Abstract
A functional evaluation of skeletal muscle oxidative metabolism during dynamic knee extension (KE) incremental exercises was carried out following a 35-day bed rest (BR) (Valdoltra 2008 BR campaign). Nine young male volunteers (age: 23.5 ± 2.2 yr; mean ± SD) were evaluated. Pulmonary gas exchange, heart rate and cardiac output (by impedance cardiography), skeletal muscle (vastus lateralis) fractional O2 extraction, and brain (frontal cortex) oxygenation (by near-infrared spectroscopy) were determined during incremental KE. Values at exhaustion were considered “peak”. Peak heart rate (147 ± 18 beats/min before vs. 146 ± 17 beats/min after BR) and peak cardiac output (17.8 ± 3.3 l/min before vs. 16.1 ± 1.8 l/min after BR) were unaffected by BR. As expected, brain oxygenation did not decrease during KE. Peak O2 uptake was lower after vs. before BR, both when expressed as liters per minute (0.99 ± 0.17 vs. 1.26 ± 0.27) and when normalized per unit of quadriceps muscle mass (46.5 ± 6.4 vs. 56.9 ± 11.0 ml·min−1·100 g−1). Skeletal muscle peak fractional O2 extraction, expressed as a percentage of the maximal values obtained during a transient limb ischemia, was lower after (46.3 ± 12.1%) vs. before BR (66.5 ± 11.2%). After elimination, by the adopted exercise protocol, of constraints related to cardiovascular O2 delivery, a decrease in peak O2 uptake and muscle peak capacity of fractional O2 extraction was found after 35 days of BR. These findings suggest a substantial impairment of oxidative function at the muscle level, “downstream” with respect to bulk blood flow to the exercising muscles, that is possibly at the level of blood flow distribution/O2 utilization inside the muscle, peripheral O2 diffusion, and intracellular oxidative metabolism.
Keywords: microgravity, muscle atrophy, physical deconditioning, exercise tolerance, near-infrared spectroscopy
bed rest (br) studies are widely utilized as a model to simulate exposure to microgravity. In more general terms, BR studies allow evaluation of the consequences on physiological functions deriving from extreme degrees of physical deconditioning. An analysis of oxidative metabolism was performed in previous BR studies (see e.g., Refs. 9, 10, 19), which found significant impairments in maximal O2 uptake (V̇o2 max) and cardiovascular function. In these studies, however, maximal O2 extraction by skeletal muscle, as inferred from the calculated maximal systemic arterial-venous O2 concentration difference [equals V̇o2 max/maximum cardiac output (Q̇)] was unaffected by BR. This appears surprising, considering that BR induces significant morphological and biochemical changes in skeletal muscles (4, 12, 19, 20, 21, 22, 35, 37), which should affect maximal systemic arterial-venous O2 concentration difference. In a previous study by our group (38), the peak capacity of fractional O2 extraction by vastus lateralis (VL) muscle during cycle ergometer exercise was markedly reduced (by ∼30%) after 35 days of BR (2007 BR-campaign); this impairment contributed to the observed reductions in peak O2 uptake (V̇o2peak) and exercise tolerance. When skeletal muscle oxidative metabolism is to be specifically evaluated, however, cycle ergometer or treadmill incremental exercise protocols are not ideal, since it is generally accepted that, during these types of exercise, the main limitation to oxidative metabolism derives from the maximal capacity of cardiovascular O2 delivery (14). This concept should apply even more strictly after BR, a condition in which, as mentioned above, cardiovascular function is substantially impaired (10, 19). In the present study, a functional evaluation of oxidative metabolism specifically aimed at skeletal muscles was carried out, before and after a 35-day BR, during a dynamic knee extension (KE) incremental exercise with one leg (1). KE exercise allows a “functional isolation” of a specific antigravity muscle group (the quadriceps femoris of one leg). The relatively small muscle mass (∼2.5 kg) involved in KE allows very high muscle blood flows (41) and significantly reduces any cardiovascular constraint to exercise tolerance, thereby allowing any impairment intrinsic to the investigated muscles to become fully manifest.
We hypothesized, after a 35-day BR, during KE incremental exercise (in which cardiovascular constraints to oxidative metabolism are significantly reduced) as follows: 1) a preserved peak capacity of cardiovascular O2 delivery (unchanged peak Q̇ and brain oxygenation); and 2) a significant impairment of skeletal muscle oxidative metabolism (lower V̇o2peak, lower peak capacity of fractional O2 extraction).
MATERIALS AND METHODS
Experimental Design
We studied nine healthy young men (age: 23.5 ± 2.2 yr; mean ± SD; body mass: 75.0 ± 9.9 kg; height: 1.79 ± 0.07 m; body mass index: 23.4 ± 1.9 kg/m2), physically active, but without a history of high athletic achievement, who participated in the 2008 Agenzia Spaziale Italiana-Osteoporosis and Muscular Atrophy BR campaign [35-day head-down (−6°) tilt BR]. None of the subjects was affected by neuromuscular or cardiovascular disorders at the time of the study. Blood hemoglobin concentration ([Hb]) was 14.8 ± 0.9 g/dl before BR and 16.3 ± 0.8 g/dl after BR.
Participants were informed about the aims and methods of the investigation and gave their written, informed consent. The experiments were carried out at the Valdoltra Orthopaedic Hospital of Ankaran, Slovenia. All procedures conformed to the declaration of Helsinki (2000) and were approved by the Slovenian National Medical Ethics Committee.
The study consisted of three different phases: 1) The first phase was baseline control experiments before BR. 2) The second phase was a 35-day BR period without countermeasures. During BR, no deviations from the lying position were permitted, and subjects were continuously monitored by video cameras. Neither exercise nor muscle contraction tests were allowed. To avoid weight gains due to the reduced activity level, the subjects followed a balanced diet during BR. 3) The third phase was the final experiments after BR. Measurements before BR were carried out during the last day before the subjects were put to bed, whereas measurements after BR were carried out during the second day after the subjects rose from bed.
