Abstract
The objective of this study was to determine the functional recovery and adaptation of dystrophic muscle to multiple bouts of contraction-induced injury. Because lengthening (i.e., eccentric) contractions are extremely injurious for dystrophic muscle, it was considered that repeated bouts of such contractions would exacerbate the disease phenotype in mdx mice. Anterior crural muscles (tibialis anterior and extensor digitorum longus) and posterior crural muscles (gastrocnemius, soleus, and plantaris) from mdx mice performed one or five repeated bouts of 100 electrically stimulated eccentric contractions in vivo, and each bout was separated by 10–18 days. Functional recovery from one bout was achieved 7 days after injury, which was in contrast to a group of wild-type mice, which still showed a 25% decrement in electrically stimulated isometric torque at that time point. Across bouts there was no difference in the immediate loss of strength after repeated bouts of eccentric contractions for mdx mice (−70%, P = 0.68). However, after recovery from each bout, dystrophic muscle had greater torque-generating capacity such that isometric torque was increased ∼38% for both anterior and posterior crural muscles at bout 5 compared with bout 1 (P < 0.001). Moreover, isolated extensor digitorum longus muscles excised from in vivo-tested hindlimbs 14–18 days after bout 5 had greater specific force than contralateral control muscles (12.2 vs. 10.4 N/cm2, P = 0.005) and a 20% greater maximal relaxation rate (P = 0.049). Additional adaptations due to the multiple bouts of eccentric contractions included rapid recovery and/or sparing of contractile proteins, enhanced parvalbumin expression, and a decrease in fiber size variability. In conclusion, eccentric contractions are injurious to dystrophic skeletal muscle; however, the muscle recovers function rapidly and adapts to repeated bouts of eccentric contractions by improving strength.
Keywords: muscle damage, muscle plasticity, dystrophin
a predominant feature of dystrophic skeletal muscle is a hypersensitivity to eccentric contractions (i.e., lengthening contractions) resulting in a loss of muscle function and cellular damage. This was first demonstrated ex vivo in muscles lacking dystrophin (mdx mice), where eccentric contractions conferred force losses exceeding 60% and were accompanied by sarcolemmal disruptions (34). Since then, the susceptibility of dystrophic muscle to eccentric contraction-induced injury has been used to measure disease severity (15) and as an index to test the efficacy of potential therapies for the human disease, Duchenne muscular dystrophy (DMD) (e.g., Refs. 22, 29, 40, and 41). While the search for a means to mitigate contraction-induced injury is justified, what has been much less investigated is the recovery from and adaptation to eccentric contraction-induced injury in dystrophic muscles. Results from these types of studies may provide new insights into the disease progression and offer alternative cellular mechanisms to exploit in the attempt to alleviate disease severity.
Skeletal muscles of individuals with DMD and mdx mice have aberrant gene coding for the cytoskeletal protein dystrophin. Normally, dystrophin acts in combination with other cytoskeletal proteins in the costameric lattice to connect the sarcomere to the extracellular matrix (11, 12). In this capacity, dystrophin helps facilitate the lateral transmission of contractile force and maintains sarcolemmal integrity (5, 33, 35) and intracellular Ca2+ homeostasis (1, 14). The general assumption is that the loss of dystrophin weakens the costameric lattice and renders fibers susceptible to eccentric contraction-induced injury. Indeed, dystrophic muscles have been shown in vivo, in situ, and ex vivo to have 20–60% greater force losses after eccentric contractions than normal muscles with functioning dystrophin (10, 26, 37). Despite an increased susceptibility to injury, recovery from eccentric contraction-induced injury is enhanced in dystrophic muscle compared with that of normal muscle. This was demonstrated in situ, where dystrophic muscles recovered all of their 70% force loss by 3 days postinjury, whereas normal muscles only recovered half of their 50% force loss in 3 days (6). The extent to which dystrophic muscle can maintain this high rate of functional recovery with multiple bouts of injury is not known and was the focus of the present study.
The mechanism(s) responsible for force loss after eccentric contraction has been better explored and documented for normal muscle than dystrophic muscle. A unique aspect of an eccentric contraction is the high force that is generated, nearly twofold greater than what is generated during an isometric contraction (17), and it is primarily this aspect of the contraction that causes the injury (24, 42). Much of the immediate force loss and that up to ∼3 days later has been attributed to the uncoupling of excitation-contraction processes (17, 45), while disruptions to contractile and cytoskeletal proteins contribute to the prolonged force decrements that persist for 5–14 days (16, 19, 21). In normal skeletal muscle, adaptations follow eccentric contractions to reinforce injury-susceptible areas, resulting in a protection against a future bout of eccentric contractions (i.e., repeated-bout effect) (30). Specifically, these adaptations manifest as increased muscle strength, attenuation of future eccentric contraction-induced force loss, and faster recovery from injury (2, 18, 28, 31). However, whether or not dystrophic muscle adapts to repeated bouts of eccentric contraction-induced injury is not known.
In this study, we investigated the functional recovery and adaptation of dystrophic muscle to repeated bouts of electrically stimulated eccentric contractions. The aim of experiment 1 was to determine the amount of time required by mdx mice to recover strength after one bout of eccentric contractions in vivo. The aims of experiments 2 and 3 were to characterize functional adaptations after repeated bouts of eccentric contractions in vivo and then investigate specific adaptations using ex vivo and biochemical analyses. The aim of experiment 4 was to determine if differences existed in the rate of recovery from contraction-induced injury between wild-type and mdx mice. Because it is well established that one bout of eccentric contractions (either in vivo, in situ, or ex vivo) is extremely injurious to dystrophic muscle, it could be predicted that multiple bouts of eccentric contractions would exacerbate the disease phenotype in mdx mice. Here, we show that muscle injury was not worsened with subsequent bouts. Paradoxically, we discovered that muscle strength improved in mdx mice as a result of repeated bouts of eccentric contractions.
MATERIALS AND METHODS
Ethical Approval, Animals, and Experimental Designs
Male mdx mice (C57Bl/10ScSn-DMDmdx) and wild-type mice (C57Bl/10ScSn) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in groups of 3–4 mice/cage on a 12:12-h light-dark cycle. Food and water were provided ad libitum. Preliminary strength loss and recovery data were collected from male mdx mice aged 6 wk (experiment 1). All other in vivo experiments were initiated when mice were 10 wk of age. This age was selected to avoid the variability associated with peak cycles of degeneration and regeneration characteristic of mdx mice aged 3–8 wk because the primary objective of this research was to investigate injury and recovery in dystrophic muscle, not mechanisms of disease onset or pathology. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and complied with guidelines set by the American Physiological Society.
