Abstract
Current surgical management of volume overload-induced heart failure (HF) leads to variable recovery of left ventricular (LV) function despite a return of LV geometry. The mechanisms that prevent restoration of function are unknown but may be related to the timing of intervention and the degree of LV contractile impairment. This study determined whether reduction of aortocaval fistula (ACF)-induced LV volume overload during the compensatory stage of HF results in beneficial LV structural remodeling and restoration of pump function. Rats were subjected to ACF for 4 wk; a subset then received a load-reversal procedure by closing the shunt using a custom-made stent graft approach. Echocardiography or in vivo pressure-volume analysis was used to assess LV morphology and function in sham rats; rats subjected to 4-, 8-, or 15-wk ACF; and rats subjected to 4-wk ACF followed by 4- or 11-wk reversal. Structural and functional changes were correlated to LV collagen content, extracellular matrix (ECM) proteins, and hypertrophic markers. ACF-induced volume overload led to progressive LV chamber dilation and contractile dysfunction. Rats subjected to short-term reversal (4-wk ACF + 4-wk reversal) exhibited improved chamber dimensions (LV diastolic dimension) and LV compliance that were associated with ECM remodeling and normalization of atrial and brain natriuretic peptides. Load-independent parameters indicated LV systolic (preload recruitable stroke work, Ees) and diastolic dysfunction (tau, arterial elastance). These changes were associated with an altered α/β-myosin heavy chain ratio. However, these changes were normalized to sham levels in long-term reversal rats (4-wk ACF + 11-wk reversal). Acute hemodynamic changes following ACF reversal improve LV geometry, but LV dysfunction persists. Gradual restoration of function was related to normalization of eccentric hypertrophy, LV wall stress, and ECM remodeling. These results suggest that mild to moderate LV systolic dysfunction may be an important indicator of the ability of the myocardium to remodel following the reversal of hemodynamic overload.
Keywords: hemodynamics, cardiac remodeling/hypertrophy, collagen
the incidence of heart failure (HF) is increasing due to an aging population and improved management of diseases that are precursors to ventricular dysfunction. The successes of therapeutic advances over the past decades have dramatically decreased mortality rates, such that the focus has now shifted towards reducing morbidities (12). As a result, cardiac remodeling has become a primary therapeutic target in patients with heart failure. Strategies to prevent or reverse adverse left ventricular (LV) remodeling include pharmacotherapy, surgical interventions, and device-based-therapies such as left ventricular assist devices (LVAD) and cardiac resynchronization therapy (CRT). Both LVAD and CRT are associated with LV reverse remodeling with varying degrees of functional recovery (1, 8, 19, 20). However, the majority of device-based therapies are initiated in advanced HF (NYA Stage III-IV) when hearts may have already transitioned to decompensated failure when functional remodeling is limited (23). There have been relatively few studies that describe structural and functional remodeling associated with reversal induced at early HF stages (13, 30). Moreover, since the cellular and molecular phenotypes are likely to be stage specific, it may be difficult to extrapolate these changes resulting from interventions at end-stage HF where the remodeling has already occurred.
The progression of LV remodeling has been widely studied in animal models, particularly in response to hemodynamic overload. Volume overload results from increased preload and leads to progressive LV dilation and pump failure. These structural and functional changes are characterized by a disproportionate decrease in the ratio of LV wall thickness to diameter (WT/diameter), contractile dysfunction, and development of congestive HF. Clinically, mitral valve regurgitation (MR) is a leading cause of pure LV volume overload and results from a primary defect in valve integrity or is secondary to dilated cardiomyopathy and end-stage heart disease. Given that hemodynamic overload drives the remodeling process in this disease, surgical intervention to repair/replace the regurgitant valve is the most common therapeutic option to restore normal hemodynamic load and limit LV remodeling and dysfunction. Results from clinical studies suggest that correction of MR leads to restoration of LV geometry with a decrease in end-diastolic chamber dimensions. However, studies regarding restoration of pump function provide conflicting reports, with increased, decreased, or unchanged ejection fraction (EF%) occurring after surgery and could be associated with time of intervention.
We and others (28, 34) have reported that chronic volume overload-induced adverse remodeling in the rat aortocaval fistula (ACF) model involves progressive changes in both LV dysfunction and extracellular matrix (ECM) turnover. Chronic contractile dysfunction in this model is associated with decreased myocardial contractility, altered excitation-contraction coupling, and impaired myocardial relaxation. Conversely, acute LV remodeling after ACF is associated with ECM degradation and an imbalance in the ratio of matrix metalloproteinases (MMP) and their tissue inhibitors (TIMPs; Ref. 28).
While this ACF model is a biventricular volume overload model used extensively to monitor LV failure, there has been only one attempt (15) to use this model to study LV remodeling following hemodynamic load reduction. In the present study, we used a custom “stent graft” to close the ACF to determine whether reduction of hemodynamic overload reverses HF progression during early compensation. Load reversal significantly improved LV structural remodeling but initially (short-term reversal, i.e., 4-wk ACF + 4-wk reversal) exacerbated LV dysfunction before eventually restoring it to control levels at a later time point (long-term reversal, i.e., 4-wk ACF + 11-wk reversal). These physiological changes were associated with dynamic ECM regulation and altered expression of hypertrophic markers.
METHODS
Animals.
Male Sprague-Dawley rats (250–300 g, Harlan) were housed in a temperature- and humidity-controlled room using a 12-h light-dark cycle, standard rat chow, and water ad libitum. All studies conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No.85-12, revised 1996). The Institutional Animal Care and Use Committee of Research Institute at Nationwide Children's Hospital approved the protocol.
ACF induction and reversal surgeries.