All tests were conducted under close medical supervision, and subjects were continuously monitored by 12-lead electrocardiography.
Exercise Protocol
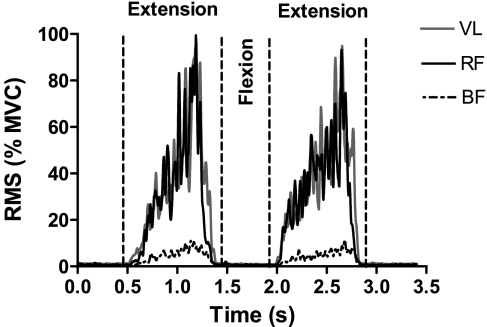
An incremental exercise protocol for V̇o2peak determination was carried out by utilizing a one-leg KE ergometer (modified Monark cycle ergometer), originally described by Andersen et al. (1). Subjects were constrained on an adjustable seat by a safety belt, which anchored the angle of the hip at ∼90°. Subjects pushed on a padded bar attached to a lever arm, extending the leg from ∼90° to ∼170° flexion. This type of exercise confines muscle contractile activity to the quadriceps femoris muscle, which is involved in the leg extension phase, while the return to the starting position is brought about passively by the momentum of the fly-wheel of the ergometer. Electromyography (EMG) (see below) excluded any significant intervention of the hamstrings muscle group during the exercise (Fig. 1).
Fig. 1.
Thigh muscle activation during an extension-flexion-extension cycle of the knee extension (KE) exercise. Electromyography (EMG) signal was processed by root mean square (RMS) algorithm and expressed as a percentage of the value obtained during maximal voluntary contraction (MVC). During the extension phase, RMS values for biceps femoris (BF) muscle were markedly lower than those for vastus lateralis (VL) and rectus femoris (RF) muscles. Substantially no muscle activation was observed for any muscle during flexion. These data confirm that knee flexors (BF) were not significantly involved during KE, and that the flexion phase was completely passive (see text for further details).
Before data collection, each subject was familiarized with the setup environment and the exercise protocol by short preliminary practice runs. After an initial 3 min of unloaded KE exercise, an incremental test to volitional exhaustion was performed with the right leg. Work rate increments were imposed every 2 min, to allow the subjects to reach exhaustion in ∼10 min. Resistance was applied to the ergometer flywheel as in a conventional cycloergometer. Throughout the test, the active KE and passive knee flexion cycle was carried out ∼40 times per minute, as imposed by a metronome. At this frequency, work rate increments corresponded to ∼10 W. During each cycle (total duration 1.5 s) KE lasted ∼1 s. That is to say, muscle contraction corresponded to ∼65% of the duty cycle. The test was terminated when the subjects were unable to continue the exercise at the required frequency, despite vigorous encouragement by the operators.
Measurements
Anthropometry.
Fat-free mass and total skeletal muscle mass were assessed by bioelectric impedance analysis performed by a tetrapolar device (Human IM, Dietosystem), in accordance with the conventional standard technique (32). The quadriceps femoris muscle mass was estimated from the thigh volume, determined with the use of thigh length, circumference, and skinfold measurements, as originally reported by Jones and Pearson (28).
Measurements during the incremental test.
For all variables, values determined at voluntary exhaustion were considered “peak” values. Time to exhaustion was taken as an index of performance. Pulmonary ventilation, tidal volume, ventilatory frequency, O2 uptake (V̇o2), and CO2 output (V̇co2) were determined on a breath-by-breath basis by means of a metabolic unit (Quark b2, Cosmed). Expiratory flow measurements were performed by a turbine flow meter calibrated before each experiment by a 3-liter syringe at three different flow rates. Calibration of O2 and CO2 analyzers was performed before each experiment by utilizing gas mixtures of known composition. The gas exchange ratio was calculated as V̇co2/V̇o2. The gas exchange threshold (GET) was determined for each subject by the V-slope method (2) on pulmonary V̇o2 and V̇co2 data, averaged every 10 s. All of the data related to GET were expressed as V̇o2 (l/min) and as a percentage of V̇o2peak.
Net mechanical efficiency was calculated for each subject as the ratio between the external mechanical power output (work rate, expressed in Watts) and the oxidative energy output. Also, this variable was expressed in Watts by assuming an energy equivalent of 20.9 kJ/l consumed O2, and the equivalence 1 W = 1 J/s.
Heart rate (HR) was determined by electrocardiography. Stroke volume (SV) was estimated beat-by-beat by impedance cardiography (Physio Flow, Manatec, Paris, France) (40). The accuracy of this device has been previously evaluated during incremental exercise in healthy subjects against the direct Fick method (40); in that study, the correlation coefficient between the two methods was r = 0.946 (P < 0.01), the mean difference was equal to −2.78 ± 12.33% (SD 2), and the accuracy of the impedance cardiography method was recognized to be “acceptable”. Q̇ was calculated as HR × SV.
