Abstract
The general purpose of this study was to test the effect of exercise training on the left ventricular (LV) pressure-volume relationship (LV/PV) and apoptotic signaling markers in normotensive and hypertensive hearts. Four-month-old female normotensive Wistar-Kyoto rats (WKY; n = 37) and spontaneously hypertensive rats (SHR; n = 38) were assigned to a sedentary (WKY-SED, n = 21; SHR-SED, n = 19) or treadmill-trained (WKY-TRD, n = 16; SHR-TRD, n = 19) group (∼60% V̇o2 peak, 60 min/day, 5 days/wk, 12 wk). Ex vivo LV/PV were established in isovolumic Langendorff-perfused hearts, and LV levels of Akt, phosphorylated Akt (AktPi), Bad, phosphorylated Bad (BadPi) c-IAP, x-IAP, calcineurin, and caspases 3, 8, and 9 were measured. Heart-to-body weight ratio was increased in SHR vs. WKY (P < 0.05), concomitant with increased calcineurin mRNA (P < 0.05). There was a rightward shift in the LV/PV (P < 0.05) and a reduction in systolic elastance (Es) in SHR vs. WKY. Exercise training corrected Es in SHR (P < 0.05) but had no effect on the LV/PV in WKY. Caspase 3 was increased in SHR-SED relative to WKY-SED, while BadPi, c-IAP, and x-IAP were significantly lower in SHR relative to WKY (P < 0.05). Exercise training increased BadPi in both WKY and SHR but did not alter caspase 9 activity in either group. While caspase 3 activity was increased with training in WKY (P < 0.05), it was unchanged with training in SHR. We conclude that moderate levels of regular aerobic exercise attenuate systolic dysfunction early in the compensatory phase of hypertrophy, and that a differential phenotypical response to moderate-intensity exercise exists between WKY and SHR.
Keywords: aerobic exercise, blood pressure, cardiovascular disease, heart
hypertension is a significant health concern as an independent risk factor for cardiovascular disease and as a precursor to heart failure (5). The hemodynamic overload presented by hypertension results in a maladaptive, pathological pattern of concentric cardiac hypertrophy and an increased rate of cardiomyocyte apoptosis (20, 26). Along these lines, cardiomyocyte apoptosis, autophagy, and necrosis are all thought to contribute to the progression of heart failure through distinct individual and shared signaling pathways (36–38).
Our laboratory has consistently reported that chronic aerobic exercise training augments cardiac remodeling in the spontaneously hypertensive rat (SHR) model (25, 26, 33, 39, 41, 42). Our studies indicate that training increases cardiomyocyte hypertrophy and cardiomyocyte proliferation, while attenuating TUNEL-positive (TUNELpos) cardiomyocytes in SHR myocardium (26). These data have led us to hypothesize that exercise training improves the overall cardiomyocyte balance in hypertensive hearts and may be a beneficial mechanism in delaying the development of heart failure (4, 13, 27, 32). Given that training-induced reductions in apoptosis might be central in maintaining cardiomyocyte number, we hypothesized that training would improve the intrinsic, antiapoptotic profile of hypertensive hearts. We also sought to determine whether moderate-intensity training induced a differential adaptive response between normotensive and hypertensive hearts.
One pathway associated with apoptosis is the intrinsic or mitochondrial death pathway. Via action on the mitochondrial membrane, proapoptotic members of the Bcl-2 family of proteins, e.g., Bad, destabilize the mitochondrial membrane, triggering the release of cytochrome c (2, 8, 40). Cytochrome c release and subsequent apoptosome formation result in the activation of initiator caspase 9, activation of downstream executioner caspases, and cell death. Through the phosphorylation of key components of the mitochondrial death pathway or through alterations in gene expression of cell death machinery, protein kinase B (Akt) has emerged as a key regulator of cell survival (8). Akt-mediated phosphorylation of Bad promotes cell survival by decreasing mitochondrial membrane destabilization and cytochrome c release (8, 9, 23). Furthermore, Akt is capable of directly phosphorylating caspase 9, thereby decreasing its catalytic ability (3). Given that phosphatidylinositol 3-kinase (PI3K)/Akt pathway has been shown to be centrally related to the development of exercise-induced physiological hypertrophy (34), we tested whether chronic aerobic training altered the protein abundance of Akt and Bad and their respective levels of phosphorylation in normotensive and hypertensive hearts. We also established whether training altered cardiac performance to a Starling challenge in normotensive and hypertensive hearts. The Wistar-Kyoto rat (WKY) and SHR models were utilized for these experiments on the basis that SHR mimic well the clinical course of untreated essential hypertension in humans and are well documented to show left ventricular (LV) chamber enlargement by 6–12 mo of age (1, 6, 11, 16, 24).
