Abstract
Alterations in epithelial cell polarity and in the subcellular distributions of epithelial ion transport proteins are key molecular consequences of acute kidney injury and intracellular energy depletion. AMP-activated protein kinase (AMPK), a cellular energy sensor, is rapidly activated in response to renal ischemia, and we demonstrate that its activity is upregulated by energy depletion in Madin-Darby canine kidney (MDCK) cells. We hypothesized that AMPK activity may influence the maintenance or recovery of epithelial cell organization in mammalian renal epithelial cells subjected to energy depletion. MDCK cells were ATP depleted through a 1-h incubation with antimycin A and 2-deoxyglucose. Immunofluoresence localization demonstrated that this regimen induces mislocalization of the Na-K-ATPase from its normal residence at the basolateral plasma membrane to intracellular vesicular compartments. When cells were pretreated with the AMPK activator metformin before energy depletion, basolateral localization of Na-K-ATPase was preserved. In MDCK cells in which AMPK expression was stably knocked down with short hairpin RNA, preactivation of AMPK with metformin did not prevent Na-K-ATPase redistribution in response to energy depletion. In vivo studies demonstrate that metformin activated renal AMPK and that treatment with metformin before renal ischemia preserved cellular integrity, preserved Na-K-ATPase localization, and led to reduced levels of neutrophil gelatinase-associated lipocalin, a biomarker of tubular injury. Thus AMPK may play a role in preserving the functional integrity of epithelial plasma membrane domains in the face of energy depletion. Furthermore, pretreatment with an AMPK activator before ischemia may attenuate the severity of renal tubular injury in the context of acute kidney injury.
Keywords: epithelial polarity, MDCK
acute kidney injury (AKI) induced by renal ischemia leads to significant morbidity and mortality (45). Despite advances in the understanding of molecular mechanisms associated with ischemic AKI, treatment remains largely supportive (55). At a molecular level, energy deprivation causes key energy-dependent membrane proteins to become displaced and dysfunctional. In particular, in the proximal tubule, the Na-K-ATPase is internalized from the basolateral membrane, disrupting the cell's capacity to maintain normal transepithelial sodium transport (13). Preservation of a polarized plasma membrane distribution of Na-K-ATPase in renal epithelia is essential for the maintenance of both solute reabsorption and volume homeostasis. It has been demonstrated that Na-K-ATPase becomes mislocalized after energy deprivation (34, 39, 40). ATP depletion also perturbs the distributions of tight junction proteins, further disrupting epithelial cell polarity and organization (16) and leading to backleak of extracellular fluid into the urinary space. Such molecular insults result in a myriad of clinical consequences, including electrolyte disturbances, fluid imbalance, and accumulation of potentially harmful toxins.
The AMP-activated protein kinase (AMPK) is a promising component of a signaling cascade that may modulate the severity of ischemic injury. AMPK is a ubiquitously expressed serine-threonine kinase that serves as an important intracellular energy sensor. It is activated by conditions that deplete ATP and alter the AMP:ATP ratio, including hypoxia, ischemia, and glucose deprivation (18). AMPK stimulates catabolic pathways such as fatty acid oxidation, glucose uptake, and glycolysis, while downregulating ATP-utilizing systems, including those associated with fatty acid and protein synthesis (22). In addition, it couples information about metabolic status to the regulation of membrane transport (18, 20). Other targets of AMPK include pathways modulating inflammation, apoptosis, angiogenesis, and blood flow (48, 54, 60). Additionally, our group and others have demonstrated that AMPK is involved in tight junction assembly (64, 66). Finally, multiple investigators have described the importance of AMPK in the maintenance of cell polarity, with or without energetic stress (29, 37).
In light of these properties, we theorized that AMPK might play a role in kidney ischemic preconditioning. The concept of ischemic preconditioning is based on the observation that brief intermittent ischemic events are protective against large ischemic attacks in canine hearts (42). Decades of study indicate that similar preconditioning phenomena operate in the kidney (3, 7). In vivo, renal ischemic preconditioning protects cell polarity, and in particular ameliorates mislocalization of Na-K-ATPase (3). Proposed mechanisms include slowing of ATP depletion and reduction in energy demand during ischemia; dedifferentiation of epithelial cells; and upregulation of protective proteins and pathways (3, 7, 42). We wished to investigate whether AMPK was a factor which, if functionally upregulated before renal ischemia, could exert a protective effect. Preactivation of AMPK before ischemia might protect cells by conserving and reallocating energy stores via downregulation of anabolic pathways and upregulation of systems generating ATP.
The importance of AMPK in the setting of ischemic cardiac injury has been well described (46, 58). Transgenic mice expressing a dominant negative AMPK isoform demonstrate impaired functional recovery when subjected to cardiac ischemia-reperfusion injury (54). Furthermore, cardiac cells from these mice were less responsive to preconditioning, as opposed to wild-type cells. Preactivation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) is also associated with reduced severity of LPS-induced lung injury (65). In the kidney, Mount et al. (41) demonstrated that AMPK is quickly and dramatically upregulated in ischemic injury, although the pathways through which it acts appear to be unique from those that transduce its influence in skeletal and cardiac muscle.
Our studies aimed to characterize the role of AMPK in the modulation of renal epithelial cell injury. We hypothesized that the AMPK cascade plays a role in preserving renal cell architecture and cell polarity in the face of energy deprivation and that its preactivation might thus be protective in the context of AKI. Drugs such as metformin, in wide clinical use for the treatment of type II diabetes mellitus, are capable of enhancing AMPK activity (56) and have been shown to have beneficial effects in vivo in the setting of cardiac ischemia (8, 57). We show that AMPK can be activated by metformin in the cultured Madin-Darby canine kidney (MDCK) line of renal tubule epithelial cells and that pretreatment before energy deprivation decreases internalization and mislocalization of Na-K-ATPase. Furthermore, AMPK preactivation at least partially ameliorates histological and biochemical consequences of kidney injury in an in vivo model.
MATERIALS AND METHODS
Cell culture.
MDCK cells were grown in a 5% CO2-95% air humidified incubator at 37°C in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, and 50 g/ml streptomycin. Cells were subjected to energy depletion via incubation in a prewarmed glucose-free DMEM containing 0.1 μM antimycin A (AA) and 25 mM 2-deoxyglucose (2-DG; Sigma-Aldrich, St. Louis, MO) to inhibit aerobic and substrate-dependent ATP generation, respectively. Cells were subjected to 30 min to 1 h of energy deprivation. Cells subjected to AMPK preactivation were treated with 1 mM metformin (Sigma-Aldrich) or 1 mM AICAR (Calbiochem, San Diego, CA) added to their media for 2 h.
Generation of AMPK-deficient cell line.
As previously described (59), a short hairpin (sh) RNA targeting the canine AMPK α1-subunit was subcloned into pLH1, a derivative of the pSUPER plasmid. The sequence was as follows: 5′-GCAGAAGTTTGTAGGGCAATT-3′. Plasmids were transfected with pVSVG into HEK-G2 cells via lentiviral packaging and then allowed to infect subconfluent MDCK cells. Stable MDCK cell clones were selected and maintained in α-MEM containing 4 μg/ml puromycin (Sigma-Aldrich). Western blot analysis was performed to screen clones for reduced expression of AMPK α1. To derive a control cell line, MDCK cells were infected with an empty pLH1 lentiviral-packaged plasmid.