Oxygenation changes in VL muscle and brain (frontal cortex) were evaluated by near-infrared spectroscopy (NIRS) (6, 15). A portable near-infrared, single-distance, continuous-wave photometer (HEO-100, Omron, Kyoto, Japan), which adopts an algorithm based on diffusion theory (43), was used for skeletal muscle measurements. The probe unit has a silicon photodiode as photodetector in the center and two light-emitting diodes (peak wavelengths 760 and 840 nm) on either side. The probe was firmly positioned to the skin overlying the lower one-third of the VL muscle (∼10–12 cm above the knee joint) of the right limb, parallel to the major axis of the thigh. The sampling frequency was set at 2 Hz. The distance between each light source and the photodetector was 3 cm, corresponding to a penetration depth of ∼1.5 cm. Concentration changes of oxygenated Hb + myoglobin (Mb) {Δ[oxy(Hb+Mb)]} and deoxygenated Hb+Mb {Δ[deoxy(Hb+Mb)]}, with respect to an initial value arbitrarily set equal to zero during the resting condition preceding the test (subject in the sitting position on the KE ergometer), were calculated and expressed in arbitrary units (43). The sum of the two variables {Δ[oxy(Hb+Mb) + deoxy(Hb+Mb)]} is related to changes in the total Hb volume in the muscle region of interest. Δ[Deoxy(Hb+Mb)] was taken as an estimate of skeletal muscle fractional O2 extraction because this variable, unlike Δ[oxy(Hb+Mb)], is relatively insensitive to changes in blood volume (18, 24). A “physiological calibration” of the Δ[deoxy(Hb+Mb)] values was performed both before and after BR during a transient limb ischemia: data obtained during exercise were expressed as a percentage of the values determined by obtaining a maximal deoxygenation of muscle, after the exercise period, by pressure cuff inflation (at 300–350 mmHg), carried out at the inguinal crease of the thigh for a few minutes until Δ[deoxy(Hb+Mb)] increase reached a plateau (for further details on the skeletal muscle NIRS measurements, see Refs. 23, 24, 30).
Δ[Deoxy(Hb+Mb)] kinetics were evaluated. Average data obtained during the last 20 s of each work rate were calculated and retained for analysis. As proposed by Ferreira et al. (16), the Δ[deoxy(Hb+Mb)] vs. work rate relationship was fitted by a sigmoid function of the type:
| (1) |
In Eq. 1, yBAS indicates the baseline, A is the amplitude of the response, and c is a constant dependent on d, the slope of the sigmoid, where c/d gives the x value corresponding to (yBAS + A)/2.
As for brain oxygenation, a different single-distance continuous wave NIRS instrument (Oxymon, Artinis, The Netherlands) was utilized. Headsets held a near-infrared emitter (laser light at 780 and 850 nm) and detector pair over the right frontal cortex region of the forehead; optodes were held in place by a plastic spacer with fixed optode distance. Spacing between optodes was 4.5 cm, corresponding to a penetration depth of 2–2.5 cm. Data were recorded at 10 Hz. The Beer-Lambert law was used to calculate micromolar (μM) changes in tissue oxygenation (Δ[oxyHb] and Δ[deoxyHb]) by using received optical densities and a differential path-length factor of 5.93 (44). Measurements obtained during exercise were normalized as changes from an initial value arbitrarily defined as 0 μM. Total (Δ[oxyHb + deoxyHb]) was taken as an index of changes in regional blood volume.
Reliability of tissue oxygenation indexes obtained by NIRS, evaluated by the intraclass correlation coefficient for repeated measurements on the same subject during different days, was recently found to be very high, for both brain and skeletal muscle (44); for brain measurements, these authors utilized the same instrument of the present study. NIRS measurements in cerebral (25) and muscle (47) tissue have been shown to be well correlated with local venous O2 saturation. Single-site NIRS measurements, as carried out in the present study, do not allow the determination of spatial heterogeneity in the dynamics of muscle oxygenation (29). Subudhi et al. (45) recently demonstrated that brain deoxygenation during high-intensity exercise occurs across the cortex, and thus directly affects also the motor areas that regulate central motor drive. Advantages and limitations of NIRS measurements in skeletal muscle and brain tissues are discussed in the reviews by Boushel et al. (6) and Ferrari et al. (15).
EMG recordings were collected from the VL, rectus femoris (RF), and biceps femoris (BF) muscles of the exercising leg by means of a four-channel EMG system (EMG100C, BIOPAC Systems). Further technical details on the EMG measurements can be found in Rejc et al. (39). EMG raw signals, recorded during the last 10 flexion-extension cycles of each work rate, were rectified, integrated, and divided by the duration of the exercise phase considered, as to obtain a mean integrated EMG (iEMG) value.
Before KE exercise, subjects performed two maximal voluntary isometric contractions (MVC) of knee extensors with the right lower limb (knee angle at 110°). Force analog output was sampled at a frequency of 1 kHz (MP100, BIOPAC Systems). During MVC, the EMG activity was defined in a 500-ms window centered at maximal force exertion.
Statistical Analysis
Results were expressed as means ± SD. Statistical significance of differences between the two conditions (after vs. before BR) was checked by two-tailed Student's t-test for paired data. When comparisons were made between data obtained in the present study with those obtained by Porcelli et al. (38) before and after BR during cycle ergometer exercise, a two-way repeated analysis of variance was utilized. A Tukey post hoc test was used to locate significant differences.
The level of significance was set at P < 0.05. Statistical analyses were carried out with a commercially available software package (Prism 4.0, GraphPad).
RESULTS
The main anthropometric and body composition parameters obtained before and after BR are given in Table 1. Body mass was ∼3% lower after BR. Percentagewise, the decrease was similar for fat-free mass, total muscle mass, and quadriceps muscle mass. Also, body fat decreased significantly after BR.
Table 1.
Anthropometric characteristics of subjects
| Before BR | After BR | Change, % | |
|---|---|---|---|
| Body mass, kg | 75.0 ± 9.9 | 72.4 ± 9.2* | −3.4 |
| BMI, kg/m2 | 23.4 ± 1.9 | 22.6 ± 1.7* | −3.4 |
| FFM, kg | 62.1 ± 6.3 | 60.6 ± 5.8* | −2.4 |
| Total muscle mass, kg | 31.9 ± 3.7 | 30.9 ± 3.4* | −3.1 |
| Quadriceps muscle mass, kg | 2.21 ± 0.16 | 2.13 ± 0.14* | −3.8 |
| Body fat, % | 17.0 ± 3.6 | 16.0 ± 3.2* | −5.1 |
Values are means ± SD for anthropometric and body composition data before and after bed rest (BR). Mean percentage of change for each parameter is also reported. BMI, body mass index; FFM, fat-free mass.
