Abstract
We have developed a general method combining photochemical grafting and copper-catalyzed click chemistry for biofunctionalization of titanium substrates. The UV-activated grafting of an α,ω-alkenyne onto TiO2/Ti substrates provided a “clickable” thin film platform. The selective attachment of the vinyl end of the molecule to the surface was achieved by masking the alkynyl end with a trimethylgermanyl (TMG) protecting group. Subsequently, various oligo(ethylene glycol) (OEG) derivatives terminated with an azido group were attached to the TMG-alkynyl modified titanium surface via a one-pot deprotection/click reaction. The films were characterized by X-ray photoelectron spectroscopy (XPS), contact angle goniometry, ellipsometry, and atomic force microscopy (AFM). We showed that the titanium surface presenting click-immobilized OEG substantially suppressed the nonspecific attachment of protein and cells as compared to the unmodified titanium substrate. Furthermore, glycine-arginine-glycine-aspartate (GRGD), a cell adhesion peptide, was coimmobilized with OEG on the platform. We demonstrated that the resultant GRGD-presenting thin film on Ti substrates can promote the specific adhesion and spreading of AsPC-1 cells.
1. INTRODUCTION
Titanium (Ti) is one of the most frequently used materials for biomedical devices and implants due to its high strength and excellent corrosion resistance.1–3 Although it is generally considered as biocompatible, many efforts have been devoted to tailor the surface properties to further enhance the desired interactions of the Ti implants with the surrounding tissues.4–9
For many biomaterial systems, it is desirable to control interfacial properties to interact selectively with cells or certain biomolecules, which requires, first of all, an antifouling or inert “background”. Nevertheless, the metallic titanium is covered with a thin film (3–6 nm) of spontaneously formed titanium dioxide (TiO2) that is shown to readily adsorb any protein (e.g., albumin, laminin, fibronectin) upon contact with biological tissues or fluids.1,10,11 This unwanted nonspecific adsorption could lead to an undesirable cascade of events that eventually result in the rejection of the implants.12,13
Poly(ethylene glycol) (PEG) and oligo(ethylene glycol) (OEG) are well-known components to suppress the nonspecific interactions between the substrate and the protein-containing media.14,15 In the case of the surface modification of Ti materials, PEG, OEG or bioactive components are typically modified with a functional group that has a high affinity for TiO2.16–18 Classic grafting methods on TiO2 surface include silanization chemistry and phosphonate attachment, but these strategies usually require multistep organic synthesis involving protecting groups,18–21 and the stability of the coatings is sensitive to the preparation techniques and generally prone to acid or base catalyzed hydrolysis.20,21
Very recently, a powerful technique for functionalizing TiO2 surfaces via photochemical grafting has been developed by Hamers and co-workers, in which the alkene-containing compounds are covalently attached to the TiO2 substrate probably via a Ti–O–C bond.22–24 Although the exact mechanism of the reaction remains unclear, the formed organic layer was chemically and thermally very stable in electrolyte solutions, thus showing great potential in the field of biomedical application. However, the photografting condition, which involves extended exposure to 254 nm UV light, does not allow direct immobilization of many bioactive molecules. It is therefore important to have a second reactive group available at the distal end to facilitate the subsequent chemical modification of the surface. In order to prevent the reactive end group from attaching to the substrate, the desired functionality usually must be protected and then deprotected after linking to the substrate. For example, Hamers and co-workers employ trifluoroacetyl protected amine as a precursor to amine-terminated surfaces that were useful for subsequent modification with DNA or proteins.23,24 However, harsh reaction was required for the deprotection of the trifluoroacetyl group to expose the amine functionality.
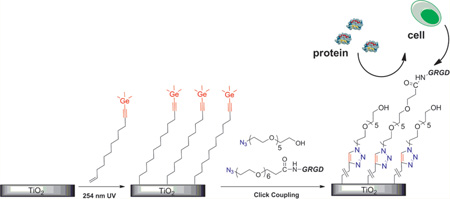
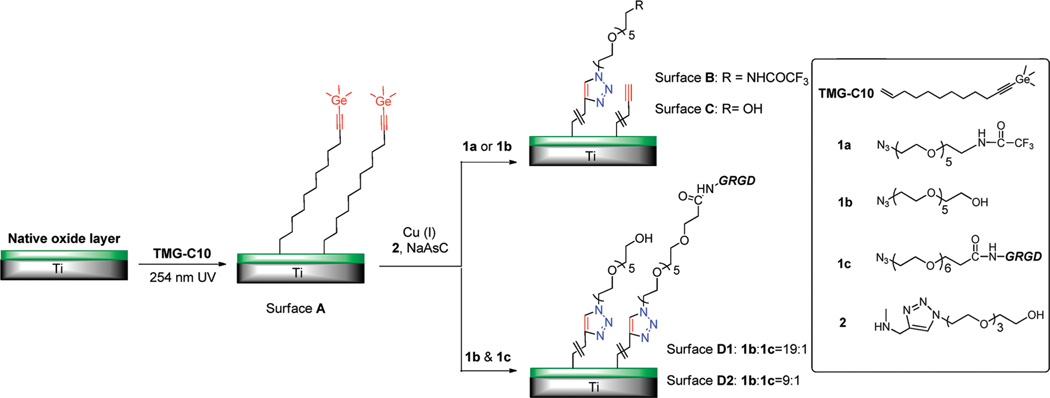
Given this background, we report here a new strategy for the preparation and photoanchoring of a “clickable” organic layer on Ti substrate.25 Our surface modification approach is outlined in Scheme 1. A bifunctionalized alkene (TMG-C10) was used as the starting material for the preparation of the TMG-alkynyl terminated surface. TMG-C10 is chosen because it can be easily detected via X-ray photoelectron spectroscopy (XPS) due to the distinct Ge signal, and because it is capable to perform one-pot deprotection/click coupling with various azido-tagged molecules.26,27 In this study, several OEG-azide derivatives (1a–1c) were used to modify the Ti substrate. As for compound 1a, the CF3-tag aids the spectroscopic characterization and facilitates the quantification for surface density of the OEG components. Functionalities introduced by neat OEG derivative 1b were expected to impart resistance toward nonspecific protein adsorption. Finally, incorporation of an azidolabled peptide 1c (GRGD) that binds to cell surface through integrin receptors yields a surface to promote receptor-mediated cell attachment.
Scheme 1. Schematic Diagram of Photochemical Grafting of TMG-C10 on Titanium Surfaces with a Native Oxide Layera.
a The TMG end group can be further modified via one-pot click coupling with OEG derivatives 1a (surface B) to quantify the surface density of the molecule immobilized, 1b (surface C) to impart protein resistance background, and the mixture of 1b and the azide-labled GRGD peptide (1c) (surface D1&D2) to promote cell adhesion and spreading.
2. EXPERIMENTAL SECTION
Chemicals
1,8-Diazabicycloundec-7-ene (DBU), triphenylphosphine 99% (PPh3), n-butylithium (n-BuLi), carbon tetrabromide 98% (CBr4), and trimethyl germanium chloride (TMGCl) were purchased from Alfa Aesar. Undecylenic aldehyde (97%) was purchased from Acros Organics. 1,11-Dodecadiyne was purchased from Chemsamp Co. and used without further purification. TMG-C10,27 1a,26 1b,26 1c,28 and 229 were synthesized according previously described protocols. 14,14,14-Trifuorotetradecanethiol (CF3-C13-SH)30 and gold wafers deposited on silicon substrates were generous gifts from Prof. T. R. Lee at the University of Houston. Ti substrates (1 × 1 cm2), >99.9% purity, one-side optical polished, were purchased from MTI corporation. The surface of the as-received samples contains mainly TiO2, with trace contamination of C, N, Si, and Ca elements.
