Abstract
An inverse relation exists between intake of flavonoid-rich foods, such as cocoa, and cardiovascular-related mortality. Favorable effects of flavonoids on the endothelium may underlie these associations. We performed a randomized, double-blind, placebo-controlled study to test the hypothesis that acute cocoa ingestion dose dependently increases endothelium-dependent vasodilation, as measured by an increase in brachial artery flow-mediated dilation (FMD), in healthy older adults. Measurements were obtained before (preingestion) and after (1- and 2-h postingestion) ingestion of 0 (placebo), 2, 5, 13, and 26 g of cocoa in 23 adults (63 ± 2 yr old, mean ± SE). Changes in brachial artery FMD 1- and 2-h postingestion compared with preingestion were used to determine the effects of cocoa. FMD was unchanged 1 (Δ−0.3 ± 0.2%)- and 2-h (Δ0.1 ± 0.1%) after placebo (0 g cocoa). In contrast, FMD increased both 1-h postingestion (2 g cocoa Δ0.0 ± 0.2%, 5 g cocoa Δ0.8 ± 0.3%, 13 g cocoa Δ1.0 ± 0.3%, and 26 g cocoa Δ1.6 ± 0.3%: P < 0.05 compared with placebo for 5, 13, and 26 g cocoa) and 2-h postingestion (2 g cocoa Δ0.5 ± 0.3%, 5 g cocoa Δ1.0 ± 0.3%, 13 g cocoa Δ1.4 ± 0.2%, and 26 g cocoa Δ2.5 ± 0.4%: P < 0.05 compared with placebo for 5, 13, and 26 g cocoa) on the other study days. A serum marker of cocoa ingestion (total epicatechin) correlated with increased FMD 1- and 2-h postingestion (r = 0.44–0.48; both P < 0.05). Collectively, these results indicate that acute cocoa ingestion dose dependently increases brachial artery FMD in healthy older humans. These responses may help to explain associations between flavonoid intake and cardiovascular-related mortality in humans.
Keywords: flavonoid, flavanol, chocolate, endothelium, endothelial function
the endothelium contributes critically to cardiovascular homeostasis by influencing factors such as vascular tone, vascular permeability, platelet aggregation, and thrombosis. As abnormal function of the endothelium is a key event in the atherosclerotic process (15), it is not surprising that endothelial dysfunction is a hallmark feature of many cardiovascular disease states (33). Moreover, it is not surprising that aging, which is a primary risk factor for the development of cardiovascular disease and atherosclerosis (29, 36), is associated with marked endothelial dysfunction. Specifically, in response to stimulation of the vascular endothelium via pharmacological substances, such as acetylcholine, or physiological stimuli, such as increases in vascular shear stress, a reduced endothelium-dependent vasodilatory response occurs in healthy older adults compared with healthy young adults (7, 11, 12, 41). As low levels of brachial artery flow-mediated dilation (FMD), a commonly used method to assess endothelium-dependent vasodilation, are predictive of increased cardiovascular morbidity and mortality in older adults (45), identification of methods/therapies that increase brachial artery FMD in this population are of biomedical importance.
High intake of flavonoid-rich foods is associated with reduced cardiovascular-related morbidity and mortality in humans (4, 25, 31). Mechanism(s) underlying these associations remain unclear but may include favorable effects of flavonoids on the endothelium (9). Previous studies established that acute (hours) and chronic (days to weeks) ingestion of flavonoid-rich foods, such as cocoa and/or chocolate, increase brachial artery FMD in healthy and diseased individuals (1, 13, 18–23, 40, 44). However, whether these responses are dose dependent has not been established in older healthy adults. Studies utilizing powerful methods to assess endothelial function in older healthy adults would be important based on the prognostic significance these measures possess (32, 37, 45). Accordingly, we tested the hypothesis that acute cocoa ingestion dose dependently increases brachial artery FMD in healthy older adults.
METHODS
Subjects
Twenty-three volunteers completed the study. All subjects were healthy based on medical history, physical examination, and routine blood chemistry. Inclusion criteria included arterial blood pressure (BP) at rest <140/90 mmHg, 12-h fasting glucose <5.55 mmol/l, nonsmoker (past 5 yr), body mass index <30 kg/m2, and total or low-density cholesterol (LDL) <6.22 and 4.15 mmol/l, respectively (33a). All subjects were sedentary to recreationally active (i.e., no subject exercised more than 3 times/wk for greater than 30 min). The Institutional Review Board at the Pennsylvania State University College of Medicine approved the study and written informed consent was obtained from all participants prior to testing.
