Abstract
Numerous studies have demonstrated upper-airway neuromuscular abnormalities during wakefulness in snorers and obstructive sleep apnea (OSA) patients. However, the functional role of sensorimotor impairment in OSA pathogenesis/disease progression and its potential effects on protective upper-airway reflexes, measures of respiratory sensory processing, and force characteristics remain unclear. This study aimed to gain physiological insight into the potential role of sensorimotor impairment in OSA pathogenesis/disease progression by comparing sensory processing properties (respiratory-related evoked potentials; RREP), functionally important protective reflexes (genioglossus and tensor palatini) across a range of negative pressures (brief pulses and entrained iron lung ventilation), and tongue force and time to task failure characteristics between 12 untreated OSA patients and 13 controls. We hypothesized that abnormalities in these measures would be present in OSA patients. Upper-airway reflexes (e.g., genioglossus onset latency, 20 ± 1 vs. 19 ± 2 ms, P = 0.82), early RREP components (e.g., P1 latency 25 ± 2 vs. 25 ± 1 ms, P = 0.78), and the slope of epiglottic pressure vs. genioglossus activity during iron lung ventilation (−0.68 ± 1.0 vs. −0.80 ± 2.0 cmH2O/%max, P = 0.59) were not different between patients and controls. Maximal tongue protrusion force was greater in OSA patients vs. controls (35 ± 2 vs. 27 ± 2 N, P < 0.01), but task failure occurred more rapidly (149 ± 24 vs. 254 ± 23 s, P < 0.01). Upper-airway protective reflexes across a range of negative pressures as measured by electromyography and the early P1 component of the RREP are preserved in OSA patients during wakefulness. Consistent with an adaptive training effect, tongue protrusion force is increased, not decreased, in untreated OSA patients. However, OSA patients may be vulnerable to fatigue of upper-airway dilator muscles, which could contribute to disease progression.
Keywords: sleep-disordered breathing, genioglossus, tensor palatini, respiratory-related evoked potential, lung
obstructive sleep apnea (OSA) is a common disorder characterized by recurrent collapse of the pharyngeal airway during sleep, yielding major adverse outcomes (50, 57, 58). The pathophysiological causes of OSA likely vary considerably between individuals (54, 55). However, inadequate upper-airway dilator muscle function during sleep plus an anatomically collapsible upper airway are hallmark characteristic traits.
The potential role of upper-airway neuropathy (impaired sensory or motor nerve function) or myopathy (muscle weakness or wasting) in OSA pathogenesis/disease progression remains uncertain (22, 30). Inadequate upper-airway sensory responsiveness or motor nerve function may lead to insufficient reflex-modulated motor output or muscle weakness and contribute to airway collapse. Furthermore, damage as a result of the mechanical/vibration trauma and/or hypoxemia associated with repetitive upper-airway collapse may further impair sensorimotor function and contribute to disease progression (35, 41). Indeed, while most of the previous studies have utilized a single neurophysiological technique in isolation, upper-airway sensory or motor function has been measured in several studies during wakefulness using a variety of approaches (1, 3, 5, 24, 26, 27, 31, 33, 37, 40, 44, 45, 48). The findings, however, have been quite disparate with many showing impairment (e.g., 3, 24, 27, 31, 33, 40, 44), others no change (e.g., 1, 26, 37), and some even increased sensorimotor responsiveness and greater potential for upper airway muscle force generation in OSA (e.g., 5, 36, 48). Recent oropharyngeal muscle exercise studies have also shown improvement in OSA (28, 42), implying a potential role for muscle weakness/dysfunction in OSA pathogenesis. Subjective sensation is also challenging to assess rigorously, particularly in studies that are difficult to blind. Finally, it remains uncertain if the sensorimotor abnormalities observed using sensitive neurophysiological techniques reported in some of the previous studies also cause impairment of important upper-airway protective reflexes to negative pressure and muscle force characteristics.
Therefore, the aim of this study was to compare sensory processing properties, functionally important upper-airway protective reflexes across a range of negative pressures, and tongue protrusion force and task failure characteristics between untreated OSA patients and healthy controls to gain physiological insight into the potential role of upper-airway sensory and motor dysfunction in OSA pathogenesis/disease progression. We hypothesized that respiratory sensory processing characteristics, upper-airway negative pressure reflexes, and tongue protrusion force and time to task failure characteristics would be impaired in the OSA patients compared with controls.
MATERIALS AND METHODS
Subjects
A total of 28 men and women not taking any medications and without known relevant comorbidities gave written informed consent to participate in our Institutional Review Board-approved study. Healthy control subjects without a history of snoring were recruited from the community. Patients were recruited following a recent diagnosis (or while undergoing evaluation) of OSA [apnea/hypopnea index (AHI) > 10/h sleep], prior to treatment.
Protocol
Subjects arrived at the laboratory at approximately 6 PM having abstained from alcohol and caffeine for at least 24 h. On arrival informed consent was obtained, a medical screen was performed by a physician, and the subject was instrumented with the recording equipment. As described (Fig. 1), measurements were performed during wakefulness before and after an 8-h polysomnogram. Briefly, upper-airway dilator muscle reflexes and respiratory-related evoked potential (RREP) waveforms were assessed in response to brief pulses of negative upper-airway pressure. Following an acclimatization period, entrained iron lung ventilation was performed to examine the response of the genioglossus muscle to negative upper-airway pressure. An 8-h sleep opportunity was provided and polysomnography was performed to document the presence or absence of sleep-disordered breathing. The entrained iron lung ventilation procedure was repeated to explore potential differences in genioglossus muscle responsiveness in the evening compared with the morning (as a result of the overnight sleep apnea). Finally, maximal tongue protrusion force and time to task failure during a repetitive isometric contraction task were assessed.
Fig. 1.
Schematic of the timeline for the experimental protocol. Pulses: brief pulses of negative upper-airway pressure were delivered to elicit upper airway reflex responses and respiratory-related evoked potential waveforms. Iron Lung: entrained iron lung ventilation was performed to examine the response of the genioglossus muscle to negative upper-airway pressure and any potential differences in responsiveness in the evening (PM) compared with the morning (AM). 8 h standard PSG: polysomnography was performed to document the presence or absence of sleep-disordered breathing (the epiglottic pressure catheter was removed). Force/Task Failure: maximal tongue protrusion force and time to task failure protocols were performed. Refer to the text for further details.
Measurements and Equipment
Upper-airway negative-pressure pulse protocol to measure upper-airway dilator muscle reflexes and RREPs.
