Abstract
The kidney plays an essential role in blood pressure regulation by controlling short-term and long-term NaCl and water balance. The thick ascending limb of the loop of Henle (TAL) reabsorbs 25–30% of the NaCl filtered by the glomeruli in a process mediated by the apical Na+-K+-2Cl− cotransporter NKCC2, which allows Na+ and Cl− entry from the tubule lumen into TAL cells. In humans, mutations in the gene coding for NKCC2 result in decreased or absent activity characterized by severe salt and volume loss and decreased blood pressure (Bartter syndrome type 1). Opposite to Bartter's syndrome, enhanced NaCl absorption by the TAL is associated with human hypertension and animal models of salt-sensitive hypertension. TAL NaCl reabsorption is subject to exquisite control by hormones like vasopressin, parathyroid, glucagon, and adrenergic agonists (epinephrine and norepinephrine) that stimulate NaCl reabsorption. Atrial natriuretic peptides or autacoids like nitric oxide and prostaglandins inhibit NaCl reabsorption, promoting salt excretion. In general, the mechanism by which hormones control NaCl reabsorption is mediated directly or indirectly by altering the activity of NKCC2 in the TAL. Despite the importance of NKCC2 in renal physiology, the molecular mechanisms by which hormones, autacoids, physical factors, and intracellular ions regulate NKCC2 activity are largely unknown. During the last 5 years, it has become apparent that at least three molecular mechanisms determine NKCC2 activity. As such, membrane trafficking, phosphorylation, and protein-protein interactions have recently been described in TALs and heterologous expression systems as mechanisms that modulate NKCC2 activity. The focus of this review is to summarize recent data regarding NKCC2 regulation and discuss their potential implications in physiological control of TAL function, renal physiology, and blood pressure regulation.
Keywords: hypertension, ion transport, kidney, Na+-K+-2Cl− cotransporter, trafficking
Role of Na+-K+-2Cl− Cotransporter in Thick Ascending Limb Function
the main function of the thick ascending limb (TAL) is to reabsorb large amounts of NaCl while reabsorbing no water, therefore diluting the forming urine in the tubule lumen (10, 19). Throughout the length of the TAL, luminal NaCl concentration decreases gradually from ∼140 mM in the inner stripe of the outer medulla to 30–60 mM at the macula densa (10, 18, 21, 102). NaCl reabsorption by the TAL is a two-step process: first, Na+, K+, and Cl− enter the cell across the apical membrane via the electroneutral Na+-K+-2Cl− cotransporter (NKCC2). These ions are transported following the electrochemical gradient generated by Na+-K+-ATPases, which mediate the second step of Na+ extrusion across the basolateral membrane (19). Cl− exits via the basolateral K+-Cl− cotransporter (KCC4) (115) or Cl− channels (CLC-Kb) (85, 93). A fraction of the K+ reabsorbed from the lumen by NKCC2 recycles across the apical membrane via apical K+ channels (ROMK) (48, 174, 175). In this way, the lumen-to-bath potential difference is positive (+5 to +10 mV), while the intracellular potential is negative (−70 mV) (20, 34, 55, 56, 105, 145).
NKCC2 plays an essential role in the TAL and renal function. It mediates 100% of the Cl− reabsorption, and although it mediates 50% of all Na+ transport apical membrane, NKCC2 activity is linked to paracellular Na+, Ca2+, and Mg2+ flux (67). A fraction of the luminal Na+ (10–20%) enters the cell via the type 3 Na+/H+ exchanger (NHE3) and diffuses across tight junctions (30–40%) following the lumen-positive potential generated by ROMK. Inhibition of NKCC2 decreases K+ exit through ROMK, disrupting the lumen-positive potential and halting not only all NaCl reabsorption but also the paracellular transport of calcium and magnesium (66, 71). In addition, NaCl reabsorption by the TAL maintains a high interstitial osmolality, necessary for countercurrent multiplication and water reabsorption by the collecting duct system. Thus NKCC2 activity is essential for salt conservation and for acute and chronic regulation of water balance.
NKCC2 dysfunction exerts negative consequences in mammals. In humans, inactivating mutations in the gene coding for NKCC2 (Slc12a1) causes Bartter syndrome type 1, characterized by severe natriuresis hypercalciuria and magnesuria, inability to concentrate urine, leading to hyponatremia, hypochloremia, metabolic alkalosis, and low blood pressure (144, 160, 166). A similar phenotype is observed in mice lacking NKCC2, most of which do not survive unless salt and volume are maintained (169). Opposite to genetic inactivation of NKCC2, enhanced activity of NKCC2 has been linked to salt-sensitive hypertension and hypertensive disorders in rodents and humans (6, 82, 88–90). Despite the importance of NKCC2 in renal function, the molecular mechanisms by which this apical cotransporter is regulated in health and disease have remained poorly understood. In this review, we have summarized recent studies pointing to the complex regulation of NKCC2 by various mechanisms. In the first part, we focus on NKCC2 gene structure and function and determinants of apical sorting and maturation. We then discuss its hormonal regulation in the TAL and the molecular mechanisms affecting NKCC2 function, including membrane trafficking, phosphorylation, and protein-protein interactions. Finally, we emphasize the role of NKCC2 in physiology and human pathology, focusing on the role of NKCC2 in blood pressure regulation and hypertension.
NKCC2 Expression and Gene Structure/Function
NKCC2 belongs to a family of integral plasma membrane proteins that mediate electroneutral symport of Na+, K+, and Cl− with a stochiometry of 1:1:2, respectively. NKCC2 is encoded by the Slc12a1 gene and was also called bumetanide-sensitive cotransporter 1 (BSC-1). NKCC2 and its closely related precursor NKCC1 (Slc12a2) are the targets of loop diuretics bumetanide and furosemide (62). NKCC2 activity and expression have only been confirmed in the medullary and cortical TAL and in macula densa cells (76, 84, 92, 123, 157). In addition to mediating all of the NaCl reabsorption in the TAL, in the specialized cells of the macula densa NKCC2 senses luminal Cl− concentration and initiates the signal for tubuloglomerular feedback, thereby controlling afferent arteriole tone and glomerular filtration rate (reviewed in Refs. 16 and 28).
NKCC1 and NKCC2 share 60% homology (35, 76, 134). NKCC1 (BSC-2) was originally cloned from the shark rectal gland (mature form: ∼195 kDa). Different from NKCC2, NKCC1 is localized primarily in the basolateral membranes of secretory epithelia (180), the mouse inner medullary collecting duct (33), and in the human colon (135), where it is responsible for transepithelial Cl− and water secretion (60). It is also present in several neuronal cell types like red blood cells and vasculature, where it is involved in cell volume regulation and ion homeostasis (59). NKCC2 was first cloned by Gamba et al. (46) from rat kidney and in the murine kidney by Igarashi et al. (76) by screening a kidney library and anchored PCR. Murine NKCC2 has 93 and 97% homology with the rabbit and rat sequence, respectively (44, 76) (Fig. 1). The topology of NKCC2, based on the cDNA sequence, predicts a protein with 12 transmembrane-spanning domains, a cytoplasmic NH2 terminus 170 amino acids long, and a large cytoplasmic COOH terminus (∼470 amino acids) (Fig. 1). While there are several phosphorylation consensus sites for protein kinase A (PKA), protein kinase C (PKC), and casein kinases, to date only a few kinases have been reported to phosphorylate NKCC2 (discussed in detail below). There are no known consensus sequences for cGMP-dependent protein kinase (PKG). Furthermore, the actual structure of NKCC2 in a double-lipid bilayer, the folding and conformation of the intracellular domains, is currently unknown.
Fig. 1.
Schematic structure of the Na+-K+-2Cl− (NKCC2) protein, highlighting established phosphorylation sites (rat sequence) and some of the protein-protein interaction identified to date.
NKCC2 Alternative Splicing
Although NKCC2 is encoded by a single gene (Slc12a1), differential splicing of NKCC2 mRNA results in the formation of alternate transcripts. The combination of two independent splicing produces at least six isoforms of NKCC2 showing species-specific expression. The first splicing produces the isoforms A, B, and F due to the variable exon 4 (17, 28, 134) (Fig. 1). The A, B, and F isoforms of NKCC2 differ in their localization along the TAL and in their ion transport characteristics (ion affinity for Na+, K+, and Cl−). The isoform NKCC2A exhibits intermediate affinity for Cl− (Km: ∼30 mM) and is located in the medullary and cortical TAL although it is most abundant in the cortex. The B isoform shows the highest affinity for Cl− (Km: ∼10–15 mM) and is primarily located in the macula densa cells and cortical portion. The NKCC2F isoform has the lowest affinity for transported ions (Km: ∼110–130 mM) and is located mainly in the medullary TAL (28, 52, 114, 138, 183). The relative distribution of these isoforms and their affinity for ions match the luminal concentration of NaCl along the TAL allowing maximal Cl− reabsorption without saturation. Mice lacking the NKCC2A isoform show slightly lower urine osmolality and a small decrease in Cl− reabsorption, and enhanced mRNA for the NKCC2B isoform, indicating compensation (125). NKCC2B isoform knockout mice show slightly lower Cl− reabsorption along the loop of Henle and blunted tubuloglomerular feedback consistent with Cl− sensing at the macula densa (28).
The large difference in ion affinity between the B and F isoforms is primarily due to amino acid changes in the intracellular linker region (ICL1) between transmembrane domains TM2 and TM3. Gimenez and Forbush (51) showed that two sets of three residues, one set in TM2 and one in ICL1, are involved in ion-affinity changes. The set of residues in ICL1 are mainly responsible for different ion affinities and seem to be part of an α-helical conformation that may be partially embedded in the plasma membrane. To date, the binding sites for Cl−, K+, and Na+ as well as for the inhibitors of the furosemide family are unknown.
A second splicing mechanism has been described that produces a stop signal in the COOH-terminal portion, producing a short NKCC2 isoform that lacks the last 327 amino acids but contains an extra 55 amino acids not present in the long isoform (114). To date, this short NKCC2 isoform has only been reported in mice (139). Expression of the short NKCC2 isoform in Xenopus laevis oocytes induces bumetanide-sensitive Na+ and Cl− entry independently of extracellular K+ (137, 139). These data suggest that the expression of a short NKCC2 isoform may explain the AVP- and K+ independent cell swelling (blocked by furosemide) observed in mouse TAL (168, 41). However, the role of the short isoform (∼770 amino acids) in TAL NaCl reabsorption is unknown, since a mutant mouse lacking the short isoform has not been generated, and its effect on apical Na+ and Cl− transport in isolated perfused TALs has not been studied in mice. Antibodies against the NKCC2 NH2 terminus fail to detect the short splice variant in rat TALs, and it is unclear whether this splice variant is present in human kidneys.
N-Linked Glycosylation and Determinants of Apical Sorting
The NKCC2 sequence predicts a protein of 1,095 amino acids with a calculated molecular mass of ∼121 kDa (76). However, NKCC2 has two N-linked glycosylation sites located between transmembrane domains 7 and 8 in the position N442 and N452, which confer an apparent molecular mass of 160 kDa when detected by immunoblotting (114, 130). Importantly, when detected by immunoblotting from kidney homogenates or isolated TALs, the immature/nonglycosylated (121 kDa) form of NKCC2 is at an extremely low abundance and only detectable after overexposure of films, indicating that in TAL most NKCC2 is efficiently glycosylated. There are additional predicted N-linked and O-linked glycosylation sites that are not likely relevant to NKCC2 function. Eliminating the two glycosylation sites between TM7 and -8 (N442 and N452) decreases but does not completely eliminate NKCC2 transport activity or plasma membrane NKCC2 levels in the X. laevis oocyte expression system (130), suggesting that a fraction of nonglycosylated NKCC2 may reach the plasma membrane. N-linked and O-linked glycosylation are involved in apical targeting (116, 152, 153) and correct maturation of several transmembrane proteins. However, the role of N-linked glycosylation in maturation, stability and trafficking of NKCC2 to the plasma membrane in TALs or polarized epithelial cells has not been studied.
While N-linked glycosylation is likely involved in maturation and membrane targeting of NKCC2, the sequence responsible for apical trafficking of NKCC2 in polarized epithelium has been mapped to the cytoplasmic C terminus. In an elegant set of studies, Carmosino et al. (26) mapped the apical targeting domain in NKCC2 by generating chimeras between basolateral NKCC1 and NKCC2 and expressed them in polarized Madin-Darby canine kidney (MDCK) cells (26). They found that a 77-amino acid portion of NKCC2 located in the COOH terminus (amino acids 708–884) is sufficient to target NKCC2, a fraction of NKCC1, and an unrelated transmembrame protein (PLAP-VSV-G, a reporter with no dominant sorting information) to the apical membrane of MDCK cells. Sequence analysis indicated that the 77-amino acid apical determinant is not present in NKCC1 while the dileucine motif that mediates basolateral targeting of NKCC1 is not conserved in NKCC2. Overall, these data suggested that NKCC2 arose later in evolution from gene duplication of an NKCC1 precursor after rearrangement (loss) of a 16-amino acid stretch on exon 21.