Experimental Protocol
This study was randomized, double-blinded, and placebo-controlled. After screening, eligible subjects were invited to participate in five experimental sessions. These sessions (i.e., study days) were separated by at least 72 h and each took place at the same time of day (morning). Subjects were fasted (overnight) and did not consume caffeine or alcohol-containing products for the previous 12 h. In addition, subjects did not ingest cocoa or chocolate-containing products in the 24-h period preceding each study day.
After arrival in the laboratory subjects were positioned supine and a catheter was inserted in an arm vein for blood sampling. Before each FMD measurement, blood pressure (BP) and heart rate (HR) were measured in triplicate and blood samples were obtained. Following at least 30 min of rest, preingestion FMD measurements were performed. Subjects then ingested 1 of 5 experimental drinks (applied randomly each study day and each was only administered once in each subject). FMD measurements were repeated 1- and 2-h postingestion each study day. About 30 min after the 2-h postingestion FMD measurement a nitroglycerin trial was performed.
Experimental drinks were prepared from individually wrapped dry powder packets packaged by a third party (Barry Callebaut, Lebbeke-Wieze, Belgium). The only identifying mark on these packets was a letter code (A-E) that was recorded on each study day. This code was held from investigators and study personnel until study completion. Drinks were similar in appearance and taste and were prepared in warm water to a final volume of 250 ml. The experimental drinks contained 0 (placebo), 2, 5, 13, or 26 g cocoa. Detailed nutritional information is provided in Table 1.
Table 1.
Nutritional composition of experimental drinks
| Variable | Placebo 0 g cocoa | 2 g cocoa | 5 g cocoa | 13 g cocoa | 26 g cocoa |
|---|---|---|---|---|---|
| Cocoa, g | 0 | 2 | 5 | 13 | 26 |
| Calories, kcal | 122 | 120 | 116 | 107 | 96 |
| Fat, g | 4.4 | 4.3 | 4.3 | 4.2 | 4.0 |
| Saturated fat, g | 4.2 | 4.1 | 4.0 | 3.6 | 3.2 |
| Carbohydrates, g | 16 | 16 | 16 | 16 | 16 |
| Sugars, g | 13 | 13 | 12 | 10 | 7 |
| Protein, g | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 |
| Dietary fiber, g | 1.1 | 1.4 | 2.0 | 3.4 | 5.1 |
| Sodium, mg | 371 | 361 | 347 | 312 | 270 |
| Vitamin A, IU | 56 | 54 | 51 | 43 | 33 |
| Vitamin C, mg | 0 | 0 | 0 | 0 | 0 |
| Calcium, mg | 261 | 252 | 238 | 204 | 164 |
| Iron, mg | 0.3 | 0.4 | 0.6 | 1.0 | 1.6 |
| Caffeine, mg | 0 | 0 | 6 | 12 | 27 |
| Theobromine, mg | 0 | 27 | 66 | 138 | 279 |
| Total polyphenols, mg | 330 | 420 | 420 | 840 | 1470 |
| Catechin, mg | 0.0 | 3.0 | 8.1 | 21.6 | 48.0 |
| Epicatechin, mg | 0.0 | 6.3 | 17.7 | 45.0 | 96.0 |
| Flavan-3-ols, mg | 0.0 | 9.3 | 25.8 | 66.6 | 146.0 |
| PAC 1-10, mg | 0.0 | 46 | 128 | 323 | 783 |
| Total PACs, mg | 0.0 | 69 | 180 | 465 | 1095 |
PAC, procyanidin.
Measurements
BP and HR.
BP (Dinamap; right arm) and HR (3-lead ECG) were determined noninvasively.
Flow-mediated dilation.
The FMD technique was used to quantify endothelium-dependent dilation in the left brachial artery (6). Established guidelines/recommendations were adhered to strictly (8, 43). A high-resolution ultrasound system (Philips iE33, Bothell, WA) utilizing a variable frequency linear array transducer (L11–3) was used to image the brachial artery. The transducer was positioned ∼5 cm proximal to the antecubital crease and held in place with a stereotactic clamp. Additionally, a 4 MHz pulsed-wave Doppler probe (model 500M, Multigon Industries, Yonkers, NY) was positioned ∼5 cm proximal to the ultrasound transducer to allow simultaneous assessment of brachial artery mean blood flow velocity (MBV). This Doppler probe was adjusted until optimal signals were obtained and then was taped in place. The insonation angle of this Doppler probe was <45°. After obtaining baseline brachial artery images and MBV (over 3 min), a cuff positioned 5 cm distal to the antecubital crease was inflated to 250 mmHg (E-20 Rapid Cuff Inflator, D.E. Hokanson, Bellevue, WA) for 5 min before being rapidly deflated to 0 mmHg. Data were collected for 3 min after cuff release. Measurements from distinct anatomical landmarks were recorded and images were printed for reference to assure similar placement of the probes and occlusion cuff throughout the studies (both trial-to-trial and day-to-day). Ultrasound images and blood flow velocities were digitally recorded for later analysis (Brachial Tools, Medical Imaging Applications, Iowa City, IA, for brachial artery diameter and Chart, ADInstruments, New Castle, Australia, for MBV). Brachial artery images were obtained at end diastole.
