Abstract
The phosphorylation of the α-subunit of the eukaryotic translation initiation factor 2 (eIF2α) occurs under many stress conditions in mammalian cells and is mediated by one of four eIF2α kinases: PERK, PKR, GCN2, and HRI. Cells of the renal medulla are regularly exposed to fluctuating concentrations of urea and sodium, the extracellular solutes responsible for the high osmolality in the renal medulla, and thus the kidneys ability to concentrate the urine in times of dehydration. Urea stress is known to initiate molecular responses that diverge from those seen in response to hypertonic stress (NaCl). We show that urea-inducible GCN2 activation initiates the phosphorylation of eIF2α and the downstream increase of activating transcription factor 3 (ATF3). Loss of GCN2 sensitized cells to urea stress, increasing the expression of activated caspase-3 and decreasing cell survival. Loss of GCN2 ablated urea-induced phosphorylation of eIF2α and reduced the expression of ATF3.
Keywords: collecting duct, activating transcription factor 3, osmotic stress
cells of the renal medulla are regularly exposed to fluctuating levels of extracellular solute concentrations and can adapt to an increase in osmotic stress by modulating cellular osmolyte content (1, 8). When local osmolality increases during anti-diuresis, organic osmolytes such as glycerophosphate choline, betaine, and sorbitol are accumulated within cells and are extruded when local osmolality drops during diuresis. The concentration of urea in the interstitial fluid of the rat renal medulla during anti-diuresis can reach levels as high as 800 mM (23), possibly higher in mice; nevertheless, renal medullary cells survive and function in this environment.
In addition to the accumulation of organic osmolytes, when faced with high osmotic stress, renal medullary cells can delay the cell cycle (18) and increase the expression of the stress-related transcription factors such as activating transcription factor 3 (ATF3), HO-1, GADD45, and CHOP10 (2, 25–27, 35). Urea and sodium chloride exert different effects on renal cells as a consequence of their differing cellular permeabilities and urea can precondition medullary cells to withstand the apoptotic effects of high sodium chloride (34). Most studies of high urea stress focused on the induction of DNA damage, the regulation of cell cycle checkpoints, and the accumulation of organic osmolytes; little is known about the signaling pathway via which urea could exert a preconditioning effect on cell survival.
When exposed to a stress signal, cells initiate a stress response whereby protein translation is rapidly inhibited via the phosphorylation of the α-subunit of the eukaryotic translation initiation factor 2 (eIF2α). The phosphorylation of eIF2α is a quantitative response; the degree of eIF2α phosphorylation determines whether cells enter growth arrest and survive the stress, or the cells enter apoptosis (17, 20, 21). The phosphorylation of eIF2α is mediated by the activation of one of four eIF2α kinases: the pancreatic endoplasmic reticulum kinase (PERK), dsRNA-activated protein kinase (PKR), general control nonderepressible 2 (GCN2), and heme-regulated inhibitor (HRI). Each kinase can be activated by a spectrum of stresses (22, 28). For example, calcium depletion or hypoxia can activate PERK (15) and amino acid depletion or UV irradiation is known to activate GCN2 (6, 30). However, recent studies demonstrate that hypoxia can also phosphorylate eIF2α via GCN2, demonstrating that eIF2α phosphorylation is a convergence point in the response to many stress signals in the global integrated stress response (22, 28), which is conserved from yeast to mammalian cells (17, 24, 33).
Our previous studies in the intact renal medulla suggested that anti-diuresis increased the expression of stress-related genes. In a concentrating defect mouse model, we identified a potential role for urea in increasing the endoplasmic reticulum (ER) stress pathway genes glucose response protein 78 (GRP78) and ATF3 and ATF4 in renal collecting ducts (5). The aim of the current study was to determine whether high urea stress directly activated these stress response genes via the eIF2α phosphorylation pathway and we addressed this using a renal medullary cell line. We then sought to determine the mechanism of activation, directly addressing the role of urea to increase the activity of eIF2α kinase GCN2 before addressing a role for GCN2 in the ability of renal cells to survive high osmotic stress.
MATERIALS AND METHODS
Materials.