In addition to the apical targeting domain, the COOH terminus of NKCC2 contains information required for endoplasmic reticulum (ER) exit, protein maturation, and ER-Golgi-plasma membrane trafficking of NKCC2 (187). By expressing mouse NKCC2 deletion mutants in nonpolarized opossum kidney (OK) or HEK-293 cells, Zaarour et al. (187) identified a trihydrophobic motif in the distal COOH terminus of NKCC2 (1081LLV1083) that is involved in NKCC2 maturation. Deletion of this motif eliminated complex glycosylation of NKCC2, resulting in a protein of 120 kDa that does not reach the plasma membrane and is retained in an ER-like compartment based on colocalization studies. While this sequence may encode an ER exit signal, it may also be required for proper dimerization, folding, and maturation. Nezu et al. (120) found that mutating this amino acid stretch in NKCC1 induced misfolding and aggregation, which activates ER quality control. Until a specific protein-protein interaction with this trihydrophobic motif is identified and ER export studied, it is accurate to indicate that this motif is important for ER export and/or maturation. Of clinical interest, these investigators also demonstrated that one of the reported NKCC2 mutations in Bartter syndrome type 1 (Y998X) falls close to this COOH-terminal ER exit motif (2, 166). This Bartter syndrome mutant (Y998X) exhibit a similar behavior as mutants lacking the ER exit signal, being retained at the ER, lacking complex glycosylation and surface expression.
Taken together, these data indicate that the COOH-terminal portion has essential information for maturation, correct expression, and functionality of NKCC2 in TALs. Nevertheless, most of the known phosphorylation sites reported have been mapped to the NH2 terminus (discussed below), suggesting that potential protein-protein interactions in this region may also be important for NKCC2 regulation.
Hormonal Regulation of NKCC2 in the TAL
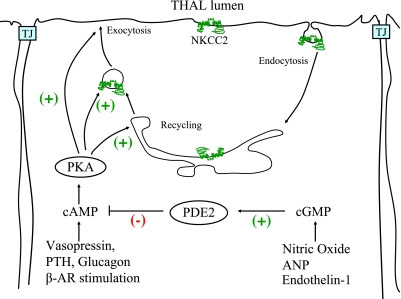
Several hormones are responsible for the accurate control of NaCl reabsorption by the TAL. In most cases, hormones and autacoids that affect TAL NaCl reabsorption do so by triggering signaling cascades that regulate NKCC2 activity in the apical membrane rather than affecting basolateral Na+-K+-ATPase or Cl− exit pathways (ClC-Kb, KCC4) (72, 81, 128, 142, 168). The best-studied stimulatory hormone in the TAL is vasopressin (AVP), which enhances NKCC2 activity primarily via an increase in intracellular cAMP (68–70, 112). Other hormones that stimulate NaCl reabsorption in the TAL do so by increasing cAMP levels (37). This is the case of parathyroid hormone (PTH), calcitonin, glucagon, as well as β-adrenergic agonists (isoproterenol, norepinephrine) (Fig. 2). However, it is not clear whether all hormones that increase cAMP in the TAL also stimulate NKCC2 since cAMP signaling is compartmentalized in other cells (12, 156, 165). The mechanisms by which cAMP enhances NKCC2 activity involves stimulation of membrane trafficking and perhaps phosphorylation and are discussed in detail in the next section.
Fig. 2.
Physiological regulatory pathways of NKCC2 in thick ascending limbs of Henle's loop (TALs). This figures highlights some of the known hormonal and signaling pathways known to modulate NKCC2 activity and trafficking in TALs via the cAMP/PKA pathway. See text for additional definitions.
Opposite to the stimulatory effect of cAMP-generating hormones, the inhibitory effect of some hormones and autacoids in NaCl reabsorption is mediated by cGMP. This is the case of atrial natriuretic peptides (ANP), endothelin, α-adrenergic agonists, and nitric oxide (NO) among others (9, 32, 118). ANP stimulates the receptor-bound guanylate cyclase (NPR-A). Endothelin and α-adrenergic agonists stimulate NO production by NOS3 which then activates soluble guanylate cyclase to increase cGMP levels in the TAL (9, 119, 142, 165). The effect of NO and cGMP is due to direct inhibition of NKCC2 activity (127, 128). We found that cGMP inhibits NaCl reabsorption by decreasing apical surface NKCC2 in TALs. This effect was mediated by the cGMP-stimulated phosphodiesterase 2 (PDE2) (4). As expected, inhibition of PDE2 blocks NO and cGMP-induced inhibition of NaCl reabsorption by the TAL (127). These data suggest that cGMP inhibits NKCC2 by affecting its trafficking, and this is caused by stimulation of PDE2 and a subsequent decrease in cAMP (4) (Fig. 2). However, in other cells, alternative signaling pathways independent of decreases in cAMP may be initiated by activation of PDE2, although these have not been studied in TALs.
In addition to cAMP- and cGMP-generating hormones, there are several arachidonic acid metabolites that inhibit NaCl reabsorption by the TAL. 20-Hydroxyeicosatetraenoic acid (20-HETE) and prostaglandin E2 (PGE2) inhibit NaCl reabsorption by the TAL (39, 57, 79). However, it is not known whether NKCC2 is their primary target since these lipid mediators may also inhibit Na+-K+-ATPase activity or ROMK. In addition, a few hormones are known to stimulate NaCl reabsorption independently from cAMP. This is the case for insulin, which stimulates various signaling pathways including tyrosine kinases, phosphatidylinositol 3P (PI3P) kinase, and others (78, 104). Despite its potential importance in type 2 diabetes, the transporter stimulated by insulin in the TAL has not been identified.
Molecular Mechanisms of NKCC2 Regulation
Although it is clear that NKCC2-dependent NaCl entry is essential for TAL function, salt homeostasis, and blood pressure control, the molecular mechanisms by which the activity of NKCC2 is regulated are poorly understood. In general transport terms, NKCC2 activity may be regulated by changes in the amount of NKCC2 in the apical surface or changes in the affinity of NKCC2 for the transported ions in the TAL. To date, only three molecular mechanisms are known to affect these transport parameters: 1) protein trafficking, 2) phosphorylation, and 3) protein-protein interactions. How these processes interact to mediate the accurate regulation of NKCC2 by hormones and cyclic nucleotides in the TAL is unknown. While in a few cases the molecular regulation of NKCC2 has been studied in the TAL, most data have been generated in the X. laevis oocyte expression system or nonpolarized cells. Some mechanisms of NKCC2 regulation are likely conserved across systems. However, we think it is important to translate and corroborate these findings to TALs as this will lead to a better understanding of the physiology and the role of NKCC2 in hypertension or salt-wasting tubulopathies.
Protein trafficking.
For most transmembrane proteins, its functional amount as well as its half-life at the plasma membrane is determined by a balance between endocytosis and exocytosis from different intracellular pools. These dynamic processes are collectively termed trafficking. The first indication that NKCC2 may be regulated by membrane trafficking came from immunolocalization and electron microscopy studies. These studies showed NKCC2 located in the apical membrane but also in abundant intracellular vesicles in the subapical space of rat TAL cells (121). The abundance of NKCC2 in subapical vesicles appears to be greater in smooth-surfaced TAL cells than in rough-surfaced cells. While this difference was not quantified, it is possible that different pools of intracellular NKCC2 exist in these two cell types in the TAL.
An important question for understanding NKCC2 trafficking was quantifying which fraction of the total pool of NKCC2 resides in the apical surface. The only methods able to address this question are electron microscopy and surface-selective protein labeling. Gimenez and Forbush (49) used electron microscopy to study NKCC2 distribution in mouse TALs and reported that ∼5–6% of total NKCC2 immunolabeling is found either within the apical membrane or 70 nm from it. They also found that up to 45% of total NKCC2 is located within 140 nm of the apical membrane (49). Since the average size of trafficking vesicles ranges from 50 to 100 nm, the large percentage of intracellular NKCC2 in proximity to the apical membrane (140 nm) may represent a pool of NKCC2 located in vesicles undergoing recycling. We used surface protein biotinylation of rat TALs to quantify the fraction of NKCC2 on the surface. We estimated that 3–5% of total NKCC2 is accessible for biotinylation at the TAL surface under baseline conditions (126). This fraction is somewhat variable depending on the antibodies used to detect NKCC2 (NH2 terminus vs. COOH terminus). These results and those from other investigators indicate that a small fraction of the total pool of NKCC2 is accessible to the TAL lumen. Confocal microscopy has been used by some investigators to estimate changes in NKCC2 trafficking after immunolabeling the total pool of NKCC2 (110, 130, 178, 187). However, the resolution of this method (250–500 nm) is too low to accurately distinguish NKCC2 in the apical membrane from NKCC2 in the subapical compartment below the membrane (where most of the NKCC2 is located). Even after labeling of the apical membrane, this method tends to overestimate NKCC2 in the apical membrane (129). The small amount of NKCC2 found in the apical membrane is not surprising and is similar to other apical transporters regulated by trafficking. This is the case of the epithelial Na+ channel (ENaC) and the inorganic phosphate cotransporter (NaPi2) in which a small percentage (1–5%) of the total pool is found at the surface (94, 170, 172). A low surface-to-total NKCC2 ratio in TALs may represent a mechanism to rapidly and efficiently increase NaCl influx by exocytosis of small amounts of intracellular NKCC2 pool. It may also suggest that maturation of an active transporter is not efficient, and a fraction of the protein never reaches the surface.
Vasopressin and other hormones that increase cAMP in the TAL are the most potent physiological stimuli of NaCl reabsorption. They act primarily by enhancing NKCC2-dependent Na+ and Cl− entry (23, 34, 45, 49, 87, 179). The molecular mechanisms by which cAMP stimulates NKCC2 are likely to be complex; however, most data indicate that trafficking to the apical membrane is required. Earlier experiments by Meade et al. (110) indicated that cAMP regulates NKCC2 trafficking in oocytes. These investigators found that dibutyryl (db)-cAMP enhanced surface expression and activity of full-length mouse NKCC2 expressed in oocytes. However, this effect was only apparent when the short splice variant of mouse NKCC2 was coexpressed (110). To study the role of AVP on apical NKCC2 levels, Gimenez and Forbush (49) infused AVP into live mice and measured NKCC2 in TALs by electron microscopy. They found that AVP enhanced the amount of NKCC2 in the apical membrane and also phosphorylation of N-terminal threonines (Thr96 and Thr101) (49). While these data indicated that trafficking was stimulated by AVP, they did not address which trafficking pathway was affected by cAMP. In 2006, Ortiz (126) showed that cAMP analogs (db-cAMP and 8-Br-cAMP) increased steady-state surface NKCC2 in an acute manner (20 min), similar to the time required for maximal cAMP-stimulated transport to plateau without changing total NKCC2 expression. We found that the vesicle-associated membrane proteins VAMP2 and VAMP3 are expressed in the subapical space of TALs. We tested whether treatment of TALs with tetanus toxin, a clostridial toxin that cleaves and inactivates VAMP2 and VAMP3, could block the effect of cAMP. We found that tetanus toxin blocked both cAMP-stimulated surface NKCC2 expression and NaCl reabsorption by the TAL (126). These data indicated that trafficking of NKCC2 enhances its levels in the apical membrane, and this mechanism is primarily responsible for enhanced NaCl reabsorption by the TAL.
Although cAMP enhances surface NKCC2 levels, the trafficking steps and the signaling cascade activated by cAMP were unknown. An increase in surface NKCC2 levels could be due to an increase in the rate of exocytic insertion, a decrease in the rate of retrieval or both. In 2009, Caceres et al. (24) showed that NKCC2 undergoes constitutive exocytosis in TALs, suggesting that trafficking of NKCC2 is a continuous dynamic process rather than a triggered event. Enhancing intracellular cAMP stimulated the rate of NKCC2 exocytosis by twofold (24). The increase in apical NKCC2 and the rate of exocytosis are most likely mediated by PKA since the PKA inhibitor H-89 blocked 65% of the increase in surface and 60% of the increase in exocytosis. A PKA-selective agonist (N6-Bnz-cAMP) enhanced NKCC2 exocytosis and surface expression. In addition to PKA, cAMP-activated guanine exchange proteins EPAC1/2 mediate cAMP signaling in other cells. However, these proteins do not seem to be involved in NKCC2 stimulation since an EPAC-selective agonist did not affect surface NKCC2 in TALs. These data indicated that stimulation of PKA enhances NKCC2 trafficking toward the apical membrane by increasing the rate of exocytosis.
It is still unclear whether PKA stimulates the biosynthetic trafficking of NKCC2, the recycling of internalized NKCC2, inhibits endocytosis, or a combination of these trafficking pathways. It is also uncertain whether cAMP-sensitive NKCC2 storage vesicles are present in the TAL. When apical membrane exocytosis was monitored in perfused TALs, cAMP produced a rapid increase in surface area within 4 min followed by a moderate and sustained increase, suggesting that some vesicles in the TAL undergo rapid apical exocytosis. Taken together, these data indicate that cAMP-stimulated NaCl reabsorption by the TAL is due to enhanced surface NKCC2 levels caused by higher rates of exocytosis (24, 126) (Fig. 3). However, PKA inhibition did not completely block cAMP-stimulated surface NKCC2, suggesting that other trafficking pathways, such as endocytosis, may be inhibited by cAMP.