Both nostrils were decongested (0.05% oxymetazoline HCl) and the more patent nostril was anesthetized (4% lidocaine HCl) for insertion of an epiglottic pressure catheter (model MCP-500, Millar, Houston, TX) to monitor pharyngeal stimulus magnitude during negative-pressure pulse delivery. The catheter was advanced 1–2 cm below the base of the tongue under direct visualization to measure pressure at the level of the epiglottis. Two Teflon-coated stainless steel fine-wire intramuscular electrodes (AS 765–36 OD 71 μm; Cooner Wire, Chatsworth, CA) with 2 mm removed from the tip were inserted via a 25-gauge needle 3–4 mm on either side of the frenulum to a depth of ∼1.5 cm following surface anesthesia (4% lidocaine HCl) to create a bipolar electromyographic (EMG) recording of genioglossus muscle activity. Two Teflon-coated fine-wire intramuscular electrodes were also inserted at a 45° angle along the lateral surface of the medial pterygoid plate, to a depth of approximately 10–15 mm into the palate to create a bipolar EMG recording of tensor palatini muscle activity (23). Each subject was fitted with a nasal mask (Gel Mask, Respironics, Murrysville, PA) with pneumotachograph (model 3700A, Hans Rudolf, Kansas City, MO) and two differential pressure transducers (Validyne, Northbridge, CA) for measurement of airflow and mask pressure.
EEG electrodes were placed over the scalp at Cz and Pz and referenced to M1-M2 to measure the early and late RREP waveform components in response to standardized brief pulses of negative airway pressure according to previously described methods (13, 18, 19, 32). Briefly, the upper-airway negative-pressure pulses that were used to elicit EMG reflex responses (Fig. 2) and RREP waveforms (Fig. 3) were calibrated to target approximately −15 cmH2O at the mask, were 250 ms in duration, and were delivered during early inspiration (∼250–300 ms after the onset of inspiration) every 4–8 breaths via a computer-controlled rapid actuating solenoid valve system (19–21, 23). The RREP provides insight into the afferent transmission and cortical processing of respiratory afferent information to a variety of respiratory stimuli (13, 18, 32). The first positive deflection (P1) signifies the arrival of the respiratory afferent information to the somatosensory cortex, and changes in the amplitude and latency of the P1 component may be indicative of sensory impairment prior to its arrival at the cortex (13, 16, 18, 32). The subsequent RREP components are believed to reflect respiratory central neural gating (N1) (11), sensory processing (N2 and P2) (7, 12), and cognitive and perceptual sensory processing (P3) (53). The RREP waveform and its various positive and negative components are displayed in Fig. 3. The genioglossus negative pressure reflex technique has been shown to be quite reproducible with an intraclass coefficient of 0.77 (36). Similarly, based on the results of a recent publication using a very similar approach to derive the RREP (19), when the first and second half of a baseline recoding period was compared, the average intraclass coefficient for the latency and amplitude of the various RREP components was high at 0.91 and 0.66, respectively.
Fig. 2.
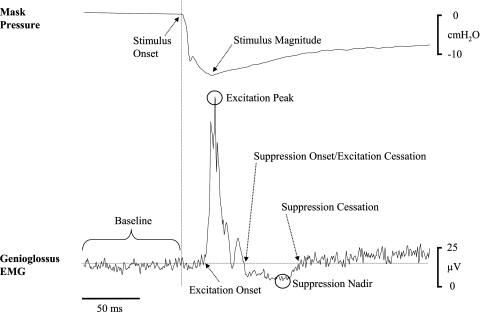
Example of the ensemble-average of the rectified raw genioglossus EMG and the corresponding mask pressure in one individual subject. The key components used to quantify reflex responses and stimulus characteristics are highlighted, including stimulus onset (vertical line), stimulus magnitude (nadir pressure), baseline genioglossus EMG (horizontal line), reflex excitation onset, excitation peak, suppression onset/excitation cessation, suppression nadir, and suppression cessation. Refer to the text for further details.
Fig. 3.
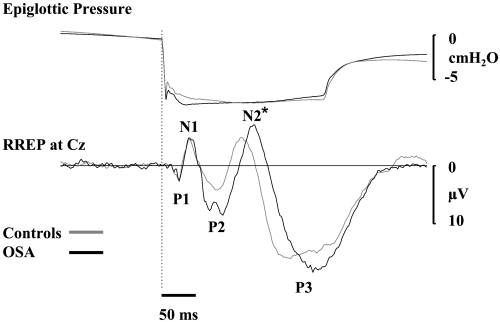
Respiratory-related evoked potential (RREP) group average waveforms at Cz and stimulus characteristics (epiglottic pressure). *Significant difference in the latency of this RREP waveform component between groups (P < 0.01).
Reflex and RREP data were not acquired until after the topical anesthetic agents had worn off, at least 90 min after their administration. EEG data were acquired using a commercially available system at a sampling rate of 500 Hz with a band-pass filter 0.3–120 Hz (Nihon Kohden, Tokyo, Japan). Analog signals (including genioglossus and tensor palatini EMGs) were also simultaneously acquired on a 1401 plus interface and Spike2 software to enable data analysis (Cambridge Electronic Design, Cambridge, UK). EMGs were sampled at 2 kHz with a band pass filter of 30–1,000 Hz.
Entrained iron lung ventilation to measure breath-to-breath local reflex mechanoreceptor input on genioglossus muscle activity.
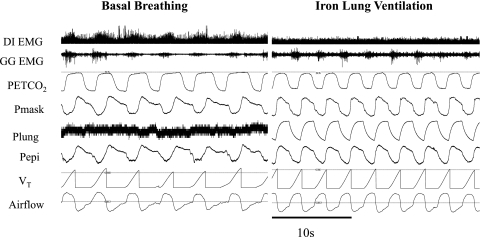
The relationship between negative epiglottic pressure to peak genioglossus EMG amplitude was measured during at least 5 min of entrained iron lung ventilation using established methods (2, 34) (Fig. 3). End-tidal CO2 was monitored at the nares and isocapnia was maintained throughout via controlled rebreathing as required. Surface EMG recordings were applied overlying the lateral chest wall muscles as described previously (8, 14) to assess respiratory drive (Fig. 4; diaphragm EMG).
Fig. 4.
Tracings of the parameters that were measured during basal breathing and entrained iron lung ventilation in one individual subject. DI EMG, surface rectified electromyogram overlaying the chest wall (diaphragm); GG EMG, raw intramuscular genioglossus muscle activity; PetCO2, end-tidal CO2 measured at the nares; Pmask, pressure at the mask; Plung, pressure inside the iron lung; Pepi, pressure at the level of the epiglottis; VT, tidal volume; Airflow, flow measured via nasal mask and pneumotachograph.
Polysomnography (PSG) to monitor sleep and quantify sleep-disordered breathing.
Electroencephalograms, electrooculograms, surface submentalis and tibialis electromyograms, a finger pulse oximeter, chest and abdominal belts and a thermister plus a nasal pressure probe were applied.
Maximal tongue protrusion force and time to task failure.
