Abstract
Hindlimb unloading of the rat causes rapid hypotrophy of the intervertebral disc (IVD) as well as reduced IVD height and glycosaminoglycan content. Here we tested the hypothesis that low-intensity mechanical vibrations (0.2 g), as a surrogate for exercise, will mitigate this degradation. Four groups of Sprague-Dawley rats (4.5 mo, n = 11/group) were hindlimb unloaded (HU) for 4 wk. In two of the HU groups, unloading was interrupted for 15 min/day by placing rats in an upright posture on a platform that was vertically oscillating at 45 or 90 Hz (HU+45, HU+90). Sham control rats stood upright on an inactive plate for 15 min/day (HU+SC). These three experimental groups were compared with HU uninterrupted by weightbearing (HU) and to normally ambulating age-matched controls. In the HU and HU+SC rats, 4 wk of unloading resulted in a 10% smaller IVD height, as well as less glycosaminoglycan in the whole IVD (7%) and nucleus pulposus (17%) and a greater collagen-to-glycosaminoglycan ratio in the whole IVD (17%). Brief daily exposure to 90 Hz mechanical oscillations mitigated this degradation; compared with HU ± SC, the IVD of HU+90 had an 8% larger height and greater glycosaminoglycan content in the whole IVD (12%) and nucleus pulposus (24%). In contrast, the 45 Hz signal failed to mitigate changes in height or glycosaminoglycan content brought with altered spinal loading, but normalized the collagen-to-glycosaminoglycan ratio to levels observed in age-matched controls. In summary, unloading caused marked phenotypic and biochemical changes in the IVD, a deterioration that was not slowed by brief weightbearing. However, low-intensity 90 Hz vibrations superimposed on weightbearing largely preserved the morphology and biochemistry of the IVD and suggest that these biomechanically based signals may help protect the IVD during long bouts of nonambulation.
Keywords: immobilization, mechanical signals, IVD morphology, IVD biochemistry, spine
the inevitable reduction in loading of the spine concomitant to immobilization, chronic bed rest, a sedentary lifestyle, or spaceflight can contribute to degradation of the intervertebral disc (IVD), serving as a potential etiologic factor in the onset of low back pain (26, 27, 36, 44). As an example of the extreme, astronauts—devoid of gravity—incur a disrupted IVD structure and nucleus pulposus (NP) herniations at a greater incidence than either the general population or an army aviator population (21, 44). While the specific etiology of back pain remains unknown, disc degeneration has been implicated as both a direct and indirect source of back pain. Stimulation of nociceptive nerves in the annulus fibrosus (AF) and anterior dura mater (9) may originate from reduced disc height, reduced water content, and/or low intradiscal pressure in moderately degenerated discs and from radial tears and rim lesions in severely degenerated discs. In the NP, degradation may initiate release of nerve-stimulating cytokines (23). In addition, degeneration of the IVD precedes herniation of the NP, which typically occurs in the more deformable posteriolateral region of the disc (48).
Although it is clear that avoiding disuse-induced disc degeneration is critical, little is known about which specific aspect of the habitual mechanical environment is critical for preserving IVD health. During daily activities, the IVD is subjected to dynamic mechanical signals of a large range of magnitudes, frequencies, and durations (43). Walking with and without loaded backpacks as well as weight training induces mechanical signals that are relatively large in magnitude but low in signal frequency (<20 Hz), and the efficacy of these signals to maintain/restore IVD morphology has been limited (19). At the other extreme, postural challenges cause a barrage of relatively small mechanical signals that arise through longer periods of time but relatively little is known about their role in preserving IVD health.
Removing mechanical loads from the spine causes the IVD to imbibe water and to swell. During reambulation, walking with or without backpack loads at 40% body wt or leg presses in the absence of weightbearing require long periods to restore the height of the IVD (19). Similarly, 45 min/day of running during negative pressure may attenuate but not fully prevent morphological alterations to the lumbar IVD (7). When using externally generated mechanical stimuli, rather than exercise per se, the frequency of the signal can be raised and there is clear evidence that the IVD has the ability to sense mechanical signals induced at frequencies that are substantially higher than those generated by aerobic or anaerobic exercise (43). For instance, the application of 10 min/day of low-intensity vibrations can preserve the morphology of the IVD and reduce the incidence of back pain exacerbated by prolonged bed rest (15), but the manner in which this protection is realized remains unknown.
While critical to understanding the interaction of mechanical signals and IVD viability, a standard nonsurgical small-animal model for altered spinal loading that may facilitate a more complete understanding of the mechanically induced changes in IVD health is yet to be established. Hindlimb unloading (HU) is commonly used to simulate the effects of weightlessness on the musculoskeletal, cardiovascular, and orthostatic systems. In the IVD, HU reduces hydrostatic pressure (12) and, similar to immobilization (27), increases the expression of the catabolic protein MMP-3 (47). In addition, HU reduces the expression of the anabolic protein aggrecan and the anti-catabolic protein TIMP-1 (47), changes that are similar to those leading to disc degeneration in humans. Other responses of the rat IVD during HU are dissimilar to clinical unloading results, likely because HU imposes a relatively static loading environment on the rodent spine rather than unloading per se. For instance, imposing HU reduces disc height (14), rather than increasing it as observed in the IVD height during bed rest. Here, using HU to alter spinal mechanical loading patterns, our aims were 1) to determine the site-specific geometric and biochemical changes of the rat IVD during the absence of dynamic compressive forces and 2) to compare the efficacy of brief ambulatory periods to two low-intensity vibration interventions in their ability to prevent IVD degradation of the rat spine during HU.
MATERIALS AND METHODS
Experimental design.