Fig. 3.
Model for NKCC2 trafficking in TALs. This figure shows known trafficking processes in the TAL, indicated with solid lines. Predicted trafficking process, not fully studied or not validated in TALs, are shown with dashed lines. TGN, trans-Golgi network.
In addition to data showing that PKA mediates part of the cAMP-stimulated increase in surface NKCC2, recent data indicate that at least two amino acids in rat NKCC2 are phosphorylated by PKA, Ser126 and Ser874 (see below for discussion) (58). Together, these results point to PKA and its potential targets as central in the stimulation of NKCC2 trafficking by cAMP. The molecular mechanisms by which PKA stimulates NKCC2 exocytosis in the TAL are unknown. PKA may stimulate exocytosis of NKCC2 by modulating the final steps of vesicle fusion with the plasma membrane or by enhancing the number of vesicles reaching/docking and fusing with the apical membrane. All of these steps involve members of the N-ethyl-maleimide-sensitive factor attachment protein receptor (SNARE) family of fusion proteins. VAMP2 and VAMP3 are present in TALs (126) as well as syntaxin-3 (15, 103), syntaxin-4 (97), and SNAP23 (77). Several SNARE-modulatory proteins are expressed in most epithelial cells. This is the case for snapin (30), tomosyn (7), complexin (22), and Munc18b/c (98), which are involved in PKA-stimulated exocytosis in other cells. Thus we speculate that PKA stimulates NKCC2 exocytosis by at least two different mechanisms: 1) induction of phosphorylation-dependent binding of vesicle-trafficking proteins to NKCC2 and 2) stimulation of SNARE-dependent fusion and vesicle trafficking to the apical membrane of TALs.
nkcc2 endocytosis.
The data showing constitutive exocytosis of NKCC2 over time suggest that steady-state surface levels are maintained by endocytosis and perhaps recycling of internalized NKCC2 in the absence of stimuli. Ares et al. (5) studied the endocytosis and recycling of NKCC2 in the absence of stimulation and found that NKCC2 undergoes constitutive retrieval from the plasma membrane at a rate that closely matches the reported exocytic insertion. In addition, a fraction of internalized NKCC2 (30–40% in 30 min) recycles back to the surface. In chase experiments, the surface NKCC2 pool showed a relatively slow rate of degradation, with 25% of the surface NKCC2 pool degraded over 60 min in TALs. These data indicate that dynamic trafficking of NKCC2 occurs under baseline conditions in the absence of stimuli. To determine whether constitutive NKCC2 endocytosis is relevant to the NaCl reabsorption by the TAL, Ares et al. tested whether acute inhibition of endocytosis enhanced surface NKCC2 expression and activity. To do this, they acutely depleted plasma membrane cholesterol with methyl-β-cyclodextrin, an agent known to extract cholesterol, thereby blocking endocytosis (91, 124, 188). Cholesterol depletion (20–30 min) completely blocked NKCC2 retrieval and enhanced surface NKCC2 and net Cl− transport by TALs (5). These data indicate that constitutive trafficking of NKCC2 is essential for the reabsorptive function of the TAL. The actual endocytic mechanism (clathrin, caveolin, Arf1) by which NKCC2 is internalized is still unknown because methyl-β-cyclodextrin blocks almost every endocytic pathway studied (29, 61, 96, 131, 151, 167). Depending on the interaction with endocytic adaptors, NKCC2 may be internalized via clathrin-mediated, caveolin-mediated, clathrin- and caveolin-independent (CLIC) pathways, or a combination of these (75). Because any of these pathways are likely to regulate NKCC2 activity, understanding which of these mediate constitutive or regulated NKCC2 endocytosis is important. To date it is not known whether any hormonal stimuli or signaling cascade regulates NKCC2 endocytosis. Potentially, any protein-protein interaction or posttranslational modifications affecting NKCC2 endocytosis are likely to impact NaCl reabsorption by the TAL.
Acute cholesterol depletion from TALs inhibited NKCC2 endocytosis and enhanced surface NKCC2, suggesting that exocytosis was not affected. These data could also indicate that the increase in surface NKCC2 after cholesterol extraction is due to exocytosis of NKCC2 located in cholesterol-rich vesicles (enriched in the endosomal and recycling compartments) with the purpose of restoring cholesterol, maintaining plasma membrane integrity. In support of this hypothesis, three groups have shown that a fraction of NKCC2 associates with cholesterol- and sphingolipid-rich membrane fractions (“lipid rafts”). Welker et al. (178) first reported that a fraction of NKCC2 was isolated from detergent-resistant membranes after sucrose gradient centrifugation of rat kidney extracts. Yu and Knepper (184) used a proteomic approach to identify lipid raft-associated proteins in the renal medulla and estimated that ∼75% of total NKCC2 fractionated to detergent-resistant membranes. Finally, Carmosino et al. (27) also found that a fraction of NKCC2 expressed in polarized MDCK cells cofractionated with detergent-resistant membranes. Interestingly, they also showed that an NKCC2/NKCC1 chimera that is targeted to the basolateral membrane also associates with this fraction, suggesting that interaction with lipid rafts is not determinant in apical targeting of NKCC2 in MDCK cells. Prolonged (2 h) or chronic (24 h) cholesterol depletion seems to decrease NKCC2 activity in oocytes and total NKCC2 expression in immortalized rabbit TAL cells (rbTAL) in culture as well as AVP-stimulated cAMP (178). In TALs, cholesterol depletion for 2 h decreased both surface and total NKCC2 expression by unknown mechanisms (unpublished observations). These data may indicate that cholesterol is required for appropriate NKCC2 folding and trafficking. However, these effects may be the consequence of impaired plasma membrane integrity and cellular metabolism resulting from prolonged cholesterol depletion.
The few data on dynamic trafficking of NKCC2 support a model in which newly synthesized NKCC2 traffics from the ER, through the Golgi, and then to the apical membrane via unknown trafficking pathways. Transmembrane proteins en route to the apical membrane follow different pathways in model epithelial cells (152, 153). The trans-Golgi network-to-plasma membrane trafficking (biosynthetic) of NKCC2 has not been studied, and the proteins involved in routing NKCC2 to the apical membrane as well as the regulation of this pathway during acute or chronic hormonal stimulation are unknown (Fig. 3). Once in the apical membrane, NKCC2 is internalized by endocytosis, a fraction of retrieved NKCC2 recycles back to the apical membrane, and a fraction is likely degraded by unknown mechanisms. The relative contribution of the biosynthetic or recycling pools of NKCC2 for the maintenance of steady-state surface NKCC2 is not clear. cAMP could act at several steps to enhance surface NKCC2. It could stimulate exocytosis from the biosynthetic or recycling pool or both via PKA. In addition, it is possible that cAMP decreases the rate of constitutive NKCC2 endocytosis, thereby contributing to enhanced surface NKCC2 levels. Opposite to the stimulatory effect of cAMP, the second messenger cGMP inhibits NaCl reabsorption in the TAL by decreasing surface NKCC2 levels via cGMP-stimulated PDE2 (4). cGMP may decrease the rate of constitutive exocytosis or enhance endocytosis. We speculate that cGMP acts at multiple steps in NKCC2 trafficking, most likely to decrease the rate of exocytic insertion, since activation of PDE2 decreases cAMP (4). However, the exact mechanism by which cGMP decreases surface NKCC2 is not clear. This pathway is of relevance because a decreased natriuretic effect of NO, cGMP, and natriuretic peptides occurs in several animal models of hypertension, in which enhanced TAL NaCl reabsorption may be involved (47, 185, 158).
NKCC2 phosphorylation.
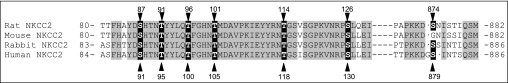
Phosphorylation sites have been demonstrated in rat, mice, rabbit, and human NKCC2 (Fig. 4). As shown in Table 1 phosphorylation has been described in both NH2 and COOH termini, in serines and threonines. In 2002, Flemmer et al. (42) described an antibody that specifically recognizes threonines 212 and 217 in human NKCC1 (R5 antibody). These threonines are conserved in a similar context in NKCC2 at positions 96 and 101 (rat and mouse sequence, equivalent to Thr100 and Thr105 in human NKCC2), and these authors predicted that phosphorylation of NKCC2 must occur at these sites during hypotonicity or low extracellular chloride as it did on NKCC1. Later, Gimenez and Forbush (49, 50) used this antibody to demonstrate for the first time that in mouse kidney, NKCC2 is phosphorylated at these sites under baseline (unstimulated) conditions. The signal for phospho-NKCC2 detected with the R5 antibody increased 100% after treating of mice with the V2 receptor agonist dDAVP for 1 h. However, phosphorylation of NKCC2 also increased after water loading mice overnight with 5% dextrose/1% ethanol, a maneuver that decreases AVP. They concluded that AVP-induced phosphorylation at Thr96 and Thr101 may be involved in enhanced NKCC2 activity and NaCl reabsorption in TALs during antidiuresis. However, it is unclear why water loading, which decreases AVP and NaCl transport in the TAL, also enhanced phosphorylation at Thr96 and Thr101.
Fig. 4.
Sequence alignment indicating reported NKCC2 phosphorylation sites in different species. (see text and Table 1 for references).
Table 1.
Reported phosphorylation sites of NKCC2 aligned to the human NKCC2 sequence
| Phospho-Site | Experimental System | Kinase | Effect | Stimuli Used | Reference No. |
|---|---|---|---|---|---|
| Ser91 | HEK-293 cells | SPAK/?? | ↔ | Low Cl/hypotonicity | 149 |
| Thr95 | HEK-293 cells | ?? | ↔ | Low Cl/hypotonicity | 149 |
| Thr100 | HEK-293 cells | SPAK, OSR-1 | ↑↑↔ | Low Cl/hypotonicity | 149 |
| HEK-293 cells | ?? | ↔ | Calyculin A | 63 | |
| Mouse TAL | ?? | ?? | AVP/water loading | 49 | |
| Oocytes | ?? | ↑↑ | Hypertonicity | 50 | |
| Thr105 | HEK-293 cells | SPAK, OSR-1 | ↑↑↔ | Low Cl/hypotonicity | 149 |
| HEK-293 cells | ?? | ↔ | Calyculin A | 63 | |
| Mouse TAL | ?? | ?? | AVP/water loading | 48 | |
| Ser130 | HEK-293 cells | AMPK | ?? | Low Cl/hypotonicity | 149 |
| Rat TAL | PKA/AMPK | ?? | dDAVP/hormone cocktail | 143 | |
| Oocytes | ?? | ↑↑ | Hypertonicity | 43 | |
| MMDD1 cells | AMPK | ?? | Hypertonicity | 43 | |
| Ser879 | HEK-293 cells | PKA | ?? | Low Cl/hypotonicity | 149 |
| Rat TAL | PKA | ?? | dDAVP | 143 |
NKCC2, Na-K-2Cl cotransporter; TAL, thick ascending limb; ↑↑, stimulation; ??, unknown/not studied; ↔, no change. Please see Fig. 4 for alignment of phosphorylation sites in other species. Throughout the text, the phosphorylation sites are noted in the NKCC2 amino acid sequence in the animal species in which they were determined.
The effect of phosphorylation at Thr96 and Thr101 on NKCC2 activity, trafficking, and protein turnover has not been studied in TALs or polarized epithelial cells. Gimenez and Forbush (50) examined the effect of mutating Thr99, Thr104, and Thr117 in rabbit NKCC2 on hypotonicity-stimulated (400–200 mosmol/kgH2O) transport and phosphorylation in oocytes. They found that hypotonicity enhanced phosphorylation at these sites and that no single threonine by itself was responsible for enhanced transport but the three threonines together were required for phosphorylation-induced activation (50). Mutation of the three threonines eliminated hypotonicity-stimulated activity and phosphorylation. The effect of these mutations on surface or total expression and maturation of NKCC2 was not studied. This behavior is different from NKCC1, where a single threonine, Thr189, is absolutely essential for phosphorylation-induced activation (31). Thus in oocytes most data indicate that phosphorylation leads to enhanced NKCC2-mediated NaCl entry.