Rats were anesthetized with 2% isoflurane, and cardiac volume overload was induced as described previously (14). Briefly, a midline abdominal incision was made and the abdominal aorta and inferior vena cava were exposed. Vascular clips were placed above the iliac bifurcation and below the iliolumbar vessels. An 18-g needle was inserted into the aorta and through the common midwall, creating an ACF. The needle was removed, and cyanoacrylate glue (Vetbond) was used to seal the aortic puncture. Shunt patency was visually confirmed by mixture of bright red arterial blood in the vena cava. The abdominal muscle and skin were closed using an absorbable suture and 9-mm surgical staples, respectively. Sham animals were treated identically except no aortic puncture was made. 4 wk following ACF surgery (Fig. 1), the fistula was closed using a custom stent graft. Rats were anesthetized, the aorta was isolated above the iliolumbar arteries, and a ligature was used to temporarily occlude blood flow at the iliac bifurcation. An 18-g short bevel needle was used as an introducer, and 1-cm long sterile, heparinized PE-190 tubing was inserted into the aorta as a stent graft. Successful stent placement was verified by echocardiography and pulsed wave Doppler velocity of the aorta. Following surgery, animals were administered an analgesic (0.05 mg/kg buprenorphine, every 12 h for 2 days). Complications of reversal surgery included intraoperative hemorrhage (5%) and hindlimb paralysis (10%).
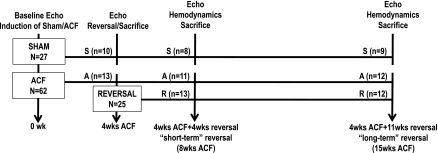
Fig. 1.
Experimental time course. S, sample from sham-operated rats; A, sample from aortocaval fistula (ACF)-induced rats; R, sample from short-term reversal rats.
Figure 1 depicts the experimental outline with ACF surgery denoted as day 1. Sham and ACF groups were studied at 4, 8, and 15 wk following ACF induction. ACF-reversal groups were studied at 4 and 11 wk postreversal surgery (4-wk ACF + 4-wk reversal and 4-wk ACF + 11-wk reversal, respectively), which corresponds to short-term and long-term reversal time points in the present study. A pilot study using echocardiography or a high-fidelity pressure-volume (PV) catheter indicated no differences between sham and sham reversal (stent placement) in structural or functional parameters (not shown). Accordingly, sham-reversal groups were not included in this study. A distinct set of animals was used for PV loop/hemodynamic studies and molecular studies. All the animals underwent echocardiography.
Echocardiography.
Transthoracic echocardiograms were performed using a Toshiba Xario (12.5-mHz transducer) while the animals were under 1.5% isoflurane. M-mode images were obtained at the level of the papillary muscles to assess chamber dimensions [LV systolic (LVDs) and diastolic dimensions (LVDd)] and posterior wall thickness [systole (PWTs) and diastole (PWTd)]. Systolic function was assessed by percent fractional shortening (%FS) = (LVDd − LVDs)/LVDd * 100 and eccentric dilation index = (2 * PWTd)/LVDd. LV wall stress is presented as circumferential end-systolic (cESS) and diastolic stress (cEDS) as described by Reichek et al. (27).
Measurement of in vivo LV hemodynamics.
Anesthetized rats were intubated with a 16-g cannula and ventilated with a pressure-controlled rodent ventilator (CWE-SAR-830) with 98.5% oxygen-1.5% isoflurane at a maximum airway pressure of 12 cmH2O and a respiratory rate of 60 breaths/min. A 1.9 F PV catheter (FTS-1912B-8018; Scisense) was placed into the right carotid artery and advanced to the aortic valve. After a 5-min equilibration period, aortic pressure was recorded, and the catheter was then advanced into the LV. Following a 10-min equilibration period, the ventilator was paused for 15 s and baseline LV hemodynamic parameters were acquired using 8–10 consecutive PV loops. Preload was varied by brief occlusion the inferior vena cava. Conductance catheter calibration was achieved using blood from each group in cuvettes of known volume to generate a standard curve. Parallel conductance was subtracted using a hypertonic (15%) saline injection (50 μl for sham and 100 μl for ACF). Data analysis was performed with iWorx Labscribe-2 acquisition and analysis software.
Measures of LV systolic function included: heart rate, stroke volume (SV), LV end-systolic elastance (Ees, slope of the end-systolic PV relation), and preload recruitable stroke work (PRSW). Measures of LV diastolic function included: left ventricular end-diastolic volume, left ventricular end-diastolic pressure, end-diastolic PV relation (EDPVR), tau, and −dp/dt. The volume scaling method of Klotz et al. (18) was used to obtain volume-normalized EDPVR.
To account for inherent nonlinearity of Ees and PRSW at low pressures (6) and for comparison between groups, we used linear regression analysis over a pressure range of 120 mmHg to 60 mmHg. Data were analyzed by analysis of covariance (ANCOVA) to account for changes in the volume-axis intercept (V0); therefore, ANCOVA adjusted marginal means (Ees and PRSW) are presented (6). The volume at 100 mmHg, V100, were presented to account for a parallel shift in Ees (6).
Immunoblot analysis.
LV free wall tissue (100 mg) lysates were prepared as described (28). Proteins (50 μg) were separated by SDS-PAGE and transferred onto PVDF membranes. Immunoblotting was performed with antibodies against MMP-2, MMP-7, MMP-13, and TIMP-4 (1:1,000; Chemicon); MMP-9 (1:1,000; Abcam); MT1-MMP and TIMP-2 (1:1,000; EMD Chemicals); TIMP-1 (1:400; Chemicon); TIMP-3 (1:200; Santa Cruz); and GAPDH (1:2,000; Abcam). Relative band densities were analyzed using GelEval (v1.22 Frog Dance Software) and were normalized to GAPDH loading controls.
Analysis of myocardial collagen volume fraction.
Interstitial collagen was measured by Picrosirius red staining under polarized light as described previously (31). Then, 20–35 images/section were obtained from the LV free wall (endocardium and epicardium) and right ventricular (RV) free wall at low power (×40). Collagen volume fraction was quantified using Olympus MicroSuite 5 software.
Quantitative real-time PCR analysis.