This study was reviewed and approved by the Institutional Use and Care Committee of Stony Brook University. Female Sprague-Dawley rats (18 wk of age, n = 44) were hindlimb unloaded (HU) for 4 wk according to the Morey-Holton method (30). A subset of HU rats (n = 11 per group) stood on a vertically oscillating plate in an upright position (24) in vertical plastic cylinders (height: 30.5 cm, inner diameter: 10.2 cm; Fig. 1). The signal was applied for 15 min/day at a peak acceleration of 0.2 g at either 45 Hz (HU+45) or 90 Hz (HU+90). Sham control (HU+SC) rats stood in an upright position in the cylinder without receiving vibrations. HU rats received no intervention. Baseline (BC; n = 11) and age-matched (AC; n = 11) rats of the same animal cohort were used as controls. Body mass was measured weekly. Animals had ad libitum access to standard rat chow and water.
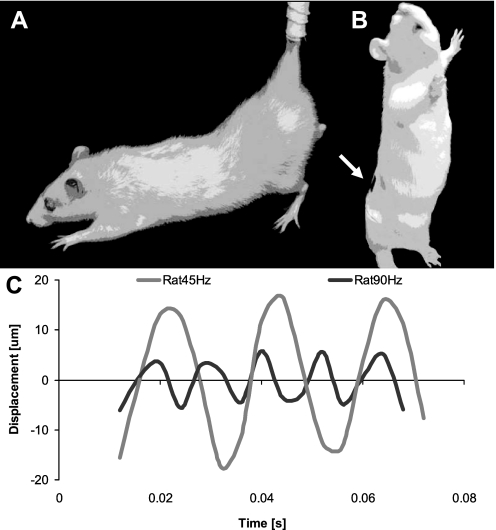
Fig. 1.
A: schematic of a hindlimb unloaded rat, demonstrating an angle of ∼30° between the torso and the ground (13). B: for 15 min/day, 3 groups of rats were released from hindlimb unloading (HU) and placed into an acrylic cylinder (height: 30.5 cm, inner diameter: 10.2 cm) in which they received a low-level, vertical vibratory signal (45 or 90 Hz) or sham loading (0 Hz). The white arrow points to the region where the motion at L5 was measured in 2 rats. C: the approximate displacement amplitude of the L5 region was 33 μm during 45 Hz vibration and 10 μm during 90 Hz vibration.
Skeletal maturity in rats is often defined by the plateauing of bone mass because the functional closing of the growth plate does not occur until 8–10 mo of age (28). In the Sprague-Dawley rat, this plateau is reached at ∼4–6 mo of age in both the femur and the spine (16). Longitudinal growth in long bones declines to very low levels at 4 mo of age (16), consistent with the observed bridging across the epiphyseal tibial growth plates that starts at 3 mo of age (28). Our rats that were 4.5 mo old at the start of the study could, therefore, be defined as “young adult rats.”
Transmission of the oscillatory signal.
Oscillatory displacements transmitted from the vibrating plate to the upright lumbar spine were estimated in two additional adult rats via computer-aided speckle interferometry (CASI) (8). Briefly, silicon carbide particles were placed on one side of two double-sided adhesive tape strips to create random speckle patterns. One tape strip was adhered to the shaved back (L5 region) of each of two rats while another tape strip was attached to the oscillating part of the vibration plate. The magnitude of the oscillatory motions of the plate and animals were measured in separate trials, and, therefore, no temporal associations between the two recordings could be made. Oscillations of speckle pattern were recorded with a Redlake Motion Scope high-speed camera (Del Imaging Systems, Cheshire, CT) at 250 fps for a total of 0.06 s while the plate was vertically oscillating at 45 and 90 Hz (0.2 g peak acceleration each). Each image frame containing a field of view of 14 mm2 was partitioned into 30 sections. Rigid body displacements between consecutive frames for each section were then mapped using CASI. The 30 displacement magnitudes, one for each partition, were averaged across the entire field of view to create a displacement profile as a function of time for the oscillatory plate as well for the L5 region. Oscillatory displacements from the L5 region at 45 and 90 Hz were determined for both rats. Transmission was calculated as the ratio of the average peak displacement of the L5 region to the average peak displacement of the plate.
Microcomputed tomography.
Before death, an in vivo two-dimensional x-ray image was taken of the spine of five anesthetized rats from each group to determine the in vivo lumbar length from superior L1 to superior S1 (“scout view” of VivaCT 75; Scanco, Brüttisellen, Switzerland). As the 3-D in vivo tomographies did not offer sufficient contrast at high resolutions, the spine was extracted to perform contrast-enhanced ex vivo microcomputed tomography (μCT) imaging. After death, the spine was stored en bloc at −20°C. Following storage, the lumbar spine was excised of extraneous tissue, soaked in a 40/60% solution of Hexabrix (Mallinckrodt, St. Louis, MO) and 0.15 M PBS for 1 h to enhance contrast for the IVD (32) and imaged by ex vivo μCT (MicroCT 40; Scanco) at a resolution of 20 μm (45 kV, 177 μA, 300 ms integration time). Ex vivo lumbar length from superior L1 to superior S1 of each lumbar spine was measured with the scanner's scout view. Inherently, the extraction of the spine led to the expansion of the IVDs and lumbar spine due to lost endogenous tension. While the distension masked the absolute in vivo change in disc morphology due to the experimental treatments, it occurred equally across all the groups (please see results) and therefore should not have influenced the relative comparisons in disc morphology between groups.
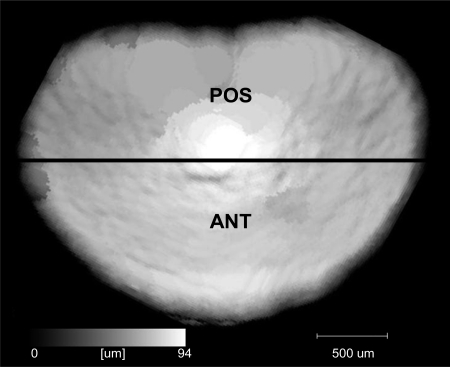
All lumbar IVD were segmented by density, and the volume, area, and height were computed. Variables from each lumbar IVD of any given spine were averaged to represent the overall lumbar IVD geometry. Anterior (ANT) and posterior (POS) disc heights were measured directly from the 3D tomographies (Fig. 2). ANT corresponded to the average IVD height of the anterior half-area of the IVD while POS corresponded to the average height of the remaining half. Average height of the entire IVD was denoted as total height. The ratio of IVD height/area represents a modified Farfan index (11), which has been proposed as a measure of disc health (34). Following scanning, all of the discs were resoaked in PBS with protease inhibitors for 1 h and then returned to −20°C.