In nonpolarized mammalian cells, the data regarding the role of threonine phosphorylation in NKCC2 activation are conflicting. Richardson et al. (149) examined the phosphorylation sites on human NKCC2 expressed in HEK-293 cells. They found novel phosphosites like Ser91 (equivalent to Ser87 in rat and mouse NKCC2), in addition to threonines in position Thr95, Thr100, Thr105, and Ser130 (equivalent to Ser126 in rat and mouse NKCC2) that were enhanced by low extracellular chloride and hypotonicity (149). However, this maneuver failed to enhance the activity of NKCC2 A, B, or F isoforms above that of endogenously expressed NKCC1, despite higher baseline activity caused by overexpression of either NKCC2 isoform. Mutation of Thr105 and Ser130 rendered NKCC2 inactive in HEK cells. Different from TALs, NKCC2 expressed in HEK cells seem to be abundantly expressed at the plasma membrane and this localization was not altered in any of the phospho-mutants. These latter data suggest that baseline NKCC2 activity is regulated by phosphorylation, consistent with experiments performed in oocytes. Recently Flatman et al. (63) expressed ferret NKCC2 in HEK-293 cells and found that enhanced phosphorylation at Thr95 and Thr100 (equivalent to Thr96 and Thr101 in rat and mouse NKCC2) caused by inhibition of protein phosphatases (calyculin A) did not result in a measurable increase in NKCC2 activity. When cells were incubated in a Na+-free medium, NKCC1 was activated whereas NKCC2 was inhibited and its phosphorylation rose slightly. These authors concluded that additional mechanisms such as trafficking or protein-protein interactions are involved in the stimulation of NKCC2. Taken together, most data suggest that phosphorylation at Thr96 and Thr101 (in rats) is important for baseline NKCC2 activity in expression systems; however, additional mechanisms must be involved in stimulated NKCC2 activity. Nevertheless, these data must be taken cautiously as activity, phosphorylation, and membrane localization were monitored in cells that express active NKCC1, and heterodimerization with NKCC2 may influence its activity, phosphorylation, and trafficking.
Recently, Mutig et al. (117) showed that NKCC2 phosphorylation is impaired in mice lacking Tamm-Horsfall protein. These mice exhibit enhanced total NKCC2 expression, unchanged apical membrane NKCC2, with decreased phosphorylation at Thr96 and Thr101, and decreased furosemide-induced diuresis suggestive of lower NKCC2 activity in TALs. Coexpression of NKCC2 with Tamm-Horsfall protein in oocytes enhanced NKCC2 activity. Thus it is possible that the urinary concentration defect observed in these mice is due to lower NKCC2 activity. However, deletion of Tamm-Horsfall protein also affects other transporters in the TAL (8) and stimulates NCC expression and phosphorylation. The molecular mechanism by which Tamm-Horsfall protein, an apical GPI-anchored protein, regulates NKCC2 activity is unknown. Given that this is the most abundant protein present in normal urine, and extremely abundant in the TAL, its deletion may affect normal TAL function.
PHOSPHORYLATION BY SPAK AND OSR1.
The kinases that mediate hypotonicity and low chloride-induced phosphorylation of the NKCC2 NH2 terminus in cultured cells have been identified as SPAK (or Ste20- and SPS1-related proline and alanine reach kinase) and OSR1 (or oxidative stress-responsive kinase). By using yeast-two hybrid screening, Piechotta et al. (136) first identified that SPAK and OSR1 directly interact with the RFQV motif present in the NH2 terminus of NKCC2 (136). SPAK and OSR1 are serine/threonines kinases of the germinal kinase family (GCK) with a structural homology of 67%. Recently, Richardson et al. (149) examined the effect of deleting the SPAK/OSR1 binding site on NKCC2 phosphorylation and found that this maneuver decreased hypotonicity-enhanced phosphorylation at Ser91, Thr95, and Thr100. Interestingly, SPAK only phosphorylated Thr95, Thr100, and Thr105 but not Ser91 and Ser130. The effect of deleting the SPAK binding site on NKCC2 activity was not examined. The role of these two kinases in the regulation of NKCC2 in vivo is not clear; however, it has been further investigated by generation of SPAK and OSR1 knockout and knocked in mice. In the kidney, SPAK and OSR1 are expressed in the TAL and distal convoluted tubule; however, these kinases are relatively ubiquitous and are expressed in other organs including the brain. A knocked in mouse expressing mutant inactive SPAK (T243A) showed decreased expression (30–40%) and phosphorylation (80%) of NKCC2 at Thr95 and also decreased expression and phosphorylation of the distal tubule thiazide-sensitive NCC (146). These mice exhibit 10-mmHg lower blood pressure on a normal- and low-salt but not on a high-salt diet. Urinary Na+ excretion was only elevated after switching of mice from high- to low-salt diets, indicating salt wasting during the adaptation period; however, NaCl reabsorption in TALs or distal convoluted tubule was not measured. Recently, a SPAK knockout mice was generated (182). The whole-animal SPAK knockout mice presented lower NCC expression and phosphorylation in distal tubules accompanied by a 15-mmHg reduction in mean blood pressure. However, total NKCC2 expression in these mice was elevated and phosphorylation at Thr96 was enhanced threefold, suggesting compensatory regulation of NKCC2 by OSR1 or other TAL-specific kinase that does not regulate NCC. In addition, this effect may be explained by a recent report (108) suggesting the existence of three SPAK isoforms in the kidney with differential expression in TAL and distal convoluted tubule. It is unclear whether the three isoforms are completely absent in SPAK knockout mice and whether OSR1 is enhanced in TALs of these mice. Furosemide-induced natriuresis was not affected in SPAK-knockout mice whereas thiazide-induced natriuresis was decreased. Taken together, these data suggest that both SPAK and OSR1 may be physiological stimulators of NKCC2 phosphorylation and perhaps maintain NKCC2 expression. However, eliminating SPAK in the whole animal may induce compensatory hormonal changes that influence the TAL. In addition, other targets for SPAK and OSR1, such as KCC3, may be present in the TAL. Thus it is still unclear by which degree, mechanism, and/or physiological condition do SPAK and OSR1 regulate NKCC2 activity and NaCl reabsorption by the TAL.
From the data presented above, it is evident that SPAK phosphorylates NKCC2. However, little is known about the physiological mechanisms that stimulate SPAK or OSR1 in the TAL or the distal tubule. Vasopressin enhances Thr96 and Thr101 phosphorylation in mouse TAL, and it also stimulates NKCC2 trafficking (49). It is not known whether AVP or cAMP stimulates SPAK or OSR1. Similarly, growth hormone (GH) enhances NKCC2 Thr96 and Thr101 phosphorylation in rat TALs but does not seem to stimulate NKCC2 activity as transepithelial potential was not enhanced after 6 h of treatment with GH (36). It is not known whether GH stimulates SPAK or OSR1. The only known upstream regulators of SPAK in the kidney are the family of with-no-lysine kinases (WNKs) WNK1, WNK3, and WNK4. Several studies showed that WNKs play a role in nephron NaCl reabsorption and blood pressure control. WNK3 is expressed in TAL and, when coexpressed in oocytes, it enhances NKCC2 activity (150). Ponce-Coria et al. (143) showed that decreasing intracellular Cl− in oocytes expressing NKCC2 induces phosphorylation at Thr96 and Thr101 and enhanced NKCC2 activity. The effect of hypotonicity/low Cl− on NKCC2 is enhanced by WNK3 in a SPAK-dependent manner. They concluded that a decrease in intracellular Cl− activates WNK3 by an unknown mechanism, which binds and activates SPAK to phosphorylate NKCC2. At least one report indicates that WNK4 is expressed in TALs whereas it is unclear whether WNK1 or the kinase-inactive kidney-specific WNK1 (KS-WNK1) are present in this nephron segment (122). Transgenic mice expressing inactive WNK4 (D561A) exhibit lower NKCC2 expression despite higher blood pressure; however, this effect may be secondary to higher NCC activity (181). Recently a KS-WNK1 knockout mouse was generated and shown to exhibit higher blood pressure, higher NKCC2 expression without a change in phosphorylation-to-total ratio (99). Conversely, transgenic mice overexpressing KS-WNK1 showed reduced blood pressure, lower NKCC2 expression (60% decrease), and unchanged phosphorylation-to-total NKCC2 ratio (99). Thus it appears that KS-WNK1 is involved in the regulation of NKCC2 expression, stability, and/or turnover, however, the mechanism by which this happens and to what extent Cl− reabsorption was altered in TALs was not studied (99). In addition to WNKs, a SPAK-interacting protein, SORLA (or sorting protein-related receptor with A-type repeats), has been recently described. SORLA seemed to regulate SPAK subcellular localization and its ability to phosphorylate NKCC2 at Thr96 and Thr101 (148). In the SORLA knockout mice, a smaller fraction of SPAK colocalized with NKCC2, and phospho-NKCC2 was severely decreased (−75%) whereas total NKCC2 was unaffected. Importantly, the amount of NKCC2 in the apical membrane was not different from wild-type mice despite the large decrease in phosphorylation, arguing against a role of threonine phosphorylation in trafficking. ROMK expression was severely decreased in the outer medulla of SORLA knockout mice while they showed enhanced NHE3, NCC, and α-ENaC expression (148). Taken together, these data support the notion that phosphorylation at Thr96 and Thr101 by SPAK, and perhaps OSR1, stimulate NKCC2 activity independently of trafficking into and out of the apical membrane but rather by a mechanism regulating protein expression, degradation, stability, and/or maturation. SPAK and OSR1 have additional targets that are present in TALs, such as KCCs and ROMK that may influence NKCC2 activity.
PHOSPHORYLATION BY PKA.
While hyposmolality and very low chloride concentration stimulate Thr96 and Thr101 phosphorylation in oocytes and cells in culture, it is unlikely that physiological or even pathological conditions produce such drastic alterations in the TAL environment. The main physiological stimulus of NKCC2 activity and trafficking is cAMP stimulated by AVP or β2AR receptor stimulation (23, 34, 45, 49, 87, 140, 161, 179). However, until recently it was not known whether cAMP stimulated NKCC2 phosphorylation and the kinases involved. Recently Gunaratne et al. (58) used mass spectrometry in TALs treated with a cocktail of hormones that stimulate cAMP to identify two novel phosphorylation sites in NKCC2. They reported that Ser126 in the NH2 terminus (equivalent to Ser130 in human NKCC2) and Ser874 in the COOH terminus (equivalent to Ser879 in human NKCC2) were phosphorylated after cAMP levels were enhanced. They raised phospho-specific antibodies and confirmed that injection of Brattleboro rats with dDAVP enhanced Ser126 and Ser874 phosphorylation in TALs. dDAVP also enhanced Thr96 phosphorylation. In vitro experiments indicated that PKA directly phosphorylates peptides encompassing the Ser126 and Ser874 sites. Different from Thr96 and Thr101, Ser126 and Ser874 phosphorylation are undetectable under baseline conditions (58). These data strongly suggest that PKA mediates the phosphorylation of NKCC2 in response to cAMP and vasopressin in TALs. While it is clear that cAMP stimulates NKCC2 trafficking by PKA, the role of these phosphosites in NKCC2 trafficking or activity itself remains unknown. When expressed in oocytes, an NKCC2 mutant lacking Ser126 shows a 70% decrease in activity, indicating that this site is stimulatory (43). However, surface expression was not assessed in these experiments, and therefore the mechanism of activation is unclear. We hypothesize that Ser126 phosphorylation is involved in cAMP-stimulated surface NKCC2 expression most likely by enhancing exocytosis and recycling by inducing the binding of accessory trafficking proteins to the NH2 terminus of NKCC2. In general, termination of signaling depends on protein dephosphorylation achieved by phosphatases. Currently, the phosphatases involved in NKCC2 regulation are unknown.
PHOSPHORYLATION BY AMPK.
Another kinase reported to phosphorylate NKCC2 is the AMP activated kinase (AMPK). Fraser et al. reported that AMPK induced in vitro phosphorylation of NKCC immunoprecipitated from a mouse macula densa cell line (MMD1), evidenced as a 120 KDa protein that was presumed to be unglycosylated NKCC2 (43). This site was identified as Ser126 (corresponding to Ser130 in human NKCC2) and an antibody raised against this site recognized a 120 KDa band from MMD1 cells. It is still unclear why in these cells only immature/nonglycosylated NKCC2 was phosphorylated. In a recent study Gunaratne et al. found that in rat TALs, phosphorylation of Ser126 was undetectable under baseline conditions, and only detectable after treatment with dDAVP. In vitro, a peptide encompassing Ser126 was phosphorylated by AMPK at much lower levels than PKA, questioning its physiological relevance. Recently, Richardson et al. reported that the AMPK activator phenformin enhanced Ser130 phosphorylation of NKCC2 expressed in HEK-293 cells and this was diminished by a non selective AMPK inhibitor (149). They also reported that hypotonic-low chloride conditions also enhanced Ser130 phosphorylation but did not stimulate AMPK activity, suggesting perhaps that the cAMP/PKA pathway maybe stimulated by low chloride. Taken together, these data suggest that AMPK could regulate NKCC2 activity in TALs. However, this has not been directly studied. Ser126 seem to be a stimulatory site since its mutation decreases NKCC2 activity expressed in oocytes. As described above, PKA phosphorylates Ser126, and it is known to stimulate NKCC2 trafficking and activity. However, it is not clear whether AMPK stimulates or inhibits NKCC2 activity in TALs. In collecting ducts, AMPK opposes the stimulatory effect of cAMP on ENaC activity (13, 25) and V-ATPase proton pump trafficking (54), indicative of an inhibitory effect on net transport.
Protein-protein interactions.