Only mdx mice were used for experiments 1–3 because contraction-induced injury and recovery have been well documented in normal muscle and the goals of experiments 1–3 were to characterize injury and recovery in dystrophic muscle. Once characterized, wild-type mice were included for comparison (experiment 4). The purpose of experiment 1 was to determine the time course of functional recovery of the anterior crural muscles [i.e., tibialis anterior (TA), extensor digitorum longus (EDL), and extensor hallicus longus] from eccentric contraction-induced injury. To do this, mdx mice performed either 10 (n = 4) or 100 (n = 4) electrically stimulated eccentric contractions in vivo. Peak electrically stimulated isometric torque of the anterior crural muscles was reassessed immediately and then 3, 7, and 10 days postinjury.
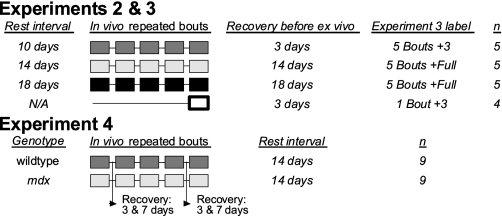
The purpose of experiment 2 was to examine the functional adaptation of the anterior crural muscles to 5 repeated bouts of 100 eccentric contractions in vivo. To do this, mdx mice aged 10 wk were separated into three groups, which were then assigned different rest intervals between repeated bouts (i.e., 10, 14, and 18 days; Fig. 1). At each bout, peak isometric torque was assessed pre- and postinjury. Isometric torque as a function of stimulation frequency was also assessed preinjury.
Fig. 1.
Design of experiments 2–4. Each box represents a bout of eccentric contractions performed in vivo. In experiment 2, mice performed five repeated bouts separated by 10, 14, or 18 days. In experiment 3, the contractility of extensor digitorum longus (EDL) muscles from 5 bouts +3 and 1 bout +3 mice was tested 3 days after the final in vivo bout. An extra interval of recovery (14–18 days) was permitted before the contractility of EDL muscles from 5 bouts +full mice was tested. In experiment 4, wild-type mice were added to compare recovery times. Isometric torque was assessed 3 and 7 days postinjury after bouts 1 and 4 as well as immediately before and after each bout.
The purpose of experiment 3 was to identify adaptations of five repeated bouts of eccentric contractions in an individual anterior crural muscle (EDL) studied ex vivo. To do this, either 3 or 14–18 days after the final in vivo bout, EDL muscles were excised from in vivo-injured left legs and contralateral control right legs to assess contractility ex vivo. These time points were selected to assess changes occurring during the recovery process (3 days) and adaptations occurring after full recovery (14–18 days). In addition to those mdx mice, a group of mdx mice that had aged and grown in parallel but only performed one bout in vivo were analyzed (1 bout +3; Fig. 1). This group was included to investigate if growth of the mice was contributing to the functional changes occurring over time within the repeated bouts.
The purpose of experiment 4 was to examine potential differences between wild-type and mdx mice in the functional recovery and adaptation of the posterior crural muscles (gastrocnemius, soleus, and plantaris) to 5 repeated bouts of 100 eccentric contraction in vivo separated by 14-day rest intervals. Additionally, the posterior crural muscles were selected for this experiment to test whether they responded to the five repeated bouts similarly to the anterior crural muscles (in terms of immediate strength loss and long-term strength improvement). At an age of 5 wk, male wild-type and mdx mice had nerve cuffs surgically implanted on the tibial nerve of their left hindlimbs. Unlike the peroneal nerve, which can be stimulated percutaneously, the tibial nerve dives deep into the posterior crural muscles after branching from the sciatic nerve; therefore, a nerve cuff was necessary to stimulate the posterior crural muscles without recruiting the anterior crural muscles. After surgical implantation of the nerve cuff, mice were allowed to recover for 5 wk, at which point isometric torque of the posterior crural muscles group was assessed to ensure a complete recovery. One week after this, the first of five bouts was performed. To determine if repeated bouts affected the rate of recovery, isometric torque was assessed 3 and 7 days after the first and fourth bouts of injury.
Experimental Methods
In vivo measurements: anterior crural muscles.
In vivo maximal isometric torque of the anterior crural muscles was assessed as previously described (3, 7). Mice were anesthetized by an intraperitoneal injection of fentanyl citrate (10 mg/kg body wt), droperidol (0.2 mg/kg body wt), and diazepam (5 mg/kg body wt), and the left leg was shaved and aseptically prepared. The foot was placed in a metal foot plate attached to the shaft of a servomotor (model 300 B-LR, Aurora Scientific, Aurora, ON, Canada). Adjustments were made so that the foot was perpendicular to the tibia and the tibia was perpendicular to the femur. All functional measurements began at this neutral position. During functional testing, body temperature was maintained at 37°C (Cole Parmer, series 16A, Vernon Hills, IL). Two platinum electrodes (model E2-12, Grass Technologies, West Warwick, RI) were inserted percutaneously on either side of the peroneal nerve. A stimulator and stimulus unit (models S48 and SIU5, respectively, Grass Technologies) stimulated the peroneal nerve via the platinum electrodes to induce a contraction of the anterior crural muscles. The voltage was adjusted from 3.0 to 9.0 V until maximal isometric torque (fused tetanus) was achieved. Torque as a function of stimulation frequency was then measured during nine isometric contractions at varying stimulation frequencies (20, 40, 60, 80, 100, 125, 150, 200, and 250 Hz) using a 0.1-ms pulse duration. Next, the anterior crural muscles were injured by performing 10 or 100 eccentric contractions. Muscles were stimulated for 119 ms using the optimal voltage and 250 Hz. During stimulation, the anterior crural muscles were stretched from 19° of ankle dorsiflexion to 19° of ankle plantarflexion at an angular velocity of 2,000°/s. Eccentric contractions were separated by 10 s, and the entire protocol lasted 18 min. A 5-min rest followed the protocol before postinjury maximal isometric torque was measured. Preliminary results confirmed that isometric torque remained depressed for at least 1 h after 100 eccentric contractions, verifying that the protocol induced injury and not fatigue.
In vivo measurements: posterior crural muscles.
