 35), might a similar mechanism be operative during the evolution of postischemic ARF? 4) Because ischemic renal injury can induce “epigenetic remodeling” (26, 27, 52, 53) which then affects subsequent gene activity, might progressive gene-activating epigenetic changes (1, 4, 24, 58), such as histone acetylation (26), occur at injury-promoting genes and thus impact renal outcomes? Finally, are these potential changes associated with activation of profibrotic pathways (e.g., TGF-β1, collagen III gene expression), and thus, lead to CKD? Results of investigations into each of these questions form the basis of this report.
METHODS
Animal Experimentation
General surgical procedures.
Male CD-1 mice (35–45 g; Charles River Laboratories, Wilmington, MA) were used for all experiments with Institutional Animal Care and Use Committee-approved surgical protocols. They were maintained under routine vivarium conditions with free food and water access throughout. All surgeries were conducted under deep pentobarbital sodium anesthesia (50 mg/kg administered by intraperitoneal injection). Once anesthetized, the mice were placed under a heating lamp to maintain a 37°C body temperature throughout surgery. Left unilateral ischemic renal injury was induced by exposing the kidney through a midline abdominal incision and then applying an atraumatic vascular clamp across the renal pedicle. The right kidney was left unperturbed. After 30 min of renal ischemia, the clamp was removed, global reperfusion was confirmed by loss of kidney cyanosis, and then the abdomen was sutured in two layers using 4–0 chromic suture. Body temperature was maintained at 37°C until recovery from anesthesia.
Postischemic time course experiments.
At either 1 day, 1 wk, or 3 wk postinduction of renal ischemia, the mice were weighed and reanesthetized with pentobarbital sodium. Both kidneys were excised through the prior midline abdominal incision. A plasma sample was obtained from the inferior vena cava for subsequent blood urea nitrogen (BUN) or cytokine analysis. Then, the kidneys were immediately weighed and quickly iced. The renal cortices were dissected and the postischemic and contralateral samples were each divided into as many as three parts. These were used for 1) RNA extraction (RNeasy+Mini kit; Qiagen), 2) protein extraction (49, 55), and 3) lipid extraction (in chloroform: methanol) (48). The results from the postischemic and contralateral (uninjured) kidneys (n = 5–7 per group at each time point) were contrasted against each other and against values observed in kidneys from six normal mice.
Biochemical Analyses
mRNA analyses.
As an index of a proinflammatory response, the RNA samples obtained at each time point were analyzed for TNF-α, monocyte chemoattractant protein-1 (MCP-1), and TGF-β1 mRNAs (49, 55). Because heme oxygenase 1 (HO-1) is a potent anti-inflammatory and cytoprotective molecule (12, 41), its mRNA and that for anti-inflammatory IL-10 (38) were also assessed. A fibrotic response was gauged by collagen III mRNA assay (52). Ongoing renal injury over the course of the experiments was assessed by measuring the mRNA for the AKI biomarker neutrophil gelatinase-associated lipocalin (NGAL) (6). Finally, because AKI can activate the intrarenal HMG CoA reductase (HMGCR) pathway (47, 48, 51, 57), HMGCR mRNA levels were determined. All eight of the above noted mRNAs (TNF-α, MCP-1, TGF-β1, HO-1, IL-10, NGAL, collagen III, HMGCR) were measured by competitive RT-PCR using previously reported primers and methods (26, 27, 47, 49, 51–53, 55). All mRNA results were factored by simultaneously obtained GAPDH product, used as a reference housekeeping gene. The results of the postischemic kidneys were compared with those obtained in normal as well as contralateral kidney samples. Because the contralateral kidneys and normal kidneys yielded highly comparable results for all of the above, statistical comparisons were run using the postischemic vs. its own contralateral kidney by paired Student's t-test.
Renal cytokine, NGAL, and cholesterol assays.
To test whether the observed mRNA levels in postischemic vs. their contralateral kidney samples correlated with their cognate protein levels, the renal cortical protein extracts that were obtained at 3-wk postischemic injury were assayed for MCP-1 (BD Biosciences, San Diego, CA), TGF-β1 (R&D Systems, Minneapolis, MN), IL-10 (R&D Systems), HO-1 (Enzo Life Sciences, Farmingdale, NY), and NGAL (R&D Systems) using commercially available ELISA kits. The results were factored by total protein content within the assayed sample. Corresponding plasma samples were also assayed for MCP-1, TGF-β1, and NGAL levels (ELISA). To determine whether the HMGCR mRNA levels correlated with renal cholesterol content, the lipid extracts obtained at each time point were assayed for free cholesterol and cholesterol esters, using the Amplex Red colorometric enzyme assay (Invitrogen, Eugene, OR). The results were expressed as ratios to total phospholipid phosphate contained in the chloroform:methanol extracts (47). Because acute ischemic renal injury acutely increases renal proximal tubule triglyceride content (13, 54), the lipid extracts were also assayed for total triglyceride content using a commercially available assay (Sigma, St. Louis, MO).
Renal histone acetylation assessments.
Progressive increases in histone H3 methylation have previously been noted at select proinflammatory genes over a 1-wk time frame postischemic or glycerol-induced AKI (52, 53). Thus, further evidence of histone modifications postischemic injury was assessed by determining the extent of another histone modification, acetylation, over 3 wk postischemia. In general, acetylation is viewed as a gene-activating histone modification (1, 24, 58). Renal cortical protein samples, obtained at either 1 day or 3 wk postischemia and from their contralateral (nonischemic) kidneys, were assayed for both total histone H3 and for acetylated H3 (H3-Ac) using two commercially available ELISAs (Cell Signaling Technology, Danvers, MA). The results were expressed as the percent of total H3 that was present in the acetylated form (H3-Ac divided by total H3 × 100%).
The above ELISAs measure global acetylation and provide no information about site- specific changes. To determine whether increased acetylation occurred at the genes of interest, chromatin samples were obtained at 1 day, 1 wk, or 3 wk postunilateral ischemia and from the contralateral kidneys. The chromatin was fixed in 1% formalin, sheared in a bioruptor, and analyzed for H3 acetylation at exon 1 of each of the test genes by chromatin immunoprecipitation assay (ChIP) (26) using an antibody that measures acetylation at lysine 9 and 14 positions, as previously described (26).