Significantly different vs. values before BR (P < 0.05).
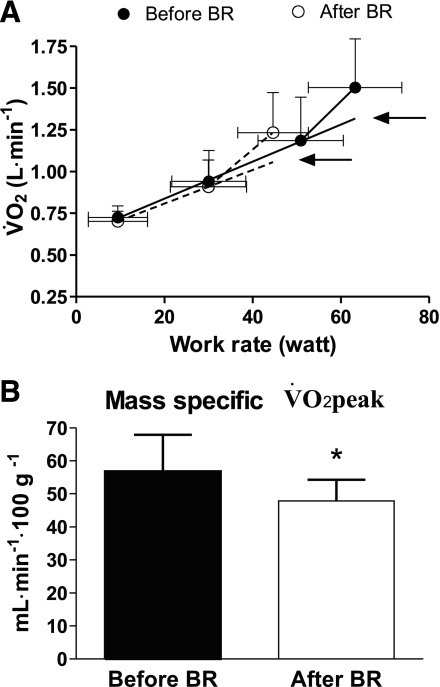
Pulmonary V̇o2 mean (±SD) values obtained during KE before and after BR are plotted as a function of work rate in Fig. 2A. To obtain this figure, individual V̇o2 values were grouped for discrete work rate intervals. Peak work rate was significantly lower after (45.8 ± 8.5 W) vs. before (63.2 ± 13.1 W) BR. V̇o2 values at the same submaximal work rates were very similar before and after BR. The values increased as a function of work rate with a biphasic pattern. A steeper rise of V̇o2 was indeed evident, starting from the work rate preceding exhaustion, at which point the slope of V̇o2 vs. work rate increased twofold (mean of individual values went from 11.1 to 25.8 ml O2·W−1·min−1 before BR and from 10.1 to 22.2 ml O2·W−1·min−1 after BR). The mean net mechanical efficiency was ∼25% at submaximal work rates and ∼11% at the highest work rate, both before and after BR.
Fig. 2.
A: mean (SD) O2 uptake (V̇o2) values (l/min) as a function of work rate (Watts) during KE incremental exercise. Knee extensor muscle-specific peak V̇o2 (V̇o2peak; indicated by the arrows) was determined by extrapolating the V̇o2 vs. work rate linear regressions, obtained before the inflection point, to peak work rate. See text for further details. B: mean (SD) V̇o2peak values normalized per unit of quadriceps femoris muscle mass, before and after bed rest (BR). *P < 0.05.
According to Richardson et al. (42), the inflection point of the V̇o2 vs. work rate relationship during incremental KE can be attributed to the intervention of ancillary muscles to stabilize the body and to the increased cost of exercise hyperpnea. Thus, to estimate KE muscle-specific V̇o2peak, the V̇o2 vs. work rate linear regression obtained before the inflection point was extrapolated to peak work rate (see Fig. 2A). The estimated V̇o2peak values are indicated by the arrows. Expressed as liters per minute, they were ∼19% lower after vs. before BR. When V̇o2peak was normalized per kilogram of quadriceps muscle mass, a percentagewise similar decrease (−16%) was found (Fig. 2B). Both before and after BR, individual GET values obtained by the V-slope method (see above) corresponded approximately to the inflection point of pulmonary V̇o2 vs. work rate; GET values were significantly lower after vs. before BR (−17%) (see Table 2). The lower V̇o2peak and GET after BR were associated with a lower exercise tolerance, as shown by the lower time to exhaustion during the incremental KE exercise (9.8 ± 2.1 min after vs. 12.3 ± 3.4 min before BR). Respiratory and cardiovascular variables at exhaustion were not affected by BR (Table 2).
Table 2.
Peak oxygen uptake and related parameters before and after bed rest
| Before BR | After BR | Change, % | |
|---|---|---|---|
| V̇e peak, l/min | 45.7 ± 6.9 | 41.4 ± 8.8 | −8.0 |
| Vt peak, liter | 1.32 ± 0.25 | 1.29 ± 0.17 | 0.0 |
| fV peak, breaths/min | 35.5 ± 5.1 | 32.3 ± 5.1 | −8.0 |
| V̇o2peak, l/min | 1.26 ± 0.27 | 0.99 ± 0.17* | −19.1 |
| V̇o2peak/QM, ml · min−1 · 100 g−1 | 56.9 ± 11.0 | 46.5 ± 6.4* | −16.2 |
| Rpeak | 1.08 ± 0.08 | 1.09 ± 0.10 | +1.7 |
| GET, l/min | 1.02 ± 0.15 | 0.84 ± 0.15* | −16.9 |
| Time to exhaustion, min | 12.3 ± 3.4 | 9.8 ± 2.1* | −18.3 |
| HRpeak, beats/min | 147 ± 18 | 146 ± 17 | −0.3 |
| SVpeak, ml | 122 ± 25 | 109 ± 16 | −7.9 |
| Q̇peak, l/min | 17.8 ± 3.3 | 16.1 ± 1.8 | −7.0 |
Values are means ± SD. Values are obtained during incremental knee extension exercise at exhaustion (peak values) before and after BR. V̇e, pulmonary ventilation; Vt, tidal volume; fV, ventilatory frequency; V̇o2peak, peak oxygen uptake; V̇o2peak/QM, oxygen uptake normalized per unit of quadriceps muscle mass; Rpeak, peak gas exchange ratio; GET, gas exchange threshold; time to exhaustion, exercise duration sustained by the subjects; HRpeak, peak heart rate; SVpeak, peak stroke volume; Q̇peak, peak cardiac output.
Significantly different from the corresponding value before BR (P < 0.05).