For surgical implantation of the stimulating nerve cuffs, mice were initially anesthetized in an induction chamber using isoflurane, and anesthesia was then maintained by an inhalation of 1.75% isoflurane mixed with oxygen by mask at a flow rate of 200 ml/min. Nerve cuffs were constructed similar to that described previously (46) using platinum-iridium wires (Medwire-Sigmund Chon 10Ir9/49T, Mt. Vernon, NY) and silastic tubing. An incision was made through the biceps femoris muscle in the left hindlimb, and the peroneal and tibial nerves were gently separated. Two platinum-iridium wires separated by 1 mm were placed around the tibial nerve; the wires were encased in a 1.75-mm segment of Silastic tubing (0.51-mm inner diameter and 0.94-mm outer diameter) to prevent stimulation of the adjacent tissue. The proximal end of the nerve cuff was externalized in the dorsal cervical region. Externalized electrode wires were connected to a stimulator and stimulator unit, and muscle contractions were elicited at 250 Hz. During functional testing, mice were anesthetized as described above, and all contractile protocols were initiated with the foot perpendicular to the tibia. Peak isometric contractions were achieved by adjusting the voltage from 3.0 to 9.0 V for a stimulation period of 150 ms. Eccentric contractions were performed under the parameters previously described with the exception that the posterior crural muscles were stretched from 19° of ankle plantarflexion to 19° of ankle dorsiflexion.
Ex vivo measurements.
Mice were anesthetized with pentobarbital sodium (100 mg/kg body wt), and the EDL muscles were excised and analyzed for force-generating capacities as previously described (3). Muscles were maintained at a resting tension of 0.4 throughout the experiment as per our previous studies (43, 44). EDL muscles were maintained at a constant 25°C throughout the experiments. Contractile characteristics tested included the following: peak twitch force, maximal isometric tetanic force, and maximal rates of tetanic contraction and relaxation.
Muscle Mass, Protein Content, Histology, and Western Blot Analysis
Total and contractile protein contents for EDL muscles were determined as previously described (8, 27). Briefly, each muscle was homogenized in 10 mM phosphate buffer (pH 7.0), and homogenates were assayed in triplicate using the bicinchoninic acid protein assay (Pierce, Rockford, IL) and then subjected to SDS-PAGE to determine the total sample myosin heavy chain and actin contents (27).
Because peak isometric torque-time tracings revealed an increase in the rate of relaxation with repeated bouts, TA muscles from 1 bout +3 and 5 bouts +full mice were analyzed for parvalbumin and sarco(endo)plasmic reticulum Ca2+-ATPase 1a (SERCA1a) by Western blot analysis to assess the contents of these proteins from muscle that had performed a single bout and muscle that had performed and recovered from five repeated bouts. Muscles were pulverized with a mortar and pestle and solubilized in 1% SDS, 5 mM EGTA, and a cocktail of protease inhibitors (7). Solubilized muscle homogenates were assessed for total protein content, and 50 μg total protein was separated on a 3–12% polyacrylamide gel and transferred to polyvinylidene difluoride membranes as previously described (7, 38). Western blot analysis was then performed using the following monoclonal antibodies: parvalbumin (1:1,000, Sigma), SERCA1a (1:2,500, Pierce), and GAPDH (1:2,000, GenScript). Secondary antibodies were diluted (1:5,000) and detected with the Odyssey Infrared Imaging System (Li-Cor Biosciences) using the 700- and 800-nm channels.
Serial sections from the midbelly of TA muscles frozen in OCT compound were cut to a thickness of 5–10 μm. Sections were stained with hematoxylin and eosin and imaged at ×100 magnification. Individual fiber cross-sectional areas were measured (20), and fibers with centralized nuclei were counted from 300 fibers within each muscle.
Statistics
For experiment 1, isometric torques were analyzed by ANOVA with the factors being group (10 and 100 electrically stimulated eccentric contractions) and time (preinjury and 0, 3, 7, and 10 days postinjury) with repeated measures on the time factor. For experiment 2, body weight and preinjury isometric torques were analyzed by two-way ANOVA with the factors being rest intervals (10, 14, and 18 days) and bout (first, second, third, fourth, and fifth) with repeated measures on the bout factor. One-way ANOVA was used to analyze the following variables in experiment 2 with repeated measures across bouts: the first eccentric contraction torque, eccentric torque loss, isometric torque loss, and preinjury torque frequency. Also, SERCA1a and parvalbumin protein contents as well as the percentage of fibers with centralized nuclei were compared between in vivo-injured and contralateral control TA muscles using paired t-tests. χ2-Analysis was used to determine differences in fiber size distribution. For experiment 3, ex vivo contractile properties and total and contractile protein contents were compared between in vivo-injured and contralateral control EDL muscles using paired t-tests. One-way ANOVA was used to analyze the following variables in experiment 4 with repeated measures on bouts for mdx mice only: preinjury isometric torque and eccentric torque loss. Recovery of isometric torque at 3 and 7 days postinjury was analyzed by two-way ANOVA, genotype (wild type vs. mdx) by bout (first and fourth), with the repeated measures on bouts. When significant main effects or interactions were found, differences were tested with Tukey's post hoc tests. An α-level of 0.05 was used for all analyses. All values are means ± SE.
RESULTS
Experiment 1
We first established the number of eccentric contractions that caused a substantial decrement in torque but from which mdx muscle could still completely recover. Groups of mice that performed either 10 or 100 eccentric contractions had equivalent preinjury peak isometric torques (1.7 ± 0.1 vs. 1.7 ± 0.2 N·mm·kg body wt−1, respectively, P = 0.887). Performing either 10 or 100 eccentric contractions affected strength loss differently, and, as a result, peak isometric torque of the anterior crural muscles differed between groups depending on the recovery time (interaction, P = 0.030). The number of eccentric contractions performed significantly affected peak isometric torque immediately postinjury as the mice subjected to 10 eccentric contractions produced 60% of preinjury torque and mice sujected to 100 eccentric contractions produced only 29% of preinjury torque (P = 0.002). At 3 days postinjury, peak torque had recovered to 96% of preinjury torque for mice subjected to 10 eccentric contractions but only to 85% of preinjury torque for mice subjected to 100 eccentric contractions (P = 0.045). At 7 and 10 days postinjury, both groups had fully recovered, and there were no differences between groups (P = 0.998 and 0.997, respectively). We chose to use 100 eccentric contractions for subsequent experiments because of the greater immediate strength decrement but equivalent recovery at 7 days postinjury.
Experiment 2
To determine whether the time between repeated bouts of eccentric contractions affected functional outcomes, individual groups of mdx mice performed 5 bouts of 100 eccentric contractions separated by either 10, 14, or 18 days. There was no difference in body weight among the groups of mice (main effect of group, P = 0.190), but, collectively, mice weighed significantly more at bout 5 compared with bout 1 (33.4 ± 0.8 vs. 27.8 ± 0.6 g, respectively, main effect of bout, P < 0.001). To accommodate for this growth, torque (in N·mm) was normalized by body weight (in kg).