Impact of Dexamethasone on Postischemic Inflammation and Loss of Renal Mass
To ascertain whether the observed postischemic proinflammatory state (see results) mechanistically contributes to ongoing renal injury, 16 mice were subjected to 30 min of unilateral renal pedicle occlusion. Approximately 4 h later, they received either dexamethasone (250 μg/mouse ip; n = 8) or its vehicle (saline; n = 8), and then repeat injections were given on a daily basis ×3 days. After 3 days, half of the mice were killed and mRNA was extracted from the postischemic and contralateral kidneys. As an index of an anti-inflammatory effect, the mRNA levels for TNF-α, MCP-1, and TGF-β1 were assessed. The remaining eight mice then received every other day dexamethasone (n = 4) or saline injection (n = 4) until 3 wk postsurgery. The mice were then killed and postischemic kidney weights were determined to ascertain whether a preservation of renal mass resulted from the steroid treatment.
Histologic Assessments
Renal histology was assessed at either 1 day or 3 wk postischemia. Three postischemic samples and three contralateral control kidneys at each time point were cut longitudinally and fixed in 10% formalin. In addition, three kidneys were obtained from three normal mice. Two-micrometer paraffin-embedded sections were cut and stained with hematoxylin and eosin for qualitative histologic analysis. As an index of collagen formation, the 3-wk postischemic kidney sections were also stained with Sirius red (23) and examined under a polarizing microscope (to detect collagen).
Calculations and Statistics
All values are presented as means ± 1 SE. Values in the postischemic kidneys were contrasted to both their contralateral kidney values and to values obtained from normal mice (by paired and unpaired Student's t-test, respectively). Between five and eight mice per group were used for each of these calculations, unless otherwise stated. Significance was judged by a P value of <0.05.
RESULTS
Kidney Weights, BUNs, and Renal Histology
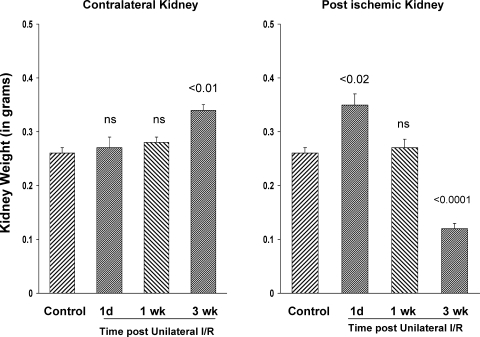
As depicted in Fig. 1, left, unilateral left renal ischemia caused an ∼15% increase in the weight of the noninjured right kidney over a 3-wk period (P < 0.01 vs. baseline weights), consistent with compensatory hypertrophy. In contrast, after an initial increase in kidney weight by the first day postischemia (presumably, tissue edema), a progressive decrease in left kidney weight ensued. Thus, by 3 wk postischemia, the injured left kidney weight was decreased to just one-third of baseline kidney weight values. BUNs of 26 ± 3, 26 ± 2, and 30 ± 2 mg/dl were observed at 1 day, 1 wk, and 3 wk postischemia, respectively (control BUNs: 23 ± 1; P < 0.01 vs. the 3-wk values). The mice retained their baseline weights as assessed at the 3-wk time point (baseline, 34 ± 1 g; 3 wk, 37 ± 1 g).
Fig. 1.
Kidney weights following unilateral ischemic injury. Following left renal ischemia, the right kidney slowly increased in weight, reaching statistical significance by the 3-wk time point (P < 0.01 vs. baseline kidney values; consistent with compensatory hypertrophy). The postischemic kidney showed an initial increase in weight at 1 day postischemia (due to edema), but then progressively decreased weight, such that by 3 wk, a 2/3rds renal weight reduction was apparent (right: consistent with progressivep fibrosis/atrophy). 1d, 1 day postischemia; 1, 3 wk, weeks postischemia.
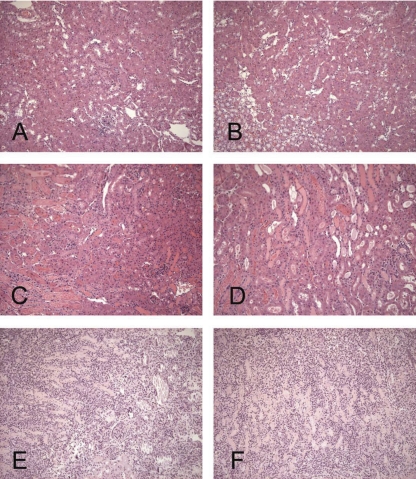
The most prominent histologic change at 1 day postischemia was widespread proximal tubular necrosis, most notable in the outer medullary stripe (Fig. 2, C and D, vs. normal sections depicted in Fig. 2, A and B). However, the outer cortex was almost completely spared. Sloughing of tubular debris into lumina was apparent with resultant cast formation. However, overt tubular dilatation was not observed. The interstitium revealed a relatively mild inflammatory cell infiltrate. The glomeruli and vasculature maintained a normal appearance. By 3 wk postischemia, there was massive tubular dropout, and marked cellular expansion of the interstitial space was observed (Fig. 2, E and F). In contrast to the changes seen at 1 day postischemia (where injury was largely confined to the outer medullary stripe), the changes at 3 wk involved the entire cortex as well as the medulla. Although the glomeruli did not appear necrotic, they were contracted and manifested periglomerular fibrosis. The residual tubule segments showed necrotic debris within the lumina. Sirius red staining manifested significant collagen deposition within the interstitium at the 3-wk time point. Its interpositioning between the tubules gave the kidney a “chicken wire” appearance (Fig. 3A). In contrast, only trace amounts of collagen were seen between tubules in the control kidney samples. As expected, the adventitia of blood vessels manifested a large amount of collagen, in a sense, serving as a positive internal control (Fig. 3B). Of note, the contralateral kidney retained normal histology (no apparent histologic differences from normal mouse kidneys), implying that the injured kidney did not cause overt contralateral kidney damage.