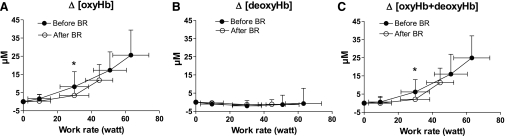
NIRS-obtained brain oxygenation data during KE are shown in Fig. 3. Mean (±SD) values of individual Δ[oxyHb], Δ[deoxyHb], and Δ[oxyHb+deoxyHb] data, grouped for discrete work rate intervals, are reported as a function of work rate. Both before and after BR, Δ[deoxyHb] values did not change significantly from the baseline, at any work rate. The Δ[oxyHb] did not increase from the baseline at the first work rate, after which it increased markedly up to exhaustion. Values after BR were significantly lower, both at submaximal (30 W) and at maximal work rates. The relationship between Δ[oxyHb+deoxyHb] and work rate was very similar to that of Δ[oxyHb].
Fig. 3.
Near-infrared spectroscopy (NIRS)-obtained brain (frontal cortex) oxygenation data during KE before and after BR. Mean (SD) values of changes in concentration of oxyhemoglobin (Δ[oxyHb]; A), changes in concentration of deoxyhemoglobin (Δ[deoxyHb]; B), and changes in concentration of total (oxy + deoxy) hemoglobin (Δ[oxyHb+deoxyHb]; C), all expressed as micromolar changes relative to an initial value arbitrarily set equal to zero, are shown as a function of work rate. *P < 0.05, before vs. after BR at the same relative work rate. See text for further details.
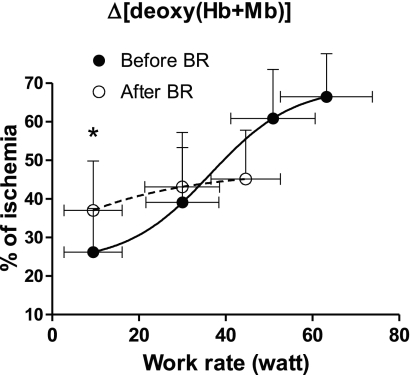
Mean (±SD) values of the NIRS-obtained muscle oxygenation index, Δ[deoxy(Hb+Mb)], which was taken as an estimate of VL fractional O2 extraction, are shown as a function of work rate in Fig. 4. Before BR, Δ[deoxy(Hb+Mb)] increased following a sigmoid pattern (Eq. 1), approaching a plateau at ∼75% of peak work rate. After BR, Δ[deoxy(Hb+Mb)] was significantly higher than before BR at the lowest work rate, whereas the ensuing Δ[deoxy(Hb+Mb)] increase was much less pronounced than before BR. The Δ[deoxy(Hb+Mb)] peak values were significantly lower after vs. before BR.
Fig. 4.
Mean (SD) values of the NIRS-obtained muscle oxygenation index {concentration changes of deoxygenated Hb + myoglobin (Mb); Δ[deoxy(Hb+Mb)]}, which was utilized to estimate VL fractional O2 extraction, are shown as a function of work rate during KE. The Δ[deoxy(Hb+Mb)] data are expressed as a percentage of those obtained during a transient limb ischemia induced at the end of the test. The sigmoid function (Eq. 1) utilized to fit the data before BR is also shown. *P < 0.05 vs. before BR data. See text for further details.
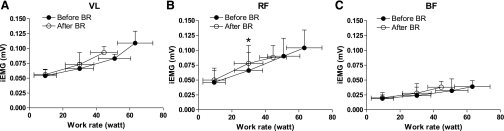
iEMG values (mV) for VL, RF, and BF during KE are shown in Fig. 5. In VL and RF, iEMG values increased as a function of work rate. Peak iEMG values were lower after vs. before BR, in association with the lower peak work rate. For the same absolute work rate, iEMG values were not different after vs. before BR, except for RF at 30 W (significantly higher values after BR). Increased iEMG is a sign of higher muscle activation, presumably attributable to recruitment of additional motor units. iEMG values for BF did not change substantially from the baseline, confirming that the knee flexors were not significantly activated during the exercise protocol (see also Fig. 1). The mean force exerted by the knee extensors during MVC was significantly lower after (530.1 ± 111.9 N) vs. before (683.2 ± 95.7 N) BR. The decrease in force was associated with iEMG values during MVC that were lower after (0.225 ± 0.076 mV for VL and 0.376 ± 0.199 mV for RF) vs. before (0.277 ± 0.05 mV for VL and 0.501 ± 0.237 mV for RF) BR.
Fig. 5.
Mean (SD) integrated EMG (iEMG) values during the last 10 flexion-extension cycles of each work rate during KE, divided by the duration of the considered phase for VL (A), RF (B), and BF (C) muscles. *P < 0.05 vs. before BR. See text for further details.
DISCUSSION
In this study, an incremental KE exercise was utilized to investigate skeletal muscle oxidative function in a group of young physically active volunteers undergoing 35 days of BR. During KE, the recruitment of a relatively small muscle mass, i.e., the quadriceps femoris of one leg, significantly reduces or eliminates any constraint to oxidative function deriving from cardiovascular O2 delivery, thereby highlighting any impairment intrinsic to skeletal muscle. As the main findings of the study, we observed after BR (vs. before) the following: 1) a significant decrease in V̇o2peak, GET, and time to exhaustion during the incremental KE exercise; 2) no change in peak cardiovascular O2 delivery, which reached levels well below those usually found during cycle ergometer exercise; 3) a preserved cerebral oxygenation; and 4) a significant decrease in the peak capacity of fractional O2 extraction by VL muscle. These findings suggest a relevant impairment of oxidative function occurring during BR at the skeletal muscle level. This impairment is a component of the microgravity-induced muscle deterioration and has a negative impact on exercise tolerance.