Western blot analysis.
Western blotting was performed according to the standard protocol used in our laboratory (64). Cells were lysed with buffer containing phosphatase and protease inhibitors [250 mM sucrose/50 mM NaCl/50 mM NaF/5 mM Na4P2O7/1 mM Na3VO4/20 mM Tris·HCl, pH 7.4/2 mM DTT/1% Triton X-100/Complete (Santa Cruz Biotechnologies, Santa Cruz, CA)]. Cell lysates were cleared by centrifugation at 18,000 g for 15 min at 4°C, and protein concentrations were determined by colorimetric assay (Bio-Rad, Hercules, CA). Proteins were resolved by SDS-PAGE on an 8% gel, electrophoretically transferred to nitrocellulose membranes (Bio-Rad), and nonspecific binding sites were blocked through incubation for 1 h at room temperature in buffer containing 20 mM Tris, pH 7.4, 150 mM NaCl, 5% powdered milk, and 0.1% Tween. Blots were then incubated with one of the following primary antibodies in 1:100 dilutions: anti-pan-α-AMPK, anti-phospho-AMPK (p-AMPK) (Cell Signaling, Boston, MA), anti- phospho-acetyl-CoA carboxylase (p-ACC; Upstate, Billerica, MA), and β-actin (Abcam, Cambridge, MA). Subsequently, membranes were probed with horseradish peroxidase-conjugated species-appropriate secondary antibodies, diluted 1:100 (Jackson ImmunoResearch Laboratories, West Grove, PA) and proteins were visualized with an enhanced chemiluminescence detection kit (Amersham, Piscataway, NJ). Band density was quantified using Image J software (National Institutes of Health, Bethesda, MD).
ATP measurement.
Measurement of ATP levels was performed as previously described (62). Briefly, MDCK cells were plated and grown to confluence. After treatment with 2-DG/AA, metformin, or AICAR, cells were quickly washed with double-distilled water, scraped off the wells into 0.5 ml of fresh double-distilled water, and then lysed by sonication for 30 s on ice. Samples were then boiled for 3 min to inactivate ATP hydrolytic activity and centrifuged at 4°C for 10 min at 15,000 rpm. The supernatant was collected, and protein concentrations were determined by colorimetric assay (Bio-Rad). ATP levels were measured using the Sigma FL-AA Bioluminescent assay kit, which involved incubating 25 μl of cell extract and 100 μl of ATP assay mix (FL-AAM) diluted in ATP assay mix dilution buffer (FL-AAB). Bioluminescence derived from the luciferin-luciferase reaction was measured in a luminometer (Beckman, Brea, CA).
Cell surface biotinylation.
MDCK cells were plated on Transwell filter inserts (Corning, Corning, NY), biotinylated with NHS-SS-biotin as described previously (17), and then subjected to treatment with metformin or incubation with α-MEM before energy deprivation. Biotin exposed at the basolateral cell surface was stripped with 100 mM 2-mercaptoethane sulfonate sodium (MesNa), and cells were washed with PBS++ (supplemented with 10 mM MgCl2 and 1 mM CaCl2). Cells were then lysed in one milliliter of lysis buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA), and incubated overnight at 4°C with streptavidin-conjugated agarose beads (Pierce, Rockford, IL). Precipitated proteins were eluted from the beads through incubation in SDS-PAGE sample buffer supplemented with 100 mM DTT and analyzed by standard SDS-PAGE and Western immunoblotting. To assess the level of Na-K-ATPase expression, equal amounts of total lysates were subjected to SDS-PAGE and Western immunoblotting using a monoclonal antibody (α5) directed against an epitope of the α-subunit of Na-K-ATPase (25). Band density was quantified using Image J software (National Institutes of Health).
Immunofluoresence analysis of MDCK cells.
Immunofluoresence analysis of MDCK cells was performed according to the standard protocol used in our laboratory (64). Cells were plated on Transwell filters, grown to confluency, and allowed to polarize fully over the course of 3 days. They were treated with AICAR, metformin, energy deprivation, or AICAR and metformin before energy deprivation, as described above. Cells were then fixed with 4% paraformaldehyde in PBS++ for 30 min at room temperature and washed with PBS++. Fixed cells were permeabilized with PBS containing 1% BSA and 3% Triton X-100 (Sigma), and then blocked with goat serum dilution buffer (16% goat serum/20 mM Na3PO4, pH 7.4/450 mM NaCl/0.3% Triton X-100). Cells were incubated in the α5 monoclonal antibody directed against the α-subunit of the Na-K-ATPase at a dilution of 1:100, and proteins were visualized with a FITC-conjugated goat-derived antibody directed against anti-IgG (Sigma), diluted 1:100. Slides were mounted with VectaShield aqueous mounting medium (Vector Laboratories, Burlingame, CA). Samples were evaluated and imaged using a Zeiss LSM 410 confocal microscope, using uniform contrast and brightness settings that were chosen to ensure that all pixels were within the linear range.
ATPase assay.
The ATPase assay was performed using a colorimetric procedure to quantify the Pi released from ATP, as previously described (35). Briefly, polarized MDCK cells were untreated (control) or treated with 1 mM of AICAR (AICAR), 2 mM of metformin (Met), energy depleted by incubating with 2DG/AA, or pretreated with AICAR (AICAR+2DG/AA) or metformin (Met+2DG/AA) for 2 h before energy depletion. After various treatments, membranes were permeabilized by incubating cells in 0.65 mg/ml deoxycholic acid, 2 mM EDTA, and 20 mM imidazole for 30 min at room temperature. ATPase measurement was accomplished by incubating 50 μl of permeabilized cells containing 10 μg of total protein with 500 μl of assay solution (3 mM MgCl2, 3 mM Tris-ATP, 25 mM imidazole, 3 mM KCl, and 100 mM NaCl, pH 7.4) for 2 h at 37°C to allow at least 4% and no more than 10% of the total ATP to be hydrolyzed under Vmax conditions. To assess the maximal specific Na-K-ATPase activity, samples were incubated in the presence or absence of 100 μM ouabain. After incubation, the reaction was stopped by adding 1 ml of fresh cold stop solution (0.5 M HCl, 3% ascorbic acid, 0.5% ammonium molybdate, 1% SDS). The color development of the phosphate assay was achieved by addition of 1.5 ml of a solution containing 2% sodium meta-arsenite, 2% sodium citrate, and 2% acetic acid. Tubes were then incubated at 37°C for 10 min. Free Pi was measured by absorbance at 850 nm on a Beckman DU-640 spectrophotometer. The specific Na-K-ATPase activity was determined by calculating the difference between samples with or without 100 μM ouabain. The experiments were performed in triplicate, and results shown correspond to the mean of three different assays.
Acute renal ischemia.
All animal protocols were approved by the Yale School of Medicine Institutional Animal Care and Use Committee. Eight-week-old male C57BL/6 mice (Charles River, Wilmington, MA) weighing 20–25 g were treated with intraperitoneal injections of metformin (Sigma) at 300 mg·kg−1·day−1 or with a similar volume of vehicle for 3 days before surgery. Animals were then anesthetized with ketamine (100 mg/kg) by intraperitoneal injection and, using aseptic techniques, subjected to a laparotomy only (“sham surgery”) or a laparotomy with bilateral renal pedicle clamping (“ischemia”) for 30 min. Ischemia was confirmed by color change observed in kidneys following clamping. For analgesia, buprenorphine (0.05 mg/kg) was injected subcutaneously before surgical procedures and then postoperatively every 12 h for 48 h. Supportive fluids were given throughout the operative period, and hypothermia was prevented by use of an isothermal heating pad and warming lights.