Fig. 2.
Renal histologic hematoxylin and eosin (H&E) sections from normal kidneys, 1 day postischemia and 3 wk postischemia. A: normal kidney section demonstrating histology of renal cortex and part of outer medulla. B: normal kidney outer medulla. C: outer medulla at 1 day postischemia. Cast formation and tubular necrosis are seen at the cortical/outer medullary junction but with preservation of most of the cortical tubular segments. D: outer medullary stripe at 1 day postischema. Widespread tubular necrosis and cast formation are apparent. E: renal cortex at 3 wk postischemia. A marked cellular interstitial infiltrate with massive tubular dropout and periglomerular fibrosis are apparent. F: outer medulla at 3 wk postischemia. As with the cortex, there is a marked cellular interstitial infiltrate, tubular dropout, and debris/casts in lumina of remaining tubules.
Fig. 3.
Sirius red staining for collagen. Kidney sections were obtained at 3 wk postischemia (A) and examined under polarized light. The postischemic kidney demonstrates interstitial collagen deposition, as evidenced by the deposition of yellow and red staining strands laced between tubules. This gave a “chicken wire” appearance. In addition, extensive tubular dilatation and marked amounts of tubular cell debris within tubular lumina were apparent. In contrast, a normal kidney section (B) showed only a minimal amount of collagen between the tubules. As a “positive internal control,” the presence of collagen in the adventitia of a medium-sized blood vessel is apparent.
NGAL Assessments of Cell Injury
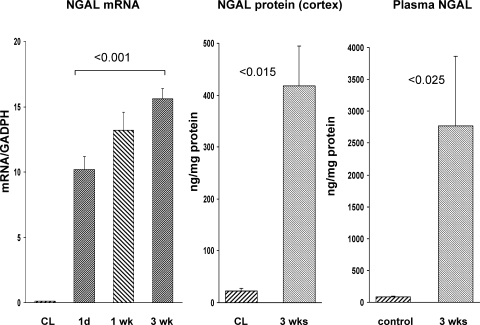
In addition to the histologic assessments, ongoing postischemic renal injury was reflected by a progressive increase in NGAL mRNA, a biomarker of AKI (Fig. 4, left). NGAL mRNA translation into protein was indicated by an ∼20-fold increase in renal cortical NGAL protein levels at the 3-wk time point (Fig. 4, middle). A corresponding marked increase in plasma NGAL levels was observed (Fig. 4, right), suggesting consistent with NGAL efflux into the systemic circulation. An alternative, albeit less likely, possibility is that the damaged kidney stimulated extrarenal NGAL induction (e.g., in lung), which then contributed to the observed elevations in plasma NGAL concentrations.
Fig. 4.
Neutrophil gelatinase-associated lipocalin (NGAL) expression postischemia. Renal cortical NGAL mRNA levels (left) progressively increased postischemia, compared with values observed in contralateral kidneys (all contralateral kidney values depicted as a single group for ease of presentation). This was associated with a marked increase in renal cortical NGAL protein levels (middle), as assessed at the 3-wk time point. CL, contralateral kidney values (which did not significantly differ from values observed in normal kidneys). Right: corresponding with the increase in renal cortical NGAL protein was a corresponding increase in plasma NGAL concentrations, compared with control plasma.
Assessments of Proinflammatory/Profibrotic Cytokine mRNAs
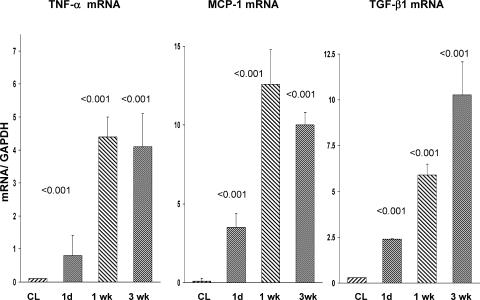
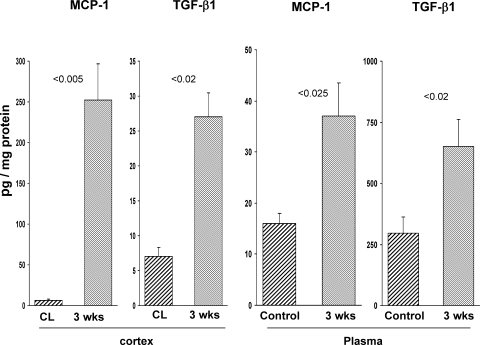
Corresponding with the progressive histologic injury and renal cortical NGAL expression were marked and progressive increases in TNF-α, MCP-1, and TGF-β1 mRNA levels (Fig. 5). These mRNA increases were associated with corresponding increases in renal cortical and plasma MCP-1 and TGF-β1 protein levels (Fig. 6), as assessed at the 3-wk time point.
Fig. 5.
Renal cortical TNF-α, monocyte chemoattractant protein-1 (MCP-1), and transforming growth factor (TGF)-β1 mRNAs following unilateral renal ischemia. By 1 day postischemia, significant increases in all 3 mRNAs were apparent, compared with values in the nonischemic CL kidneys. Further increases in each were observed at the 1-wk time point. TGF-β1 mRNA levels continued to increase throughout the course of the experiment. The contralateral kidney values did not significantly differ over time, and these results are presented as a single group. P values were derived by comparing postischemic vs. contralateral kidney values at each time point.
Fig. 6.
MCP-1 and TGF-β1 protein levels in renal cortex and plasma after unilateral ischemia. Renal cortical MCP-1 and TGF-β1 protein levels were measured at 3 wk in the postischemic and CL kidneys, and dramatic increases in both protein levels were observed (left). There were associated increases in plasma MCP-1 and TGF-β1 levels (compared to values in normal mouse plasma), suggesting renal efflux of these cytokines into the systemic circulation.
