Abstract
Previous studies have suggested that a reduction in cerebral oxygen delivery may limit motor drive, particularly in hypoxic conditions, where oxygen transport is impaired. We hypothesized that raising end-tidal Pco2 (PetCO2) during incremental exercise would increase cerebral blood flow (CBF) and oxygen delivery, thereby improving peak power output (Wpeak). Amateur cyclists performed two ramped exercise tests (25 W/min) in a counterbalanced order to compare the normal, poikilocapnic response against a clamped condition, in which PetCO2 was held at 50 Torr throughout exercise. Tests were performed in normoxia (barometric pressure = 630 mmHg, 1,650 m) and hypoxia (barometric pressure = 425 mmHg, 4,875 m) in a hypobaric chamber. An additional trial in hypoxia investigated effects of clamping at a lower PetCO2 (40 Torr) from ∼75 to 100% Wpeak to reduce potential influences of respiratory acidosis and muscle fatigue imposed by clamping PetCO2 at 50 Torr. Metabolic gases, ventilation, middle cerebral artery CBF velocity (transcranial Doppler), forehead pulse oximetry, and cerebral (prefrontal) and muscle (vastus lateralis) hemoglobin oxygenation (near infrared spectroscopy) were monitored across trials. Clamping PetCO2 at 50 Torr in both normoxia (n = 9) and hypoxia (n = 11) elevated CBF velocity (∼40%) and improved cerebral hemoglobin oxygenation (∼15%), but decreased Wpeak (6%) and peak oxygen consumption (11%). Clamping at 40 Torr near maximal effort in hypoxia (n = 6) also improved cerebral oxygenation (∼15%), but again limited Wpeak (5%). These findings demonstrate that increasing mass cerebral oxygen delivery via CO2-mediated vasodilation does not improve incremental exercise performance, at least when accompanied by respiratory acidosis.
Keywords: altitude, fatigue, cerebral blood flow, near infrared spectroscopy, cerebral oxygenation, muscle oxygenation
during high-intensity aerobic exercise, increased cerebral oxygen demand (34) is paradoxically matched with decreased cerebral oxygen delivery as hyperventilation-induced hypocapnia constricts cerebral blood vessels and restricts cerebral blood flow (17, 42). Whether the reduction in cerebral oxygen delivery limits central motor drive is unknown. Evidence in support of this hypothesis comes largely from studies in which peak power output (Wpeak) (38) and time to exhaustion (5, 20) in hypoxia were rapidly improved by administration of supplemental oxygen near the point of fatigue. We have argued that such rapid changes in performance happen too quickly to reverse the accrual or depletion of metabolites associated with peripheral (muscle) fatigue and, therefore, may be mediated by other mechanisms, such as increased central (brain) oxygen delivery (5, 20, 38). Unfortunately, conclusions based on the supplemental oxygen model are limited, because improvements in arterial oxygenation are systemic and hence not localized to brain tissue. A specific method to alter cerebral oxygenation while holding all other physiological variables constant is needed to definitively test the hypothesis, but may be unrealistic to achieve in human subjects with available technology. Fortunately, important insight may still be gained with existing methods that alter cerebral oxygenation. In this study, we theorized that inhaled carbon dioxide (CO2), a potent cerebral but not muscle vasodilator (22), could be utilized to evaluate the effects of increased cerebral oxygen delivery during exhaustive exercise.
Our laboratory recently demonstrated that a simple rebreathing circuit can elevate end-tidal Pco2 (PetCO2) during exercise and prevent the drop in cerebral blood flow near maximal exertion. Our laboratory's preliminary results showed that the rebreathing circuit effectively elevated cerebral blood flow but reduced Wpeak in normoxia (27). These findings were contrary to our hypothesis and raised concerns that limitations with the circuit (e.g., high inspiratory resistance) may have influenced our results. We subsequently designed a new, low-resistance, non-rebreathing system to overcome previous limitations and continued our investigation.
In this study, we used this new, non-rebreathing system to evaluate the effects of increased cerebral blood flow in normoxic and hypoxic conditions, where large reductions in cerebral oxygen delivery may approach a theoretical limit necessary to maintain voluntarily motor output (30, 31, 34, 42). We hypothesized that, if cerebral oxygen delivery was the primary variable limiting motor output, raising PetCO2 during exercise would improve peak aerobic power by increasing cerebral blood flow and oxygen delivery.
METHODS
Subject recruitment and screening.
Following approval from our local institutional review board, amateur cyclists between the ages of 18 and 45 yr were solicited through local cycling clubs. Those giving voluntary written consent were scheduled for physical examinations to assess general health. Subjects were excluded if they reported prescription drug use or presented with respiratory or cardiovascular illnesses, such as asthma or hypertension. Aerobic capacity was assessed using incremental cycling tests to maximal exertion (30-min self-paced warm up followed by 25 W/min ramp) at two barometric pressures (Pb) inside a hypobaric chamber. The first test was performed under normobaric normoxia (Pb ∼630 mmHg or ∼1,650 m), and the second under hypobaric hypoxia to simulate high altitude (Pb ∼425 mmHg or ∼4,875 m) with 30 min of rest between tests. Those achieving a minimum of 300-W Wpeak in normoxia were scheduled for the experimental protocol.
On a separate day, each cyclist performed a set of two additional incremental tests (30-min rest interval), in either normoxia or hypoxia, to compare the normal, poikilocapnic response (control) against an experimental condition, in which PetCO2 was held (clamped) at 50 Torr from rest to maximal exertion (clamp50). We reasoned that clamping PetCO2 at 50 Torr would provide a supraphysiological, yet tolerable, stimulus for cerebral vasodilation during intense exercise, thereby maximizing our ability to detect an effect on Wpeak as a first step in our investigation. Approximately 1 wk later, subjects were asked to repeat a second set of tests in the other environmental condition. The order of tests (control and clamp50) and environmental conditions (normoxia and hypoxia) were randomly assigned and counterbalanced. The CO2 clamp was achieved by manually adding compressed gas (10% CO2, 21% O2, 69% N2 in normoxia and 15% CO2, 21% O2, 64% N2 in hypoxia) to room air in a custom-made, low-resistance (<0.02 cmH2O·l−1·s added), 5-liter (10-cm diameter × 64-cm long), open-ended inspiratory mixing chamber. The flow of compressed gas was manually adjusted based on breath-by-breath feedback of PetCO2 (Medical Graphics, Ultima CPX, St. Paul, MN). During the control trial, no compressed gas was added to the inspiratory mixing chamber. Cyclists were blinded to the intervention and naive to the hypothesis that CO2 would improve performance.