RNA was isolated from LV and RV tissue (50 mg) using a commercially available kits and protocols (Qiagen RNeasy microarray tissue mini kit). RNA was reverse transcribed using the RevertAid first strand cDNA synthesis kit and protocol (Fermentas). First strand equivalent to 100 ng input RNA (LV) or 50 ng input RNA (RV) was amplified in duplicate for each animal using appropriate Roche universal probe/primer pairs for each target gene: collagen-1a1(Col1a1), collagen-3a1(Col3a1), elastin, α-myosin heavy chain (α-MHC), β-MHC, atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and transforming growth factor (TGF-β; see Table 1 for primer sequences) and Maxima Probe quantitative real-time PCR master mix (Fermentas). Amplifications were carried out for 40 cycles using an Eppendorf MasterCycler-ep Realplex thermocycler. Parallel amplifications using nonreverse transcribed samples were performed to rule out genomic DNA contamination. Data were analyzed for relative expression using the 2−ΔΔCt method, with ribosomal protein Rpl13a serving as the internal control and the average sham value at each time point serving as a second normalizer.
Table 1.
Primer sequences
| Target | NCBI Accession No. | Forward Primer | Reverse Primer | Roche Universal Probe | Average Ct Value (4-wk sham) |
|---|---|---|---|---|---|
| Col1a1 | NM_053304 | tctggtctccagggtcctc | gtccatctttgccaggagaa | 21 | 18.5 |
| Col3a1 | NM_032085 | tcccctggaatctgtgaatc | tgagtcgaattggggagaat | 49 | 20.4 |
| Elastin | NM_012722 | gctgatcctcttgctcaacc | gggaactccaccaggaagtc | 64 | 24.2 |
| α-MHC | NM_017239 | gccaagagccgtgacatt | tttattgtgggatagcaacagc | 120 | 11.0 |
| β-MHC | NM_017240 | gccaagagccgtgacatt | tgcctaaggtgctgtttcaa | 85 | 14.0 |
| ANP | NM_012612 | cacagatctgatggatttcaaga | cctcatcttctaccggcatc | 25 | 18.8 |
| BNP | NM_031545.1 | agaacaatccacgatgcaga | gctgtctctgagccatttcc | 120 | 16.5 |
| TGF-β | NM_021578 | tcagacattcgggaagcagt | acgccaggaattgttgctat | 56 | 20.2 |
| Rpl13a | NM_173340.2 | ccctccaccctatgacaaga | ggtacttccacccgacctc | 41 | 16.1 |
MHC, myosin heavy chain; ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide;TGF-β, transforming growth factor-β.
Statistics.
Data are expressed as means ± SE. Statistical analyses were performed using GraphPad Prism V5.0 Software. One-way ANOVA followed by Bonferroni's post hoc test were used to measure differences between groups. Quantitative real-time PCR data were analyzed using nonparametric one-way ANOVA followed by Dunn's post hoc test. ANCOVA of Ees and PRSW variables was performed using a pair-wise comparison and a Bonferroni post hoc test (SPSS-v.18.0; SPSS, Chicago, IL).
The expression of mRNAs involved in structural and functional remodeling of the heart at short-term reversal time point were subjected to Pearson's correlation analysis using Graphpad Prism software to identify associations between gene transcripts and LV structural and functional parameters (LVDd or %FS). In all cases, P < 0.05 was considered statistically significant.
RESULTS
ACF reversal reduces LV eccentric dilation.
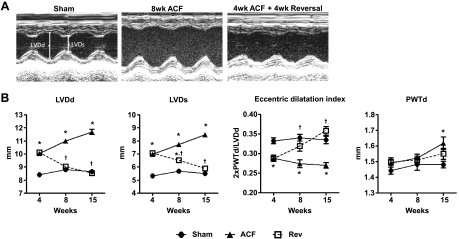
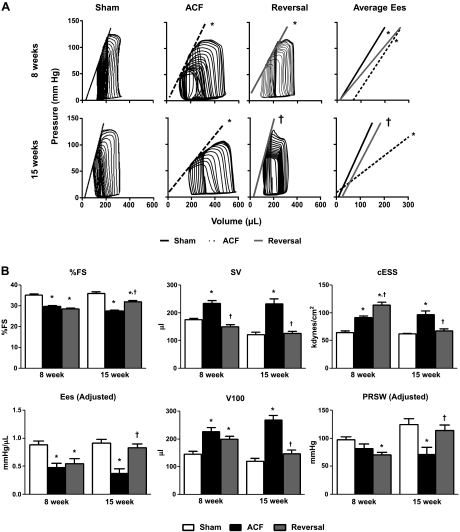
ACF-induced volume overload caused progressive increases in LVDd and LVDs (Fig. 2A), resulting in eccentric dilation (Fig. 2B). These changes were detected at 4 wk and persisted throughout the 15-wk time course. However, during short-term reversal (4-wk ACF + 4-wk reversal), LV chamber size returned to sham values due to a decrease in LVDd without changes in wall thickness (Fig. 2B). This pattern of remodeling remained throughout the time course of the study.
Fig. 2.
ACF reversal improves left ventricular (LV) structure. A: representative M-mode images from sham, ACF (8 wk), and reversal (4-wk ACF + 4-wk Rev) rats. B: echocardiographic measurements: LVDd is LV diameter at end-diastole, LVDs is LV diameter at end-systole, 2*PWTd/LVDd is eccentric dilation, HR is heart rate, %FS is fractional shortening, and PWT is posterior wall thickness diastole. Data are means ± SE (n = 8–12 per group). *P < 0.05 vs. sham; †P < 0.05 vs. ACF.
ACF reversal attenuates interstitial fibrosis.