Collagen III mRNA, HMGCR mRNA, and Renal Cortical Cholesterol/Triglyceride Assessments
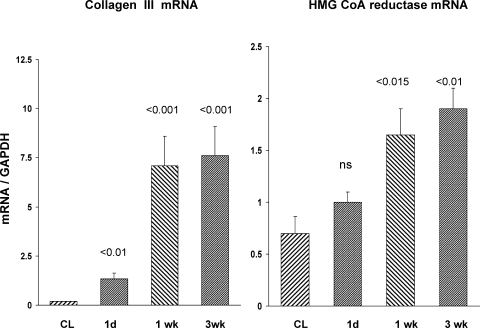
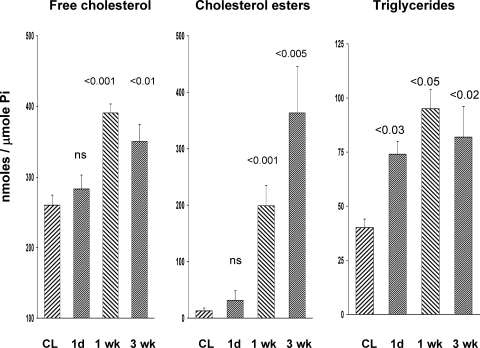
The above evidence of ongoing renal injury was also supported by progressive increases in collagen III mRNA (Fig. 7, left). This was consistent with the above described histologic assessments indicating increased renal interstitial collagen deposition (Fig. 3). Progressive increases in HMGCR mRNA levels were noted (Fig. 7, right). As shown in Fig. 8, this was associated with elevated renal cortical unesterified (free) cholesterol levels, and with progressive increases in the cholesterol ester “storage” pool (>20-fold higher than baseline cholesterol ester values). As with cholesterol, progressive triglyceride accumulation was also observed (Fig. 8, right).
Fig. 7.
Collagen III and HMG CoA reductase mRNA levels postischemia. These 2 mRNAs were measured in postischemic and CL kidneys at 1 day, 1 wk, and 3 wk postischemia. Progressive increases in both mRNA levels were observed over time. The CL kidney results did not significantly differ over time, and these results are presented as a single group. P values were derived by comparing postischemic vs. contralateral kidney values at each time point.
Fig. 8.
Free cholesterol, esterified cholesterol, and total triglyceride levels in renal cortex postischemia. By 1 wk postischemia, an ∼35% increase in free cholesterol levels were observed (left), compared with values observed in the nonischemic CL kidneys. These elevated levels remained relatively stable thereafter. Conversely, cholesterol ester levels continued to increase over time, such that by 3 wk an ∼20-fold increase was observed, compared with the values seen in CL kidneys (middle). Triglyceride levels were significantly elevated at 1 day postischemia, further increases were seen by 1 wk, and they remained elevated at the 3-wk time point. The CL kidney results did not differ significantly over time for any of these measurements, and, thus, the CL kidney results are presented as a single group. P values were derived by comparing postischemic vs. CL kidney results at each time point.
HO-1 and IL-10 Gene Expression
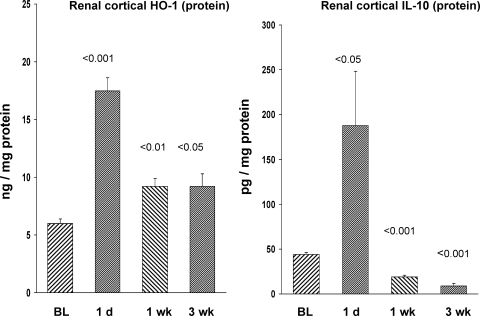
As shown in Fig. 9, HO-1 mRNA values peaked at 1 day postischemia. Although the HO-1 mRNA levels remained significantly elevated throughout the course of the experiment, the values had declined to ∼50% of the 1-day postischemia values. HO-1 protein levels tracked the mRNA results: the greatest increase was observed at 1 day postischemia, and then declined by ∼50% by 1 wk, and remained at this reduced level for the remainder of the 3-wk experiment (Fig. 10, left).
Fig. 9.
Heme oxygenase 1 (HO-1) mRNA and IL-10 mRNA levels postischemia. Both mRNAs were significantly elevated by 1 day postischemia. HO-1 mRNA remained elevated over the course of the experiments, albeit at just 50% of the 1-day value. Conversely, IL-10 mRNA levels manifested further increases from 1 day vs. 1 wk postischemia. The CL kidney results did not differ over time, and these results are presented as a single group. P values were derived from comparing ischemic vs. contralateral kidney values at each time point.
Fig. 10.
Renal cortical HO-1 and IL-10 protein levels postischemia. Both HO-1 and IL-10 protein levels were markedly elevated at 1 day postischemia. At 1 and 3 wk, the HO-1 levels remained elevated, albeit at just 50% of the 1-day values. Thus, HO-1 protein expression almost exactly paralleled its cognate mRNA. In contrast, IL-10 levels manifested a drastic fall at 1 wk postischemia, and by 3 wk postischemia, the values were significantly less than those observed in normal or CL kidneys. The CL kidney results did not significantly differ over time, and these results are presented as a single group. P values were derived from ischemic vs. CL kidneys at each time point.
IL-10 mRNA manifested marked increases at 1 day postischemia and remained elevated thereafter (Fig. 9, right). At 1 day postischemia, there was a corresponding increase in IL-10 protein in renal cortex (Fig. 10, right). However, beyond 1 day, renal cortical IL-10 levels progressively fell, even below normal kidney values (Fig. 10, right). In sum, the dissociation of rising IL-10 mRNA and falling IL-10 protein levels suggested an mRNA translation block.