Protocol of Photochemical Grafting on Ti Substrate
Prior to the photochemical grafting, the substrates were first rinsed ultrasonically in acetone, ethanol, and Milli-Q water successively for 10 min each, dried in a stream of nitrogen, and exposed for 20 s to oxygen-based plasma (Harricks plasma cleaner, RF power 7.2W). Then, the Ti substrate was placed inside a N2-filled box, facing a droplet (~1 mg) of the alkene (TMG-C10) that was placed on freshly cleaned and dried quartz. The Ti substrate was allowed to contact with the droplet, sandwiching a thin and homogeneous layer of the alkene between the substrate and the quartz wall. The substrate was then illuminated with a hand-held 254 nm UV-lamp (model UVLS-28, UVP, 9.8 mW/cm2) for a specific time, followed by washing sequentially with CH2Cl2 and ethanol, and finally dried with a stream of nitrogen.
Click Modification on the TMG-Acetylene Terminated Ti Substrate
A stock solution of ligand 2 in H2O (100 mM, 0.1 mL) and azide derivatives in MeOH (50 mM, 0.1 mL) were combined in a reaction vial containing the TMG-terminated Ti surface (1 × 1 cm2) and a mixture of MeOH/H2O (1:1, 1 mL). Subsequently, sodium ascorbate (2.64 mg, 15 µmol) and Cu(MeCN)4PF6 (0.22 mg, 1.5 µmol) were added to the reaction mixture as the catalytic system. For the preparation of surface D1 and D2, the compound 1b and 1c was premixed in solution at specific ratio before the click reaction. The reaction vial was backfilled with N2, sealed with a cap and covered with aluminum foil Then it was placed on an orbital shaker (VWR, DS-500) with slight shaking for 4 h. After that, the Ti wafer was rinsed with CH2Cl2 and EtOH, ultrasonic cleaned in EDTA solution (0.15%) for 10 min, washed again with Milli-Q water, and dried with a stream of Ar. The Ti substrate was stored in PBS buffer (pH = 7.4) before XPS and biological study. The sonication in EDTA solution (0.15%) is important to remove the adsorbed copper residue from the surface, and the sample was stored in PBS buffer without the exposure to light in order to suppress the degradation of OEG component.31,32
Preparation of Self-Assembled Monolayers on Au(111) Substrate
Gold substrates were cut, washed several times with methylene chloride and ethanol, and exposed to hydrogen plasma (Harricks plasma cleaner, RF power 7.2W) for 15 s. Freshly cleaned gold substrate was incubated in an ethanolic solution of CF3-C13-SH (1 mM) for 48 h to ensure that a well-defined monolayer was developed on the surface. The gold substrates were removed from the ethanolic solution, washed with ethanol, and dried under a stream of N2.
X-ray Photoelectron Spectroscopy (XPS)
XPS data was obtained by a PHI 5700 X-ray photoelectron spectrometer equipped with a monochromatic Al Kα X-ray source (hν = 1486.7 eV) incident at 90° relative to the axis of a hemispherical energy analyzer. XPS data was obtained at a photoelectron take-off angle of 45° from the surface, and an analyzer spot diameter of 1.1 mm. The survey spectra were collected from 0 to 1400 eV, and the high-resolution spectra were obtained for photoelectrons emitted from C 1s, O 1s, Ti 2p, N 1s, Ge 3d, and Ge 2p3/2. All spectra were collected at room temperature with a base pressure of less than 1 × 10−8 Torr. Electron binding energies were calibrated with respect to the C 1s line at 284.8 eV for C–C bonds. The free software XPSpeak41, developed by Dr. Raymond Kwok (The Chinese University of Hong Kong), was used for all data processing. The high-resolution data were analyzed first by background subtraction using the Shirley routine and a subsequent nonlinear fitting by mixed Gaussian–Lorentzian functions. The sensitivity-corrected peak areas were used to estimate the atomic ratios.
For the characterization of film thickness, we carefully measured the XPS spectra of a pristine Ti sample and a sample with grafted organic layer. The two Ti samples (bare and grafted) were placed side by side on a single sample holder to ensure identical alignment. The high-resolution spectra of Ti 2p signal for these two Ti substrates were collected at 45° take-off angle.
Ellipsometry
The ellipsometric thicknesses were measured with a Rudolph Research (Auto EL III) ellipsometer, operating at 632.8 nm (He–Ne laser) and an angle of incidence of 70°. First, the average optical constants of the Ti substrate were determined with three pieces of freshly etched TiO2/Ti substrates (n = 2.313 and k = 3.074). The thickness was then determined with an assumed refractive index of 1.45 for the grafted organic layer. The reported values are the average of at least three measurements taken on different samples.
Contact Angle Measurements
Contact angles of water were measured at room temperature and ambient relative humidity using a Ramé-Hart model 100 contact angle goniometer. The data were collected and averaged over at least three measurements on different sample replicates.
Surface Roughness Measurements
Surface roughness (Ra) was determined by atomic force microscopy (AFM) (MultiMode Nanoscope IIIa AFM Digital Instruments Inc., Santa Barbara, CA). These analyses were performed in the tapping mode with a scan rate of 1.37 Hz.
Protein-Resistance Measurements
The Ti substrates were immersed in a solution of 1 mg fibrinogen in 1 mL of 0.01 M phosphate buffered saline (PBS) at 20–25 °C for 1 h with slight shaking and then rinsed with Millipore water for 30 s to remove the weakly adsorbed protein and salts, followed by drying with a stream of N2. The sample obtained was immediately measured by XPS, and the increase of the N 1s signal intensity was normalized with pristine Ti substrate.
Cell Culture
AsPC-1 cells (human pancreatic tumor cell line) transformed with a GFP plasmid were kindly provided by Dr. Changyi Chen (Molecular Surgeon Research Center, Division of Vascular Surgery and Endovascular Therapy, Michael E. Debakey Department of Surgery, Baylor College of Medicine, Houston, TX). The cells were maintained in Dulbecco’s modified Eagle;s medium (DMEM) with 10% fetal bovine serum (FBS) and were incubated at 37 °C in a humidified atmosphere of 5% CO2. The adhered cells were detached by trypsinization and resuspended in fresh culture medium for subsequent experiments described below.
Cell Attachment
Cell attachment on the various titanium substrates was evaluated by counting the number of attached cells 12 h after cell seeding. The substrates were placed into a 24-microwell plate (Nalge, Nunc International) and seeded with AsPC-1 cells at a density of about 10 000 cells/cm2. DMEM with 1% FBS was used as the culture medium. At the time of cell counting, loosely attached cells were washed away by rinsing twice with PBS. Adherent cells were then detached by trypsinization and counted using a hemocytometer. The number of the attached cells is reported as number of cells/cm2.
To evaluate the cell spreading on the substrates, the samples were incubated with the cells for 16 h and visualized using a Nikon H600L microscope with a FITC filter at 200× magnification.
3. RESULTS AND DISCUSSION
Photochemical Grafting of TMG-C10 on Ti substrate
The technique to generate a TMG-C10 derived organic layer on the native oxide layer of Ti substrates is straightforward, in conditions close to the previous described photochemical grafting on hydrogen or hydroxyl-terminated silicon substrates.27,33 To minimize the possible photochemical oxidation, this process was conducted in a N2 glovebox. Successful modification of the Ti substrate was confirmed by analyzing the chemical composition, thickness, and wettability of the TMG-C10 grafted surfaces.