To partially control for respiratory acidosis and increased ventilatory drive induced in clamp50 trials, we invited cyclists completing hypoxic trials described above to perform one follow-up incremental test in which we clamped PetCO2 at a lower level only as subjects neared exhaustion (clamp40). From rest to their previously identified respiratory compensation point (∼75% Wpeak), cyclists breathed room air. Above the respiratory compensation point, PetCO2 was clamped at 40 Torr by surreptitiously raising inspired fraction of CO2 using the same system.
Measurements.
All testing was performed on a magnetically braked cycle ergometer (Velotron Dynafit, Seattle, WA). To obtain breath-by-breath measurements of pulmonary ventilation and gas exchange, air flowed from the inspiratory mixing chamber through 0.5 m of tubing to a standard two-way non-rebreathing valve (Hans Rudolph 2700 series) that was modified to hold a Medical Graphics pneumotach and gas sampling line (Medical Graphics, Ultima CPX, St. Paul, MN). Cerebral and muscle oxygenation measurements were obtained as previously described (37, 39). Briefly, mean cerebral blood flow velocity (CBFv) was measured in the left middle cerebral artery (49 ± 5 mm deep) using a 2-MHz transcranial Doppler (Spencer Technologies, Seattle, WA). Cerebral oxygenation in the left prefrontal lobe was assessed by monitoring changes in oxy- (O2Hb), deoxy- (HHb), and total hemoglobin (THb) and a tissue saturation index [TSI; O2Hb/(O2Hb + HHb)] obtained via spatially resolved, continuous wave near infrared spectroscopy (NIRS; Artinis Oxymon, MKIII, Zetten, the Netherlands). Source-detector spacing was set at 3.5, 4.0, and 4.5 cm for spatially resolved measurements of TSI, and data obtained from the optode pair 4.5 cm apart were used to calculate changes in O2Hb, HHb, and THb with a differential path-length factor of 5.93 (40). We interpreted increases in TSI to reflect better cerebral oxygenation and increases in O2Hb and THb as evidence of cerebral vasodilation and improved oxygen delivery, with the underlying assumption that arterial blood is responsible for ∼30% of total near infrared absorbance in cerebral tissue (8). Muscle oxygenation in the left vastus lateralis (∼15 cm proximal and ∼5 cm lateral to the superior border of the patella) was measured with an additional NIRS channel on the same instrument using a source-detector spacing of 4.0 cm and differential pathlength factor of 4.95 (13). Thigh skinfold measurements were taken between optodes to determine adipose tissue thickness above the region of interest. Arterial pulse oximetry (SpO2; Nellcor N-595, Boulder, CO) was monitored over the right prefrontal lobe. Blood pressure was obtained using finger photoplethysmography (Nexfin HD, Louisville, KY). Rating of perceived exertion (RPE) scores using a 6–20 Borg scale (7) were obtained at 1-min intervals during each test. In a subset of subjects (n = 4), arterial blood samples (2 × 1 ml) were drawn from the right radial artery (22-gauge catheter) at rest and at 25, 75, and 100% of Wpeak during control and clamp50 trials only. Samples were analyzed for arterial Pco2 (PaCO2), pH, and lactate (iStat, Abbott, Princeton, NJ) in duplicate and averaged. Because the ambient Po2 at Pb of 425 mmHg was outside the blood-gas analyzer's limit of detection, the device was unable to calibrate arterial Po2 (PaO2) during acute hypobaric hypoxia, and these data could not be measured.
Analysis and statistics.
Data were recorded continuously throughout trials. To avoid potential errors introduced by NIRS and transcranial Doppler placement/replacement, sensors were left in place between trials. Data were reduced for analysis across 15-s average windows immediately before exercise and at relative (25, 50, 75, and 100% Wpeak) and absolute (100 and 200 W) work rates. NIRS data were expressed as changes from the 15-s period immediately before each exercise test (arbitrarily defined as 0 μM). Paired t-tests were used to evaluate differences between control and clamp trials at each work rate. The Holm's procedure was used to control for type I error, considering comparisons at 100% Wpeak significant at the P < 0.05 and other comparisons at the P < 0.01 level. Trends were considered when P < 0.10. All data are presented as means ± SD.
RESULTS
Thirteen mountain bike and road cyclists (32 ± 8 yr, 180 ± 8 cm, 76 ± 11 kg, 12 ± 5 mm thigh skinfold) consented to the protocol and met the inclusion criteria. Nine male subjects completed the normoxic and 11 (8 men, 3 women) completed the hypoxic trials. Six subjects (3 men, 3 women) completed the follow-up clamp40 trial in hypoxia.
Effects of clamping PetCO2 at 50 Torr in normoxia.
Before exercise, raising PetCO2 from 33 ± 4 to 50 ± 1 Torr increased expired minute ventilation (V̇e: 184 ± 106%) end-tidal Po2 (PetO2) (31 ± 7%) and arterial SpO2 (2 ± 1%). Elevating PetCO2 increased middle cerebral artery CBFv (63 ± 23%) and cerebral oxygenation (9 ± 5% TSI), but had no effect on muscle oxygenation (Table 1).
Table 1.