At each specified time point, the animals were once again anesthetized with ketamine (100 mg/kg) and subjected to a laparotomy. Kidneys were collected after being snap frozen in situ. Frozen kidneys were stored at −80°C or immediately homogenized in 800 μl of cold homogenization buffer containing phosphatase and protease inhibitors [250 mM sucrose/50 mM NaCl/50 mM NaF/5 mM Na4P2O7/1 mM Na3VO4/20 mM Tris·HCl, pH 7.4/2 mM DTT/1% Triton X-100/Complete (Santa Cruz Biotechnologies)]. Homogenates were then prepared as previously published (41). Specimens were rotated for 1 h at 4°C, centrifuged at 16,000 g for 5 min at 4°C to separate nuclei and tissue debris, and then ultracentrifuged at 100,000 g for 60 min at 4°C to remove cytoskeletal proteins. Protein concentrations of supernatants were determined by Bradford assay (Bio-Rad) and subjected to Western blot analysis.
Fixation and preparation of kidney tissue.
Kidney tissue was fixed according to our laboratory's standard protocol (50). Briefly, mice were fixed via whole body ventricular perfusion of PBS, pH 7.4 for 15 s followed with freshly prepared 4% formaldehyde in PBS for 2 min. Kidneys were removed, dissected into transverse slices 1 mm thick, and postfixed for 1 h at room temperature. This fixation was followed by three 15-min washes in PBS. The tissue was then prepared for cryostat sectioning or further postfixed in PBS 1% glutaraldehyde followed by osmication and embedding in EPOX-812 resin. Epoxy-embedded tissue was sectioned at 2 μm with a Reichert Ultracut-E ultramicrotome and stained with a mixture of 1% azure I and II in water and 0.5% sodium borate.
Immunofluoresence analysis of mouse kidneys.
Cryostat-prepared sections were subjected to 15 min of antigen retrieval through incubation in 10 mM, pH 6.0 citrate buffer. Following multiple washes with TBS and quenching with 0.5 M NH4Cl, sections were washed with 1% SDS and then blocked with 0.1% BSA in 10% goat serum buffer. Primary antibody to p-AMPK or to the α5-antibody of the Na-K-ATPase was applied at a dilution of 1:500, followed by secondary antibody Alexa Fluor goat anti-rabbit 594 or 488, respectively, at a 1:200 dilution (Molecular Probes, Carlsbad, CA).
Measurement of biochemical parameters.
Urine was obtained by bladder puncture, and blood was obtained by retro-orbital bleed. Urine neutrophil gelatinase-associated lipocalin (NGAL) measurements were obtained via ELISA (38) and were performed in the laboratory of Dr. Prasad Devarajan (Cincinnati Children's Medical Center, Cincinnati, OH). Fresh whole blood capillary samples were measured on a coulometric electrochemical sensor to obtain glucose levels (Abbott, Alameda, CA). Serum creatinine measurements were performed by the Mouse Metabolic Phenotyping Center (MMPC) at Yale (New Haven, CT).
Statistical evaluation.
All experiments were carried out in at least three independent replicates. Values are expressed as means ± SE. Comparisons between experimental groups were made using Student's t-test.
RESULTS
AMPK activation in MDCK cells.
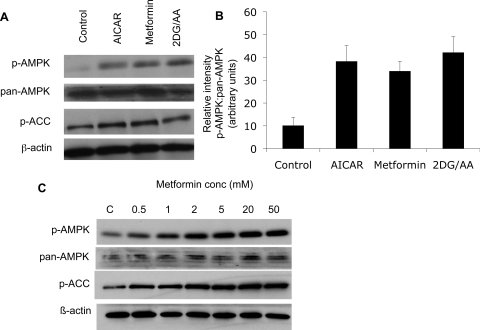
AMPK activity responds to alterations in the ratio of AMP:ATP. Thus we anticipated that subjecting MDCK cells to energy depletion would lead to an increase in p-AMPK (activated) levels. ATP depletion was achieved by incubation in a glucose-free media containing 2DG/AA. This treatment led to AMPK activation, as detected by Western blotting using an antibody directed against p-AMPK at Thr172. We also probed blots with an anti- p-ACC antibody, which revealed that energy deprivation led to increased levels of p-ACC in MDCK cells (Fig. 1A). AMPK activation was detected within 1 min of energy deprivation initiation and was reversed after 1 h of recovery in standard glucose-containing media (data not shown). Metformin and AICAR, known activators of AMPK in other cell types (9, 11, 67), also activated AMPK, as detected by Western blotting (Fig. 1A). The activation of AMPK by metformin is dose dependent (Fig. 1C).
Fig. 1.
AMP-activated protein kinase (AMPK) activation occurs with ATP depletion and with administration of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and metformin in Madin-Darby canine kidney (MDCK) cells. MDCK cells were treated with AICAR, or metformin, or energy depleted using a combination of 2-deoxy-d-glucose (2-DG) and antimycin A (AA) in a glucose-free media. Cell lysates were prepared as described in the text, and 10 μg of protein was loaded in each well. A: representative Western blot of MDCK cell lysates demonstrates AMPK activation, as revealed by increases in the level of phosphorylated (p)-AMPK in response to treatment with AICAR, metformin, or to energy depletion produced by incubation with 2-DG/AA. Phospho-acetyl-CoA carboxylase (p-ACC) is also upregulated under these conditions. B: quantification of p-AMPK relative to total AMPK expression by densitometry. C: treatment with metformin activates AMPK in a dose-dependent fashion. Results are representative of at least 3 independent studies and reflect means ± SE.
Energy depletion results in internalization of Na-K-ATPase, and preactivation of AMPK attenuates this effect.
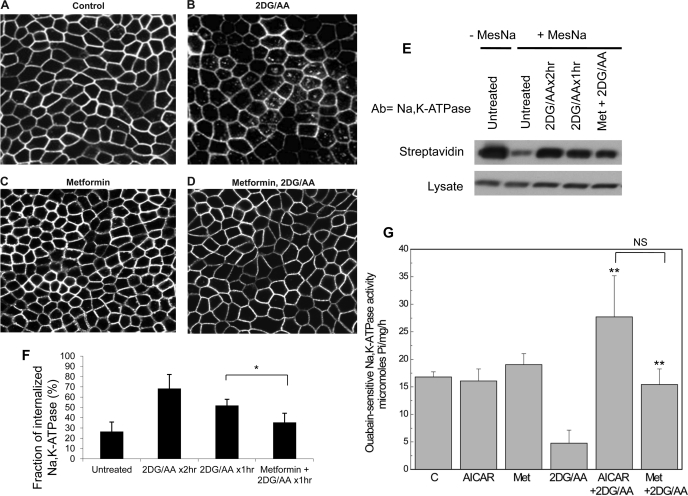
Multiple investigators have shown that energy depletion results in internalization of Na-K-ATPase from the cell surface into intracellular compartments (28, 39). We wondered whether AMPK preactivation would reduce this effect. To test this possibility, MDCK cells were incubated with 2DG/AA and Na-K-ATPase localization was assessed by immunofluorescence microscopy and by cell surface biotinylation. As expected, ATP depletion resulted in internalization of Na-K-ATPase to endocytic compartments (Fig. 2, B, E, and F). Metformin treatment alone produced no effect on Na-K-ATPase distribution (Fig. 2C), but pretreatment with metformin substantially reduced this effect (Fig. 2, D–F). Pretreatment with an AMPK activator (either metformin or AICAR) before energy depletion preserved the level of total cell-associated, ouabain-sensitive Na-K-ATPase activity (Fig. 2G). AICAR administered in association with energy depletion seems to result in slightly higher activity levels of Na-K-ATPase than does metformin pretreatment, but this difference was not statistically significant.