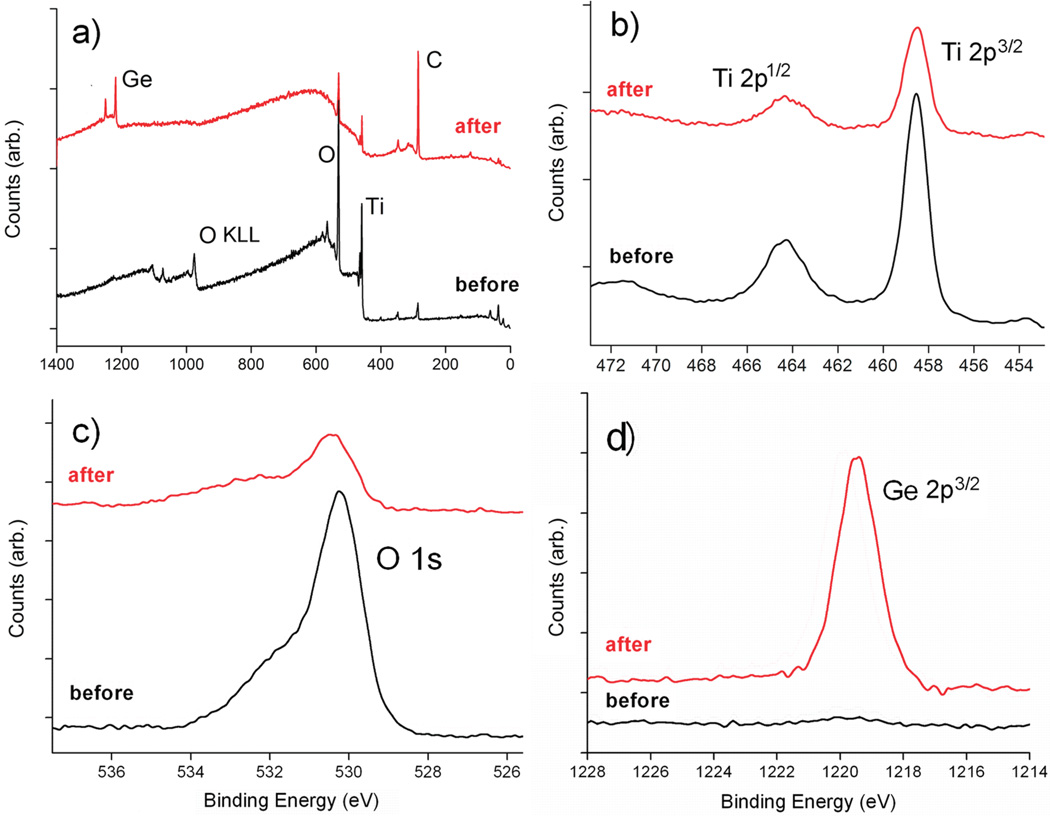
The chemical composition of the surfaces was characterized by XPS at each stages of surface functionalization (Table 1). Only Ti and O elements were present as the predominant components on the freshly cleaned Ti substrate. Adsorption of adventitious oxygen and water on the surface of TiO2 might be the cause of the discrepancy between the measured (2.3:1) and expected (2:1) O/Ti ratios. A small amount of carbon contamination was also present, and it was used as an internal reference at 284.8 eV for the XPS. Figures 1a shows XPS survey spectra of Ti substrate before (bottom) and after (top) 4 h of UV grafting of TMG-C10 to the surface. A notable increase of the C contents was observed after grafting TMG-C10 on the Ti substrate. Analysis of the high-resolution spectra of Ti 2p (Figure 1b) indicates that Ti element on the surface was mainly in Ti4+–O form, showing two major spin–orbit components unique to TiO2 at about 458.2 and 464.2 eV, respectively.22–24 In Figure 1, the main contribution of O 1s (binding energy 530.1 eV) originates from the oxygen in the TiO2 substrate.34 The shoulder peak near 531.5 eV is similar to those reported previously from physisorbed water, oxidized carbon species, and partially hydroxylated TiO2.35 However, the resolution of the O 1s peak does not allow us to distinguish between all these contributions, and therefore, no effort for further deconvolution of the O 1s signal was made. Both the Ti 2p and O 1s spectra show no significant changes upon grafting of TMG-C10 except for the attenuation of the photoelectrons by the organic layer. The presence of the TMG groups on the film was further confirmed by the unique Ge 2p3/2 peak at about 1219.5 eV.36 The atomic ratio of C/Ge of the freshly prepared surface A was calculated to be about 16 ± 2, which was slightly higher than the theoretical ratio (15). The excess of carbon content in the resulting organic layer is likely to be contributed by the initial carbon contamination before the photochemical grafting.
Table 1.
Average Concentrations (atom %) of Elements Present on Surface of Titanium Samples as Determined by XPSa
| Ti | O | C | N | Ge | F | |
|---|---|---|---|---|---|---|
| pristine Tib | 26.33 | 59.53 | 14.09 | 0.05 | 0.00 | 0.00 |
| surface A | 11.33 | 31.71 | 53.68 | 0.0 | 3.28 | 0.00 |
| surface B | 5.37 | 25.31 | 63.56 | 3.19 | 0.12 | 2.45 |
| surface C | 6.64 | 30.46 | 60.69 | 2.12 | 0.09 | 0.00 |
| surface D1 | 5.28 | 24.05 | 66.88 | 3.24 | 0.05 | 0.00 |
| surface D2 | 4.82 | 22.25 | 68.92 | 3.97 | 0.04 | 0.00 |
After correction by the corresponding elemental sensitivity factors and expressed in percent.
Depending on samples, traces of Si and Ca elements may be present on the surface.
Figure 1.
XPS data for pristine Ti (black) and the TMG-C10 grafted Ti substrate (red): (a) survey scan, (b) Ti 2p spectrum, (c) O 1s spectrum, and (d) Ge 2p3/2 spectrum.
Although the grafting of TMG-C10 on TiO2 has not been reported before, it probably occurs in a similar manner as the other reported alkenes, which was proposed through the addition of a surface OH group to a C═C bond to form a C–O bond.22–24 A set of control experiments was performed to test whether it was necessary to protect the alkyne by TMG group during the photochemical grafting (see Supporting Information, Figure S1). The results showed that if the vinyl group in TMG-C10 was replaced by an ethynyl group, the molecule could also attach on the TiO2 substrate under identical irradiation conditions. Whereas if both of the alkyne termini were protected by the TMG groups, no appreciable increase of C 1s signal was detected on the substrate under identical experimental condition. Thus, it can be concluded that the TMG group, which offers a strong protection for the alkyne functionality, is critical to achieve the desired selectivity during the photochemical grafting process.
Thickness of the TMG-C10 Derived Organic Layer
Ellipsometry thicknesses of 1.3 and 1.6 nm were obtained for irradiation times of 2.5 and 4 h, respectively. The results are less than the length of one TMG-C10 molecule (1.7 nm, as estimated with MM2, Chem3D), indicating that the layer formed is still a monolayer. After 6 h of modification, the layer thickness increased to 2.4 nm, which indicates the formation of more than a monolayer. However, since single wavelength ellipsometry gives only semiquantitative values,37,38 the thickness of the growing organic layer at intermediate irradiation times were also calculated from XPS data using
| (1) |
In eq 1, Ti2pgrafted is the absolute integrated area of the Ti 2p peak, Ti2pclean is the absolute Ti 2p peak area of a unmodified, clean Ti substrate, L is the thickness of the grafted organic layer, λTiC is the attenuation length of Ti 2p electrons in the hydrocarbon layer, and θ is the electron take-off angle (45°).