FMD was calculated as the percent change in brachial artery diameter from baseline (i.e., in the 2-min period before cuff inflation) to the peak increase after cuff release, using a five-point moving average (12, 17). Shear rate (4 × brachial artery MBV/brachial artery diameter) area under the curve was used to quantify the stimulus for FMD. This was done by summing shear rate, on a beat-by-beat basis, from cuff release to the time in which the peak brachial artery dilator response was observed (34). Forearm blood flow was calculated as brachial artery MBV × (brachial artery diameter/2)2 × 60.
Endothelium-independent dilation.
Endothelium-independent dilation was quantified by measuring the brachial artery dilator response to sublingual nitroglycerin (0.4 mg). Brachial artery images were obtained for 3 min prior (baseline) to and for 8 min after nitroglycerin administration using the methods described above. Peak changes (%change from baseline) in brachial artery diameter after nitroglycerin are reported.
Biochemical parameters.
Blood samples collected during the study (preingestion and 2-h postingestion) were analyzed for norepinephrine (HPLC), oxidized LDL (ELISA, Mercodia, Uppsala Sweden), glucose (EIA), and insulin (chemiluminescent assay). This was done as these markers, or systems they provide insight into, may be have been influenced by our intervention and can influence endothelium-dependent vasodilation (2, 5, 26, 28). Additionally, we also measured serum total epicatechin to document the effect of cocoa ingestion (preingestion, 1-h postingestion, and 2-h postingestion). This was done using a rapid liquid chromatography tandem mass spectrometry assay, as described in detail elsewhere (38).
Statistical Analysis
Linear mixed effects models were fit to assess changes from preingestion values in continuous outcomes (e.g., FMD) with respect to dose [placebo (0), 2, 5, 13, 26 g cocoa] and time point (preingestion, 1- and 2-h postingestion). As there were two repeated factors in this study (dose and time), the Kronecker product of an unstructured matrix (for dose) and a first-order auto-regressive matrix (for time) were used in the mixed effects model to specify the variance-covariance structure. Residual diagnostics were used to evaluate model fit. To account for multiple hypotheses testing, the adaptive Holm adjustment to the step-down Bonferroni method was used to correct P values (27). All hypotheses tests were two sided and all analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC). Pearson product moment correlations were used to determine strength of relations between variables of interest. Statistical significance was accepted at P < 0.05. All data are reported as means ± SE.
RESULTS
Subject Characteristics
Subject characteristics are presented in Table 2.
Table 2.
Subject characteristics
| Variable | (n = 23) |
|---|---|
| Sex | 9 male/14 female |
| Age, yr | 63 ± 2 |
| Height, cm | 170.6 ± 1.6 |
| Body mass, kg | 72.7 ± 2.3 |
| BMI, kg/m2 | 24.9 ± 0.6 |
| Systolic BP, mmHg | 124 ± 2 |
| Diastolic BP, mmHg | 71 ± 1 |
| Mean BP, mmHg | 91 ± 1 |
| Heart rate, beats/min | 59 ± 2 |
| Total cholesterol, mmol/l | 5.27 ± 0.14 |
| LDL cholesterol, mmol/l | 3.18 ± 0.14 |
| HDL cholesterol, mmol/l | 1.50 ± 0.08 |
| Glucose, mmol/l | 4.63 ± 0.03 |
Values are means±SE. BMI, body mass index; BP, blood pressure; LDL, low-density cholesterol; HDL, high-density cholesterol.