Fig. 2.
AMPK preactivation decreases the extent of Na-K-ATPase internalization induced by ATP depletion, as detected by immunofluoresence microscopy and cell surface biotinylation as described in materials and methods. A: confocal image of untreated MDCK cells labeled with an antibody directed toward the α-subunit of the Na-K-ATPase. Energy depletion induced by 2DG/AA results in internalization of the Na-K-ATPase (B), while preincubation of cells with AICAR (C) or metformin (D) ameliorates this effect. E: cell surface biotinylation was performed, and cells were subsequently subjected to energy depletion or treated with metformin before energy depletion. 2-Mercaptoethane sulfonate sodium (MesNa) was used to strip the biotin tag from proteins exposed at the basolateral plasma membrane, and the remaining intracellular biotinylated proteins were recovered with streptavidin. F: quantification of 5 independent experiments. Bars represent percentage of total Na-K-ATPase recovered with streptavidin after MesNa stripping. G: total cellular levels of ouabain-sensitive Na-K-ATPase activity are preserved in cells pretreated with AMPK activators AICAR or metformin before energy depletion. Values are means ± SE. NS, not statistically significant.*P < 0.05, **P < 0.01 compared with 2-DG/AA.
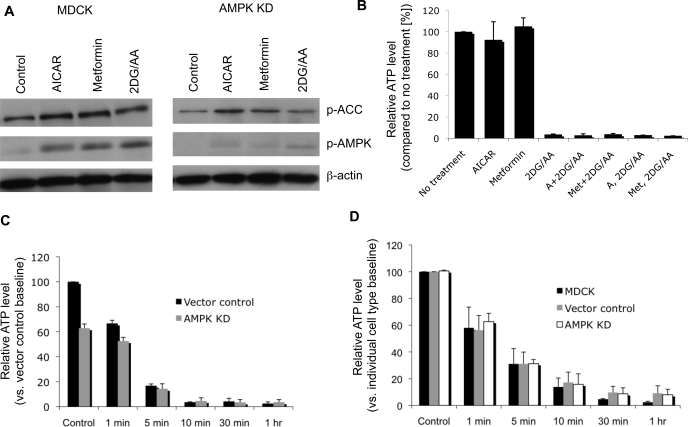
Effects of energy depletion on an AMPK-deficient cell line.
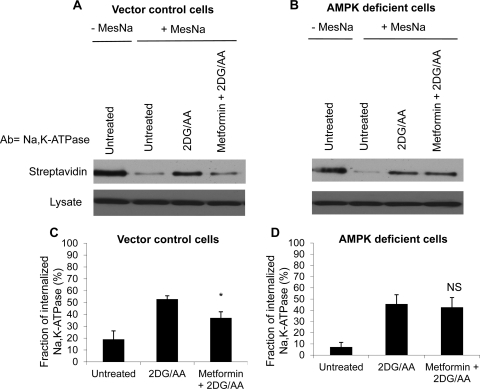
To determine the significance of AMPK in the cellular response to energy depletion, we created a cell line with reduced expression of the AMPK α1-subunit, the predominant isoform in MDCK cells. This AMPK α1-line has been previously characterized (59). As expected, upon exposure to ATP depletion, AICAR incubation, and metformin incubation, levels of activated p-AMPK were significantly decreased, but not completely abolished, in the AMPK-α1 knockdown cells compared with control cells (Fig. 3). At baseline, AMPK knockdown cells had slightly, but significantly, lower ATP levels. The effect of energy depletion on ATP levels, however, was similar to that seen in control cell lines. In wild-type cells, incubation with AICAR or metformin did not significantly alter ATP levels (Fig. 3B). In contrast to wild-type MDCK cells and cells transfected with an empty viral vector, AMPK-deficient cells preincubated with metformin still exhibited significant endocytosis of Na-K-ATPase in response to energy deprivation (Fig. 4).
Fig. 3.
AMPK-deficient (KD) cell lines demonstrate dramatically reduced AMPK activation following treatment with AMPK activators and energy depletion, as revealed by assessing levels of p-AMPK by Western blotting. A: representative immunoblot demonstrating wild-type MDCK cell and AMPK KD cell response to AICAR, metformin, and energy deprivation treatments. AMPK and ACC are both activated in these settings, but to a substantially reduced degree in AMPK-deficient cells. AMPK KD cells also have significantly reduced p-AMPK at baseline and blunted response to AMPK activators. B: treatment with AICAR or metformin before or during energy deprivation does not significantly alter ATP levels. C: cells deficient in AMPK have lower baseline ATP levels but (D) the response of ATP levels to energy depletion is similar to that of control cell lines. Blots and figures are representative of at least 3 independent experiments. Values are means ± SE.
Fig. 4.
Metformin pretreatment does not rescue Na-K-ATPase endocytosis in AMPK-deficient cells. We assessed whether AMPK preactivation would alter Na-K-ATPase internalization in AMPK-deficient cells compared with cells expressing a vector control via cell surface biotinylation. A: and B: Western blots of streptavidin-recovered Na-K-ATPase or Na-K-ATPase in the total cell lysates. Cells were biotinylated, then treated with 2DG/AA or with metformin before 2DG/AA. Surface-exposed biotin groups stripped with MesNa and the remaining intracellular biotinylated proteins were recovered with streptavidin. Blots were probed with antibody directed against the Na-K-ATPase α-subunit. Quantification (C and D) reflects at minimum 4 separate experiments. Results are means ± SE. *P < 0.05 compared with 2DG/AA alone; NS, not significant compared with 2DG/AA.
Localization of p-AMPK in mouse kidneys.