Maximal voluntary tongue protrusion force was assessed via force transducer and time to task failure (fatigability) via a repeated isometric contraction protocol (5 s on at 70% maximal force and 5 s off until task failure) using established techniques (6, 17, 37, 46, 47). Briefly, subjects anchored their teeth in a set position on the force transducer and lingual force was measured by having the subject protrude their tongue anteriorly against the force transducer (for a photograph of a similar transducer see figure 1B in Ref. 6). Subjects were given the following instructions immediately prior to testing: “We are going to perform two tests. One measures the maximal force of your tongue, the other measures the fatigability of your tongue. It is very important that you try as hard as you can during these tests and be aware that your tongue and possibly your jaw may get tired but that this is normal and will not last for long.” Verbal encouragement and clear visual feedback of performance was provided throughout the testing.
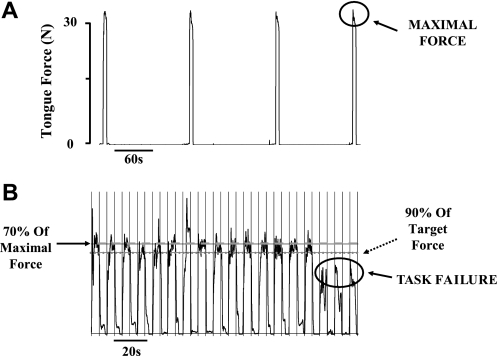
Maximal voluntary tongue protrusion force was derived as the largest value obtained during at least four coached attempts separated by ≥2 min intervals (Fig. 5A). Time to task failure during the repetitive isometric contraction task was defined as three consecutive attempts in which >90% of the target force could not be maintained for at least half of the required contraction time (i.e., 2.5 s) (Fig. 5B). Using similar techniques, both the maximal tongue protrusion force and time to task failure during sustained isometric tongue protrusion tasks have previously been shown to be highly reproducible with coefficient of variation values ranging from 3 to 11% (39).
Fig. 5.
A: representative example during the maximal tongue protrusion force task. In this case, the maximum force achieved by the subject was ∼35 N. B: an example of the repetitive isometric contraction task that was used to examine time to task failure and the criteria used to define task failure. In this instance, time to task failure occurred after ∼2.5 min.
Data Analysis and Statistical Procedures
Sleep stages and respiratory events were scored using standard criteria (4, 43). The latency and amplitude of upper-airway reflex and RREP waveform components to negative-pressure stimuli were quantified using previously described criteria (18–21, 23). Briefly, the point in mask pressure at which the rate of change in pressure was most negative during pulse presentation was identified to align each individual pulse to an accurately identifiable and highly reproducible reference point for EMG and RREP event-related analyses. Negative-pressure pulses were excluded from analysis if 1) delivered during, or in the breath following, a sigh or a swallow; 2) delivered in a 30-s epoch immediately following any intrusion of sleep (as determined by EEG); 3) there was loss of signal integrity. Raw genioglossus and tensor palatini EMG recordings were full-wave rectified and ensemble-averaged to derive reflex responses for each subject. EEG signals were ensemble-averaged to derive Cz and Pz RREP waveforms. Negative-pressure pulse stimulus magnitude was calculated for each subject as the nadir pressure observed in the ensemble-averaged waveform in the mask and epiglottic pressure channels. Stimulus onset (time zero) was defined in the conventional manner as the last point preceding the sudden decrement in the ensemble-averaged mask pressure following solenoid activation.
Using custom designed semiautomated software, individual subject's ensemble-averaged responses were visually inspected to measure the presence, timing, and amplitude of each positive and negative component of the EMG and RREP response, respectively, as previously described (13, 18–21, 23, 32, 51). EMG reflex amplitude data were expressed as a percentage of the baseline average EMG activity for the 100 ms preceding pulse onset. Excitation onset was defined as the point at which the rectified EMG signal crossed baseline before the first sustained (lasting >5 ms) positive EMG peak. Where present, suppression onset was defined as the first point at which the rectified EMG recording crossed the baseline level for a sustained period of >5 ms following the peak of the excitation response. Suppression cessation was defined as the first point at which the rectified EMG returned to baseline levels following the suppression nadir (Fig. 2).
The slope of negative epiglottic pressure vs. peak genioglossus activity during entrained iron lung ventilation was quantified as previously described (2, 34). Briefly, each breath during entrained iron lung ventilation was visually inspected to ensure entrainment (as assessed via flattening of the surface EMG response overlying the diaphragm and abolition of preinspiratory genioglossus EMG activity; Fig. 4). Each entrained breath was then ensemble-averaged from the start of inspiration for each subject, and the slope and intercept for the relationship between peak genioglossus EMG activity (rectified with a 100 ms moving-time average) and epiglottic pressure during inspiration were quantified.
ANOVA for repeated measures was used to examine time of day (evening vs. morning), group (OSA vs. control) and time of day × group interaction effects for the slope of the peak genioglossus EMG activity vs. epiglottic pressure during entrained iron lung ventilation (SPSS, Chicago, IL). Remaining comparisons between OSA patients and controls were performed using unpaired Student's t-tests. Statistical significance was defined as P < 0.05. Data are reported as means ± SE.
RESULTS
Baseline Anthropometric and Sleep Characteristics
Of the 28 subjects recruited, no data were obtained in 3 subjects. One subject signed the consent from but was excluded from participating in the study as it was revealed during the physician screening that the patient had mild diabetes. One subject experienced discomfort during the fine-wire electrode insertion procedure and as a result the wires were removed and the experiment was terminated. An additional subject experienced feelings of claustrophobia while breathing through the nasal mask and therefore the study was terminated.
The mean age and the body mass index (BMI) for the 13 controls (6 men) and 12 OSA patients (8 men) that participated in study were 43 ± 3 vs. 48 ± 2 yr (P = 0.07) and 26 ± 1 vs. 32 ± 1 kg/m2 (P < 0.01), respectively. Baseline sleep characteristics of the participants are displayed in Table 1.
Table 1.
Group polysomnography sleep parameters
| Controls (n =13; 6 males) | OSA Patients (n =12; 8 males) | |
|---|---|---|
| AHI. no. of events/h sleep | 4 ± 1 | 36 ± 6* |
| Arousal index, no. of arousals/h sleep | 13 ± 1 | 35 ± 6* |
| Minimum O2 saturation, % | 89 ± 1 | 71 ± 4* |
| Total sleep time, min | 427 ± 31 | 409 ± 11 |
| Sleep efficiency, % total sleep time | 89 ± 2 | 86 ± 3 |
| Stage 1 sleep, % total sleep time | 12 ± 2 | 24 ± 5* |
| Stage 2 sleep, % total sleep time | 61 ± 3 | 57 ± 3 |
| Slow wave sleep, % total sleep time | 8 ± 2 | 7 ± 3 |
| REM sleep, % total sleep time | 19 ± 1 | 12 ± 1* |
Values are means ± SE OSA, obstructive sleep apnea; AHI, apnea/hypopnea index, REM, rapid eye movement.
Significant difference compared with controls.
