Abstract
We examined the role of AMP-activated protein kinase (AMPK) in modulating the viability of cultured kidney proximal tubular cells subjected to metabolic stress induced by either dextrose deprivation, inhibition of glycolysis, or inhibition of mitochondrial respiration. We used BU.MPT cells, a conditionally immortalized kidney epithelial cell line derived from the proximal tubules of transgenic mice bearing a temperature-sensitive mutation of the simian virus 40 large-tumor antigen. All three forms of metabolic stress increased the phosphorylation and activity of AMPK. Activation of AMPK led to changes in the phosphorylation of two downstream targets of AMPK, acetyl coenzyme A carboxylase and p70 S6 kinase. Inhibition of AMPK, either pharmacologically with compound C (CC) or by gene silencing, significantly increased the amount of apoptosis in response to all three forms of metabolic stress. Although the amount of apoptosis was directly related to the severity of ATP depletion, inhibition of AMPK had no effect on cellular ATP levels. Notably, metabolic stress increased the phosphorylation and activity of Akt. Furthermore, inhibition of AMPK, with CC or gene silencing, abrogated the ability of metabolic stress to activate Akt. The augmentation of apoptosis induced by inhibition of AMPK was comparable to that induced by inhibition of Akt. We conclude that activation of AMPK following acute metabolic stress plays a major role in promoting the viability of cultured proximal tubular cells. Protection by AMPK appears to be due not to AMPK-mediated conservation of cell energy stores, but rather, at least in part, to AMPK-mediated activation of Akt.
Keywords: AMP-activated protein kinase, ATP depletion, Akt, mammalian target of rapamycin (mTOR)
AMP-activated protein kinase (AMPK) is a ubiquitous serine/threonine kinase that acts as a highly sensitive sensor of cell energy stores (8, 21, 26, 64). AMPK is a heterotrimeric protein composed of a catalytic α-subunit and two regulatory (β and γ) subunits (21, 23, 64). There are two isoforms of the catalytic subunit (α1 and α2), two isoforms of the β-subunit (β1 and β2), and three isoforms of the γ-subunit (γ1, γ2, and γ3). All of these isoforms are encoded by distinct genes (21, 23, 64). Theoretically, there are 12 possible heterotrimeric combinations of AMPK, with gene splicing offering further potential diversity (21, 23, 64). The functional differences between the various heterotrimeric forms of AMPK remain uncertain.
The primary event in activation of AMPK is phosphorylation by liver kinase B1 (LKB1) of an amino acid residue (Thr172) within the activation loop of the α-subunit of AMPK (4, 24, 68). Since LKB1 is constitutively active, AMPK is continuously being phosphorylated (4, 24, 68). However, when cell energy stores are replete, AMPK is maintained in an inactive state by phosphatases such as the phosphatase-2Cα (PP2Cα) (4, 24). During periods of cellular stress, hydrolysis of ATP leads to a fall in ATP levels and an increase in cytosolic concentrations of ADP and AMP (4, 24). The regulatory γ-domain of AMPK has binding sites for all three nucleotides (51, 70). Binding of ADP or AMP activates AMPK by two mechanisms: inhibiting the dephosphorylation of Thr172 and facilitating allosterically the phosphorylation of AMPK by LKB1 (4, 24). In contrast, binding of ATP to the γ-domain has opposite effects to those of ADP and AMP. The net result of these opposing interactions is that the activity of AMPK is inversely related to the cell's energy charge, defined as the ratio of the concentration of ATP to that of either ADP or AMP (4, 24, 51).
Upon activation, AMPK acts as a metabolic “switch” with profound effects on intermediary cell metabolism. These are brought about through phosphorylation of several downstream kinases (8, 23) and through alterations in gene expression (25). Many of these changes serve to conserve ATP stores by promoting ATP production, inhibiting ATP consumption, and facilitating the cellular uptake of nutrients (8, 21–23, 64). For example, by phosphorylating and inhibiting acetyl-CoA carboxylase (ACC), AMPK inhibits lipid synthesis and promotes fatty acid oxidation (26, 27, 30). AMPK also inhibits the activity of the mammalian target of rapamycin (mTOR), an essential kinase for cell growth and proliferation (19, 28). Other AMPK-mediated effects that serve to increase ATP stores include facilitation of the cellular uptake of metabolic nutrients (such as glucose and fatty acids) and stimulation of ATP production by glycolysis (23).
A substantial body of evidence supports a role for AMPK in multiple diseases, including diabetes mellitus (18, 46), the metabolic syndrome (37, 43, 49), and cancer (33). By contrast, information regarding the role of AMPK in renal disease is sparse. A few studies suggest that AMPK may participate in sodium homeostasis, possibly by coupling sodium transport to cellular energy stores (7, 15, 20). Although substantial evidence exists that AMPK participates in the response to ischemia by the heart (2, 9, 39, 59, 65) and brain (41, 45), no studies have explored the possibility that AMPK may modulate the response of the kidney to acute ischemic injury.
Here, we used a cell culture model to investigate the role of AMPK in acute metabolic stress of renal proximal tubular epithelial cells. Metabolic stress was induced by three distinct methods: dextrose deprivation, inhibition of glycolysis with 2-deoxyglucose (DOG), and inhibition of mitochondrial respiration with antimycin A. We found that AMPK was activated during acute metabolic stress and that active AMPK modulated the phosphorylation and activity of two downstream substrates, ACC and p70 S6 kinase (p70S6K). Moreover, inhibition of AMPK exacerbated apoptosis of proximal tubular cells subjected to all three forms of metabolic stress, suggesting that AMPK plays an important role in maintaining cell survival during energy depletion. The effects of AMPK inhibition on cell survival were independent of changes in cell ATP, implying that conservation of cellular energy stores does not play a major role in the prosurvival activity of AMPK. Finally, activation of AMPK was associated with increased phosphorylation of Akt on both its known activation sites. Taken together, our data indicate that activation of AMPK during acute metabolic stress promotes the viability of energy-depleted tubular epithelial cells and that enhanced viability is mediated, at least in part, by AMPK-mediated activation of Akt.
EXPERIMENTAL PROCEDURES
Materials
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise stated. IFN-γ was purchased from Invitrogen (Carlsbad, CA). Compound C (CC) was purchased from Calbiochem (San Diego, CA). Soybean trypsin inhibitor was purchased from GIBCO (Grand Island, NY). Rabbit polyclonal antibody to total AMPK, mouse monoclonal antibody to phosphorylated AMPK (Thr172), rabbit polyclonal antibodies against total-α-AMPK, rabbit polyclonal antibody to total Akt, and two monoclonal antibodies to phosphorylated Akt (Thr308 and Ser473) were all purchased from Cell Signaling Technologies (Danvers MA). Rabbit polyclonal antibody to phosphorylated ACC (Thr79) was purchased from Upstate (Lake Placid, NY). Rabbit polyclonal antibody to phosphorylated p70S6K (Thr389) and rabbit polyclonal antibodies specific for the α- and α2-catalytic domains of AMPK were purchased from Abcam (Cambridge, MA). Monoclonal antibody to β-actin was purchased from Chemicon (Temecula, CA).
Cell Culture
Boston University Mouse Proximal Tubular (BU.MPT) cells were grown in plastic tissue cultures plates in a humidified air/CO2 incubator (5% vol/vol) as previously described (62, 63). BU.MPT cells are proximal tubular cells obtained originally from kidneys of transgenic mice bearing a temperature-sensitive mutation (tsA58) of the simian virus 40 (SV40) large-tumor antigen under the control of the mouse major histocompatibility complex H-2Kb class I promoter (31). BU.MPT cells were grown in DMEM-F12 medium containing 5.5 mM dextrose, 10% (vol/vol) heat-inactivated FBS, 2 mM l-glutamine, 10 mM HEPES, and 100 U/ml penicillin/streptomycin. All cells were initially grown under “permissive” conditions, defined as an ambient temperature of 37°C in the presence of IFN-γ. Under these conditions, the tsA58 transgene is expressed and leads to cell proliferation (31, 62, 63). When cells approached confluence, they were switched to “nonpermissive” conditions, defined as an ambient temperature of 39.5°C in the absence of IFN-γ. Nonpermissive conditions inhibit tsA58 expression by >95%. Before all experiments, BU.MPT cells were incubated in the presence of FBS under nonpermissive conditions for 24 h, and then in the absence of FBS under nonpermissive conditions for an additional 24 h (62, 63). Under nonpermissive conditions, confluent BU.MPT cells behave like primary cultures of mouse kidney proximal tubular cells (62, 63).
OK (opossum kidney) cells, a proximal tubular cell line, were grown in plastic tissues culture plates in DMEM containing 5 mM dextrose, 10% (vol/vol) heat-inactivated FBS, 2 mM l-glutamine, 10 mM HEPES, and 100 U/ml penicillin/streptomycin.
Induction of Metabolic Cell Stress
BU.MPT cells were subjected to three forms of metabolic stress using methods we have previously described (38, 61): 1) dextrose deprivation, induced by incubation in dextrose-free DMEM; 2) inhibition of glycolysis, induced by addition of varying concentrations of DOG in dextrose-free DMEM; and 3) inhibition of mitochondrial respiration, induced by addition of antimycin A (2 μM) in the presence of varying concentrations of dextrose.