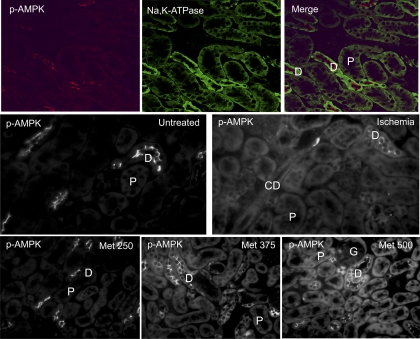
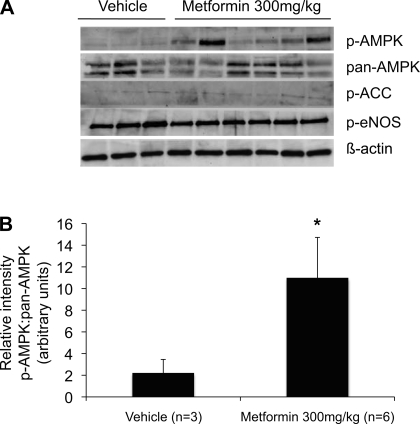
Localization of p-AMPK at baseline and after acute kidney ischemia has been characterized in the rat (15, 41). In murine kidneys, we demonstrate similar localization of p-AMPK under these conditions. Upon immunofluorescence staining using a p-AMPK-specific antibody, p-AMPK was detected in the apical membranes of distal tubules of mice at baseline. Following ischemia, there was a diffuse increase in p-AMPK fluorescence globally, including within proximal tubules and glomeruli (Fig. 5). Metformin has been demonstrated to activate AMPK in the heart, vascular tissue, and kidney homogenates (30, 57, 68). We observed that treatment with metformin delivered by intraperitoneal (ip) injection results in increased fluorescence of p-AMPK in all cortical tubule segments (Fig. 5). Furthermore, this activation appears to be dose dependent, as assessed by immunofluoresence microscopy. Western blot analysis of p-AMPK levels also confirmed that AMPK activation occurs in response to the metformin dosing regimen employed in the in vivo studies that follow (Fig. 6).
Fig. 5.
Localization of p-AMPK in mouse kidneys is diffuse but prominent at the apical surfaces of distal tubules. Immunofluorescent localization of p-AMPK following treatment of mice with varying amounts of metformin (expressed in mg·kg−1·day−1) demonstrates dose-dependent increases in p-AMPK staining. Ischemia also upregulates p-AMPK levels. P, proximal tubule; D, distal tubule; CD, collecting duct; G, glomerulus. Images are reflective of 3 independent experiments and are photographed at uniform contrast and brightness settings.
Fig. 6.
Metformin treatment induces AMPK activation in murine kidneys, but ACC and endothelial nitric oxide synthase (eNOS) do not appear to be downstream targets. A: representative Western blot of mouse kidney homogenates probed with p-AMPK, pan-AMPK, p-ACC, p-eNOS1177, and β-actin antibodies. The first 3 lanes represent kidney tissue prepared from animals treated with vehicle, and the next 6 lanes represent tissue prepared from mice that received injections of metformin at a dose of 300 mg·kg−1·day−1 ip for 3 days. Treatment of mice with this dose of metformin induces AMPK activation. ACC and eNOS, downstream targets of AMPK in cardiac and vascular tissue, do not appear to be activated in the kidney in response to metformin treatment. Lack of p-ACC activation by metformin in kidney tissue is distinct from the response that metformin produces in MDCK cells. B: quantification by densitometry. Values are means ± SE. *P < 0.05.
Metformin effects on serum glucose concentration.
Reportedly, high doses of metformin induce hypoglycemia in rodents (49), but doses in the range of 330 mg·kg−1·day−1 given orally have no significant effect on serum glucose levels (67). In our model, doses of up to 375 mg·kg−1·day−1 given ip did not significantly alter blood glucose levels. At higher doses, however, there was a trend toward lower glucose levels (Table 1). Chronic metformin dosing at 300 mg·kg−1·day−1 ip for 3 days did not result in hypoglycemia (Table 2).
Table 1.
Serum glucose measurements in animals treated with metformin
| Condition | Serum Glucose, mg/dl |
|---|---|
| Vehicle | 247 ± 26.9 |
| Metformin (50 mg/kg) | 226 ± 34.5 |
| Metformin (125 mg/kg) | 196 ± 37.7 |
| Metformin (250 mg/kg) | 224 ± 27.6 |
| Metformin (375 mg/kg) | 174 ± 13.4 |
| Metformin (500 mg/kg) | 137 ± 27.2* |
| Metformin (650 mg/kg) | 113 ± 33.2† |
Values are means ± SE; n = ≥5 animals/group. Measurements were taken 4 hours after intraperitoneal injection of metformin at the indicated dose. All doses were delivered in equivalent volume of vehicle.
P < 0.05 compared with vehicle alone.
P < 0.01 compared with vehicle alone.
Table 2.
Serum glucose measurements in animals treated with metformin (300 mg/kg/dose once daily for 3 consecutive days) compared with vehicle
| Condition | Serum Glucose (mg/dl) After Third Daily Dose |
|---|---|
| Vehicle | 230 ± 19.3 |
| Metformin | 221 ± 31.1NS |
Values are means ± SE; n = ≥13 animals/group. NS, not significantly different compared with vehicle alone.
Na-K-ATPase mislocalization after renal ischemia in vivo is attenuated by metformin pretreatment.
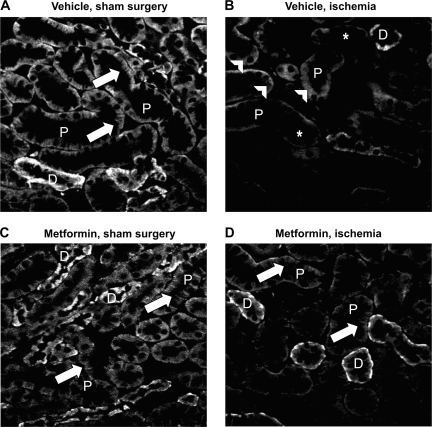
Loss of cell polarity and mislocalization of Na-K-ATPase are well-described consequences of energy depletion in renal epithelia in situ (3, 39). Immunofluorescence analysis revealed that this behavior was also observed in the kidneys of mice subjected to renal ischemia. We observed that in animals pretreated with metformin for 3 days before the imposition of renal ischemia, Na-K-ATPase localization was largely preserved compared with in vehicle-treated ischemic mice (Fig. 7).
Fig. 7.
Mislocalization of Na-K-ATPase after ischemia is attenuated by metformin pretreatment. Immunofluorescent images of kidney cortex, prepared as described in the text, probed with an antibody to the α-subunit of the Na-K-ATPase are presented here. P, proximal tubule; D, distal tubule. A: vehicle-treated animal exposed to sham surgery. B: vehicle-treated animal subjected to 30 min of bilateral renal pedicle clamping. C: metformin treated animal exposed to sham surgery. D: metformin-treated animal subjected to renal ischemia. The expected distribution of Na-K-ATPase along distinct basolateral membrane surfaces and infoldings (arrows) is observed in untreated animals (A) and in animals treated with metformin (C), but in animals subjected to ischemia-reperfusion injury (B) it appears to be diffuse (arrowheads) and is diminished in intensity (asterisks). Na-K-ATPase localization appears less perturbed in animals pretreated with metformin before ischemia (D). Images were taken at uniform contrast and brightness settings and are representative at least 6 animals/group.
NGAL levels impacted by metformin pretreatment.
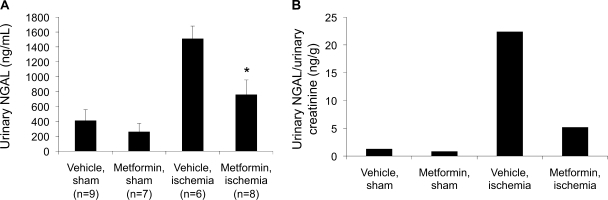
NGAL, an early, sensitive marker of renal tubular injury, is a small, secreted, protease-resistant polypeptide that is easily detected in the urine and is upregulated within hours of renal ischemia (38). Urinary NGAL levels, measured by ELISA, were lower in metformin-pretreated animals subjected to ischemia compared with their vehicle-treated counterparts (Fig. 8). When normalized to average urinary creatinine, NGAL:creatinine ratios were also significantly reduced in animals pretreated with metformin before being subjected to renal ischemia compared with vehicle-treated animals that underwent renal ischemia. Serum creatinine levels 24 and 48 h postischemia were not significantly different in the two groups (data not shown).