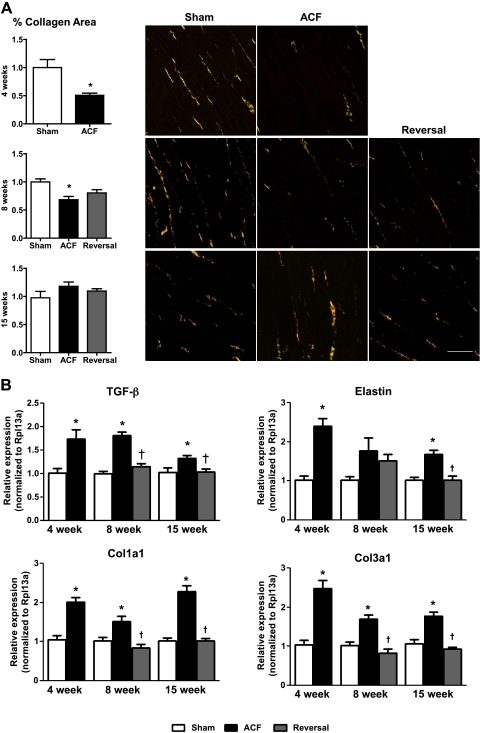
We next determined whether ECM changes were associated with LV remodeling following ACF reversal. In ACF rats, total LV collagen content was significantly decreased at 4- and 8-wk ACF compared with sham but was not different from sham at 15 wk (Fig. 3A). A similar pattern was observed in the RV free wall with no differences between the epicardial and endocardial layers (data not shown). There was no change in total collagen content in the short-term reversal or long-term (4-wk ACF + 11-wk reversal) reversal groups compared with ACF. ACF increased gene expression of Col1a1, Col3a1, and elastin at 4 and 15 wk that was blunted at the long-term reversal stage (Fig. 3B).
Fig. 3.
Extracellular matrix (ECM) composition. A: cumulative %collagen area and representative micrographs of LV interstitial Picrosirius red staining (n = 6–8 per group). B: mRNA expression of transforming growth factor (TGF-β), elastin, Col1a1, and Col3a1. Data are means ± SE (n = 4–7 per group). Scale bar = 50 μm. *P < 0.05 vs. sham; †P < 0.05 vs. ACF.
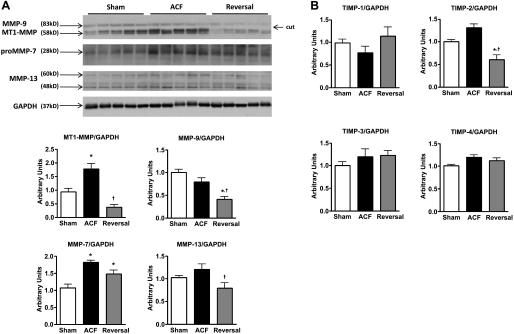
To gain further insight in ECM changes, we measured protein expression of MMPs (Fig. 4A) and TIMPs (Fig. 4B). The MMPs and TIMPs were chosen based on their relative contributions to ECM remodeling in volume overload hypertrophy. We were specifically interested in MMPs that have broad relevance such as the gelatinases (MMP2/9) and collagenases (MT1-MMP and MMP13). We chose TIMPs that are commonly associated with ECM remodeling and those that have broad specificity for MMPs. The collagenase MMP-13 and plasma membrane bound MT1-MMP were increased following 8-wk ACF but returned towards sham values at the short-term reversal time point. In contrast, elevated matrilysin MMP-7 levels following 8-wk ACF remained increased in short-term reversal rats. Neither MMP-9 nor TIMP-2 levels were increased by ACF at this time point but were decreased below sham levels by volume overload reversal. There were no significant changes in MMP-2 (P = 0.26; data not shown) or TIMP-1, -3, and -4 among groups.
Fig. 4.
Changes in LV matrix metalloproteinases (MMP) and their tissue inhibitors (TIMPs) 4 wk postreversal. Representative immunoblots and cumulative data for MMPs (A) and cumulative data for TIMPs (B). Protein was normalized to GAPDH. Data are means ± SE (n = 4–7 per group) *P < 0.05 vs. sham; †P < 0.05 vs. ACF.
ACF reversal normalizes hypertrophy induced by volume overload.
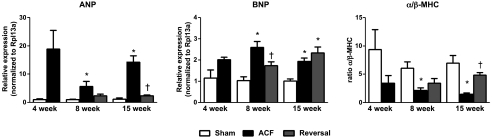
As reported (28), mechanical stretch can activate signaling cascades that lead to LV hypertrophy. At 8 and 15 wk, ACF increased LV weight and the LV weight-to-body weight ratio, these increases were normalized at both short-term and long-term reversal groups (Table 2). Gene expression for markers of LV hypertrophy, ANP and BNP, were increased by ACF throughout the time course of the study: 4 (P = 0.053), 8, and 15 wk (Fig. 5A). ACF reversal normalized ANP expression in both short-term and long-term reversal groups; however, BNP gene expression remained elevated in long-term reversal group (Fig. 5A).
Table 2.
Morphological parameters
| 8 wk |
15 wk |
|||||
|---|---|---|---|---|---|---|
| Sham | ACF (8-wk ACF) | Rev (4-wk ACF + 4-wk reversal) | Sham | ACF (15-wk ACF) | Rev (4-wk ACF + 11-wk reversal) | |
| n | 6 | 6 | 6 | 6 | 6 | 10 |
| BW, g | 410 ± 27 | 445 ± 13 | 411 ± 7 | 468 ± 15 | 458 ± 9 | 451 ± 11 |
| LV wt, g | 0.87 ± 0.03 | 1.38 ± 0.05* | 0.97 ± 0.04† | 1.05 ± 0.07 | 1.57 ± 0.04* | 1.04 ± 0.11† |
| LV:BW | 2.56 ± 0.16 | 4.06 ± 0.18* | 2.86 ± 0.09† | 2.76 ± 0.13 | 4.20 ± 0.19* | 2.82 ± 0.19† |
Data are means ± SE. ACF, aortocaval fistula; BW, body weight; LV, left ventricular.
P < 0.05 vs. sham;
P < 0.05 vs. ACF.
Fig. 5.
Changes in hypertrophic markers. mRNA expression of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) and changes in the α/β-myosin heavy chain (MHC) ratio. Data are means ± SE (n = 4–7 per group). *P < 0.05 vs. sham; †P < 0.05 vs. ACF.
Reexpression of fetal β-MHC and an altered α/β-MHC ratio in the LV of ACF rats are markers for phenotypic reprogramming resulting in LV hypertrophy. Our data clearly show that the decreased α/β-MHC ratio induced by ACF at 8 and 15 wk was restored by volume overload reversal (Fig. 5), suggesting that fetal gene reexpression was attenuated by load reduction.