METHODS
Experimental paradigm.
Seventy-five 4-mo-old female WKY (n = 37) and SHR (n = 38) animals were obtained from Charles River Laboratories (St-Constant, QC, Canada). Animals from each group were randomly stratified into sedentary (WKY-SED, n = 21; SHR-SED, n = 19), or exercise-trained (WKY-TRD, n = 16; SHR-TRD, n = 19) groups. All rats were housed three per cage, maintained on a 12:12-h light-dark cycle, and fed ad libitum (Harlan Teklad Global Diets, 18% Protein Diet, Madison, WI). Exercise consisted of moderate-intensity endurance training at a speed of 25 m/min, 0% grade, 60 consecutive min/day, 5 days/wk for a period of 12 wk (25, 26, 33, 39, 41, 42). We have previously shown that this training paradigm significantly increases soleus citrate synthase activity in SHR (25). Also, we have shown that the identical protocol enhances myocyte length and width in hypertensive hearts (26). To account for handling and noise-induced stress, both WKY-SED and SHR-SED were placed near the treadmill during exercise sessions. Blood pressure and heart rate (HR) were collected prior to death with a tail-cuff apparatus (Kent Scientific, Torrington, CT, XBP 1000) (33). Animals were acclimated to the tail cuff apparatus two times prior to blood pressure determination. Fifty-one animals were killed at 7 mo of age for ex vivo LV functional studies (WKY-SED, n = 15; WKY-TRD, n = 10; SHR-SED, n = 13; SHR-TRD, n = 13), while hearts from the remaining animals (n = 6/group) were used for caspase measurements and Western blots. All animals received humane care according to protocols reviewed and approved by the University Institutional Animal Care and Use Committee and in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Pub. No. 85-23, revised 1985).
Langendorff experiments.
To determine the effects of exercise on LV mechanical function, LV pressure-volume relationships were determined in isolated, isovolumic buffer-perfused hearts. Rats were anesthetized with pentobarbital sodium (60 mg/kg ip) and heparinized (500 U iv). A thoracotomy was performed, and the heart was excised and then perfused retrograde with a Langendorff apparatus as previously described (39, 41, 42). During equilibration, all hearts were loaded with an initial balloon volume yielding an LV end-diastolic pressure (LVEDP) of 10 mmHg. LV systolic pressure (LVSP), LVEDP, and coronary perfusion pressure (CPP) were continuously recorded by means of a data acquisition system (Powerlab/8SP, ADI Instruments, Colorado Springs, CO). LV developed pressure (LVdevP) was calculated by subtracting LVEDP from LVSP. Hearts were paced at 5 Hz (Grass Instruments, Quincy, MA) and immersed in a water-jacketed organ chamber at 37°C throughout the protocol.
Left ventricular pressure-volume relationship.
After 20 min of equilibration, the LV pressure-volume relationship was established over a wide range of LV filling volumes as previously described (31, 42). Briefly, with a Hamilton syringe, LV balloon volume was increased in 5- to 10-μl increments until an LVEDP of ∼30 mmHg was achieved. To compare the LV pressure-volume relationship between groups, LV volumes were determined from each pressure-volume curve at 5-mmHg intervals (31). The LVSP-volume relationship (LVSP/V) and the LVEDP-volume relationship (LVEDP/V) were curve fit with a best fit analysis, with the linear slope of the LVSP/V indicating the systolic elastance (Es). The slope of the LVEDP/V relationship was used to reflect the LV diastolic chamber stiffness. LV capacitance (V25) was measured as the LV volume that elicited an LVEDP of 25 mmHg.
Protein assay/Western blot analysis.
Frozen tissue was weighed and homogenized on ice in PBS lysis buffer containing 2% sodium dodecyl sulfate (SDS; FisherBiotech), 1% IGEPAL CA-630 (Sigma), 0.5% deoxycholate (Sigma), 5 mM EDTA, and proteinase inhibitors (aprotinin, leupeptin, 1 mM phenylmethylsulfonyl fluoride, and pepstatin A, calpain inhibitor I and II, and benzamidine). Tissue protein abundance and phosphorylation levels in isolated protein [Akt, phosphorylated Akt (AktPi), Bad, phosphorylated Bad (BadPi) c-IAP, and x-IAP] were analyzed by Western blot analysis. Target antigens were probed with phosphorylation-specific polyclonal antibodies for Akt and Bad. Blots were measured with densitometry using Image J software (National Institutes of Health).