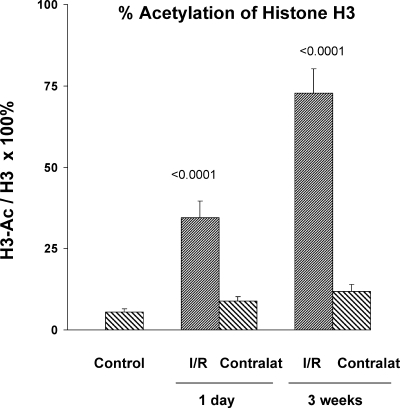
Histone H3 Acetylation
Under normal (baseline) conditions, ∼5% of total histone 3 (H3) was in an acetylated form (Fig. 11). Ischemia reperfusion increased the amount of acetylation in a time-dependent manner. Thus, by 1 day and 3 wk postischemia, the extent of H3 acetylation increased dramatically, reaching ∼35 and 75%, respectively (Fig. 11). In contrast, no significant increase in H3 acetylation was observed in the contralateral nonischemic kidneys. ChIP assay confirmed marked acetylation at exon 1 of each of the target genes. The greatest increases in acetylation were noted between 1 day and 1 wk postischemia, with relatively stable values being maintained until the 3-wk time point (Table 1).
Fig. 11.
Percent histone 3 (H3) acetylation in normal kidneys, in postischemic kidneys, and in CL controls. Nonacetylated and acetylated H3 values were measured by independent ELISAs, and the relative % acetylation was calculated. No increase in acetylation was observed in the CL (contralat) kidneys, compared with values in normal (control) kidneys. Conversely, the postischemic kidneys manifested a dramatic increase in % H3 acetylation at 1 day postischemia and by 3 wk postischemia, ∼75% of total H3 was in an acetylated form.
Table 1.
Percent increase in H3 lysine 9/14 acetylation at exon 1 of the test genes, as assessed by ChIP assay
| 1 Day | 1 Wk | 3 Wk | |
|---|---|---|---|
| TNF-α | 56 ± 18% | 339 ± 48% | 315 ± 99% |
| MCP-1 | 77 ± 43% | 370 ± 45% | 342 ± 130% |
| TGF-β1 | 18 ± 8% | 140 ± 25% | 226 ± 72% |
| IL-10 | 38 ± 24% | 277 ± 57% | 236 ± 93% |
| HO-1 | 6 ± 8% | 233 ± 27% | 129 ± 15% |
| Collagen III | 52 ± 40% | 235 ± 48% | 171 ± 78% |
| NGAL | 15 ± 21% | 243 ± 53% | 206 ± 62% |
| HMGCR | 10 ± 17 | 197 ± 55 | 204 ± 71 |
Values are means ± SE. Chromatin immunoprecipitation (ChIP) assay was used to assess the percent increase in acetylation of histone 3 (H3) at the lysine 9 (K9) + and lysine 14 (K14) positions at exon 1 of each of the test genes. The percent increases were derived by comparing the absolute levels in the postischemic kidneys vs. those in contralateral nonischemic kidneys. There were significant (P < 0.01) postischemic increases in acetylation at each gene and at each time point. An approximate tripling of acetylation was observed from 1 day to 1 wk, with the value remaining relatively stable thereafter. TGF, transforming growth factor; HO, heme oxygenase; NGAL, neutrophil gelatinase-associated lipocalin.
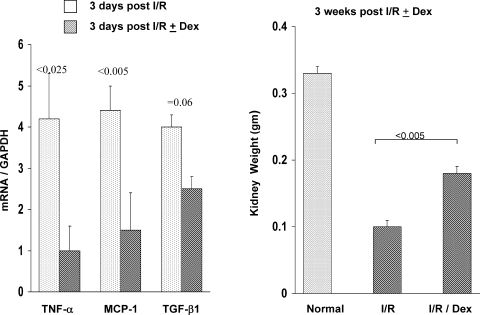
Dexamethasone Treatment
Steroid therapy substantially blunted postischemic TNF-α, MCP-1, and TGF-β1 mRNA increases, as assessed at the 3-day time point (Fig. 12, left). Partial renal protection was also apparent at 3 wk postischemia, as indicated by an ∼50% greater postischemic kidney weight in the dexamethasone-treated mice vs. their controls (Fig. 12, right). In contrast to the postischemic kidney, steroid therapy significantly decreased the weight of the contralateral kidneys (0.29 ± 0.01 vs. 0.34 ± 0.01 g; P < 0.005; steroid vs. nonsteroid treatment, respectively). This is consistent with less renal injury in the postischemic kidney, leading to less of a stimulus for compensatory hypertrophy.
Fig. 12.
Effect of dexamethasone (Dex) treatment on postischemic-reperfusion (I/R) cytokine expression and renal mass reduction, as assessed by renal weight. Cytokine mRNAs were measured at 3-day postunilateral ischemic injury with and without Dex treatment. Renal weights at 3 wk postischemia with and without Dex treatment were also assessed. Dex lowered postischemic cytokine expression and conferred partial protection against renal injury, as assessed by a relative preservation of kidney weight at the 3-wk time point. I/R, ischemia-reperfusion.
DISCUSSION
As noted above, studies performed more than 25–50 years ago demonstrated that prolonged (60–120 min) bouts of unilateral ischemic renal injury, induced in rats, caused sustained reductions in renal function and renal atrophy, as assessed over a 4- to 8-wk period (7, 9, 10, 17). Two factors were defined that dictated the ultimate severity of the resulting renal damage: the length of the initial ischemic insult, and whether a normal contralateral kidney was left in place. The latter was posited to worsen contralateral ischemic renal injury by two mechanisms: first, the presence of a normal kidney intensified immediate postischemic renal vasoconstriction, potentially prolonging the ischemic insult; and second, by lowering single nephron glomerular filtration rate, the vasoconstriction worsened tubular cast formation by limiting tubular debris washout (7, 9, 10). Thus, when these factors were considered together, it was assumed that events within the immediate ischemic/postischemic periods dictated the subsequent course of the renal damage.
The results of the present study expand on these prior findings and provide important new insights. Most notable in these regards are the following: first, we show that the course of unilateral ischemic renal injury is not simply dictated by early ischemic/postischemic events; rather, active injury pathways are initially triggered by ischemia that then progresses for wk into the postischemic period. Second, we demonstrate, for the first time, that activation of this postischemic injury cascade does not require a profound initial ischemic event. Unlike prior studies that used a 60- to 120-min ischemic insult in rats, we observed that just 30 min of renal ischemia, imposed in CD-1 mice, can initiate progressive renal damage that is characterized by severe tubular injury, tubular atrophy/tubular dropout, interstitial inflammation, and ultimately renal fibrosis. Indeed, that a bout of relatively modest experimental ischemia can initiate progressive nephron loss provides support for the emerging clinical concept that even relatively modest AKI may initiate CKD.