Upper-Airway Dilator Muscle Reflex Responses to Negative-Pressure Pulse Stimuli
Negative-pressure pulse stimuli produced a short-latency excitatory reflex peak in both the genioglossus and tensor palatini muscles in all participants. A secondary reflex suppression phase below baseline was present in 16 of the 25 participants in genioglossus (Fig. 2). Conversely, a very brief secondary suppression phase was present in only 3 of the 25 participants in tensor palatini. A detailed description of these reflex characteristics involving some of the control subjects has recently been reported (23).
EMG peak reflex amplitudes, latency characteristics, the number of subjects in whom reflex suppression was present, stimulus properties, and the number of artifact-free negative-pressure pulse stimuli used to derive ensemble-averaged reflex responses in the controls and OSA patients are summarized in Table 2. Reflex amplitude and latency components to negative pressure pulse stimuli were not different between controls and OSA patients (Table 2).
Table 2.
Group genioglossus and tensor palatini reflex and stimulus characteristics
| Reflex Characteristics |
||||
|---|---|---|---|---|
| Genioglossus |
Tensor palatini |
|||
| Controls | OSA | Controls | OSA | |
| Excitation phase | ||||
| Onset latency, ms | 19 ± 2 | 20 ± 1 | 8 ± 2 | 11 ± 3 |
| Peak amplitude, % baseline EMG | 317 ± 51 | 473 ± 108 | 305 ± 26 | 289 ± 41 |
| Peak latency, ms | 30 ± 4 | 34 ± 2 | 29 ± 5 | 24 ± 4 |
| Duration, ms | 28 ± 3 | 35 ± 5 | N/Aa | 14 ± 2a |
| Suppression phase | ||||
| No. of subjects in whom suppression occurred | 9/13 | 7/12 | 0/13 | 3/12 |
| Onset latency, ms | 44 ± 4 | 55 ± 4 | 19 ± 1 | |
| Nadir amplitude, % baseline EMG | 64 ± 5 | 60 ± 7 | 77 ± 5 | |
| Nadir latency, ms | 57 ± 7 | 62 ± 5 | 24 ± 2 | |
| Duration, ms | 25 ± 4 | 28 ± 9 | 11 ± 4 | |
| Stimulus Properties | ||||
| Controls | OSA | |||
| Nadir Pmask, cmH2O | −15.8 ± 0.7 | −16.5 ± 0.8 | ||
| Nadir Pepi, cmH2O | −9.2 ± 0.5 | −9.0 ± 0.6 | ||
| No. of artifact-free pulse presentations | 75 ± 5 | 81 ± 4 | ||
Values are means ± SE. There were no significant differences in reflex amplitude or latency components between groups for either muscle.
Given that excitation duration was defined as the time from excitation onset to suppression onset, excitation duration could not be quantified for tensor palatini in the control group due to the absence of a suppression phase. Note also that tensor palatini excitation duration data for the OSA group are derived from the 3 patients in which a brief suppression phase was observed. Refer to the text for further details.
RREPs to Negative-Pressure Pulse Stimuli
Negative-pressure pulse stimulus profile and magnitude at the level of the mask and epiglottis were similar between controls and OSA patients (Table 2, Fig. 3). The latency and amplitude of the P1 component of the RREP at Cz and Pz were not different between controls and OSA patients (Table 3, Fig. 3). Similarly, the latency and amplitude for the majority of the latter RREP waveform components (N1, P2, and P3) were not different between controls and OSA patients. However, N2 latency was delayed in the OSA patients at Cz and Pz (Table 3, Fig. 3).
Table 3.
Respiratory-related evoked potential waveform components mean latency and amplitude data
| RREP Latencies at Cz, ms |
RREP Latencies at Pz, ms |
|||
|---|---|---|---|---|
| Controls | OSA | Controls | OSA | |
| P1 | 25 ± 1 | 25 ± 2 | 28 ± 2 | 29 ± 2 |
| N1 | 46 ± 4 | 44 ± 2 | 46 ± 4 | 44 ± 2 |
| P2 | 82 ± 5 | 88 ± 4 | 81 ± 4 | 89 ± 6 |
| N2 | 121 ± 3 | 138 ± 3* | 123 ± 3 | 140 ± 3* |
| P3 | 218 ± 15 | 225 ± 7 | 221 ± 16 | 230 ± 9 |
| RREP Amplitudes at Cz, μV |
RREP Amplitudes at Pz, μV |
|||
|---|---|---|---|---|
| Controls | OSA | Controls | OSA | |
| P1 | 4 ± 1 | 4 ± 1 | 5 ± 1 | 4 ± 1 |
| N1 | −9 ± 2 | −8 ± 1 | −4 ± 1 | −5 ± 1 |
| P2 | 10 ± 2 | 13 ± 2 | 11 ± 2 | 10 ± 1 |
| N2 | −9 ± 2 | −8 ± 3 | −6 ± 2 | −6 ± 2 |
| P3 | 21 ± 2 | 23 ± 3 | 17 ± 1 | 20 ± 2 |
Values are means ± SE.
Significant difference in the respiratory-related evoked potential (RREP) waveform component in the OSA patients compared with controls.
Entrained Iron Lung Ventilation Before and After an 8-h Sleep Opportunity
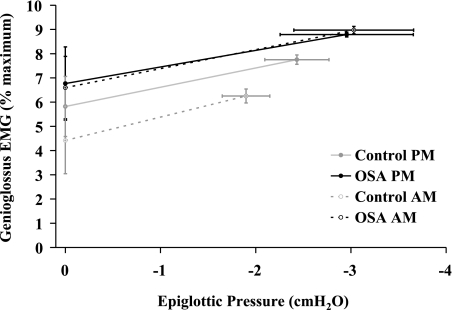
A robust relationship between peak genioglossus EMG activity and epiglottic pressure during entrained iron lung ventilation was observed in all participants (r2 = 0.94 ± 0.01, range 0.67–0.99). The slope of this relationship was not different between controls and OSA patients, or in the evening compared with the morning, and there were no group × time interaction effects (Fig. 6). Similarly, the y-intercept (i.e., tonic genioglossus EMG activity) was not different between controls and OSA patients, or in the evening vs. the morning (Fig. 6). However, the y-intercept tended to be lower in the morning vs. the evening in the controls (P = 0.07, Fig. 6) but not in the OSA patients (P = 0.83, Fig. 6).
Fig. 6.
Relationship between genioglossus activity and epiglottic pressure during entrained iron lung ventilation before and after an 8-h sleep opportunity. The slope of peak genioglossus activity vs. negative epiglottic pressure during entrained iron lung ventilation was not different between groups (patients vs. controls) or in the evening vs. the morning. Similarly, there were no statistically significant differences in the y-intercept of this relationship although there was a tendency for a decrease in the morning compared with the evening in the controls but not in the OSA patients. Refer to the text for further details.