Determination of ATP Levels
Cellular ATP levels were determined using previously described methods (42). ATP content was measured by luciferase assay and normalized to total cellular protein, as assessed in cell lysates by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, NY). Control ATP levels (obtained in cells incubated in medium containing 10 mM dextrose in the absence of any inhibitors) were expressed as nanomoles of ATP per milligram of cell protein. ATP levels obtained during metabolic stress were expressed as a percentage of ATP levels under control conditions.
Freezing of Mouse Organs
Two male 8-wk-old C57BL/6 mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (55 mg/kg). Following anesthesia, the heart, left lateral lobe of the liver, both kidneys, and both gastrocnemius muscles were removed. After being snap frozen in liquid nitrogen, tissues were ground into ice-cold lysis buffer containing 20 mM Tris·HCl, pH 7.5, 130 mM NaCl, 1% vol/vol Triton X-100, 0.5% wt/vol deoxycholate, 0.1% wt/vol SDS, 1 mM DTT, 10 mM sodium pyrophosphate, 5 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 200 μM sodium orthovanadate. Lysates were subjected to immunoprecipitation and immunoblotting as described below. Approval for these studies was obtained from the Institutional Animal Care and Use Committee (IACUC) at Stony Brook Medical Center.
Immunoblotting
Immunoblotting was performed, as previously described (63). Briefly, BU.MPT cells were washed with ice-cold PBS, and then lysed in ice-cold cell lysis buffer (20 mM Tris·HCl, pH 7.5, containing 130 mM NaCl, 1% vol/vol Triton X-100, 0.5% wt/vol deoxycholate, 0.1% wt/vol SDS, 1 mM DTT, 10 mM sodium pyrophosphate, 5 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 200 μM sodium orthovanadate). Lysates were centrifuged at 10,000 g for 10 min at 4°C, and the supernatants stored at −70°C. Protein samples (20 μg/lane), as determined by BCA protein assay, were boiled in 6× reducing sample buffer, electrophoresed on SDS-polyacrylamide gels, and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked with either 2.5% BSA or 5% dry milk in TBS before probing with primary antibody. After incubation with the appropriate secondary antibody, immunoreactive bands were visualized by Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Boston, MA). Immunoblots were quantified by densitometry using Image J software from the National Institutes of Health as previously described (17).
Immunoprecipitation
Comparison of the relative amounts of the α1- and α2-isoforms of the catalytic subunit of AMPK was performed in lysates of snap-frozen tissues taken from the liver, heart, skeletal muscle, and kidney of the mice as well as in lysates of cultured BU.MPT cells. Lysates (0.5 mg/sample) were immunoprecipitated using Sepharose A beads (Healthcare Biosciences, Uppsala, Sweden) to which the appropriate antibody was prebound. Immunoprecipitates were then immunoblotted with the appropriate antibody.
Quantitation of Apoptosis
Apoptosis was quantified by previously described methods (54). Briefly, after trypsinization and washing, BU.MPT or OK cells were stained with propidium iodide (PI) and FITC-conjugated annexin V (Invitrogen). Stained cells were analyzed by flow cytometry (FACScan, BD Biosciences), and data were analyzed using CELLQuestPro Version 3.3 (BD Biosciences). Cells were analyzed by forward and side scatter and gated to remove debris, cell fragments, and aggregates of cells. Viable cells were defined as both annexin V and PI negative. Early apoptotic cells were defined as annexin V positive and PI negative (indicating an intact plasma membrane). Late apoptotic cells were defined as both annexin V and PI positive (indicating loss of plasma membrane integrity). Necrotic cells were defined as annexin V negative and PI positive. Separation of apoptotic and necrotic cells was confirmed by analysis of their forward scatter. Apoptotic cells were smaller than viable cells, whereas necrotic cells were larger. Since the proportion of necrotic cells never exceeded 5–10% of the population, they were excluded from all FACS analyses. The total number of apoptotic cells (early plus late) was expressed as a percentage of the number of cells analyzed.
Quantitation of Proliferation
Proliferation was assessed by incorporation of 5-bromodeoxyuridine (BrdU), a synthetic nucleoside and analog of thymidine. BrdU incorporation was measured using a colorimetric ELISA assay according to the manufacturer's instructions (Roche Pharmaceuticals).
Knockdown of AMPK Using Gene Silencing
RNA interference with “short hairpin” RNA (shRNA) was used to “knock down” expression of the α2-catalytic subunit of AMPK. HuSH pRS plasmid vectors were purchased from Origene (Rockville, MD) and used according to the manufacturer's instructions. BU.MPT cells were transiently infected with shRNA-containing vectors using a replication-deficient retroviral system (Phoenix cells; Orbigen, San Diego, CA), according to the manufacturer's instructions. Three plasmids, each encoding a distinct shRNA “sense” sequence of 29 base pairs, were targeted to inhibit expression of the α2-catalytic subunit of AMPK. The sequences of the “sense” portion of these shRNAs were shRNA 1: GACTATCTCAACCGTTCTGTCGCCACTCT; shRNA 2: GTGGAGCAGAGGTCTGGTTCTTCAACACC; and shRNA 3: TTGACAATCGGAGAATAATGAACCAAGCC. As a control, we used a plasmid that expressed a nontargeting shRNA. The sequence of the “sense” portion of this nontargeting shRNA was TGACCACCCTGACCTACGGCGTGCAGTGC.
Statistics
All data are presented as means ± SE. Statistical differences were determined using ANOVA, followed by the Bonferroni correction. All P values <0.05 were considered statistically significant.
RESULTS
Pharmacological Inhibition of AMPK Increases Apoptosis of BU.MPT Cells Subjected to Metabolic Stress
To test the hypothesis that AMPK contributes to cell survival during acute metabolic stress, we used CC, a pharmacological inhibitor of AMPK. CC, which inhibits AMPK by reversible competition with AMP for binding to AMPK, has been used to explore the role of AMPK in multiple tissues and cells (16, 28, 35, 45). The effect of CC on cell survival was examined in BU.MPT cells subjected to three forms of metabolic stress: 1) dextrose deprivation; 2) inhibition of glycolysis with DOG in the absence of dextrose; and 3) inhibition of mitochondrial respiration by antimycin A.
Dextrose deprivation.
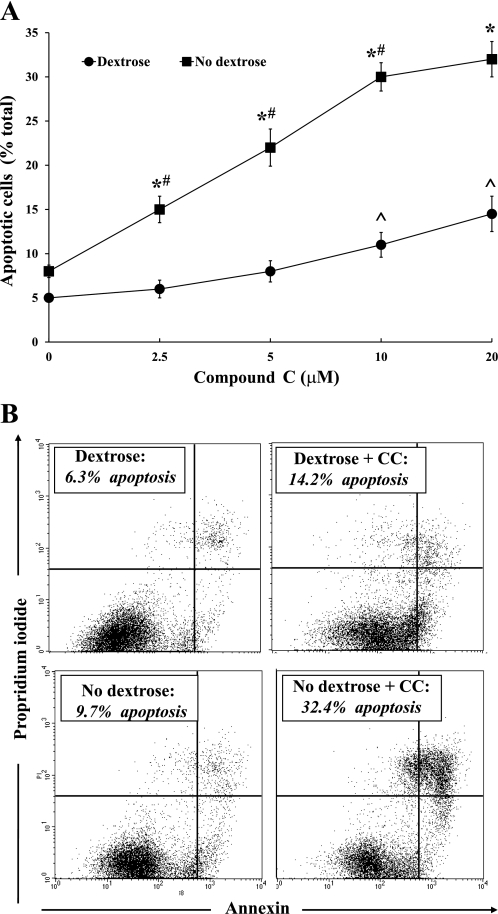
BU.MPT cells were incubated for 16–18 h in the presence of varying concentrations of CC in either dextrose-containing (10 mM) or dextrose-free medium. Apoptosis was quantified by flow cytometry (n = 5). Basal levels of apoptosis (in the absence of CC) were comparable in the absence and presence of dextrose (8.2 ± 0.7 vs. 5.5 ± 0.4%, respectively) (Fig. 1A). In the absence of dextrose, CC induced a dose-dependent increase in apoptosis (Fig. 1A). In the presence of dextrose, however, apoptosis was induced only at the highest concentrations of CC (5 and 10 μM) (Fig. 1A). Notably, at each dose of CC, the degree of apoptosis was greater in the absence than in the presence of dextrose (Fig. 1A). A representative flow cytometric analysis is shown in Fig. 1B, in which BU.MPT cells were incubated in dextrose-containing (top) or dextrose-free (bottom) medium in the absence (left) or presence (right) of 20 μM CC.
Fig. 1.