Before the “inflection point” (see Fig. 2), presumably attributable to the intervention of ancillary muscles to stabilize the body (42), V̇o2 values at the same submaximal work rate and the slope of the V̇o2 vs. work rate relationship were unaffected by BR, suggesting an unchanged efficiency of oxidative metabolism.
On the other hand, V̇o2peak values were significantly lower after BR. Percentagewise (−19%), the reduction was very similar to that observed in previous studies after the same BR period, but during cycle ergometer exercise (19, 38). Thus elimination of cardiovascular constraints did not influence the impairment of oxidative metabolism at peak exercise observed after BR. This appears in agreement with recent data obtained by Bringard et al. (7), who observed lower V̇o2peak values after BR in subjects cycling in the supine posture, which allowed V̇o2peak to be substantially unaffected by BR.
The extent of decrease in V̇o2peak did not differ substantially (−16%) once the BR-induced reduction in specific muscle mass was taken into account, and it was also very similar to that observed for GET (−17%). Lower values of V̇o2peak and GET after BR were associated with (and presumably were responsible for) a significant impairment of exercise tolerance, as shown by the significantly lower peak work rate and time to exhaustion values.
Peak cardiovascular O2 delivery was unaffected by BR. A small decrease in V̇o2peak after BR was indeed offset by a small increase in [Hb]. Peak cardiovascular O2 delivery, estimated as the product of V̇o2peak and arterial O2 concentration (calculated on the basis of the measured [Hb], after assuming an arterial Hb saturation of 98% and an O2 binding coefficient for Hb of 1.34 ml/g, and after neglecting the small contribution of physically dissolved O2), was 3.51 l O2/min before and 3.43 l O2/min after BR. This scenario is different from the substantial decrease in V̇o2peak after BR observed by previous authors during cycling exercise (9, 10, 19, 22, 38). In the present study, cardiovascular function at exhaustion, as represented by V̇o2peak values, was exploited for ∼70–75% of the peak levels reached during incremental cycle ergometer exercise, carried out before and after the same BR period of the present study [Porcelli et al. (38)]. This indirectly confirms that KE exercise, by involving only relatively small muscle masses (on average, 2.1–2.2 kg in the present study), did not represent a significant burden for the cardiovascular system.
Brain (frontal cortex) oxygenation, as estimated by NIRS, increased with work rate during KE, reaching the highest values at maximal exertion. This is suggested by the increased oxy signal (Δ[oxyHb]), by the unchanged deoxy signal (Δ[deoxyHb]), and by the increased total Hb signal (Δ[oxyHb+deoxyHb]). These patterns were unaffected by BR. The data suggest vasodilatation in cerebral tissue, mainly represented by oxygenated blood. Thus they allow exclusion of any increased O2 extraction or tissue hypoxia, possibly limiting exercise tolerance, as it has been described during exhausting cycle ergometer exercise (36). The results of the present study are not surprising, considering the relatively small muscle mass involved in KE and the consequent lack of cardiovascular constraints.
NIRS-obtained muscle Δ[deoxy(Hb+Mb)] has been frequently utilized to estimate fractional O2 extraction (the ratio between O2 utilization and O2 delivery) in skeletal muscle (11, 16, 23, 24, 30, 38). The dynamics of Δ[deoxy(Hb+Mb)] as a function of work rate during incremental cycle ergometer exercise have been described by Ferreira et al. (16) by fitting a sigmoid function. A similar pattern was observed in the present study for the data obtained before BR. After BR, on the other hand, Δ[deoxy(Hb+Mb)] values were higher than before BR at the lowest work rate and increased only slightly at higher work rates. Higher Δ[deoxy(Hb+Mb)] for the same work rate, and for the same V̇o2 (efficiency of KE was unaffected by BR, see above), would suggest an enhanced tissue O2 extraction to sustain the needed V̇o2 (30), assuming an identical optode placement and muscle penetration depth. Increased muscle fractional O2 extraction at submaximal work rates has been observed during KE in acute hypoxic exposure (41); in that study, the increased fractional O2 extraction compensated for the reduction in O2 delivery. In that study, moreover, fractional O2 extraction rose rapidly with work rate, attaining a plateau at values matching those found in normoxic condition. This was not the case for the present study after BR: Δ[deoxy(Hb+Mb)] increased only slightly as a function of work rate, reaching peak values (∼45% of the maximal values obtained by a transient ischemia), which were significantly lower than those obtained before BR (∼65%). The impairment in peak fractional O2 extraction observed in the present study after BR is very similar to that observed, after a similar BR exposure, by Porcelli et al. (38) during incremental cycle ergometer exercise. Thus, as also discussed above for V̇o2peak, with the present exercise protocol, in which constraints imposed by O2 delivery were minimized, a BR-induced impairment in peak capacity for muscle fractional O2 extraction was still evident.
In normal subjects, in normoxia and during whole body exercise, V̇o2 max is often considered to be mainly limited by the maximal capacity of O2 delivery to the exercising muscles (13, 14, 31). As discussed above, cardiovascular function (maximum Q̇) during incremental cycle ergometer exercise is significantly affected by BR (9, 19, 22, 38). However, as also mentioned above, in the present study, the KE exercise protocol eliminated the constraints to V̇o2 max related to cardiovascular O2 delivery, which, moreover, was unaffected by BR. It should be noted that the studies that examined muscle blood flow during KE [see e.g., Richardson et al. (41)] measured it in the vein draining from the muscles. To the best of our knowledge, the spatial distribution of blood in the muscle has never been determined during KE. Moreover, the distribution of blood flow should be evaluated in relation to the distribution of metabolism. The ratio between O2 utilization and O2 delivery is reflected by the Δ[deoxy(Hb+Mb)] signal obtained by NIRS. Koga et al. (29) recently described by multichannel NIRS a significant heterogeneity of Δ[deoxy(Hb+Mb)] kinetics in the quadriceps muscle during constant-load cycle ergometer exercise. The authors did not report if this heterogeneity was present also at steady state. We do not know of similar studies carried out during KE exercise or after BR. Maximal fractional O2 extraction during KE, however, was found to be very similar to that obtained during cycle ergometer exercise (38). Thus it may be assumed that the relationship between distribution of blood flow/distribution of metabolism is substantially unaffected by the high blood flows observed during KE. We recognize, however, that this relationship could, in theory, be altered by BR. At the same submaximal work rate and pulmonary V̇o2, the higher O2 extraction estimated by NIRS after BR (see Fig. 4) would suggest an overall impairment of O2 delivery. Any alteration of O2 delivery and/or of the O2 delivery/O2 utilization distribution [the latter variable would belong to the “peripheral” factors (FP) in the model by di Prampero (see below)] could impair oxidative metabolism independently from intramyocyte function.