Fig. 2.
Example of a height map of an intervertebral disc (IVD) constructed via microcomputed tomography scanning. ANT corresponded to the average IVD height of the anterior half of the IVD while POS corresponded to the average IVD height of the posterior half of the IVD.
Biochemical analysis.
Once thawed, the NP of each IVD from 5 (of 11) lumbar spines in each group was sectioned with a 1.5-mm dermal biopsy punch (Miltex, York, PA). The remaining AF was sectioned medially into the anterior (AAF) and posterior (PAF) annulus fibrosus. NP, AAF, PAF, and uncut IVD were dried at 65°C for 18 h to determine the stable dry weight (DW). The temperature was selected based on previous investigations (4), but it is possible that dehydration at a higher temperature or of an intact segment would have more accurately quantified disc constituents (3). The samples were then digested in equal volumes of papain (Sigma-Aldrich, St. Louis, MO) for 18 h at 60°C. The content of sulfated glycosaminoglycans (sGAG) was estimated by a 1,9-dimethylmethylene blue assay (Sigma-Aldrich) (4). Upon hydrolysis in HCl for 18 h at 110°C, collagen (Col) content was estimated by a hydroxyproline assay. The sGAG and Col content was expressed with DW as a referent (4). In addition, sGAG content was expressed with Col content as a referent (33). Total IVD content was obtained from unsectioned IVD as well as sectioned IVD in which total content was expressed as the sum of its parts.
Statistics.
In vivo and ex vivo lumbar length within rats was compared with a paired t-test. One-way ANOVA with Tukey post hoc tests were used to compare differences between groups. Because the baseline control group was not critical for directly testing the hypotheses, but served to quantify age-related changes in control rats, two-tailed t-tests were used to compare AC to BC rats. Longitudinal data of body mass within any given group were compared with a repeated-measure ANOVA and pairwise comparisons. All data were presented as means ± SD. Relative differences between group means were denoted as percent difference ± SD of the sampling distribution of the relative difference (relative standard error of the difference). Statistical significance for all tests was considered at <5% (SPSS 18; SPSS, Chicago, IL).
RESULTS
Animals.
All animals tolerated the hindlimb unloading protocol without any visible discomfort. The average initial body mass of each group was between 291 and 293 g (Table 1). Normally ambulating age-matched controls gained 7% (P < 0.01) body mass over the 4-wk protocol while body mass of unloaded rats did not change significantly (Table 1). As a result, body mass of the hindlimb unloaded animals was consistently less than of ambulating controls except for HU+90 at day 14 (P = 0.067). There were no significant differences in body mass between any of the HU groups at any given point in time, indicating that neither placing the rats into cylinders nor the application of vibrations raised stress hormones to a level that caused altered body mass. In addition to body mass, t-tests indicated that there were also no significant differences in any outcome variable between HU and HU+SC groups. In an effort to preserve statistical power (and avoid raising the probability of committing a type II error), these two groups were pooled and referred to as HU ± SC.
Table 1.
Body mass of the six groups of rats
| Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| BC | 292 ± 29 | — | — | — | — |
| AC | 291 ± 16 | 303 ± 21* | 303 ± 20* | 310 ± 23*† | 311 ± 23*† |
| HU | 295 ± 19 | 277 ± 25‡ | 273 ± 23‡ | 284 ± 15‡ | 283 ± 21‡ |
| HU+SC | 292 ± 15 | 279 ± 16‡ | 282 ± 16‡ | 289 ± 21‡ | 287 ± 22‡ |
| HU+45 | 292 ± 14 | 280 ± 10‡ | 282 ± 13‡ | 285 ± 11‡ | 286 ± 13‡ |
| HU+90 | 291 ± 16 | 278 ± 13‡ | 284 ± 12 | 286 ± 14‡ | 286 ± 15‡ |
Values are means ± SD in grams. BC, baseline; HU, hindlimb unloading; SC, sham control, HU+45 and HU+90, HU rats at 40 and 90 Hz signals, respectively.
Significant difference vs. day 0;
significant difference vs. day 7;
significant difference vs. age-matched controls (AC); P < 0.05.
Signal transmissibility.
After two trials, the measured frequency and amplitude of the plate when configured for the 45 Hz group was 46 ± 1 Hz with a 51.3 ± 0.2 μm displacement, and, when set for 90 Hz, the platform oscillated at 87 ± 4 Hz with a 12.5 ± 0.8 μm displacement, matching the mathematically predicted plate displacement of 48 μm at 45 Hz and 12 μm at 90 Hz. Video recordings of the L5 spinal regions, made from the computer-aided speckle interferometry, revealed displacement magnitudes of 32.8 ± 3.2 and 9.6 ± 0.6 μm at 45 and 90 Hz, respectively, suggesting a 64 and 77% transmission of the vibration signal from the platform to the appendicular skeleton. As measured by CASI, the vibration frequency at L5 (47 ± 6 Hz; 91 ± 2 Hz) was similar to the frequency of the plate (Fig. 1). The static signal (0 Hz) did not generate a measurable oscillatory displacement amplitude at the L5 region.
Extraction effect on lumbar spine length.
Comparison of the in vivo and ex vivo lumbar length for the mean of all animals showed that excision of the lumbar spine from the body increased the lumbar length by 1.8%, from 44.9 ± 1.0 to 45.7 ± 1.1 mm (P < 0.001). When stratified into groups, the percent difference between the in vivo and ex vivo lumbar length was not different between any of the groups (P ≥ 0.41).
Age-related IVD changes in normally ambulating rats.