Inhibition of AMP-activated protein kinase (AMPK) by compound C (CC) enhances apoptosis of Boston University Mouse Proximal Tubular (BU.MPT) cells during dextrose deprivation. A: varying concentrations of CC were added to BU.MPT cells incubated in either dextrose-containing (10 mM; ●) or dextrose-free (■) medium for 16–18 h. Apoptosis was quantified by flow cytometry. *P < 0.001, dextrose vs. no dextrose medium at each concentration of CC. #P < 0.01, each CC concentration vs. preceding CC concentration in no dextrose medium. ^P < 0.02, 20 and 10 μM CC vs. 2.5 μM and no CC in dextrose-containing medium. B: representative flow cytometric analysis of BU.MPT cells incubated in dextrose-containing (top) or dextrose-free (bottom) in the absence (left) or presence (right) of CC (20 μM). Apoptosis was quantified following staining with annexin V (x-axis) and propidium iodide (PI; y-axis). For each condition, the percentage of apoptotic cells was obtained by summing the percentages of early apoptotic (annexin V-positive, PI-negative) and late apoptotic (annexin V-positive, PI-positive) cells.
Inhibition of glycolysis by DOG.
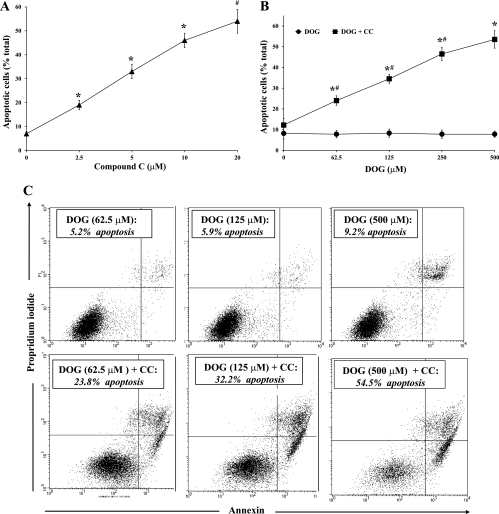
We next examined the effect of CC during inhibition of glycolysis by DOG. In our first experiment, BU.MPT cells, incubated in the absence of dextrose, were exposed to a single concentration of DOG (500 μM) and varying concentrations of CC for 16–18 h (n = 5). The basal level of apoptosis (without CC) was 7.0 ± 1.1% (Fig. 2A). CC induced a dose-dependent increase in apoptosis, reaching 54.0 ± 5.1% at the highest concentration of CC (20 μM). In the second experiment, BU.MPT cells, incubated in the absence of dextrose, were exposed to a maximal concentration of CC (20 μM) and varying concentrations of DOG for 16–18 h (n = 5) (Fig. 2B). In the absence of CC, no apoptosis occurred at any concentration of DOG. In the presence of CC, however, DOG induced a dose-dependent increase in apoptosis, reaching 53.4 ± 6.0% at the highest concentration of DOG (500 μM) (Fig. 2B). A representative flow cytometric analysis is shown in Fig. 2C, in which BU.MPT cells incubated in dextrose-free medium were exposed to different concentrations of DOG in the absence (top) or presence (bottom) of 20 μM CC.
Fig. 2.
Inhibition of AMPK by CC induces apoptosis of BU.MPT cells treated with 2-deoxyglucose (DOG) in the absence of dextrose. A: varying concentrations of CC were added to BU.MPT cells incubated in dextrose-free medium containing DOG (500 μM) for 16–18 h. Apoptosis was quantified by flow cytometry. *P < 0.01, each CC concentration vs. preceding CC concentration. #P < 0.01, 20 μM CC vs. 5 μM CC. B: varying concentrations of DOG were added to BU.MPT cells incubated in dextrose-free medium in the absence (●) or presence (■) of CC (20 μM). *P < 0.001, presence vs. absence of CC at each concentration of DOG. #P < 0.01, each DOG concentration vs. preceding DOG concentration for cells incubated in the presence of CC. C: representative flow cytometric analysis of BU.MPT cells incubated in dextrose-free medium and varying concentrations of DOG in either the absence (top) or the presence (bottom) of CC (20 μM). Apoptosis was quantified following staining with annexin V (x-axis) and PI (y-axis). For each condition, the percentage of apoptotic cells was obtained by summing the percentages of early apoptotic (annexin V-positive, PI-negative) and late apoptotic (annexin V-positive, PI-positive) cells.
Inhibition of mitochondrial respiration by antimycin A.
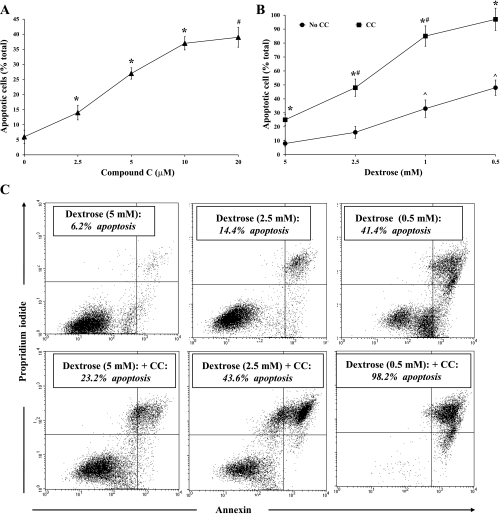
Finally, we examined the effect of CC during metabolic stress induced by antimycin A (2 μM), an inhibitor of mitochondrial respiration. In our first experiment, BU.MPT cells, incubated in the presence of a reduced concentration of dextrose (2.5 mM), were exposed to antimycin A and varying concentrations of CC for 16–18 h (n = 5). The basal level of apoptosis (without CC) was 6.1 ± 2.2%, comparable to that seen in untreated BU.MPT cells, despite the presence of antimycin A (Fig. 3A). The addition of CC led to a dose-dependent increase in the percentage of apoptotic cells, reaching 39.1 ± 3.3% at the highest concentration of CC (20 μM) (Fig. 3A). In the second experiment, BU.MPT cells, incubated in the presence of antimycin A and the highest concentration of CC (20 μM), were exposed to varying concentrations of dextrose (n = 5). In the presence of both antimycin A and CC, decreasing concentrations of dextrose were associated with a progressive increase in the percentage of apoptotic cells, with almost all (98.2 ± 6.0%) BU.MPT cells dying at the lowest concentration of dextrose (0.5 mM) (Fig. 3B). In the absence of CC, the percentage of apoptotic cells also increased as the dextrose concentration was reduced. However, at every dextrose concentration, the amount of apoptosis was greater in the presence than in the absence of CC (Fig. 3B). A representative flow cytometric analysis is given in Fig. 3C, in which BU.MPT cells treated with antimycin A were exposed to varying concentrations of dextrose in the absence (top) or presence (bottom) of CC (20 μM).
Fig. 3.
Inhibition of AMPK by CC induces apoptosis of BU.MPT cells treated with antimycin A. A: varying concentrations of CC were added to BU.MPT cells incubated in reduced dextrose (2.5 mM) and antimycin A (2 μM) for 16–18 h. Apoptosis was quantified by flow cytometry. *P < 0.01, each CC concentration vs. preceding CC concentration. #P < 0.01, 20 μM CC vs. 5 μM CC. B: BU.MPT cells were exposed to antimycin A (2 μM) in the presence of varying concentrations of dextrose in either the presence (■) or the absence (●) of CC (20 μM). *P < 0.001, presence vs. absence of CC at each concentrations of dextrose. #P < 0.01, each dextrose concentration vs. preceding dextrose concentration for cells incubated in the presence of CC. ^P < 0.02, 0.5 and 1.0 mM dextrose vs. 2.5 and 5.0 mM dextrose in the absence of CC. C: representative flow cytometric analysis of BU.MPT cells incubated in the presence of antimycin A and varying concentrations of dextrose, in either the absence (top) or the presence (bottom) of CC (20 μM). Apoptosis was quantified following staining with annexin V (x-axis) and PI (y-axis). For each condition, the percentage of apoptotic cells was obtained by summing the percentages of early apoptotic (annexin V-positive, PI-negative) and late apoptotic (annexin V-positive, PI-positive) cells.
Comparable results were obtained using OK cells subjected to the same three models of metabolic stress (data not shown). Thus, in two separate cell lines, and for all three models of metabolic stress, pharmacological inhibition of AMPK by CC significantly increased the degree of apoptosis. We conclude that AMPK plays an important role in maintaining cell survival under conditions of acute metabolic stress. Our data also indicate that the prosurvival effects of AMPK contribute minimally to cell survival under energy-replete states, since, in the absence of metabolic stress, inhibition of AMPK with CC did not produce an increase in apoptotic death.
Pharmacological Inhibition of AMPK Does Not Affect ATP Levels in Cells Subjected to Metabolic Stress
One potential mechanism by which pharmacological inhibition of AMPK may alter the apoptotic response to acute metabolic stress is through conservation of cellular ATP stores (21, 23, 64). To address this possibility, ATP levels were measured in BU.MPT cells in the absence and presence of CC during each of our three experimental models of metabolic stress.
Dextrose deprivation.