Three pieces of evidence support the concept that a 30-min ischemic insult can, indeed, lead to progressive renal damage. First, NGAL expression, a highly sensitive biomarker of AKI, manifested stepwise increments throughout the 3-wk postischemic period. Indeed, at 3 wk postischemia, NGAL mRNA levels were 50% higher than they were at 1 day postischemia, the latter being the time at which tubular injury would be expected to be at, or near, its height. These stepwise NGAL mRNA increases, and the corresponding 20- to 50-fold elevations in renal cortical and plasma NGAL protein levels, support the presence of active, ongoing, renal damage. Second, accompanying this progressive NGAL increase were stepwise reductions in renal mass. Thus, at the 3-wk time point, a 2/3rds reduction in renal weight was observed. Third, the renal histologic sections confirmed the presence of active tubular damage. Even at 3 wk, cell sloughing into tubular lumina and cast formation were observed. Thus, three sets of complementary observations (increasing NGAL expression, falling renal weight, morphologic injury) support the concept that active and progressive renal injury followed the initial ischemic event.
There were undoubtedly multiple, and interactive, mechanisms that were responsible for this ongoing postischemic renal damage. The available data provide insights into at least three of them. First, as evidenced by renal histology, the delayed postischemic period was characterized by an active inflammatory response. Notable in this regard were that the mRNAs for TNF-α, MCP-1, and TGF-β1 progressively rose from 1 to 7 days postischemia and either remained at these elevated levels (TNF-α, MCP-1) or continued to increase (TGF-β1) thereafter. The functional significance of the mRNA increases was underscored by the corresponding 5- to 25-fold increases in renal cortical MCP-1 and TGF-β1 protein levels, respectively, indicating that mRNA translation had occurred. Of interest, these high intrarenal cytokine levels apparently effluxed into the systemic circulation. This suggests the possibility that unilateral renal injury has the potential to impact extrarenal tissue homeostasis, i.e., so called “organ cross talk.”
A second pathway that may have contributed to ongoing renal injury was a failure of expected compensatory increases in anti-inflammatory defenses, specifically, HO-1 and IL-10. Despite the fact that both HO-1 and IL-10 gene induction were observed at 1 day postischemia, as reflected by both their mRNA and protein levels, the trend was for falling, not rising, levels over time. In the case of HO-1, at both 1 and 3 wk postischemia, its mRNA and protein levels were reduced by ∼50%, compared with the 1-day postischemic values. This was in sharp contrast to the proinflammatory cytokines (e.g., TNF-α/MCP-1) that manifested stable or stepwise increases. In the case of IL-10, although its mRNA remained elevated over time, IL-10 protein levels were paradoxically suppressed, falling to 50% of even normal kidney values at the 3-wk time point. We previously reported that AKI can induce an IL-10 mRNA translation block, possibly due to microRNA induction (55). The present observation of postischemic IL-10 mRNA increases, with paradoxical stepwise IL-10 protein reductions, is consistent with this concept. Thus, when the HO-1/IL-10 results are contrasted with the TNF-α/MCP-1/TGF-β1 results, it appears that the relative “pro- vs. anti-inflammatory balance” was “tipped” toward a proinflammatory state. That this proinflammatory state played a mechanistic role in disease progression is underscored by the fact that dexamethasone, which was able to decrease postischemic TNF-α, MCP-1, and TGF-β1 mRNA levels, partially preserved renal mass over the course of these experiments.
The third pathway that may have contributed to the progressive renal injury is so called “lipotoxicity.” It has been well-documented that diverse forms of AKI, including ischemia- reperfusion, cause both cholesterol and triglyceride accumulation within the kidney (13, 26, 47, 48, 51, 54, 57). To variable degrees, increased lipid uptake, decreased lipid efflux, decreased lipid catabolism, and increased lipid synthesis may each be involved (14, 50). During the early phases of AKI, increased cholesterol and triglyceride synthesis may serve renal protective functions (21, 48, 57). For example, cholesterol accumulation increases plasma membrane stability, protecting cells against further ischemic or toxic damage (48, 57). In contrast, triglyceride formation is cytoprotective by incorporating cytotoxic, PLA2-generated, free fatty acids into triacylglycerol synthesis (43). However, if left unchecked, these initially beneficial actions can lead to “lipotoxicity” and contribute to progressive renal damage (14). For example, increased HMGCR-driven cholesterol synthesis, as evidenced in the present study by increasing HMGCR mRNA levels over time, generates geranyl and farensyl pyrophosphates, which then prenylate signaling molecules that stimulate a proinflammatory state (56). Cholesterol, per se, can directly increase renal tubular cell inflammatory responses (51). Similarly, excess triglyceride levels can enhance inflammation and cytotoxicity, either by activating cytotoxic T lymphocytes (2), or more directly by impacting tubular cell injury pathways (20, 25, 39). The present findings that the postischemic period was associated with progressive activation of the HMGCR/cholesterol axis and triglyceride accumulation underscore the potential for lipotoxicity to occur.
A fundamental question emerges from the above observations: namely, what factors are initiated by a self-limited bout of renal ischemia that can then produce stable activation of the above noted pathways? One concept that we recently advanced is that a bout of AKI can activate histone-modifying enzymes that lead to stable, or advancing, chromatin (“epigenetic”) remodeling at proinflammatory genes (26, 27, 52, 53). Histone acetylation is one such change. By relaxing chromatin structure at target genes, increased transcription factor and RNA polymerase II recruitment to promoter and transcribed gene regions result in driving gene transcription. Given these considerations, we assessed whether the postischemic period was associated with increased histone acetylation. As an initial probe into this question, the percent of global H3 acetylation was assessed by ELISA. By 1 day postischemia, the overall percent H3 acetylation rose from a baseline of 5 to 35%, and by 3 wk postischemia, ∼75% of total H3 pool appeared to be in the acetylated form. To obtain gene-specific results, we probed the start exon of the above noted target genes using ChIP assay. As presented in Table 1, progressive histone acetylation was observed from 1 to 7 days postischemia, and these elevated levels persisted throughout the course of the experiments. Thus, these findings raise the intriguing possibility that at least one reason for ongoing postischemic cytokine and cholesterol synthesis is ischemia-initiated chromatin remodeling that facilitates ongoing gene activity.