The 4 wk age difference between age-matched and baseline control rats was associated with a 10 ± 3% greater (P = 0.013) posterior disc height, a 7 ± 1% greater (P = 0.012) area, and a 8 ± 2% (P = 0.032) smaller height-to-area ratio (Table 2). The IVD in the older AC rats also had an 81 ± 27% greater (P = 0.017) NP collagen content and 18 ± 8% greater (P = 0.048) PAF collagen content.
Table 2.
Intervertebral disc morphology of the 5 groups of rats
| Group | Height/Area, mm−1 | Area, mm2 | Volume, mm3 |
|---|---|---|---|
| BC | 0.072 ± 0.002 | 10.9 ± 0.7 | 6.4 ± 1.2 |
| AC | 0.066 ± 0.004* | 11.7 ± 0.6* | 6.9 ± 0.4 |
| HU±SC | 0.069 ± 0.005 | 10.8 ± 0.8† | 5.6 ± 0.8† |
| HU+45 | 0.067 ± 0.005 | 11.1 ± 0.7 | 5.8 ± 0.6† |
| HU+90 | 0.066 ± 0.004 | 11.4 ± 0.4 | 6.5 ± 0.6‡ |
Values are means ± SD.
AC vs. BC controls;
significant difference vs. AC;
significant difference vs. HU with/without sham loading (HU±SC); P < 0.05.
Geometric and biochemical changes of the IVD during HU.
Compared with AC, the IVD of rats subject to 4 wk of unloading (±sham loading) were 10 ± 3% shorter (P < 0.001) in total height, 10 ± 3% shorter (P = 0.001) in ANT height, 10 ± 3% shorter (P = 0.009) for POS height, 8 ± 2% less (P = 0.008) in area, and 19 ± 3% lower (P < 0.001) in IVD volume (Fig. 3, Table 2). For specific disc levels, the IVD volume of HU ± SC was 20 ± 7% lower (P = 0.024) than AC at L2-L3, 19 ± 5% (P = 0.007) lower at L3-L4, and 20 ± 4% (P = 0.001) lower at L4-L5. No significant differences were evident at either L1-L2 or L6-S1 (P ≥ 0.225; Table 3).
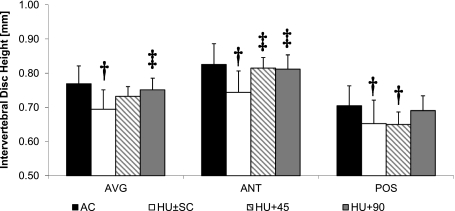
Fig. 3.
Height (mean + SD) of the entire IVD (TOTAL), as well as ANT and POS regions; age-matched (AC; n = 11), HU ± sham control (SC; n = 22), HU+45 (n = 11) and HU+90 (n = 11). †Significant difference vs. AC; ‡Significant difference vs. HU ± SC; P < 0.05.
Table 3.
Volume and Col/sGAG for individual rat IVD
| Level | BC | AC | HU±SC | HU+45 | HU+90 | |
|---|---|---|---|---|---|---|
| Volume, mm3 | L1-L2 | 4.5 ± 1.3 | 4.9 ± 1.9 | 4.0 ± 1.2 | 4.0 ± 0.7 | 4.8 ± 1.7 |
| L2-L3 | 5.1 ± 0.9 | 5.9 ± 1.2 | 4.7 ± 1.1† | 4.8 ± 0.7 | 5.4 ± 1.1 | |
| L3-L4 | 6.6 ± 1.7 | 6.4 ± 1.0 | 5.1 ± 0.6† | 5.5 ± 0.7 | 5.5 ± 0.4 | |
| L4-L5 | 6.9 ± 1.2 | 7.0 ± 1.0 | 5.6 ± 0.8† | 5.9 ± 0.9† | 6.4 ± 0.5 | |
| L5-L6 | 8.7 ± 2.2 | 7.5 ± 1.1 | 7.0 ± 0.7 | 7.2 ± 0.8 | 7.7 ± 0.5 | |
| L6-S1 | 8.0 ± 1.5 | 8.6 ± 1.8 | 7.5 ± 1.6 | 8.1 ± 1.3 | 8.5 ± 1.1 | |
| Col/sGAG, μg/μg | L1-L2 | 2.5 ± 1.0 | 2.5 ± 1.0 | 3.1 ± 1.0† | 2.3 ± 0.4‡ | 3.1 ± 0.1 |
| L2-L3 | 3.2 ± 0.7 | 3.5 ± 1.6 | 3.7 ± 1.5 | 2.8 ± 0.9 | 3.1 ± 0.6 | |
| L3-L4 | 2.7 ± 1.0 | 3.1 ± 0.9 | 3.9 ± 0.8† | 3.2 ± 1.1 | 3.2 ± 0.8 | |
| L4-L5 | 3.4 ± 0.9 | 3.1 ± 1.4 | 3.0 ± 0.5 | 4.5 ± 0.8 | 3.1 ± 1.0 | |
| L5-L6 | 2.6 ± 0.7 | 3.0 ± 0.8 | 3.6 ± 0.6 | 3.5 ± 0.6 | 4.0 ± 0.8 | |
| L6-S1 | 3.3 ± 0.7 | 4.6 ± 1.7 | 3.6 ± 1.1 | 4.3 ± 0.9 | 4.0 ± 1.0 |
Values are means ± SD. Each sulfated glycosaminoglycan (sGAG) and collagen (Col) value was normalized by dry weight. Volume: sample size for HU±SC was n = 22 and for other groups was n = 11. Col/sGAG: sample size for HU±SC was n = 20 and for other groups was n = 10.
Significant difference vs. AC.
significant difference vs. HU±SC; P < 0.05.