Mouse inner medullary collecting duct (mIMCD3) cell line was purchased from ATCC (Manassas, VA) and GCN2+/+ and −/− MEF cells were generously provided by Dr. Wek (Indiana University School of Medicine). Protease inhibitor (no. 1836153), phosphatase inhibitor (04906845001), WST-1 reagent (no. 11644807001) were from Roche Applied Science (Indianapolis, IN). Antibody ATF3 (no. sc-188, C19) and aquaporin-2 (AQP2; no. 9882, C17) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody phospho-eIF2α (no. 9721S), eIF2α (no. 9722), phospho-GCN2 (no. 3301S), GCN2 (no. 3302), phosphor-PERK (no. 3179), cleaved caspase 3 (no. 9661), and horseradish peroxidase-conjugated goat anti-rabbit IgG (no. 7074) were all from Cell Signaling Technology (Beverly, MA). Alternative phosph-GCN2 antibody (no. ab75836) is also from Abcam (Cambridge, MA). Supersignal West Femto Maximum Sensitivity Substrate (no. 34095) was from Thermo Scientific (Rockford, IL). RNeasy Mini Kit (no. 74104) was from Qiagen (Valencia, CA) and PerfeCTa SYBR Green FastMix (no. 95072–012) was from Quanta Biosciences (Gaithersburg, MD).
Cell culture.
mIMCD3 cells were cultured at 37°C in 5% CO2 atmosphere in 45% low-glucose DMEM and 45% F-12 plus 10% FBS, 2 mM glutamine, and 10 mM HEPES. Confluent cells (95–100%) were used for experiments. MEF cells were cultured in DMEM supplemented with 10% FBS, 2 mM glutamine, 1 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin. Osmolality of control media was 300 mosmol/kgH2O. Hyperosmotic medium was made by adding urea into the control medium.
Protein sample preparation and Western blot.
To prepare protein samples, cultured cells were rinsed twice in cold PBS and harvested in RIPA lysis buffer (150 mM NaCl, 50 mM Tris, 1% NP-40, 1 mM EDTA, pH 7.4) plus protease inhibitor and phosphatase inhibitor. Equal amounts of proteins were loaded and separated on SDS-PAGE gels, and then they were transferred to PVDF membrane. The membrane was blocked in 5% milk and then incubated with GCN2, p-GCN2, phosphor-PERK, eIF2α, p-eIF2α, cleaved caspase 3, and ATF3 antibodies overnight at 4°C, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG for 1 h at room temperature, which was visualized using Supersignal West Femto Maximum Sensitivity Substrate. Images were obtained and quantified by Quantity One (Bio-Rad, Hercules, CA).
RNA isolation and real-time quantitative PCR.
RNA isolation, cDNA synthesis, and quantitative PCR were performed as previously described (4). Briefly, total RNA was isolated from mouse inner medullas using an RNeasy Mini Kit followed by cDNA synthesis. Real-time quantitative PCR was performed using the RotorGene RG3000 sequence detection system and PerfeCTa SYBR Green FastMix. PCR mixture contained 5 μl of SYBR FastMix, 1.0 μl of RNAse-free water, 2.5 pmol of forward and reverse primers, and 16 ng of cDNA in a volume of 10 μl. Each reaction was performed in triplicate at 95°C, 15 s and 60°C, 30 s for 40 cycles. This was followed by a melt cycle that consisted of a stepwise increase in temperature from 60 to 99°C.
Cell survival study.
To determine the role of GCN2 in osmotic tolerance, GCN2+/+ and −/− MEF cells were plated into 96-well plates and exposed to hyperosmotic medium. Sixteen hours after stress, WST-1 reagent was used to evaluate cell survival following the manufacturer's instruction.
Statistical analysis.
Statistical analysis was performed using Student's t-test. To facilitate comparisons, data were normalized such that the mean for the control group was defined as 100%. Results were presented as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Urea induces the phosphorylation of eIF2α in mIMCD3 cells.