Across the five bouts, there was no difference in peak isometric torque measured preinjury among groups of mice with different rest intervals (main effect of group, P = 0.482; Fig. 2A). Two-way ANOVA power analysis revealed this main effect may have been underpowered (power = 0.167). Nonetheless, groups of mice with different rest intervals had the same functional outcome, so groups were collapsed for the further in vivo functional outcomes investigated in experiment 2.
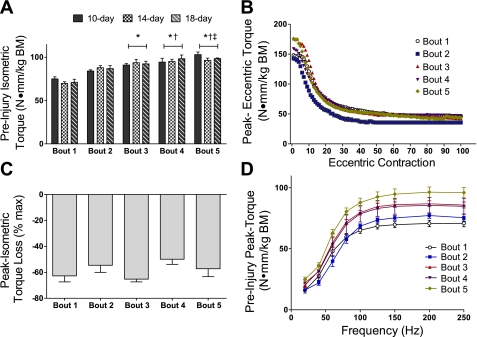
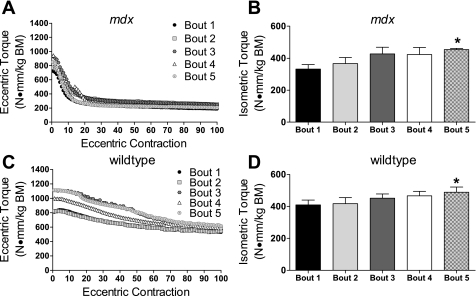
Fig. 2.
Maximal anterior crural muscle torques for bouts 1–5. A: means ± SE of peak isometric torques produced preinjury at bouts 1–5 for groups of mice with different rest intervals between bouts. Peak torques improved with each bout regardless of rest interval. *P < 0.001, greater than bout 1; †P = 0.034, greater than bout 2; ‡P = 0.007, greater than bout 3. B–D: means ± SE for groups combined across rest intervals. B: the first peak eccentric torque at each bout was ∼210% of preinjury peak isometric torque at each bout. Thereafter, eccentric torque was loss during the contraction protocol. Peak torque loss during the eccentric contraction protocol did not differ from bouts 1 to 5 (P = 0.229). C: percentage of peak isometric torque loss after the eccentric injury protocol did not differ among bouts (P = 0.262). D: preinjury peak isometric torque as a function of stimulation frequency at bouts 1–5. During bout 5, preinjury peak torques at several submaximal frequencies were greater than those during bouts 1 and 2. BM, body mass.
One hundred eccentric contractions drastically injured muscles at each bout (Fig. 2B). By the end of each bout of eccentric contractions, there was an average loss of 69 ± 2% of eccentric torque with no difference across bouts (P = 0.229; Fig. 2B). Additionally, immediate torque loss, as indicated by comparing peak pre- and postinjury isometric torques, did not differ across the repeated bouts, averaging between 50% and 62% loss of isometric torque (P = 0.262; Fig. 2C). Despite the persistent torque loss due to contraction-induced injury, preinjury isometric torques showed a complete recovery at each subsequent bout. Moreover, with subsequent bouts, peak torques improved (Fig. 2A). By bout 5, maximal isometric torque was 38% greater than at bout 1 (99 ± 2 vs. 72 ± 2 N·mm·kg body wt−1, respectively, P < 0.001).
Isometric torques as a function of stimulation frequency, before injurious eccentric contractions were performed, for each of the five bouts are shown in Fig. 2D. There were many significant improvements across subsequent bouts in isometric torques that were assessed preinjury. Specifically, at frequencies of >80 Hz, all torques were greater at bout 5 compared with bouts 1 and 2 (P ≤ 0.011).
To consider changes in torque that might be attributed to the growth of mice during the experimental protocols, a group of mdx mice that were aged in parallel to the mice that performed five repeated bouts were studied. These mice performed one in vivo bout (Fig. 1: study design). Their uninjured peak isometric torque was 18% less than preinjury torques produced by mice that performed five repeated bouts (81 ± 3 vs. 99 ± 2 N·mm·kg body wt−1, respectively, P = 0.002), suggesting that it was primarily the repeated bouts, not just aging, that resulted in the higher torques.
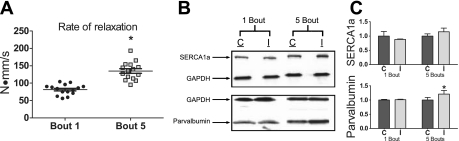
Isometric torque-time tracings were analyzed from peak isometric torques to determine if repeated bouts affected torque contractile properties (i.e., maximal contraction and relaxation rates). The maximal rate of relaxation (in N·mm·s−1) was enhanced by 70% at bout 5 compared with bout 1 (P < 0.001; Fig. 3A). When changes in body weight were accounted for, the maximal rate of relaxation was enhanced by 38% at bout 5 compared with bout 1 [4.0 ± 0.2 vs. 2.9 ± 0.1 N·mm·s−1·kg body wt−1, P < 0.001]. When changes in peak isometric torque were accounted for, the maximal rate of relaxation was enhanced by 20% at bout 5 compared with bout 1 (1.35 ± 0.11 vs. 1.12 ± 0.09 kg body wt/s, P = 0.006). Because the TA muscle is responsible for ∼89% of anterior crural muscle torque (23), we analyzed TA muscles from 1 bout +3 and 5 bout +full mice for the expression of proteins known to affect muscle relaxation. Bouts of eccentric contractions had no affect on SERCA1a expression, although there was a trend for the injured muscles from 5 bout mice to have a greater level than contralateral muscles (P = 0.061; Fig. 3, B and C). Parvalbumin was 21% greater in in vivo-injured TA muscles from 5 bout mice compared with contralateral controls (P = 0.038).
Fig. 3.
A: anterior crural muscle relaxation rates of peak isometric torques assessed during bouts 1 and 5. *P < 0.001, bout 5 > bout 1. B: representative Western blots probed for sarco(endo)plasmic reticulum Ca2+-ATPase 1a (SERCA1a), parvalbumin, and GAPDH for control (C) and injured (I) muscles. C: comparisons of protein contents between contralateral control and in vivo-injured muscles from 1 bout +3 (1 bout) and 5 bouts +full (5 bouts) mice. All protein contents were normalized to GAPDH and are shown relative to contralateral control muscles for each group, respectively. *P = 0.038, greater than the contralateral control muscle.