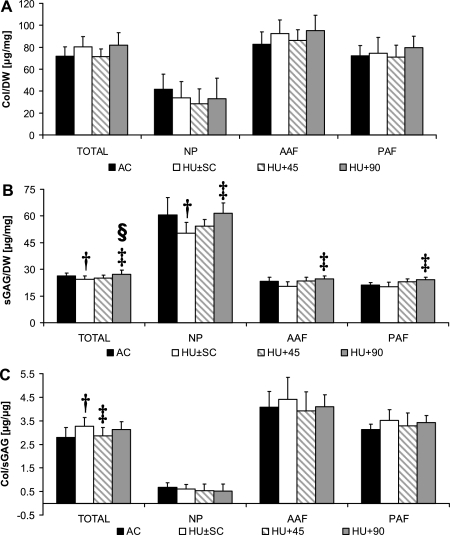
No significant differences in Col content of the IVD were observed between AC and HU ± SC (P ≥ 0.082; Fig. 4). In contrast, total sGAG content of the IVD was 7 ± 2% lower (P = 0.039) in the unloaded (±sham loading) than the AC group (Fig. 4). When stratified by region, sGAG in the NP was 17 ± 8% less (P = 0.041) in HU ± SC, but was not different between the two groups in the AAF (P = 0.143) or PAF (P = 0.206; Fig. 4). The Col-to-sGAG ratio was 17 ± 5% (P = 0.009) greater in unloaded than AC rats for the entire IVD, but no significant statistical differences were detected in specific regions (P ≥ 0.386; Fig. 4). For specific disc levels and compared with AC, the IVD Col-to-sGAG ratio of HU ± SC was significantly greater at L1-L2 (35 ± 14%, P = 0.020) and at L3-L4 (35 ± 11%, P = 0.009) but not at L2-L3, L4-L5, L5-L6, or L6-S1 (P ≥ 0.066; Table 3).
Fig. 4.
Collagen (A; Col, mean+SD), sulfated-glycosaminoglycan (B; sGAG), and (c) Col/sGAG content (C) of the whole IVD (TOTAL), nucleus pulposus (NP), anterior annulus fibrosus (AAF), and posterior annulus fibrosus (PAF) of AC (A, C: TOTAL n = 10; B: TOTAL n = 11; A–C: Region n = 5), HU ± SC (A, C: TOTAL n = 20; B: TOTAL n = 22; A–C: Region n = 10), HU+45 (A, C: TOTAL n = 10; B: TOTAL n = 11; A–C: Region n = 5), and HU+90 (A, C: TOTAL n = 10; B: TOTAL n = 11; A–C: Region n = 5). †Significant difference vs. AC; ‡significant difference vs. HU ± SC; §significant difference vs. HU+45; P < 0.05.
Efficacy of 45 vs. 90 Hz vibration.
The ability of the two mechanical interventions to attenuate unloading-induced hypotrophy of the lumbar IVD was differential. Overall, the ability of the 45 Hz signal to influence the degradation of the IVD was minimal, contrasting with the application of the 90 Hz signal that partially preserved both IVD morphology and sGAG content. The only geometric benefit to the IVD of the 45 Hz signal was reflected in the anterior height, which was 10 ± 2% greater (P = 0.006) than HU ± SC and not significantly different from AC (Fig. 3). The inability of the 45 Hz signal to preserve morphology was evident by the 9 ± 3% smaller (P = 0.009) posterior height and 16 ± 3% smaller (P = 0.008) IVD volume compared with AC (Table 3, Fig. 3). Furthermore, the individual IVD volume at L4-L5 was 16 ± 6% lower (P = 0.035) in HU+45 than in AC (Table 3). In contrast, IVD morphology from HU+90 compared with HU showed total IVD height to be 8 ± 2% greater (P = 0.022), anterior height 9 ± 2% greater (P = 0.011), and IVD volume 15 ± 4% (P = 0.029) greater (Table 2, Fig. 3). For any given morphological parameter, the IVD of HU+90 were not statistically different from those of AC (P ≥ 0.266).
Biochemically, sGAG content of the IVD subjected to the 45 Hz signal was not different from HU ± SC for any given region (P ≥ 0.078), but the total Col-to-sGAG ratio of HU+45 discs was 13 ± 4% (P = 0.034) smaller than HU ± SC and not significantly different from AC (Fig. 4). For specific disc levels and compared with HU ± SC, Col-to-sGAG ratio of HU+45 discs was significantly greater (35 ± 10%, P = 0.052) only at L1-L2. By increasing the signal frequency from 45 to 90 Hz, most biochemical changes associated with hindlimb unloading were prevented. Compared with HU ± SC, the sGAG content of HU+90 rats was 12 ± 3% greater (P < 0.001) in the whole IVD, 22 ± 6% greater (P = 0.025) in the NP, 21 ± 5% greater (P = 0.013) in the AAF, and 20 ± 5% greater (P = 0.013) for PAF (Fig. 4). None of the IVD biochemical properties of HU+90 was significantly different from values in AC. Comparing the two different vibrated groups directly, total IVD sGAG content of HU+45 was 9 ± 3% (P = 0.038) less than that measured in HU+90 (Fig. 4).
DISCUSSION
The purpose of the study was to determine the consequences of hindlimb unloading, a model of disc degeneration, on the overall and regional morphology and biochemistry of lumbar IVD and then quantify the ability of low-intensity vibration (45 or 90 Hz) to attenuate changes in the IVD induced by the removal of dynamic loading. Four weeks of hindlimb unloading induced morphological and compositional degradation of the intervertebral discs, deterioration that was not slowed by short daily periods of upright postural weight bearing. Superimposing very low-magnitude mechanical signals, induced by 90 Hz vibrations, on the short period of upright weightbearing helped preserve both the disc morphology and sGAG content, with a greater benefit to the sGAG content of the NP than the AF. In contrast to the protective effects of the 90 Hz signal, the 45 Hz signal provided few morphologic or biochemical benefits. As the IVD was preferentially protected by the 90 Hz signal, which induced displacements significantly smaller than the 45 Hz signal, the mechanism by which the IVD perceived and responded to the oscillatory signal is likely associated with characteristics particular to the higher frequency (e.g., cellular sensitivity to a specific frequency band), rather than a displacement based physical mechanism (e.g., extracellular matrix strain).