Protein-protein interactions are an important component on every biological process. In the case of NKCC2, interaction with kinases (SPAK, OSR1) is known to regulate its phosphorylation which in turn may regulate its activity. However, other interactions with NKCC2 that primarily affect its surface expression or membrane trafficking have been reported. As in most cases involving the kinase-dependent regulation of NKCC2, all of the protein interactions described to date have been studied in heterologous expression systems and its role in controlling TAL function remains unclear.
ALDOLASE B.
The first non-kinase protein described to bind NKCC2 was the glycolitic enzyme aldolase B. This interaction was detected in a yeast-two-hybrid assay using the COOH terminus of NKCC2 (amino acids 661–768) as bait (11) and confirmed by immunoprecipitation in opossum kidney (OK) cells expressing exogenous NKCC2. In OK cells, enhancing aldolase B expression decreased surface NKCC2 without affecting the total NKCC2 pool. As expected, the decrease in surface NKCC2 levels was accompanied by a decrease in NKCC2 activity measured as NH4+ entry. While the relevance of this interaction in TAL physiology is unknown, these authors found that adding the substrate of aldolase (fructose 1,6-biphosphate) to the cell media decreased the interaction with NKCC2 and blocked the inhibition of surface NKCC2 by overexpression of aldolase. These data suggest that changes in the relative concentration of intracellular fructose 1,6-biphosphate in TALs may regulate the interaction with aldolase B; however, this has not been tested. The mechanism by which aldolase B inhibits surface NKCC2 is unknown. Other investigators showed that aldolase B binds with high affinity to sorting nexin 9 (SNX9), decreasing SNX9 membrane binding and inhibiting endocytosis in other cells (147). It is unlikely that this is the mechanism used by aldolase B in OK cells since overexpression of aldolase B should decrease endocytosis by sequestering SNX9, enhancing surface NKCC2. Aldolase B also interacts with V-ATPase (100), which is involved in exocytosis and recycling in other cells, and also with GLUT4, mediating its exocytosis (83). Thus this glycolytic enzyme is most likely involved in regulating the constitutive exocytic insertion of NKCC2.
SECRETORY CARRIER MEMBRANE PROTEIN 2.
Recently, the same group that identified the interaction with aldolase B reported that the vesicle scaffolding protein secretory carrier membrane protein 2 (SCAMP2) also binds the C terminus of NKCC2. SCAMP2 was identified in a yeast-two-hybrid screen with the proximal region of the C terminus (amino acids 661–768) and confirmed in OK cells expressing NKCC2. Overexpression of SCAMP2 in OK cells decreases steady-state surface NKCC2 and decreases the rate of exocytic insertion from intracellular pools but does not affect endocytosis. A fraction of SCAMP2 colocalizes with NKCC2 in Rab11-positive intracellular structures but not at the plasma membrane in nonpolarized OK cells. These data suggest that SCAMP2 retains NKCC2 in a recycling compartment (186). These results are in agreement with the reported constitutive NKCC2 recycling in TALs (5). It is of interest that SCAMP2 also interacts with the COOH terminus of NCC (Slc12a3), NHE5, NHE7, as well as with the serotonin and dopamine transporters in other cells. In endocrine PC12 cells, SCAMP2 interacts with SNAP-23, the VAMP2/3 partner in SNARE complex formation. However, in OK or HEK cells little colocalization of SCAMP2 and NKCC2 was observed at the plasma membrane (186), suggesting that SCAMP2 works primarily by retaining NKCC2 in an intracellular compartment rather than inhibiting fusion of NKCC2-containing vesicles with the plasma membrane.
MAL/VIP17.
An additional interacting partner with the COOH terminus of NKCC2 is the tetraspan protein MAL/VIP17 (27). MAL/VIP17 is a lipid raft-associated protein implicated in apical delivery of some transmembrane proteins and in endocytosis of aquaporin-2 in collecting ducts. MAL/VIP17 is expressed in TALs, distal convoluted tubule, and collecting ducts (106). In mouse renal medullary lysates, NKCC2 immunoprecipitates with MAL/VIP17. However, MAL/VIP17 does not appear to mediate apical NKCC2 targeting since a NKCC1 chimera containing the COOH-terminal apical targeting sequence of NKCC2 (NKCC1/CtermNKCC2) expressed in LLC-PK1 or MDCK cells was routed to the apical membrane independently of MAL/VIP17 expression. However, endocytosis of the NKCC1/CtermNKCC2 chimera was decreased by MAL/VIP17 expression in LLC-PK1 cells and enhanced by silencing MAL/VIP17 in MDCK cells, suggesting that MAL/VIP17 inhibits NKCC2 endocytosis. Interestingly, phosphorylation of NH2 terminal Thr96 and Thr101 was enhanced by expression of MAL/VIP17 both in the NKCC1 chimera and in transgenic mice overexpressing MAL/VIP17. The molecular mechanism by which MAL/VIP17 regulates NKCC2 phosphorylation was not studied. MAL/VIP17-overexpressing mice show neurological defects but also renal cysts with apical membrane labeling for phosho-NKCC1/NKCC2 in cells lining the cysts' lumen. Because the antibody used reacts with both phospho-NKCC1 and phospho-NKCC2, these data suggest that some cysts are of TAL origin or NKCC1 is targeted apically in cysts of collecting duct origin. The physiological and pathological implications of the interaction with MAL/VIP17 remain to be studied in TALs since it is unclear whether all of these alterations in NKCC2 trafficking result in differences in NaCl transport (predicted to be enhanced). It is also unclear whether the trafficking mechanism affected by MAL/VIP17 is the same for full-length NKCC2 expressed in TAL cells as it is for the NKCC1 chimera in model systems.
A general consideration to take into account is that differences have been reported for the relative ratios of immature (120 kDa) to mature (160 kDa) NKCC2 depending on the expression system. In kidney homogenates or TAL total lysates, the 120/160-kDa ratio is ∼0.1–0.01 (14, 38, 40, 86, 101, 109, 171), and in the surface fraction of rat TALs only the 160-kDa band is detected. In OK cells expressing myc-tagged NKCC2, the 120/160-kDa ratio is ∼0.5 in total lysates, and only mature NKCC2 is detectable on the surface fraction (11, 27, 113, 186, 187). In LLC-PK1 and MDCK cells expressing the NKCC1/CtermNKCC2 chimera, the 120/160-kDa ratio is inverted to ∼1–2, and both the immature and mature forms are evident on the surface (27). It is likely that differences in the ratio of immature/mature NKCC2 in total lysates are due to decreased efficiency of maturation in heterologous expression systems. However, it is unclear why in some cells NKCC2 that is not maturely glycosylated reaches the plasma membrane. Perhaps this is specific to trafficking of the NKCC1 portion of the chimeric protein or it may be specific to this cell culture system. This phenomenon seems to occur in X. laevis oocytes, as discussed above, showing decreased but not absent NKCC2-mediated Rb+ uptake in NKCC2 mutants lacking glycosylation sites (130).
NKCC2 Dysfunction and Its Role in Blood Pressure Regulation
The kidneys play an essential role in short- and long-term control of blood pressure. NKCC2 reabsorbs 20–30% of the filtered NaCl load and maintains a high interstitial osmolality. Thus there is a direct relationship between NKCC2 activity and blood pressure. This was first evident in a rare human disorder caused by mutations in the NKCC2 gene (SLC12A1) that result in decreased or absent NKCC2 activity (Bartter's syndrome) (144, 160, 173), causing a massive salt loss and hypotension. Opposite to salt-losing syndromes, enhanced NKCC2 activity is related to hypertension, a disease affecting 30% of the adult population with an upward trend over time. Up to half of hypertensive patients exhibit salt sensitivity. That is, their blood pressure is lowered by decreasing salt intake in their diet, indicating abnormally enhanced salt reabsorption. Animal models have demonstrated that salt sensitivity is due to an inability of the kidney to excrete a salt load. Salt-sensitive hypertension is a complex polygenic disease. The molecular and genetic mechanisms leading to salt sensitivity are poorly understood, but they involve enhanced NaCl reabsorption along the nephron which decreases the sensitivity of the pressure-natriuretic mechanism. In humans, there are race-dependent differences in salt sensitivity. In African-Americans, the incidence of salt-sensitive hypertension is higher than in whites (176, 177). The latter racial group produces more concentrated urine, retains more Na+, Ca2+, and Mg2+ and has higher TAL NaCl reabsorption based on furosemide-induced diuresis and water-loading studies reviewed by Jung et al. (82). While these data point to defective TAL transport in humans, animal models of salt-sensitive hypertension clearly indicate enhanced NKCC2 activity before and after the development of hypertension.
NKCC2 in animal models of hypertension.
NaCl absorption by the TAL is abnormally enhanced in rat models of salt-sensitive hypertension (47, 90, 189). In the Dahl salt-sensitive rat, enhanced Cl− reabsorption is evident in TALs perfused in vitro (79, 155) or during in vivo micropuncture (88, 89). In this strain, proximal Cl− reabsorption is unchanged whereas distal tubule Cl− reabsorption is decreased, perhaps to compensate higher TAL Cl− reabsorption (88, 89). Na+ and water reabsorption in isolated perfused cortical collecting duct is not enhanced in Dahl salt-sensitive rats (65, 95). Thus all data indicate that in the Dahl salt-sensitive rat enhanced TAL Cl− reabsorption is involved in the development of hypertension. Alvarez-Guerra and Garay (3) found that bumetanide-sensitive NKCC2 activity was higher in Dahl salt-sensitive rats. Salt-sensitive rats exhibited higher bumetanide-induced diuresis and natriuresis, consistent with enhanced NKCC2 activity. However, the molecular mechanisms by which NKCC2 activity is enhanced in Dahl salt-sensitive rats are not known. We studied the effect of a high-salt diet on surface, total, and Thr96 and Thr101 phosphorylation of NKCC2 in Dahl salt-sensitive and control Dahl salt-resistant rats. We found that a high salt intake decreased NKCC2-dependent TAL transport, and surface NKCC2 without affecting total NKCC2 expression in TALs from Dahl salt-resistant rats, suggesting that decreased NKCC2 trafficking contributes to salt excretion. However, in Dahl salt-sensitive rats, high salt intake not only failed to decrease NKCC2-dependent transport but enhanced surface NKCC2 (64). Under a normal-salt diet, phosphorylation of NKCC2 at Thr96 and Thr101 was enhanced in Dahl salt-sensitive rats despite lower NKCC2 total expression. phosphorylation of Thr96 and Thr101 was not affected by high salt intake in Dahl salt-resistant or Dahl salt-sensitive rats. These data indicated that NKCC2 trafficking is enhanced in Dahl salt-sensitive rats, suggesting that the possible compensatory mechanism that protects normotensive rats from becoming hypertensive is absent in Dahl salt-sensitive rats. There have been several reports with disparate data regarding total NKCC2 expression in Dahl salt-sensitive rats. Alvarez-Guerra (3) reported decreased total NKCC2 expression in salt-sensitive rats, and we found lower total NKCC2 expression that was not affected by dietary salt (64). Hoagland et al. (74) found increased NKCC2 expression in Dahl salt-sensitive rats compared with Brown Norway (BN) or consomic salt-sensitive rats with BN chromosome 13. The reason for these discrepancies is not clear, but they are probably due to different rat strains and different types of NKCC2 antibodies used. Nevertheless, it is the amount of NKCC2 at the apical membrane and its phosphorylation at the membrane which determine NKCC2 activity, and these mechanisms are enhanced in Dahl salt-sensitive rats.
Altered NaCl handling by the TAL has been shown in others animal models of hypertension like the spontaneously hypertensive rat (SHR) (154, 163). In 2004, Sonalker et al. (162) showed that NKCC2 expression was fourfold higher in SHR whereas NCC tended to be lower. Enhanced expression of the α1-subunit of the Na+-K+-ATPase was also found in the outer and inner medulla of SHR (162). This group later measured membrane and total NKCC2 in the outer medulla by subcellular fractionation and found that there were not differences in the surface-to-intracellular ratio of NKCC2 when rats were normotensive (163). However, progression from prehypertensive to hypertensive (5–8 wk) in SHR was accompanied by a two- to fourfold increase in the surface-to-intracellular ratio of NKCC2 compared with control rats in which membrane NKCC2 was not changed (33, 49). These data indicate that in genetic animal models of hypertension NKCC2 trafficking may be affected in such a way that enhanced membrane NKCC2 levels maintain a direct relationship with the development of hypertension.