TA muscles from 5 bout mice allowed to fully recover were assessed for muscle mass and fiber cross-sectional area to investigate whether these contributed to increases in torque-generating capacity. In vivo-injured TA muscle weighed 8% less than contralateral control muscles (2.24 ± 0.07 vs. 2.44 ± 0.03 mg/g body wt, P = 0.039). The frequency distribution of fiber size showed that in vivo-injured TA muscles from 5 bout mice was skewed toward smaller fibers. Small fibers were categorized as those being <1,000 μm2, and large fibers were categorized as those being >5,000 μm2. In vivo-injured TA muscles had nearly twice as many small fibers (13% vs. 7% of all fibers, P < 0.001) and 60% fewer large fibers (6% vs. 16% of all fibers, P < 0.001) compared with uninjured contralateral TA muscles. The frequency distribution for fibers ranging from 1,000 to 5,000 μm2 was not different (P ≥ 0.205). TA muscles that had recovered and adapted from the repeated bouts of in vivo injury had 7% fewer centrally nucleated fibers compared with control TA muscles (221 ± 4 vs. 238 ± 4 fibers of 300 counted fibers, respectively, P = 0.009).
Experiment 3
To further investigate dystrophic muscle recovery and improved strength, right and left EDL muscles from the mdx mice described in experiment 2 were tested ex vivo for contractility. Mice that performed one bout of eccentric contractions and then had EDL muscles tested 3 days later (1 bout +3) were used to show a typical ex vivo contractile response of the EDL muscle after in vivo injury of the entire anterior crural muscle group. In contrast, 5 bouts +full mice were allowed a complete recovery after the final in vivo bout to determine whether five repeated bouts could improve EDL contractile properties, as was observed in vivo for torque.
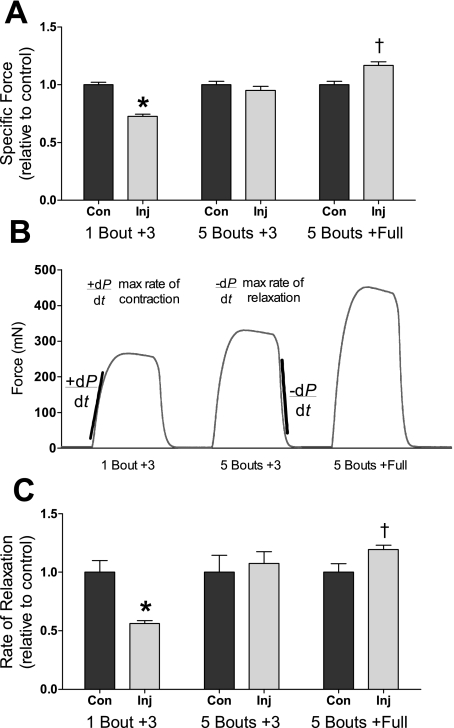
Three days after one bout of in vivo eccentric contractions, in vivo-injured EDL muscles generated significantly lower specific force compared with contralateral EDL muscles (Fig. 4A, 1 bout +3). However, that low force was not apparent after 5 repeated bouts of 100 eccentric contractions in vivo. Specifically, the specific force of in vivo-injured EDL muscles was 1) 28% less than that of contralateral control muscles in 1 bout +3 mice (7.9 ± 0.2 vs. 10.9 ± 0.7 N/cm2, P = 0.003); 2) not different from control muscles in 5 bouts +3 mice (10.4 ± 0.2 vs. 10.7 ± 0.5 N/cm2, P = 0.434); and 3) 17% greater than control muscles in 5 bouts +full mice (12.2 ± 0.3 vs. 10.4 ± 0.5 N/cm2, P = 0.005; Fig. 4A). Contralateral control EDL muscles from 1 bout +3, 5 bouts +3, and 5 bouts +full mice had equivalent specific forces (10.9 ± 0.7, 10.7 ± 0.5, and 10.4 ± 0.5, respectively, P = 0.595). Also, maximal tetanic force-time tracings were analyzed to determine maximal contraction and relaxation rates (Fig. 4B). The rate of relaxation of injured muscles was 40% slower in 1 bout +3 mice (P = 0.019), not different in 5 bouts +3 mice (P = 0.604), and 20% faster in 5 bouts +full mice relative to control muscles (P = 0.049; Fig. 4C and Table 1). Maximal rates of relaxation produced by EDL muscles from 5 bouts +full mice were normalized by force, and injured/recovered EDL muscles still had 13% greater rates than contralateral control EDL muscles (55 ± 1 vs. 49 ± 1 s−1, P = 0.003).
Fig. 4.
Ex vivo contractility of EDL muscles. A: injured (Inj) EDL muscle peak specific force for 1 bout +3, 5 bouts +3, and 5 bouts +full mice relative to contralateral control (Con) EDL muscles. B: representative isometric force tracings (force vs. time) for the three experimental groups. The maximal rate of contraction (+dP/dt) was determined from the greatest slope during a maximal isometric tetanic contraction. The maximal rate of relaxation (−dP/dt) was determined from the greatest slope during relaxation from a maximal isometric tetanic contraction. C: tetanic rate of relaxation in injured EDL muscles relative to contralateral control muscles. *P ≤ 0.019, less than the contralateral control muscle; †P ≤ 0.049, greater than the contralateral control muscle.
Table 1.
Extensor digitorum longus muscle protein contents and contractility 3 and 14–18 days after either one or five bouts of eccentric contractions were performed in vivo by mdx mice
| 1 Bout +3 |
5 Bouts +3 |
5 Bouts +Full |
||||
|---|---|---|---|---|---|---|
| Control | Injured | Control | Injured | Control | Injured | |
| Weight, mg | 21.0 ± 1.9 | 19.8 ± 1.4 | 17.5 ± 0.9 | 19.2 ± 1.0 | 22.5 ± 1.1 | 21.7 ± 0.8 |
| Total protein, mg | 3.9 ± 0.2 | 3.3 ± 0.2* | 3.1 ± 0.2 | 3.5 ± 0.2 | 3.9 ± 0.1 | 4.1 ± 0.3 |
| Total contractile protein, mg | 1.7 ± 0.2 | 1.2 ± 0.1* | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 |
| Peak twitch force, mN | 65.1 ± 7.0 | 52.2 ± 3.1* | 50.0 ± 9.4 | 55.8 ± 7.9 | 69.9 ± 7.3 | 77.7 ± 4.4 |
| Peak isometric tetanic force, mN | 417 ± 39 | 290 ± 13* | 370 ± 21 | 372 ± 32 | 425 ± 27 | 469 ± 12 |
| Maximal rate of force development, N/s | 10.7 ± 0.9 | 7.3 ± 0.4* | 7.2 ± 0.9 | 8.4 ± 0.7 | 10.1 ± 0.9 | 11.4 ± 0.4 |
| Maximal rate of relaxation, N/s | −24.2 ± 2.4 | −13.6 ± 0.6* | −15.9 ± 2.3 | −17.1 ± 1.6 | −21.6 ± 1.6 | −25.8 ± 0.8* |
Values are means ± SE. Full, 14 or 18 days after the final bout of eccentric contractions.