Caspase activity assay.
The tissue was washed with PBS and scraped off in ice-cold lysis buffer provided with caspase assay kits used to detect caspases 3, 8, and 9 (EMD4Biosciences). After sonication, the lysate was centrifuged for 20 min at 14,000g at 4°C. The supernatants were analyzed for protein content and used for caspase colorimetric enzymatic activity assays per the manufacturer's instructions with 96-well plates. Absorbance was recorded on a plate reader immediately after the start of the assay and after 10–16 h of incubation at 37°C. The net increase in absorbance represented enzyme activity.
RT-PCR mRNA.
Calcineurin A mRNA expression was assessed by RT-PCR. Calcineurin primers were 5-CCACAGGGATGTTGCCTAGTG (forward) and 5-GTCCCGTGGTTCTCAGTGGTA (reverse). RT-PCR reactions were performed along with 1 μg of cDNA created from RNA with iScript (Bio-Rad) followed by 22 cycles of PCR amplification (annealing temperature 62.5°C) with a Bio-Rad iCycler. Appropriate melt curves were also run. Individual samples were normalized to levels of 28S: 5-TTGAAA-ATCCGGGGGAGAG (forward) and 5-ACATTGT-TCCAACATGCCAG (reverse) with group differences (RQ) expressed relative to an individual animal from the WKY-SED group.
Data analysis and interpretation.
In vivo hemodynamics, animal characteristics, LVEDP/V, LVSP/V, LV capacitance, diastolic slope, peak and relative LVdevP, CPP, and molecular analyses were compared with 2 × 2 ANOVA (hypertension vs. exercise main effects) followed by Tukey post hoc analysis when appropriate. All analyses were performed with SPSS version 15.0 (SPSS, Chicago, IL). Statistical significance was set at an α level of P < 0.05. All data are reported as means ± SE.
RESULTS
Hemodynamic parameters and animal characteristics.
Table 1 illustrates the systolic blood pressure (SBP), heart rate (HR), and rate-pressure product (RPP) in WKY and SHR animals at 7 mo of age. Significant differences between WKY and SHR existed for SBP, HR, and RPP (main effects; P < 0.05). Exercise training also significantly reduced both HR and RPP in SHR-TRD compared with SHR-SED animals (P < 0.05). SBP, HR, and RPP were similar in WKY-SED and WKY-TRD animals.
Table 1.
In vivo hemodynamics
| WKY-SED | WKY-TRD | SHR-SED | SHR-TRD | |
|---|---|---|---|---|
| n = 21 | n = 16 | n = 19 | n = 19 | |
| SBP, mmHg | 141 ± 3 | 146 ± 6 | 180 ± 2* | 175 ± 3* |
| HR, beats/min | 389 ± 7 | 377 ± 12 | 503 ± 6* | 454 ± 10*† |
| RPP | 54,857 ± 1,643 | 55,607 ± 3,655 | 90,340 ± 1,477* | 79,055 ± 2,759*† |
Data are presented as means ± SE. WKY, Wistar-Kyoto rat; SHR, spontaneously hypertensive rat; SED, sedentary; TRD, trained; SBP, systolic blood pressure; HR, heart rate; RPP, rate-pressure product.
Main effect for WKY vs. SHR;
P < 0.01 vs. SHR-SED.
The physical characteristics of the experimental groups are presented in Table 2. Prior to death, body weight was lower in SHR versus WKY (main effect; P < 0.05). Tibial length was greater in TRD versus SED (treatment effect; P < 0.05), and heart weight-to-body weight ratio was increased in SHR relative to WKY (main effect; P < 0.05). There were no differences for heart weight or heart weight-to-tibial length ratio among groups.
Table 2.
Physical characteristics
| WKY-SED | WKY-TRD | SHR-SED | SHR-TRD | |
|---|---|---|---|---|
| Body weight, g | 232 ± 5 | 250 ± 6 | 209 ± 4* | 217 ± 5* |
| Heart weight, g | 1.05 ± 0.04 | 1.09 ± 0.03 | 1.09 ± 0.04 | 1.15 ± 0.04 |
| Tibial length, cm | 3.56 ± 0.03 | 3.70 ± 0.04† | 3.63 ± 0.04 | 3.64 ± 0.05† |
| HW/BW, mg/g | 4.54 ± 0.16 | 4.37 ± 0.13 | 5.23 ± 0.15* | 5.31 ± 0.12* |
| HW/TL, g/cm | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.32 ± 0.01 |
Data are presented as means ± SE. HW/BW, heart-to-body weight ratio; HW/TL, heart weight-to-tibial length ratio.