Maximal Tongue Protrusion Force and Fatigability Task
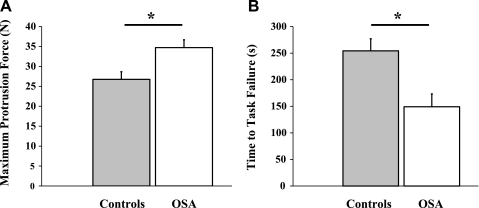
Maximum tongue protrusion force during the final four attempts in each subject was highly reproducible with an average coefficient of variation of 6.3%. Tongue protrusion force was significantly greater in the OSA patients compared with the controls (Fig. 7A, P < 0.01). Similarly, the absolute peak genioglossus EMG activity during the maximal tongue protrusion maneuver was greater in OSA patients vs. controls (568 ± 65 vs. 361 ± 48 μV, P = 0.02). Maximal tongue protrusion force did not differ between males and females (31 ± 2 vs. 29 ± 3 N, P = 0.52). Time to task failure during the repetitive isometric contraction task occurred earlier in the OSA patients compared with the controls (Fig. 7B, P < 0.01). The average peak genioglossus EMG activity (as a percent of maximum) was similar between OSA patients and controls during the 5-s contractions (72 ± 3 vs. 74 ± 3% maximum, P = 0.82). Similarly, the average force (as a percent of maximum) achieved during the 5-s contraction task prior to task failure was not different in OSA patients vs. controls (62 ± 1 vs. 63 ± 1% maximum, P = 0.32). Given that tongue protrusion force was higher in OSA patients than controls, we performed a subanalysis in 5 controls and 5 OSA patients in whom maximal tongue protrusion force was similar (31 ± 2 vs. 29 ± 2, P = 0.37). Time to task failure remained significantly earlier in the OSA patients compared with the controls (279 ± 17 vs. 138 ± 34 s, P < 0.01).
Fig. 7.
Maximal voluntary tongue protrusion force (A) and time to task failure (B). *Significant difference between groups (P < 0.01).
DISCUSSION
The main findings of this study are that during wakefulness, OSA patients are able to generate greater tongue protrusion force, but time to task failure during a repetitive isometric contraction task occurs ∼50% earlier compared with non-OSA controls. Thus, while untreated OSA patients are capable of generating large amounts of tongue protrusion force, they may have greater propensity for upper-airway muscle fatigue, which could contribute to OSA disease progression. Conversely, genioglossus and tensor palatini negative-pressure reflexes, the early P1 component of the RREP, and the relationship between genioglossus activity and negative epiglottic pressure during entrained iron lung ventilation are not different between OSA patients and controls, indicating preservation of these important protective responses during wakefulness. However, consistent with abnormal cortical sensory processing to respiratory stimuli, the N2 component of the RREP is delayed in untreated OSA patients compared with controls.
Measures of Sensorimotor Function in OSA
Upper-airway negative-pressure reflexes.
Only two studies have examined upper-airway muscle reflex responses to negative pressure stimuli between OSA patients and controls (5, 38). Mortimore and Douglas (38) demonstrated reduced palatal muscle EMG activation in response to brief negative-pressure pulses in patients with OSA. Conversely, Berry and colleagues (5) observed quantitatively similar EMG activation of the genioglossus muscle at mask pressures of approximately −20 cmH2O, and increased activation at pressures <−15 cmH2O between OSA patients and controls. However, the use of EMG smoothing techniques that were employed in these earlier studies tends to distort short latency reflex components (21). In addition, the relatively wide averaging windows as adopted in the Mortimore and Douglas study can introduce a significant voluntary component to the observed EMG response making it difficult to draw firm conclusions regarding reflex sensorimotor integrity. We have recently examined the genioglossus negative pressure reflex without the use of EMG smoothing techniques and revealed the presence of a short latency excitation phase followed by a state-dependent inhibition (21). Employing similar techniques in the current study, we observed no difference in the latency or amplitude of the short latency excitation or suppression phase of the genioglossus negative pressure reflex between patients and controls. Similarly, the excitation phase of the tensor palatini reflex response was not different between patients and controls indicating that reflex function of these two important protective upper-airway dilator muscles is preserved in untreated OSA patients during wakefulness.
RREP waveform components.
While one study revealed reductions in early RREP activity (indicative of sensory afferent transmission) in OSA patients (3), and others reported impairment of later RREP components (15) (reflective of cortical sensory processing of respiratory afferent information), particularly during sleep (1, 26), the majority of RREP studies, including the present study, do not show any change in the latency or amplitude of the P1 component of the RREP (1, 15, 26). Thus the majority of data suggest that the sensory pathways that respond to transient respiratory load are functionally intact in patients with OSA during wakefulness. However, consistent with a recent report (15), cortical sensory processing (N2 latency) of respiratory stimuli is delayed in OSA patients compared with controls. Unlike the early P1 and late P3 components(13, 16, 18, 32, 52, 53), little is known regarding the functional significance of the N2 component of the RREP but it is believed to be a marker of sensory processing. Thus, functional interpretation of a delay in N2 latency is uncertain but may reflect cognitive impairment due to hypoxia or sleep fragmentation/deprivation and if also present during sleep may result in altered arousal responses to respiratory stimuli.
Genioglossus muscles responsiveness during entrained iron lung ventilation.
Entrained iron lung ventilation is a useful tool to examine negative-pressure reflex activation of the genioglossus muscle during relatively small excursions of pharyngeal pressure associated with tidal breathing (greater than −5cmH2O) (34). Consistent with an earlier report, the slope of the relationship between genioglossus muscle activity and pharyngeal pressure was not different between OSA patients and controls. These findings suggest that reflex responsiveness of the genioglossus muscle to relatively small levels of negative airway pressure as measured by electromyography is functionally intact in OSA patients during wakefulness. Furthermore, if the proposed deleterious effects of OSA (repetitive airway closure, hypoxia, etc.) acutely impair upper-airway reflex function, then it would be predicted that muscle responsiveness would be most impaired immediately following a night of repetitive apnea with the potential for some recovery throughout the day. However, this finding was not observed in the present study. The slope and y-intercept of the relationship between epiglottic pressure and genioglossus activity (indicative of tonic genioglossus activity when preactivation is absent) during entrained iron lung ventilation was not different in the evening vs. the morning in the OSA patients or controls. There was, however, a tendency toward a reduced y-intercept in the morning vs. evening in the controls that was not apparent in the OSA patients. While the reasons for this potential difference are uncertain, should this finding be reproducible, it may be the result of a circadian phenomenon whereby tonic upper-airway dilator muscle activity is reduced in the morning vs. the evening, an effect that may have been masked in the OSA patients due to their compromised anatomy and continual reliance on upper-airway dilator muscle activity to maintain airway patency. Consistent with our experience, and a neurocompensatory response (36), the OSA patients tended to produce more pronounced negative pharyngeal pressures and muscle activation during entrained iron lung ventilation compared with controls. Additional studies are required to address these issues definitively. However, these data indicate that reflex responsiveness of the genioglossus muscle as measured by electromyography is preserved during small levels of negative upper-airway pressure during wakefulness.