LV systolic function remains compromised at 4 wk post-ACF reversal. %FS, a load-dependent parameter of contractility measured by echocardiography, was significantly decreased in ACF compared with sham and was not improved by ACF reversal at the short-term reversal time point (Fig. 6B). We next used PV analysis to study load-independent parameters to more thoroughly characterize in vivo LV function (Fig. 6). As expected, SV was significantly increased in ACF compared with sham (Fig. 6B), and the reduction in preload by ACF reversal resulted in decreased SV but did not improve %FS (Fig. 6B). Compared with sham, ACF had decreased contractility (Ees adjusted), indicated by a rightward shift of the line that defines Ees and an increased V100 and V0 (Fig. 6B). However, short-term reversal rats showed no improvement in LV contractility; however, there was a decrease in the slope of the line that defines Ees, with a corresponding increase in V100 but no change in V0. Although there were no differences in PRSW (adjusted) between sham and 8-wk ACF, short-term reversal caused a decrease in PRSW (adjusted) compared with sham. Unadjusted means for PRSW, Ees and V0 are presented in Table 3 for reference. Finally, short-term reversal increased mean arterial pressure (Fig. 6B) and effective arterial elastance (Ea; Table 3) compared with ACF, resulting in increased cESS (Fig. 6B).
Fig. 6.
Indexes of LV-systolic function. A: representative pressure-volume loops and average end-systolic elastance (Ees). B: LV functional measurements: FS, fractional shortening; SV, stroke volume; cESS, circumferential end-systolic wall stress; EesV100, volume at 100 mmHg; PRSW, preload recruitable stroke work; PRSW V0, volume axis intercept of PRSW. Data are means ± SE (n = 6–10 per group). *P < 0.05 vs. sham; †P < 0.05 vs. ACF.
Table 3.
In vivo indexes of LV hemodynamics
| 8 wk |
15 wk |
|||||
|---|---|---|---|---|---|---|
| Sham | ACF (8-wk ACF) | Rev (4-wk ACF + 4-wk reversal) | Sham | ACF (15-wk ACF) | Rev (4-wk ACF + 11-wk reversal) | |
| n | 6 | 6 | 6 | 6 | 6 | 10 |
| HR, beats/min | 327 ± 10 | 326 ± 4 | 333 ± 5 | 339 ± 10 | 329 ± 6 | 324 ± 5 |
| MAP, mmHg | 103 ± 3 | 96 ± 3 | 112 ± 6† | 117 ± 5 | 100 ± 1 | 124 ± 5† |
| V0, μl | 12 ± 12 | 69 ± 7* | 10 ± 5 | 9 ± 8 | −24 ± 18 | 24 ± 12 |
| Ees, mmHg/μl (unadj) | 0.75 ± 0.04 | 0.70 ± 0.06 | 0.55 ± 0.02* | 0.92 ± 0.06 | 0.35 ± 0.02* | 0.87 ± 0.08† |
| PRSW, mmHg (unadj) | 83.8 ± 18.5 | 106.9 ± 6.7 | 65.3 ± 5.8*† | 111.8 ± 12.0 | 77.6 ± 12.5* | 121.2 ± 14.7† |
| ESP, mmHg | 119 ± 5 | 100 ± 3* | 132 ± 6† | 125 ± 4 | 110 ± 5 | 127 ± 6 |
| SV, μl | 180 ± 2 | 234 ± 10* | 150 ± 7*† | 205 ± 7 | 264 ± 12* | 178 ± 10† |
| Ea, mmHg/μl | 0.64 ± 0.03 | 0.41 ± 0.01* | 0.86 ± 0.07*† | 0.61 ± 0.02 | 0.41 ± 0.03* | 0.74 ± 0.04*† |
Data are means ± SE. HR, heart rate; mean arterial pressure; V0, volume-axis intercept; Ees, end-systolic elastance; PRSW, preload recruitable stroke work; ESP, end-systolic pressure; SV, stroke volume; Ea, arterial elastance.
P < 0.05 vs. sham;
P < 0.05 vs. ACF.
LV diastolic function remains compromised at 4 wk postreversal.
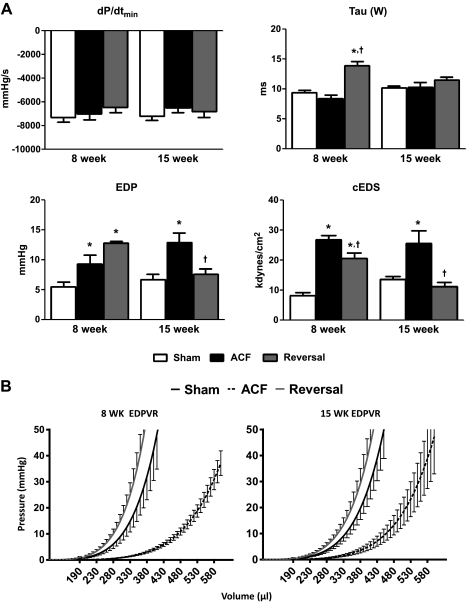
ACF significantly increased cEDS at 8 wk; however, no changes were observed in dp/dtmin or tau (Weiss; Fig. 7A), suggesting increased LV compliance with no changes in the rate of relaxation. This was confirmed by a downward shift in EDPVR (Fig. 7B). However, at the short-term reversal time point, EDP, tau, and cEDS were increased compared with sham and ACF groups, with a trend towards decreased dp/dtmin that did not reach significance. Additionally, short-term reversal resulted in an upward shift in EDPVR compared with sham values.
Fig. 7.
Indexes of LV-diastolic function. A: EDP, end-diastolic pressure; −dP/dt, first derivative of LV pressure decline; tau, Weiss calculation; cEDS, circumferential end-diastolic wall stress. B: LV diastolic function shown as volume-normalized EDPVR. Data are means ± SE (n = 6–10 per group). *P < 0.05 vs. sham; †P < 0.05 vs. ACF.
LV function is improved at 11 wk postreversal.