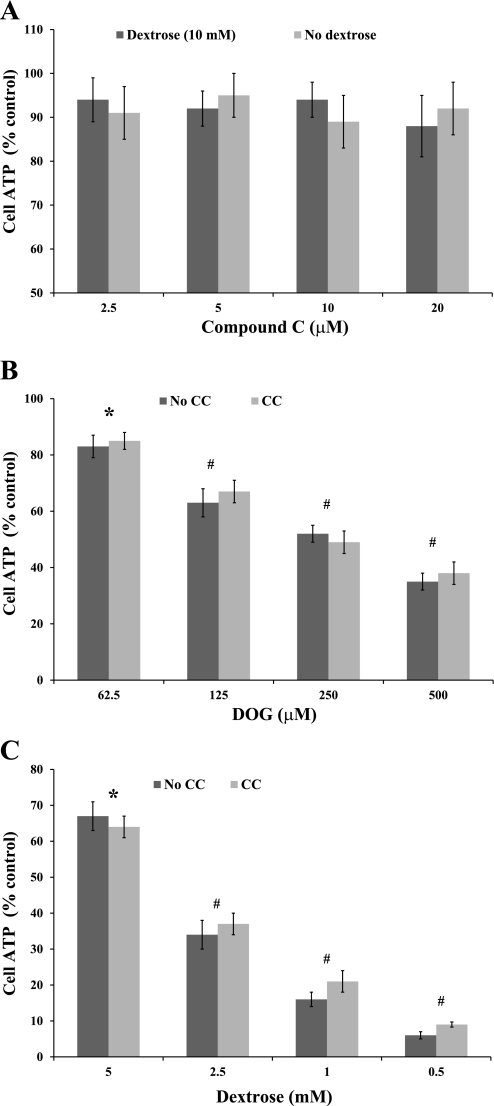
BU.MPT cells were incubated for 8 h in dextrose-containing (10 mM) or dextrose-free medium in the presence of varying concentrations of CC (n = 3) (Fig. 4A). Absolute ATP levels under control conditions (dextrose-containing medium without CC) were 24 ± 3 nM/mg cell protein. Cellular ATP levels were not reduced at any concentration of CC, either in the presence or in the absence of dextrose (Fig. 4A).
Fig. 4.
CC does not alter cell ATP levels in BU.MPT cells subjected to metabolic stress. ATP levels were measured (nM ATP/mg cell protein). ATP levels in cells subjected to metabolic stress were expressed as a percentage of ATP levels under control conditions (defined as 10 mM dextrose, without CC). A: ATP levels in BU.MPT cells were measured after incubation for 8 h in dextrose-containing (dark gray bars) or dextrose-free (light gray bars) medium containing varying concentrations of CC. B: ATP levels were measured in BU.MPT cells after incubation for 8 h in dextrose-free medium containing varying concentrations of DOG in the absence (dark gray bars) or the presence (light gray bars) of CC (20 μM). *P < 0.001, DOG at all concentrations, in both the presence and the absence of CC vs. control unstressed cells. #P < 0.01, each DOG concentration vs. preceding DOG concentration, in both the absence and the presence of CC (20 μM). C: BUMPT cells were incubated with antimycin A (2 μM) and varying concentrations of dextrose in the absence (dark gray bars) or the presence (light gray bars) of CC (20 μM) for 8 h, after which ATP levels were measured. *P < 0.001, antimycin A vs. control unstressed cells, at all dextrose concentrations, in both the presence and the absence of CC (20 μM). #P < 0.01, each dextrose concentration vs. preceding dextrose concentration in both the presence and the absence of CC.
Inhibition of glycolysis by DOG.
BU.MPT cells were incubated for 8 h in dextrose-containing (10 mM) medium or in dextrose-free medium with and without CC (20 μM) in the presence of varying concentrations of DOG (n = 4) (Fig. 4B). Absolute ATP levels under control conditions (dextrose-containing medium without DOG or CC) were 28 ± 5 nM/mg cell protein. Exposure to increasing concentrations of DOG induced a dose-dependent decrease in cell ATP levels. However, CC (20 μM) did not reduce ATP levels further (Fig. 4B).
Inhibition of mitochondrial respiration by antimycin A.
Finally, we determined the effect of antimycin A on cellular ATP levels. Absolute ATP levels under control conditions (dextrose-containing medium without CC or antimycin) was 22 ± 4 nM/mg cell protein. The effect of antimycin A (2 μM) on ATP levels was determined in the presence of varying concentrations of dextrose with and without CC (20 μM) (n = 4) (Fig. 4C). In the presence of antimycin A, decreasing the concentration of dextrose from 5 to 0.5 mM led to a progressive decrease in cellular ATP levels (Fig. 4C). However, CC (20 μM) had no further effect on ATP levels (Fig. 4C).
Comparable results were obtained with OK cells (data not shown). As in BU.MPT cells, for all three models of acute metabolic stress, cellular ATP levels were unaltered by inhibition of AMPK with CC. We conclude that inhibition of AMPK with CC does not alter cell ATP levels beyond that induced by the acute metabolic stress itself. These data argue strongly against modulation of cellular ATP stores as the mechanism by which AMPK protects cells from apoptosis during metabolic stress.
Metabolic Stress Activates the AMPK Signaling Pathway
We next examined the effect of metabolic stress on AMPK-dependent signaling events. While AMPK has many known downstream substrates, we focused on two: ACC and mTOR (19, 26, 28, 30). Activated AMPK inhibits ACC by phosphorylation at Thr79, leading to β-oxidation of fatty acids and inhibition of lipid synthesis (1). AMPK also inhibits mTOR, a kinase essential for cell growth and proliferation (28, 47). As a readout of mTOR activity, we used p70S6K, which lies downstream of mTOR and is phosphorylated by mTOR at Thr389 (28).
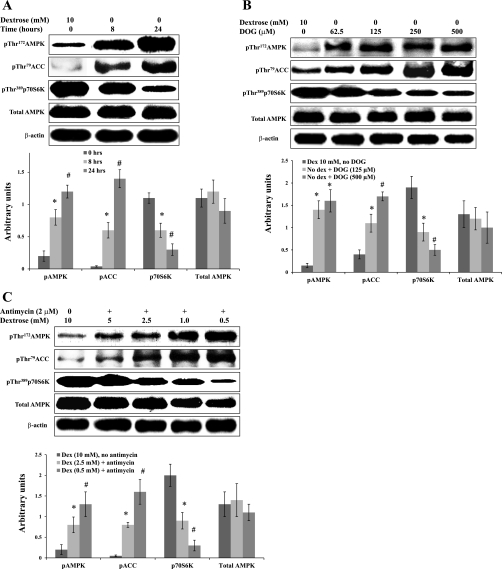
Dextrose deprivation induced a time-dependent increase in phosphorylation of both AMPK and its downstream target ACC, as well as a progressive decrease in phosphorylation of p70S6K (Fig. 5A). DOG in the absence of dextrose also induced a dose-dependent increase in phosphorylation of AMPK and ACC and a dose-dependent decrease in phosphorylation of p70S6K (Fig. 5B). Finally, exposure to antimycin A in the presence of decreasing concentrations of dextrose replicated the results of dextrose deprivation and DOG-induced stress. As the concentration of dextrose was decreased, we observed a progressive increase in phosphorylation of AMPK and ACC and a progressive decrease in phosphorylation of p70S6K (Fig. 5C). In all cases, whenever metabolic stress was of sufficient severity to induce phosphorylation of AMPK, activation of AMPK was associated with changes in phosphorylation of both ACC and p70S6K. Similar results were obtained when the same experiments were done using OK cells (data not shown). Thus, in two different proximal tubular cell lines, all three forms of metabolic stress led to increased phosphorylation and activity of AMPK, accompanied by increased phosphorylation (and consequent inhibition) of ACC and decreased phosphorylation (and consequent inhibition) of p70S6K.
Fig. 5.
Metabolic stress activates the AMPK signaling pathway. A: BU.MPT cells were incubated in dextrose-containing (10 mM) or dextrose-free medium for 8 or 24 h. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose control at 8 h. #P < 0.05, vs. dextrose control at 24 h. B: BU.MPT cells were incubated for 6 h in either medium containing 10 mM dextrose (control cells) without DOG or in dextrose-free medium with varying concentrations of DOG. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose, no DOG control. #P < 0.05, vs. no dextrose+DOG (125 μM). C: BU.MPT cells were incubated for 6 h in either dextrose-containing (10 mM) medium in the absence of antimycin A (control cells) or in medium containing varying concentrations of dextrose plus antimycin A (2 μM). Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. For all experiments, cell lysates were probed with antibodies against phosphorylated (active) AMPK, phosphorylated (inactive) ACC, phosphorylated (active) p70S6K, total AMPK, and total β-actin. *P < 0.05, vs. dextrose, no antimycin. #P < 0.05, vs. dextrose (2.5 mM)+antimycin.