Previous studies of self-assembled monolayers on various substrates found that the attenuation lengths (λ) of the photoelectrons from the underlying substrate through the hydrocarbon layers could be estimated by the following empirical equation, if monochromatic Al Kα is used as the X-ray source.38
| (2) |
In eq 2, E is the binding energy of the considered substrate element, yielding λTiC as 3.2 nm for Ti 2p photoelectron with bonding energy of 458 eV. Using the equations described above, the thickness of the organic layer was calculated to be 1.1, 1.4, and 2.0 nm for irradiation times of 2.5, 4 and 6 h, respectively. Within the experimental uncertainties, these calculated values agree with the measured ellipsometry thickness. Thus, uncontrolled overgrowth of the films was observed under prolonged radiation with the 254 nm UV light, probably due to the presence of radical intermediates generated in the film. Similar results were reported for the photografting of alkenes on other hydroxyl-terminated substrates.33,39
Stability of the Photochemically Grafted Organic Layer
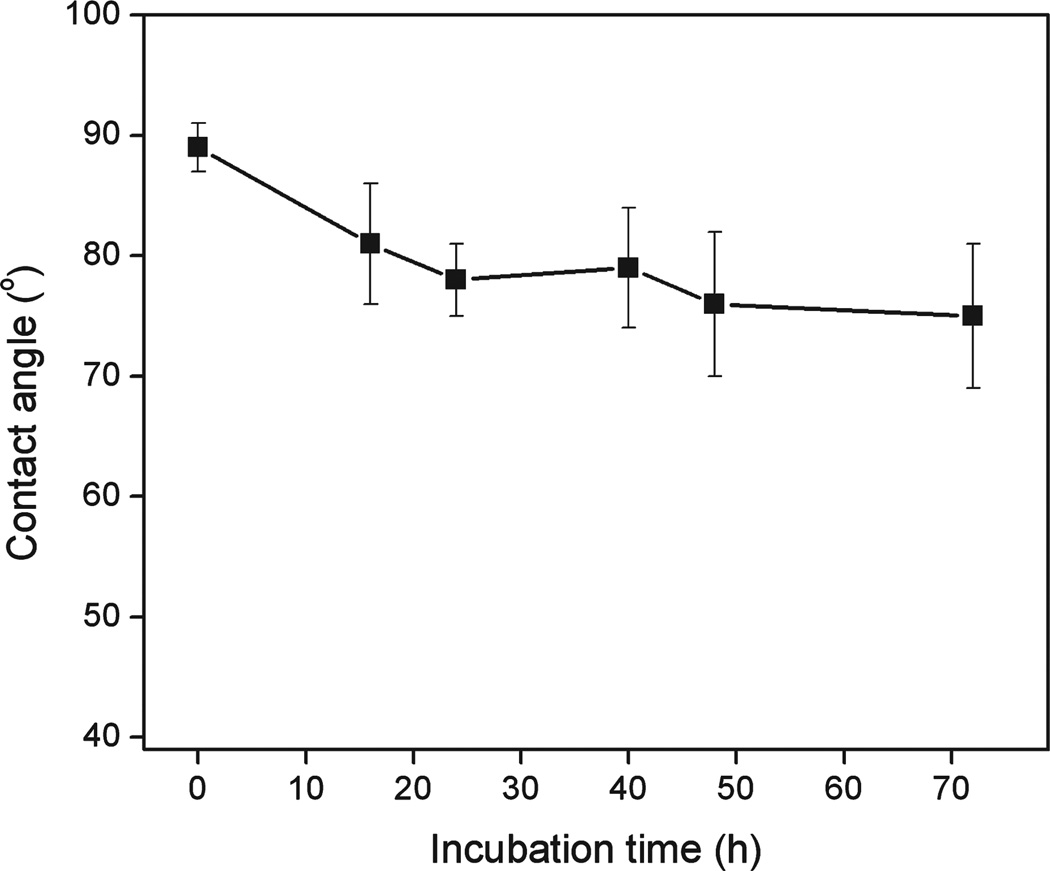
The thermal stability of TMG-C10 modified Ti substrate (surface A) in aqueous solution was characterized. The sample that was functionalized with TMG-C10 by 4 h of illumination was immersed in deionized water at 70 °C, and at various intervals of time the sample was removed for contact angle analysis. The freshly prepared surface A was hydrophobic, exhibiting water contact angle around 89°. As shown in Figure 2, despite the initial drop of the value in the first 16 h, the sample retained water contact angle higher than 75° even after 3 days incubation, which is contrast to the slight hydrophilic surface of unmodified Ti substrate (θ < 40°). Additionally, based on the C/Ti ratio in the XPS spectra, there was no significant change (<5%) for the chemical composition of the samples upon storage in dark under ambient conditions for 2 weeks.
Figure 2.
Water contact angle measured on TMG-C10 modified Ti surface exposed to deionized water at 70 °C.
Modification of the TMG-Terminated Ti Substrate via Click Chemistry
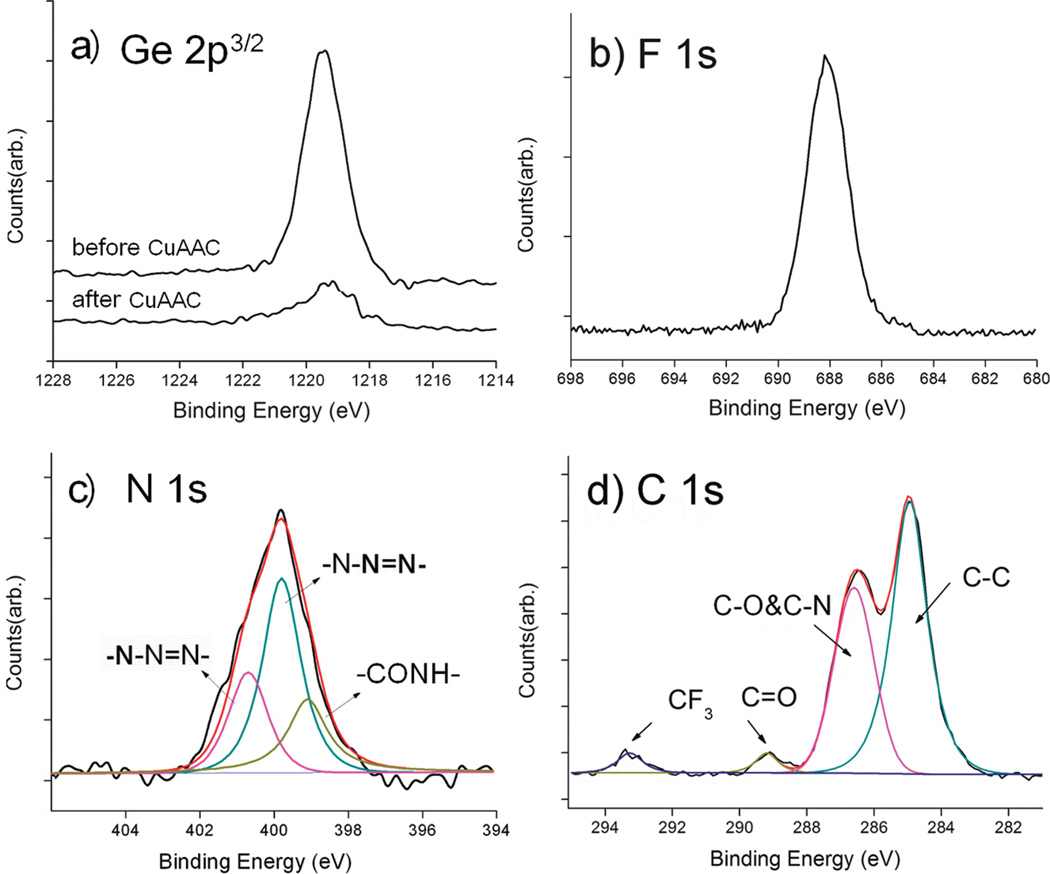
The good stability of TMG-C10 derived thin films on Ti substrates makes it amendable to further functionalization. The in situ deprotection/click reaction between the TMG-masked alkyned surface and the azides was carried out in MeOH/H2O (1:1), in the presence of Cu(MeCN)4PF6, the monotriazole Cu(I)-ligand 2, and ascorbic acid (Scheme 1), similar to our previous reported conditions for click immobilization on other substrates.26,27,29 The CF3-terminated azide 1a was grafted first to facilitate XPS characterization and yield the surface B. As shown in Figure 3a, significant reduction of the Ge 2p3/2 signal intensity (~90%) was observed after 4 h of incubation in the reaction mixture containing Cu(I), indicating the successful deprotection of TMG. In the meantime, the F 1s and N 1s signals appeared at 688 eV (Figure 3b) and 401 eV (Figure 3c), as expected after the conversion of TMG-alkynes to the CF3-terminated triazole. The narrow N 1s scans in Figure 3c showed a broad signal centered at 401 eV that could be fit to three peaks assigned to CONH (400.1 eV), N—N═N (400.8 eV), and N—N═N (401.7 eV). The assignment of the N 1s signals from the triazole ring is supported by the reported XPS data and density functional theory calculation for some aromatic compounds containing sp2 N atoms bonded to two or three atoms.40 The ratio of the peak areas was about 1:2:1, consistent with the expected structure of the surface B. The absence of the azido N 1s signal at ~405 eV indicated the absence of physically adsorbed 1a on the surface.25–27 The C 1s signal could be deconvoluted into four peaks and assigned to C–C (284.8 eV), C–O and C–N (286.5 eV), C═O (290.0 eV), and CF3 (293.2 eV) in Figure 3d. In particular, the broad C 1s peak at 286.5 eV was attributed to the overlapping signals of C–O (from OEG) and C–N (from triazole) in the organic layer, and the signal at 293.2 eV characteristic for fluorine-bonded carbons (C–F) further supports the successful immobilization of the CF3-tagged OEG-azide 1a.