Fig. 8.
Levels of urine neutrophil gelatinase-associated lipocalin (NGAL), a biomarker of renal epithelial cell injury, are decreased in ischemic animals that were pretreated with metformin. NGAL measurements were performed by ELISA on urine specimens collected 24 h following post-ischemia-reperfusion (A). When NGAL levels were normalized to average urinary creatinine levels, metformin still appears to confer a protective effect (B). Measurements were performed in at least 6 animals/group. Values are means ± SE. *P < 0.05.
Improvement in histological appearance with metformin pretreatment.
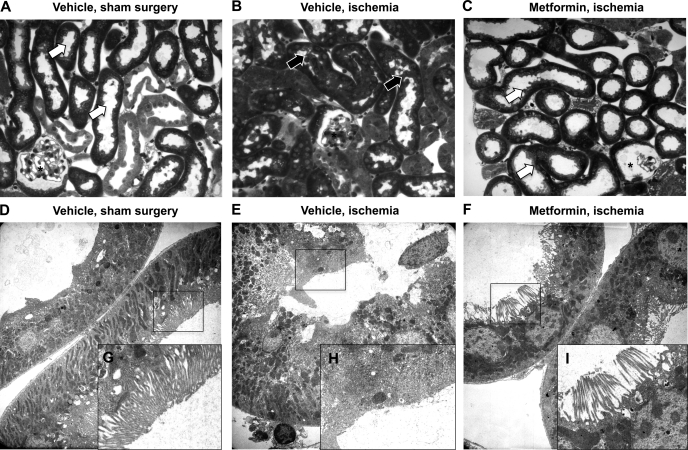
We next evaluated histological correlates of kidney injury in animals subjected to vehicle or metformin treatment before renal ischemia. Saline-treated animals demonstrated normal proximal tubule segments with open lumens and well-defined brush borders (Fig. 9A). Electron micrographs (EMs) (Fig. 9, D and G) showed a normal tubular ultrastructure and brush border. Kidneys from vehicle-treated animals following ischemia-reperfusion (Fig. 9B) had partially collapsed lumens with loss of brush borders, apical blebbing, and intraluminal tubular debris. By EM, we observed swollen epithelial cells with brush border loss and apical blebbing (Fig. 9, E and H). Metformin-treated animals subjected to ischemia (Fig. 9C) exhibited dilated tubules with preserved cellular architecture and brush borders. Occasional cells showed vacuolization. At the EM level, tubules demonstrated preserved cellular architecture with some attenuation of the brush border (Fig. 9, F and I).
Fig. 9.
Metformin treatment before renal ischemia results in preservation of cellular architecture as demonstrated in light and electron micrographs of mouse kidney sections. Animals were subjected to vehicle or metformin treatment before induction of renal ischemia and then perfusion-fixed as described in the text. Light micrographs were stained with toluidine blue (A–C), and alternate sections of the same kidney were prepared for electron microscopy (D–F). G–I represent higher magnifications of the original electron microscopy (EM) image, outlined in black. A: control kidneys demonstrate normal proximal tubular segments with open lumens and well-defined brush borders. EMs (D) show a normal tubular ultrastructure and brush border (zoom, G). Following ischemia-reperfusion, light microscopic images reveal (B) partially collapsed tubular lumens with loss of brush borders and apical blebbing, and tubules containing cellular debris within the lumen, while EM images (E and H) show swollen epithelial cells with loss of brush borders and apical blebbing. Metformin-treated animals subjected to ischemia-reperfusion (C) show dilated tubules with preservation of cellular architecture and brush borders. Occasional cells show vacuolization. At the EM level (F and I), tubules show preservation of cellular architecture with some attenuation of the brush border. Results are representative of at least 3 independent experiments with at least 2 animals/group.
DISCUSSION
We report that AMPK can be activated in MDCK cells by energy deprivation and through pharmacological stimulation with AICAR and metformin. ACC, a known downstream target of AMPK in cardiac cells, skeletal muscle, and hepatic epithelia (27, 43, 67), is also phosphorylated in response to AMPK stimulation in MDCK cells.
Metformin activates AMPK in Sprague-Dawley rat kidney lysates (30), but immunocytochemical localization of metformin-induced AMPK activity in the kidney has not been previously described. We demonstrate that metformin treatment increases detectable p-AMPK in a dose-dependent manner and that metformin-induced AMPK activation occurs in proximal tubules as well as in distal segments. Mount et al. (41) described in rats a diffuse increase in renal cortical p-AMPK within 5 min of renal ischemia, and our investigations recapitulate this finding in the murine kidney. ACC and endothelial nitric oxide synthase (eNOS) do not appear to be downstream targets of metformin-induced AMPK activation in our model (Fig. 6), consistent with the finding that ischemia-induced AMPK activation in the rat kidney is also not associated with phosphorylation of eNOS or ACC (41). Interestingly, this behavior is distinct from the response that metformin produces in MDCK cells.
Metformin is theorized to act by inhibiting oxidative phosphorylation through blockade of a mitochondrial phosphorylation complex 1 (12, 47). This effect would be expected to reduce cellular ATP and increase AMP levels, which would lead to AMPK activation as a consequence of AMP binding to the γ-subunit and subsequent phosphorylation of the α-subunit at Thr-172 by an upstream AMPK. However, this model conflicts with reports indicating that total cellular ATP levels are not affected by standard doses of metformin (47, 67). More subtle changes in the ATP:AMP ratio, or even an alteration of local ATP concentrations may activate AMPK. Consistent with this interpretation, our data indicate that metformin and AICAR treatment produce no significant changes in cellular ATP levels. We suspect, therefore, that AMPK's observed effect on Na-K-ATPase localization is not simply due to its impact on cellular energy supply.
Given the potential importance that the AMPK cascade has in generating and maintaining epithelial cell polarity, we evaluated the effect of AMPK preactivation on the distribution and activity of the Na-K-ATPase in response to energy depletion. Our studies confirm that in vitro energy depletion and in vivo renal ischemia result in mislocalization of Na-K-ATPase. We also observed that AMPK preactivation may preserve the appropriate subcellular localization of the Na-K-ATPase as well as the morphological organization of renal epithelial cells. In MDCK cells, AMPK preactivation by metformin decreases Na-K-ATPase internalization after energy depletion, as detected by immunofluoresence microscopy and cell surface biotinylation, and preserves the level of cell-associated ouabain-sensitive Na-K-ATPase activity. AICAR pretreatment seems to result in even higher levels of total cellular Na-K-ATPase activity than does metformin pretreatment, but this difference was not statistically significant. Most gratifyingly, in murine kidneys, basolateral localization of Na-K-ATPase is largely preserved in animals treated with metformin before the imposition of renal ischemia.
In α1-AMPK-deficient cells, this metformin-induced protection of Na-K-ATPase localization is not observed. It should be noted that a small quantity of p-AMPK activity is detectable in the α1-AMPK knockdown cells. This residual AMPK activity is likely associated with α2-AMPK, which is expressed at similarly low levels in both control and α1-AMPK knockdown cells (59). These data suggest that this small pool of α2-AMPK is insufficient or unable to prevent Na-K-ATPase internalization in response to energy depletion.