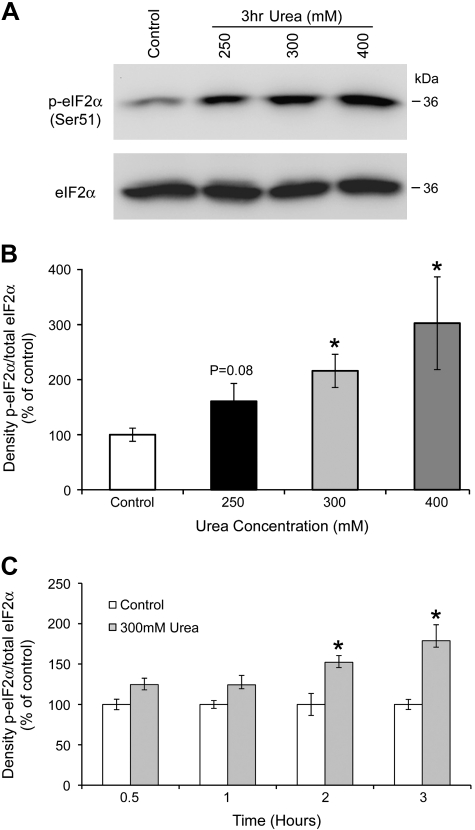
Cells respond to stress by rapidly inhibiting protein synthesis via the phosphorylation of the α-subunit of eIF2α on serine 51. To determine whether collecting duct cells respond to an increase in urea concentration by activating the phosphorylation of eIF2α, mIMCD3 cells were exposed to increasing concentrations of urea (250, 300, and 400 mM) for 3 h, thus raising the osmolality of the culture medium from 300 to 550, 600, and 700 mosmol/kgH2O, respectively. eIF2α phosphorylation (on serine 51) was examined by Western blot analysis. As shown in Fig. 1, A and B, addition of urea to mIMCD3 cells caused a significant increase in eIF2α phosphorylation, in a concentration-dependent manner. Urea-induced phosphorylation of eIF2α was time-dependent, shown graphically in Fig. 1C.
Fig. 1.
Urea induces the phosphorylation of eukaryotic translation initiation factor 2 (eIF2α) in mouse inner medullary cells. A: Western blot analysis of whole cell lysates from mouse inner medullary collecting duct (mIMCD3) after treatment with 250, 300, or 400 mM urea for 3 h, probed for phospho- (top) and total eIF2α (bottom). Total eIF2α was used as loading control for densitometry. B: densitometry results of dose response with phospho-eIF2α normalized to total eIF2α. C: time course of urea-induced eIF2α phosphorylation. mIMCD3 cells were exposed to 300 mM urea for 0.5, 1, 2, and 3 h; data presented as ratio of densitometric values of phospho-eIF2α normalized to total eIF2α. *Significant difference between control and urea-treated cells (t-test; P < 0.05, n = 3).
GCN2, an eIF2α kinase, is activated in response to high urea.
Four kinases have been identified that can phosphorylate eIF2α resulting in an inhibition of translation. PERK is activated in response to the accumulation of unfolded proteins or depletion of intracellular calcium stores (via thapsigargin) and is hence the classic ER stress kinase. We examined the activity of PERK in mIMCD3 cells after the addition of urea at 250, 300, and 400 mM; however, no change in PERK activity was observed (data not shown).
GCN2 is known to be activated in cells in response to amino acid deprivation (28) with activity measured by the phosphorylation of GCN2 at threonine 898. Data in Fig. 1 demonstrated that exposure to 300 mM urea for 3 h increased phospho-eIF2α in mIMCD3 cells. To determine whether GCN2 was activated at 300 mM urea, we exposed mIMCD3 cells for 30 min to 16 h and examined the expression of phospho-GCN2 by Western blot. Data shown in Fig. 2 demonstrate that 300 mM urea rapidly increases the amount of phospho-GCN2 in mIMCD3 cells. GCN2 kinase activity was elevated at 30 min, and remained elevated through 6 h, returning to a basal level after 16 h of urea stress (Fig. 2, A and C). Data in Fig. 2B demonstrate that urea activation of GCN2 was dose dependent, with an increase in phospho-GCN2 observed at the lowest dose of urea tested, 250 mM.
Fig. 2.
Urea induces the phosphorylation of eIF2α kinase, GCN2. Western blot analysis of GCN2 phosphorylation (top) and GCN2 total (bottom) in whole cell lysates from mIMCD3 following: A: time course treatment with 300 mM urea, in intervals from 30 min to 16 h, as indicated; B: dose-response treatment with 250, 300, or 400 mM urea for 3h. C: densitometry results of A. D: densitometry results of B. Significant difference *control vs. urea-treated cells, #300 vs. 400 mM urea-treated cells (t-test; P < 0.05, n = 3).