Figure 3.
Narrow scans of Ge 2p3/2 (a), F 1s (b), N 1s (c), and C 1s (d) regions of the XPS spectra of surface B prepared by attaching the azide component 1a on the TMG-alkynyl-presenting Ti substrate via CuAAC reaction.
The density of the CF3-tagged OEG surface B on the Ti substrate was estimated by XPS using a well-defined CF3-terminated self-assembled monolayer (SAM) on Au(111) as a reference (see Figure S2 in the Supporting Information). The obtained value (2.27 molecules/nm2) was similar to the reported density (3.31 molecules/nm2) of the densely packed CF3-OEG-terminated SAM on Au(111) surface,41 indicating high functional density was introduced via this one-pot deprotection/click coupling reaction.
Efficient click immobilization on the TMG-alkynyl-presenting surface A was also demonstrated using other OEG-azide derivatives (Scheme 1). The immobilization of the OEG-azide 1b on surface A via click reaction rendered the surface hydrophilic, reducing the water contact angle from 89° ± 2° to 51° ± 3°, which is in accordance with other substrates presenting click immobilized OH-terminated OEG chains.26,27 Assuming the surface density of the component 1b was similar to its analogue 1a (2.27 molecules/nm2), the ethylene glycol (EG) surface density on surface C was estimated to 11 EG/nm2, which is expected to show remarkable reduction for the nonspecific protein adsorption on TiO2 surface.42
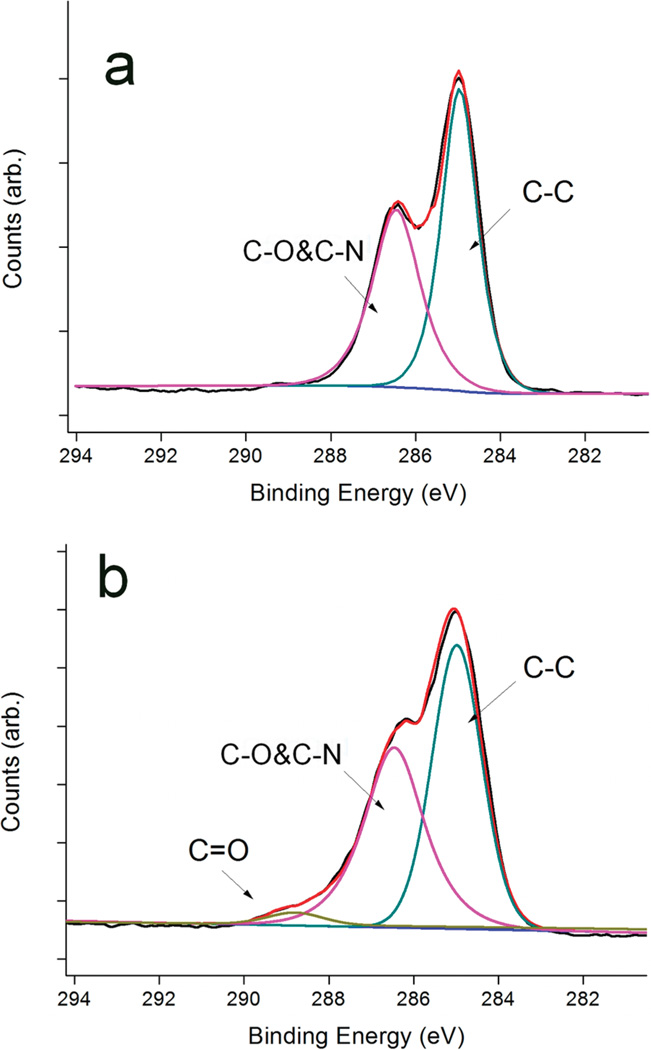
Since the CuAAC coupling is highly specific, and generally not affected by the substituents,28 multiple functional moieties can be attached onto the substrate in a controllable manner via the one-pot reaction. As an example, we coimmobilized the OEG-azide 1b with the azido-OEG-tagged peptide GRGD (1c) at a 1b/1c ratio of 19:1 onto the alkynyl-TMG surface A to provide the surface D1 presenting GRGD on an inert OEG background. XPS was performed on surface C and surface D1. As shown in Figure 4, deconvolution of the recorded C1s narrow scan indicates that photoelectrons emitted from oxygen-bonded carbons (C–O) at 286.5 eV are present on both substrates. However, the contribution centered at 290.0 eV with a large dispersion value (1.52 eV fwhm) was only observed on surface D1. This peak was designated to carbonyl carbon (C═O) that was contributed by the amide-carbonyl and ester-carbonyl moieties in the peptide, which confirms that GRGD was covalently attached on the Ti surface. With a higher ratio of 1c/1b used in the reactants, the ratio of N/C in XPS was also increased for the resulting film (surface D2 in Table 1), suggesting that more peptides was immobilized on the substrate. It is also worth noting that we assume the relative mole fractions of 1b and 1c on the surface and in solution to be the same. This assumption may be subjected to large error due to the larger size and higher polarity of GRGD-azide 1c compared to OEG-azide 1b, but substantial higher peptide density on the surface D2 than D1 is expected.
Figure 4.
High-resolution C 1s spectra of (a) surface C and (b) surface D1, showing peak fits for C–C at 284.8 eV, C–O and C–N at 286.5 eV, and C═O at 290.0 eV.
After the heterogeneous click reaction, the harmful Cu(I) residue adsorbed on the surface was effectively removed by sonication in 0.15% EDTA solution, as evidenced by the significant drop of the Cu 2p signal intensity (see Figure S3 in the Supporting Information). The bound OEG components remained intact on the substrate after the sonication cleaning as indicated by the negligible decrease of N 1s signal intensity (see Figure S4 in the Supporting Information).