The multifactorial model of V̇o2 max limitation proposed by di Prampero (for details, see Refs. 13, 14) was applied to the present KE data as an attempt to quantify the contribution of “central” factors (FQ) and FP in setting the upper limit to oxidative metabolism after BR. FP included peripheral vascular O2 delivery, peripheral O2 diffusion, and intramyocyte factors {represented here by the NIRS-obtained Δ[deoxy(Hb+Mb)] in the VL muscle}. FQ included cardiovascular O2 transport (represented here by the product of Q̇peak times [Hb]). Fp and FQ amounted to 0.56 and to 0.44, respectively, supporting the notion that FP are the main determinant of V̇o2peak during normoxic exercise with small muscle mass. It may be of interest to observe that, upon the data described above, the reduction in V̇o2peak following BR observed in the present study can be fully explained by the impairment in the peak capacity of fractional O2 extraction by skeletal muscles. By accounting for ∼60% in limiting V̇o2peak during KE, the observed ∼30% decrease in fractional O2 extraction led to a V̇o2peak decrease of ∼18–20%.
Our findings are similar to those obtained by McGuire et al. (34) in aged subjects; in both studies, a profound physical deconditioning, either BR or age related, determined a decline of oxidative function, which involved primarily the skeletal muscles.
Impairments of peripheral O2 diffusion and/or O2 utilization by muscle fibers could be attributable to structural and functional changes, which may involve both slow- and fast-twitch fibers, such as decreases in volume density of mitochondria, muscle oxidative enzymes activity, muscle capillary length and density (4, 19, 26); muscle fiber atrophy, greater in postural muscle groups and in slow type I vs. fast type II fibers (5, 20, 21); and substantial downregulation of proteins involved in oxidative metabolism (35). Moreover, qualitative changes, such as a shift in the contractile and metabolic phenotype toward that of a fast-twitch muscle, with significant reductions in myosin heavy chain-1 and increases in myosin heavy chain-2X relative content, and changes in motor unit recruitment profiles (12) toward a preferential recruitment of fast-twitch fibers, have been described after BR (5, 8, 27). It is noteworthy that changes in muscle oxidative capacity, vascular control, and fiber-type composition and/or recruitment may be associated with alterations in the O2 delivery to O2 utilization relationship. Previous studies, for instance, measured higher O2 extractions and lower microvascular O2 pressures, at low metabolic rates, in fast-twitch muscles, or in muscle regions in which these fibers predominate, compared with slow-twitch muscles (3, 17, 33).
Conclusions
We recognize that the quantitative analysis of the upper limits to oxidative metabolism (13, 14) described above may represent an oversimplification, since “central” and “peripheral” constraints to V̇o2 max are closely interrelated, as demonstrated by Peter Wagner's group over the years [see, e.g., Wagner (46)]. Our reasoning, however, should be straightforward. By utilizing the KE exercise protocol (relatively small muscle mass), 1) peak cardiovascular O2 delivery was not affected by BR; and 2) on the other hand, V̇o2peak and peak fractional O2 extraction in the exercising muscles were substantially lower after BR. Thus these decreases should be attributable to factors “downstream” with respect to bulk blood flow to the exercising muscles that is at the level of blood flow distribution/O2 utilization inside the muscle, peripheral O2 diffusion, and intracellular oxidative metabolism. Besides being relevant from the point of view of microgravity-induced muscle deterioration and for the definition of countermeasures aimed at reversing it, the present results appear of interest also for rehabilitation purposes following extreme physical deconditioning.
GRANTS
Financial support by the Agenzia Spaziale Italiana (ASI-OSMA Contract I/007/06/0-Workpackage 1B-32-1) and by Telethon-UILDM Italy (project GUP08007) is acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.S., S.L., M.M., S.P., E.R., and B.S. performed experiments; D.S., S.L., M.M., S.P., E.R., and B.S. analyzed data; D.S., R.P., P.E.d.P., and B.G. interpreted results of experiments; D.S. prepared figures; D.S. and B.G. drafted manuscript; D.S., P.E.d.P., and B.G. edited and revised manuscript; D.S. and B.G. approved final version of manuscript; B.G. conception and design of research.
ACKNOWLEDGMENTS
The authors are grateful to Gianni Biolo and Dr. Francesco Agostini (University of Trieste, Italy) for the excellent organization and coordination of the Valdoltra 2008 BR campaign, to the staff of the Valdoltra Hospital for the excellent technical collaboration and supervision of the subjects during the BR, and to the volunteers of the study for kind determination to perform our experiments. The authors also thank Ranieri Burelli and Luca Bazzichetto, whose technical assistance during the implementation of the KE ergometer was invaluable.