Fifteen weeks of ACF exacerbated contractile dysfunction as indicated by a progressive decrease in %FS (Fig. 6B), a dramatic decrease in Ees (adjusted) and PRSW (adjusted), and an increase in V100 (Fig. 6B). 11 wk of reversal restored %FS, Ees (adjusted), V100, PRSW (adjusted), and cESS to sham levels. Furthermore, long-term reversal improved diastolic function, indicated by a normalization of EDP, tau, and cEDS to sham levels.
Gene transcripts for LV remodeling correlate with LV structure but not function.
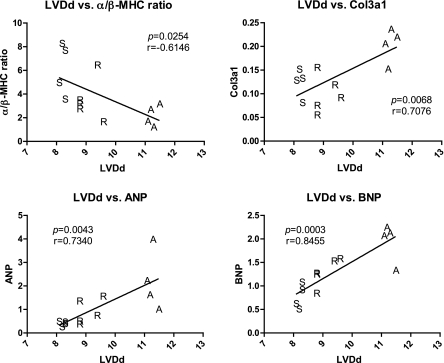
Because structural but not functional improvements are seen in the short-term reversal rats, we tested different gene markers of LV remodeling/hypertrophy for correlation with both structural (LVDd) and functional (%FS) physiological parameters. The gene transcripts for Col3a1, ANP, and BNP positively correlated with, and the α/β-MHC ratio negatively correlated with LVDd (Fig. 8), but not %FS (data not shown). This suggests that the return of structure with hemodynamic load reduction is associated with changes in gene expression that regulate cardiac remodeling. In addition, no correlations were seen between indexes of structural remodeling and functional parameters.
Fig. 8.
Correlation analysis of LV diastolic dimensions (LVDd) vs. different expression markers. Correlation coefficient (r) and P value are shown. Relative gene expression values are shown in the y-axis following normalization to Rpl13a, whereas LV dilation (LVDd) is shown in the x-axis.
DISCUSSION
This study provides the first comprehensive in vivo functional assessment of LV structural and functional remodeling during HF progression in the rat ACF-induced volume overload model. The “stent graft” approach to close the ACF allowed us to monitor structural and functional changes due to load reduction at key time points by in vivo analysis. Finally, we related these changes to alterations in ECM homeostasis and markers of LV hypertrophy. We chose the 4-wk time point in the rat ACF model to close the fistula as our previous studies (28) indicate that this represents the compensatory stage of HF progression that is characterized by significant LV eccentric dilation with moderately impaired pump function (Figs. 2A and 6A). The results of the present study demonstrate that the reverse cardiac remodeling process following load reduction is associated with improved LV chamber geometry and a gradual restoration of pump function.
ACF reversal is initially characterized by LV structural remodeling that precedes improved function.
Upon volume overload reversal, a new hemodynamic state is established characterized by a normalization of preload and increased afterload. We observed decreased LVDd and decreased compliance in short-term reversal rats compared with 8-wk ACF rats. Since decreased LVDd was not accompanied by a change in wall thickness, ACF reversal normalized diastolic chamber dimensions to sham values. We also observed a restoration of LV wall stiffness depicted by an upward shift in the slope of EDPVR (Fig. 7B), reflecting changes in the passive mechanical properties of the myocardial wall. The changes in systolic chamber dimensions were more complex. LVDs was partially (although not significantly) affected by reversal with a value between that of sham and ACF. This is likely attributed to the observed decreases in systolic pump function and increased afterload (Ea; Table 3), as reflected in increased cESS.
A compensatory decrease in LV stiffness in an attempt to normalize increased wall stress and eccentric hypertrophy are hallmarks of the compensatory phase of volume overload HF progression (5, 25). Collagen and titin (33) are the primary determinants of myocardial passive stiffness; however, we found no changes in titin isoform expression amongst groups (K. R. Hutchinson and M. Greaser, unpublished observations). Conversely, ACF resulted in progressive changes in collagen content with net degradation at early time points that correspond with eccentric LV dilation (see Ref. 16 for a recent review). Since we observed a significant increase in LV stiffness (upward shift in the EDPVR) at the short-term reversal time point primarily due to an increase in EDP, we hypothesized that ACF reversal would increase collagen content, especially in the presence of increased afterload. Interestingly, there were no changes in collagen volume fraction between 8-wk ACF and short-term reversal groups even though the ACF-induced Col1a1 and Col3a1 mRNA levels were normalized by ACF reversal. In addition, there were actually no statistical differences in cumulative data among groups in the collagen volume percent at the 15-wk time point. We and others (10, 28) suggest that this stage is characterized by profound chamber dilation and increased ECM turnover with net matrix deposition, perhaps in an attempt to compensate for the increase in compliance. We also observed significant decreases in MT1-MMP, MMP-9, and MMP-13 protein levels in the short-term reversal group compared with ACF indicating decreased ECM degradation. The decreases in MT1-MMP, MMP-9, MMP-13, and TIMP-2 in short-term reversal rats to levels below that of shams were not expected and suggest that reversal leads to a distinct pattern of ECM homeostasis. We propose that these proteins play pivotal and distinct regulatory roles following reversal. Taken together, these data suggest that dynamic changes in the composition of the ECM, as well as remodeling of the existing matrix by changes in MMPs and TIMPs, resulted in a viscoelastic ECM that rendered the overall LV less compliant in short-term reversal rats. It is also important to note that this time point in volume overload progression represents a composite “snapshot” of the ECM that reflects both causative and compensatory changes in matrix regulatory proteins. Future studies of LV proteomics will allow us to more fully define how LV molecular changes contribute to the unique hemodynamic state established by load reduction.
Several molecular markers showed significant correlations with LV dilation. Col3a1, the more compliant collagen isoform, expression positively correlated with LVDd, suggesting that an increase may be permissive for LV dilation. As expected there was also a positive correlation between ANP/BNP levels and LVDd, which is due to hemodynamic load-induced mechanical stretch. Conversely, there was a negative correlation between the α/β-MHC ratio and LVDd. Taken together, these data are consistent with induction of a fetal hypertrophic phenotype in response to excessive hemodynamic overload. It is also interesting to note that all gene markers of LV remodeling in ACF reversal were clustered towards sham, suggesting that volume overload reversal normalized ACF-induced LV remodeling.