Resistant to the Nonspecific Adsorption of Proteins
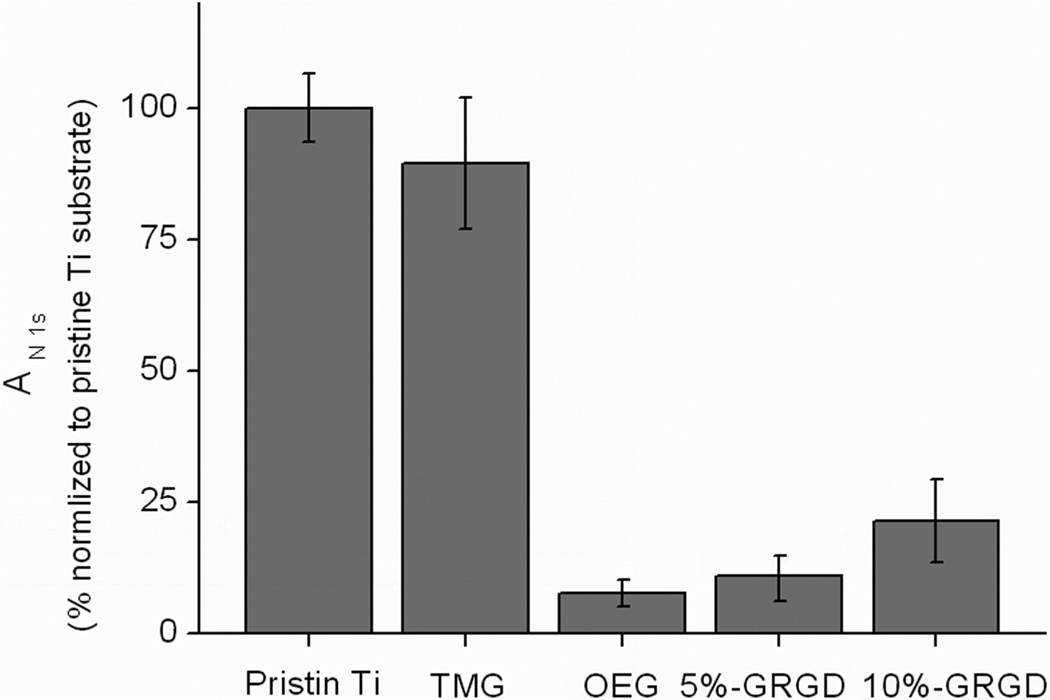
One of the major prerequisites for ideal biomaterial interface is the absence of nonspecific binding of proteins, thus permitting the specific binding of biological targets to the interface presenting capturingmolecules (ligands).43 We used a standard protocol26,31 to evaluate the protein resistance of the OEG-modified Ti substrates, and found that the adsorption of fibrinogen was significantly suppressed. As shown in Figure 5, upon click immobilization of 1b onto the TMG-alkynyl-presenting surface A to form the OEG-presenting surface C, although total protein resistant was not achieved, adsorption of fibrinogen was significantly decreased by 92%. Remarkably, codeposition of 5% GRGD-azide 1c with OEG-azide 1b still preserved a satisfactory antifouling property (11% compared to pristine Ti). However, further increasing the ratio of 1c/1d to 1:9 used for the click immobilization, the protein resistant property was deteriorated and the amount of fibrinogen adsorbed on the resulting organic film was almost tripled compared to the surface presenting only OH-terminated OEG component.
Figure 5.
Evaluation of the protein resistant property on various Ti substrates after incubation in fibrinogen solution (PBS buffer, 1 mg/mL) for 1 h (measured by the increase of N 1s intensity and normalized with pristine Ti substrate). Data represent the mean with standard deviation of measurements performed on three replicated samples.
Cells Adhesion and Spreading on the Modified Ti Substrates
After validating the protein resistant property of modified Ti substrates, we next characterized the cell adhesion mediated by the specific binding of the GRGD ligand presenting on a bioinert OEG background. This binding is known to trigger the clustering of integrin receptors, leading to focal adhesion that is characteristic for cell spreading on surfaces.44,45
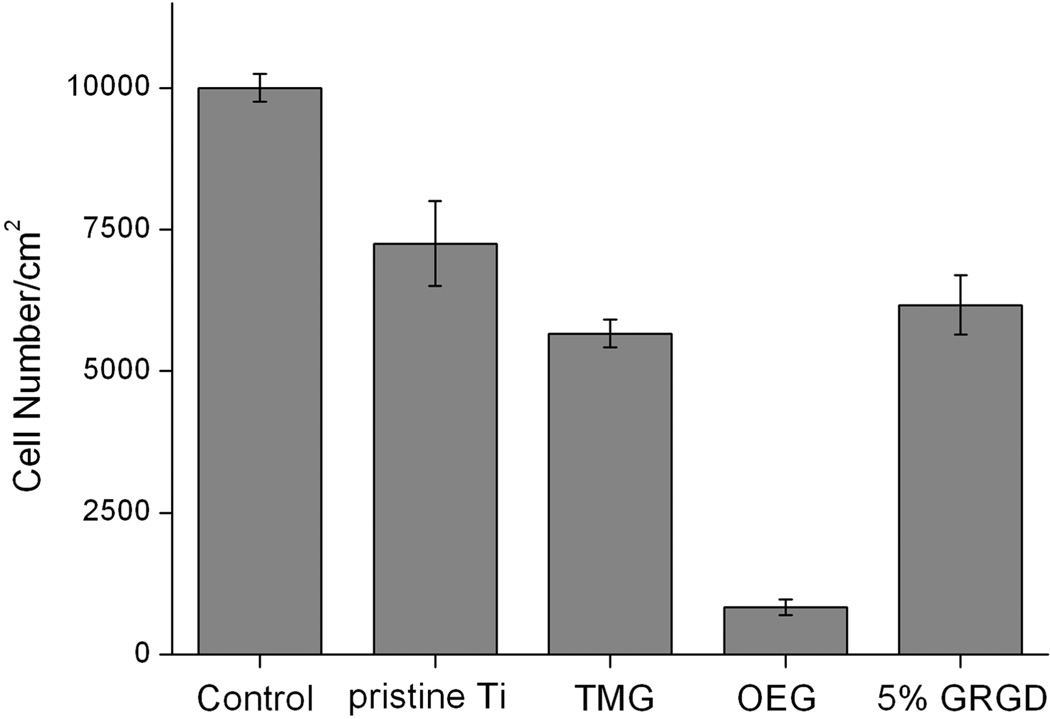
After allowing various Ti substrates to incubate with cells in the culture media for a specific time, adherent cells were then detached by trypsinization and counted using a hemocytometer. From the result shown in Figure 6, the cell density on the pristine Ti substrate was 75% of the density (10 000/cm2) on the polystyrene cell culture well as a control surface. After photografting TMG-C10 onto the Ti substrate, the surface density of the cell was slightly decreased by 10% to around 6000/cm2. In contrast, there are very few cells attached to the neat OEG coated substrate, which is consistent with the result of protein resistant test and previous studies.46–49 After 5% GRGD-OEG-N3 was codeposited with OEG-N3, the number of adherent cells was increased by 7-fold. This result is in agreement with other reported studies where bioinert substrates presenting 0.1 mol % growth factor were sufficient to promote cell attachment.48,49
Figure 6.
Number of adherent AsPC-1 cells/cm2 on surfaces of control (the bottom of the culture well), pristine Ti, TMG (surface A), OEG (surface C), and Ti presenting 5% GRGD (surface D1) (n = 3).