REFERENCES
- 1. Andersen P, Adams RP, SjØgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59: 1647–1653, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549: 597–605, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29: 197–206, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Borina E, Pellegrino MA, D'Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports 20: 65–73, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Boushel R, Langberg H, Olesen J, Gonzales-Alonso J, Bülow J, Kjær M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11: 213–222, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bringard A, Pogliaghi S, Adami A, De Roia G, Lador F, Lucini D, Pizzinelli P, Capelli C, Ferretti G. Cardiovascular determinants of maximal oxygen consumption in upright and supine posture at the end of prolonged bed rest in humans. Respir Physiol Neurobiol 172: 53–62, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Caiozzo VJ, Haddad F, Lee S, Baker M, Paloski W, Baldwin KM. Artificial gravity as a countermeasure to microgravity: a pilot study examining the effects on knee extensor and plantar flexor muscle groups. J Appl Physiol 107: 39–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capelli C, Antonutto G, Azabji Kenfack A, Cautero M, Lador F, Moia C, Tam E, Ferretti G. Factors determining the time course of V̇o2 max decay during bedrest: implications for V̇o2 max limitation. Eur J Appl Physiol 98: 152–160, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Convertino VA. Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc 29: 191–196, 1997 [DOI] [PubMed] [Google Scholar]
- 11. DeLorey DS, Kowalchuck JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- 12. di Prampero PE, Narici MV. Muscles in microgravity: from fibres to human motion. J Biomech 36: 403–412, 2003 [DOI] [PubMed] [Google Scholar]
- 13. di Prampero PE. Metabolic and circulatory limitations to V̇o2 max at the whole animal level. J Exp Biol 115: 319–331, 1985 [DOI] [PubMed] [Google Scholar]
- 14. di Prampero PE. Factors limiting maximal performance in humans. Eur J Appl Physiol 90: 420–429, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near-infrared spectroscopy. Can J Appl Physiol 29: 463–487, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 103: 1999–2004, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Ferreira LF, McDonough P, Behnke BJ, Musch TI, Poole DC. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fiber types. Respir Physiol Neurobiol 153: 237–249, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Ferreira LF, Poole DC, Barstow TJ. Muscle blood flow-O2 uptake interaction and their relation to on-exercise dynamics of O2 exchange. Respir Physiol Neurobiol 147: 91–103, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Ferretti G, Antonutto G, Denis C, Hoppeler H, Minetti AE, Narici MV, Desplanches D. The interplay of central and peripheral factors in limiting maximal O2 consumption in man after prolonged bed rest. J Physiol 501: 677–686, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitts RH, Riley DA, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol 588: 3567–3592, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fortney SM, Schneider VS, Greenleaf JE. The physiology of bed rest. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996. sect. 4, vol. II, chapt. 39, p. 889–939 [Google Scholar]
- 23. Grassi B, Marzorati M, Lanfranconi F, Ferri A, Longaretti M, Stucchi A, Vago P, Marconi C, Morandi L. Impaired oxygen extraction in metabolic myopathies: detection and quantification by near-infrared spectroscopy. Muscle Nerve 35: 510–520, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transition in humans. J Appl Physiol 95: 149–158, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Henson LC, Calalang C, Temp JA, Ward DS. Accuracy of a cerebral oximeter in healthy volunteers under conditions of isocapnic hypoxia. Anesthesiology 88: 58–65, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc 35: 95–104, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Hortobagyi T, Dempsey L, Fraser D, Zheng D, Hamilton G, Lambert J, Dohn L. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization. J Physiol 524: 293–304, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones PRM, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63p–66p, 1969 [PubMed] [Google Scholar]
- 29. Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lanfranconi F, Borrelli E, Ferri A, Porcelli S, Maccherini M, Chiavarelli M, Grassi B. Noninvasive evaluation of skeletal muscle oxidative metabolism after heart transplant. Med Sci Sports Exerc 38: 1374–1383, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Levine BD. V̇o2 max: what do we know, and what do we still need to know. J Physiol 586: 25–34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical measurements to assess human body composition. J Appl Physiol 60: 1327–1332, 1986 [DOI] [PubMed] [Google Scholar]
- 33. McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study. II. Effect of age on cardiovascular adaptation to exercise training. Circulation 104: 1358–1366, 2001 [PubMed] [Google Scholar]
- 35. Moriggi M, Vasso M, Fania C, Capitanio D, Bonifacio G, Salanova M, Blottner D, Rittweger J, Felsenberg D, Cerretelli P, Gelfi C. Long term bed rest with and without vibration exercise countermeasures: effects in human muscle protein dysregulation. Proteomics 10: 3758–3774, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol Heart Circ Physiol 277: H1045–H1052, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Pavy-Le Traon A, Heer M, Narici MV, Rittweger M, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies. Eur J Appl Physiol 101: 143–194, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Porcelli S, Marzorati M, Lanfranconi F, Vago P, Pišot R, Grassi B. Role of skeletal muscles impairment and brain oxygenation in limiting oxidative metabolism during exercise after bed rest. J Appl Physiol 109: 101–111, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Rejc E, Lazzer S, Antonutto G, Isola M, di Prampero PE. Bilateral deficit and EMG activity during explosive lower limb contractions against different overloads. Eur J Appl Physiol 108: 157–165, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Bucheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B, Lonsdorfer J. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 85: 202–207, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise V̇o2 during single leg knee-extensor exercise in humans. Am J Physiol Heart Circ Physiol 268: H1453–H1461, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75: 1911–1916, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Shiga T, Yamamoto K, Tanabe K, Nakase Y, Chance B. Study of an algorithm based on model experiments and diffusion theory for a portable tissue oximeter. J Biomed Opt 2: 154–161, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Subudhi AW, Dimmen AC, Roach RC. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol 103: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Subudhi AW, Miramon BR, Granger ME, Roach RC. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol 106: 1153–1158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58: 21–50, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Wilson JR, Mancini DM, McCully K, Ferraro N, Lanoce V, Chance B. Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation 80: 1668–1674, 1989 [DOI] [PubMed] [Google Scholar]