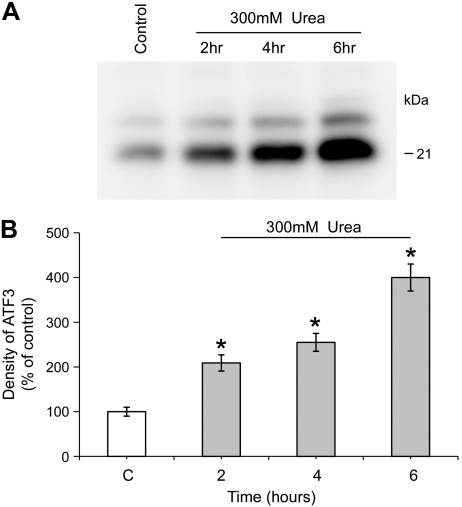
Urea increases the abundance of ATF3 but not ATF4 in mIMCD3 cells.
ATF3 and ATF4 regulation are known to be dependent on eIF2α phosphorylation (12, 14, 17). Urea is known to increase ATF3 mRNA and protein abundance in mIMCD3 cells (27). Data in Fig. 3 confirm this observation and demonstrate that urea increased the protein expression of ATF3 in a time-dependent manner, with a significant increase in ATF3 protein expression observed after 2-h incubation with 300 mM urea. In contrast, while urea increased the expression of ATF3 mRNA in mIMCD3 cells, urea did not increase the expression of ATF4 as shown in Fig. 4.
Fig. 3.
Urea increases the expression of activating transcription factor 3 (ATF3) protein in mIMCD3 cells. A: Western blot analysis of ATF3 expression in whole cell lysates from mIMCD3 following treatment with 300 mM urea for 2, 4, and 6 h. B: densitometry results of A. *Significant difference between control and urea-treated cells (t-test; P < 0.05, n = 3).
Fig. 4.
Urea increases the abundance of ATF3 mRNA in mIMCD3 cells. SYBR Green I real-time PCR assay validation for expression of ATF3 and ATF4 in mIMCD3 cells following the addition of 250, 300, and 400 mM urea for 4 h. The data shown are the mean relative fold-change vs. control (C) cells. *Significant difference between control and urea-treated cells (t-test; P < 0.05, n = 3). Assays for β-actin were run in parallel on each sample for subsequent normalization of the data.
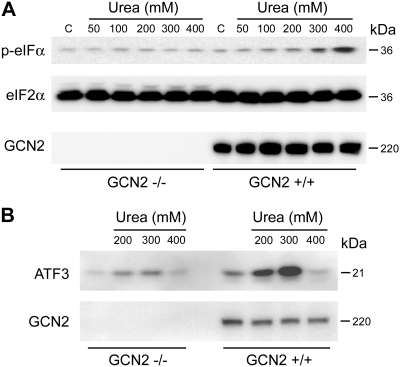
Loss of GCN2 reduces the ability of urea to activate the eIF2α pathway.
To determine whether GCN2 is the primary kinase responsible for the urea-induced phosphorylation of eIF2α, we exposed GCN2−/− MEF cells to varying concentrations of urea. In the absence of GCN2, urea-induced eIF2α phosphorylation was significantly reduced (Fig. 5A). In the absence of GCN2, urea-induced expression of ATF3 was significantly reduced (Fig. 5B). These data suggest that GCN2 is the primary eIF2α kinase responsible for the phosphorylation of eIF2α and the activation of ATF3, following urea stress.
Fig. 5.
Loss of GCN2 reduces urea-induced eIF2α phosphorylation and ATF3 expression. Western blot analysis of whole cell lysates from GCN2+/+ and GCN2−/− MEF cells after treatment with A: 50, 100, 200, 300, or 400 mM urea for 1 h, probed for phospho-eIF2α (top) and total eIF2α (middle) and total GCN2 (bottom). B: ATF3 expression following treatment with 200, 300, and 400 mM urea for 6 h.