Effects of clamping PetCO2 at 50 Torr throughout incremental exercise in normoxia
| Control Trial |
Clamp at 50 Torr from 0 W to Wmax |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 0 W | 100 W | 200 W | Wmax | Preclamp 0 W | 0 W | 100 W | 200 W | Wmax |
| Power, W | 0 ± 0 | 100 ± 0 | 200 ± 0 | 366 ± 45 | 0 ± 0 | 0 ± 0 | 100 ± 0 | 200 ± 0 | 344 ± 42.6‡ |
| HR, beats/min | 82 ± 13 | 108 ± 5 | 139 ± 10 | 183 ± 8 | 78 ± 15 | 81 ± 13 | 111 ± 13 | 141 ± 17 | 175 ± 8‡ |
| V̇e, l/min | 19.5 ± 7.1 | 42.7 ± 4.2 | 74.1 ± 5.4 | 169.4 ± 17.7 | 18.7 ± 3.4 | 51.3 ± 17.9* | 86.4 ± 20.5‡ | 113.7 ± 17.7‡ | 159.7 ± 25.7 |
| RR, breaths/min | 16 ± 6 | 22 ± 7 | 29 ± 5 | 58 ± 6 | 16 ± 7 | 20 ± 5 | 29 ± 6† | 35 ± 6‡ | 56 ± 10 |
| Vt, l/breath | 1.3 ± 0.3 | 2.1 ± 0.5 | 2.7 ± 0.5 | 2.9 ± 0.2 | 1.3 ± 0.3 | 2.5 ± 0.6* | 3.0 ± 0.5‡ | 3.2 ± 0.3‡ | 2.9 ± 0.4 |
| PetCO2, Torr | 34 ± 4 | 42 ± 15 | 46 ± 16 | 33 ± 3 | 33 ± 4 | 50 ± 1* | 51 ± 1‡ | 50 ± 2† | 52 ± 5‡ |
| PetO2 Torr | 84 ± 7 | 75 ± 7 | 78 ± 5 | 91 ± 4 | 84 ± 4 | 110 ± 5* | 98 ± 7‡ | 92 ± 6‡ | 92 ± 5† |
| V̇e/V̇co2 | 34 ± 9 | 23 ± 5 | 26 ± 6 | 40 ± 9 | 33 ± 6 | 116 ± 35* | 51 ± 11‡ | 41 ± 6‡ | 43 ± 7 |
| V̇e/V̇co2 | 36 ± 9 | 28 ± 7 | 26 ± 6 | 32 ± 8 | 37 ± 3 | 177 ± 59* | 62 ± 18‡ | 39 ± 9‡ | 37 ± 6 |
| V̇co2, l/min | 0.6 ± 0.2 | 1.9 ± 0.4 | 3.0 ± 0.4 | 4.3 ± 0.6 | 0.6 ± 0.2 | 0.5 ± 0.1 | 1.7 ± 0.3 | 2.8 ± 0.2 | 3.8 ± 0.7† |
| V̇co2, l/min | 0.6 ± 0.4 | 1.6 ± 0.6 | 3.1 ± 1.1 | 5.5 ± 1.5 | 0.5 ± 0.1 | 0.3 ± 0.1* | 1.4 ± 0.1 | 3.0 ± 0.3 | 4.4 ± 0.7 |
| CBFv, cm/s | 47 ± 9 | 55 ± 10 | 59 ± 13 | 56 ± 11 | 43 ± 5 | 71 ± 14* | 77 ± 17‡ | 76 ± 12‡ | 78 ± 13‡ |
| SpO2, % | 97 ± 2 | 96 ± 3 | 95 ± 3 | 91 ± 5 | 97 ± 2 | 99 ± 2* | 99 ± 2 | 98 ± 3 | 91 ± 5 |
| Cerebral oxygenation | |||||||||
| ΔTSI, % | 0 ± 0 | −3.6 ± 4.1 | −4.8 ± 4.7 | −18.9 ± 11.4 | 0 ± 0 | 9.5 ± 4.6* | 9.1 ± 5.5‡ | 5.9 ± 7.0‡ | −5.9 ± 10.8‡ |
| ΔO2Hb, μM | 0 ± 0 | −4.5 ± 2.0 | −2.6 ± 4.7 | −1.7 ± 6.3 | 0 ± 0 | 5.1 ± 2.4* | 3.3 ± 2.9‡ | 1.8 ± 3.3‡ | 2.6 ± 6.4‡ |
| ΔHHb, μM | 0 ± 0 | 1.1 ± 1.2 | 1.9 ± 2.7 | 7.7 ± 5.4 | 0 ± 0 | −1.7 ± 1.5* | −1.6 ± 1.6‡ | −0.6 ± 2.7‡ | 6.7 ± 5.7 |
| ΔTHb, μM | 0 ± 0 | −3.4 ± 1.8 | −0.7 ± 3.0 | 6.0 ± 6.8 | 0 ± 0 | 3.4 ± 2.7* | 1.8 ± 2.8‡ | 1.2 ± 3.6 | 9.0 ± 8.0 |
| Muscle oxygenation | |||||||||
| ΔO2Hb, μM | 0 ± 0 | −8.8 ± 3.2 | −12.8 ± 4.5 | −16.3 ± 5.8 | 0 ± 0 | −1.0 ± 1.5 | −6.5 ± 3.4 | −10.4 ± 4.9 | −14.1 ± 3.0 |
| ΔHHb, μM | 0 ± 0 | 6.9 ± 4.7 | 13.8 ± 6.1 | 18.5 ± 6.3 | 0 ± 0 | 0.5 ± 2.3 | 6.1 ± 4.2 | 12.5 ± 6.0 | 18.3 ± 5.1 |
| ΔTHb, μM | 0 ± 0 | −1.8 ± 4.8 | 1.0 ± 5.6 | 2.2 ± 6.1 | 0 ± 0 | −0.5 ± 2.6 | −0.5 ± 3.4 | 2.7 ± 3.4 | 4.2 ± 4.3 |
Values are means ± SD; n = 9 subjects. Wmax, maximal power output; HR, heart rate; V̇e, expired volume; RR, respiratory rate; Vt, tidal volume; PetCO2, end-tidal Pco2; PetO2, end-tidal Po2; V̇e/V̇o2, ventilatory equivalent for oxygen; V̇e/V̇co2, ventilatory equivalent for carbon dioxide; V̇o2, volume of oxygen consumed; V̇co2, volume of carbon dioxide produced; CBFv, mean cerebral blood flow velocity of the middle cerebral artery; SpO2, arterial pulse saturation; ΔTSI, change in tissue saturation index; ΔO2Hb, change in oxygenated hemoglobin; ΔHHb, change in deoxygenated hemoglobin; ΔTHb, change in total hemoglobin.
Different from preclamp (P < 0.01).
Different from control trial (P < 0.05).
Different from control trial (P < 0.01).
During submaximal exercise, clamping PetCO2 at 50 Torr increased V̇e, PetO2, and arterial SpO2. These effects, combined with CO2-mediated vasodilation, improved CBFv and cerebral oxygenation across submaximal intensities (Fig. 1), without affecting muscle oxygenation (Fig. 2). Elevation of PetCO2 increased RPE compared with the control trial (9.6 ± 0.5 vs. 8.6 ± 0.9 at 100 W and 14.4 ± 1.5 vs. 13.3 ± 1.0 at 200 W; P < 0.05).
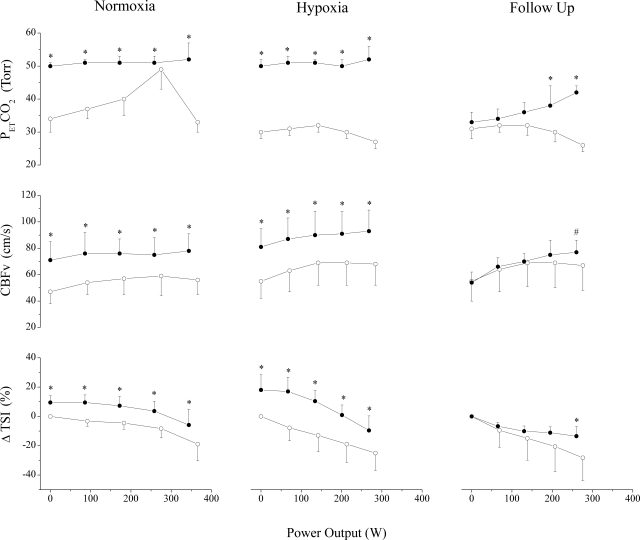
Fig. 1.