Significantly different than controls (within group; P < 0.05). The age at euthanization was 124 days for 1 bout +3, 116 days for 5 bouts +3, 140 days for 5 bouts+Full (14-day interval), and 160 days for 5 bouts +Full (18-day interval).
In addition to specific force and the rate of relaxation, other contractile parameters were detrimentally altered in injured EDL muscles that were analyzed 3 days after just one bout of eccentric contractions (Table 1). On the contrary, there were no contractile differences between control and in vivo-injured EDL muscles for mice that performed five repeated bouts (5 bouts +3 and 5 bouts +full groups; Table 1). These data support that repeated bouts of eccentric contractions caused beneficial remodeling in dystrophic muscle despite the constant degree of strength loss from bout to bout.
Total and contractile proteins of EDL muscles were measured to determine if those contributed to impairments (1 bout +3) or improvements (5 bouts) in EDL force generation. The composition of in vivo-injured EDL muscles from 1 bout +3 mdx mice differed from contralateral uninjured muscles (Table 1). Specifically, EDL muscles that performed one bout of eccentric contractions had 15% less total protein and 29% less contractile protein than contralateral muscles (P ≤ 0.046). In contrast, these difference were not detected between control and in vivo-injured EDL muscles from mice that recovered with five bouts.
Experiment 4
The aim of experiment 4 was to directly compare the functional recovery between wild-type and mdx mice across multiple bouts of injury and to determine if the injury response and adaptation in the posterior crural muscle group was similar to that observed in the anterior crural muscles. By the end of each eccentric contraction bout, there was an average loss of 71 ± 2% of eccentric torque with no difference across bouts (P = 0.543; Fig. 5A). Despite the persistent torque loss due to contraction-induced injury across bouts, preinjury isometric torques improved, similar to the strength improvements found with the anterior crural muscles. By bout 5, isometric torque of the posterior crural muscles was 36% greater than that during bout 1 (453 ± 6 vs. 332 ± 27 N·mm·kg body wt−1, P = 0.022; Fig. 5B). These results indicate that the dystrophic muscle adaptation to repeated eccentric contractions is independent of the muscle group (anterior vs. posterior crural muscles). Similar to mdx mice, the average loss of eccentric torque did not differ across bouts for wild-type mice (−40 ± 2%, P = 0.206; Fig. 5C), but there was a 19% increase in preinjury peak isometric torques with repeated bouts (bout 1: 410 ± 28 N·mm·kg body wt−1 vs. bout 5: 489 ± 32 N·mm·kg body wt−1, P = 0.025; Fig. 5D). A recovery score for peak isometric torque was calculated to determine the preclinical relevancy of this adaptation to repeated bouts for mdx mice relative to wild-type mice [(453–332 N·mm·kg body wt−1)/(410–332 N·mm·kg body wt−1) = recovery score of 155%] (as per the TREAT-NMD-recommended standard protocol “The recovery score to evaluate therapy efficiency in NMD: a common, quantitative and comparative scoring system M.1.1_001”; http://www.treat-nmd.eu/research/preclinical/dmd-sops/).
Fig. 5.
Maximal posterior crural muscle torques for bouts 1–5 for mdx (A and B) and wild-type (C and D) mice. A: peak eccentric torque generated by the posterior crural muscles during bouts 1–5 for mdx mice. There was no difference in torque loss during the injury protocol across bouts (P = 0.543). B: preinjury peak isometric torque of the posterior crural muscle from mdx mice improved across bouts. C: peak eccentric torque generated by the posterior crural muscle during bouts 1–5 for wild-type mice. There was no difference in eccentric torque loss during the injury protocol across bouts (P = 0.206). D: preinjury peak isometric torque of the posterior crural muscles from wild-type mice improved across bouts. *P ≤ 0.025, bout 5 > bout 1. All data are means ± SE. Error bars not seen are contained within the symbol.
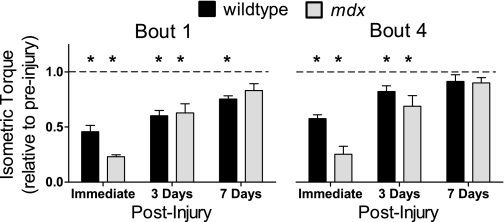
Eccentric contractions elicited torques twice that produced by isometric contractions for wild-type and mdx mice (201 ± 10% vs. 211 ± 10%). However, the isometric torque loss after one bout was greater in mdx mice compared with wild-type mice, such that after the injury protocol mdx mice generated 22% of preinjury torque, whereas wild-type mice generated 47% (P < 0.001). However, mdx mice demonstrated a remarkable capacity to recover from injury as isometric torque 7 days postinjury was not significantly different than preinjury torque, whereas wild-type mice still showed a 25% decrement (Fig. 6, bout 1). Moreover, relative to peak torques generated immediately postinjury, there was a 270% improvement in peak isometric torque within 3 days postinjury and a 360% improvement in peak isometric torque by 7 days postinjury for mdx mice (76 ± 9 vs. 201 ± 25 vs. 270 ± 26 N·mm·kg body wt−1, immediately, 3 days, and 7 days postinjury, respectively). This demonstrates the rapid rate of recovery from contraction-induced injury in dystrophic muscle. Furthermore, the high recovery rate of dystrophic muscle was not mitigated by multiple bouts of injury as the rate of recovery was similar for mdx mice after bout 4 compared with bout 1 (i.e., +325% and +450% improvement in peak-isometric torque, 3 and 7 days postinjury, respectively).
Fig. 6.
Posterior crural muscle recovery of isometric torque relative to preinjury peak torques after bouts 1 and 4 for wild-type and mdx mice. *Significantly different from preinjury peak torque (within genotype; P ≤ 0.011).
DISCUSSION
We designed four experiments to comprehensively test the recovery and adaptation of dystrophic muscle to repeated bouts of eccentric contractions. There were three primary findings. First, after one bout of eccentric contractions in vivo, there was a rapid recovery (within 7 days) of muscle contractile function in mdx mice. Second, repeated bouts of eccentric contractions did not exacerbate muscle injury during subsequent bouts. Third, repeated bouts of injurious eccentric contractions paradoxically increased strength in dystrophic muscle. Each outcome will be considered in the following sections.