P < 0.05 main effect WKY vs. SHR;
P < 0.05 main effect SED vs. TRD.
Langendorff isolated heart performance.
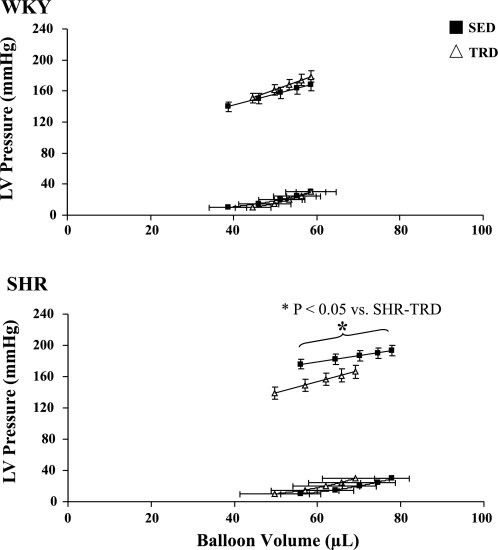
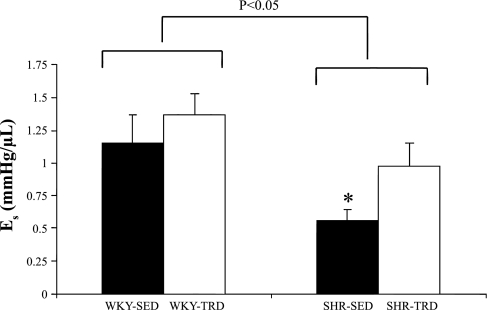
Figure 1 illustrates the pressure-volume relationships in both WKY and SHR animals. The LVEDP/V was shifted slightly rightward in SHR relative to WKY (main effect; P < 0.05) but was not statistically different between TRD and SED. There was also an increased LV capacitance (main treatment effect; P < 0.05) in SHR relative to WKY (WKY-SED: 55 ± 6 μl, WKY-TRD: 56 ± 4 μl, SHR-SED: 75 ± 4 μl, SHR-TRD: 66 ± 8 μl), with no differences in LV diastolic chamber stiffness among groups. As Fig. 2 illustrates, the slope of the LVSP/V, i.e., Es, was depressed in SHR relative to WKY (main effect and interaction; P < 0.05) but was increased with training in SHR-TRD vs. SHR-SED (P < 0.05). We also measured CPP at an LVEDP of 10 mmHg as a marker of coronary resistance. CPP was greater in SHR relative to WKY (main effect; P < 0.05) (WKY-SED: 82 ± 3 mmHg, WKY-TRD: 100 ± 6 mmHg, SHR-SED: 126 ± 9 mmHg, SHR-TRD: 95 ± 4 mmHg), illustrating greater coronary resistance with hypertension.
Fig. 1.
Left ventricular (LV) systolic and LV diastolic pressure (LVEDP)-volume relationships. Systolic and end-diastolic pressure-volume relationships in Wistar-Kyoto rats (WKY; top) and spontaneously hypertensive rats (SHR; bottom) are illustrated. The LVEDP-pressure relationship (LVEDP/V) was shifted slightly rightward in SHR relative to WKY (main effect; P < 0.05) but was not statistically different between exercise-trained (TRD) and sedentary (SED) animals of either strain. The depressed slope of the end-systolic pressure volume relationship (systolic elastance, Es) in SHR-SED was improved with training (SHR-TRD). Data are presented as means ± SE.
Fig. 2.
Es in WKY and SHR. Es was depressed in SHR relative to WKY (main effect; P < 0.05) but improved with training in SHR (P < 0.05). Data are presented as means ± SE. *P < 0.05 for SHR-SED vs. SHR-TRD.
Apoptotic markers and caspases.