Control (○) and clamp (●) experiments in normoxia (n = 9), hypoxia (n = 11), and follow-up (n = 6) trials. Values are means and SDs at 0 W, 25, 50, 75, and 100% of peak work rate. End-tidal Pco2 (PetCO2) was clamped at 50 Torr in normoxia and hypoxia throughout exercise. In follow-up trials, PetCO2 was clamped at 40 Torr from ∼75 to 100% peak work rate. Clamping increased cerebral blood flow velocity (CBFv) and cerebral oxygenation, but decreased maximal power output. ΔTSI, change in tissue saturation index. Different from control (*P < 0.05) and trend (#P < 0.10).
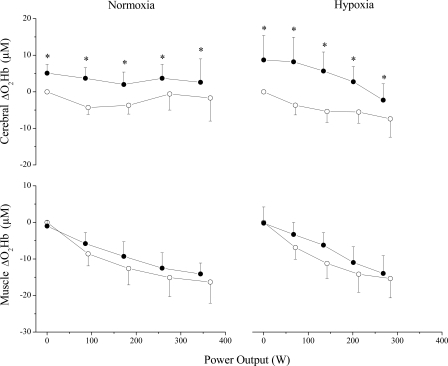
Fig. 2.
Control (○) and clamp at 50 Torr (●) trials in normoxia (n = 9) and hypoxia (n = 11). Values are means and SDs at 0 W, 25, 50, 75, and 100% of peak work rate expressed as changes from the 15-s period immediately before exercise. Clamping increased oxyhemoglobin (ΔO2Hb) in cerebral but not muscle tissue. Different from control, *P < 0.01.
At exhaustion (i.e., RPE of 20), V̇e between trials was similar, reaching 95 ± 16% of estimated maximal voluntary ventilation (MVV) (21, 23). Although at maximum exertion PetO2 was slightly elevated (2 ± 2%) in the clamp50 trial, SpO2 was not different from control. Clamping CO2 effectively elevated CBFv (43 ± 24%) and cerebral oxygenation (13 ± 10% TSI), without affecting muscle oxygenation (Fig. 2). Despite these findings, Wpeak, peak O2 consumption and heart rate were reduced (6 ± 5, 11 ± 12, and 5 ± 2%, respectively) during the clamp50 trial (Table 1).
Arterial blood samples from two subjects confirmed that clamping raised PaCO2 vs. the control trial, at rest (50 and 46 vs. 35 and 33 Torr) and at maximal exertion (43 and 43 vs. 35 and 27 Torr). These levels of hypercapnia were accompanied by both lower blood pH (7.19 and 7.21 vs. 7.20 and 7.25) and lactate concentrations (8.8 and 9.5 vs. 15.8 and 15.9 mM) at maximal exertion.
Effects of clamping PetCO2 at 50 Torr in hypoxia.
Before exercise, raising PetCO2 from 30 ± 4 to 50 ± 3 Torr increased V̇e (197 ± 90%), PetO2 (53 ± 11%), and arterial SpO2 (9 ± 6%). Elevation of PetCO2 increased middle cerebral artery CBFv (68 ± 31%) and cerebral oxygenation (18 ± 10% TSI) without affecting muscle oxygenation (Table 2).
Table 2.
Effects of clamping PetCO2 at 50 Torr throughout incremental exercise in hypoxia
| Control Trial |
Clamp at 50 Torr from 0 W to Wmax |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 0 W | 100 W | 200 W | Wmax | Preclamp 0 W | 0 W | 100 W | 200 W | Wmax |
| Power, W | 0 ± 0 | 100 ± 0 | 200 ± 0 | 284 ± 31 | 0 ± 0 | 0 ± 0 | 100 ± 0 | 200 ± 0 | 268 ± 34† |
| HR, beats/min | 86 ± 29 | 123 ± 14 | 155 ± 10 | 170 ± 11 | 89 ± 11 | 80 ± 14* | 119 ± 14 | 152 ± 10 | 170 ± 9 |
| V̇e, l/min | 19.0 ± 6.4 | 51.2 ± 6.9 | 104.7 ± 14.0 | 155.1 ± 22.6 | 19.0 ± 7.6 | 57.4 ± 20.3* | 101.4 ± 6.9‡ | 144.2 ± 14.0‡ | 155.4 ± 25.2 |
| RR, breaths/min | 18 ± 5 | 27 ± 7 | 44 ± 15 | 60 ± 9 | 16 ± 6 | 23 ± 6* | 34 ± 7‡ | 45 ± 14 | 55 ± 8‡ |
| Vt, l/breath | 1.1 ± 0.3 | 2.0 ± 0.4 | 2.5 ± 0.5 | 2.6 ± 0.4 | 1.2 ± 0.3 | 2.5 ± 0.8* | 3.0 ± 0.4‡ | 3.2 ± 0.5‡ | 2.9 ± 0.5† |
| PetCO2, Torr | 30 ± 2 | 31 ± 3 | 30 ± 3 | 27 ± 2 | 30 ± 4 | 50 ± 2* | 50 ± 1‡ | 50 ± 2‡ | 52 ± 4‡ |
| PetO2 Torr | 45 ± 3 | 45 ± 2 | 52 ± 2 | 57 ± 1 | 46 ± 3 | 70 ± 3* | 62 ± 2‡ | 60 ± 2‡ | 61 ± 2‡ |
| V̇e/V̇o2 | 36 ± 4 | 33 ± 2 | 42 ± 5 | 54 ± 3 | 36 ± 5 | 113 ± 31* | 65 ± 2‡ | 59 ± 6‡ | 61 ± 5‡ |