Pharmacological Inhibition of AMPK Prevents Metabolic Stress-Induced Signaling Events
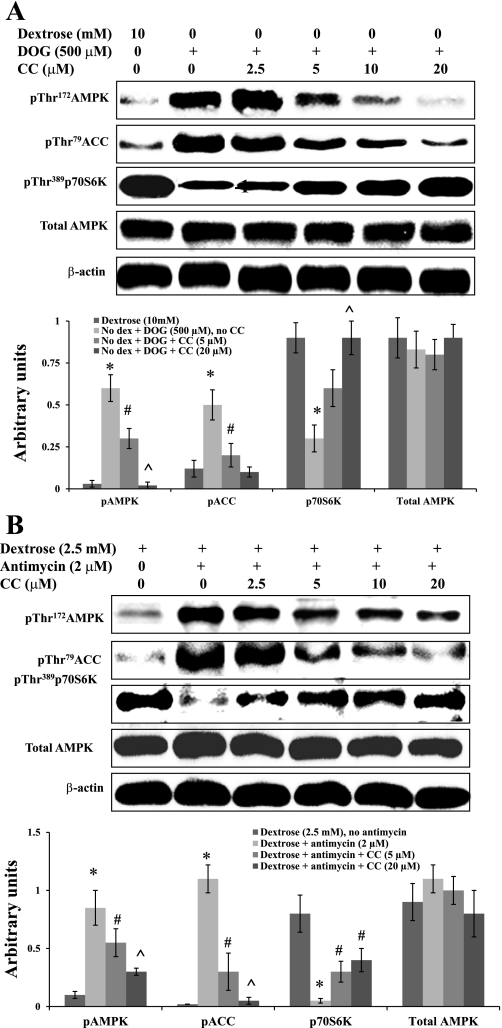
To establish a causal relationship between AMPK activation and the observed changes in phosphorylation of ACC and p70S6K during metabolic stress, we determined the effect of CC on these events. BU.MPT cells were subjected to metabolic stress by incubation in dextrose-free medium containing DOG (500 μM) (Fig. 6A). CC reversed the DOG-induced changes in phosphorylation of AMPK, ACC, and p70S6K in a dose-dependent manner (Fig. 6A). Similar results were obtained when BU.MPT cells were subjected to metabolic stress by culture in the presence of antimycin A (2 μM) and reduced dextrose (2.5 mM dextrose) (Fig. 6B).
Fig. 6.
Pharmacological inhibition of AMPK prevents metabolic stress-induced signaling events. A: BU.MPT cells were incubated in dextrose-containing medium (10 mM) without DOG and CC (control cells) or dextrose-free medium containing DOG (500 μM) and varying concentrations of CC. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose 10 mM. #P < 0.05, vs. no dextrose+DOG, no CC. ^P < 0.05, vs. no dextrose+DOG+CC (5 μM). B: BU.MPT cells were incubated in the presence of reduced dextrose (2.5 mM) and antimycin A (2 μM) plus varying concentrations of CC. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. For all experiments, cell lysates were probed with antibodies against phosphorylated (active) AMPK, phosphorylated (inactive) ACC, phosphorylated (active) p70S6K, total AMPK, and total β-actin. *P < 0.05, vs. dextrose, no antimycin. #P < 0.05, vs. dextrose+antimycin. ^P < 0.05, vs. dextrose+antimycin+CC (5 μM).
Comparable results were obtained using OK cells (data not shown). Taken together, these results indicate that metabolic stress induces the phosphorylation and activation of AMPK, which then inhibits the activity of both ACC and p70S6K. Thus inhibition of ACC is mediated directly through phosphorylation of ACC by AMPK, whereas inhibition of p70S6K occurs indirectly through inhibition of mTOR.
The α2-Isoform of AMPK is Expressed to a Greater Extent than the α1-Isoform in Renal Tissues
Although CC has been widely used to study the pathophysiological role of AMPK in multiple organs (16, 28, 35, 45, 72), the use of pharmacological inhibitors has potential problems of nonspecificity. We therefore sought a second independent approach to inhibit AMPK. To this end, we used gene silencing to knock down the expression and activity of the catalytic α-subunit of AMPK (see experimental procedures).
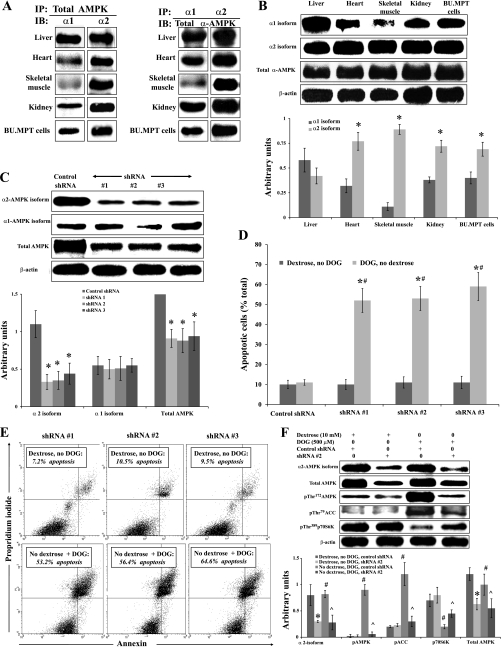
To determine which isoform of the α-subunit to target by gene silencing, we examined the relative expression of the α1- and α2-isoforms of AMPK in lysates of cultured BU.MPT cells and in whole organ lysates of mouse kidney, liver, heart, and skeletal muscle using immunoprecipitation followed by immunoblotting. In the first immunoprecipitation/immunoblotting experiment, we used antibodies to total AMPK for immunoprecipitation and then immunoblotted the precipitates with antibodies to the α1- and α2-isoforms of the catalytic domain of AMPK (Fig. 7A, left). To control for the possibility that these proportions may have been skewed by differences in the affinities of our antibodies for the α1- and α2-isoforms, we confirmed these results by a second experiment in which we used an excess of antibody to the α1- and α2-isoforms of the catalytic domain of AMPK for immunoprecipitation and then immunoblotted the precipitates with antibody to the total catalytic domain of AMPK (i.e., total α-AMPK). This ensured that comparisons were made using the same blotting antibody (Fig. 7A, right) Similar results were obtained using both techniques. We found that the liver contained about equal amounts of both isoforms, while the heart, kidney and BU.MPT cells contained a predominance of the α2-isoform (Fig. 7A). In skeletal muscle, almost all of the catalytic domain was made up of the α2-isoform (Fig. 7A).
Fig. 7.
Inhibition of AMPK by gene silencing with short hairpin (sh) RNA induces apoptosis and prevents metabolic stress-induced signaling events in BU.MPT cells subjected to acute metabolic stress. A: whole organ lysates of mouse liver, heart, skeletal muscle, and kidney and lysates of cultured BU.MPT cells were obtained, and the relative expression of the α1- and α2-isoforms of AMPK was determined by immunoprecipitation (IP) followed by immunoblotting (IB). Left: IP was performed using an antibody to total AMPK followed by IB with antibodies to the α1- and α2-isoforms of AMPK (n = 4). Right: IP was performed using antibodies to either the α1- or the α2-isoform of AMPK followed by IB using an antibody to the total catalytic domain (i.e., total α-AMPK; n = 3). B: whole organ lysates of mouse liver, heart, skeletal muscle, and kidney and lysates of cultured BU.MPT cells were probed with antibodies to the α1- and α2-isoforms of the catalytic domain of AMPK and to the total catalytic domain (total α-AMPK). Top: representative immunoblot. Bottom: results of 4 experiments by densitometric analysis using β-actin as a loading control. *P < 0.01, vs. α1-isoform in the corresponding tissue. C: BU.MPT cells were infected with vectors expressing either a nontargeting shRNA or 1 of 3 distinct shRNAs (denoted shRNA #1, #2, and #3) designed to knock down expression of the α2-isoform of AMPK. Lysates from unstressed BU.MPT cells (incubated in 10 mM dextrose) were probed with antibodies specific for the α1- and α2-isoforms of AMPK, total AMPK, or total β-actin. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. control shRNA for the same antibody. D: BU.MPT cells, infected with control shRNA or 1 of the 3 shRNAs targeted to α2, were incubated in dextrose-containing medium (10 mM) without DOG (dark gray bars) or in dextrose-free medium plus DOG (500 μM; light gray bars). After 16–18 h, apoptosis was assessed by flow cytometry. *P < 0.001, presence vs. absence of DOG for each targeted shRNA. #P < 0.001, targeted vs. control shRNA in the presence of DOG. E: representative flow cytometric analysis of BU.MPT cells infected with each of 3 shRNAs targeted to the α2-isoform of AMPK and incubated in either dextrose-containing medium (10 mM) without DOG (top) or in dextrose-free medium with DOG (500 μM; bottom). Apoptosis was quantified following staining with annexin V (x-axis) and PI (y-axis). For each condition, the percentage of apoptotic cells was obtained by summing the percentages of early apoptotic (annexin V-positive, PI-negative) and late apoptotic (annexin V-positive, PI-positive) cells. F, top: BUMPT cells were infected with either control shRNA or shRNA #2, specific for the α2-isoform of AMPK, and either cultured under control conditions (10 mM dextrose) or subjected to metabolic stress for 6 h [no dextrose+DOG (500 μM)]. Cell lysates were probed with antibodies against the α2-isoform of the catalytic domain, total AMPK, phosphorylated (active) AMPK, phosphorylated (inactive) ACC, phosphorylated (active) p70S6K, and total β-actin. A representative immunoblot is shown. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose, no DOG, control shRNA. #P < 0.05, vs. dextrose, no DOG, shRNA #2. ^P < 0.05, vs. no dextrose, DOG, control shRNA.