Tongue force/time to task failure.
Upper-airway muscle weakness or insufficient contractile force in individuals with vulnerable upper-airway anatomy is likely to lead to greater vulnerability to airway closure. Indeed, one study revealed a weak but statistically significant negative correlation (r2 = 0.04) between maximal tongue protrusion force and the AHI (37). However, in the same study, maximum tongue protrusion force did not differ between nonsnoring controls and patients with mild-moderate OSA (37). In an earlier study (36), maximal lingual force was not statistically different in a group of 4 controls vs. 4 patients with very severe OSA, although maximal force did tend to be greater in the OSA patients (30 vs. 38 N). The present study demonstrated significantly increased tongue protrusion force in moderately severe OSA patients compared with non-OSA controls. Similar measurement techniques were employed and comparable absolute levels of force were obtained compared with the prior studies (36, 37). Thus the reason for the apparent discrepancy is unclear but may relate to subtle differences in patient characteristics. Consistent with the finding of increased upper-airway dilator muscle force in OSA, Series and colleagues (48) demonstrated increased capacity for tension production (e.g., greater maximum tetanic tensions and muscle cross-sectional area) from musculus uvulae biopsy samples compared with snorers. While maximal twitch tension did not differ between groups, Carrera et al. (9), examined biopsy samples from genioglossus and showed an increased proportion of fast twitch fibers (Type II) in a group of untreated OSA patients compared with healthy controls and a group of OSA patients who had received CPAP therapy for at least a year. Together, these findings suggest that the upper-airway muscles of untreated OSA patients are highly trained and have increased force production capacity. While stronger, not weaker, upper-airway muscles in OSA indicate a lack of myopathy per se (as defined as muscle weakness or wasting), the apparent training effect could conceivably lead to muscle hypertrophy which, in an already crowded upper airway, might be predicted to worsen pharyngeal mechanics. Thus these changes may have disparate effects with the evolution of disease whereby force output is initially increased in response to repetitive airway closure and may yield benefit in pharyngeal mechanics but over time the accompanying hypertrophy may have the opposite effect. Furthermore, assuming the increased tongue protrusion force observed in the present study is associated with a shift towards a greater proportion of genioglossus type II fibers, untreated OSA patients may be at greater risk of developing upper-airway dilator muscle fatigue, which could further contribute to disease progression.
Indeed, as evidenced by decreased time to task failure in the present study, even when matched for absolute force, and consistent with an in vitro genioglossus functional study (9), patients with untreated OSA appear to be more vulnerable to genioglossus muscle fatigue. The findings from the in vitro genioglossus study suggest that this observation may be a secondary phenomenon rather than the cause of OSA given that the patients who were treated with OSA had a similar time course of reduced force production to repetitive stimulation compared with controls (9). However, prospective follow-up studies are required to confirm this finding. Conversely, Series et al. (49) did not find differences in genioglossus enzymes or Type I fibers between OSA patients and snorers. Indeed, the proportion of Type IIA and IIB fibers was larger and smaller, respectively, in OSA patients, implying that the genioglossus may be more fatigue resistant (49). Time to fatigue during a 50% maximum sustained isometric tongue protrusion task was also not different between OSA patients and nonsnoring controls in a previous study (37), an apparent discrepancy that may relate to differences in the tasks employed (sustained vs. repetitive, 50% vs. 70% maximum effort). We would argue that a repetitive task may have more functional relevance to OSA, although more work is clearly needed. What does appear to be consistent across studies, although not directly measured in the present study, is that the upper-airway muscles have a high proportion of anaerobic muscle fibers (type Type IIA and IIB) compared with other skeletal muscles (46, 48, 49). Furthermore, while upper-airway dilator muscle activity is reduced at sleep onset (56), considerable levels of muscle activation do occur during respiratory events and periods of spontaneous breathing stability during sleep in patients with OSA, well beyond the levels obtained during spontaneous breathing in wakefulness (29). Thus this situation may place the upper-airway dilator muscles of untreated OSA patients vulnerable to fatigue during sleep. There is also evidence to suggest that hypoxia may lead to greater vulnerability to upper-airway muscle fatigue (25).
Finally, the observed increased tongue protrusion strength in patients with OSA would suggest that the recent findings of reduced OSA severity with upper-airway training exercises (28, 42) are not likely to be mediated via improvements in muscle strength, although the duration of OSA may be an important confounding factor. It is also possible that these training techniques reduce the potential for fatigue and/or improve the functional mechanical efficiency of upper-airway muscles. However, this conjecture remains to be tested.
Methodological Considerations
Given the complexity of the multiple physiological measurements performed in this study the sample size was relatively small. However, between-group differences examining various components of the parameters measured in this study have been observed in other studies with similar or smaller sample sizes (5, 9, 18). Indeed, as recently reported in a study using very similar methodology (19), on the order of 10 subjects per group should have been sufficient to detect meaningful changes in RREP components with 80% power. Nonetheless, the lack of between-group differences observed for many parameters in this study may be due to insufficient statistical power. While this possibility cannot be discounted, should true differences be present they would appear to be small and may be of limited physiological importance. For example, our results suggest that to detect significant differences between controls and OSA patients with 80% power in genioglossus and tensor palatini reflex onset latency would require on the order of 1,000 and 200 subjects in each group, respectively (based on a true differences of 0.8 and 2.3 ms, and SDs of 7.8 and 4.7, and 7.8 and 8.8, respectively, as measured in this study). In addition, while the direction of change observed in the present study is toward increased, not decreased reflex excitation in untreated OSA patients, we estimate that ∼55 subjects in each group would be required to observe differences in peak genioglossus reflex amplitude between controls and OSA patients should they exist (based on a true difference of 156% baseline and SDs of 177 and 357, respectively). Finally, we estimate that to detect a difference in peak tensor palatini reflex amplitude of 16% baseline would require ∼700 subjects in each group to achieve 80% power based on the SDs of 90 for the controls and 122 for the OSA patients as observed in this study. Clearly, such physiological studies are not feasible.
Our goal in the present study was to provide insight into the function of important protective reflexes and sensorimotor characteristics during wakefulness in untreated OSA patients. However, given the observed differences in muscle force, time to task failure, and the N2 component of the RREP, the next logical step would be to perform similar comprehensive measurements during sleep, as well as before and after sleep, to determine the state dependence of these neuromuscular responses and their potential functional significance with respect to OSA. While RREP, iron lung, and reflex studies are feasible during sleep, muscle force and time to task failure assessments are not. In addition, measurement of time to task failure responses across a range of forces, assessments of length-tension relationships (particularly given that that the OSA patients likely have increased tongue volume), electrical twitch stimulation studies, and the addition of muscle biopsies would also likely yield additional important mechanistic insights. These measurements were beyond the scope of the present protocol. Thus, while the techniques utilized in the present study are indirect measures of sensorimotor function, and need to be interpreted in the context of the various other direct and indirect measures performed in previous studies, the present data provide important new insight and pave the way for multiple new lines of investigation.