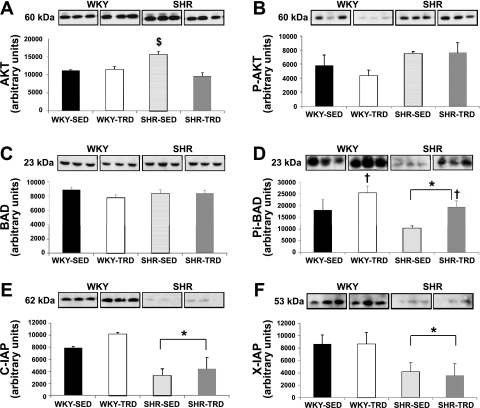
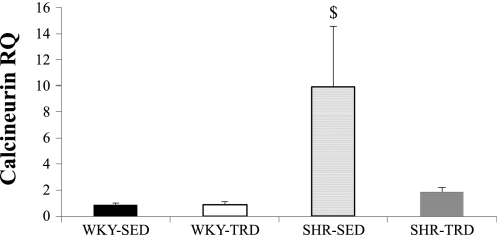
As Fig. 3A illustrates, the abundance of Akt was greatest in SHR-SED relative to the other groups (P < 0.05; following a significant interaction effect), despite no significant differences in AktPi (Fig. 3B). The abundance of Bad was not different (Fig. 3C) across groups, while BadPi was greater in WKY versus SHR (main effect; P < 0.05) and increased with training in both WKY and SHR (P < 0.05; following a significant main effect) (Fig. 3D). Both c-IAP and x-IAP were lower in SHR relative to WKY hearts (main effect; P < 0.05), with no training effect induced for either marker (Fig. 3, E and F). Calcineurin mRNA was significantly increased in SHR-SED (P < 0.05; following a significant interaction effect) and mitigated with training in SHR-TRD (Fig. 4). Table 3 summarizes caspase 3, 8, and 9 activities in our study sample. Caspase 3 activity was significantly lower in WKY-SED versus WKY-TRD and SHR-SED (P < 0.05; following a significant interaction effect). There were no significant treatment or training effects for caspase 8 or 9 among groups.
Fig. 3.
Abundance of key apoptotic signaling molecules in WKY and SHR. The abundance of Akt (A) was significantly higher in SHR-SED vs. the other groups (P < 0.05; following a significant interaction effect), while phosphorylated Akt (AktPi) (B) was similar across groups. Bad (C) was also similar across groups, but phosphorylated Bad (BadPi) was lower in SHR vs. WKY (D) (main effect; P < 0.05). Exercise training increased BadPi in both WKY and SHR (P < 0.05; following a significant main effect; D). Both c-IAP (E) and x-IAP (F) were lower in SHR vs. WKY (main effect; P < 0.05). Brackets indicate statistical differences. *P < 0.05 for WKY vs. SHR; †P < 0.05 for SED vs. TRD; $P < 0.05 vs. all other groups.
Fig. 4.
RT-PCR. Calcineurin A mRNA was significantly increased in SHR-SED (P < 0.05; following a significant interaction effect) and mitigated with training in SHR-TRD. Data are expressed relative to an individual animal from the WKY-SED group to reflect fold change. RQ, group difference. $P < 0.05 vs. all other groups.
Table 3.
Myocardial caspases
| WKY-SED | WKY-TRD | SHR-SED | SHR-TRD | |
|---|---|---|---|---|
| Caspase 3 | 102 ± 3* | 124 ± 7 | 123 ± 7 | 122 ± 3 |
| Caspase 8 | 203 ± 49 | 322 ± 77 | 207 ± 47 | 326 ± 86 |
| Caspase 9 | 121 ± 9 | 110 ± 12 | 118 ± 11 | 109 ± 12 |
Data (in rfu·min−1·mg−1) are presented as means ± SE..
P < 0.05 vs. WKY-TRD and SHR-SED. rfu, relative fluorescence units.
DISCUSSION
In this study, we found an impaired Es, increased calcineurin mRNA, decreased antiapoptotic protein abundance, and increased caspase 3 activity in hearts harvested from sedentary SHR relative to WKY. We also found that exercise training improved Es and decreased calcineurin mRNA in SHR but not in WKY. While exercise improved Bad phosphorylation in both SHR and WKY, it did not translate into a lower caspase 9 or 3 activity in either strain. In fact, moderate-intensity exercise increased caspase 3 activity in WKY-TRD relative to WKY-SED, without altering the pressure-volume relationship in WKY. We conclude that 1) moderate levels of regular treadmill exercise attenuate systolic dysfunction early in the compensatory phase of hypertension-induced hypertrophy independent of lowering caspase 3 activity and 2) a differential apoptotic and functional phonotypical response to moderate-intensity exercise exists between WKY and SHR.
Apoptosis is an important component of pathological cardiac remodeling and is central in the pathogenesis of heart failure (38). Apoptosis has been reported to be increased in the LV of young, adult, and senescent SHR (1, 11, 15, 26) as well as in other models of pressure overload (7, 18). Results of the present study are in accord with these previous reports. Morphological alterations such as cell shrinkage, membrane blebbing, DNA fragmentation, and chromatin condensation (10, 36–37) are typical features of apoptotic cardiomyocytes. Apoptosis may be induced either by a death ligand (extrinsic pathway) or by mitochondrion-related mechanisms (intrinsic pathway) (10) and is triggered by a host of cues, including reactive oxygen species, elevated intracellular Ca2+ concentration, and tumor necrosis factor.