To obtain quantitative data of the relative amounts of the two isoforms of the catalytic domain in the various mouse organs and in BU.MPT cells, we next subjected all lysates to immunoblotting (without prior immunoprecipitation) using antibodies to each of the α1- and α2-isoforms and to total AMPK. The relative expression of each isoform and of total α-AMPK was determined by densitometry using β-actin as the loading control (n = 4) (Fig. 7B). Hepatic tissue contained approximately equal amounts of the α1- and α2-isoforms, whereas cardiac tissue contained roughly twofold more α2 than α1. The catalytic domain in skeletal muscle was almost entirely of the α1-isoform. Whole kidney and BU.MPT cells expressed somewhat more of the α2-isoform (63 ± 3 and 65.0 ± 3.2%, respectively) than the α1-isoform (39 ± 4 and 36.0 ± 4%, respectively). The distribution of the two isoforms of the catalytic domain in OK cells was similar to that in whole kidney and BU.MPT cells (data not shown).
Inhibition of AMPK by Gene Silencing Prevents Metabolic Stress-Induced Signaling Events Within the AMPK Pathway
Since the α2-isoform is present in greater amounts in BU.MPT cells and whole kidney, we chose to knock down its expression in BU.MPT cells. We used three distinct “targeted” shRNAs to knock down expression of the α2-isoform of AMPK in BU.MPT cells. All three shRNAs performed equivalently, reducing expression of the α2-isoform by ∼70%, compared with expression of the α2-isoform in the presence of control nontargeted shRNA (Fig. 7C). The targeted shRNAs also reduced total AMPK by ∼50% compared with control shRNA (Fig. 7C). As expected, the shRNAs designed to knock down the α2-isoform had no effect on the expression of the α1-isoform (Fig. 7C).
Infection of BU.MPT cells, cultured under control conditions (10 mM dextrose), with either targeted or control shRNA, had no effect on cell viability (Fig. 7D). In marked contrast, when BU.MPT cells were subjected to metabolic stress by exposure to DOG (500 μM) in the absence of dextrose, targeted shRNA induced substantial apoptosis (∼50–60%) (Fig. 7D). No increase in apoptosis was induced in cells infected with the control shRNA. A representative flow cytometric analysis is given in Fig. 7E, in which BU.MPT cells infected with each of the three shRNA were cultured in either dextrose-containing medium without DOG (top) or dextrose-free medium with DOG (500 μM, bottom). Thus inhibition of AMPK during DOG-induced metabolic stress, using either CC (Fig. 2B) or gene silencing (Fig. 7D), produced comparable increases in apoptosis of BU.MPT cells.
We next determined the effect of gene silencing on AMPK-dependent signaling. For these studies, BU.MPT cells were infected with either control shRNA or targeted shRNA 2 (see above) and then subjected to metabolic stress by exposure to DOG (500 μM) in the absence of dextrose for 6 h. Cells lysates were probed using antibodies to the α2-isoform of AMPK, total AMPK, and the phosphorylated forms of AMPK, ACC, and p70S6K (Fig. 7F). The targeted shRNA knocked down expression of the α2-isoform by ∼65% under both control and experimental conditions. Infection with shRNA 2 also reduced expression of total AMPK by ∼50%. As expected, the reduction in expression of the α2-isoform was greater than that of total AMPK. In accord with our previous results, metabolic stress increased phosphorylation of AMPK and ACC and reduced phosphorylation of p70S6K in cells infected with the control shRNA (Fig. 7F). Knock down of the α2-isoform of AMPK with shRNA 2 partially reversed the stress-induced changes in phosphorylation of AMPK, ACC, and p70S6K (Fig. 7F). Thus inhibition of AMPK during DOG-induced metabolic stress, using either CC (Fig. 6A) or gene silencing (Fig. 7F), reversed AMPK-associated changes in phosphorylation of ACC and p70S6K. These data provide support to a causal role for AMPK.
Finally, we examined the effect of knockdown of AMPK activity on cell ATP levels during DOG-induced metabolic stress (n = 3). As with pharmacological inhibition of AMPK by CC (Fig. 4B), infection with shRNA 2 did not alter cell ATP levels compared with infection with control shRNA (34 ± 4 vs. 38 ± 5% of control levels, respectively).
We conclude that, regardless of the mechanism of inhibition, decreasing AMPK activity in BU.MPT cells subjected to metabolic stress leads to a substantial increase in apoptosis and an abrogation of downstream AMPK-dependent signaling events, with both changes occurring in the absence of an alteration in cell ATP levels.
Metabolic Stress Inhibits Proliferation of Renal Tubular Cells by Activating AMPK
AMPK has been reported to inhibit cell cycle progression and proliferation by inhibiting mTOR activity (14, 73). We assessed cell proliferation by BrdU uptake in confluent BU.MPT cells, either grown in control dextrose-containing medium or subjected to DOG-induced metabolic stress for 6 h in the presence and absence of CC. BrdU uptake was expressed as a percentage of uptake by cells under control conditions. In the absence of CC, DOG-induced stress reduced proliferation to 22 ± 8% of control levels (P < 0.0001). In the presence of CC, proliferation was reduced only to 68 ± 7% of control (P < 0.005 vs. control; P < 0.01 vs. no CC) (n = 5). Comparable results were obtained with OK cells (data not shown). Thus, in two different proximal tubular cell lines, inhibiting AMPK with CC significantly ameliorated stress-induced inhibition of proliferation.
Inhibiting AMPK by knocking down expression of the α2-isoform in BU.MPT cells gave similar results. Confluent BU.MPT cells infected with either control shRNA or shRNA 2 were subjected to DOG-induced metabolic stress. For cells infected with control shRNA, BrdU uptake was reduced to 26 ± 7% of control levels (P < 0.001). For cells infected with shRNA 2, BrdU uptake was reduced to only 56 ± 7% of control (P < 0.001 vs. control; P < 0.01 vs. nontargeted shRNA).
We conclude that metabolic stress markedly inhibits cell proliferation and that inhibition of proliferation can be ameliorated by inhibition of AMPK, either pharmacologically with CC or by knocking down expression of the α2-isoform of AMPK. Together, these data indicate an important role for AMPK in mediating the inhibition of cell proliferation during metabolic stress.
Activation of AMPK by Metabolic Stress Enhances Survival Through Activation of Akt
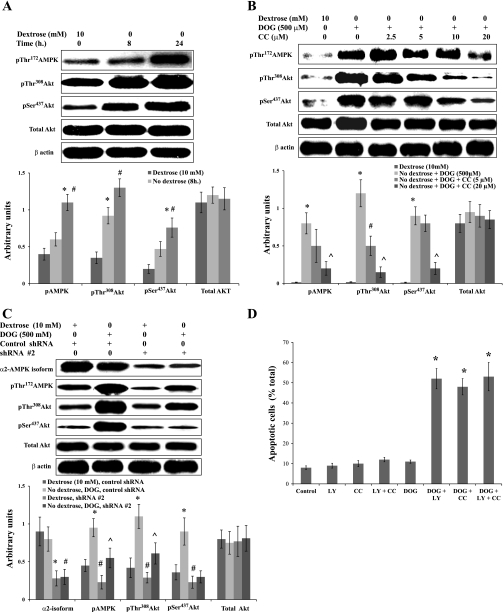
Akt is a well-characterized kinase that protects cells from cell death in response to a wide variety of stresses (6, 13, 57, 66). We therefore examined the role of Akt in survival of BU.MPT cells subjected to acute metabolic stress. Metabolic stress induced by dextrose deprivation alone (Fig. 8A) or by dextrose deprivation in the presence of DOG (500 μM) (Fig. 8B) increased phosphorylation of Akt at its two major regulatory sites (Thr308 and Ser437) (6, 13, 57, 66). Notably, for both forms of metabolic stress, increasing concentrations of CC inhibited phosphorylation progressively of both Thr308 and Ser437 (Fig. 8B). We repeated these studies in BU.MPT cells infected with control shRNA or shRNA 2 (Fig. 8C). Infection with shRNA 2 reduced expression of the α2-isoform of AMPK and also reduced phosphorylation of Akt at both sites in response to acute metabolic stress. Control shRNA had no effect on Akt phosphorylation.
Fig. 8.