Despite data in humans and animals pointing to enhanced NKCC2 activity, no mutations have been reported in the SLC12A1 gene that could explain this defect. Genetic linkage studies have suggested the possibility that the NKCC2 locus cosegregates with the gene for the Na+-K+-ATPase and the susceptibility to hypertension in Dahl salt-sensitive rats (73) and in hypertensive humans (53). However, gain-of-function mutations are not necessarily responsible for enhanced NKCC2 activity in humans and animals but rather genetic differences leading to altered NKCC2 trafficking, phosphorylation, or both. Several of the hormonal and second messenger-mediated regulatory pathways for NKCC2 are altered in salt-sensitive hypertension. Inhibitory signaling pathways that decrease NKCC2 activity and trafficking such as NO, natriuretic peptides, endothelin, cGMP, and 20-HETE are impaired in animal models of hypertension (47, 111, 132, 133, 185). Stimulatory signaling pathways such as those induced by norepinephrine, AVP, cAMP, angiotensin II, and superoxide are also known to be affected in TALs from hypertensive animals. Disruption of the balance between factors inducing NKCC2 stimulation and its inhibition is associated with hypertension. For example, renal medullary infusion of a subpressor dose of AVP does not cause hypertension in normal rats but increases blood pressure in salt-sensitive rats (185). Based on the literature, we hypothesize that several inhibitory pathways are absent in these rats, enhancing the effect of cAMP on NKCC2 trafficking. This would not only increase the sensitivity of the TAL to AVP but also to renal nerve stimulation, which is enhanced in some forms of hypertension and during salt loading (107, 164). β-Adrenergic stimulation enhances NaCl reabsorption (37, 140, 141) and surface NKCC2 expression (unpublished results) in the TAL, and increased catecholamines are involved in hypertension in Dahl salt-sensitive rats (159). There are several genes and entire chromosomal regions that have been implicated in the development of salt-sensitive hypertension in animals; however, in the majority of cases the functional role of these genes is unknown. Given that NKCC2 activity is controlled by protein trafficking, phosphorylation, and perhaps hundreds of protein interactions, it is imperative to identify the molecular mechanisms of NKCC2 regulation in the TAL to systematically study the genes involved in blood pressure control as they relate to NKCC2.
NKCC2 dysfunction in humans.
Gain-of-function mutations have not been identified in NKCC2; however, additional evidence of NKCC2 involvement in blood pressure regulation comes from an association study performed in the Framingham Heart Study cohort (80). Ten allelic NKCC2 variants were identified in the SLC12A1 gene whose carriers have reduced blood pressure and lowered the incidence of hypertension. One of those mutations is a frame shift already known to cause Bartter syndrome. The remaining nine variants were newly described mutations, predicted to code NKCC2 proteins with impaired function based on the degree of evolutionary conservation of the residues involved. The functional characterization of those variants has been recently addressed by two independent groups (1, 113). The results obtained by both teams are consistent with the hypothesis of reduced NKCC2 activity on these alleles that may contribute to decreased blood pressure in humans. There are some minor discrepancies, however, most surely attributed to technical details. Acuña et al. (1) used rat cRNA for experiments in X. laevis oocytes, while Monette et al. (113) used the human sequence mostly in HEK cells and also in oocytes. For example, the variant N399S (human NKCC2) had increased activity in Acuña et al. (2) while it had lower activity than the wild-type in Monette et al. (113). Also, a variant at the distal COOH terminus (Y1070C) showed decreased NKCC2 activity in Acuña's study but normal NKCC2 activity in Monette's. There are two variants (R302W and L505V) with very low NKCC2 activity in both studies, causing impaired protein translocation to the membrane and reduced expression levels (113). The overall conclusion of these studies is that rare allelic variants or mutations in NKCC2 in the normal population may protect against the development of hypertension. The mechanism by which this occurs may be related to decreased NKCC2 function either by intrinsic activity, protein expression levels, and/or trafficking to the plasma membrane. The particular cause appears to be specific for each variant and needs to be studied individually.
Several loss-of-function mutations in NKCC2 causing type I Bartter syndrome have been described in humans (2, 144, 166). These findings, supported by animal models, provide proof of the importance of NKCC2 in the TAL function. While we have not reviewed the individual NKCC2 mutations and animal models of Bartter syndrome, we would like to emphasize the fact that without exceptions, loss of NKCC2 function leads to decreased blood pressure, as well as salt and water loss in urine. This phenotype is apparent despite maximal compensatory increases in distal tubule and collecting duct transport capacity, and during activation of the renin-angiotensin-aldosterone system. These findings together with the reviewed data showing enhanced NKCC2 activity in salt-sensitive and spontaneous hypertension clearly support a role of NKCC2 in the development of hypertension and as a target in the control of blood pressure.
Concluding Remarks
Compared with other apical renal transporters, little is known about the molecular regulation of NKCC2. This is in part due to difficulties in expressing a full-length NKCC2 in mammalian cells. However, at least three groups have now reported functional expression of most NKCC2 splice variants in nonpolarized cultured cells. This model will be useful in studying some molecular aspects of NKCC2 regulation but may not be suitable for understanding polarized trafficking or the physiological regulation of NKCC2. With few exceptions, NKCC2 expression is reportedly restricted to the TAL and macula densa cells. Most likely, additional proteins specifically expressed in these nephron cell types are required for correct apical trafficking, maturation, functional expression, and regulation of NKCC2. Importantly, the hormonal and intrinsic control of NKCC2 is specific to the cell where it is expressed and thus it is better studied in freshly isolated TALs or primary cultures of TAL cells. While this is labor intensive, several advances in subcellular imaging, reporter fluorescent proteins, biochemical methods, and protein labeling could be used to address the molecular regulation of NKCC2 in the TAL.
Our data and that of others indicate that several aspects of NKCC2 regulation are affected in animal models of hypertension. In humans, enhanced NaCl reabsorption by the TAL is associated with hypertension and salt sensitivity. Despite the important role that NKCC2 may play in hypertension, the molecular mechanisms affected are unknown. This is perhaps due to the fact that the regulation of NKCC2 is only now starting to be understood in mammals under physiological conditions. However, several questions remain unanswered, such as the role of the different phospho-sites in NKCC2 trafficking and activity; the protein interactions that drive regulated and constitutive NKCC2 trafficking; whether SPAK/OSR1 are the only kinases that bind and phosphorylate NKCC2; how PKA stimulates trafficking; which are the binding sites for Na, K, Cl, and loop diuretics; what are the mechanisms by which different splice variants regulate activity; and which of these mechanisms contribute to the development of hypertension or salt sensitivity in metabolic syndrome. Understanding the molecular regulation of NKCC2 in TALs is likely to shed light on the mechanisms causing hypertension and provide new, specific, and effective targets for pharmacological intervention.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO-1 HL080409 and PO1 HL090550 (subproject 2) to P. A. Ortiz, G. Ares was supported in part by an American Heart Association fellowship grant (0715579Z).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G. R. A. prepared figures; G. R. A. and P. S. C. drafted manuscript; G. R. A. and P. A. O. edited and revised manuscript; G. R. A. and P. A. O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elizabeth Furest for secretarial assistance.
REFERENCES
- 1.Acuña R, Martinez-de-la-Maza L, Ponce-Coria J, Vazquez N, Ortal-Vite P, Pacheco-Alvarez D, Bobadilla NA, Gamba G. Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. J Hypertens 293: 475–483, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Adachi M, Asakura Y, Sato Y, Tajima T, Nakajima T, Yamamoto T, Fujieda K. Novel SLC12A1 (NKCC2) mutations in two families with Bartter syndrome type 1. Endocr J 54: 1003–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Guerra M, Garay RP. Renal Na-K-Cl cotransporter NKCC2 in Dahl salt-sensitive rats. J Hypertens 20: 721–727, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Ares GR, Caceres P, Alvarez-Leefmans FJ, Ortiz PA. cGMP decreases surface NKCC2 levels in the thick ascending limb: role of phosphodiesterase 2 (PDE2). Am J Physiol Renal Physiol 295: F877–F887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ares GR, Ortiz PA. Constitutive endocytosis and recycling of NKCC2 in rat thick ascending limbs. Am J Physiol Renal Physiol 299: F1193–F1202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension 43: 707–713, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Baba T, Sakisaka T, Mochida S, Takai Y. PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2+-dependent exocytosis of neurotransmitter. J Cell Biol 170: 1113–1125, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bailly C. Effect of luminal atrial natriuretic peptide on chloride reabsorption in mouse cortical thick ascending limb: inhibition by endothelin. J Am Soc Nephrol 11: 1791–1797, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Bennett CM, Brenner BM, Berliner RW. Micropuncture study of nephron function in the rhesus monkey. J Clin Invest 47: 203–216, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benziane B, Demaretz S, Defontaine N, Zaarour N, Cheval L, Bourgeois S, Klein C, Froissart M, Blanchard A, Paillard M, Gamba G, Houillier P, Laghmani K. NKCC2 surface expression in mammalian cells: down-regulation by novel interaction with aldolase B. J Biol Chem 282: 33817–33830, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Berrera M, Dodoni G, Monterisi S, Pertegato V, Zamparo I, Zaccolo M. A toolkit for real-time detection of cAMP: insights into compartmentalized signaling. Handb Exp Pharmacol 186: 285–298, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4–2. J Biol Chem 281: 26159–26169, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Bickel CA, Knepper MA, Verbalis JG, Ecelbarger CA. Dysregulation of renal salt and water transport proteins in diabetic Zucker rats. Kidney Int 61: 2099–2110, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Breton S, Inoue T, Knepper MA, Brown D. Antigen retrieval reveals widespread basolateral expression of syntaxin 3 in renal epithelia. Am J Physiol Renal Physiol 282: F523–F529, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 49: 251–273, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Brunet GM, Gagnon E, Simard CF, Daigle ND, Caron L, Noel M, Lefoll MH, Bergeron MJ, Isenring P. Novel insights regarding the operational characteristics and teleological purpose of the renal Na+-K+-2Cl cotransporter (NKCC2s) splice variants. J Gen Physiol 126: 325–337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burg M, Good D. Sodium chloride coupled transport in mammalian nephrons. Annu Rev Physiol 45: 533–547, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Burg MB. Tubular chloride transport and the mode of action of some diuretics. Kidney Int 9: 189–197, 1976 [DOI] [PubMed] [Google Scholar]
- 20.Burg MB, Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol 224: 659–668, 1973 [DOI] [PubMed] [Google Scholar]
- 21.Burg MB, Papper S, Rosenbaum JD. Factors influencing the diuretic response to ingested water. J Lab Clin Med 57: 533–545, 1961 [PubMed] [Google Scholar]
- 22.Butterworth MB, Frizzell RA, Johnson JP, Peters KW, Edinger RS. PKA-dependent ENaC trafficking requires the SNARE-binding protein complexin. Am J Physiol Renal Physiol 289: F969–F977, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Cabral PD, Silva GB, Baigorria ST, Juncos LA, Juncos LI, Garcia NH. 8-Iso-prostaglandin-F2α stimulates chloride transport in thick ascending limbs: role of cAMP and protein kinase A. Am J Physiol Renal Physiol 299: F1396–F1400, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na+-K+-2Cl− cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Carmosino M, Gimenez I, Caplan M, Forbush B. Exon loss accounts for differential sorting of Na-K-Cl cotransporters in polarized epithelial cells. Mol Biol Cell 19: 4341–4351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmosino M, Rizzo F, Procino G, Basco D, Valenti G, Forbush B, Schaeren-Wiemers N, Caplan MJ, Svelto M. MAL/VIP17, a new player in the regulation of NKCC2 in the kidney. Mol Biol Cell 21: 3985–3997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castrop H, Schnermann J. Isoforms of renal Na-K-2Cl cotransporter NKCC2: expression and functional significance. Am J Physiol Renal Physiol 295: F859–F866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Chen L, Wang G, Tang H. Cholesterol-dependent and -independent CD40 internalization and signaling activation in cardiovascular endothelial cells. Arterioscler Thromb Vasc Biol 27: 2005–2013, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Chheda MG, Ashery U, Thakur P, Rettig J, Sheng ZH. Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. Nat Cell Biol 3: 331–338, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Darman RB, Forbush B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem 277: 37542–37550, 2002 [DOI] [PubMed] [Google Scholar]