In the present study, we focused our attention on the role that training exerts on the intrinsic apoptotic pathway, because of previous published reports showing that PI3K/Akt signaling is upregulated with training (34). Through the phosphorylation of key components of the mitochondrial death pathway or through alterations in gene expression of cell death machinery, Akt is appreciated as a key regulator of cell survival (8). Akt-mediated phosphorylation of Bad prevents the inactivation of prosurvival factors, thereby promoting cell survival by decreasing mitochondrial membrane destabilization and cytochrome c release (8, 9, 23). Furthermore, Akt is capable of directly phosphorylating caspase 9, thereby decreasing its catalytic ability (3). Here we report that despite similarities in phosphorylated Akt, the level of phosphorylated Bad (i.e., inactivated Bad) was significantly greater in WKY compared with SHR. Moreover, treadmill training increased Bad phosphorylation in both WKY and SHR without altering caspase 9 activity in either strain. These observations may suggest that other protein kinases, such as protein kinase C, or phosphatases such as calcineurin mediate Bad phosphorylation and that Bad phosphorylation in and of itself does not translate into an attenuation of caspase 9 activity.
Calcineurin, a Ca2+-calmodulin-dependent protein phosophatase, has been reported to oppose Akt-mediated phosphorylation of Bad (43, 51) and is reproducibly increased in pressure-overload hypertrophy (35). In the present study, we report that training mitigated gene expression of calcineurin in SHR. These results are consistent with our previous work showing that calcineurin protein abundance and activity were increased in SHR (26) and reduced in SHR animals undergoing 3 mo of treadmill training (26). Thus a plausible mechanism for increased Bad phosphorylation in SHR may be a training-induced mitigation of calcineurin. However, Bad phosphorylation was also elevated in WKY with training, without a training effect on calcineurin, suggesting the involvement of other regulatory factors.
While the phosphorylation of Bad is likely to mitigate downstream activation of proteolytic caspases, the catalytic potential of these caspases can also be regulated directly by a family of proteins called the inhibitors of apoptosis (IAPs) (44). Of the IAPs, x-IAP is the best characterized and appears to directly inhibit the catalytic ability of caspase 9 (50). c-IAP is also an antiapoptotic molecule capable of binding caspase 9 and inhibiting caspase 3 (12, 45). In the present investigation, the abundance of c-IAP and x-IAP were decreased in SHR relative to WKY without any training effect for either protein. The reduction of c-IAP and x-IAP in SHR is also consistent with the increased caspase 3 activity observed in SHR-SED relative to WKY-SED.
Our finding of greater caspase 3 activity in WKY-TRD relative to WKY-SED is of interest, given that several previous reports have shown that exercise training attenuates apoptosis in normotensive rodent myocardium (19, 28, 48, 49). Moreover, previous studies on pressure overload have also reported a reduction in various antiapoptotic markers following training (26, 29, 30). In the present study, caspase 3 was similar between SHR-SED and SHR-TRD. While we previously reported that exercise training reduced TUNELpos cardiomyocytes in SHR myocardium (26), we do not view the lack of a training effect on caspase 3 activity in SHR in the present study as contradictory with these previous findings. Measurement of caspase 3 activity is temporally indicative of only one point in time, whereas the number of TUNELpos cardiomyocytes is summative. Moreover, TUNELpos cells reflect broken DNA strands and are not specific for apoptosis exclusively. Thus other modes of cell death, e.g., necrosis, can also yield TUNELpos cardiomyocytes and factor into regulating cardiomyocyte number in hypertension. Additional experimental paradigm variations in animal strain, sex, training status, and age also factor into differences related to exercise in various reports across the literature. Clearly, the impact of exercise on myocardial apoptosis requires further study, particularly with respect to its specific regulatory role on cardiac function.
Systolic cardiac function has been shown to be altered in SHR, with some reports showing systolic function to be decreased (24, 42), increased (6), or unchanged (47) early in the course of hypertension. In the present study, Es was significantly lower in SHR compared with WKY. These results are directionally consistent with our previous report of a decreased absolute Es in older SHR hearts compared with WKY (42). Taken together, these data suggest that a blunted Starling response occurs very early in the development of compensatory hypertrophy. The Starling relationship is predicated on the influence of myofiber length on myofilament activation, a process known as length-dependent activation (14). Both optimal actin-myosin overlap and increased myofilament calcium responsiveness are thought to mechanistically explain this effect (17). Moreover, cardiomyocyte activation number, which is attenuated with apoptosis in hypertension, may play a role. Apoptotic cardiomyocyte loss is associated with a progressive decline in LV function and heart failure (36–38). In the present study, exercise lessened the decline in Es in SHR but did so independently of reducing caspase 3 activity. These data suggest that the favorable functional profile observed in trained SHR hearts was not secondary to an attenuation of apoptosis.