These findings are intriguing for several reasons. As the Na-K-ATPase is a highly energy-dependent pump, it seems counterintuitive that AMPK, which generally turns off ATP-consuming processes, would allow maintenance of Na-K-ATPase activity by prohibiting its internalization from the basolateral membrane (24). Recent studies by Vadász et al. (61) have shown that AMPK activation actually promotes internalization of Na-K-ATPase in alveolar epithelial cells. Hallows et al. (19) also demonstrated that AMPK inhibits apical accumulation of vacuolar H+-ATPase in epididymal clear cells. Both groups logically posit that by reducing the cell surface population of an ATP-consuming pump, energy utilization is decreased. The data presented here suggest that in renal epithelial cells, metformin-induced AMPK activation sends a different message. In a separate study, our group has also observed that inhibition of AMPK with compound C actually induces Na-K-ATPase internalization in MDCK cells, possibly via AMPK's association with AS160, a Rab-GTPase-activating protein (2). If renal epithelial cells substantially reduced basolateral Na-K-ATPase pumping capacity, then transepithelial fluid reabsorption would be severely compromised, leading to massive diuresis and volume depletion. Although each individual renal epithelial cell might logically be expected to respond to metformin-induced AMPK activation by reducing ATP utilization via internalization of Na-K-ATPase, these cells appear to defer this self-preserving action and instead ensure the capacity of the kidney to maintain the organism's volume homeostasis. Interestingly, in the original characterization of ischemic preconditioning, it was postulated that compromising the activities of certain key ATPases, including the Na-K-ATPase, might substantially reduce cell viability. Thus the Na-K-ATPase may not be a primary target of preconditioning's energy-conserving consequences (42).
The magnitude of serum creatinine elevation induced by renal ischemia was not significantly impacted by metformin pretreatment, indicating that AMPK activation, at least as achieved in the present studies, is by no means a panacea for the prevention of functional consequences of AKI. It is possible that preservation of renal function might be achievable with different dosing and delivery regimens for metformin. We did observe, however, that levels of NGAL, a biomarker of renal tubular injury, are impacted by metformin pretreatment. It is also encouraging that metformin-induced AMPK activation before renal ischemia appears to partially ameliorate the histological and biochemical consequences of ischemia-induced cellular injury. Lin et al. (32) also reported that treatment with a regimen combining the AMPK activator AICAR and N-acetylcysteine, an antioxidant, attenuates ischemia-reperfusion injury in a canine model of autologous renal transplantation. AMPK activation before, during, and after cardiac ischemia has a protective effect (8, 57) via AMPK-induced GLUT4 translocation, activation of eNOS, and upstream stimulation of AMPK by macrophage migration inhibitory factor (8, 46, 52). However, since renal epithelial cells do not express GLUT4, and eNOS does not appear to be regulated by AMPK in the kidney, the pathways through which preactivation of AMPK induces partial protection from renal ischemic damage may differ from those in the heart.
Several factors could be responsible for the finding that metformin pretreatment ameliorates at least a subset of the pathological consequences of renal ischemia. AMPK levels change in response to salt intake (15), and the kinase plays a role in regulating solute transport (20) by inhibiting CFTR (21), impacting ubiquitination of the epithelial sodium channel (1, 4), and phosphorylating the Na-K-2Cl cotransporter (14). AMPK preactivation with metformin may decrease active solute transport before injury, reducing the energy demands to be faced in the metabolically vulnerable milieu conferred by ischemia.
Another potential mechanism for AMPK's protective action may lie in its anti-inflammatory activity. Recent studies propose that variations in certain inflammatory cytokines and inflammation-related proteins, such as TNF-α, IL-6, TLR-6, and TLR-4 contribute significantly to the pathophysiology of AKI (10, 23, 26, 31, 33, 51, 63). AMPK activation is associated with anti-inflammatory properties and, in particular, downregulation of TNF-α, inducible nitric oxide synthase, and IL-6 (44, 48). It will be interesting to examine the impact of AMPK activation on kidney inflammation in the setting of ischemia.
Finally, AMPK activation may also facilitate the activities of heat shock proteins (HSPs) in the kidney (11). HSPs are molecular chaperones that impact the preservation and restitution of polarity in the setting of renal ischemia. Riordan et al. (53) demonstrated that HSP70 directly interacts with Na-K-ATPase, and others have described that HSP70, HSP25, and HSP90 stabilize Na-K-ATPase in the ischemic rat kidney (5, 6). The potential relationship between the AMPK cascade and HSP activity will be a topic of future analysis.
Much remains to be learned about the role of AMPK in the generation and maintenance of renal epithelial polarity at baseline and in response to ischemia. Our data suggest that its activity may be exploited to prevent at least some of the epithelial cell damage associated with renal ischemic injury. This is especially interesting since metformin is a drug in wide clinical use that can induce AMPK activation in humans (43). Future studies will focus on determining whether the doses of metformin that appear to confer partial protection from AKI achieve this effect primarily through AMPK activation in vivo. The possible risk of lactic acidosis in patients with AKI taking metformin also needs to be assessed. In addition, it will be important to determine whether clinically relevant human metformin-dosing regimens are sufficient to induce sufficient AMPK activation. Were this to be so, it may be possible to translate our findings into clinical trials testing the potential of metformin pretreatment in reducing the effects of predictable renal ischemic injury, such as that associated with cardiothoracic surgery and renal transplantation.
GRANTS
This study was supported by a fellowship from the National Kidney Foundation (P. Seo-Mayer) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK17433 and DK072612 (M. J. Caplan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors acknowledge SueAnn Mentone for invaluable technical expertise. We also thank Dr. Prasad Devarajan and his laboratory for performing the NGAL measurements, and members of the Caplan Laboratory for helpful discussions and our late friend and colleague, Dr. Norman Siegel.