| V̇e/V̇co2 | 40 ± 4 | 35 ± 3 | 37 ± 4 | 42 ± 3 | 41 ± 5 | 251 ± 188* | 66 ± 3‡ | 48 ± 4‡ | 44 ± 6 |
| V̇o2, l/min | 0.5 ± 0.2 | 1.6 ± 0.2 | 2.5 ± 0.3 | 2.9 ± 0.5 | 0.5 ± 0.2 | 0.5 ± 0.2 | 1.6 ± 0.2 | 2.5 ± 0.3 | 2.6 ± 0.4‡ |
| V̇co2, l/min | 0.5 ± 0.2 | 1.5 ± 0.2 | 2.8 ± 0.3 | 3.7 ± 0.4 | 0.5 ± 0.2 | 0.3 ± 0.2* | 1.6 ± 0.2 | 3.0 ± 0.3 | 3.6 ± 0.7 |
| CBFv, cm/s | 55 ± 13 | 65 ± 17 | 70 ± 17 | 68 ± 16 | 45 ± 19 | 81 ± 14* | 88 ± 17‡ | 91 ± 17‡ | 93 ± 16‡ |
| SpO2, % | 84 ± 6 | 75 ± 5 | 72 ± 5 | 70 ± 5 | 85 ± 7 | 94 ± 3* | 88 ± 5‡ | 78 ± 5‡ | 72 ± 7 |
| Cerebral oxygenation | |||||||||
| ΔTSI, % | 0 ± 0 | −9.8 ± 8.5 | −17.6 ± 12.1 | −25.0 ± 11.9 | 0 ± 0 | 18.1 ± 10.4* | 14.3 ± 9.1‡ | 1.9 ± 6.8‡ | −9.6 ± 9.9‡ |
| ΔO2Hb, μM | 0 ± 0 | −4.7 ± 2.3 | −6.1 ± 3.1 | −7.4 ± 5.0 | 0 ± 0 | 8.7 ± 6.7* | 7.2 ± 6.3‡ | 2.6 ± 4.8‡ | −2.3 ± 4.5† |
| ΔHHb, μM | 0 ± 0 | 3.7 ± 3.7 | 6.6 ± 5.5 | 11.2 ± 8.4 | 0 ± 0 | −4.9 ± 4.1* | −3.4 ± 3.8‡ | 2.1 ± 3.2‡ | 7.8 ± 5.6† |
| ΔTHb, μM | 0 ± 0 | −0.9 ± 1.8 | 0.5 ± 3.7 | 3.8 ± 9.8 | 0 ± 0 | 3.9 ± 3.1* | 3.8 ± 3.6‡ | 4.8 ± 4.1‡ | 5.5 ± 6.2 |
| Muscle oxygenation | |||||||||
| ΔO2Hb, μM | 0 ± 0 | −8.4 ± 3.0 | −13.7 ± 4.6 | −15.4 ± 5.3 | 0 ± 0 | −0.2 ± 4.4 | −4.1 ± 3.0‡ | −10.7 ± 3.7‡ | −14.0 ± 4.8 |
| ΔHHb, μM | 0 ± 0 | 7.8 ± 5.3 | 15.5 ± 7.3 | 17.6 ± 8.4 | 0 ± 0 | −0.6 ± 3.4 | 4.3 ± 3.3† | 12.9 ± 5.7 | 16.3 ± 7.7 |
| ΔTHb, μM | 0 ± 0 | −0.7 ± 5.0 | 1.7 ± 4.7 | 2.2 ± 6.1 | 0 ± 0 | 0.4 ± 4.7 | 0.2 ± 3.5 | 2.3 ± 5.0 | 2.3 ± 6.9 |
Values are means ± SD; n = 9 subjects.
Different from preclamp (P < 0.01).
Different from control trial (P < 0.05).
Different from control trial (P < 0.01).
During submaximal exercise, clamping PetCO2 at 50 Torr increased V̇e, PetO2, and SpO2. These effects, combined with CO2-mediated vasodilation, improved CBFv and cerebral oxygenation across submaximal intensities (Fig. 1). Muscle oxygenation was improved slightly at low intensities, but this effect diminished as work rate increased (Table 2). Elevation of PetCO2 did not affect RPE compared with the control trial in hypoxia.
At exhaustion, V̇e was similar between clamp50 and control trials, reaching 97 ± 12% of estimated MVV. Although PetO2 was increased (5 ± 4%) in the clamp50 trial, SpO2 was not different from control. Clamping CO2 successfully elevated CBFv (39 ± 19%) and cerebral oxygenation (15 ± 11% TSI) without affecting muscle oxygenation at maximal exertion (Fig. 2). Despite these findings, Wpeak and peak O2 consumption were reduced (6 ± 8 and 11 ± 8%, respectively) during the CO2 clamp trial. Responses were similar in male and female cyclists.
Arterial blood samples from two subjects confirmed elevation of PaCO2 during the clamped vs. control trials at rest (46 and 43 vs. 24 and 29 Torr) and at maximal exertion (41 and 48 vs. 24 and 26 Torr). Hypercapnia was accompanied by a reduction in both blood pH (7.29 and 7.24 vs. 7.37 and 7.35) and lactate concentrations (6.7 and 9.7 vs. 11.1 and 10.9 mM) at maximal exertion.
Effects of clamping PetCO2 at 40 Torr in hypoxia.
When subjects breathed room air up to ∼75% maximal power output (Wmax), then were clamped at a PetCO2 of 40 Torr until exhaustion, no differences were detected in V̇e, PetO2, or SpO2 compared with the poikilocapnic control trial (Table 3). Clamping PetCO2 at 40 Torr tended to increase CBFv (36 ± 22%: P = 0.09) and improved cerebral oxygenation (17 ± 10% TSI) at maximal exertion, but Wmax still tended to be reduced (5 ± 6%: P = 0.06).
Table 3.