Rapid Recovery of Dystrophic Muscle
The main functional consequence of eccentric contraction-induced injury is the immediate and then sustained reduction in the muscle's ability to generate force (2, 9, 18). Here, we used an in vivo method to elicit eccentric contraction injury and then to assess muscle strength, allowing us to track the functional recovery over time in the same mdx mice. We observed that in response to one bout of eccentric contractions, dystrophic muscle function was completely recovered by 7 days postinjury (Fig. 6). This is in contrast to what we observed in wild-type mice, whose muscles still exhibited a 25% decrement at 7 days postinjury. This is also in stark contrast to the rates of recovery observed in wild-type mice, where force decrements induced by an analogous eccentric contraction protocol can persist out to 28 days postinjury (16). The prompt recovery of muscle strength for mdx mice is congruent with two previous reports. First, Ridgley et al. (37) showed that 7 days after 20 eccentric contractions, anterior crural muscles from mdx mice generated isometric torques that were not significantly different than preinjury, whereas muscles of C57/FVB wild-type mice still showed a 25% decrement. Second, Brooks (6) assessed the injury and recovery of a single anterior crural muscle (i.e., EDL) using an in situ method. Immediately after 10 eccentric contractions, force produced by EDL muscles from mdx mice was 30% of preinjury force and wild-type muscles produced 50% of preinjury. By 72 h postinjury, EDL muscles from mdx mice were completely recovered and capable of generating 100% of preinjury force, whereas wild-type control muscles were capable of only generating 75% of preinjury force.
To further investigate recovery after injury, we explored adaptations in the anterior crural muscles after one bout and five repeated bouts of injury. It appears that one adaptation of dystrophic muscle after injury is the sparing of total and contractile protein content in response to repeated bouts. There was a 29% reduction in contractile protein after one injurious bout (1 bout +3; Table 1) but no difference in contractile protein content between in vivo-injured and contralateral control EDL muscles after five repeated bouts (5 bouts +3; Table 1). Reductions in myofibrillar proteins 5–14 days postinjury are common in wild-type mice (16, 18), but until this study, the extent to which contractile protein loss contributes to force decrements after contraction-induced injury in dystrophic muscle was unclear. Low tetanic force immediately and up to 3 days after eccentric contractions has been attributed to aspects of excitation-contraction uncoupling in wild-type mice (16, 46) and may play a role in the early strength deficits in mdx mice as well (47).
We also investigated if the regenerative capacity of dystrophic muscle was exhausted by multiple bouts of contraction-induced injury and found that it was not because recovery rates were equivalent after bouts 1 and 4 (Fig. 6). We also found that the functional recovery for normal muscle was faster with repeated bouts of injury, confirming a previous report (18) (Fig. 6: bout 1 vs. bout 4). In reference to bout 1, normal, healthy muscle has minimal ongoing regeneration, which is in contrast to dystrophic muscle, which undergoes continual injury and repair. These different basal levels of regeneration may explain the relative slow response to initial injury in wild-type mice compared with the faster response in mdx mice. In other words, dystrophic muscle rapidly regains contractile function due to regenerative processes already being in an elevated state. This is supported by the greater basal expression of myogenic regulatory factors (i.e., myogenin and MyoD) in dystrophic muscle compared with normal muscle (4, 25, 32, 48). The earlier recovery of muscle function occurs in normal muscle but only after repeated bouts of injury. More investigation is needed to further elucidate the mechanisms of functional recovery after muscle injury in dystrophic muscle.
Repeated Bouts Do Not Exacerbate Muscle Injury
The strength that was lost immediately after eccentric contractions was not worsened by repeated bouts of the injurious protocol. That is, the anterior and posterior crural muscles of mdx mice lost ∼70% of eccentric torque-generating capacity in response to each of the 5 bouts of 100 eccentric contractions (Figs. 2B and 5A). Given the amount of injury that occurs from our eccentric contraction protocol, it was surprising that the mdx phenotype was not exacerbated with repeated bouts. Because torque-generating capacity is significantly less in mdx mice compared with wild-type mice immediately afte eccentric contractions (Fig. 6), we can deduce that dystrophin is probably essential for mitigating eccentric contraction-induced injury in vivo, but dystrophin in not essential for all adaptations, such as those required to improve strength in dystrophic muscle.
Improved Contractility as a Result of Repeated Bouts of Eccentric Contractions
The most significant functional outcome of the five repeated bouts of eccentric contractions was the improved strength of dystrophic muscle. We observed a 38% increase in dorsiflexion torque (Fig. 2A), a 35% increase in plantarflexion torque (Fig. 5B), and a 17% improvement in EDL muscle specific force (Fig. 4A).
Improvements in torque and EDL specific force were independent of muscle hypertrophy as muscle mass and protein contents were not increased after five bouts (Table 1). This suggests that repeated bouts caused an adaptation that affected the intrinsic capacity of dystrophic gastrocnemius, TA, and EDL muscles to generate or transmit force. Previously, we (13) have shown that TA muscles from mdx mice have 72% more fluid and 52% more collagen compared with wild-type mice. These alterations can affect the transmission of force and confound the comparisons between normal and dystrophic muscle of force normalized to physiological cross-sectional area, which take into account muscle mass and length. This latter point was exemplified by Sharp and co-workers (40), who observed that absolute forces of TA muscles from mdx mice exceed those of wild-type mice (20% greater). However, when forces were normalized by physiological cross-sectional area, the results were reversed, reflecting dystrophic muscle weakness. These results indicate that at least one factor contributing to force differences between normal and dystrophic muscle is the deposition of noncontractile materials that do not generate force but do contribute to increased muscle mass and increased fiber cross-sectional area. Adaptations after repeated bouts may have altered fluid and collagen contents in dystrophic muscle. Indeed, after full recovery, in vivo-injured TA muscles weighed less than contralateral control muscles and in vivo-injured TA muscles had a shift toward fibers with smaller cross-sectional areas, which has previously been reported as beneficial (20).
In addition to torque and force generation being improved in dystrophic muscle in response to repeated bouts of eccentric contractions, maximal rates of relaxation by the anterior muscle group and EDL muscle were also greater (Figs. 3A and 4C). Analysis of this contractile property led us to investigate SERCA and parvalbumin expression. Indeed, greater rates of relaxation may reflect enhanced expression of Ca2+-handling proteins such as parvalbumin. Parvalbumin is a Ca2+-binding protein present in the cytosol, and in fast-twitch muscles such as the TA, the expression of parvalbumin is reduced starting at disease onset in mdx mice (39). Mdx:parvalbumin double-knockout mice display greater decrements in force-generating capacity of EDL muscles compared with mdx mice, demonstrating further that reductions in Ca2+-handling proteins can negatively affect muscle function (36). Therefore, the increased parvalbumin that was measured after repeated bouts may be an adaptation that is beneficial for dystrophic muscle in terms of both strength and relaxation (Fig. 3, B and C).