As for bare Ti substrate, nonspecifically adhered proteins (e.g., vitronectin and fibronectin) from the culture medium will foul the surfaces and mediate cell adhesion.50 However, we have shown that the modification of the Ti substrate with 5% GRGD-OEG-N3 in OEG-N3 decreased the nonspecific adsorption of fibrinogen by ~90% (Figure 5). Therefore, the cell attachment on this GRGD/OEG modified surface must be due to the specific interactions of the cells with the GRGD peptide.48,49
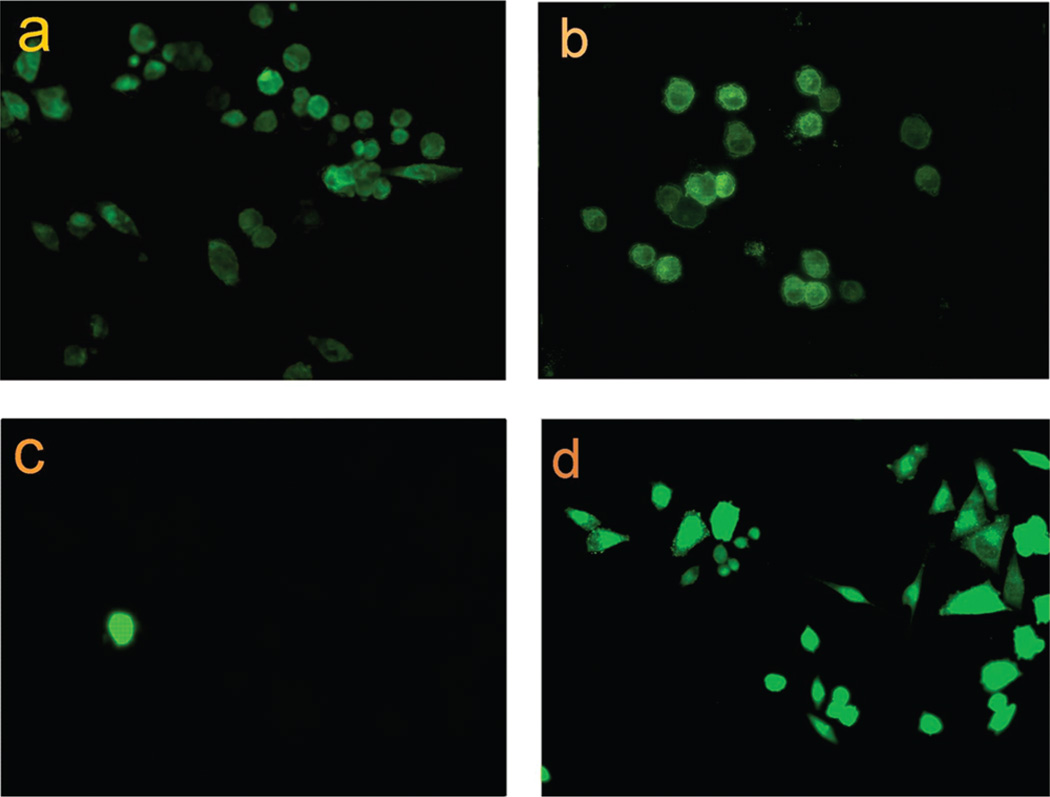
The ability of the immobilized GRGD on the substrate to stimulate appropriate cellular responses is also of crucial importance. Figure 7 shows the fluorescent images of the cells attached on various kinds of Ti substrates after 16 h of incubation. The majority (~80%) of the cells attached on the unmodified Ti substrate were spherical in shape, and only a few displayed the spindle shape indicative of the presence of focal adhesion. On the other hand, cells attached on the TMG-terminated substrate did not spread and the majority of them remained isolated, suggesting the poor biocompatibility of the precursor hydrophobic surface. In contrast, many well-spread cells were easily found on the surface D1 prepared by coimmobilization of 5% GRGD-OEG-N3 with OEG-N3 (Figure 7d). Therefore, the immobilized GRGD peptide promoted the focal adhesion as expected.
Figure 7.
Microscopy images (200× magnification) of adhered cells on pristine Ti (a), surface A (b), surface C (c), and surface D1 (d). All of the substrate slides were seeded with AsPC-1 cells at a density of about 10 000 cells/cm2 and incubated in DMEM with 1% FBS for 16 h.
It is important to note that the surface roughness of the substrates may also have a direct effect on the adhesion and spreading of the cells.51,52 As characterized by atomic force microscopy (AFM), the surface roughness (Ra) of surface A and surface D1 was 4.9 ± 0.6 and 5.6 ± 0.6 nm, respectively. Thus, there is no significant change for roughness of the substrates after click immobilization. However, remarkable changes in the cell behavior were observed. Therefore, the enhanced adhesion and spreading of the cells on the OEG films incorporating GRGD can be attributed by to the presence of the GRGD peptide.
4. CONCLUSION
In summary, we have explored a stepwise strategy to modify Ti substrates in order to resist the nonspecific adsorption of proteins and to promote the specific interactions of cells with ligands presented on an inert OEG background. The TMG-protected alkyne was compatible with the photochemical grafting, allowing the formation of a thin film platform presenting the TMG-alkynyl groups. Azido-tagged OEG and biomolecules could be readily attached to this “clickable” platform via a one-pot deprotection/click reaction. According to the initial biological test, the cell adhesion GRGD peptide retained its activity upon the immobilization and promoted the adhesion and spreading of AsPC-1 cells on the substrates. In addition, we have shown that the “clickable” platforms were quite stable in aqueous solution at 70 °C for days, and we expect that the immobilized OEG and biomolecules will display a higher stability against hydrolytic cleavage at room temperature.
We further anticipate that the “clickable” thin film platform described in this Article can be used to modify TiO2/Ti based materials potentially for other applications, including photovoltaics, nanoelectronics, and energy storage devices.
Supplementary Material
ACKNOWLEDGMENT
We thank the Alliance for NanoHealth (W81XWH-09-2-0139), NSF (DMR-0706627), NIH (R21HD058985 and R21EY018303), The Welch Foundation (E-1498), and the Texas Center for Superconductivity at the University of Houston for financial support, and Dr. Changyi Chen for providing the AsPC-1 cells. We would also like to thank Prof. T. R. Lee for the generous gift of 14,14,14-trifuorotetradecanethiol and gold wafers.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Additional XPS data analysis, including control experiments for the photografting on TiO2; density estimation for the click immobilized OEG component; and the verification for the removal of the copper residue after click coupling. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Brunette DM, Tengvall P, Textor M, Thomsen P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses, and Medical Applications. Berlin: Springer; 2001. [Google Scholar]
- 2.Pan J, Thierry D, Leygraf C. J. Biomed. Mater. Res. 1994;28:113–122. doi: 10.1002/jbm.820280115. [DOI] [PubMed] [Google Scholar]
- 3.Kokubo T, Kim HM, Kawashita M. Biomaterials. 2003;24:2161. doi: 10.1016/s0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 4.Poh CK, Shi Z, Lim TY, Neoh KG, Wang W. Biomaterials. 2010;31:1578–1585. doi: 10.1016/j.biomaterials.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Raynor JE, Petrie TA, García AJ, Collard DM. Adv. Mater. 2007;19:1724–1728. [Google Scholar]
- 6.Fan X, Lin L, Dalsin JL, Messersmith PB. J. Am. Chem. Soc. 2005;127:15843–15847. doi: 10.1021/ja0532638. [DOI] [PubMed] [Google Scholar]
- 7.Griep-Raming N, Karger M, Menzel H. Langmuir. 2004;20:11811–11814. doi: 10.1021/la0485327. [DOI] [PubMed] [Google Scholar]
- 8.Xiao SJ, Textor M, Spencer ND. Langmuir. 1998;14:5507–5516. [Google Scholar]
- 9.Viornery C, Chevolot Y, Léonard D, Aronsson BO, Péchy P, Mathieu HJ, Descouts P, Grätzel M. Langmuir. 2002;18:2582–2589. [Google Scholar]
- 10.Born R, Scharnweber D, Rössler S, Stölzel M, Thieme M, Wolf C, Worch H. J. Anal. Chem. 1998;361:697–700. [Google Scholar]
- 11.Giacomelli CE, Esplandiu MJ, Ortiz PI, Avena MJ, Pauli CP. J. Colloid Interface Sci. 1999;218:404–411. doi: 10.1006/jcis.1999.6434. [DOI] [PubMed] [Google Scholar]