Effects of clamping PetCO2 at 40 Torr as subjects neared exhaustion in hypoxia
| Control Trial |
Clamp at 40 Torr from ∼75% to Wmax |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | 0 W | 100 W | 200 W | Wmax | 0 W | 100 W | 200 W | Wmax |
| Power, W | 0 ± 0 | 100 ± 0 | 200 ± 0 | 278 ± 0 | 0 ± 0 | 100 ± 0 | 200 ± 0 | 260 ± 33§ |
| HR, beats/min | 75 ± 10 | 125 ± 13 | 158 ± 10 | 173 ± 7 | 84 ± 15 | 131 ± 14 | 160 ± 8 | 171 ± 11 |
| V̇e, l/min | 18.8 ± 7.8 | 49.1 ± 7.0 | 103.2 ± 6.7 | 148.5 ± 25.3 | 16.9 ± 9.9 | 50.9 ± 8.2 | 107.8 ± 7.6 | 150.4 ± 29.0 |
| RR, breaths/min | 18 ± 8 | 25 ± 4 | 42 ± 8 | 60 ± 6 | 16 ± 8 | 25 ± 4 | 41 ± 7 | 55 ± 6 |
| Vt, l/breath | 1.0 ± 0.3 | 2.1 ± 0.4 | 2.5 ± 0.5 | 2.5 ± 0.5 | 1.1 ± 0.3 | 2.2 ± 0.4 | 2.7 ± 0.4 | 2.8 ± 0.5† |
| PetCO2, Torr | 31 ± 3 | 32 ± 3 | 30 ± 3 | 26 ± 2 | 33 ± 3 | 35 ± 3 | 38 ± 5‡ | 42 ± 2‡ |
| PetO2 Torr | 44 ± 4 | 44 ± 1 | 52 ± 2 | 57 ± 1 | 43 ± 3 | 43 ± 2 | 52 ± 3 | 57 ± 1 |
| V̇e/V̇o2 | 34 ± 4 | 32 ± 2 | 43 ± 5 | 55 ± 2 | 32 ± 2 | 31 ± 2 | 42 ± 5 | 55 ± 6 |
| V̇e/V̇co2 | 40 ± 4 | 34 ± 2 | 36 ± 3 | 42 ± 3 | 35 ± 3 | 32 ± 3 | 38 ± 8 | 41 ± 7 |
| V̇o2, l/min | 0.6 ± 0.2 | 1.5 ± 0.3 | 2.4 ± 0.3 | 2.7 ± 0.6 | 0.5 ± 0.3 | 1.7 ± 0.3 | 2.6 ± 0.3 | 2.7 ± 0.5 |
| V̇co2, l/min | 0.5 ± 0.2 | 1.4 ± 0.2 | 2.9 ± 0.3 | 3.5 ± 0.5 | 0.5 ± 0.3 | 1.6 ± 0.3 | 2.9 ± 0.5 | 3.7 ± 0.7 |
| CBFv, cm/s | 55 ± 15 | 67 ± 18 | 69 ± 19 | 67 ± 18 | 54 ± 8 | 68 ± 7 | 71 ± 9 | 78 ± 8§ |
| SpO2, % | 82 ± 7 | 74 ± 4 | 71 ± 4 | 70 ± 4 | 83 ± 8 | 70 ± 7 | 70 ± 3 | 70 ± 6 |
| Cerebral oxygenation | ||||||||
| ΔTSI, % | 0 ± 0 | −5.8 ± 4.4 | −14.1 ± 9.8 | −28.2 ± 15.7 | 0 ± 0 | −9.3 ± 2.9 | −11.3 ± 5.9 | −13.5 ± 6.6† |
| ΔO2Hb, μM | 0 ± 0 | −2.4 ± 0.7 | −4.0 ± 2.2 | −8.2 ± 5.7 | 0 ± 0 | −4.8 ± 2.5 | −5.0 ± 1.3 | −6.0 ± 3.7 |
| ΔHHb, μM | 0 ± 0 | 2.1 ± 1.9 | 5.3 ± 4.5 | 12.6 ± 10.8 | 0 ± 0 | 4.6 ± 1.7 | 6.3 ± 1.5 | 8.2 ± 3.4 |
| ΔTHb, μM | 0 ± 0 | −0.3 ± 1.5 | 1.3 ± 4.5 | 4.4 ± 11.1 | 0 ± 0 | −0.2 ± 1.5 | 1.3 ± 0.8 | 2.2 ± 4.6 |
| Muscle oxygenation | ||||||||
| ΔO2Hb, μM | 0 ± 0 | −2.3 ± 0.8 | −6.5 ± 2.1 | −12.4 ± 5.0 | 0 ± 0 | −7.1 ± 1.2 | −10.3 ± 2.4 | −13.7 ± 10.8 |
| ΔHHb, μM | 0 ± 0 | 3.3 ± 1.6 | 9.3 ± 3.4 | 14.6 ± 6.5 | 0 ± 0 | 7.5 ± 4.4 | 12.3 ± 7.0 | 14.2 ± 6.4 |
| ΔTHb, μM | 0 ± 0 | 1.0 ± 1.4 | 2.8 ± 2.0 | 2.1 ± 5.1 | 0 ± 0 | 0.4 ± 4.4 | 2.0 ± 7.7 | 4.8 ± 7.6 |
Values are means ± SD; n = 6 subjects.
Different from preclamp (P < 0.01).
Different from control trial (P < 0.05).
Different from control trial (P < 0.01).
Trend away from control trial (P < 0.10).
DISCUSSION
This study advances an approach to study the effect of cerebral oxygen delivery on exercise performance. To the best of our knowledge, it is the first study that attempts to improve exercise performance by increasing cerebral blood flow. By clamping PetCO2 during exercise, we were able to selectively increase oxygen delivery to the brain during exhaustive exercise, yet peak performance was impaired rather than improved. Results suggest that cerebral oxygen delivery may not be a primary determinant limiting central motor drive during incremental exercise, at least when accompanied by respiratory acidosis.
Effectiveness of the approach.
Our first major challenge in this study was to develop a method that could selectively alter cerebral oxygen delivery. We accomplished this by administering supplemental CO2, which has long been known to increase cerebral blood flow more than muscle blood flow (22). This method effectively increased cerebral but not muscle tissue oxygenation, thereby directly addressing a major limitation of previous studies (5, 20, 38). During exercise, elevation of PetCO2 to 50 Torr increased cerebral blood flow in the middle cerebral artery and elevated cerebral hemoglobin concentrations (O2Hb and THb) in the prefrontal cortex, indicators of cerebral vasodilation and improved oxygen delivery (8). On the contrary, no changes in muscle hemoglobin concentrations were detected in vastus lateralis muscle, which, assuming no changes in muscle blood flow, would indicate unchanged muscle oxygen delivery (Fig. 2). These data are in line with previous results demonstrating that vasoreactivity to CO2 is greater in cerebral than in muscle vasculature (1) and provide experimental evidence that we augmented cerebral relative to muscle oxygen delivery.
Since mass oxygen delivery is a product of blood flow and arterial oxygen content (CaO2), maintaining CaO2 between trials was a concern. While our non-rebreathing method was effective at clamping PetCO2, the resulting increase in respiratory drive elevated SpO2, and therefore CaO2, during submaximal work rates up to 75% Wmax. Yet at maximal exertion, the additive respiratory effect of elevated CO2 was diminished, evidenced by the fact that V̇e and SpO2 were similar between clamp and control trials (Tables 1 and 2). Since CaO2 is largely the product of SpO2 when blood Hb concentration is constant, CaO2 was likely similar between trials at maximal exertion. Elevations in PetO2 were evident throughout exercise during CO2 clamp trials, but these changes were too small at peak exercise to appreciably affect PaO2 and hence CaO2. These results substantiate our claim to have augmented cerebral oxygen delivery primarily by increasing cerebral blood flow, rather than CaO2, at maximal exertion and represent an important step in developing an experimental model to manipulate cerebral oxygen delivery during exhaustive exercise.
Does increased cerebral oxygen delivery improve maximal performance?
Despite the efficacy of the method to increase cerebral oxygen delivery during intense exercise, maximal aerobic power was impaired in normoxia. These results are similar to our laboratory's pilot study (27), in which we reported decreased Wpeak during incremental exercise when we prevented the fall in cerebral blood flow above the respiratory compensation point. Taken together with our previous studies in normoxia, which demonstrate no effect on maximal aerobic performance when CaO2 is rapidly increased with supplemental oxygen (38), nor evidence of a critical level of cerebral hemoglobin concentration at exhaustion (37, 39), our new observation that increased cerebral oxygenation through CO2-mediated vasodilation does not improve performance suggests that mass cerebral oxygen delivery (blood flow × CaO2) may not limit maximal aerobic performance in normoxia.