While tests of maximum voluntary tongue protrusion force and time to task failure are effort dependent, considerable care was taken to minimize the potential confounding effects of variability in subject motivation in our experiments. This strategy included employing standardized instructions, following a standardized protocol with strong verbal encouragement by two investigators, and performing multiple measures (i.e., maximum force for which the coefficient of variation was very low at 6.3%). Nonetheless, as is the case with all measures of this type (e.g., spirometry, exercise testing), motivation remains a potential confounding factor for these parameters.
In addition, our cases and controls were not well matched for BMI, since obese individuals without at least some apnea are quite rare (particularly among men). Furthermore, although the age of the controls and cases were not statistically different, OSA patients tended to be slightly older than controls. While precise matching for BMI and age would be desirable, higher BMI and age in the OSA patients would be predicted to worsen sensorimotor function and lead to reduced, not increased muscle force characteristics. While one study did reveal differences in muscle endurance capacity in lean OSA patients, the obese patients were indistinguishable from healthy controls (10). Indeed, muscle physiology research indicates that the cross-sectional area of a muscle is the key determinate of muscle force capacity such that obesity per se should not play an influential role. Thus, in the context of testing the hypotheses in the present experiments, we believe that the degree of sleep-disordered breathing is the most important stimulus. However, we cannot separate obesity effects from OSA effects and our conclusions about sleep apnea cannot necessarily be generalized to lean OSA patients. Finally, the healthy controls and OSA patients were both middle-aged within 5 years of each other on average. Accordingly, we feel that this approach is appropriate to test our hypotheses. Nonetheless, it is acknowledged that closer matching for age between groups in future studies would be desirable.
Furthermore, while the control subjects did not have clinically defined sleep apnea as measured by the AHI, it remains possible that periods of undetected flow-limitation may have been present in some subjects which could have affected sensorimotor function and contributed to the lack of between group differences for the majority of the variables assessed. To minimize potential sleep disruption confounding effects and optimize conditions in which to test our primary hypotheses, subjects slept in as minimally invasive environment as possible. Thus we were unable to quantify accurately flow-limitation indexes in our present study. However, this possibility warrants further investigation in future carefully designed experiments.
Finally, patients with other comorbidities such as diabetes that may lead to sensorimotor damage were strictly excluded in the present study. Thus statements regarding the results of this study are limited to untreated obese OSA patients without other major comorbidities. Indeed, it will be interesting to examine protective reflexes and sensorimotor function carefully in patients with comorbidities given the high prevalence of conditions such as diabetes in patients with OSA.
Sensorimotor Function in OSA: Summary and Implications
Consistent with the disparate results observed in previous studies that utilized a single neurophysiological technique in isolation, the results of the present study utilizing a variety of neurophysiological tasks in the same subjects revealed similar contrasting findings with reductions in some of the measured components (e.g., increased time to task failure), preservation of other important protective responses (e.g., upper-airway negative-pressure reflexes across a range of pressures before and after sleep), and increased responsiveness (e.g., greater tongue protrusion force). Thus the potential functional role of impaired sensorimotor function in OSA pathogenesis/disease progression is clearly complex and requires additional careful investigation. What is clear from the present study is that the integrity of the sensorimotor pathways that underpin the upper-airway negative pressure reflex responses and early P1 component of the RREP are either 1) unchanged in untreated OSA patients or 2) sufficient neurocompensatory mechanisms are present to preserve their function during wakefulness. Thus these data suggest that other factors may be more influential in contributing to OSA pathogenesis than impairment of the primary sensorimotor pathways mediating these responses. For example, state-dependent reductions in neural output to upper-airway muscles, which occurs in all individuals during sleep, is likely to lead to airway closure in those with vulnerable anatomy. However, it remains possible that state-related impairment of upper-airway sensory function during sleep is more pronounced in OSA patients than controls, which could further contribute to the propensity for airway collapse. Clearly, further work is required to differentiate these various possibilities. However, the results of the present study reveal that important protective upper-airway reflexes across a wide range of negative pressures are functionally intact as measured via electromyography during wakefulness in obese OSA patients without major comorbidities.
Disease progression due to the mechanical trauma and/or hypoxemia associated with repetitive upper-airway collapse has been hypothesized to impair sensorimotor function. While the present study reveals that the muscles of the tongue appear to be highly capable of producing force in the short term, possibly due to structural changes in muscle fiber type, their ability to generate force over time was greatly reduced, which could contribute to disease progression. Furthermore, increased cross-sectional area (hypertrophy) that underpins increased maximal force generation within the limited confinement of the upper airway would also be predicted to reduce pharyngeal lumen size and contribute to disease progression. Finally, whether oropharyngeal muscle exercises improve the endurance capabilities or mechanical efficiency of these muscles remains unclear.
GRANTS
This study was supported by National Institutes of Health Grants HL-73146, R01-HL-085188-01A2, R01-HL-090897-01A2, K24-HL-093218-01A1, and 1P01-HL-095491-01A1. The Harvard Catalyst is funded by UL1-RR-025758-01. D. J. Eckert is supported by a National Health and Medical Research Council of Australia Biomedical (CJ Martin) Fellowship (510392) and the American Heart Association (10SDG3510018).
DISCLOSURES
D. J. Eckert and A. S. Jordan have received consulting income from Apnex Medical. Y. L. Lo and J. P. Saboisky have no conflicts to declare. D. P. White is Chief Medical officer for Philips Respironics. A. Malhotra has received consulting and/or research income from Philips, Medtronic, Apnicure, Apnex, Ethicon, Pfizer, Sepracor, Galleon, Merck, Cephalon, SGS, and SHC. While a number of the authors have some industry affiliations related to the treatment of sleep apnea, we do not see these as relevant to this original research investigation that was funded via peer review grant mechanisms. Nonetheless, these potential conflicts have been listed in this section of the manuscript.
AUTHOR CONTRIBUTIONS
D.J.E., Y.L.L., A.S.J., D.P.W., and A.M. conception and design of research; D.J.E., Y.L.L., J.P.S., and A.S.J. performed experiments; D.J.E. and A.S.J. analyzed data; D.J.E., J.P.S., A.S.J., D.P.W., and A.M. interpreted results of experiments; D.J.E. prepared figures; D.J.E. drafted manuscript; D.J.E., Y.L.L., J.P.S., A.S.J., D.P.W., and A.M. edited and revised manuscript; D.J.E., Y.L.L., J.P.S., A.S.J., D.P.W., and A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen Stevenson, Lauren Hess, Erik Smales, and Scott Smith for valuable technical support. All of the work contained within the manuscript was performed at the Brigham and Women's Hospital, Boston, MA.
Present address for Y. L. Lo: Dept. of Thoracic Medicine, Chang Gang Memorial Hospital, Chang Gang Univ. College of Medicine, Taipei, Taiwan.