There are obvious limitations in using the IVD of a quadrupedal rat as a model of the impact of the removal of weightbearing for the bipedal human (38). Nevertheless, when normalized to the ratio of IVD height to area, biomechanical properties of lumbar IVD of rodents are similar to those of humans (4). The adult rat IVD retains notochordal cells that disappear by adulthood in humans, but unlike most other species and similar to humans, chondrocyte-like cells of the rat IVD dominate the cell population of the IVD (18). And while the higher metabolism and shorter life span permit adaptive changes in IVD properties to occur more rapidly, the existence of (slow) rates of bone growth in our young adult rats may also have to be considered when translating results of this study to adult humans. Consistent with stress levels that increase during the first few days of unloading and then normalize to ambulatory control levels (30), body mass of our unloaded rats was significantly less than in ambulating controls after the first week, upon which the rate of body mass gain normalized. While constraining the rats to an upright posture in the acrylic tubes did not alter body mass, it is possible that the temporary confinement altered the biochemical profile.
Furthermore, it is important to consider that hindlimb unloading does not necessarily unload the spine per se, but instead alters the mechanical environment of the spine by substituting the dynamic mechanical signals engendered by locomotion with a relatively static loading environment. Suspension at 30° subjects the tail to an unusual tension of 50% body wt (13), but the resulting load distribution at the lumbar IVD remains unclear because the applied load is distributed among ligaments, zygapophysial joints, muscle, bone, and IVD of the spine and has not been measured directly. It is possible that the disparity between the hypotrophic response in the HU rat and the hypertrophic response of the human IVD during bed rest and space flight is directly related to differences in the imposed mechanical environment, although 5 days of spaceflight also caused a nonsignificant increase in rat disc height (37). Together, the hindlimb unloaded rat represents a model of the impact of altered loading on disc degeneration, but cellular and functional disparities need to be kept in mind when interpreting the consequences of the removal of weightbearing and the potential benefits of providing surrogates for load bearing such as vibration.
To facilitate direct transmission from the inferior to the superior region of the spine, the low-magnitude oscillatory mechanical signals were applied in the longitudinal direction of the spine (15) while the rats were standing upright on their hindlimbs in an acrylic cylinder (24). Similar to the translation of vibrations to the L4 vertebra of human subjects standing on an oscillating plate with 20° knee flexion (35), the level of transmissibility in the rats reached ∼65–75%. Bipedal stance is a routine activity of rats roaming their cages, and because of the habitual nature of this activity (2a), it may not be surprising that 15 min/day of upright standing in the absence of the low-intensity vibrations provided no protective benefit to the IVD compared with hindlimb unloading alone. Comparable to studies of immobilization, unloading, and spaceflight (27, 33), the sGAG content in the IVD of hindlimb unloaded rats decreased by ∼20%, a difference as large as the decline over 20 yr in human adulthood (41). Thus our data emphasize that changing the mechanical environment of the IVD from dynamic habitual loading to a predominantly static state is anti-anabolic/catabolic and that reintroducing mechanical challenges “simply” by return to function for short periods were not sufficient to maintain disc morphology and biochemistry at those levels measured in age-matched controls (14, 19).
In contrast to the degradation measured in the IVD of the hindlimb unloaded, or those subject to 15 min of weightbearing, superimposing vertical 90 Hz vibrations for 15 min/day effectively served to maintain height and volume of the IVD. In contrast, rats exposed to the 45 Hz signal had IVD morphological properties that resembled those of rats with or without sham loading. Increasing the vibration frequency from 45 to 90 Hz also preserved growth-related changes in most geometric variables of the IVD. These data derived from an animal model of disc degeneration caused by unloading support the findings of bed rest studies, which show, in the human, the consequences of nonweightbearing and the potential benefits of introducing mechanical signals via vibration (16–30 Hz and 0.3–0.7 g), with or without exercise, toward maintaining IVD shape and volume (5, 15). Unlike the current study, clinical studies have not directly compared different vibration frequencies and it remains to be investigated whether mechanical signals at frequencies are more efficacious in preserving the morphology of the human IVD as well.
Inherently, there are very little data on altered glycosaminoglycan or collagen content of human IVD during prolonged unloading of the spine with or without countermeasures. A significant advantage of animal models involves the ability to directly associate regional biochemical changes in the IVD to mechanical loading or its removal. While maintaining erect posture, exposure to daily bouts of 0.2 g vibrations at 90 Hz, but not 45 Hz, maintained sGAG content of the lumbar IVD during unloading. These observations are supported by in vitro investigations that showed that high-frequency oscillations (100 Hz) can increase sGAG synthesis in chondrocytes even at acceleration magnitudes of far less than 0.1 g (40). Thus the loss of sGAG content modulated by the removal of weightbearing forces may be prevented by the anabolic nature of mechanical signals reintroduced in a higher frequency domain as vibrations.
The response of the IVD was not only sensitive to the specific vibration frequency but also dependent on the specific region within the disc and on the disc level within the spine. The homogenously smaller height of the IVD in hindlimb unloaded animals here contrasted with a HU study of younger rodents that showed a greater difference in the posterior height of the IVD than the anterior portion (14), suggesting an age-dependent manner in which regions of the disc modulate unloading. Similar to the greater loss of glycosaminoglycan in the NP of degenerated discs (1), the difference in sGAG content of discs that were unloaded with and without brief weightbearing tended to be greater in the NP than AF. Fewer glycosaminoglycans in the NP may reduce hydration and propagate degradation by increasing the range of motion of the disc, consequently delivering excessive strain to the annulus (39). It appears that these undesirable outcomes were partially prevented by the daily introduction of 90 Hz vibration during brief periods of weightbearing, which normalized the regionally dependent sGAG difference compared with both unloading and the application of 45 Hz oscillations.