- 32.de Jesus Ferreira MC, Bailly C. Luminal and basolateral endothelin inhibit chloride reabsorption in the mouse thick ascending limb via a Ca2+-independent pathway. J Physiol 505: 749–758, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na+-K+-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem 269: 25677–25683, 1994. [PubMed] [Google Scholar]
- 34.de Rouffignac C, Quamme G. Renal magnesium handling and its hormonal control. Physiol Rev 74: 305–322, 1994 [DOI] [PubMed] [Google Scholar]
- 35.de Rouffignac C, Corman B, Roinel N. Stimulation by antidiuretic hormone of electrolyte tubular reabsorption in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 244: F156–F164, 1983 [DOI] [PubMed] [Google Scholar]
- 36.Dimke H, Flyvbjerg A, Bourgeois S, Thomsen K, Frokiaer J, Houillier P, Nielsen S, Frische S. Acute growth hormone administration induces antidiuretic and antinatriuretic effects and increases phosphorylation of NKCC2. Am J Physiol Renal Physiol 292: F723–F735, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Dublineau I, Pradelles P, de RC, Elalouf JM. Differential short-term desensitization to vasopressin, isoproterenol, glucagon, parathyroid hormone and calcitonin in the thick ascending limb of rat kidney. Pflügers Arch 420: 16–22, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Ecelbarger CA, Kim GH, Wade JB, Knepper MA. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol 171: 227–234, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Escalante B, Erlij D, Falck JR, McGiff JC. Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol Cell Physiol 266: C1775–C1782, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Esteva-Font C, Ars E, Guillen-Gomez E, Campistol JM, Sanz L, Jimenez W, Knepper MA, Torres F, Torra R, Ballarin JA, Fernandez-Llama P. Ciclosporin-induced hypertension is associated with increased sodium transporter of the loop of Henle (NKCC2). Nephrol Dial Transplant 22: 2810–2816, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Eveloff JL, Calamia J. Effect of osmolarity on cation fluxes in medullary thick ascending limb cells. Am J Physiol Renal Fluid Electrolyte Physiol 250: F176–F180, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+-K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405: 85–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Gamba G. The nanopeptide hormone vasopressin is a new player in the modulation of renal Na+-Cl− cotransporter activity. Kidney Int 78: 127–129, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994 [PubMed] [Google Scholar]
- 47.Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension 34: 508–513, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Giebisch G. Diuretic action of potassium channel blockers. Eur J Clin Pharmacol 44, Suppl 1: S3–S5, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Gimenez I, Forbush B. The residues determining differences in ion affinities among the alternative splice variants F, A, and B of the mammalian renal Na-K-Cl cotransporter (NKCC2). J Biol Chem 282: 6540–6547, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Gimenez I, Isenring P, Forbush B. Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem 277: 8767–8770, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Glorioso N, Filigheddu F, Troffa C, Soro A, Parpaglia PP, Tsikoudakis A, Myers RH, Herrera VL, Ruiz-Opazo N. Interaction of alpha1-Na,K-ATPase and Na,K,2Cl-cotransporter genes in human essential hypertension. Hypertension 38: 204–209, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 390: 30–37, 1981 [DOI] [PubMed] [Google Scholar]
- 56.Greger R. Chloride reabsorption in the rabbit cortical thick ascending limb of the loop of Henle. A sodium dependent process. Pflügers Arch 390: 38–43, 1981 [DOI] [PubMed] [Google Scholar]
- 57.Grider JS, Falcone JC, Kilpatrick EL, Ott CE, Jackson BA. P450 arachidonate metabolites mediate bradykinin-dependent inhibition of NaCl transport in the rat thick ascending limb. Can J Physiol Pharmacol 75: 91–96, 1997 [PubMed] [Google Scholar]
- 58.Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci USA 107: 15653–15658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas M. The Na-K-Cl cotransporters. Am J Physiol Cell Physiol 267: C869–C885, 1994 [DOI] [PubMed] [Google Scholar]
- 60.Haas M, Forbush B., III The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res 39: 369–379, 1998 [PubMed] [Google Scholar]
- 62.Hannaert P, Alvarez-Guerra M, Pirot D, Nazaret C, Garay RP. Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs. Naunyn Schmiedebergs Arch Pharmacol 365: 193–199, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Hannemann A, Flatman PW. Phosphorylation and transport in the Na-K-2Cl cotransporters, NKCC1 and NKCC2A, compared in HEK-293 cells. PLoS One 6: e17992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haque MZ, Ares GR, Caceres PS, Ortiz PA. High salt differentially regulates surface NKCC2 expression in thick ascending limbs of Dahl salt-sensitive and salt-resistant rats. Am J Physiol Renal Physiol 300: F1096–F1104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawk CT, Schafer JA. Effects of AVP and deoxycorticosterone on Na+ and water transport in the Dahl salt-sensitive rat CCD. Am J Physiol Renal Fluid Electrolyte Physiol 260: F471–F478, 1991 [DOI] [PubMed] [Google Scholar]
- 66.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 246: F745–F756, 1984 [DOI] [PubMed] [Google Scholar]
- 67.Hebert SC, Andreoli TE. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl− absorption. J Gen Physiol 87: 567–590, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol Renal Fluid Electrolyte Physiol 241: F412–F431, 1981 [DOI] [PubMed] [Google Scholar]
- 69.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport: origin of transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F432–F442, 1981 [DOI] [PubMed] [Google Scholar]
- 70.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. III. Modulation of the ADH effect by peritubular osmolality. Am J Physiol Renal Fluid Electrolyte Physiol 241: F443–F451, 1981 [DOI] [PubMed] [Google Scholar]
- 71.Hebert SC, Friedman PA, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle. I. ADH increases transcellular conductance pathways. J Membr Biol 80: 201–219, 1984 [DOI] [PubMed] [Google Scholar]
- 72.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem 284: 1454–1460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herrera VL, Lopez LV, Ruiz-Opazo N. Alpha1 Na,K-ATPase and Na,K,2Cl− cotransporte/D3mit3 loci interact to increase susceptibility to salt-sensitive hypertension in Dahl S(HSD) rats. Mol Med 7: 125–134, 2001 [PMC free article] [PubMed] [Google Scholar]
- 74.Hoagland KM, Flasch AK, hly-Vernon AJ, dos Santos EA, Knepper MA, Roman RJ. Elevated BSC-1 and ROMK expression in Dahl salt-sensitive rat kidneys. Hypertension 43: 860–865, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, Hernandez-Deviez DJ, Hadzic G, McCluskey A, Bashir R, Liu L, Pilch P, McMahon H, Robinson PJ, Hancock JF, Mayor S, Parton RG. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol 190: 675–691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Igarashi P, Vanden Heuvel GB, Payne JA, Forbush B, III Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am J Physiol Renal Fluid Electrolyte Physiol 269: F405–F418, 1995 [DOI] [PubMed] [Google Scholar]
- 77.Inoue T, Nielsen S, Mandon B, Terris J, Kishore BK, Knepper MA. SNAP-23 in rat kidney: colocalization with aquaporin-2 in collecting duct vesicles. Am J Physiol Renal Physiol 275: F752–F760, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Ito O, Kondo Y, Takahashi N, Kudo K, Igarashi Y, Omata K, Imai Y, Abe K. Insulin stimulates NaCl transport in isolated perfused MTAL of Henle's loop of rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F265–F270, 1994 [DOI] [PubMed] [Google Scholar]
- 79.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension 33: 419–423, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Jung J, Basile DP, Pratt JH. Sodium reabsorption in the thick ascending limb in relation to blood pressure: a clinical perspective. Hypertension 57: 873–879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kao AW, Noda Y, Johnson JH, Pessin JE, Saltiel AR. Aldolase mediates the association of F-actin with the insulin-responsive glucose transporter GLUT4. J Biol Chem 274: 17742–17747, 1999 [DOI] [PubMed] [Google Scholar]
- 84.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC. Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int 49: 40–47, 1996 [DOI] [PubMed] [Google Scholar]
- 85.Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ. Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci USA 91: 6943–6947, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim GH, Choi NW, Jung JY, Song JH, Lee CH, Kang CM, Knepper MA. Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2. Am J Physiol Renal Physiol 294: F702–F709, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 276: F96–F103, 1999 [DOI] [PubMed] [Google Scholar]
- 88.Kirchner KA. Greater loop chloride uptake contributes to blunted pressure natriuresis in Dahl salt sensitive rats. J Am Soc Nephrol 1: 180–186, 1990 [DOI] [PubMed] [Google Scholar]
- 89.Kirchner KA. Increased loop chloride uptake precedes hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 262: R263–R268, 1992 [DOI] [PubMed] [Google Scholar]
- 90.Kirchner KA, Crosby BA, Patel AR, Granger JP. Segmental chloride transport in the Dahl-S rat kidney during l-arginine administration. J Am Soc Nephrol 5: 1567–1572, 1995 [DOI] [PubMed] [Google Scholar]
- 91.Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34: 13784–13793, 1995 [DOI] [PubMed] [Google Scholar]
- 92.Knepper MA, Brooks HL. Regulation of the sodium transporters NHE3, NKCC2 and NCC in the kidney. Curr Opin Nephrol Hypertens 10: 655–659, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F. Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12: 1327–1334, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Konstas AA, Korbmacher C. The γ-subunit of ENaC is more important for channel surface expression than the beta-subunit. Am J Physiol Cell Physiol 284: C447–C456, 2003 [DOI] [PubMed] [Google Scholar]
- 95.Kudo LH, Hawk CT, Schafer JA. Sodium and water transport in cortical collecting duct of Dahl salt-resistant rat. Am J Physiol Renal Fluid Electrolyte Physiol 267: F583–F591, 1994 [DOI] [PubMed] [Google Scholar]
- 96.Le PU, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem 277: 3371–3379, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Li X, Low SH, Miura M, Weimbs T. SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am J Physiol Renal Physiol 283: F1111–F1122, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Liu Y, Ding X, Wang D, Deng H, Feng M, Wang M, Yu X, Jiang K, Ward T, Aikhionbare F, Guo Z, Forte JG, Yao X. A mechanism of Munc18b-syntaxin 3-SNAP25 complex assembly in regulated epithelial secretion. FEBS Lett 581: 4318–4324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 20: 855–866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu M, Ammar D, Ives H, Albrecht F, Gluck SL. Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J Biol Chem 282: 24495–24503, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Lytle C, Xu JC, Biemesderfer D, Forbush B, III Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol Cell Physiol 269: C1496–C1505, 1995 [DOI] [PubMed] [Google Scholar]
- 102.Malnic G, Klose RM, Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 211: 529–547, 1966 [DOI] [PubMed] [Google Scholar]
- 103.Mandon B, Nielsen S, Kishore BK, Knepper MA. Expression of syntaxins in rat kidney. Am J Physiol Renal Physiol 273: F718–F730, 1997 [DOI] [PubMed] [Google Scholar]
- 104.Mandon B, Siga E, Chabardes D, Firsov D, Roinel N, de Rouffignac C. Insulin stimulates Na+, Cl−, Ca2+, and Mg2+ transports in TAL of mouse nephron: cross-potentiation with AVP. Am J Physiol Renal Fluid Electrolyte Physiol 265: F361–F369, 1993 [DOI] [PubMed] [Google Scholar]
- 105.Mandon B, Siga E, Roinel N, de Rouffignac C. Ca2+, Mg2+ and K+ transport in the cortical and medullary thick ascending limb of the rat nephron: influence of transepithelial voltage. Pflügers Arch 424: 558–560, 1993 [DOI] [PubMed] [Google Scholar]
- 106.Marazuela M, Alonso MA. Expression of MAL and MAL2, two elements of the protein machinery for raft-mediated transport, in normal and neoplastic human tissue. Histol Histopathol 19: 925–933, 2004 [DOI] [PubMed] [Google Scholar]
- 107.Mark AL. Sympathetic neural contribution to salt-induced hypertension in Dahl rats. Hypertension 17, Suppl: I86–I90, 1991 [DOI] [PubMed] [Google Scholar]
- 108.McCormick JA, Delpire E, Ellison DH. SPAK isoforms differentially regulate renal sodium transport. FASEB J 25: 1038, 2011 [Google Scholar]
- 109.McKee JA, Kumar S, Ecelbarger CA, Fernandez-Llama P, Terris J, Knepper MA. Detection of Na+ transporter proteins in urine. J Am Soc Nephrol 11: 2128–2132, 2000 [DOI] [PubMed] [Google Scholar]
- 110.Meade P, Hoover RS, Plata C, Vazquez N, Bobadilla NA, Gamba G, Hebert SC. cAMP-dependent activation of the renal-specific Na+-K+-2Cl− cotransporter is mediated by regulation of cotransporter trafficking. Am J Physiol Renal Physiol 284: F1145–F1154, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Miyata N, Zou AP, Mattson DL, Cowley AW., Jr Renal medullary interstitial infusion of l-arginine prevents hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 275: R1667–R1673, 1998 [DOI] [PubMed] [Google Scholar]
- 112.Molony DA, Reeves WB, Hebert SC, Andreoli TE. ADH increases apical Na+, K+, 2Cl− entry in mouse medullary thick ascending limbs of Henle. Am J Physiol Renal Fluid Electrolyte Physiol 252: F177–F187, 1987 [DOI] [PubMed] [Google Scholar]