A consideration of great pathophysiologic interest is why unilateral ischemic injury leads to progressive renal disease, whereas bilateral renal injury is associated with near complete renal functional recovery. Although previously discussed studies implicate the influence of a normal contralateral kidney on early hemodynamic factors in the postischemic-reperfusion period (7, 9, 10), we believe that two recent publications (46, 55) from our laboratory provide important new insights into this issue. The most obvious difference between bilateral vs. unilateral ischemic injury is the presence or absence of uremia. Thus, we previously tested whether the uremic milieu, per se, impacts some of the determinants of postischemic disease progression, as noted in the present experiments. In one of these studies (46), we documented that the presence of uremia confers direct cytoprotective effects on residual nephrons. For example, when isolated proximal tubules, or cultured proximal tubule HK-2 cells, were exposed to an experimental uremic milieu, the cells were protected against superimposed hypoxic attack (46). These observations suggest the intriguing possibility that the absence of a cytoprotective “uremic brake” in the setting of unilateral ischemic renal damage allows for ongoing cell death to develop. In a second recent study (55), we demonstrated that uremia, per se, can exert a potent intrarenal anti-inflammatory effect. Thus, when mice were subjected to unilateral vs. bilateral ischemic injury, or unilateral ischemia ± contralateral ureteral transection (i.e., unilateral ischemic injury in the presence or absence of uremia), the uremic state suppressed the expression of proinflammatory (TNF-α, MCP-1) and profibrotic (TGF-β1) genes. Furthermore, when cultured proximal tubule (HK-2) cells were exposed to a uremic milieu, TNF-α, MCP-1, and TGF-β1 gene expression were also suppressed. Based on these two studies, we hypothesize that with unilateral ischemic renal injury, the absence of a uremic brake creates a permissive environment that allows for ongoing tubular injury, cytokine generation, and ultimately progressive nephron loss to occur.
The relative roles of the above noted postischemic injury pathways in the observed progressive nephron loss remain to be defined. Indeed, it is conceivable that at least some of the observed changes, e.g., cholesterol accumulation, could be reflections of ongoing tissue injury (e.g., analogous to NGAL increases), and not necessarily primary mediators of it. Thus, future studies directed at inhibiting specific injury pathways will be required to dissect out what are likely to be highly complex and interactive mechanisms. Such experiments could include the use of specific antagonists of proinflammatory cytokines, or provision of exogenous anti-inflammatory cytokines, e.g., IL-10, and test for disease mitigation. Alternatively, it might be that an upregulator of HO-1 activity, such as bardoxolone methyl (32, 44), might attenuate disease activity. Alternatively, combined therapies to block lipotoxicity (e.g., statins + peroxisome proliferator-activated receptor ligands + fatty acid-binding proteins) might exert beneficial effects. Finally, the impact of histone acetylase inhibitors on gene activity following AKI remains to be defined. Each of these represents fertile areas for future study.
In conclusion, this study demonstrates that a relatively modest (30 min) ischemic insult imposed on CD-1 mice leads to progressive tubular damage, tubular dropout, interstitial inflammation, interstitial fibrosis, and an associated 2/3rds reduction in renal mass over a 3-wk period. Correlates, and likely active participants, in this ongoing renal injury are progressive activation of proinflammatory pathways (e.g., TNF-α, MCP-1), increased expression of profibrotic (TGF-β1, collagen III) pathways, a downregulation of anti-inflammatory IL-10 expression, and the absence of a sustained, robust HO-1 response. A progressive increase in cholesterol and triglyceride accumulation suggests that so called “lipotoxicity” may result. The reasons for the sustained, or progressive, activation of these disease pathways over 3 wk, despite what is a brief and finite renal insult, remain to be defined. However, the presence of marked “gene-activating” histone acetylation at proinflammatory genes suggests that “epigenetic remodeling” may be involved. Future studies will be needed to dissect out the mechanistic roles played by each of the above.
GRANTS
This work was supported by National Institutes of Health Research Grants DK 83310, DK 83315, and DK 38432.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.A.Z. conception and design of research; R.A.Z., A.C.J., and K.B. analyzed data; R.A.Z., A.C.J., and K.B. interpreted results of experiments; R.A.Z. prepared figures; R.A.Z. drafted manuscript; R.A.Z. edited and revised manuscript; R.A.Z. approved final version of manuscript; A.C.J. and K.B. performed experiments.