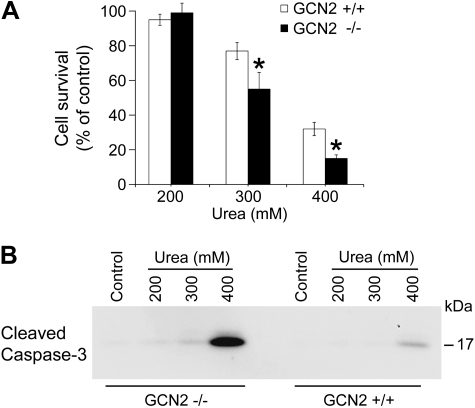
Loss of GCN2 decreases cell survival to osmotic stress.
To determine whether GCN2 plays a role in the ability of cells to survive high urea stress, GCN2−/− and GCN2+/+ MEF cells were exposed to increasing concentrations of urea. Cell survival was measured at each urea concentration. As shown in Fig. 6A, GCN2−/− cells had a decreased ability to survive in the face of high osmotic stress at 300 and 400 mM urea. Following exposure to 300 mM urea for 16 h, 55% of GCN2−/− cells survived, vs. 77% of GCN2 +/+ cells, a significant decrease in cell survival. Following exposure to 400 mM urea for 16 h, cell survival of GCN2−/− cells was also significantly decreased compared with control GCN2 +/+ cells.
Fig. 6.
Loss of GCN2 decreases cell survival to osmotic stress. A: GCN2+/+ and −/− MEF cells were exposed to 200, 300, and 400 mM urea for 16 h. Cells were incubated with WST-1 to evaluate cell survival. The percentage of viable cells was calculated as control cells were defined as 100% viable. *Significant difference between GCN2+/+ and GCN−/− cells (t-test; P < 0.05, n = 7). B: Western blot analysis of activated caspase-3 levels in whole cell lysates from GCN2+/+ and GCN−/− MEF cells after treatment with 200, 300, and 400 mM urea for 6 h.
Previous studies demonstrated that high urea causes cell death by apoptosis (18). Caspase 3 is activated by the protein’s cleavage, which plays a critical role in the executive stage of apoptosis. To confirm that GCN2−/− cells were entering apoptosis following exposure to high urea stress, we used Western blots to determine the expression of cleaved caspase 3 in both GCN2−/− and GCN2 cells. The expression of activated caspase 3, following a urea stress of 400 mM for 6 h, was significantly increased in GCN2−/− MEF cells compared with GCN2 +/+ MEF cells as shown in Fig. 6B. Activated caspase 3 was also detected in cells treated with 300 mM urea when GCN2 was absent, but not in control cells. These data suggest that GCN2 is necessary for cells to survive high urea-related osmotic stress.
DISCUSSION
The current study demonstrates that high urea causes the phosphorylation of eIF2α in a renal medullary collecting duct epithelial cell line primarily via the activation of the general nonderepressible control kinase, GCN2. The activation of GCN2 and the phosphorylation of eIF2α by urea were shown to be concentration and time dependent. This study also demonstrates that loss of GCN2 reduced the ability of high urea to significantly increase the expression of the transcription factor, ATF3. GCN2-negative cells were more susceptible to the proapoptotic effects of high urea than wild-type GCN2 cells. These data from the medullary collecting duct cell line suggest that urea activation of GCN2 contributes to the ability of renal collecting duct cells to tolerate high urea-induced osmotic stress.
Studies in yeast and in mammalian cells have demonstrated that GCN2 is activated by amino acid depletion (30) and UV irradiation (6, 21). Under conditions of amino acid starvation, the accumulation of uncharged t-RNAs activates GCN2 phosphorylation of eIF2α; global protein synthesis is downregulated, and yeast adapts to the starvation stress (29, 36). Following the inhibition of protein synthesis, the translation of a transcription factor, GCN4 in yeast, is promoted, which in turn activates the transcription of genes encoding amino acid biosynthetic enzymes. In mammalian cells, the expression of transcription factors ATF3 and ATF4 is promoted during the inhibition of protein synthesis, thus performing a similar function in response to the phosphorylation of eIF2α. GCN2 phosphorylation of eIF2α also occurs when yeast is subjected to salt stress (osmotic stress). However, loss of GCN2 function improved salt tolerance in yeast, and induction of GCN2 was associated with an increase in apoptosis (9). In contrast, data presented here suggest activation of GCN2 protects renal cells against high urea osmotic stress. In our studies, loss of GCN2 decreased the cell survival rate when cells were exposed to high urea concentrations.