Acute Effect of Cocoa Ingestion on Brachial Artery FMD
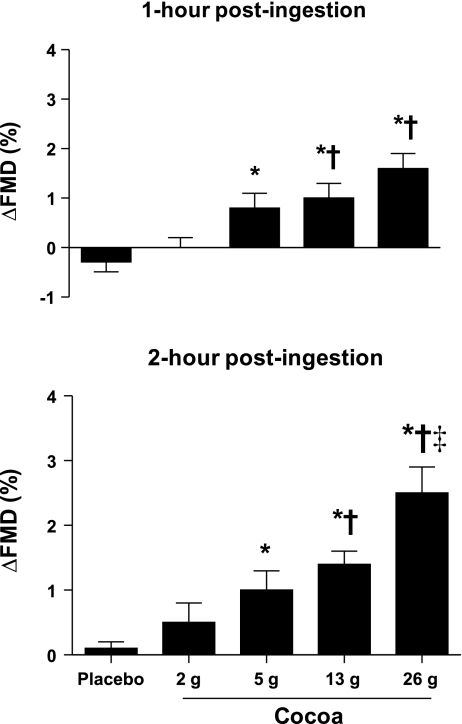
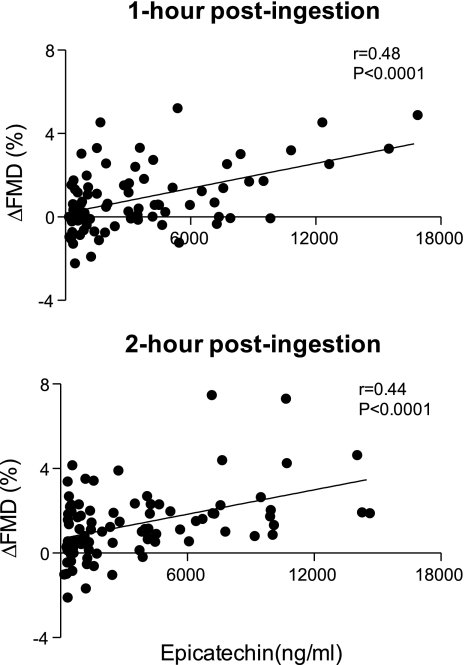
Brachial artery diameters at rest were similar before drink ingestion (i.e., preingestion) each study day. Additionally, brachial artery diameters at rest were unaltered by the interventions (Table 3). FMD values on the control (i.e., placebo) day did not differ from preingestion values measured on the other study days (Table 3). FMD was similar before (preingestion) and after drink ingestion (at 1- and 2- h postingestion) on the placebo (0 g cocoa) day. In contrast, FMD increased from preingestion levels on the other study days (at both 1- and 2-h postingestion), reaching statistical significance at the three highest levels of cocoa ingestion (5, 13, and 26 g cocoa) compared with responses on the placebo day (Table 3 and Fig. 1). The largest increase in FMD was observed after ingestion of the highest quantity of cocoa (26 g cocoa). Total epicatechin, measured 1- and 2-h postingestion correlated with increases in FMD at the same time points (Fig. 2).
Table 3.
Brachial artery responses to drink ingestion
| Study day | Time point | FBF, ml | Brachial Diameter, mm | FMD, Δ | FMD, % | Shear, AUC au |
|---|---|---|---|---|---|---|
| Placebo | Preingestion | 18 ± 2 | 4.0 ± 0.1 | 0.17 ± 0.01 | 4.2 ± 0.3 | 3,487 ± 281 |
| (0 g cocoa) | 1 h post | 17 ± 1 | 4.0 ± 0.1 | 0.15 ± 0.01 | 3.9 ± 0.2 | 3,525 ± 266 |
| 2 h post | 17 ± 1 | 3.9 ± 0.1 | 0.17 ± 0.01 | 4.2 ± 0.3 | 3,824 ± 411 | |
| 2 g cocoa | Preingestion | 18 ± 2 | 4.1 ± 0.1 | 0.18 ± 0.01 | 4.6 ± 0.3 | 3,383 ± 311 |
| 1 h post | 17 ± 1 | 4.0 ± 0.1 | 0.18 ± 0.01 | 4.6 ± 0.3 | 3,761 ± 259 | |
| 2 h post | 17 ± 1 | 4.0 ± 0.1 | 0.20 ± 0.02 | 5.1 ± 0.4 | 3,992 ± 433 | |
| 5 g cocoa | Preingestion | 18 ± 2 | 4.0 ± 0.1 | 0.16 ± 0.01 | 4.0 ± 0.3 | 3,508 ± 308 |
| 1 h post | 17 ± 2 | 4.0 ± 0.1 | 0.19 ± 0.01 | 4.9 ± 0.3 | 3,302 ± 315 | |
| 2 h post | 18 ± 2 | 4.0 ± 0.1 | 0.20 ± 0.01† | 5.0 ± 0.4† | 3,926 ± 325 | |
| 13 g cocoa | Preingestion | 18 ± 2 | 4.0 ± 0.1 | 0.14 ± 0.01 | 3.6 ± 0.2* | 3,494 ± 280 |
| 1 h post | 16 ± 1 | 4.1 ± 0.1 | 0.19 ± 0.01†‡ | 4.6 ± 0.3†‡ | 3,498 ± 341 | |
| 2 h post | 17 ± 1 | 4.0 ± 0.1 | 0.20 ± 0.01†‡ | 5.1 ± 0.2† | 4,111 ± 325 | |
| 26 g cocoa | Preingestion | 17 ± 2 | 4.0 ± 0.1 | 0.14 ± 0.01 | 3.6 ± 0.2 | 3,538 ± 250 |
| 1 h post | 18 ± 2 | 4.0 ± 0.1 | 0.21 ± 0.02†‡§ | 5.2 ± 0.3†‡ | 3,865 ± 275 | |
| 2 h post | 18 ± 1 | 4.0 ± 0.1 | 0.24 ± 0.02†‡§ | 6.1 ± 0.4†§ | 3,782 ± 337 |