The physical mechanism whereby such low-magnitude mechanical signals influence the morphology and biochemistry of the IVD remains unclear. It is conceivable that a physical mechanism based on fluid motion may reflect some of the “benefits” of vibration, as the mechanical signal had a greater impact on sGAG content in the highly fluid NP. Removing dynamic mechanical loading may have initiated degradation by decreasing the hydrostatic pressure of the NP (12) and expelling fluid from the NP. Compared with 45 Hz vibration, 15 min of 90 Hz oscillations would have generated more numerous and more rapid loading cycles, a physical challenge that may have more effectively influenced fluid characteristics and, ultimately, contributed to protecting the disc from degradation. While this hypothesis will ultimately be tested via direct fluid measurements, it is also consistent with the greater impact of 90 Hz vibrations on anterior disc height than posterior disc height. As indicated by a similarity in regional collagen structure (29) to human discs, the rat IVD may mimic the tension-compression disparity in the anterior-posterior region of the human disc (42). Therefore, the 90 Hz signals might have induced a mechanical environment that drew fluid anteriorly from the fluid-filled spinal cord (31) and promoted the response in this region.
Alternative to a tissue-deformation or fluid-dependent mechanism that is typically associated with the mechanical stimulation of viscoelastic cells incumbent in the IVD, the apparent protective benefit of low-intensity vibrations may have been realized by a cellular mechanism (10). Indeed, vibrations of much higher frequencies (300–350 Hz) and even lower displacements (1.5–3 μm) than studied here have been shown to stimulate chondrocyte proliferation and proteoglycan synthesis (23, 25). The physical means by which a cell can sense a mechanical signal this small is unknown but perhaps involves out-of-phase acceleration of the nucleus relative to the membrane of the cell, with adaptive responses triggered by mechanical challenges transmitted through the cytoskeleton (2). If a mechanism based on intracellular interactions accounts for the response found here it will likely be influenced by a number of factors such as cytoskeletal functionality (6) or IVD structure. It could also be speculated that, at the level of the cell and the molecules, the signaling pathways by which cells across the musculoskeleton perceive and respond to low-intensity vibrations are similar and involve mechanosensors distinct from those that sense much larger forces at lower frequencies. Future studies identifying molecular pathways as well as altered kinetics of cellular components induced by vibrations will not only uncover the underlying mechanisms but may also facilitate optimization of the vibratory signal.
Relatively large, dynamic loads are presumed to be critical for maintaining the compositional and metabolic health of the IVD (45). Here we show that extremely low-magnitude mechanical signals, applied using low-intensity vibration for only brief periods every day, can serve to slow the morphological and biochemical deterioration of the disc during hindlimb unloading. Similar to previous data in the skeleton (22), the ability of these signals to preserve the IVD was frequency dependent, with the 90 Hz signal being more effective than the 45 Hz signal, although the displacement of the higher frequency signal was much smaller. We do not know if the benefits of the 90 Hz signal were realized through a physical-transduction mechanism dependent on mechanical deformation of the matrix or a direct triggering of a biologic response through signaling of the cells within the IVD. Nevertheless, these data may indicate the potential benefit of a “surrogate” for high-frequency, low-magnitude endogenous muscle contractions otherwise removed by hindlimb unloading (17) and indirectly benefited the IVD by challenging the structure and biology of the disc with a physiologically relevant barrage of mechanical signals (46). Together, these data emphasize the risk to the intervertebral disc of removing mechanical loading, as well as the ability of extremely low-magnitude mechanical signals to contribute to the protection of an IVD otherwise devoid of function.
GRANTS
This work was kindly supported by National Institutes of Health, National Aeronautics and Space Administration (NASA), Alliance for Graduate Education and Professoriate, and the NASA-Harriett G. Jenkins Pre-Doctoral and W. Burghardt Turner Fellowships.
DISCLOSURES
Clinton Rubin is a founder of Marodyne Medical. Both S. Judex and C. Rubin own (provisional) patents regarding the application of vibrations to the musculoskeletal system.
AUTHOR CONTRIBUTIONS
Author contributions: N.H. and S.J. conception and design of research; N.H., G.U., F.-P.C., and S.J. performed experiments; N.H., G.U., F.-P.C., and S.J. analyzed data; N.H., G.U., C.T.R., and S.J. interpreted results of experiments; N.H. and S.J. prepared figures; N.H. drafted manuscript; N.H., C.T.R., and S.J. edited and revised manuscript; N.H., C.T.R., and S.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Svetlana Lublinsky for technical contributions.