In conclusion, our study shows that systolic dysfunction occurs very early in the compensatory phase of pressure-overload hypertrophy, without evidence of diastolic dysfunction. Consistent with other studies, increased apoptosis appears to be associated with the impaired systolic function in hypertensive hearts. While moderate levels of regular exercise attenuated systolic dysfunction early in the compensatory phase pressure-overload hypertrophy, the effect appears independent of mitigating apoptosis. Moderate-intensity exercise did not improve the Starling response in normotensive hearts, suggesting that a differential phenotypical response to exercise exists between normotensive and hypertensive rodent myocardium. More work exploring varying intensities and durations of exercise training on apoptosis and function is needed.
GRANTS
This study was supported by the American Heart Association (J. R. Libonati) and by National Heart, Lung, and Blood Institute Grant HL-76799 (A. Sabri).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.L., A.S., S.M.M., and B.F.R. conception and design of research; J.R.L., A.S., S.M.M., and B.F.R. performed experiments; J.R.L., A.S., C.X., S.M.M., and B.F.R. analyzed data; J.R.L., A.S., C.X., and B.F.R. interpreted results of experiments; J.R.L., A.S., S.M.M., and B.F.R. prepared figures; J.R.L. and B.F.R. drafted manuscript; J.R.L., A.S., S.M.M., and B.F.R. edited and revised manuscript; J.R.L., A.S., S.M.M., and B.F.R. approved final version of manuscript.
REFERENCES
- 1. Bing OH, Ngo HQ, Humphries DE, Robinson KG, Lucey EC, Carver W, Brooks WW, Conrad CH, Hayes JA, Goldstein RH. Localization of alpha1(I) collagen mRNA in myocardium from the spontaneously hypertensive rat during the transition from compensated hypertrophy to failure. J Mol Cell Cardiol 29: 2335–2344, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bratton SB, Cohen GM. Apoptotic death sensor: an organelle's alter ego? Trends Pharmacol Sci 22: 306–315, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318–1321, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-intensity exercise training delays heart failure and improves survival in female hypertensive heart failure rats. Hypertension 51: 1096–1102, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart L, Blood Institute Joint National Committee on Prevention, Detection, and Evaluation, and Treatment of High Blood Pressure, and National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cingolani OH, Yang XP, Cavasin MA, Carretero OA. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension 41: 249–254, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, de Rienzo A, Roncarati R, Trimarco B, Lembo G. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 99: 3071–3078, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241, 1997 [DOI] [PubMed] [Google Scholar]
- 9. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Delhalle S, Duvoix A, Schnekenburger M, Morceau F, Dicato M, Diederich M. An introduction to the molecular mechanisms of apoptosis. Ann NY Acad Sci 1010: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Diez J, Panizo A, Hernandez M, Vega F, Sola I, Fortuno MA, Pardo J. Cardiomyocyte apoptosis and cardiac angiotensin-converting enzyme in spontaneously hypertensive rats. Hypertension 30: 1029–1034, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem 281: 3254–3260, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol 289: H2030–H2038, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Fabiato A, Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature 256: 54–56, 1975 [DOI] [PubMed] [Google Scholar]
- 15. Fortuno MA, Gonzalez A, Ravassa S, Lopez B, Diez J. Clinical implications of apoptosis in hypertensive heart disease. Am J Physiol Heart Circ Physiol 284: H1495–H1506, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Garciarena CD, Pinilla OA, Nolly MB, Laguens RP, Escudero EM, Cingolani HE, Ennis IL. Endurance training in the spontaneously hypertensive rat: conversion of pathological into physiological cardiac hypertrophy. Hypertension 53: 708–714, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Hofmann PA, Fuchs F. Effect of length and cross-bridge attachment on Ca2+ binding to cardiac troponin C. Am J Physiol Cell Physiol 253: C90–C96, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Ikeda S, Hamada M, Qu P, Hiasa G, Hashida H, Shigematsu Y, Hiwada K. Relationship between cardiomyocyte cell death and cardiac function during hypertensive cardiac remodelling in Dahl rats. Clin Sci (Lond) 102: 329–335, 2002 [PubMed] [Google Scholar]
- 19. Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Van Peborgh J, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 279: H2994–H3002, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol 287: H72–H80, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kavantzas NG, Lazaris AC, Agapitos EV, Nanas J, Davaris PS. Histological assessment of apoptotic cell death in cardiomyopathies. Pathology 32: 176–180, 2000 [PubMed] [Google Scholar]