We also evaluated the effect of increased cerebral blood flow in acute hypoxia because the greater challenge to oxygen delivery may approach a critical level, incompatible with maintaining central motor drive (3, 26, 30, 31, 34, 42). Results again did not support the hypothesis. Clamping PetCO2 at 50 Torr increased cerebral blood flow and oxygenation, but decreased maximal aerobic power output and oxygen consumption to a similar extent as in normoxia (Tables 1 and 2). While these findings were robust, we were concerned that the level of hypercapnia may have been excessive (PetCO2 >10 Torr above poikilocapnic control) and could have led to accumulated acidosis (9) or respiratory muscle fatigue (4, 6, 33) that might have outweighed any potential benefits of increased cerebral oxygen delivery. We subsequently performed a follow-up trial in which we clamped PetCO2 at a lower target (40 Torr) only after cyclists reached their respiratory compensation point. Reducing the degree and duration of respiratory acidosis (∼20 min at 50 Torr vs. ∼2 min at 40 Torr) did not affect results. Performance in acute hypoxia still tended to be impaired, despite an increase in cerebral oxygenation.
Collectively, these results argue against a limiting role of cerebral blood flow on maximal aerobic performance and lead us back to question the relative influence of CaO2 and PaO2 on exercise performance in hypoxia (2, 32). As stated earlier, CaO2 was similar between trials, but PetO2 was elevated (∼4 Torr) at the point of fatigue during the CO2-clamp trials in hypoxia. Assuming that oxygen transport across alveoli was not affected by supplemental CO2, elevation in PetO2 may have increased PaO2 slightly in the clamp trials. Furthermore, the induced respiratory acidosis accompanying hypercapnia would have been expected to shift the O2Hb dissociation curve rightward, thereby facilitating unloading of oxygen from Hb. The combination of these effects may have increased muscle and brain tissue Po2 during the CO2-clamp trials, yet maximal aerobic performance was impaired rather than improved. These findings are in agreement with previous studies citing minimal effects of PaO2 on exercise performance (2, 32), but should be interpreted with caution, since any changes in arterial or tissue Po2 with our approach would have been small.
Why might performance have been impaired?
We were surprised by the finding that performance was impaired with this approach, because others have not reported a change in peak aerobic power when cyclists inspired a fixed concentration of CO2 (4%) during incremental exercise in normoxia (14, 25). The differences between studies may be explained by the relative degrees of respiratory acidosis. Although peak PaCO2 values (∼54 Torr) in the previous studies were slightly greater than our PetCO2 values at submaximal work rates, they did not titrate the delivery of CO2 to account for relative hyperventilation near exhaustion, so PaCO2 at maximal exertion may have fallen below what we were able to maintain by clamping PetCO2. A study with higher inspired concentrations of CO2 (6%) reported results similar to ours (19), in line with findings that respiratory acidosis affects muscle contractility in a dose-dependent manner (41). While hypercapnia did appear to reduce arterial pH at maximal exertion in our limited number of arterial blood samples, this explanation is difficult to reconcile with more recent studies showing that exposure of muscle fibers to reduced extracellular pH actually increases contractile ability when muscle temperature is increased (9, 28). In our study, we did not detect a degree or duration-dependent effect, as the decrement in performance was similar during severe, sustained (clamp50) and mild, brief (clamp40) hypercapnia. We acknowledge that these observations cannot rule out pH-mediated impairment of performance. Future studies that clamp both PetCO2 and arterial pH, with bicarbonate infusion (29), are needed to further isolate effects of increased cerebral oxygen delivery during exercise.
Further inspection of available data does not point to a clear physiological mechanism by which hypercapnia impaired performance (19, 41). Vianna et al. (41) concluded the deleterious effect of hypercapnia on muscle contractility was likely mediated by factors other than intracellular pH. We reasoned that, if a variable potentially posed a limit to performance, it would reach the same value at maximal exertion, regardless of the trial. V̇e and muscle oxygenation were the only two physiological variables that consistently appeared to reach similar maximums and minimums, respectively, between trials. Our trained athletes may have been limited by the finite structure of their respiratory system, since ventilatory reserves and expiratory flow are limited and may restrict the ventilatory response to high-intensity effort (12). Our subjects reached ∼95% of their estimated MVV at maximal exertion and may have approached such functional limits. Additionally, during PetCO2 clamp trials in hypoxia, maximal V̇e was similar to control trials, despite occurring at lower work rates (Tables 2 and 3). The accentuated ventilatory drive due to hypercapnia may have increased oxygen demand within respiratory muscles, thereby diverting blood flow away from exercising limb muscles (15, 16), and reduced muscle oxygenation prematurely. We propose that such a “steal” mechanism could have been compounded by the degree of hypercapnia-induced cerebral vasodilation imposed by our experimental conditions and led to an even greater proportion of blood flow being diverted away from contracting muscle, thereby limiting performance (10, 11, 24). Alternatively, since hypercapnia tended to increase subjects' RPE during exercise, subjects may have quit exercising independent of a failure of any single physiological system (36).
Limitations.
Despite our success in selectively increasing cerebral oxygen delivery during exercise, our approach has several limitations. Foremost, we acknowledge that administration of supplemental CO2 induced respiratory acidosis, which may have influenced fatigue and confounded our ability to detect an effect of increased cerebral oxygen delivery on performance. Although reducing the degree and duration of respiratory acidosis in our follow-up trials did not affect performance, clamping blood pH with bicarbonate infusion is a logical next step to improve the method.
Our approach was not expected to clamp PaCO2, the direct effector of cerebrovascular tone, since the alveolar to arterial Pco2 difference widens with exercise intensity and exhibits a great degree of intraindividual variability (18). We chose to clamp PetCO2 because it could be noninvasively monitored and adjusted breath by breath during ramped exercise. While less precise than controlling PaCO2, clamping PetCO2 raised PaCO2 enough to achieve the desired cerebral vasodilation.
Transcranial Doppler measurements of flow velocity are widely accepted indexes of cerebral blood flow, but are dependent on the assumption that vessel radius does not change. While the radius of the middle cerebral artery remains constant at rest (35), this has not been experimentally validated during exercise with supplemental CO2. We appreciate by Poiseuille's Law that even a small degree of vasodilation will greatly increase blood flow, yet we do not see this as a major limitation of our method because hypercapnia-induced vasodilation of the middle cerebral artery would have caused us to underestimate the increase in cerebral blood flow and not affected our interpretation of the results.