Conclusions
In summary, a significant amount of strength is lost when dystrophic muscles perform eccentric contractions. However, the dystrophic phenotype in mdx mice is not worsened when eccentric contractions are performed on a regular basis. In fact, muscle torque and force improved after multiple bouts of eccentric contractions, showing that substantial strength gains are possible without the presence of dystrophin.
GRANTS
This study was supported by National Institutes of Health Grants T32-AR-07612, P30-AR-0507220, and K02-AG-036827.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.C., K.A.B., G.L.W., and D.A.L. conception and design of research; J.A.C. and M.D.E. performed experiments; J.A.C. and M.D.E. analyzed data; J.A.C., K.A.B., G.L.W., and D.A.L. interpreted results of experiments; J.A.C. and M.D.E. prepared figures; J.A.C. drafted manuscript; J.A.C., M.D.E., K.A.B., G.L.W., and D.A.L. edited and revised manuscript; J.A.C., M.D.E., K.A.B., G.L.W., and D.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Greg Cochrane for technical assistance and James Ervasti for critical reading.
REFERENCES
- 1. Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88: 83–91, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Balnave CD, Thompson MW. Effect of training on eccentric exercise-induced muscle damage. J Appl Physiol 75: 1545–1551, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve 40: 443–454, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beilharz MW, Lareu RR, Garrett KL, Grounds MD, Fletcher S. Quantitation of muscle precursor cell activity in skeletal muscle by Northern analysis of MyoD and myogenin expression: application to dystrophic (mdx) mouse muscle. Mol Cell Neurosci 3: 326–331, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev 31: 73–78, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Brooks SV. Rapid recovery following contraction-induced injury to in situ skeletal muscles in mdx mice. J Muscle Res Cell Motil 19: 179–187, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Call JA, McKeehen JN, Novotny SA, Lowe DA. Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve 42: 871–880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, Talmadge RJ, Grange RW. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol 105: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarkson PM, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 65: 1–6, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil 22: 467–475, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta 1772: 108–117, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell 66: 1121–1131, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Garlich MW, Baltgalvis KA, Call JA, Dorsey LL, Lowe DA. Plantarflexion contracture in the mdx mouse. Am J Phys Med Rehab 89: 976–985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 121: 1044–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grange RW, Gainer TG, Marschner KM, Talmadge RJ, Stull JT. Fast-twitch skeletal muscles of dystrophic mouse pups are resistant to injury from acute mechanical stress. Am J Physiol Cell Physiol 283: C1090–C1101, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Ingalls CP, Warren GL, Armstrong RB. Dissociation of force production from MHC and actin contents in muscles injured by eccentric contractions. J Muscle Res Cell Motil 19: 215–224, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol 85: 58–67, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Ingalls CP, Wenke JC, Nofal T, Armstrong RB. Adaptation to lengthening contraction-induced injury in mouse muscle. J Appl Physiol 97: 1067–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Koh TJ, Escobedo J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am J Physiol Cell Physiol 286: C713–C722, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve 38: 1290–1303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25% strain. J Appl Physiol 70: 2498–2507, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 11: 245–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowe DA, Warren GL, Ingalls CP, Boorstein DB, Armstrong RB. Muscle function and protein metabolism after initiation of eccentric contraction-induced injury. J Appl Physiol 79: 1260–1270, 1995 [DOI] [PubMed] [Google Scholar]
- 24. McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol 61: 293–299, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Meadows E, Flynn JM, Klein WH. Myogenin regulates exercise capacity but is dispensable for skeletal muscle regeneration in adult mdx mice. PLoS One 6: e16184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil 14: 446–451, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol 40: 966–975, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol 63: 1381–1386, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Ng R, Metzger JM, Claflin DR, Faulkner JA. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am J Physiol Cell Physiol 295: C146–C150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc 27: 1263–1269, 1995 [PubMed] [Google Scholar]
- 31. Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol 63: 70–76, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Okano T, Yoshida K, Nakamura A, Sasazawa F, Oide T, Takeda S, Ikeda S. Chronic exercise accelerates the degeneration-regeneration cycle and downregulates insulin-like growth factor-1 in muscle of mdx mice. Muscle Nerve 32: 191–199, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80: 1008–1012, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raymackers JM, Debaix H, Colson-Van Schoor M, De Backer F, Tajeddine N, Schwaller B, Gailly P, Gillis JM. Consequence of parvalbumin deficiency in the mdx mouse: histological, biochemical and mechanical phenotype of a new double mutant. Neuromuscul Disord 13: 376–387, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Ridgley JA, Pinniger GJ, Hamer PW, Grounds MD. The physiological effects of IGF-1 (class 1:Ea transgene) over-expression on exercise-induced damage and adaptation in dystrophic muscles of mdx mice. Pflugers Arch 457: 1121–1132, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Rybakova IN, Patel JR, Davies KE, Yurchenco PD, Ervasti JM. Utrophin binds laterally along actin filaments and can couple costameric actin with sarcolemma when overexpressed in dystrophin-deficient muscle. Mol Biol Cell 13: 1512–1521, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sano M, Yokota T, Endo T, Tsukagoshi H. A developmental change in the content of parvalbumin in normal and dystrophic mouse (mdx) muscle. J Neurol Sci 97: 261–272, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Sharp PS, Bye AJ, Wells DJ. Physiological characterization of muscle strength with variable levels of dystrophin restoration in mdx mice following local antisense therapy. Mol Ther 19: 165–171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sonnemann KJ, Heun-Johnson H, Turner AJ, Baltgalvis KA, Lowe DA, Ervasti JM. Functional substitution by TAT-utrophin in dystrophin-deficient mice. PLoS Med 6: e1000083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Warren GL, Hayes DA, Lowe DA, Armstrong RB. Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J Physiol 464: 457–475, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Warren GL, Hayes DA, Lowe DA, Williams JH, Armstrong RB. Eccentric contraction-induced injury in normal and hindlimb-suspended mouse soleus and EDL muscles. J Appl Physiol 77: 1421–1430, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Warren GL, Ingalls CP, Armstrong RB. Temperature dependency of force loss and Ca2+ homeostasis in mouse EDL muscle after eccentric contractions. Am J Physiol Regul Integr Comp Physiol 282: R1122–R1132, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exerc Sport Sci Rev 29: 82–87, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Warren GL, Ingalls CP, Shah SJ, Armstrong RB. Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J Physiol 515: 609–619, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woods CE, Novo D, DiFranco M, Capote J, Vergara JL. Propagation in the transverse tubular system and voltage dependence of calcium release in normal and mdx mouse muscle fibres. J Physiol 568: 867–880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn 235: 203–212, 2006 [DOI] [PubMed] [Google Scholar]