- 113.Monette MY, Rinehart J, Lifton RP, Forbush B. Rare mutations in the human Na-K-Cl cotransporter (NKCC2) associated with lower blood pressure exhibit impaired processing and transport function. Am J Physiol Renal Physiol 300: F840–F847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mount DB, Baekgaard A, Hall AE, Plata C, Xu J, Beier DR, Gamba G, Hebert SC. Isoforms of the Na-K-2Cl cotransporter in murine TAL. I. Molecular characterization and intrarenal localization. Am J Physiol Renal Physiol 276: F347–F358, 1999 [DOI] [PubMed] [Google Scholar]
- 115.Mount DB, Gamba G. Renal potassium-chloride cotransporters. Curr Opin Nephrol Hypertens 10: 685–691, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Muth TR, Caplan MJ. Transport protein trafficking in polarized cells. Annu Rev Cell Dev Biol 19: 333–366, 2003 [DOI] [PubMed] [Google Scholar]
- 117.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castaneda-Bueno M, Gamba G, Bachmann S. Activation of the bumetanide-sensitive Na+,K+,2Cl− cotransporter NKCC2 is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 286: 30200–30210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neant F, Bailly C. Luminal and intracellular cGMP inhibit the mTAL reabsorptive capacity through different pathways. Kidney Int 44: 741–746, 1993 [DOI] [PubMed] [Google Scholar]
- 119.Neant F, Imbert-Teboul M, Bailly C. Cyclic guanosine monophosphate is the mediator of platelet-activating factor inhibition on transport by the mouse kidney thick ascending limb. J Clin Invest 94: 1156–1162, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nezu A, Parvin MN, Turner RJ. A conserved hydrophobic tetrad near the C terminus of the secretory Na+-K+-2Cl− cotransporter (NKCC1) is required for its correct intracellular processing. J Biol Chem 284: 6869–6876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol Renal Physiol 275: F885–F893, 1998 [DOI] [PubMed] [Google Scholar]
- 122.O'Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17: 2402–2413, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Obermuller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest 98: 635–640, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem 186: 17–22, 1989 [DOI] [PubMed] [Google Scholar]
- 125.Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J Am Soc Nephrol 18: 440–448, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Ortiz PA, Garvin JL. NO Inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001 [DOI] [PubMed] [Google Scholar]
- 128.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 129.Ortiz PA. A small fraction of total NKCC2 is located in the apical membrane of thick ascending limbs under basal conditions (Abstract). FASEB J 20: A1223, 2006 [Google Scholar]
- 130.Paredes A, Plata C, Rivera M, Moreno E, Vazquez N, Munoz-Clares R, Hebert SC, Gamba G. Activity of the renal Na+-K+-2Cl− cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl− transport. Am J Physiol Renal Physiol 290: F1094–F1102, 2006 [DOI] [PubMed] [Google Scholar]
- 131.Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem 276: 9670–9678, 2001 [DOI] [PubMed] [Google Scholar]
- 132.Patel A, Layne S, Watts D, Kirchner KA. l-Arginine administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension 22: 863–869, 1993 [DOI] [PubMed] [Google Scholar]
- 133.Patel AR, Granger JP, Kirchner KA. l-Arginine improves transmission of perfusion pressure to the renal interstitium in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 266: R1730–R1735, 1994 [DOI] [PubMed] [Google Scholar]
- 134.Payne JA, Forbush B., III Alternatively spliced isoforms of the putative renal Na-K-Cl cotransporter are differentially distributed within the rabbit kidney. Proc Natl Acad Sci USA 91: 4544–4548, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Payne JA, Xu JC, Haas M, Lytle CY, Ward D, Forbush B, III Primary structure, functional expression, and chromosomal localization of the bumetanide-sensitive Na-K-Cl cotransporter in human colon. J Biol Chem 270: 17977–17985, 1995 [DOI] [PubMed] [Google Scholar]
- 136.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002 [DOI] [PubMed] [Google Scholar]
- 137.Plata C, Meade P, Hall A, Welch RC, Vazquez N, Hebert SC, Gamba G. Alternatively spliced isoform of apical Na+-K+-Cl− cotransporter gene encodes a furosemide-sensitive Na+-Cl− cotransporter. Am J Physiol Renal Physiol 280: F574–F582, 2001 [DOI] [PubMed] [Google Scholar]
- 138.Plata C, Meade P, Vazquez N, Hebert SC, Gamba G. Functional properties of the apical Na+-K+-2Cl− cotransporter isoforms. J Biol Chem 277: 11004–11012, 2002 [DOI] [PubMed] [Google Scholar]
- 139.Plata C, Mount DB, Rubio V, Hebert SC, Gamba G. Isoforms of the Na-K-2Cl cotransporter in murine TAL. II. Functional characterization and activation by cAMP. Am J Physiol Renal Physiol 276: F359–F366, 1999 [DOI] [PubMed] [Google Scholar]
- 140.Plato CF. α-2 And β-adrenergic receptors mediate NE's biphasic effects on rat thick ascending limb chloride flux. Am J Physiol Regul Integr Comp Physiol 281: R979–R986, 2001 [DOI] [PubMed] [Google Scholar]
- 141.Plato CF, Garvin JL. α2-Adrenergic-mediated tubular NO production inhibits thick ascending limb chloride absorption. Am J Physiol Renal Physiol 281: F679–F686, 2001 [DOI] [PubMed] [Google Scholar]
- 142.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000 [DOI] [PubMed] [Google Scholar]
- 143.Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los HP, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pressler CA, Heinzinger J, Jeck N, Waldegger P, Pechmann U, Reinalter S, Konrad M, Beetz R, Seyberth HW, Waldegger S. Late-onset manifestation of antenatal Bartter syndrome as a result of residual function of the mutated renal Na+-K+-2Cl− co-transporter. J Am Soc Nephrol 17: 2136–2142, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Quamme GA, de RC. Epithelial magnesium transport and regulation by the kidney. Front Biosci 5: D694–D711, 2000 [DOI] [PubMed] [Google Scholar]
- 146.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rangarajan ES, Park H, Fortin E, Sygusch J, Izard T. Mechanism of aldolase control of sorting nexin 9 function in endocytosis. J Biol Chem 285: 11983–11990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reiche J, Theilig F, Rafiqi FH, Carlo AS, Militz D, Mutig K, Todiras M, Christensen EI, Ellison DH, Bader M, Nykjaer A, Bachmann S, Alessi D, Willnow TE. SORLA/SORL1 functionally interacts with SPAK to control renal activation of Na+-K+-Cl− cotransporter 2. Mol Cell Biol 30: 3027–3037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Richardson C, Sakamoto K, de Los HP, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rinehart J, Kahle KT, de Los HP, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA 102: 16777–16782, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodal SK, Skretting G, Garred O, Vilhardt F, van DB, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 10: 961–974, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6: 233–247, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Rodriguez-Boulan E, Musch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim Biophys Acta 1744: 455–464, 2005 [DOI] [PubMed] [Google Scholar]
- 154.Roman RJ, Cowley AW., Jr Abnormal pressure-diuresis-natriuresis response in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 248: F199–F205, 1985 [DOI] [PubMed] [Google Scholar]
- 155.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991 [DOI] [PubMed] [Google Scholar]
- 156.Sayner SL. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol 300: L667–L678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schmitt R, Ellison DH, Farman N, Rossier BC, Reilly RF, Reeves WB, Oberbaumer I, Tapp R, Bachmann S. Developmental expression of sodium entry pathways in rat nephron. Am J Physiol Renal Physiol 276: F367–F381, 1999 [DOI] [PubMed] [Google Scholar]
- 158.Simchon S, Manger W, Blumberg G, Brensilver J, Cortell S. Impaired renal vasodilation and urinary cGMP excretion in Dahl salt-sensitive rats. Hypertension 27: 653–657, 1996 [DOI] [PubMed] [Google Scholar]
- 159.Simchon S, Manger WM, Shi GS, Brensilver J. Impaired renal vascular reactivity in prehypertensive Dahl salt-sensitive rats. Hypertension 20: 524–532, 1992 [DOI] [PubMed] [Google Scholar]
- 160.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 [DOI] [PubMed] [Google Scholar]
- 161.Sonalker PA, Jackson EK. Norepinephrine, via beta-adrenoceptors, regulates bumetanide-sensitive cotransporter type 1 expression in thick ascending limb cells. Hypertension 49: 1351–1357, 2007 [DOI] [PubMed] [Google Scholar]
- 162.Sonalker PA, Tofovic SP, Jackson EK. Increased expression of the sodium transporter BSC-1 in spontaneously hypertensive rats. J Pharmacol Exp Ther 311: 1052–1061, 2004 [DOI] [PubMed] [Google Scholar]
- 163.Sonalker PA, Tofovic SP, Jackson EK. Cellular distribution of the renal bumetanide-sensitive Na-K-2Cl cotransporter BSC-1 in the inner stripe of the outer medulla during the development of hypertension in the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol 34: 1307–1312, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Sripairojthikoon W, Oparil S, Wyss JM. Renal nerve contribution to NaCl-exacerbated hypertension in spontaneously hypertensive rats. Hypertension 14: 184–190, 1989 [DOI] [PubMed] [Google Scholar]
- 165.Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G, Zaccolo M. cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ Res 108: 929–939, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Starremans PG, Kersten FF, Knoers NV, van den Heuvel LP, Bindels RJ. Mutations in the human Na-K-2Cl cotransporter (NKCC2) identified in Bartter syndrome type I consistently result in nonfunctional transporters. J Am Soc Nephrol 14: 1419–1426, 2003 [DOI] [PubMed] [Google Scholar]
- 167.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA 96: 6775–6780, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sun A, Grossman EB, Lombardi M, Hebert SC. Vasopressin alters the mechanism of apical Cl- entry from Na+:Cl− to Na+:K+:2Cl− cotransport in mouse medullary thick ascending limb. J Membr Biol 120: 83–94, 1991 [DOI] [PubMed] [Google Scholar]
- 169.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc Natl Acad Sci USA 97: 5434–5439, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Traebert M, Kohler K, Lambert G, Biber J, Forster I, Murer H. Investigating the surface expression of the renal type IIa Na+/Pi-cotransporter in Xenopus laevis oocytes. J Membr Biol 180: 83–90, 2001 [DOI] [PubMed] [Google Scholar]
- 171.Turban S, Wang XY, Knepper MA. Regulation of NHE3, NKCC2, and NCC abundance in kidney during aldosterone escape phenomenon: role of NO. Am J Physiol Renal Physiol 285: F843–F851, 2003 [DOI] [PubMed] [Google Scholar]
- 172.Valentijn JA, Fyfe GK, Canessa CM. Biosynthesis and processing of epithelial sodium channels in Xenopus oocytes. J Biol Chem 273: 30344–30351, 1998 [DOI] [PubMed] [Google Scholar]
- 173.Vargas-Poussou R, Feldmann D, Vollmer M, Konrad M, Kelly L, van den Heuvel LP, Tebourbi L, Brandis M, Karolyi L, Hebert SC, Lemmink HH, Deschenes G, Hildebrandt F, Seyberth HW, Guay-Woodford LM, Knoers NV, Antignac C. Novel molecular variants of the Na-K-2Cl cotransporter gene are responsible for antenatal Bartter syndrome. Am J Hum Genet 62: 1332–1340, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290: F14–F19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang WH, White S, Geibel J, Giebisch G. A potassium channel in the apical membrane of rabbit thick ascending limb of Henle's loop. Am J Physiol Renal Fluid Electrolyte Physiol 258: F244–F253, 1990 [DOI] [PubMed] [Google Scholar]
- 176.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996 [DOI] [PubMed] [Google Scholar]
- 177.Weinberger MH, Luft FC, Bloch R, Henry DP, Pratt JH, Weyman AE, Rankin LI, Murray RH, Willis LR, Grim CE. The blood pressure-raising effects of high dietary sodium intake: racial differences and the role of potassium. J Am Coll Nutr 1: 139–148, 1982 [DOI] [PubMed] [Google Scholar]
- 178.Welker P, Bohlick A, Mutig K, Salanova M, Kahl T, Schluter H, Blottner D, Ponce-Coria J, Gamba G, Bachmann S. Renal Na+-K+-Cl− cotransporter activity and vasopressin-induced trafficking are lipid raft-dependent. Am J Physiol Renal Physiol 295: F789–F802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wittner M, Di SA, Mandon B, Roinel N, de Rouffignac C. Stimulation of NaCl reabsorption by antidiuretic hormone in the cortical thick ascending limb of Henle's loop of the mouse. Pflügers Arch 419: 212–214, 1991 [DOI] [PubMed] [Google Scholar]
- 180.Xu JC, Lytle C, Zhu TT, Payne JA, Benz E, Jr, Forbush B., III Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci USA 91: 2201–2205, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang SS, Hsu YJ, Chiga M, Rai T, Sasaki S, Uchida S, Lin SH. Mechanisms for hypercalciuria in pseudohypoaldosteronism type II-causing WNK4 knock-in mice. Endocrinology 151: 1829–1836, 2010 [DOI] [PubMed] [Google Scholar]
- 182.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931–F939, 1996 [DOI] [PubMed] [Google Scholar]
- 184.Yu MJ, Pisitkun T, Wang G, Aranda JF, Gonzales PA, Tchapyjnikov D, Shen RF, Alonso MA, Knepper MA. Large-scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am J Physiol Cell Physiol 295: C661–C678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yuan B, Cowley AW., Jr Evidence that reduced renal medullary nitric oxide synthase activity of Dahl s rats enables small elevations of arginine vasopressin to produce sustained hypertension. Hypertension 37: 524–528, 2001 [DOI] [PubMed] [Google Scholar]
- 186.Zaarour N, Defontaine N, Demaretz S, Azroyan A, Cheval L, Laghmani K. Secretory carrier membrane protein 2 regulates exocytic insertion of NKCC2 into the cell membrane. J Biol Chem 286: 9489–9502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zaarour N, Demaretz S, Defontaine N, Mordasini D, Laghmani K. A highly conserved motif at the COOH terminus dictates endoplasmic reticulum exit and cell surface expression of NKCC2. J Biol Chem 284: 21752–21764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta 1768: 1311–1324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zou AP, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension 27: 631–635, 1996 [DOI] [PubMed] [Google Scholar]