High-salt osmotic stress has been shown to cause the phosphorylation of eIF2α in mouse reticulocytes; when exposed to a high-salt osmotic stress (0.5 M NaCl), eIF2α phosphorylation occurred along with the activation of the eIF2α kinase, HRI (16). However, HRI was not the only eIF2α kinase responsible for eIF2α phosphorylation under osmotic stress; data in HRI−/− and PKR−/− reticulocytes showed that eIF2α phosphorylation still occurred. The authors concluded that it was possible that GCN2 or PERK may be the additional eIF2α kinase activated in response to this high-sodium osmotic stress but they did not proceed to directly study GCN2 or PERK. Our studies presented here demonstrate that high urea osmotic stress activates GCN2 but does not activate PERK. In GCN2−/− cells, eIF2α phosphorylation was significantly reduced following urea osmotic stress; this reduction in eIF2α was accompanied by a significant reduction in ATF3 expression, and an increase in cleaved caspase-3 expression.
ATF3 is a transcriptional regulator that is a member of the ATF/CREB subfamily of the basic region leucine zipper family (10). ATF3 levels are known to be induced in response to a variety of stress conditions in many different tissues (10, 11). In the kidney, ATF3 is induced in response to renal ischemia (32) and the response is a protective one for proximal tubule cells. In a microarray study aimed at identifying osmotically regulated genes in renal medullary cells, ATF3 was demonstrated to increase in response to urea and to a lesser degree with sodium chloride (27). Our data, presented here, suggests that the activation of ATF3 maybe a key element of the signaling pathway necessary for cells to survive a urea-induced osmotic stress. However, in contrast to eIF2α phosphorylation, urea-induced ATF3 expression was not completely blocked by the loss of GCN2. ATF3 expression was also rapidly induced in mIMCD3 cells by urea, with a comparable time frame as eIF2α phosphorylation. Combined, these data suggest that the GCN2/eIF2α pathway is not the only signal increasing ATF3 expression in response to urea.
Several genes have been identified as target genes of ATF3 and these include GADD153 (7) and E-selectin (19). ATF3 can also regulate cellular functions through protein-protein interactions (31). It is likely that ATF3 target genes vary in different tissues and in response to different stress signals and considering the array of stress responses that can activate ATF3, specific studies should be performed to identify ATF3 targets following high osmotic stress in the renal medulla.
As mentioned, GCN2 was originally found to be activated by the accumulation of uncharged t-RNA caused by amino acid starvation (16). Protein translation is an energy-consuming process. Under stressful conditions, eIF2α is associated with the global reduction of translation, thus energy is conserved while cells activate mechanisms to overcome the stress encountered. However, persistent eIF2α phosphorylation associated with protein translation inhibition is not compatible with normal cell activity. It is unclear at this time how high urea activates GCN2 to increase eIF2α phosphorylation, although it can be hypothesized that the resulting inhibition of translation protects cells during conditions of hyperosmolality. Further studies will explore this protective mechanism of GCN2 activation in the face of high urea.
Finally, ATF4 is a transcription factor that is activated during many stress responses and it is known to activate the specific translation of stress genes during the eIF2α phosphorylation-dependent inhibition of global translation (13). ATF4 activation requires PERK-dependent eIF2α phosphorylation (17). Neither PERK nor ATF4 was activated by urea stress in our study. In vivo, we reported that ATF4 expression increases in the renal medulla of water-restricted mice (3) and in the renal medulla of dDAVP-infused, AQP1-null mice (5).
In summary, urea increases the phosphorylation of eIF2α, via the activation of kinase GCN2, in renal medullary cells. Urea is known to increase the expression of ATF3, and we demonstrate here that loss of GCN2 reduces urea-induced ATF3 expression. Loss of GCN2 reduces the ability of cells to tolerate urea-induced osmotic stress.