Values are means ± SE. FBF, forearm blood flow at rest; FMD, brachial artery flow-mediated dilation; brachial diameterm brachial artery diameter at rest; Shear, area under the curve (AUC) for shear (4*brachial artery mean blood velocity/brachial artery diameter) from cuff release to the point of maximal brachial dilation during the FMD measurement.
P < 0.05 compared with preingestion (2 g cocoa);
P < 0.05 compared with preingestion (same study day);
P < 0.05 compared with 2 g cocoa response (same time point);
P < 0.05 compared with 5 g cocoa response (same time point).
Fig. 1.
Brachial artery flow-mediated dilation (FMD) measured during the studies. Values are presented as the changes from preingestion levels on each study day. FMD was assessed 3 time each study day: 1) preingestion, 2) 1-h postingestion, and 3) 2-h postingestion. FMD increased significantly after ingestion of the 3 highest quantities of cocoa (i.e., 5, 13, and 26 g cocoa) compared with responses observed on the placebo day at both the 1 (top graph)- and 2- h (bottom) time points. Values are means ± SE. *P < 0.05 vs. placebo; †P < 0.05 vs. 2 g cocoa; ‡P < 0.05 vs. 5 g cocoa.
Fig. 2.
Total serum epicatechin measured 1- and 2-h postingestion plotted as a function of the change in brachial artery FMD. Change in brachial artery FMD represents the change from preingestion levels. The strength of the relation (i.e., the Pearson product moment correlations) was similar at both 1- and 2-h postingestion.
The sum of the brachial artery shear rate after cuff release to the peak dilatory response (i.e., the stimulus for FMD) was similar preingestion and postingestion at all time points (Table 3). Endothelium-independent dilation (sublingual nitroglycerin) was similar after ingestion each study day (17.3 ± 1.0, 16.2 ± 1.1, 17.3 ± 1.0, 17.3 ± 1.0, and 15.1 ± 0.8% for placebo, 2, 5, 13, and 26 g cocoa, respectively). Responses to the experiments were similar in men and women (data not shown).
Acute Effect of Cocoa Ingestion on Hemodynamic and Biochemical Parameters
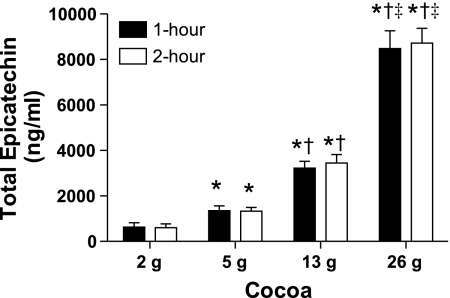
Small changes in BP were present at various time points during the experiments, but responses were similar on placebo and experimental days (Table 4). Biochemical parameters were unchanged with the experiments for measures of insulin, norepinephrine, and oxidized LDL. Small, but significant decreases were noted at some time points for glucose (Table 5). Total epicatechin was below the level of detection (<150 ng/ml) preingestion (all study days) and postingestion on the placebo day. In contrast, total epicatechin increased in a dose-dependent fashion on the other study days (Fig. 3).
Table 4.