REFERENCES
- 1.Adcock IM, Ito K, Barnes PJ. Histone deacetylation: an important mechanism in inflammatory lung diseases (Review). COPD 2: 445–455, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Alves BN, Marshall K, Tamang DL, Leong J, Redelman D, Elliott V, Lowe ME, Hudig D. Lipid-dependent cytotoxicity by the lipase PLRP2 and by PLRP2-positive cytotoxic T lymphocytes (CTLs). Cell Biochem Funct 27: 296–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV. Pathophysiology of acute kidney injury: roles of potential inhibitors of inflammation (Review). Contrib Nephrol 156: 39–46, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chen F, Smith R, Gu YZ, Collins ND, Nioi P. Toxicoepigenetic alteration of the kidney injury molecule 1 gene in gentamicin-exposed rat kidney. Toxicol Sci 117: 375–380, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Christo JS, Rodrigues AM, Mouro MG, Cenedeze MA, Simoes MJ, Schor N, Higa EM. Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide 24: 77–83, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr 23: 194–200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn WF. Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ Res 46: 440–448, 1980 [DOI] [PubMed] [Google Scholar]
- 8.Finn WF. Recovery from acute renal failure. In: Acute Renal Failure, edited by Brenner BM, Lazarus JM. New York: Churchill Livingstone, 1988, p. 875–918 [Google Scholar]
- 9.Finn WF, Chevalier RL. Recovery from postischemic acute renal failure in the rat. Kidney Int 16: 113–123, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Finn WF, Fernandez-Repollet E, Goldfarb D, Iaina A, Eliahou HE. Attenuation of injury due to unilateral renal ischemia: delayed effects of contralateral nephrectomy. J Lab Clin Med 103: 193–203, 1984 [PubMed] [Google Scholar]
- 11.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarmi T, Agarwal A. Heme oxygenase and renal disease (Review). Curr Hypertens Rep 11: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Johnson AC, Stahl A, Zager RA. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int 67: 2196–2209, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol 296: F1297–F1306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kjellstrand CM, Ebben J, Davin T. Time of death, recovery of renal function, development of chronic renal failure and need for chronic hemodialysis in patients with acute tubular necrosis. Trans Am Soc Artif Intern Organs 27: 45–50, 1981 [PubMed] [Google Scholar]
- 16.Kjellstrand CM, Gornick C, Davin T. Recovery from acute renal failure. Clin Exp Dial Apheresis 5: 143–161, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Koletsky S. Effects of temporary interruption of renal circulation in rats. AMA Arch Pathol 58: 592–603, 1954 [PubMed] [Google Scholar]
- 18.Koyama T, Kume S, Koya D, Araki SI, Isshiki K, Chin-Kanasaki M, Sugimoto T, Haneda M, Sugaya T, Kashiwagi A, Maegawa H, Uzu T. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med prepublished online May 30, 2011; doi:10.1016/j.freeradbiomed.2011.05.028, 9999 [DOI] [PubMed] [Google Scholar]
- 19.Lakhal K, Ehrmann S, Chaari A, Laissy JP, Régnier B, Wolff M, Pajot O. Acute Kidney Injury Network definition of contrast-induced nephropathy in the crically ill: incidence and outcome. J Crit Care prepublished online July 5, 2011; doi:10.1016/j.crci2011.05.010, 9999 [DOI] [PubMed] [Google Scholar]
- 20.Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol 289: F469–F480, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100: 3077–3082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malkusch W, Rehn B, Bruch J. Advantages of Sirius Red staining for quantitative morphometric collagen measurements in lungs. Exp Lung Res 21: 67–77, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Mifsud KR, Gutièrrez-Mecinas M, Trollope AF, Collins A, Saunderson EA, Reul JM. Epigenetic mechanisms in stress and adaption. Brain Behavior Immunity prepublished online June 14, 2011; doi:10.1016/jbbi.2011.06.005, 9999 [DOI] [PubMed] [Google Scholar]
- 25.Nagothu KK, Bhatt R, Kaushal GP, Portilla D. Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int 68: 2680–2693, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Naito M, Bomsztyk K, Zager RA. Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol 174: 54–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito M, Zager RA, Bomsztyk K. BRG1 increases transcription of proinflammatory genes in renal ischemia. J Am Soc Nephrol 20: 1787–1796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues (Review). Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol prepublished online April 27, 2011; doi:10.1152/ajprenal.00162.2011, 9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med prepublished online June 24, 2011; doi:10.1056/NEJMoa1105351, 9999 [DOI] [PubMed] [Google Scholar]
- 33.Perman JC, Bostrom P, Lindbom M, Lidberg U, Stahlman M, Hagg D, Lindskog H, Scharin TM, Omerovic E, Mattsson HL, Jeppsson A, Petursson P, Herlitz J, Olivecrona G, Strickland DK, Ekroos K, Olofsson SO, Boren J. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest 121: 2625–2640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation (Review). J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Schaffer JE. Lipotoxicity: when tissues overeat (Review). Curr Opin Lipid 14: 281–287, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nature Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Tadagavadi RK, Reeves WB. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J Immunol 185: 4904–4911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Sugimoto T, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Fenofibrate, a PPARalpha agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int 79: 871–882, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol 170: 1820–1830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Weinberg JM. Lipotoxicity (Review). Kidney Int 70: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, Winterberg P, Chen B, Agarwal A, Lu CY. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARgamma, and HO-1. Am J Physiol Renal Physiol 300: F1180–F1192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Zager RA. Uremia induces proximal tubular cytoresistance and heme oxygenase-1 expression in the absence of acute kidney injury. Am J Physiol Renal Physiol 296: F362–F368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zager RA, Andoh T, Bennett WM. Renal cholesterol accumulation: a durable response after acute and subacute renal insults. Am J Pathol 159: 743–752, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zager RA, Burkhart KM, Johnson ACM, Sacks BM. Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance”. Kidney Int 56: 1788–1797, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Zager RA, Johnson AC, Hanson SY, Lund S. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int 69: 1181–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Zager RA, Johnson AC, Hanson SY, Shah VO. Acute tubular injury causes dysregulation of cellular cholesterol transport proteins. Am J Pathol 163: 313–320, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zager RA, Johnson AC, Lund S. “Endotoxin tolerance”: TNF-alpha hyperreactivity and tubular cytoresistance in a renal cholesterol loading state. Kidney Int 71: 496–503, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Zager RA, Johnson ACM. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol 296: F1032–F1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zager RA, Johnson ACM. Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury. Am J Physiol Renal Physiol 298: F827–F837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zager RA, Johnson ACM, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int 67: 111–121, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Zager RA, Johnson ACM, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol Renal Physiol 297: F961–F970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zager RA, Shah VO, Shah HV, Zager PG, Johnson AC, Hanson S. The mevalonate pathway during acute tubular injury: selected determinants and consequences. Am J Pathol 161: 681–692, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zager RA, Johnson A, Anderson K. Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int 58: 193–205, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Zhu X, Zhang Y, Bjornsdottir G, Liu Z, Quan A, Constanzo M, Dávila López M, Westholm JO, Ronne H. Histone modifications influence mediator interactions with chromatin. Nucleic Acids Res prepublished online July 8, 2011; doi:10.1093/nar/gkr551, 9999 [DOI] [PMC free article] [PubMed] [Google Scholar]