GRANTS
This work was funded by National Institutes of Health Grant RO1-DK073611 (to H. L. Brooks) and by the American Physiological Society's Postdoctoral Fellowship in Physiological Genomics (to Q. Cai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J Biol Chem 283: 7309–7313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai Q, Dmitrieva NI, Ferraris JD, Michea LF, Salvador JM, Hollander MC, Fornace AJ, Jr, Fenton RA, Burg MB. Effects of expression of p53 and Gadd45 on osmotic tolerance of renal inner medullary cells. Am J Physiol Renal Physiol 291: F341–F349, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Cai Q, Keck M, McReynolds MR, Klein JD, Greer KA, Sharma K, Hoying JB, Sands JM, Brooks HL. Effects of water restriction on gene expression in mouse renal medulla: identification of 3β-HSD4 as a collecting duct protein. Am J Physiol Renal Physiol 291: F218–F224, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Renal Physiol 293: F1858–F1864, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cai Q, Nelson SK, McReynolds MR, Diamond-Stanic MK, Elliott D, Brooks HL. Vasopressin increases expression of UT-A1, UT-A3, and ER chaperone GRP78 in the renal medulla of mice with a urinary concentrating defect. Am J Physiol Renal Physiol 299: F712–F719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, Kaufman RJ, Ron D, Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 12: 1279–1286, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339: 135–141, 1999 [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Perez A, Burg MB. Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol 119: 1–13, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Goossens A, Dever TE, Pascual-Ahuir A, Serrano R. The protein kinase Gcn2p mediates sodium toxicity in yeast. J Biol Chem 276: 30753–30760, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr 7: 321–335, 1999 [PMC free article] [PubMed] [Google Scholar]
- 12. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24: 1365–1377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22: 7405–7416, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol 21: 7971–7980, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by preemptive conditional phosphorylation of translation initiation factor 2. EMBO J 23: 169–179, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michea L, Ferguson DR, Peters EM, Andrews PM, Kirby MR, Burg MB. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am J Physiol Renal Physiol 278: F209–F218, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Nawa T, Nawa MT, Cai Y, Zhang C, Uchimura I, Narumi S, Numano F, Kitajima S. Repression of TNF-alpha-induced E-selectin expression by PPAR activators: involvement of transcriptional repressor LRF-1/ATF3. Biochem Biophys Res Commun 275: 406–411, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Niknejad N, Morley M, Dimitroulakos J. Activation of the integrated stress response regulates lovastatin-induced apoptosis. J Biol Chem 282: 29748–29756, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Parker SH, Parker TA, George KS, Wu S. The roles of translation initiation regulation in ultraviolet light-induced apoptosis. Mol Cell Biochem 293: 173–181, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Sadowski J, Dobrowolski L. The renal medullary interstitium: focus on osmotic hypertonicity. Clin Exp Pharmacol Physiol 30: 119–126, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Sood R, Porter AC, Ma K, Quilliam LA, Wek RC. Pancreatic eukaryotic initiation factor-2alpha kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem J 346: 281–293, 2000 [PMC free article] [PubMed] [Google Scholar]
- 25. Tian W, Boss GR, Cohen DM. Ras signaling in the inner medullary cell response to urea and NaCl. Am J Physiol Cell Physiol 278: C372–C380, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Tian W, Cohen DM. Signaling and gene regulation by urea in cells of the mammalian kidney medulla. Comp Biochem Physiol A Mol Integr Physiol 130: 429–436, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Tian W, Cohen DM. Urea stress is more akin to EGF exposure than to hypertonic stress in renal medullary cells. Am J Physiol Renal Physiol 283: F388–F398, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal 9: 2357–2371, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Wek RC, Jackson BM, Hinnebusch AG. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci USA 86: 4579–4583, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 15: 4497–4506, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan C, Lu D, Hai T, Boyd DD. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J 24: 2425–2435, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshida T, Sugiura H, Mitobe M, Tsuchiya K, Shirota S, Nishimura S, Shiohira S, Ito H, Nobori K, Gullans SR, Akiba T, Nitta K. ATF3 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19: 217–224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, Tian W, Cohen DM. Urea protects from the proapoptotic effect of NaCl in renal medullary cells. Am J Physiol Renal Physiol 279: F345–F352, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z, Yang XY, Cohen DM. Urea-associated oxidative stress and Gadd153/CHOP induction. Am J Physiol Renal Physiol 276: F786–F793, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Zhu S, Sobolev AY, Wek RC. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J Biol Chem 271: 24989–24994, 1996 [DOI] [PubMed] [Google Scholar]