BP and heart rate responses to drink ingestion
| Cocoa |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (0 g cocoa) |
2 g |
5 g |
13 g |
26 g |
|||||||||||
| Time point | Pre | 1 h | 2 h | Pre | 1 h | 2 h | Pre | 1 h | 2 h | Pre | 1 h | 2 h | Pre | 1 h | 2 h |
| Variable | |||||||||||||||
| Heart rate, beats/min | 58 ± 2 | 60 ± 1 | 57 ± 2 | 60 ± 2 | 59 ± 2 | 57 ± 2 | 59 ± 2 | 58 ± 2 | 57 ± 2 | 59 ± 2 | 58 ± 2 | 57 ± 2 | 58 ± 2 | 58 ± 2 | 58 ± 2 |
| Δ Heart rate, beats/min | 2 ± 1 | −1 ± 1 | −1 ± 1 | −3 ± 1 | −1 ± 1 | −2 ± 1 | −1 ± 1 | −2 ± 1 | 0 ± 1 | 0 ± 1 | |||||
| Systolic BP, mmHg | 124 ± 2 | 127 ± 3 | 127 ± 2 | 123 ± 2 | 125 ± 3 | 128 ± 2* | 127 ± 2 | 127 ± 2 | 128 ± 3 | 123 ± 2 | 124 ± 2 | 126 ± 2 | 123 ± 2 | 126 ± 2 | 129 ± 3* |
| ΔSystolic BP, mmHg | 3 ± 2 | 3 ± 2 | 2 ± 1 | 5 ± 2* | 0 ± 1 | 1 ± 2 | 0 ± 2 | 3 ± 2 | 3 ± 1 | 6 ± 2* | |||||
| Diastolic BP, mmHg | 72 ± 2 | 72 ± 1 | 74 ± 1* | 71 ± 1 | 72 ± 1 | 75 ± 1* | 72 ± 1 | 73 ± 1 | 73 ± 2 | 70 ± 1 | 72 ± 1 | 73 ± 1* | 72 ± 1 | 74 ± 2* | 75 ± 1* |
| ΔDiastolic BP, mmHg | 1 ± 1 | 2 ± 1* | 0 ± 1 | 4 ± 1* | 1 ± 1 | 1 ± 1 | 2 ± 1 | 3 ± 1* | 3 ± 1 | 3 ± 1 | |||||
| Mean BP, mmHg | 91 ± 2 | 92 ± 1 | 93 ± 2 | 91 ± 1 | 92 ± 1* | 94 ± 1 | 92 ± 1 | 93 ± 1 | 93 ± 2 | 90 ± 1 | 91 ± 1 | 93 ± 1* | 91 ± 1 | 93 ± 2* | 95 ± 2* |
| ΔMean BP, mmHg | 1 ± 1 | 2 ± 1 | 1 ± 1* | 3 ± 1 | 1 ± 1 | 2 ± 1 | 1 ± 1 | 3 ± 1* | 2 ± 1* | 4 ± 1* | |||||
Values are means ± SE. BP, blood pressure; Pre, before drink ingestion; Post, 2-h postdrink ingestion.
P < 0.05 from Pre.
Table 5.
Biochemical responses to drink ingestion
| Cocoa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (0 g cocoa) |
2 g |
5 g |
13 g |
26 g |
||||||
| Time point | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Variable | ||||||||||
| Glucose, mmol/l | 4.76 ± 0.11 | 4.57 ± 0.09* | 4.73 ± 0.11 | 4.48 ± 0.11* | 4.75 ± 0.10 | 4.56 ± 0.08* | 4.65 ± 0.11 | 4.54 ± 0.10 | 4.74 ± 0.09 | 4.61 ± 0.09 |
| Insulin, μU/ml | 3.7 ± 0.7 | 4.2 ± 0.7 | 3.8 ± 0.7 | 4.1 ± 0.6 | 3.4 ± 0.5 | 3.7 ± 0.6 | 3.9 ± 0.6 | 4.3 ± 0.7 | 3.1 ± 0.6 | 4.0 ± 0.6 |
| Norepinephrine, nM | 2.1 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.2 | 2.0 ± 0.1 | 2.5 ± 0.2 | 2.2 ± 0.1 |
| Oxidized LDL, ng/ml | 1,659 ± 363 | 1,659 ± 362 | 1,565 ± 353 | 1,514 ± 339 | 1,682 ± 370 | 1,705 ± 368 | 1,555 ± 365 | 1,594 ± 386 | 1,720 ± 377 | 1,693 ± 365 |
Values are means ± SE.
P < 0.05 from Pre.
Fig. 3.
Total serum epicatechin measured 1- and 2-h post-cocoa ingestion. Preingestion values, as well as values 1- and 2-h postingestion on the placebo day, are not reported, as most samples were found to contain levels below the minimal level of detection (150 ng/ml). It is clear, at both time points, that cocoa ingestion dose dependently increased serum total epicatechin levels. *P < 0.05 compared with 2 g cocoa (same time point); †P < 0.05 compared with 5 g cocoa (same time point); ‡P < 0.05 compared with 13 g cocoa (same time point).