ACF reversal leads to early systolic and diastolic dysfunction.
ACF resulted in the expected increase in SV, due to increased preload, however, decreased the %FS. Short-term reversal did not improve %FS compared with ACF nor did it result in an increase in load-independent parameters (Ees and PRSW) of LV function. The observation that Ees is decreased and V0 shifted to the left supports our argument that ACF reversal creates a new hemodynamic state in which both preload and contractility are reduced. A lack of functional improvement following reversal, at first glance, may be a negative consequence of corrective surgery; however, the shift in hemodynamic state (changes in Ees dynamics) may actually be beneficial to a heart that is undergoing active repair.
These changes at short-term reversal were associated with increased cEDS, which is consistent with reported increased cEDS and reduced systolic function following mitral repair in canine hearts (36), adults (3, 26), and children with congenital MR (22). The underlying mechanisms responsible for decreased systolic performance are unclear and may be attributed to changes in gene and protein expression, force generation, or electromechanical coupling. However, there was no correlation between any of the molecular markers of LV remodeling/hypertrophy with indexes of LV function (%FS; data not shown), suggesting that the normalization of structure does not correlate with functional recovery.
Long-term reversal restores LV function.
Development of decompensated HF occurred during 8- and 15-wk ACF, signified by progressive LV systolic and diastolic dysfunction and exacerbated LV eccentric dilation. At this time point, the values for the extrapolated V0 were negative, likely due to the inherent curvilinearity of the end-systolic PV relationship in vivo as originally described by Kass et al. (17). Long-term reversal restored load-dependent and -independent function to sham levels, indicated by improvements in %FS, end-systolic PV relationship, PRSW, and tau. These functional changes were associated with a reduction in wall stress, normalization of collagen and elastin gene expression, and restoration of the α/β-MHC ratio.
Similar patterns of LV structural and functional remodeling are observed following replacement of regurgitant mitral valves in dogs (29) and humans (2, 7, 11), even though MR is a model of pure LV volume overload. Moreover, gradual restoration of pump function has been observed clinically in asymptomatic patients with mild to moderate MR and LV systolic dysfunction before intervention (32).
The gene expression profiles of hypertrophic markers at the long-term reversal stage were similar to those observed at the short-term reversal time point. However, the persistent increase in BNP expression level in the long-term reversal group was unexpected and did not correlate with any structural indexes. There are several potential explanations for this observation. BNP may be a more sensitive marker for pressure overload than for volume overload (9), and its expression is regulated by adrenergic stimulation, endothelin-1 (24), and hypoxia (35). A recent study (21) raised the intriguing possibility that increased BNP was associated with improved LV function following infarction or angiotensin II-induced hypertension through distinct molecular mechanisms, which is consistent with our finding that long-term reversal improves LV function. Finally, it is possible that LV BNP mRNA expression does not necessarily correlate with increased BNP protein levels. Future studies are warranted to distinguish between these possibilities.
Differential effects of ACF-induced cardiac remodeling on left vs. right ventricles.
Previous studies using this volume overload model have not compared ECM regulation between LV and RV. Therefore, we performed additional PCR-based analyses of mRNA expression for Col1a1, Col3a1, elastin, ANP, BNP, and TGF-β in the RV (data not shown). Interestingly, we noted differences in basal expression of several mRNAs encoding ECM regulatory proteins. For example, although Col3a1 expression was equivalent between ventricles, Col1a1 mRNA expression was 10-fold lower in the RV vs. the LV and elastin was 6-fold lower. Overall, this pattern is consistent with a more compliant RV ECM (i.e., decreased Col1a1/Col3a1 ratio). ACF and reversal caused the same relative changes compared with sham in the LV and RV with one notable exception. The RV responded to chronic ACF (i.e., 8–15 wk) with a 40- to 55-fold induction of ANP and BNP compared with a 5- to 12-fold induction in the LV at the same time points. Since ANP expression is induced by mechanical stretch, these differences may suggest that the thinner walled, more compliant RV is more sensitive to hemodynamic load compared with the LV. We also did not observe any differences in the autophagy markers light chain-3 (LC3A/B-I/II) and beclin-1 between LV and RV at any of the time points in the present study (data not shown).
We cannot, however, rule out the possibility that RV overload in the rat ACF model creates a distinct hemodynamic influence on LV function that is not observed clinically in MR. Finally, although there have been numerous studies describing molecular and morphological changes in the myocardium following LVAD placement and mechanical unloading, the majority of these studies were performed on tissues collected from adult patients with end-stage HF in which LVADs were placed as a bridge to transplant (4). Secondly, LVADs can cause LV hemodynamic unloading to below physiological levels, which may result in pathophysiological rather than physiological remodeling.
In conclusion, hemodynamic unloading by volume overload reversal at early stage HF leads to reverse LV remodeling followed by a restoration of LV systolic and diastolic function. These improvements were associated with changes in ECM remodeling and markers of LV hypertrophy.
GRANTS
Support for this work was provided by National Heart, Lung, and Blood Institute Grants 2RO1-HL-5604-12 and 2RO1-HL-63318-11 (to P. A. Lucchesi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.R.H., A.G., K.C.L., and P.A.L. conception and design of research; K.R.H., A.G., M.J.C., J.A.S., and X.Z. performed experiments; K.R.H., A.G., M.J.C., M.L.G., T.A.W., and J.A.S. analyzed data; K.R.H., A.G., M.J.C., K.C.L., and P.A.L. interpreted results of experiments; K.R.H., A.G., M.L.G., T.A.W., and J.A.S. prepared figures; K.R.H. and A.G. drafted manuscript; K.R.H., A.G., M.J.C., M.L.G., T.A.W., J.A.S., and P.A.L. edited and revised manuscript; K.R.H., A.G., M.J.C., M.L.G., T.A.W., J.A.S., X.Z., K.C.L., and P.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Marion Greaser for titin isoform analysis and Drs. Loren Wold and Timothy Hoffman for insightful manuscript review.