- 12.Ratner BD, Bryant SJ. Annu. Rev. Biomed. Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 13.Turner RFB, Harrison DJ, Rojotte RV. Biomaterials. 1991;12:361–368. doi: 10.1016/0142-9612(91)90003-s. [DOI] [PubMed] [Google Scholar]
- 14.Harris JM, Zalipsky S. Poly (ethylene glycol): Chemistry and Biological Applications. Washington, DC: American Chemical Society; 1997. p. 489. [Google Scholar]
- 15.Palegrosdemange C, Simon ES, Prime KL, Whitesides GM. J. Am. Chem. Soc. 1991;113:12–20. [Google Scholar]
- 16.Porté-Durrieu MC, Guillemot F, Pallu S, Labrugère C, Brouillaud B, Bareille R, Amédée J, Barthe N, Dard M, Baquey C. Biomaterials. 2004;25:4837–4846. doi: 10.1016/j.biomaterials.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Bozzini S, Petrini P, Tanzi MC, Zrcher S, Tosatti S. Langmuir. 2010;26:6529–6534. doi: 10.1021/la904066y. [DOI] [PubMed] [Google Scholar]
- 18.Silverman BM, Wieghaus KA, Schwartz J. Langmuir. 2004;21:225–228. doi: 10.1021/la048227l. [DOI] [PubMed] [Google Scholar]
- 19.Adden N, Gamble LJ, Castner DG, Hoffmann A, Gross G, Menzel H. Langmuir. 2006;22:8197–8204. doi: 10.1021/la060754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adden N, Gamble LJ, Castner DG, Hoffman A, Gross G, Menzel H. Biomacromolecules. 2006;7:2552–2559. doi: 10.1021/bm060473j. [DOI] [PubMed] [Google Scholar]
- 21.Marcinko S, Fadeev AY. Langmuir. 2004;20:2270–2273. doi: 10.1021/la034914l. [DOI] [PubMed] [Google Scholar]
- 22.Franking RA, Landis EC, Hamers RJ. Langmuir. 2009;25:10676–10684. doi: 10.1021/la901116c. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Franking R, Landis EC, Kim H, Hamers RJ. ACS Appl. Mater. Interfaces. 2009;1:1013–1022. doi: 10.1021/am900001h. [DOI] [PubMed] [Google Scholar]
- 24.Hamers RJ, Chambers SA, Evans PE, Franking R, Gerbec Z, Gopalan P, Kim H, Landis EC, Li B, McCoy MW, Ohsawa T, Ruther R. Phys. Status Solidi C. 2010;7:200–205. [Google Scholar]
- 25.During the preparation of this manuscript, a “clickable” platform on Ti substrate was reported, which was prepared from azide-terminated catechol anchor Watson MA, Lyskawa J, Zobrist C, Fournier D, Jimenez M, Traisnel M, Gengembre L, Woisel P. Langmuir. 2010;26:15920–15924. doi: 10.1021/la102688m.
- 26.Qin G, Santos C, Zhang W, Li Y, Kumar A, Erasquin UJ, Liu K, Muradov P, Trautner BW, Cai C. J. Am. Chem. Soc. 2010;132:16432–16441. doi: 10.1021/ja1025497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Wang J, Cai C. Langmuir. 2011;27:2437–2445. doi: 10.1021/la104060j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Erasquin UJ, Qin G, Li K, Cai C. Chem. Commun. 2010;46:5746–5748. doi: 10.1039/c0cc00784f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos CM, Kumar A, Zhang W, Cai C. Chem. Commun. 2009:2854–2856. doi: 10.1039/b821148e. [DOI] [PubMed] [Google Scholar]
- 30.Graupe M, Koini T, Wang VY, Nassif GM, Colorado R, Jr, Villazana RJ, Dong H, Miura YF, Shmakova OE, Lee TR. J. Fluorine Chem. 1999;93:107–115. [Google Scholar]
- 31.Qin GT, Cai C. Chem. Commun. 2009:5112–5114. doi: 10.1039/b911155g. [DOI] [PubMed] [Google Scholar]
- 32.Zoulalian V, Zürcher S, Tosatti S, Textor M, Monge S, Robin JJ. Langmuir. 2010;26:74–82. doi: 10.1021/la902110j. [DOI] [PubMed] [Google Scholar]
- 33.ter Maat J, Regeling R, Yang M, Mullings MN, Bent SF, Zuilhof H. Langmuir. 2009;25:11592–11597. doi: 10.1021/la901551t. [DOI] [PubMed] [Google Scholar]
- 34.Spori DM, Venkataraman NV, Tosatti SGP, Durmaz F, Spencer ND, Zürcher S. Langmuir. 2007;23:8053–8060. doi: 10.1021/la700474v. [DOI] [PubMed] [Google Scholar]
- 35.Wang LQ, Baer DR, Engelhard MH, Shultz AN. Surf. Sci. 1995;344:237–250. [Google Scholar]
- 36.Barr TL, Mohsenian M, Chen LM. Appl. Surf. Sci. 1991;51:71–87. [Google Scholar]
- 37.Linford MR, Fenter P, Eisenberger PM, Chidsey CED. J. Am. Chem. Soc. 1995;117:3145–3155. [Google Scholar]
- 38.Wallart X, de Villeneuve CH, Allongue P. J. Am. Chem. Soc. 2005;127:7871–7878. doi: 10.1021/ja0430797. [DOI] [PubMed] [Google Scholar]
- 39.Rosso M, Giesbers M, Arafat A, Schroën K, Zuilhof H. Langmuir. 2009;25:2172–2180. doi: 10.1021/la803094y. [DOI] [PubMed] [Google Scholar]
- 40.Ito E, Oji H, Araki T, Oichi K, Ishii H, Ouchi Y, Ohta T, Kosugi N, Maruyama Y, Naito T, Inabe T, Seki K. J. Am. Chem. Soc. 1997;119:6336–6344. [Google Scholar]
- 41.Bonnet N, Hagan D, Hahner G. Phys. Chem. Chem. Phys. 2010;12:4367–4374. doi: 10.1039/b923065n. [DOI] [PubMed] [Google Scholar]
- 42.Dalsin JL, Lin L, Tosatti S, Vörös J, Textor M, Messersmith PB. Langmuir. 2005;21:640–646. doi: 10.1021/la048626g. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Johnson RW, Desai TA. Langmuir. 2004;20:348–356. doi: 10.1021/la034753l. [DOI] [PubMed] [Google Scholar]
- 44.Massia SP, Hubbell JA. J. Cell Biol. 1991;114:1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruoslahti E, Pierschbacher MD. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 46.Liu D, Xie Y, Shao H, Jiang X. Angew. Chem, Int. Ed. 2009;48:4406–4408. doi: 10.1002/anie.200901130. [DOI] [PubMed] [Google Scholar]
- 47.Guan B, Ciampi S, Le Saux G, Gaus K, Reece PJ, Gooding JJ. Langmuir. 2011;27:328–334. doi: 10.1021/la102599m. [DOI] [PubMed] [Google Scholar]
- 48.Roberts C, Chen CS, Mrksich M, Martichonok V, Ingber DE, Whitesides GM. J. Am. Chem. Soc. 1998;120:6548–6555. [Google Scholar]
- 49.Hudalla GA, Murphy WL. Langmuir. 2009;25:5737–5746. doi: 10.1021/la804077t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai T, Larsen M, Yamada KM. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 51.Jayaraman M, Meyer U, Buhner M, Joos U, Wiesmann HP. Biomaterials. 2004;25:625–631. doi: 10.1016/s0142-9612(03)00571-4. [DOI] [PubMed] [Google Scholar]
- 52.Spiteri CG, Pilliar RM, Kandel RA. J. Biomed. Mater. Res., Part A. 2006;78:676–683. doi: 10.1002/jbm.a.30746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.