DISCUSSION
The results of this randomized, double-blinded, placebo-controlled study indicate that acute cocoa ingestion dose dependently increases brachial artery FMD in healthy older adults. Increases in FMD with cocoa ingestion correlated with a serum biomarker of cocoa ingestion (total epicatechin), suggesting a possible mechanistic link between the two effects. These findings may be important as brachial FMD is a measure of endothelial function with prognostic significance, and thus interventions that increase FMD could reduce individual risk (32, 37, 43, 45).
Previous studies indicate that cocoa/chocolate ingestion increases endothelium-dependent vasodilation in various groups. Specifically acute cocoa/chocolate ingestion increases brachial artery FMD in young healthy adults (44), adults with risk factors for cardiovascular disease (13, 19), smokers (22, 23), and diabetics (1). However, acute intake does not increase endothelium-dependent vasodilation in all populations (14). In most of these previous studies the quantities of cocoa/chocolate ingested were very high, being roughly equivalent to 13 (13) or 26 g of cocoa (19, 22, 23, 44). In only one of these previous studies was the effect of lower quantities of cocoa ingestion determined (75, 371, and 963 mg of total flavanols) and this determination was made only in medicated type 2 diabetics (1). In that study (1) the total flavanol content ingested approximated levels in our 2 g (69 mg), 13 g (465 mg), and 26 g cocoa trials (1,095 mg). Thus the present study confirms these prior findings that higher quantities of total flavanols intake acutely increase FMD and extends them by showing that positive effects occur at even lower levels of intake in a distinctly different subject population. It is possible that the positive effect we observed at lower intakes of cocoa may not be observed in other subject populations. These findings are important as they emphasize the importance of considering subject population and quantity of chocolate/cocoa ingested when considering responses to chocolate/cocoa ingestion. These findings should be of interest as depressed levels of brachial artery FMD measures have prognostic significance in various populations, including healthy older adults (32, 37, 45).
The mechanisms underlying the acute effect of cocoa ingestion on increased FMD in the present study are not known. However, prior studies suggest that cocoa/chocolate ingestion enhances vascular production of nitric oxide (NO). Specifically, increased FMD resulting from cocoa/chocolate ingestion are reversed by NO synthase inhibition (16, 22) and circulating biomarkers of NO synthesis are increased (19, 22, 42). Additionally, flavanols appear to increase endothelial-derived NO synthase activity (30, 35) and lower vascular arginase activity (39). If NO production was increased by cocoa, we have no evidence that this was mediated by an antioxidant effect of cocoa, as we observed no change in oxidized LDL levels. However, it is possible that cocoa increased antioxidant potential and thus increased NO bioavailability independent of changes in oxidized LDL. Also of importance is our observation that vascular responses to nitroglycerin were similar after cocoa ingestion (0–26 g cocoa) on all study days, consistent with prior findings (1, 20, 44). Collectively, these findings suggest that cocoa-induced increases in brachial artery FMD are likely to be mediated by enhanced production rather than enhanced vascular responsiveness to NO.
In the present study we chose to administer a wide range of cocoa quantities to our subjects. This approach precludes us from identifying the exact chemical substance(s) underlying the observed response, as cocoa is a complex food containing a large number of nutritive compounds (Table 1). This issue would persist if we had chosen to have subjects ingest an alternative food source, such as chocolate. In our opinion this is not a limitation, as cocoa will continue to be a major ingredient in many foods that are widely available and thus understanding responses to cocoa ingestion are important. Previous studies suggest that specific compounds contained in cocoa, such as −(-)epicatechin increases endothelium-dependent vasodilation (i.e., improve endothelial function) in humans (40). Consistent with these data we observed a modest correlation between serum total epicatechin after cocoa ingestion and increases in FMD (r = 0.44–0.48). Thus, −(-)epicatechin, or a related compound, may have contributed to the increases in brachial artery FMD we observed.