Present address for A. S. Jordan: Sleep Laboratory, Psychological Sciences, The Univ. of Melbourne, Grattan St. Parkville, VIC 3010, Australia.
REFERENCES
- 1. Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol 136: 221–234, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol 531: 677–691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akay M, Leiter JC, Daubenspeck JA. Reduced respiratory-related evoked activity in subjects with obstructive sleep apnea syndrome. J Appl Physiol 94: 429–438, 2003 [DOI] [PubMed] [Google Scholar]
- 4. American Sleep Disorders Association EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 173–184, 1992 [PubMed] [Google Scholar]
- 5. Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol 94: 1875–1882, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bittner EA, Martyn JA, George E, Frontera WR, Eikermann M. Measurement of muscle strength in the intensive care unit. Crit Care Med 37: S321–S330, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Bloch-Salisbury E, Harver A, Squires NK. Event-related potentials to inspiratory flow-resistive loads in young adults: stimulus magnitude effects. Biol Psychol 49: 165–186, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Butler JE, McKenzie DK, Crawford MR, Gandevia SC. Role of airway receptors in the reflex responses of human inspiratory muscles to airway occlusion. J Physiol 487: 273–281, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med 159: 1960–1966, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Santos C, Agusti AG. Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. Eur Respir J 23: 425–429, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol 105: 1106–1113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol 115: 732–744, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Davenport PW, Friedman WA, Thompson FJ, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol 60: 1843–1848, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Demoule A, Verin E, Locher C, Derenne JP, Similowski T. Validation of surface recordings of the diaphragm response to transcranial magnetic stimulation in humans. J Appl Physiol 94: 453–461, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Donzel-Raynaud C, Redolfi S, Arnulf I, Similowski T, Straus C. Abnormal respiratory-related evoked potentials in untreated awake patients with severe obstructive sleep apnoea syndrome. Clin Physiol Funct Imaging 29: 10–17, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Donzel-Raynaud C, Straus C, Bezzi M, Redolfi S, Raux M, Zelter M, Derenne JP, Similowski T. Upper airway afferents are sufficient to evoke the early components of respiratory-related cortical potentials in humans. J Appl Physiol 97: 1874–1879, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol 94: 1849–1858, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Eckert DJ, Catcheside PG, McDonald R, Adams AM, Webster KE, Hlavac MC, McEvoy RD. Sustained hypoxia depresses sensory processing of respiratory resistive loads. Am J Respir Crit Care Med 172: 1047–1054, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Eckert DJ, Elgar NJ, McEvoy RD, Catcheside PG. Alcohol alters sensory processing to respiratory stimuli in healthy men and women during wakefulness. Sleep 33: 1389–1395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Effects of hypoxia on genioglossus and scalene reflex responses to brief pulses of negative upper-airway pressure during wakefulness and sleep in healthy men. J Appl Physiol 104: 1426–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol 581: 1193–1205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eckert DJ, Saboisky JP, Jordan AS, Malhotra A. Upper airway myopathy is not important in the pathophysiology of obstructive sleep apnea. J Clin Sleep Med 3: 570–573, 2007 [PMC free article] [PubMed] [Google Scholar]
- 23. Eckert DJ, Saboisky JP, Jordan AS, White DP, Malhotra A. A secondary reflex suppression phase is present in genioglossus but not tensor palatini in response to negative upper airway pressure. J Appl Physiol 108: 1619–1624, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorer's disease in an upper airway muscle. Am J Respir Crit Care Med 157: 586–593, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. J Appl Physiol 88: 2123–2130, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Gora J, Trinder J, Pierce R, Colrain IM. Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am J Respir Crit Care Med 166: 1225–1234, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Guilleminault C, Li K, Chen NH, Poyares D. Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest 122: 866–870, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med 179: 962–966, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32: 361–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kimoff RJ. Upper airway myopathy is important in the pathophysiology of obstructive sleep apnea. J Clin Sleep Med 3: 567–569, 2007 [PMC free article] [PubMed] [Google Scholar]
- 31. Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med 164: 250–255, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Knafelc M, Davenport PW. Relationship between resistive loads and P1 peak of respiratory-related evoked potential. J Appl Physiol 83: 918–926, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Larsson H, Carlsson-Nordlander B, Lindblad LE, Norbeck O, Svanborg E. Temperature thresholds in the oropharynx of patients with obstructive sleep apnea syndrome. Am Rev Respir Dis 146: 1246–1249, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 165: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Mayer P, Dematteis M, Pepin JL, Wuyam B, Veale D, Vila A, Levy P. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med 159: 213–219, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571–1579, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res 9: 389–393, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Mortimore IL, Douglas NJ. Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am J Respir Crit Care Med 156: 867–873, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatiguability in male and female subjects. Eur Respir J 14: 191–195, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 28: 585–593, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Petrof BJ, Hendricks JC, Pack AI. Does upper airway muscle injury trigger a vicious cycle in obstructive sleep apnea? A hypothesis. Sleep 19: 465–471, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Puhan MA, Suarez A, Lo Cascio C, Zahn A, Heitz M, Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ 332: 266–270, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute UCLA, 1968 [Google Scholar]
- 44. Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol 585: 135–146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saboisky JP, Stashuk DW, Hamilton-Wright A, SN, Carusona AL, Campana LM, Trinder JA, McSharry DG, David WS, Malhotra A. Automated decomposition based quantitative electromyography (dqemg) in the examination of genioglossus motor unit potentials. Am J Respir Crit Care Med 183: A3687, 2011 [Google Scholar]
- 46. Scardella AT, Krawciw N, Petrozzino JJ, Co MA, Santiago TV, Edelman NH. Strength and endurance characteristics of the normal human genioglossus. Am Rev Respir Dis 148: 179–184, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Schmitt K, DelloRusso C, Fregosi RF. Force-EMG changes during sustained contractions of a human upper airway muscle. J Neurophysiol 101: 558–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Series F, Cote C, Simoneau JA, Gelinas Y, St Pierre S, Leclerc J, Ferland R, Marc I. Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J Clin Invest 95: 20–25, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Series FJ, Simoneau SA, St Pierre S, Marc I. Characteristics of the genioglossus and musculus uvulae in sleep apnea hypopnea syndrome and in snorers. Am J Respir Crit Care Med 153: 1870–1874, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med 340: 847–851, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Webster KE, Colrain IM. Multichannel EEG analysis of respiratory evoked-potential components during wakefulness and NREM sleep. J Appl Physiol 85: 1727–1735, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Webster KE, Colrain IM. P3-specific amplitude reductions to respiratory and auditory stimuli in subjects with asthma. Am J Respir Crit Care Med 166: 47–52, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Webster KE, Colrain IM. The relationship between respiratory-related evoked potentials and the perception of inspiratory resistive loads. Psychophysiology 37: 831–841, 2000 [PubMed] [Google Scholar]
- 54. Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol 110: 1627–1637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 172: 1363–1370, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol 85: 908–920, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]