Our assertion that muscle blood flow and oxygen delivery were not affected by CO2 was based on NIRS measurements of O2Hb and HHb rather than direct measurements of leg blood flow and CaO2. Future studies documenting distribution of blood flow with this approach are necessary to elucidate potential hemodynamic effects in respective tissues.
Last, our results are restricted to the conditions of testing. All subjects had lived at ∼1,650 m for at least 1 yr. This degree of acclimatization may have an effect on the physiological response to higher altitudes and limit the generalizability of our findings.
Conclusions.
Administration of supplemental CO2 allowed us to augment cerebral, but not muscle, oxygenation during incremental exercise, thereby addressing a limitation of previous studies. Despite improvements in cerebral oxygen delivery, peak performance was impaired. These results suggest that mass cerebral oxygen delivery may not be the primary factor limiting incremental exercise performance, at least when accompanied by respiratory acidosis. Future refinements to the method are needed to control for changes in acid-base balance to determine whether small improvements in performance were potentially masked by deleterious effects of reduced pH.
GRANTS
Funding was provided by National Heart, Lung, and Blood Institute Grant HL-070362 and the Altitude Research Center. B. Kayser was supported by the Swiss National Research Foundation (Grant 320030-127536).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.W.S., J.T.O., A.C.D., B.K., and R.C.R. conception and design of research; A.W.S., J.T.O., A.C.D., and D.M.P. performed experiments; A.W.S., J.T.O., and A.C.D. analyzed data; A.W.S., J.T.O., A.C.D., D.M.P., B.K., and R.C.R. interpreted results of experiments; A.W.S. prepared figures; A.W.S. drafted manuscript; A.W.S., J.T.O., A.C.D., D.M.P., B.K., and R.C.R. edited and revised manuscript; A.W.S., J.T.O., A.C.D., D.M.P., B.K., and R.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We express our gratitude to David Gottlieb for help with data collection.
REFERENCES
- 1. Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol (Lond) 566: 613–624, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amann M, Calbet JAL. Convective oxygen transport and fatigue. J Appl Physiol 104: 861–870, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Amann M, Kayser B. Nervous system function during exercise in hypoxia. High Alt Med Biol 10: 149–164, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293: R2036–R2045, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol (Lond) 581: 389–403, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol 93: 201–206, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Borg GA. Perceived exertion. Exerc Sport Sci Rev 2: 131–153, 1974 [PubMed] [Google Scholar]
- 8. Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bülow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11: 213–222, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Cairns SP. Lactic acid and exercise performance: culprit or friend? Sports Med 36: 279–291, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Calbet JAL, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on V̇o2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291: R447–R453, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Calbet JAL, Rådegran G, Boushel R, Saltin B. On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol (Lond) 587: 477–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dempsey JA, McKenzie DC, Haverkamp HC, Eldridge MW. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest 134: 613–622, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Graham T, Wilson BA, Sample M, Van Dijk J, Bonen A. The effects of hypercapnia on metabolic responses to progressive exhaustive work. Med Sci Sports Exerc 12: 278–284, 1980 [PubMed] [Google Scholar]
- 15. Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82: 1573–1583, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 85: 609–618, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Hellström G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol 81: 413–418, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial Pco2 in exercise. J Appl Physiol 47: 954–960, 1979 [DOI] [PubMed] [Google Scholar]
- 19. Kato T, Tsukanaka A, Harada T, Kosaka M, Matsui N. Effect of hypercapnia on changes in blood pH, plasma lactate and ammonia due to exercise. Eur J Appl Physiol 95: 400–408, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kayser B, Narici M, Binzoni T, Grassi B, Cerretelli P. Fatigue and exhaustion in chronic hypobaric hypoxia: influence of exercising muscle mass. J Appl Physiol 76: 634–640, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Kory RC, Callahan R, Boren HG, Syner JC. The Veterans Administration-Army cooperative study of pulmonary function. I. Clinical spirometry in normal men. Am J Med 30: 243–258, 1961 [DOI] [PubMed] [Google Scholar]
- 22. Lennox WG, Gibbs EL. The blood flow in the brain and the leg of man, and the changes induced by alteration of blood gases. J Clin Invest 11: 1155–1177, 1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindall A, Medina A, Grismer JT. A re-evaluation of normal pulmonary function measurements in the adult female. Am Rev Respir Dis 95: 1061–1064, 1967 [DOI] [PubMed] [Google Scholar]
- 24. Lundby C, Boushel R, Robach P, Møller K, Saltin B, Calbet JAL. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol (Lond) 586: 123–130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLellan TM. The influence of a respiratory acidosis on the exercise blood lactate response. Eur J Appl Physiol Occup Physiol 63: 6–11, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Nybo L, Rasmussen P. Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev 35: 110–118, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Olin JT, Dimmen AC, Subudhi AW, Roach RC. Cerebral blood flow and oxygenation at maximal exercise: the effect of clamping carbon dioxide. Respir Physiol Neurobiol 175: 176–180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and beta2-agonist. J Physiol (Lond) 551: 277–286, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Péronnet F, Meyer T, Aguilaniu B, Juneau CE, Faude O, Kindermann W. Bicarbonate infusion and pH clamp moderately reduce hyperventilation during ramp exercise in humans. J Appl Physiol 102: 426–428, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen P, Nielsen J, Overgaard M, Krogh-Madsen R, Gjedde A, Secher NH, Petersen NC. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol (Lond) 588: 1985–1995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rasmussen P, Dawson EA, Nybo L, van Lieshout JJ, Secher NH, Gjedde A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J Cereb Blood Flow Metab 27: 1082–1093, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol 104: 879–888, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Seifert T, Rasmussen P, Secher NH, Nielsen HB. Cerebral oxygenation decreases during exercise in humans with beta-adrenergic blockade. Acta Physiol (Oxf) 196: 295–302, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 36. St. Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med 38: 797–806, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Subudhi AW, Dimmen AC, Roach RC. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol 103: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 294: H164–H171, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Subudhi AW, Miramon BR, Granger ME, Roach RC. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol 106: 1153–1158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EO. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 316: 143–153, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Vianna LG, Koulouris N, Lanigan C, Moxham J. Effect of acute hypercapnia on limb muscle contractility in humans. J Appl Physiol 69: 1486–1493, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Vogiatzis I, Louvaris Z, Habazettl H, Athanasopoulos D, Andrianopoulos V, Cherouveim E, Wagner H, Roussos C, Wagner PD, Zakynthinos SG. Frontal cerebral cortex blood flow, oxygen delivery and oxygenation during normoxic and hypoxic exercise in athletes. J Physiol 589: 4027–4039, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]