Activation of AMPK induces phosphorylation of Akt. A: BU.MPT cells were incubated in control dextrose-containing medium (10 mM) or in dextrose-free medium for 8 or 24 h. Cell lysates were probed with antibodies specific for phosphorylated (active) AMPK, phosphorylated (active) Akt (pThr308 and pSer437), total Akt, and β-actin. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose (10 mM). #P < 0.05, vs. no detxrose (8 h). B: BU.MPT cells were incubated in control, dextrose-containing medium (10 mM dextrose) or in dextrose-free medium plus DOG (500 μM) with varying concentrations of CC for 6 h. Cell lysates were probed with antibodies specific for phosphorylated (active) AMPK, phosphorylated (active) Akt (pThr308 and pSer437), total Akt, and β-actin. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose (10 mM). #P < 0.05, vs. no dextrose+DOG. ^P < 0.05, vs. dextrose+DOG+CC (5 μM). C: BU.MPT cells, infected with either the control (nontargeting) shRNA or shRNA #2 (targeted against the α2-isoform of AMPK), were cultured in control dextrose-containing medium (10 mM) or in dextrose-free medium plus DOG (500 μM) for 6 h. Cell lysates were probed with antibodies to the α2-isoform of AMPK, phosphorylated (active) AMPK, phosphorylated (active) Akt (pThr308 and pSer437), total Akt, and β-actin. Top: representative immunoblot. Bottom: results of 3 experiments by densitometric analysis using β-actin as a loading control. *P < 0.05, vs. dextrose, control shRNA. #P < 0.05, vs. no dextrose, DOG, control shRNA. ^P < 0.05, vs. dextrose, shRNA #2. D: BU.MPT cells were cultured for 16–18 h in either control dextrose-containing medium (10 mM dextrose) or in dextrose-free medium plus the indicated combinations of CC (20 μM), LY294002 (10 μM), and DOG (500 μM). Apoptosis was quantified by flow cytometry. *P < 0.001, vs. control.
To provide functional evidence that Akt plays a prosurvival role during metabolic stress, we used LY294002, a pharmacological inhibitor of Akt (12, 58). Inhibition of Akt with LY294002 (10 μM) or inhibition of AMPK with CC (20 μM), as well as combined inhibition of both Akt and AMPK, increased apoptosis to equivalent degrees in BU.MPT cells subjected to DOG-induced metabolic stress (Fig. 8D). The lack of a synergistic interaction between these two inhibitors suggests that activation of AMPK during metabolic stress leads to activation of Akt and that Akt, at least in part, mediates the prosurvival effect of AMPK activation.
Finally, we attempted to determine whether phosphorylation of Akt occurs directly by AMPK or indirectly through intermediate kinases, using immunoprecipitation to look for evidence of a physical interaction between AMPK and Akt under either basal conditions or after 6 h of DOG-induced metabolic stress. We immunoprecipitated with antibody to total AMPK and immunoblotted with antibody to total Akt (n = 3). We also immunoprecipitated with antibody to total Akt and immunoblotted with antibody to total AMPK (n = 3). No Akt was detected in precipitates of total AMPK and, similarly, no AMPK was detected in precipitates of total Akt (data not shown). While not definitive, these findings argue against direct phosphorylation of Akt by AMPK and suggest the involvement of intermediary pathways.
DISCUSSION
AMPK is a serine/threonine kinase that is highly sensitive to small decreases in cell energy charge. When activated, AMPK modulates the activity of multiple downstream kinases as well as the expression of multiple genes (8, 21, 26, 64). Many of these effects of AMPK serve to preserve cell energy stores in response to energy depletion. This has led investigators to propose that AMPK acts as a “guardian” of cell energy stores, helping to maintain cell survival during ischemic insults (8, 23). Although multiple studies in the heart (2, 9, 39, 44, 59, 65), brain (41, 45), and liver (55) have shown that AMPK is activated during ischemia, definitive evidence that AMPK plays a role in modulating the fate of cells subjected to ischemic stress has remained elusive. While some studies have reported a cytoprotective role for AMPK during ischemic injury, others have found that AMPK either has no effect on cell viability or may even promote cell death (21). In the kidney, there are few data available regarding either the response of the AMPK signaling pathway to ischemic injury or whether AMPK modulates the survival of renal tubular cells to metabolic stress.
The objective of this study was to examine the hypothesis that AMPK is activated and promotes cell survival in renal tubular cells subjected to metabolic stress. We used a cell culture model of proximal tubular cells and subjected cells to three different forms of acute metabolic stress: 1) dextrose deprivation; 2) inhibition of glycolysis by DOG in the absence of dextrose; and 3) inhibition of mitochondrial respiration by antimycin A in the presence of reduced concentrations of dextrose. We inhibited AMPK activity by two independent means, either pharmacologically with CC or by gene silencing with shRNA targeted against the α2-isoform of AMPK.
We report here several major findings. First, acute metabolic stress, induced by three different models, led to the phosphorylation and activation of AMPK as well as alterations in the phosphorylation of ACC and p70S6K, two downstream substrates of AMPK. Since inhibition of AMPK, both pharmacologically with CC and by knockdown of the α2-isoform of AMPK, prevented these changes in phosphorylation of ACC and p70S6K, we deduce that these effects derive from upstream activation of AMPK. This conclusion is strengthened considerably by the fact that comparable results were observed with two independent methods of inhibiting AMPK.
Second, inhibition of AMPK during acute metabolic stress led to a substantial increase in apoptosis. This was true for both methods of inhibiting AMPK and with all three forms of metabolic stress. Together, these data suggest a critical role for AMPK in maintaining the viability of proximal tubular cells during metabolic stress. Notably, while inhibition of AMPK markedly impaired the survival of tubular epithelial cells, inhibition of AMPK had no effect on cellular ATP levels. Thus, regardless of the model or the severity of acute metabolic stress, cellular ATP stores were equivalently reduced in the presence vs. the absence of AMPK inhibition. This implies that AMPK-mediated amelioration of apoptosis during metabolic stress cannot be explained by conservation of cell ATP stores.
A third major finding of our studies is that Akt, a kinase with profound antiapoptotic effects (13, 32, 57), contributes to the prosurvival activity of AMPK during metabolic stress. Both dextrose deprivation and inhibition of glycolysis with DOG increased phosphorylation of Akt at Thr308 and Ser437, the two sites required for full activation of Akt (13, 32, 57). Importantly, inhibition of AMPK prevented phosphorylation of Akt. A causal link between AMPK activation and phosphorylation of Akt is further suggested by the fact that inhibition of AMPK alone with CC and inhibition of Akt alone with LY294002 (36), as well combined inhibition of AMPK and Akt with the two inhibitors together, induced comparable degrees of apoptosis. A degree of synergistic interaction would be expected if activation of Akt was not a consequence of AMPK activation.
Such a role for AMPK in activating Akt is consistent with several studies in the literature. AICAR, a cell-permeable nucleotide used extensively in vivo and in vitro to activate AMPK (11), has been shown to increase phosphorylation of Akt at residues Thr308 and Ser347 in lymphocytes (40). In the same study, inhibition of AMPK with CC reduced the phosphorylation of Akt at both residues, suggesting a causal role for AMPK in Akt activation (40). Also, a constitutively active AMPK mutant increased glucose uptake by cardiomyocytes in an Akt-dependent manner (3). Furthermore, AMPK has been reported to be responsible for Akt-mediated production of nitric oxide in human umbilical vein endothelial cells (10). Finally, Akt-mediated increases in angiogenesis, induced by both hypoxic stress (50) and adiponectin (52), have been shown to depend upon activation of AMPK. Thus our results add to a growing body of evidence suggesting that under appropriate circumstance AMPK is capable of activating Akt.
Finally, we report that whole mouse kidney and BU.MPT cells express substantial amounts of both the α1- and the α2-isoforms of AMPK, with a slight excess of α2 over α1, on the order of 60 to 40%. While our results describing the relative expression of these two isoforms in other organs (liver, heart, and skeletal muscle) are in accord with those obtained by other investigators in rodent and human tissues (34, 56, 67, 69, 71), our results for the kidney differ from those of Fraser et al. (15), who found a predominance of the α1-isoform. The reason for this discrepancy is unclear, as similar techniques were used in both studies. One possibility is a species difference, since our studies were performed in mice and Fraser et al. worked with rats. In this regard, it is worth noting that the expression patterns of these two isoforms in the kidney vary substantially among available studies and across species, with the previously described study in rats showing a predominance of the α1-isoform (15) and another in humans showing approximately equal amounts of the two isoforms (56).
Several other aspects of our studies merit comment. A finding of interest was that activation of AMPK occurred during dextrose deprivation without a detectable fall in cell ATP levels. We speculate that activation of AMPK, despite apparently normal cell ATP levels, reflects the extreme sensitivity of AMPK to very small changes in cell nucleotide levels. AMPK may be activated not only by small decreases in cell ATP levels but also by small increases in ADP or AMP (4, 24, 51). Thus it is possible that tiny combined changes in the concentrations of AMP, ADP, and ATP were sufficient to activate AMPK, even though changes in ATP fell below the sensitivity of our assay.
In addition, it is worthy of notice that a direct relationship existed between the degree to which cell energy stores were reduced and the extent to which apoptosis was augmented by inhibition of AMPK with CC. For example, a maximal concentration of CC (20 μM) induced ∼30% apoptosis during dextrose deprivation (Fig. 1A), a situation in which cell ATP levels were not measurably altered (Fig. 4A). When cell ATP levels were approximately halved by the combination of DOG and dextrose-free medium (Fig. 4B), the same concentration of CC resulted in almost twice as much apoptosis (∼60%) (Fig. 2A). Finally, when cell energy stores were depleted to <10% of control levels by antimycin A (Fig. 4C), the same concentration of CC induced virtually complete apoptosis of the cell population (Fig. 3B).