- 22. Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928–H935, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 19: 5800–5810, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kokubo M, Uemura A, Matsubara T, Murohara T. Noninvasive evaluation of the time course of change in cardiac function in spontaneously hypertensive rats by echocardiography. Hypertens Res 28: 601–609, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kolwicz SC, Kubo H, MacDonnell SM, Houser SR, Libonati JR. Effects of forskolin on inotropic performance and phospholamban phosphorylation in exercise-trained hypertensive myocardium. J Appl Physiol 102: 628–633, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kolwicz SC, MacDonnell SM, Renna BF, Reger PO, Seqqat R, Rafiq K, Kendrick ZV, Houser SR, Sabri A, Libonati JR. Left ventricular remodeling with exercise in hypertension. Am J Physiol Heart Circ Physiol 297: H1361–H1368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res 98: 540–548, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J 20: 791–793, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Lajoie C, Calderone A, Beliveau L. Exercise training enhanced the expression of myocardial proteins related to cell protection in spontaneously hypertensive rats. Pflügers Arch 449: 26–32, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Lee YI, Cho JY, Kim MH, Kim KB, Lee DJ, Lee KS. Effects of exercise training on pathological cardiac hypertrophy related gene expression and apoptosis. Eur J Appl Physiol 97: 216–224, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Libonati JR. Exercise and diastolic function after myocardial infarction. Med Sci Sports Exerc 35: 1471–1476, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Libonati JR, Gaughan JP. Low-intensity exercise training improves survival in Dahl salt hypertension. Med Sci Sports Exerc 38: 856–858, 2006 [DOI] [PubMed] [Google Scholar]
- 33. MacDonnell SM, Kubo H, Crabbe DL, Renna BF, Reger PO, Mohara J, Smithwick LA, Koch WJ, Houser SR, Libonati JR. Improved myocardial beta-adrenergic responsiveness and signaling with exercise training in hypertension. Circulation 111: 3420–3428, 2005 [DOI] [PubMed] [Google Scholar]
- 34. McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100: 12355–12360, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narula J, Hajjar RJ, Dec GW. Apoptosis in the failing heart. Cardiol Clin 16: 691–710, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxena S, Kharbanda S. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 96: 8144–8149, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Reger PO, Barbe MF, Amin M, Renna BF, Hewston LA, MacDonnell SM, Houser SR, Libonati JR. Myocardial hypoperfusion/reperfusion tolerance with exercise training in hypertension. J Appl Physiol 100: 541–547, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol 38: 3–13, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Renna BF, Kubo H, MacDonnell SM, Crabbe DL, Reger PO, Houser SR, Libonati JR. Enhanced acidotic myocardial Ca2+ responsiveness with training in hypertension. Med Sci Sports Exerc 38: 847–855, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Renna BF, MacDonnell SM, Reger PO, Crabbe DL, Houser SR, Libonati JR. Relative systolic dysfunction in female spontaneously hypertensive rat myocardium. J Appl Physiol 103: 353–358, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Saito S, Hiroi Y, Zou Y, Aikawa R, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I. beta-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J Biol Chem 275: 34528–34533, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 3: 401–410, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Scheubel RJ, Bartling B, Simm A, Silber RE, Drogaris K, Darmer D, Holtz J. Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium: fragile balance of myocyte survival? J Am Coll Cardiol 39: 481–488, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Schultz RL, Swallow JG, Waters RP, Kuzman JA, Redetzke RA, Said S, de Escobar GM, Gerdes AM. Effects of excessive long-term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension 50: 410–416, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Shorofsky SR, Aggarwal R, Corretti M, Baffa JM, Strum JM, Al-Seikhan BA, Kobayashi YM, Jones LR, Wier WG, Balke CW. Cellular mechanisms of altered contractility in the hypertrophied heart: big hearts, big sparks. Circ Res 84: 424–434, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J 18: 1150–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Siu PM, Bryner RW, Murlasits Z, Alway SE. Response of XIAP, ARC, and FLIP apoptotic suppressors to 8 wk of treadmill running in rat heart and skeletal muscle. J Appl Physiol 99: 204–209, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410: 112–116, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284: 339–343, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]