REFERENCES
- 1. Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98: 996–1003, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bacabac RG, Smit TH, Van Loon JJ, Doulabi BZ, Helder M, Klein-Nulend J. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J 20: 858–864, 2006 [DOI] [PubMed] [Google Scholar]
- 2a. Bailey AS, Adler F, Min Lai S, Asher MA. A comparison between bipedal and quadrupedal rats: do bipedal rats actually assume an upright posture? Spine (Phila Pa 1976) 26: E308–313, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bass EC, Wistrom EV, Diederich CJ, Nau WH, Pellegrino R, Ruberti J, Lotz JC. Heat-induced changes in porcine annulus fibrosus biomechanics. J Biomech 37: 233–240, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine 33: E166–173, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Belavy DL, Hides JA, Wilson SJ, Stanton W, Dimeo FC, Rittweger J, Felsenberg D, Richardson CA. Resistive simulated weightbearing exercise with whole body vibration reduces lumbar spine deconditioning in bed-rest. Spine 33: E121–131, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Benya PD, Brown PD, Padilla SR. Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol 106: 161–170, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao P, Kimura S, Macias BR, Ueno T, Watenpaugh DE, Hargens AR. Exercise within lower body negative pressure partially counteracts lumbar spine deconditioning associated with 28-day bed rest. J Appl Physiol 99: 39–44, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chiang F. Micro-/nano-speckle method with applications to materials, tissue engineering and heart mechanics. Strain 44: 27–39, 2008 [Google Scholar]
- 9. Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of “painful” lumbar discs. Spine 22: 2342–2350, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Desmoulin GT, Reno CR, Hunter CJ. Free axial vibrations at 0 to 200 Hz positively affect extracellular matrix messenger ribonucleic acid expression in bovine nucleus pulposi. Spine (Phila Pa 1976) 35: 1437–1444, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Farfan HF, Cossette JW, Robertson GH, Wells RV, Kraus H. The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration. J Bone Joint Surg Am 52: 468–497, 1970 [PubMed] [Google Scholar]
- 12. Hargens AR, Mahmood M. Decreased swelling pressure of rat nucleus pulposus associated with simulated weightlessness. Physiologist 32: S23–24, 1989 [PubMed] [Google Scholar]
- 13. Hargens AR, Steskal J, Johansson C, Tipton CM. Tissue fluid shift, forelimb loading, and tail tension in tail-suspended rats. Physiologist 27: S37–38, 1984 [Google Scholar]
- 14. Holguin N, Judex S. Rat intervertebral disc health during hindlimb unloading: brief ambulation with or without vibration. Aviat Space Environ Med 81: 1078–1084, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Holguin N, Muir J, Rubin C, Judex S. Short applications of very low-magnitude vibrations attenuate expansion of the intervertebral disc during extended bed rest. Spine J 9: 470–477, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Horton JA, Bariteau JT, Loomis RM, Strauss JA, Damron TA. Ontogeny of skeletal maturation in the juvenile rat. Anat Rec (Hoboken) 291: 283–292, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci 54: B352–357, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat 205: 357–362, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutton WC, Malko JA, Fajman WA. Lumbar disc volume measured by MRI: effects of bed rest, horizontal exercise, and vertical loading. Aviat Space Environ Med 74: 73–78, 2003 [PubMed] [Google Scholar]
- 21. Johnston SL, Campbell MR, Scheuring R, Feiveson AH. Risk of herniated nucleus pulposus among US astronauts. Aviat Space Environ Med 81: 566–574, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40: 1333–1339, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kaupp JA, Waldman SD. Mechanical vibrations increase the proliferation of articular chondrocytes in high-density culture. Proc Inst Mech Eng H 222: 695–703, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Legerlotz K, Schjerling P, Langberg H, Bruggemann GP, Niehoff A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J Appl Physiol 102: 564–572, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Sekiya I, Asai K, Tada T, Kato T, Matsui N. Biosynthetic response of cultured articular chondrocytes to mechanical vibration. Res Exp Med (Berl) 200: 183–193, 2001 [PubMed] [Google Scholar]
- 26. Macias BR, Cao P, Watenpaugh DE, Hargens AR. LBNP treadmill exercise maintains spine function and muscle strength in identical twins during 28-day simulated microgravity. J Appl Physiol 102: 2274–2278, 2007 [DOI] [PubMed] [Google Scholar]
- 27. MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine 28: 973–981, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Martin EA, Ritman EL, Turner RT. Time course of epiphyseal growth plate fusion in rat tibiae. Bone 32: 261–267, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Maynard JA. The effects of space flight on the composition of the intervertebral disc. Iowa Orthop J 14: 125–133, 1994 [PMC free article] [PubMed] [Google Scholar]
- 30. Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol 92: 1367–1377, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Nahhas Rodacki CL, Luiz Felix Rodacki A, Ugrinowitsch C, Zielinski D, Budal da Costa R. Spinal unloading after abdominal exercises. Clin Biomech (Bristol, Avon) 23: 8–14, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci USA 103: 19255–19260, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedrini-Mille A, Maynard JA, Durnova GN, Kaplansky AS, Pedrini VA, Chung CB, Fedler-Troester J. Effects of microgravity on the composition of the intervertebral disk. J Appl Physiol 73: 26S–32S, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Pfirrmann CW, Metzdorf A, Elfering A, Hodler J, Boos N. Effect of aging and degeneration on disc volume and shape: a quantitative study in asymptomatic volunteers. J Orthop Res 24: 1086–1094, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine 28: 2621–2627, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Saberi H, Rahimi L, Jahani L. A comparative MRI study of upper and lower lumbar motion segments in patients with low back pain. J Spinal Disord Tech 22: 507–510, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Sinha RK, Shah SA, Hume EL, Tuan RS. The effect of a 5-day space flight on the immature rat spine. Spine J 2: 239–243, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Smit TH. The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J 11: 137–144, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine 29: 2724–2732, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takeuchi R, Saito T, Ishikawa H, Takigami H, Dezawa M, Ide C, Itokazu Y, Ikeda M, Shiraishi T, Morishita S. Effects of vibration and hyaluronic acid on activation of three-dimensional cultured chondrocytes. Arthritis Rheum 54: 1897–1905, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine 13: 179–187, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Wang S, Xia Q, Passias P, Wood K, Li G. Measurement of geometric deformation of lumbar intervertebral discs under in-vivo weightbearing condition. J Biomech 42: 705–711, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976) 24: 755–762, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Wing PC, Tsang IK, Susak L, Gagnon F, Gagnon R, Potts JE. Back pain and spinal changes in microgravity. Orthop Clin North Am 22: 255–262, 1991 [PubMed] [Google Scholar]
- 45. Wuertz K, Godburn K, MacLean JJ, Barbir A, Donnelly JS, Roughley PJ, Alini M, Iatridis JC. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res 27: 1235–1242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol 2008 [DOI] [PubMed] [Google Scholar]
- 47. Yasuoka H, Asazuma T, Nakanishi K, Yoshihara Y, Sugihara A, Tomiya M, Okabayashi T, Nemoto K. Effects of reloading after simulated microgravity on proteoglycan metabolism in the nucleus pulposus and anulus fibrosus of the lumbar intervertebral disc: an experimental study using a rat tail suspension model. Spine 32: E734–740, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Zou J, Yang H, Miyazaki M, Morishita Y, Wei F, McGovern S, Wang JC. Dynamic bulging of intervertebral discs in the degenerative lumbar spine. Spine 34: 2545–2550, 2009 [DOI] [PubMed] [Google Scholar]