Last, we wish to point out an important difference between our results and those of other investigators (48) with respect to the effects of metabolic stress on AMPK-dependent signaling events in the kidney. In contrast to our findings, ischemia of the rat kidney, induced by pedicle clamping, was shown to induce activation of AMPK without changes in the phosphorylation of two of AMPK's downstream substrates (ACC and endothelial nitric oxide synthase) (48). The authors concluded that stress-induced activation of AMPK in the kidney results in a pattern of substrate phosphorylation different from that in other organs, such as the heart, brain, liver, and skeletal muscle (2, 21, 23, 29, 53, 55, 64). Our results, in which activation of AMPK by acute metabolic stress of the kidney resulted in changes in phosphorylation in two of its downstream targets (ACC and p70S6K), are fully consistent with results by multiple investigators in other organs (2, 21, 23, 29, 53, 55, 64).
In conclusion, we report that activation of AMPK plays an important role in maintaining the survival of proximal tubular cells during acute metabolic stress and that this effect of AMPK is mediated, at least in part, by activation of Akt. In consideration of the role of AMPK within the broader context of acute kidney injury, it may be important to differentiate between AMPK's role during acute ischemia vs. its role during recovery and repair (5, 60). Activation of AMPK during acute ischemia appears to promote a state of cellular quiescence, during which viability is maintained but growth and proliferation are suppressed. This combination of events minimizes energy expenditure while maximizing the pool of viable cells. We speculate that in the period following transient ischemia, reoxygenation could lead to suppression of AMPK and reactivation of mTOR. This would allow quiescent tubular cells (kept alive by AMPK-mediated activation of Akt) to reenter the cell cycle and initiate proliferation. In this way, through the actions of AMPK, a larger pool of viable cells would be available, thereby hastening recovery of kidney function.
GRANTS
W. Lieberthal was supported by a Veterans Affairs Merit Award and by awards from Dialysis Clinic, Inc. (DCI; C-3176 and C-2243). V. A. Patel was supported by National Institutes of Health Grant DK071678. J. S. Levine was supported by a GRIP Renal Innovations Program Award from Genzyme, Inc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.L. and J.S.L. provided conception and design of research; W.L., L.Z., and V.A.P. performed experiments; W.L., L.Z., and V.A.P. analyzed data; W.L., L.Z., V.A.P., and J.S.L. interpreted results of experiments; W.L. prepared figures; W.L. drafted manuscript; W.L. and J.S.L. edited and revised manuscript; W.L., L.Z., V.A.P., and J.S.L. approved final version of manuscript.
REFERENCES
- 1. Abbate M, Brown D, Bonventre JV. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am J Physiol Renal Physiol 277: F454–F463, 1999. [DOI] [PubMed] [Google Scholar]
- 2. Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res 100: 474–488, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol 291: H239–H250, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Bland ML, Birnbaum MJ. Cell biology. ADaPting to energetic stress. Science 332: 1387–1388, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14, Suppl 1: S55–S61, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Cantley LC. The phosphoinositide 3-kinase pathway. Science 296: 1655–1657, 2002. [DOI] [PubMed] [Google Scholar]
- 7. Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Carling D. The AMPK-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci 29: 18–24, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the α2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol 292: H3136–H3147, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995. [DOI] [PubMed] [Google Scholar]
- 12. Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem 277: 35364–35370, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene 24: 7401–7409, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285: 14071–14077, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp BE, Power DA. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem 281: 25956–25964, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Gassmann M, Grenacher B, Rohde B, Vogel J. Quantifying Western blots: pitfalls of densitometry. Electrophoresis 30: 1845–1855, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Gruzman A, Babai G, Sasson S. Adenosine monophosphate-activated protein kinase (AMPK) as a new target for antidiabetic drugs: a review on metabolic, pharmacological and chemical considerations. Rev Diabet Stud 6: 13–36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hallows KR. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Hallows KR, Mount PF, Pastor-Soler NM, Power DA. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol 298: F1067–F1077, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hardie DG. AMP-activated protein kinase: the guardian of cardiac energy status. J Clin Invest 114: 465–468, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Hardie DG. Signal transduction: how cells sense energy. Nature 472: 176–177, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997. [DOI] [PubMed] [Google Scholar]
- 26. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67: 821–855, 1998. [DOI] [PubMed] [Google Scholar]
- 27. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30: 1064–1070, 2002. [DOI] [PubMed] [Google Scholar]
- 28. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49: 527–531, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Hue L, Beauloye C, Bertrand L, Horman S, Krause U, Marsin AS, Meisse D, Vertommen D, Rider MH. New targets of AMP-activated protein kinase. Biochem Soc Trans 31: 213–215, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA 88: 4171–4175, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 21: 6082–6090, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Kim M, Tian R. Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol 71: 1637–1647, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koh JS, Lieberthal W, Heydrick S, Levine JS. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Invest 102: 716–727, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab 17: 205–215, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Kroshian VM, Sheridan AM, Lieberthal W. Functional and cytoskeletal changes induced by sublethal injury in proximal tubular epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 266: F21–F30, 1994. [DOI] [PubMed] [Google Scholar]
- 39. Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270: 17513–17520, 1995. [DOI] [PubMed] [Google Scholar]
- 40. Leclerc GM, Leclerc GJ, Fu G, Barredo JC. AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia. J Mol Signal 5: 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38: 2992–2999, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol Renal Physiol 274: F315–F327, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26: 69–76, 2005. [DOI] [PubMed] [Google Scholar]
- 44. Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255, 2000. [DOI] [PubMed] [Google Scholar]
- 45. McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280: 20493–20502, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Mori H, Inoki K, Masutani K, Wakabayashi Y, Komai K, Nakagawa R, Guan KL, Yoshimura A. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun 384: 471–475, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574: 63–71, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005. [DOI] [PubMed] [Google Scholar]
- 49. Musi N, Goodyear LJ. Targeting the AMP-activated protein kinase for the treatment of type 2 diabetes. Curr Drug Targets Immune Endocr Metabol Disord 2: 119–127, 2002. [PubMed] [Google Scholar]
- 50. Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003. [DOI] [PubMed] [Google Scholar]
- 51. Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science 332: 1433–1435, 2011. [DOI] [PubMed] [Google Scholar]
- 52. Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304–1309, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem 277: 32571–32577, 2002. [DOI] [PubMed] [Google Scholar]
- 54. Patel VA, Lee DJ, Feng L, Antoni A, Lieberthal W, Schwartz JH, Rauch J, Ucker DS, Levine JS. Recognition of apoptotic cells by epithelial cells: conserved versus tissue-specific signaling responses. J Biol Chem 285: 1829–1840, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpi E, Rosello-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology 34: 1164–1173, 2001. [DOI] [PubMed] [Google Scholar]
- 56. Quentin T, Kitz J, Steinmetz M, Poppe A, Bar K, Kratzner R. Different expression of the catalytic alpha subunits of the AMP activated protein kinase - an immunohistochemical study in human tissue. Histol Histopathol 26: 589–596, 2011. [DOI] [PubMed] [Google Scholar]
- 57. Ruggero D, Sonenberg N. The Akt of translational control. Oncogene 24: 7426–7434, 2005. [DOI] [PubMed] [Google Scholar]
- 58. Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem 279: 2632–2639, 2004. [DOI] [PubMed] [Google Scholar]
- 59. Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Safirstein R. Renal regeneration: reiterating a developmental paradigm. Kidney Int 56: 1599–1600, 1999. [DOI] [PubMed] [Google Scholar]
- 61. Sheridan AM, Schwartz JH, Kroshian VM, Tercyak AM, Laraia J, Masino S, Lieberthal W. Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol Renal Fluid Electrolyte Physiol 265: F342–F350, 1993. [DOI] [PubMed] [Google Scholar]
- 62. Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488–F497, 2003. [DOI] [PubMed] [Google Scholar]
- 63. Sinha D, Wang Z, Ruchalski KL, Levine JS, Krishnan S, Lieberthal W, Schwartz JH, Borkan SC. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am J Physiol Renal Physiol 288: F703–F713, 2005. [DOI] [PubMed] [Google Scholar]
- 64. Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009. [DOI] [PubMed] [Google Scholar]
- 65. Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 25: 9554–9575, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene 24: 7391–7393, 2005. [DOI] [PubMed] [Google Scholar]
- 67. Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: lessons from transgenic and knockout animals. Front Biosci 14: 19–44, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008, 2003. [DOI] [PubMed] [Google Scholar]
- 69. Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett 397: 347–351, 1996. [DOI] [PubMed] [Google Scholar]
- 70. Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, Vanoverschelde JL, Bertrand L. Role of the α2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol 291: H2875–H2883, 2006. [DOI] [PubMed] [Google Scholar]
- 72. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]