This study has several limitations. First, we did not determine how long the beneficial effects of acute cocoa ingestion persisted. Second, we did not test whether chronic ingestion results in similar increases in FMD, at perhaps lower levels of ingestion. Third, the mechanism(s) responsible for the beneficial effects of cocoa on FMD may differ between acute and chronic ingestion. For example, in the present study we observed no decrease in BP at rest with acute cocoa ingestion. In contrast longer term ingestion does appear to decrease BP (10). These data suggest that different mechanisms may underlie the beneficial effects of cocoa/chocolate when considered in the context of acute vs. chronic ingestion. Fourth, the observed responses may be specific to the study population and thus the results of this study should not be applied to other populations without careful consideration. Last, endothelium-independent vasodilation (i.e., responses to sublingual nitroglycerin) was assessed only once per study day (end of the protocol) to avoid long-standing nitroglycerin effects on subsequent measurements. Thus we cannot determine if responses to nitroglycerin changed on any individual study day (pre- to postingestion), but rather can only say that they were the same after ingestion of all cocoa quantities tested (0–26 g cocoa).
In summary, our findings provide experimental support for the concept that acute cocoa ingestion dose dependently increases brachial artery FMD in healthy older adults. Increases in FMD may be mechanistically linked with increased circulating total epicatechin. The importance of these findings may lie in the fact that low levels of FMD in older adults are associated with increased cardiovascular-related mortality (45) and thus strategies that increase FMD in this population may reduce this risk. The present study provides further evidence that the endothelium may serve as an important target responsible for mediating some of the health benefits of cocoa. These effects on the endothelium may help explain the inverse relation that exists between cocoa/chocolate/flavonoid intake and cardiovascular-related morbidity and mortality in humans (4, 24).
GRANTS
The Hershey Company provided financial support for this study through an unrestricted grant. Additional support was provided by a Grant from the National Institutes of Health (M01-RR10732 and C06-RR016499).
DISCLOSURES
At the time of the study M.E.J.L., A.G.P., and D.L.M. had equity (stock) ownership in The Hershey Company and A.G.P. and D.L.M were employees of The Hershey Company.
ACKNOWLEDGMENTS
We thank the nursing staff of the General Clinical Research Center for technical assistance and Barry Callebaut for providing the drink packets used in this study.
REFERENCES
- 1. Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol 51: 2141–2149, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 103: 1618–1623, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med 166: 411–417, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol 286: H76–H82, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation 119: 1433–1441, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Desch S, Schmidt J, Kobler D, Sonnabend M, Eitel I, Sareban M, Rahimi K, Schuler G, Thiele H. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens 23: 97–103, 2010 [DOI] [PubMed] [Google Scholar]
- 11. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr 88: 58–63, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Farouque HM, Leung M, Hope SA, Baldi M, Schechter C, Cameron JD, Meredith IT. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study. Clin Sci (Lond) 111: 71–80, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291: H985–H1002, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 46: 398–405, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 290: 1030–1031, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol 49: 74–80, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Heiss C, Jahn S, Taylor M, Real WM, Angeli FS, Wong ML, Amabile N, Prasad M, Rassaf T, Ottaviani JI, Mihardja S, Keen CL, Springer ML, Boyle A, Grossman W, Glantz SA, Schroeter H, Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol 56: 218–224, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hermann F, Spieker LE, Ruschitzka F, Sudano I, Hermann M, Binggeli C, Luscher TF, Riesen W, Noll G, Corti R. Dark chocolate improves endothelial and platelet function. Heart 92: 119–120, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342: 1007–1011, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H, Feskens EJM, Hollman PCH, Katan MB. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155: 381–386, 1995 [PubMed] [Google Scholar]
- 26. Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 9: 811–818, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Kelishadi R, Hashemi M, Mohammadifard N, Asgary S, Khavarian N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clin Chem 54: 147–153, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Leikert JF, Rathel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 106: 1614–1617, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 85: 895–909, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40: 505–510, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Moncada S, Palmer RM, Higgs Nitric oxide: physiology EA, pathophysiology, pharmacology. Pharmacol Rev 43: 109–142, 1991 [PubMed] [Google Scholar]
- 33a. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421, 2002 [PubMed] [Google Scholar]
- 34. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (-)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 55: 1398–1405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Roura E, Andres-Lacueva C, Jauregui O, Badia E, Estruch R, Izquierdo-Pulido M, Lamuela-Raventos RM. Rapid liquid chromatography tandem mass spectrometry assay to quantify plasma (-)-epicatechin metabolites after ingestion of a standard portion of cocoa beverage in humans. J Agric Food Chem 53: 6190–6194, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Schnorr O, Brossette T, Momma TY, Kleinbongard P, Keen CL, Schroeter H, Sies H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch Biochem Biophys 476: 211–215, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103: 1024–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA 298: 49–60, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vlachopoulos C, Aznaouridis K, Alexopoulos N, Economou E, Andreadou I, Stefanadis C. Effect of dark chocolate on arterial function in healthy individuals. Am J Hypertens 18: 785–791, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]