REFERENCES
- 1. Abraham WT. Cardiac resynchronization therapy. Progr Cardiovasc Dis 48: 232–238, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Acker MA, Bolling S, Shemin R, Kirklin J, Oh JK, Mann DL, Jessup M, Sabbah HN, Starling RC, Kubo SH. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg 132: 568–577, 577.e561–564, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Jr, Powell P, McGiffin DC, Dell'Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J Am Coll Cardiol 55: 671–679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baba HA, Wohlschlaeger J. Morphological and molecular changes of the myocardium after left ventricular mechanical support. Curr Cardiol Rev 4: 157–169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol Heart Circ Physiol 271: H2071–H2078, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol 52: 319–326, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Castellant P, Fatemi M, Bertault-Valls V, Etienne Y, Blanc JJ. Cardiac resynchronization therapy: “nonresponders” and “hyperresponders”. Heart Rhythm 5: 193–197, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cavallero S, Gonzalez GE, Puyo AM, Roson MI, Perez S, Morales C, Hertig CM, Gelpi RJ, Fernandez BE. Atrial natriuretic peptide behaviour and myocyte hypertrophic profile in combined pressure and volume-induced cardiac hypertrophy. J Hypertens 25: 1940–1950, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Chen YW, Pat B, Gladden JD, Zheng J, Powell P, Wei CC, Cui X, Husain A, Dell'italia LJ. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol 300: H2251–H2260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Bonis M, Alfieri O. Surgery Insight: surgical methods to reverse left ventricular remodeling. Nat Clin Pract Cardiovasc Med 3: 507–513, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Eapen Z, Rogers JG. Strategies to attenuate pathological remodeling in heart failure. Curr Opin Cardiol 24: 223–229, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Francis GS, Wilson Tang WH. Early cardiac resynchronization therapy and reverse remodeling in patients with mild heart failure: is it time? Circulation 120: 1845–1846, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res 24: 430–432, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Gerdes AM, Clark LC, Capasso JM. Regression of cardiac hypertrophy after closing an aortocaval fistula in rats. Am J Physiol Heart Circ Physiol 268: H2345–H2351, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Hutchinson KR, Stewart JA, Jr, Lucchesi PA. Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol 48: 564–569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kass DA, Beyar R, Lankford E, Heard M, Maughan WL, Sagawa K. Influence of contractile state on curvilinearity of in situ end-systolic pressure-volume relations. Circulation 79: 167–178, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Klotz S, Dickstein ML, Burkhoff D. A computational method of prediction of the end-diastolic pressure-volume relationship by single beat. Nat Protoc 2: 2152–2158, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Komoda T, Komoda S, Dandel M, Weng Y, Hetzer R. Explantation of INCOR left ventricular assist device after myocardial recovery. J Card Surg 23: 642–647, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Krabatsch T, Schweiger M, Dandel M, Stepanenko A, Drews T, Potapov E, Pasic M, Weng YG, Huebler M, Hetzer R. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg 91: 1335–1340, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Moilanen AM, Rysa J, Mustonen E, Serpi R, Aro J, Tokola H, Leskinen H, Manninen A, Levijoki J, Vuolteenaho O, Ruskoaho H. Intramyocardial BNP gene delivery improves cardiac function through distinct context-dependent mechanisms. Circ Heart Fail 4: 483–495, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Murakami T, Nakazawa M, Nakanishi T, Momma K. End-systolic wall stress is a major determinant of postoperative left ventricular dysfunction in patients with congenital mitral regurgitation. Cardiol Young 12: 236–239, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Ogletree ML, Sweet WE, Talerico C, Klecka ME, Young JB, Smedira NG, Starling RC, Moravec CS. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 29: 554–561, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol 42: 498–507, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 20: 248–254, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Pearlman AS. Mitral regurgitation: timing of valve surgery. Am Heart Hosp J 5: 48–52, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Reichek N, Wilson J, St John Sutton M., Plappert TA, Goldberg S, Hirshfeld JW. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation 65: 99–108, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Ryan TD, Rothstein EC, Aban I, Tallaj JA, Husain A, Lucchesi PA, Dell'Italia LJ. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol 49: 811–821, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Spinale FG, Ishihra K, Zile M, DeFryte G, Crawford FA, Carabello BA. Structural basis for changes in left ventricular function and geometry because of chronic mitral regurgitation and after correction of volume overload. J Thorac Cardiovasc Surg 106: 1147–1157, 1993 [PubMed] [Google Scholar]
- 30. St. John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, Linde C, and on Behalf of the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction Study Group Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association Class I/II heart failure. Circulation 120: 1858–1865, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Stewart JA, Jr, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell'Italia LJ. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol 35: 311–319, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Takeda K, Matsumiya G, Sakaguchi T, Miyagawa S, Yamauchi T, Shudo Y, Izutani H, Sawa Y. Impact of untreated mild-to-moderate mitral regurgitation at the time of isolated aortic valve replacement on late adverse outcomes. Eur J Cardiothorac Surg 37: 1033–1038, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Warren CM, Jordan MC, Roos KP, Krzesinski PR, Greaser ML. Titin isoform expression in normal and hypertensive myocardium. Cardiovasc Res 59: 86–94, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Weber KT, Pick R, Silver MA, Moe GW, Janicki JS, Zucker IH, Armstrong PW. Fibrillar collagen and remodeling of dilated canine left ventricle. Circulation 82: 1387–1401, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Weidemann A, Klanke B, Wagner M, Volk T, Willam C, Wiesener MS, Eckardt KU, Warnecke C. Hypoxia, via stabilization of the hypoxia-inducible factor HIF-1alpha, is a direct and sufficient stimulus for brain-type natriuretic peptide induction. Biochem J 409: 233–242, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Zile MR, Tomita M, Nakano K, Mirsky I, Usher B, Lindroth J, Carabello BA. Effects of left ventricular volume overload produced by mitral regurgitation on diastolic function. Am J Physiol Heart Circ Physiol 261: H1471–H1480, 1991 [DOI] [PubMed] [Google Scholar]