REFERENCES
- 1.Almaça J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral M, Kunzelmann K. AMPK controls epithelial Na+ channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflügers Arch 458: 713–721, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Alves DS, Farr GA, Seo-Mayer P, Caplan MJ. AS160 associates with the Na,K-ATPase and mediates the AMPK-dependent regulation of sodium pump surface expression. Mol Biol Cell 21: 4400–4408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel N, Kashgarian M, Van Why S. Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res 51: 722–727, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bhalla V, Oyster N, Fitch A, Wijngaarden M, Neumann D, Schlattner U, Pearce D, Hallows K. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4–2. J Biol Chem 281: 26159–26169, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bidmon B, Endemann M, Müller T, Arbeiter K, Herkner K, Aufricht C. Heat shock protein-70 repairs proximal tubule structure after renal ischemia. Kidney Int 58: 2400–2407, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bidmon B, Endemann M, Müller T, Arbeiter K, Herkner K, Aufricht C. HSP-25 and HSP-90 stabilize Na,K-ATPase in cytoskeletal fractions of ischemic rat renal cortex. Kidney Int 62: 1620–1627, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bonventre J. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 11: 43–48, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Calvert J, Gundewar S, Jha S, Greer J, Bestermann W, Tian R, Lefer D. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 57: 696–705, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Corton J, Gillespie J, Hawley S, Hardie D. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Cunningham P, Dyanov H, Park P, Wang J, Newell K, Quigg R. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Davis B, Xie Z, Viollet B, Zou M. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496–505, 2006 [DOI] [PubMed] [Google Scholar]
- 12.El-Mir M, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275: 223–228, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Fish E, Molitoris B. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med 330: 1580–1588, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Fraser S, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp B, Power D. Regulation of the renal-specific Na+-K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405: 85–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp B, Power D. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gopalakrishnan S, Hallett M, Atkinson S, Marrs J. aPKC-PAR complex dysfunction and tight junction disassembly in renal epithelial cells during ATP depletion. Am J Physiol Cell Physiol 292: C1094–C1102, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gottardi C, Dunbar L, Caplan M. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol Renal Fluid Electrolyte Physiol 268: F285–F295, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Hallows K. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hallows K, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler N. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallows K, Mount P, Pastor-Soler N, Power D. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol 298: F1067–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallows K, Raghuram V, Kemp B, Witters L, Foskett J. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie D. The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci 117: 5479–5487, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Jaber B, Rao M, Guo D, Balakrishnan V, Perianayagam M, Freeman R, Pereira B. Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine 25: 212–219, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Jaitovich A, Bertorello A. Na+,K+-ATPase: an indispensable ion pumping-signaling mechanism across mammalian cell membranes. Semin Nephrol 26: 386–392, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kashgarian M, Biemesderfer D, Caplan M, Forbush Br. Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int 28: 899–913, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Krüger B, Krick S, Dhillon N, Lerner S, Ames S, Bromberg J, Lin M, Walsh L, Vella J, Fischereder M, Krämer B, Colvin R, Heeger P, Murphy B, Schröppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci USA 106: 3390–3395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo N, Gillespie J, Kung L, Witters L, Schulz R, Clanachan A, Lopaschuk G. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta 1301: 67–75, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Kwon O, Corrigan G, Myers B, Sibley R, Scandling J, Dafoe D, Alfrey E, Nelson W. Sodium reabsorption and distribution of Na+/K+-ATPase during postischemic injury to the renal allograft. Kidney Int 55: 963–975, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Koh H, Kim M, Kim Y, Lee S, Karess R, Lee S, Shong M, Kim J, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447: 1017–1020, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Lee M, Feliers D, Mariappan M, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg J, Choudhury G, Kasinath B. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Leemans J, Stokman G, Claessen N, Rouschop K, Teske G, Kirschning C, Akira S, van der Poll T, Weening J, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin A, Sekhon C, Sekhon B, Smith A, Chavin K, Orak J, Singh I, Singh A. Attenuation of ischemia-reperfusion injury in a canine model of autologous renal transplantation. Transplantation 78: 654–659, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Coca S, Patel U, Cantley L, Parikh C, TRIBE-AKI Consortium. Searching for genes that matter in acute kidney injury: a systematic review. Clin J Am Soc Nephrol 4: 1020–1031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandel L, Doctor R, Bacallao R. ATP depletion: a novel method to study junctional properties in epithelial tissues. II. Internalization of Na+,K+-ATPase and E-cadherin. J Cell Sci 107: 3315–3324, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Mense M, Dunbar LA, Blostein R, Caplan MJ. Residues of the fourth transmembrane segments of the Na,K-ATPase and the gastric H,K-ATPase contribute to cation selectivity. J Biol Chem 275: 1749–1756, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Mirouse V, Christoforou C, Fritsch C, St Johnston D, Ray R. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev Cell 16: 83–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Mirouse V, Swick L, Kazgan N, St, Johnston D, Brenman J. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol 177: 387–392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Molitoris B, Geerdes A, McIntosh J. Dissociation and redistribution of Na+,K+-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. J Clin Invest 88: 462–469, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molitoris B, Wilson P, Schrier R, Simon F. Ischemia induces partial loss of surface membrane polarity and accumulation of putative calcium ionophores. J Clin Invest 76: 2097–2105, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mount P, Hill R, Fraser S, Levidiotis V, Katsis F, Kemp B, Power D. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Murry C, Jennings R, Reimer K. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 43.Musi N, Hirshman M, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson J, Ljunqvist O, Efendic S, Moller D, Thorell A, Goodyear L. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Myerburg M, King JJ, Oyster N, Fitch A, Magill A, Baty C, Watkins S, Kolls J, Pilewski J, Hallows K. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 42: 676–684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res 61: 610–619, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Owen M, Doran E, Halestrap A. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614, 2000 [PMC free article] [PubMed] [Google Scholar]
- 48.Peairs A, Radjavi A, Davis S, Li L, Ahmed A, Giri S, Reilly C. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin Exp Immunol 156: 542–551, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pénicaud L, Hitier Y, Ferré P, Girard J. Hypoglycaemic effect of metformin in genetically obese (fa/fa) rats results from an increased utilization of blood glucose by intestine. Biochem J 262: 881–885, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pluznick J, Zou D, Zhang X, Yan Q, Rodriguez-Gil D, Eisner C, Wells E, Greer C, Wang T, Firestein S, Schnermann J, Caplan M. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulskens W, Teske G, Butter L, Roelofs J, van der Poll T, Florquin S, Leemans J. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One 3: e3596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, Young L. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest 119: 3807–3816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riordan M, Sreedharan R, Wang S, Thulin G, Mann A, Stankewich M, Van Why S, Kashgarian M, Siegel N. HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol 288: F1236–F1242, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Russell RR, 3rd, Li J, Coven D, Pypaert M, Zechner C, Palmeri M, Giordano F, Mu J, Birnbaum M, Young L. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrier R, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5–14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw R, Lamia K, Vasquez D, Koo S, Bardeesy N, Depinho R, Montminy M, Cantley L. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solskov L, Løfgren B, Kristiansen S, Jessen N, Pold R, Nielsen T, Bøtker H, Schmitz O, Lund S. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 hours after administration. Basic Clin Pharmacol Toxicol 103: 82–87, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Sukhodub A, Jovanović S, Du Q, Budas G, Clelland A, Shen M, Sakamoto K, Tian R, Jovanović A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol 210: 224–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takiar V, Nishio S, Seo-Mayer P, King JD, Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108: 2462–2467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan B, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, Lehnert H, Randeva H. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res 83: 566–574, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Vadász I, Dada L, Briva A, Trejo H, Welch L, Chen J, Tóth P, Lecuona E, Witters L, Schumacker P, Chandel N, Seeger W, Sznajder J. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest 118: 752–762, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent A, Lim B, Tan J, Whiteman M, Cheung N, Halliwell B, Wong K. Sulfite-mediated oxidative stress in kidney cells. Kidney Int 65: 393–402, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Wu H, Chen G, Wyburn K, Yin J, Bertolino P, Eris J, Alexander S, Sharland A, Chadban S. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Li J, Young L, Caplan M. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA 103: 17272–17277, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X, Zmijewski J, Lorne E, Liu G, Park Y, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L497–L504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng B, Cantley L. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA 104: 819–822, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman M, Goodyear L, Moller D. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou M, Kirkpatrick S, Davis B, Nelson J, Wiles Wt Schlattner U, Neumann D, Brownlee M, Freeman M, Goldman M. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279: 43940–43951, 2004 [DOI] [PubMed] [Google Scholar]